Abstract
PURPOSE
To determine whether horizontal angle diameter and sulcus diameter can be accurately estimated by conventional external measurements in high myopic eyes.
METHODS
Ten horizontal anterior segment scans were obtained with the Artemis 1 very high-frequency (VHF) digital ultrasound arc-scanner in 40 eyes of 20 patients. Angle and sulcus diameters were measured and descriptive statistics and within-eye repeatability were calculated. Linear regression was performed between each permutation of white-to-white, angle diameter, and sulcus diameter. Multivariate regression also included anterior chamber depth (ACD), age, manifest refraction, keratometry, and central corneal thickness (CCT). The standard deviation and 95% confidence interval (CI) of the residuals were calculated.
RESULTS
The population mean ±standard deviation (95% CI) was 12.88±0.42 mm [12.74, 13.02] for angle diameter and 12.85±0.69 mm [12.63, 13.07] for sulcus diameter. Within-eye repeatability was 0.13 mm for angle diameter and 0.23 mm for sulcus diameter. A weak correlation was noted between white-to-white and angle diameter (r2=0.59) with a 95% Cl of ±0.53 mm. Multivariate regression found white-to-white, CCT, and minimum keratometry predicted angle diameter (r2=0.69) with a 95% Cl of ±0.46 mm. For predicting sulcus diameter, there were weak correlations between white-to-white (r2=0.32) with a 95% Cl of ±1.11 mm and angle diameter (r2=0.46) with a 95% Cl of ±0.99 mm. Multivariate regression found angle diameter and ACD predicted sulcus diamter (r2=0.57) with 95% Cl of ±0.88 mm.
CONCLUSIONS
Regression modeling found weak correlations among all combinations of white-to-white, angle diameter, and sulcus diameter. Given the relative accuracy of direct measurement of angle and sulcus diameter compared to the potential accuracy of these regression equations, it appears that direct measurement would increase the safety of anterior and posterior chamber phakic IOL sizing.
Lens sizes for anterior chamber phakic intraocular lenses (IOL) have commonly been estimated by adding 0.50 to 1.00 mm to the horizontal white-to-white corneal diameter obtained externally.1 However, many postoperative complications associated with anterior chamber IOLs are due to poor lens sizing; an oversized lens can cause pupil ovalization2 and iritis,2,3 whereas an undersized lens can become mobile and result in endothelial cell loss2,4 and secondary glaucoma.2 Recently, a number of instruments capable of measuring the anterior segment horizontal angle-to-angle diameter (angle diameter) have been developed including the Orbscan II (Bausch & Lomb, Salt Lake City, Utah), Pentacam (Oculus, Wetzlar, Germany), and Visante Optical Coherence Tomography (OCT; Carl Zeiss Meditec, Jena, Germany). Goldsmith et al5 reported that using white-to-white to estimate angle diameter would result in an error of >0.50 mm in 22% of cases, which could explain many of the sizing-related complications observed. Goldsmith et al5 reported that using an OCT direct measurement of angle diameter would reduce this error to 0.02%.
Similar to anterior chamber phakic IOLs, lens sizes for posterior chamber phakic IOLs have commonly been estimated by adding 0.50 to 1.00 mm to the horizontal white-to-white corneal diameter obtained externally. 6,7 Posterior chamber phakic IOLs have recently been approved by the US Food and Drug Administration using a sizing criteria of adding 0.50 mm to the white-to-white in eyes where the anterior chamber depth (ACD) was 2.80 to 3.50 mm, and adding 1.00 mm to the white-to-white in eyes where the ACD was >3.50 mm.7 Similar to anterior chamber phakic IOLs, the common theme regarding postoperative complications of posterior chamber phakic IOL surgery is that of lens sizing—an oversized lens can cause angle closure leading to malignant glaucoma8,9 or the lens can chafe the iris leading to pigment dispersion10-12; an undersized lens can cause cataract12-15 or damage to the zonules with dislocation of the phakic IOL.12,16
The haptics of posterior chamber phakic IOLs may rest in the ciliary sulcus and the horizontal sulcus-to-sulcus diameter (sulcus diameter) has been traditionally used for determining lens sizing. However, published studies have not found any anatomical correspondence between white-to-white and sulcus diameter,17-20 suggesting that some lens sizing issues may be explained by the assumption that sulcus diameter can be estimated from white-to-white.
Optical instruments capable of directly measuring the angle diameter have improved lens sizing for anterior chamber phakic IOLs; however, optical instruments are not capable of directly measuring the sulcus diameter because the optical path is blocked by the pigment epithelium of the iris. It has been suggested that angle diameter might be used to estimate sulcus diameter more accurately.5,20
Using a direct measurement of the sulcus diameter would likely improve lens sizing for posterior chamber phakic IOLs. The sulcus diameter width can be directly measured using magnetic resonance imaging,17 analog ultrasound biomicroscopy,18,20-22 and very high-frequency (VHF) digital ultrasound.1,19,23,24 Some surgeons are now using direct sulcus diameter measurements for lens sizing.1,22 Choi et al22 used analog ultrasound biomicroscopy to image the nasal and temporal sulcus and measured the distance to the sulcus from external indentations made using calipers to mark the position of the limbus. These measurements were combined with the white-to-white measurement to estimate sulcus diameter and resulted in better control of the vault than traditional lens sizing using white-to-white.
In this study, we used the Artemis 1 VHF digital ultrasound arc-scanner (ArcScan Inc, Morrison, Colo) to directly measure angle diameter and sulcus diameter. The Artemis 1 VHF digital ultrasound arc-scanner has previously been shown to obtain measurements on a test object with lateral dimensions of the size commonly found in the anterior segment with accuracy of 0.00 mm, repeatability of 0.04 mm, and reproducibility of 0.01 mm.25 We set out to investigate the accuracy of estimating angle diameter and sulcus diameter from external measurements by analyzing the direct correlations between white-to-white, angle diameter, and sulcus diameter as well as multivariate correlations including other conventional external measurements—manifest refraction, ACD, corneal curvature, central corneal thickness (CCT), and age.
PATIENTS AND METHODS
This prospective study included preoperative data from 40 eyes of 20 patients with high myopia who presented to be assessed for phakic IOL surgery. Patients underwent an ocular examination including manifest refraction (CSV-1000; VectorVision Inc, Greenville, Ohio), wavefront analysis (WASCA, Carl Zeiss Meditec), Orbscan II topography, pupillometry (Procyon Instruments, Wales, United Kingdom), and Artemis VHF digital ultrasound arc-scanning.
Informed consent was obtained from the participants. The study adhered to the tenets of the Declaration of Helsinki and was performed under an Institutional Review Board approved protocol.
Ultrasound Scanning
Artemis VHF digital ultrasound uses sterile normal saline (at 33°C) as the acoustic coupling medium between the object being measured and the transducer. The patient rests his/her head in a head rest prone at 45° while the orbital margin fits into a swimming goggle style eye-cup, which seals the saline-filled scanning compartment. A broad-band 50 MHz VHF ultrasound transducer (bandwidth 10 to 60 MHz) sweeps in a reverse arc high-precision mechanism to acquire B-scans. The scan radius is adjustable to follow the contours of either the cornea or globe, thus maintaining transducer perpendicularity (and signal-to-noise ratio) to the curved structures being scanned. The ultrasonic elements of Artemis technology include raw ultrasonic radiofrequency data digitization and storage for digital signal processing. The digitized ultrasound data are then transformed using proprietary digital signal processing technology, which increases signal-to-noise ratio and optimizes image quality and measurement precision.26
Ten horizontal B-scans with the focal plane approximately at a depth corresponding to the iris plane were acquired for each eye. Each individual scan plane comprised 512 pulse/echo vectors made at 70-μm intervals, for an overall scan width of 15 mm. Each vector consisted of 4096 samples obtained at a sample rate of 500 MHz in a gated region corresponding to the area of interest (ie, around the focal plane) with an anterior to posterior depth of 6.3 mm for each scan. The patient fixates on a narrowly focused aiming beam, which is coaxial with the infra-red camera, corneal vertex, and center of rotation of the scanning system. The technician adjusts the center of rotation of the system until it is coaxial with the corneal vertex. In this manner, the position of each scan plane is maintained about a single point on the cornea and corneal mapping is therefore centered on the corneal vertex. The digitized ultrasound data were displayed using proprietary software developed at Cornell University and the angle and sulcus diameters were measured on screen by a trained observer (D.Z.R.) by placing crosshair cursors on the reflections of the angle and sulcus. In cases where a cyst was present in the ciliary body, sulcus diameter was measured from the medial edge of the cyst. Scans with significant eye movement or in which the angle or sulcus was not clearly visible were excluded.
Statistical Analysis
The mean and standard deviation of the angle diameter and sulcus diameter measurements for each eye were calculated. The 95% confidence interval (CI) of the mean was calculated as 1.96 times the standard error. Mean values were used for population analysis. The variance of the 10 measurements of each eye was calculated for both angle diameter and sulcus diameter. The within-eye repeatability was calculated as the square root of the mean variance.27 Student paired t tests were performed to test for significant differences between white-to-white and angle diameter, white-to-white and sulcus diameter, and angle diameter and sulcus diameter. The mean difference between white-to-white and angle diameter (white-to-white – angle diameter), white-to-white and sulcus diameter (white-to-white – sulcus diameter), and angle diameter and sulcus diameter (angle diameter – sulcus diameter) were calculated.
Linear regression analysis was performed between white-to-white and angle diameter measurements, white-to-white and sulcus diameter measurements, and angle diameter and sulcus diameter measurements. The regression equation and Pearson’s correlation coefficient were calculated for each pair of parameters. Residual analysis was performed for each pair of parameters to represent the potential error in using white-to-white to predict angle diameter, white-to-white to predict sulcus diameter, and angle diameter to predict sulcus diameter. Residuals were calculated as the difference between the measured value and the value predicted using the corresponding linear regression equation. The standard deviation and 95% CI of the residuals were calculated for each pair of parameters.
Stepwise multivariate linear regression analysis was performed to determine whether angle diameter could be predicted by conventional external measurements and whether sulcus diameter could be predicted by angle diameter combined with the same conventional external measurements. The conventional external measurements included age, manifest sphere, manifest cylinder, manifest spherical equivalent refraction, Orbscan white-to-white, Orbscan ACD (measured from the endothelium), Orbscan mean keratometry, and Orbscan CCT. The independent variable with the highest non-significant P value was excluded before repeating the analysis until the remaining variables were all statistically significant. A P value <.05 was deemed to be statistically significant. The regression equation, Pearson’s correlation coefficient, and residuals were calculated. The standard deviation and 95% CI of the residuals were calculated to represent the potential error in predicting angle diameter and sulcus diameter using the statistically significant independent variables. All statistics were calculated using Microsoft Excel 2003 (Microsoft, Redmond, Wash).
RESULTS
Descriptive statistics for age, manifest sphere, manifest cylinder, manifest spherical equivalent refraction, ACD, mean keratometry, CCT, and white-to-white are shown in Table 1.
TABLE 1.
Descriptive Statistics of 40 Eyes That Underwent Artemis 1 Very High-frequency Digital Ultrasound Arc Scanning
| Mean | Standard Deviation | Minimum | Maximum | Range | |
|---|---|---|---|---|---|
| Age (y) | 34.5 | 7.0 | 24.4 | 47.8 | 23.4 |
| Sphere (D) | −10.76 | 3.56 | −4.50 | −19.75 | 15.25 |
| Cylinder (D) | −1.17 | 0.98 | −0.20 | −3.50 | 3.30 |
| Spherical equivalent refraction (D) | −11.35 | 3.63 | −5.00 | −20.25 | 15.25 |
| Anterior chamber depth (mm) | 3.30 | 0.28 | 2.79 | 3.90 | 1.10 |
| Minimum keratometry (D) | 43.04 | 1.65 | 40.80 | 45.80 | 5.00 |
| Maximum keratometry (D) | 44.30 | 1.86 | 41.90 | 48.60 | 6.70 |
| Central corneal thickness (μm) | 487.96 | 47.52 | 384.00 | 554.00 | 170.00 |
| White-to-white (mm) | 12.06 | 0.37 | 11.40 | 12.70 | 1.30 |
| Angle diameter (mm) | 12.88 | 0.42 | 11.90 | 13.65 | 1.74 |
| Sulcus diameter (mm) | 12.85 | 0.69 | 11.35 | 14.34 | 2.99 |
Figure 1 presents horizontal B-scan images of the anterior segment obtained with the Artemis 1 VHF digital ultrasound arc scanner for right and left eyes of six patients. These examples demonstrate the variability in angle and sulcus dimension and morphology between patients. Anterior segment B-scans were obtained for all patients; angle was visible in 95% and sulcus was visible in 94% of all scans from all patients.
Figure 1.
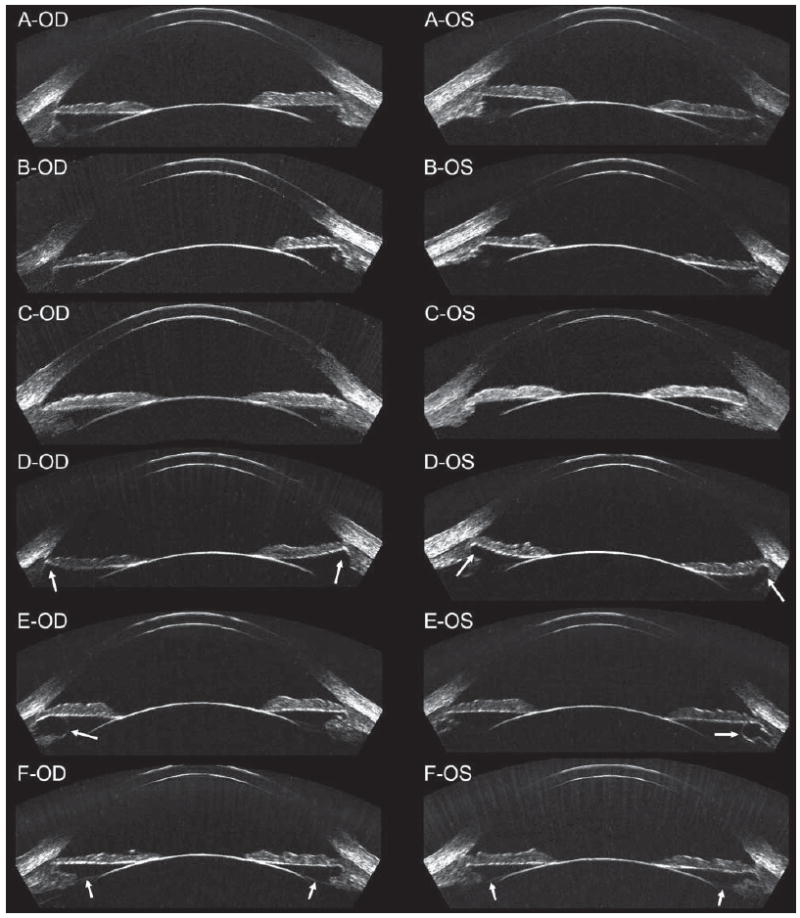
Geometrically corrected horizontal B-scan images of the anterior segment of right/left pairs in six patients. A) The angle diameter was similar to the sulcus diameter in this patient. B) The angle diameter was smaller than the sulcus diameter in this patient. C) The angle diameter was larger than the sulcus diameter in this patient. D) Anterior segment imaging showed the sulcus to be recessed in this patient (indicated by white arrows). E) Anterior segment imaging found cysts within the ciliary body of each eye (indicated by white arrows). F) The zonules were also visible in this patient (indicated by white arrows).
The within-eye repeatability was 0.13 mm for angle diameter and 0.23 mm for sulcus diameter. The mean and 95% CI of the mean was 12.88 mm [12.74, 13.02] for angle diameter and 12.85 mm [12.63, 13.07] for sulcus diameter. The population standard deviation was 0.42 mm for angle diameter and 0.69 mm for sulcus diameter. The mean±standard deviation of the difference between white-to-white and angle diameter was −0.92±0.28 mm, which was statistically significant (P<.001). The mean±standard deviation of the difference between white-to-white and sulcus diameter was −0.89±0.57 mm, which was statistically significant (P<.001). The mean±standard deviation of the difference between angle diameter and sulcus diameter was +0.02±0.51 mm, which was not statistically significant (P=.128). Sulcus diameter was more than 0.50 mm larger than angle diameter in 2.5% of eyes and more than 0.50 mm smaller than angle diameter in 27.5% of eyes. Figure 1B-OS shows an example image of an eye where the sulcus diameter was 0.73 mm larger than the angle diameter. Figure 1C-OS shows an example image of an eye where the sulcus diameter was 1.37 mm smaller than the angle diameter.
Linear regression analysis found a statistically significant correlation between white-to-white and angle diameter (P<.001) and Pearson’s correlation coefficient of 0.587 (Fig 2). The linear regression equation was
Figure 2.
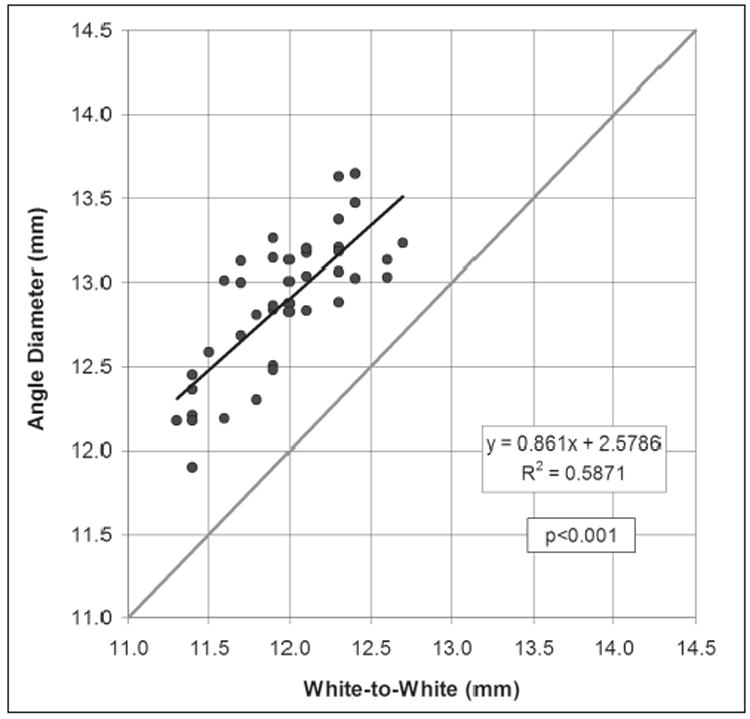
Scatterplot of the Orbscan measurement of white-to-white against the Artemis direct measurement of angle diameter. The regression line is plotted as a continuous black line and the regression equation and Pearson’s correlation coefficient are presented. The gray line represents equality between the two measurements.
| (1) |
The standard deviation of the residuals was 0.27 mm. The 95% CI of estimating angle diameter using Eq.(1) was ±0.53 mm.
Stepwise multivariate regression analysis found the white-to-white (P<.001), CCT (P=.007), and minimum keratometry (P=.009) to be statistically significant variables. The multivariate linear regression equation was
| (2) |
Figure 3 shows the Artemis measured angle diameter plotted against the angle diameter as predicted by Eq.(2). Pearson’s correlation coefficient for the multivariate regression was 0.691. The standard deviation of the residuals was 0.23 mm. The 95% CI of estimating angle diameter using Eq.(2) was ±0.46 mm.
Figure 3.
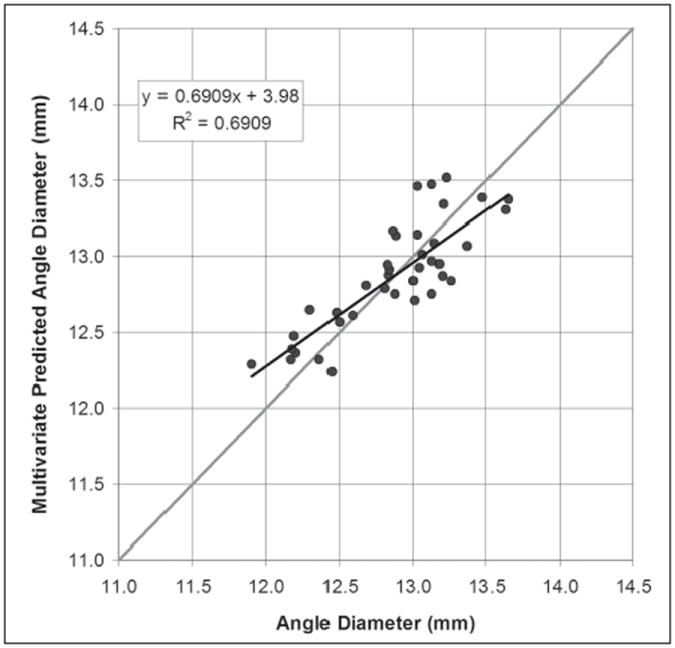
Scatterplot of the Artemis direct measurement of angle diameter against the angle diameter value predicted by the multivariate regression Eq.(2). The regression line is plotted as a continuous black line and the regression equation and Pearson’s correlation coefficient are presented. The gray line represents equality.
Linear regression analysis found a statistically significant correlation between white-to-white and sulcus diameter (P<.001) and a Pearson’s correlation coefficient of 0.323 (Fig 4). The linear regression equation was
Figure 4.
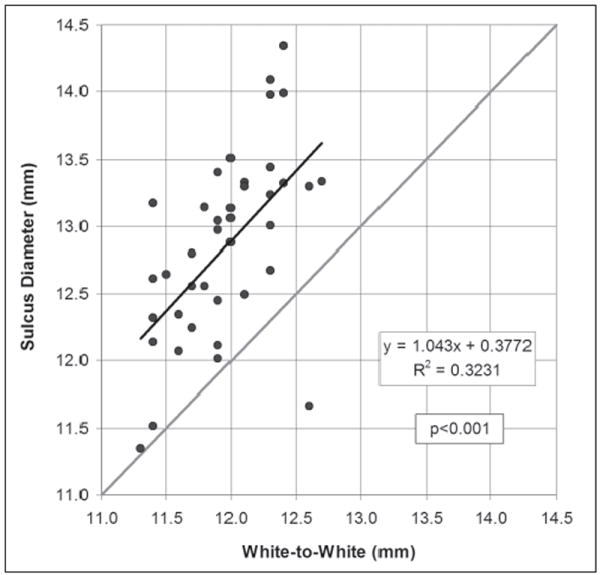
Scatterplot of the Orbscan measurement of white-to-white against the Artemis direct measurement of sulcus diameter. The regression line is plotted as a continuous black line and the regression equation and Pearson’s correlation coefficient are presented. The gray line represents equality between the two measurements.
| (3) |
The standard deviation of the residuals was 0.57 mm. The 95% CI of estimating sulcus diameter using Eq.(3) was ±1.11 mm.
Linear regression analysis found a statistically significant correlation between angle diameter and sulcus diameter (P<.001) and Pearson’s correlation coefficient of 0.458 (Fig 5). The linear regression equation was
Figure 5.
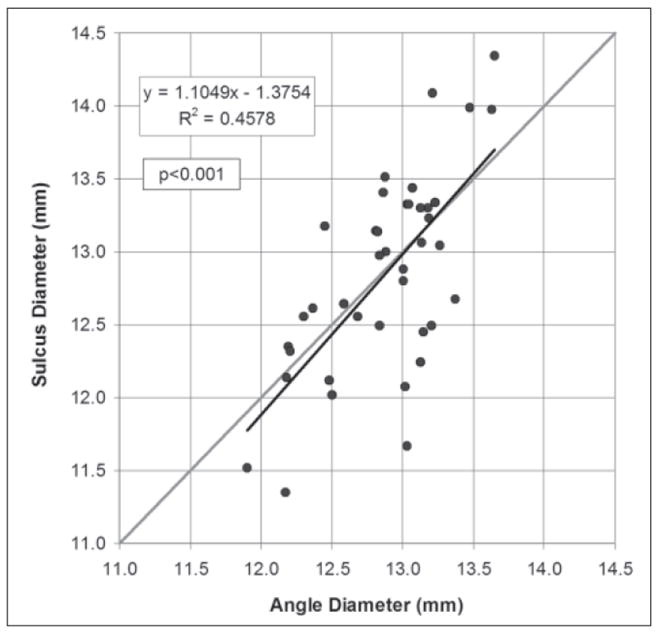
Scatterplot of the Artemis direct measurement of angle diameter against the Artemis direct measurement of sulcus diameter. The regression line is plotted as a continuous black line and the regression equation and Pearson’s correlation coefficient are presented. The gray line represents equality between the two measurements.
| (4) |
The standard deviation of the residuals was 0.51 mm. The 95% CI of estimating sulcus diameter using Eq.(4) was ±0.99 mm.
Stepwise multivariate regression analysis found the angle diameter (P<.001) and ACD (P=.006) to be statistically significant variables. The multivariate linear regression equation was
| (5) |
Figure 6 shows the Artemis measured sulcus diameter plotted against the sulcus diameter as predicted by Eq.(5). Pearson’s correlation coefficient for the multivariate regression was 0.571. The standard deviation of the residuals was 0.45 mm. The 95% CI of estimating sulcus diameter using Eq.(5) was ±0.88 mm.
Figure 6.
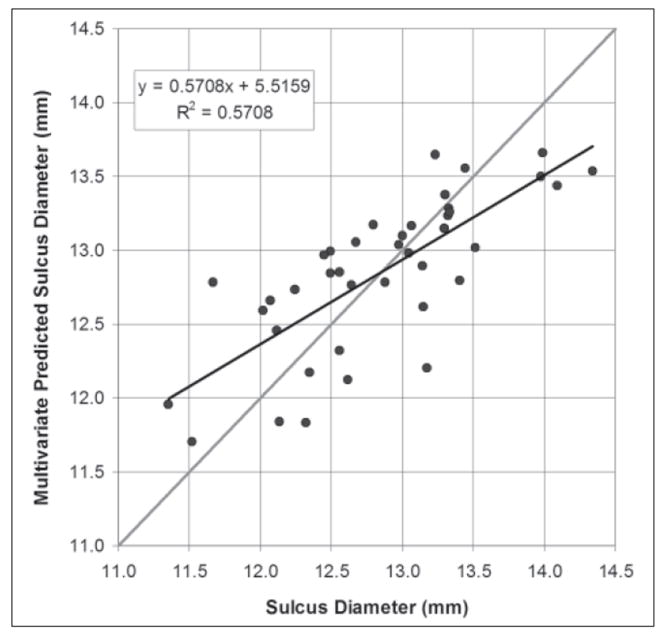
Scatterplot of the Artemis direct measurement of sulcus diameter against the sulcus diameter value predicted by the multivariate regression Eq.(5). The regression line is plotted as a continuous black line and the regression equation and Pearson’s correlation coefficient are presented. The gray line represents equality.
The results of the linear regression analysis are summarized in Table 2.
TABLE 2.
Results of the Linear Regression Analysis in 40 Eyes That Underwent Artemis 1 Very High-frequency Digital Ultrasound Arc Scanning
| Variable/Predictors | Mean Difference (mm)
(Predictor - Variable) (P Value) |
Correlation Significance
(P Value) |
Pearson’s r2 | 95% Confidence Interval of the Residuals (mm) |
|---|---|---|---|---|
| Angle diameter/White-to-white | −0.92±0.28 (<.001) | <.001 | 0.587 | ±0.53 |
| Angle diameter/ | ||||
| White-to-white | <.001 | 0.691 | ±0.46 | |
| Central corneal thickness | .007 | |||
| Minimum keratometry | .009 | |||
| Sulcus diameter/White-to-white | −0.89±0.57 (<.001) | <.001 | 0.323 | ±1.11 |
| Sulcus diameter/Angle diameter | −0.02±0.51 (<.128) | <.001 | 0.458 | ±0.99 |
| Sulcus diameter/ | ||||
| Angle diameter | <.001 | 0.571 | ±0.88 | |
| Anterior chamber depth | .003 |
DISCUSSION
This study demonstrated the ability of the Artemis 1 VHF digital ultrasound arc-scanner to directly measure both angle and sulcus diameters. This study found statistically significant, but weak, linear correlations between white-to-white and angle diameter, white-to-white and sulcus diameter, and angle diameter and sulcus diameter, and statistically significant multivariate correlations between angle diameter and white-to-white, CCT, and minimum keratometry and between sulcus diameter and angle diameter and ACD. Thus, the power of using these correlations to predict angle diameter and sulcus diameter was weak.
A linear model based on white-to-white was found to predict angle diameter to within 0.53 mm in 95% of cases. The multivariate regression equation, including white-to-white, CCT, and minimum keratometry, improved the predictability of angle diameter to within 0.46 mm in 95% of cases. This means that the predicted angle diameter would be more than 0.46 mm larger or smaller than the actual angle diameter dimension in 5% of eyes. A linear model based on white-to-white was found to predict sulcus diameter to within 1.11 mm in 95% of cases, whereas using angle diameter improved the predictability to within 0.99 mm in 95% of cases. The multivariate regression equation, including angle diameter and ACD, improved the predictability of sulcus diameter to within 0.88 mm in 95% of cases. This means that the predicted sulcus diameter would be more than 0.88 mm larger or smaller than the actual sulcus diameter dimension in 5% of eyes.
The within-eye standard deviation for both angle diameter (0.13 mm) and sulcus diameter (0.23 mm) was higher than that which we reported when measuring an inanimate test object (0.04 mm) using the same scanner that was used for the present study.25 Although every effort was made to obtain scans in the same horizontal meridian, this variability is likely due to slight variation in the scan plane between scans and measurement error caused by observer judgment of the fiducial points. The test object in our repeatability study was a metal bolt, which has clearly defined edges, whereas the location of the fiducial points to measure angle and sulcus dimensions are less clearly defined. The fiducial points for measuring angle dimensions are easier to identify than those for measuring sulcus dimensions, which is reflected in the somewhat greater uncertainty of sulcus measurements. This difference might also reflect a greater variability of sulcus anatomy, affected by the position of the ciliary processes.
The systematic error of measuring angle diameter and sulcus diameter using VHF digital ultrasound could not be measured as no alternative method of validating anterior segment dimensions in vivo exists currently. However, the systematic error of measuring an inanimate test object with the Artemis has previously been shown to be 0.00 mm.25 The use of arc-scanned ultrasound for measurement of internal ocular dimensions is ideal from the standpoint of refraction-induced measurement error. Optical techniques must contend with a refractive index change of approximately 38% at the cornea–air interface versus approximately 6% at the normal saline–cornea interface for ultrasound. Furthermore, the arc-scan geometry produces a near-normal angle of incidence between the ultrasound beam and the eye. These two factors make geometric distortion of internal ocular structures negligible in Artemis 1 examinations.
The confidence of direct anterior segment measurements with the Artemis 1 can be improved by obtaining an average of multiple measurements. The repeatability of angle diameter measurement was found to be 0.13 mm. Therefore, calculating the standard error of the mean indicates that an average of 5 measurements would be expected to be within ±0.06 mm of the actual value and an average of 10 measurements would be expected to be within ±0.04 mm of the actual value. The repeatability of sulcus diameter measurement was found to be 0.23 mm. Therefore, an average of 5 measurements would be expected to be within ±0.10 mm of the actual value and an average of 10 measurements would be expected to be within ±0.07 mm of the actual value.
The present study found a statistically significant correlation between white-to-white and angle diameter, which concurs with previously published reports.5 Goldsmith et al5 reported that the standard deviation of the residuals with their model was 0.41 mm and therefore using white-to-white to estimate angle diameter would result in an error of >0.50 mm in 22% of cases. Using the linear regression model described in the present study, an error of >0.50 mm would be expected in 6.5% of cases. This error would be improved to 3.3% if the multivariate regression equation was used. As suggested by Goldsmith et al, 5 it appears that using white-to-white to size anterior chamber IOLs could explain a number of sizing-related complications, such as pupil ovalization, lens movement, and inflammatory response.
The present study found a statistically significant correlation between white-to-white and sulcus diameter, in contrast to a number of previous studies where no correlation was reported.17-20 Fea et al17 used magnetic resonance imaging to measure horizontal sulcus diameter. Werner et al19 measured the horizontal sulcus diameter with digital calipers after dissection in 12 cadaver eyes. Pop et al18 used analog ultrasound microscopy, in which lateral scan width was insufficient to image sulcus diameter in one scan sweep and sulcus diameter measurement required a combination of images to be pasted together. Oh et al20 used a HiScan 35 MHz analog UBM (Optikon, Roma, Italy) to measure the horizontal sulcus diameter. The higher precision of the measurement technique used in the present study compared with previous studies might be the reason that a correlation between white-to-white and sulcus diameter was found in the present study. However, the correlation found between white-to-white and sulcus diameter in the present study was not clinically significant with a Pearson’s correlation coefficient of 0.323. If lens sizes were calculated using the regression model for sulcus diameter based on white-to-white alone, the error of the predicted sulcus dimension would be >0.5 mm in 37.7% of cases and >1.0 mm in 7.7% of cases. As posterior chamber IOLs are currently sized to the nearest 0.5 mm, an error of this magnitude would mean that an inappropriate lens size may be selected, which would in turn increase the risk of complications such as angle closure, iris pigment dispersion, or cataract.
Using analog ultrasound biomicroscopy (35 MHz) to measure angle diameter and sulcus diameter, Oh et al20 reported a statistically significant correlation between angle diameter and sulcus diameter, as was found in the present study, and suggest that angle diameter might be helpful for estimating sulcus size. However, if lens sizes were calculated using the best-case multivariate regression model described in this study and angle diameter measurements were obtained using VHF digital ultrasound, the error of the predicted sulcus dimension would be >0.5 mm in 27.3% of cases and >1.0 mm in 2.8% of cases. As with the white-to-white model, such an error could likely lead to complications associated with lens sizing. A residual analysis was not reported for the correlation found by Oh et al, therefore the clinical relevance of the reported correlation cannot be ascertained. Further study is required to determine the magnitude of this error had angle diameter measurements been obtained by commercially available optical methods.
We are currently collecting data for a comparative population to test the clinical predictability of the model. The results presented in this study are based on a residual analysis of the same data that were used to generate the regression models and so represent a best-case scenario. For this reason, we hypothesize that the error of applying such regression models clinically will result in a larger error in sulcus diameter prediction than reported in this study.
Another application of anterior segment imaging could be in IOL power calculations for cataract surgery. These calculations depend on knowledge of the position of the IOL with respect to the nodal point of the eye. Some formulae use an “A constant” together with ACD and lens/cataract anteroposterior thickness to estimate the final resting position of the IOL to be inserted. In principle, a preoperative anterior segment scan can provide the ACD measured to the zonular plane. This could in principle provide a more reliable parameter to use for predicting IOL position relative to the cornea and improve IOL power predictions.
This study demonstrated that Artemis 1 VHF digital ultrasound provides repeatable direct measurements of angle and sulcus dimensions. Regression modeling showed that white-to-white alone and white-to-white combined with CCT and minimum keratometry can provide statistically significant, but weak, estimates of angle diameter dimension. Regression modeling also showed that angle diameter alone and angle diameter combined with ACD can provide statistically significant, but weak, estimates of sulcus diameter dimension. Given the far greater accuracy of direct measurement of angle diameter and sulcus diameter, it seems clear that current IOL sizing protocols based on estimating angle and sulcus diameters using the white-to-white corneal diameter (with or without other parameters) will eventually be replaced by protocols utilizing direct measurement of internal ocular anatomy.
Acknowledgments
Supported in part by NIH grants EB00238; Research to Prevent Blindness, New York, NY; and the Dyson Foundation, Millbrook, NY.
Footnotes
Drs Reinstein, Silverman, Rondeau, and Coleman have a proprietary interest in the Artemis technology (ArcScan Inc, Morrison, Colo). Mr Archer has no proprietary or financial interest in the materials presented.
Prepared in part as fulfillment of the requirements for Dr Reinstein’s doctoral thesis, University of Cambridge.
Some aspects of this study were presented at the European Society of Cataract and Refractive Surgeons Annual Meeting; September 9-13, 2006; London, United Kingdom.
References
- 1.Lovisolo CF, Reinstein DZ. Phakic intraocular lenses. Surv Ophthalmol. 2005;50:549–587. doi: 10.1016/j.survophthal.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 2.Alió JL, de la Hoz F, Pérez-Santonja JJ, Ruiz-Moreno JM, Quesada JA. Phakic anterior chamber lenses for the correction of myopia: a 7-year cumulative analysis of complicationsin 263 cases. Ophthalmology. 1999;106:458–466. doi: 10.1016/S0161-6420(99)90103-3. [DOI] [PubMed] [Google Scholar]
- 3.Perez-Santonja JJ, Iradier MT, Benitez del Castillo JM, Serrano JM, Zato MA. Chronic subclinical inflammation in phakic eyes with intraocular lenses to correct myopia. J Cataract Refract Surg. 1996;22:183–187. doi: 10.1016/s0886-3350(96)80216-1. [DOI] [PubMed] [Google Scholar]
- 4.Javaloy J, Alio JL, Iradier MT, Abdelrahman AM, Javaloy T, Borras F. Outcomes of ZB5M angle-supported anterior chamber phakic intraocular lenses at 12 years. J Refract Surg. 2007;23:147–158. doi: 10.3928/1081-597X-20070201-07. [DOI] [PubMed] [Google Scholar]
- 5.Goldsmith JA, Li Y, Chalita MR, Westphal V, Patil CA, Rollins AM, Izatt JA, Huang D. Anterior chamber width measurement by high-speed optical coherence tomography. Ophthalmology. 2005;112:238–244. doi: 10.1016/j.ophtha.2004.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanders DR, Vukich JA. Incidence of lens opacities and clinically significant cataracts with the implantable contact lens: comparison of two lens designs. J Refract Surg. 2002;18:673–682. doi: 10.3928/1081-597X-20021101-03. [DOI] [PubMed] [Google Scholar]
- 7.Sanders DR, Vukich JA, Doney K, Gaston M. U.S. Food and Drug Administration clinical trial of the Implantable Contact Lens for moderate to high myopia. Ophthalmology. 2003;110:255–266. doi: 10.1016/s0161-6420(02)01771-2. [DOI] [PubMed] [Google Scholar]
- 8.Kodjikian L, Gain P, Donate D, Rouberol F, Burillon C. Malignant glaucoma induced by a phakic posterior chamber intraocular lens for myopia. J Cataract Refract Surg. 2002;28:2217–2221. doi: 10.1016/s0886-3350(02)01213-0. [DOI] [PubMed] [Google Scholar]
- 9.Reed JE, Thomas JV, Lytle RA, Simmons RJ. Malignant glaucoma induced by an intraocular lens. Ophthalmic Surg. 1990;21:177–180. [PubMed] [Google Scholar]
- 10.Brandt JD, Mockovak ME, Chayet A. Pigmentary dispersion syndrome induced by a posterior chamber phakic refractive lens. Am J Ophthalmol. 2001;131:260–263. doi: 10.1016/s0002-9394(00)00606-1. [DOI] [PubMed] [Google Scholar]
- 11.Kohnen T, Kasper T, Terzi E. Intraocular lenses for the correction of refraction errors. Part II. Phakic posterior chamber lenses and refractive lens exchange with posterior chamber lens implantation [German] Ophthalmologe. 2005;102:1105–1117. doi: 10.1007/s00347-005-1274-7. [DOI] [PubMed] [Google Scholar]
- 12.Mastropasqua L, Toto L, Nubile M, Falconio G, Ciancaglini M. Long-term complications of bilateral posterior chamber phakic intraocular lens implantation. J Cataract Refract Surg. 2004;30:901–904. doi: 10.1016/j.jcrs.2003.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Brauweiler PH, Wehler T, Busin M. High incidence of cataract formation after implantation of a silicone posterior chamber lens in phakic, highly myopic eyes. Ophthalmology. 1999;106:1651–1655. doi: 10.1016/S0161-6420(99)90352-4. [DOI] [PubMed] [Google Scholar]
- 14.El-Sheikh HF, Tabbara KF. Cataract following posterior chamber phakic intraocular lens. J Refract Surg. 2003;19:72–73. doi: 10.3928/1081-597X-20030101-17. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Galeana CA, Smith RJ, Sanders DR, Rodriguez FX, Litwak S, Montes M, Chayet AS. Lens opacities after posterior chamber phakic intraocular lens implantation. Ophthalmology. 2003;110:781–785. doi: 10.1016/s0161-6420(02)01973-5. [DOI] [PubMed] [Google Scholar]
- 16.Gimbel HV, Condon GP, Kohnen T, Olson RJ, Halkiadakis I. Late in-the-bag intraocular lens dislocation: incidence, prevention, and management. J Cataract Refract Surg. 2005;31:2193–2204. doi: 10.1016/j.jcrs.2005.06.053. [DOI] [PubMed] [Google Scholar]
- 17.Fea AM, Annetta F, Cirillo S, Campanella D, De Giuseppe M, Regge D, Grignolo FM. Magnetic resonance imaging and Orb-scan assessment of the anterior chamber. J Cataract Refract Surg. 2005;31:1713–1718. doi: 10.1016/j.jcrs.2005.02.040. [DOI] [PubMed] [Google Scholar]
- 18.Pop M, Payette Y, Mansour M. Predicting sulcus size using ocular measurements. J Cataract Refract Surg. 2001;27:1033–1038. doi: 10.1016/s0886-3350(00)00830-0. [DOI] [PubMed] [Google Scholar]
- 19.Werner L, Izak AM, Pandey SK, Apple DJ, Trivedi RH, Schmidbauer JM. Correlation between different measurements within the eye relative to phakic intraocular lens implantation. J Cataract Refract Surg. 2004;30:1982–1988. doi: 10.1016/j.jcrs.2003.10.041. [DOI] [PubMed] [Google Scholar]
- 20.Oh J, Shin HH, Kim JH, Kim HM, Song JS. Direct measurement of the ciliary sulcus diameter by 35-megahertz ultrasound biomicroscopy. Ophthalmology. 2007;114:1685–1688. doi: 10.1016/j.ophtha.2006.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Lebaillif S, Roussel B, Cochener B. Assessment of the Quantel Medical 20-Mhz Ultrasound Cinescan in intraocular measurements [French] J Fr Ophtalmol. 2006;29:820–828. doi: 10.1016/s0181-5512(06)73853-0. [DOI] [PubMed] [Google Scholar]
- 22.Choi KH, Chung SE, Chung TY, Chung ES. Ultrasound biomicroscopy for determining visian implantable contact lens length in phakic IOL implantation. J Refract Surg. 2007;23:362–367. doi: 10.3928/1081-597X-20070401-08. [DOI] [PubMed] [Google Scholar]
- 23.Kim DY, Reinstein DZ, Silverman RH, Najafi DJ, Belmont SC, Hatsis AP, Rozakis GW, Coleman DJ. Very high frequency ultrasound analysis of a new phakic posterior chamber intraocular lens in situ. Am J Ophthalmol. 1998;125:725–729. doi: 10.1016/s0002-9394(98)00025-7. [DOI] [PubMed] [Google Scholar]
- 24.Rondeau MJ, Barcsay G, Silverman RH, Reinstein DZ, Krishnamurthy R, Chabi A, Du T, Coleman DJ. Very high frequency ultrasound biometry of the anterior and posterior chamber diameter. J Refract Surg. 2004;20:454–464. doi: 10.3928/1081-597X-20040901-08. [DOI] [PubMed] [Google Scholar]
- 25.Reinstein DZ, Archer TJ, Silverman RH, Coleman DJ. Accuracy, repeatability, and reproducibility of Artemis very high-frequency digital ultrasound arc-scan lateral dimension measurements. J Cataract Refract Surg. 2006;32:1799–1802. doi: 10.1016/j.jcrs.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reinstein DZ, Silverman RH, Coleman DJ. High-frequency ultrasound measurement of the thickness of the corneal epithelium. Refract Corneal Surg. 1993;9:385–387. [PubMed] [Google Scholar]
- 27.Bland JM, Altman DG. Measurement error. BMJ. 1996;313:744. doi: 10.1136/bmj.313.7059.744. [DOI] [PMC free article] [PubMed] [Google Scholar]


