Abstract
objective
It has been hypothesized that excessive fatty acid availability contributes to steatosis and the metabolic abnormalities associated with nonalcoholic fatty liver disease (NAFLD). The purpose of this study was to evaluate whether adipose tissue lipolytic activity and the rate of fatty acid release into plasma are increased in obese adolescents with NAFLD.
Methods
Palmitate kinetics were determined in obese adolescents with normal (n = 9; BMI = 37 ± 2 kg/m2; intrahepatic triglyceride (IHTG) ≤5.5% of liver volume) and increased (n = 9; BMI = 36 ± 2 kg/m2; IHTG ≥ 10% of liver volume) IHTG content during the basal state (postabsorptive condition) and during physiological hyperinsulinemia (postprandial condition). Both groups were matched on body weight, BMI, percent body fat, age, sex, and Tanner stage. The hyperinsulinemic-euglycemic clamp procedure, in conjunction with a deuterated palmitate tracer infusion, was used to determine free-fatty acid (FFA) kinetics, and magnetic resonance spectroscopy was used to determine IHTG content.
Results
The rate of whole-body palmitate release into plasma was greater in subjects with NAFLD than those with normal IHTG content during basal conditions, (87 ± 7 vs. 127 ± 13 μmol/min; P < 0.01) and during physiological hyperinsulinemia, (24 ± 2 vs. 44 ± 8 μmol/min; P < 0.01).
Discussion
These results demonstrate that adipose tissue lipolytic activity is increased in obese adolescents with NAFLD and results in an increase in the rate of fatty acid release into plasma throughout the day. This continual excess in fatty acid flux supports the hypothesis that adipose insulin resistance is involved in the pathogenesis of steatosis and contributes to the metabolic complications associated with NAFLD.
INTRODUCTION
The marked increase in childhood obesity (1,2) has led to an increase in pediatric nonalcoholic fatty liver disease (NAFLD). Approximately 3% of all children and adolescents have NAFLD, and the prevalence rate increases to 23-53% in those who are overweight or obese (3,4). The increase in NAFLD has important clinical implications because of the potential progression to severe liver disease and the association between NAFLD and metabolic complications. In adults, excessive intrahepatic triglyceride (IHTG) content is associated with insulin resistance in multiple organs (5-9), and is now considered part of the constellation of abnormalities associated with the metabolic syndrome (10). We have recently found that NAFLD is associated with insulin-resistant glucose metabolism in both liver and skeletal muscle in obese adolescents (11).
The precise mechanisms responsible for the development of steatosis and the metabolic complications associated with NAFLD are not known. However, it has been hypothesized that increased release of fatty acids from adipose tissue and delivery of excessive free-fatty acid (FFA) to the liver and skeletal muscle leads to IHTG accumulation and impaired insulin action in both organs (12-15). Therefore, insulin resistance in adipose tissue and failure to adequately suppress lipolysis of adipose tissue triglycerides could be a key factor in the pathogenesis and pathophysiology of NAFLD. However, this issue has not been carefully evaluated in obese children and adolescents, and it is not known whether NAFLD is associated with excessive lipolytic rates or adipose tissue insulin resistance in childhood obesity.
The purpose of this study was to evaluate whether adipose tissue insulin sensitivity and lipolytic activity are altered in obese adolescents with NAFLD. The hyperinsulinemiceuglycemic clamp procedure, in conjunction with stable isotopically labeled tracer infusion was used to determine basal whole-body lipolytic rates and adipose tissue sensitivity to physiological hyperinsulinemia in vivo in obese adolescents who had either normal or increased IHTG content, assessed by using magnetic resonance spectroscopy.
Methods And Procedures
subjects
Eighteen obese (BMI ≥ 95th percentile) adolescents participated in this study; nine subjects had normal IHTG content (IHTG ≤ 5.5% of liver volume) (16) and nine subjects had NAFLD (IHTG >10% of liver volume) (Table 1). A value of ≥10% IHTG content was used to define subjects who had NAFLD to ensure a clear separation from the normal IHTG group. Groups with normal and increased IHTG content were matched on sex, age, Tanner stage, and BMI (Table 1). Data on hepatic and skeletal muscle insulin action with respect to glucose metabolism in these subjects has been published previously (11).
Table 1. Characteristics of subjects with normal intrahepatic triglyceride (IHTG) content and with nonalcoholic fatty liver disease (NAFLD).
| Normal IHTG | NAFLD | |
|---|---|---|
| n (M/F) | 9 (7/2) | 9 (7/2) |
| Age (yr) | 15 ± 0 | 16 ± 0 |
| Tanner stage | 4.1 ± 0.2 | 4.4 ± 0.2 |
| Body weight (kg) | 106 ±7 | 109 ± 6 |
| BMI (kg/m2) | 37 ± 2 | 36 ± 2 |
| BMI percentile | 98 ± 1 | 98 ± 0 |
| Fat mass (% body weight) | 40 ± 2 | 39 ± 2 |
| Intra-abdominal fat (cm3) | 631 ± 56 | 1,074 ± 152* |
| Subcutaneous abdominal fat (cm3) | 4,509 ± 256 | 3,662 ± 300 |
| IHTG content (%) | 3.3 ±0.6 | 26.5 ± 3.5* |
Values are means ± s.e.m.
Value significantly different from the corresponding Normal IHTG group value, P < 0.01.
All subjects completed a comprehensive medical evaluation, which included a history, physical examination and blood tests. No subject had any history or evidence of liver disease other than NAFLD, and none had impaired fasting glucose concentration, diabetes, or severe hypertriglyceridemia (> 400 mg/dl). In addition, none of the subjects consumed alcohol, smoked tobacco products, or took medications that are known to cause steatosis or alter glucose or lipid metabolism.
The study was approved by the Human Research Protection Office and the Intensive Research Unit (IRU) Advisory Committee of Washington University School of Medicine (St. Louis, MO). All subjects agreed to participate in the study after a detailed explanation of the study was provided to them and their parents. Written informed consent was obtained from each subject’s parent(s) and written informed assent was obtained from each subject before being enrolled in the study.
Body composition analyses
Body fat (FM) and fat-free mass (FFM) were determined by using dual-energy X-ray absorptiometry (Delphi W-densitometer equipped with version 12.4 software; Hologic, Waltham, MA) (17). Total abdominal, subcutaneous abdominal and intra-abdominal fat volumes were determined by using magnetic resonance imaging with a 1.5 T scanner (Siemens, Iselin, NJ) (18). Eight 10-mm-thick axial images were obtained beginning at the L4-L5 interspace, and analyzed for subcutaneous and intra-abdominal fat content by using Analyze 6.0 software (Mayo Foundation, Biomedical Imaging Resource, Rochester, MN); the volume of fat was calculated for each slice and the values were added. Readings were verified by a second investigator blinded to the initial readings; the values obtained by each of the two investigators were averaged for data analyses.
IHTG content was measured by using proton magnetic resonance spectroscopy (1.5 T Siemens Magneton Vision scanner; Siemens, Erlanger, Germany), as we have previously described (19). Three 2 × 2 × 2 cm3 voxels were examined in each subject, and the values were averaged to determine IHTG content. The coefficient of variation of replicate values of the triplicate determinations for 3 voxels was 1.5%.
Hyperinsulinemic-euglycemic clamp
A one-stage hyperinsulinemic-euglycemic clamp procedure (20) was performed ∼1 week after assessment of body composition. Female subjects were studied during the follicular phase of their menstrual cycle. Subjects were instructed to adhere to their regular diet and to refrain from exercise for three days before the study, to avoid caffeine for one day before the study, and to fast (except for water) for 12 h before their admission to the Intensive Research Unit at 0600 on the morning of the study. At 0700, a catheter was inserted into an antecubital vein to infuse a stable isotopically labeled palmitate tracer, insulin and dextrose. Another catheter was inserted into a contralateral hand vein, which was heated to 55 °C by using a thermostatically controlled box, to obtain arterialized blood samples.
At 0800, after a baseline blood sample was obtained, a continuous infusion of [2,2-2H2]palmitate (infusion rate: 0.035 μmol/kg/min), bound to human albumin, was started and maintained for 4.5 h (through the end of the euglycemic-hyperinsulinemic clamp procedure).
At 90 min, a hyperinsulinemic-euglycemic clamp procedure was started and maintained for 180 min. Insulin was infused at a rate of 40 mU/m2/min (initiated with a two-step priming dose of 160 mU/m2/min for 5 min followed by 80 mU/m2/min for 5 min) to achieve normal postprandial plasma insulin concentrations (21). Blood samples were taken every 10 min to monitor plasma glucose concentrations and 20% dextrose was infused at a variable rate to maintain plasma glucose concentration at 100 mg/dl. The infusion rate of [2,2-2H2]palmitate was decreased by 50% (to 0.0175 μmol/kg/min) during the clamp procedure (90-270 min after the start of the study) to account for the expected decline in adipose tissue lipolytic rate. Blood samples were taken every 10 min during the last 30 min of the basal period and the clamp procedure to determine plasma glucose and insulin concentrations and plasma palmitate tracer-to-tracee ratio.
After the clamp procedure was completed, palmitate tracer and insulin infusions were stopped and subjects were given a standard meal. The dextrose infusion was stopped after subjects ate lunch. Subjects were discharged from the Intensive Research Unit after confirming that blood glucose concentrations were stable for at least 1 h after stopping the dextrose infusion.
Analyses of blood samples
Plasma glucose concentration was determined by using an automated glucose analyzer (YSI 2300 STAT Plus; Yellow Spring Instrument, Yellow Springs, OH). Plasma insulin concentration was measured by radioimmunoassay (Linco Research, St Louis, MO). Plasma FFA concentrations were quantified by using gas chromatography (HP 5890 Series II GC; Hewlett-Packard, Palo Alto, CA) after adding heptadecanoic acid to plasma as an internal standard (22). Plasma palmitate tracer-to-tracee ratio was determined by using electron impact ionization gas chromatography/mass spectroscopy (MSD 5973 system with capillary column; Hewlett-Packard) as previously described (22).
Calculations
Metabolic and isotopic steady states were achieved during the last 30 min of the basal period (i.e., between 60 and 90 min) and the hyperinsulinemic-euglycemic clamp procedure (i.e., between 240 and 270 min). Therefore, palmitate rate of appearance (Ra) in plasma, an index of adipose tissue lipolytic rate, was calculated by using Steele’s equation for steady-state conditions (23). Palmitate Ra was expressed in μmol/min to reflect total body lipolytic rates and in μmol/kg fat mass per min, which indicates the amount of palmitate released into the circulation in relation to the amount of endogenous fat stored in adipose tissue (i.e., index of the lipolytic activity per unit of adipose tissue). Palmitate rate of disappearance from plasma was calculated by adding the amount of infused palmitate tracer to endogenous palmitate Ra. Palmitate rate of disappearance was expressed in μmol/kg fat-free mass per min, which indicates the amount of palmitate released into the circulation in relation to the amount of lean tissues that utilize FFAs (i.e., index of FFA availability).
The homeostatic model assessment, based on basal glucose and insulin concentrations, was used to provide an index of insulin resistance (24).
Statistical analyses
Statistical analyses were performed using SPSS (version 13.0; SPSS, Chicago, IL). All data sets were tested for normality. Differences in baseline subject characteristics and metabolic variables between NAFLD and normal IHTG groups were evaluated by using Student’s t-test for normally distributed data. ANOVA was used to evaluate possible differences between NAFLD and normal IHTG groups in palmitate kinetics during basal and hyperinsulinemic conditions. A P value of ≤0.05 was considered statistically significant. Data in the text are presented as means ± s.e.m.
RESULTS
Body composition
Subjects with increased IHTG content and those with normal IHTG content were matched on sex, age, Tanner stage, and BMI. The amount of IHTG ranged from 2.7 to 3.9% in the control group and from 23 to 30% in the NAFLD group. Total body weight, FM, FFM and subcutaneous abdominal fat were similar between groups, whereas intra-abdominal fat volume in subjects with NAFLD was almost double the value in those with normal IHTG content (Table 1).
Metabolic variables
Basal plasma glucose concentration was not different between groups. However, basal insulin concentration and homeostatic model assessment of insulin resistance values were greater in subjects with increased IHTG content than in those with normal IHTG content (Table 2). As we found in our previous study (11), the relative increase in glucose uptake during insulin infusion was blunted in subjects who had high (67.4 ± 9.6%) compared with those who had normal (168.6 ± 28.1%) IHTG content (P < 0.01). Mean plasma triglyceride and low-density lipoprotein-cholesterol concentrations were greater and high-density lipoprotein-cholesterol concentration was lower in subjects with NAFLD than subjects with normal IHTG content (Table 2).
Table 2. Metabolic variables in subjects with normal intrahepatic triglyceride (IHTG) content and with nonalcoholic fatty liver disease (NAFLD).
| Normal IHTG | NAFLD | |
|---|---|---|
| Glucose (mg/dl) | 87 ± 1 | 89 ±3 |
| Insulin (μU/ml) | 19 ± 3 | 34 ± 6* |
| HOMA-IR | 4.1 ± 0.5 | 7.6 ± 1.4* |
| Free-fatty acids (μmol/ml) | 0.414 ± 0.038 | 0.481 ± 0.032 |
| Triglyceride (mg/dl) | 78 ± 7 | 172 ± 22* |
| HDL-cholesterol (mg/dl) | 45 ± 3 | 36 ± 3* |
| LDL-cholesterol (mg/dl) | 73 ± 7 | 102 ± 8* |
Values are means ± s.e.m.
HDL, high-density lipoprotein; HOMA-IR, homeostasis model assessment of insulin resistance; LDL, low-density lipoprotein.
Value significantly different from the corresponding Normal IHTG group value, P < 0.05.
Fatty acid kinetics
Total palmitate Ra was higher in subjects with increased IHTG than normal IHTG content during both basal (127 ± 13 μmol/min vs. 87 ± 7 μmol/min; P < 0.01) and hyperinsulinemic clamp conditions (44 ± 8 μmol/min vs. 24 ± 2 μmol/min; P < 0.01) (Figure 1a). During insulin infusion, plasma fatty acid concentrations decreased to 0.060 ± 0.008 and 0.094 ± 0.016 μmol/ml in the normal and high IHTG groups, respectively, but the differences between groups were not statistically significant because of the variability in values and small number of subjects. Palmitate Ra expressed per unit of FM was greater in the NAFLD than the normal IHTG group during basal conditions (3.06 ± 0.34 vs. 2.10 ± 0.19 μmol/kg FM/min; P = 0.01) and hyperinsulinemia (1.09 ± 0.22 vs. 0.59 ± 0.08 μmol/kg FM/min; P = 0.01) (Figure 1b). Palmitate rate of disappearance per unit of FFM was also greater in the NAFLD than normal IHTG group during both basal conditions (1.99 ± 0.15 vs. 1.47 ± 0.13 μmol/kgFFM/min; P < 0.01) and hyperinsulinemia (0.69 ± 0.11 vs. 0.40 ± 0.05 μmol/kg FFM/min; P < 0.01) (Figure 1c).
Figure 1.
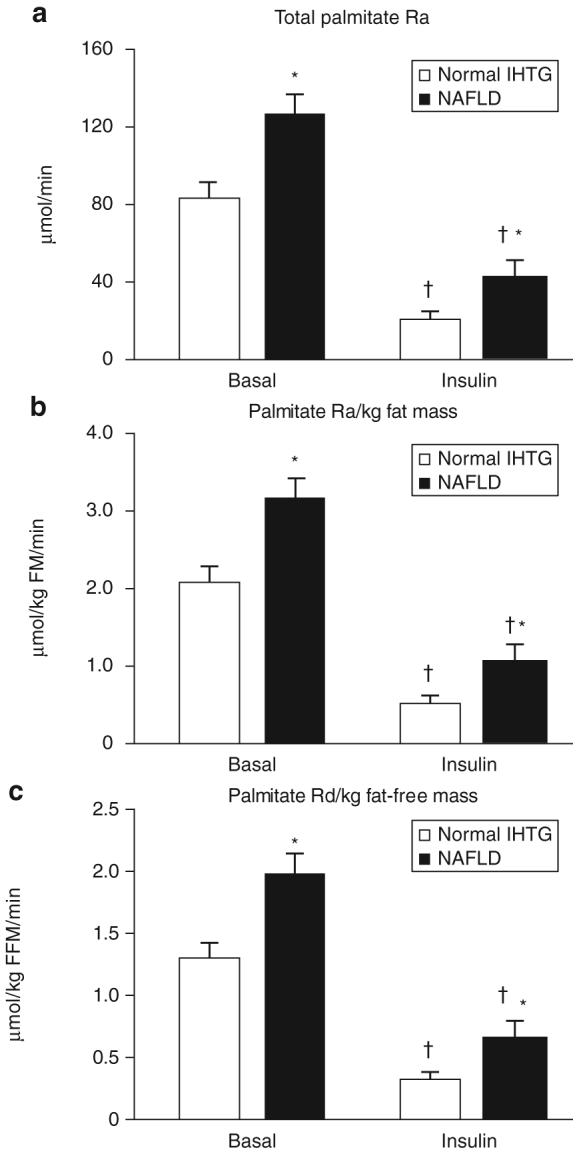
Whole-body palmitate kinetics during basal conditions and insulin infusion in subjects with normal and increased (nonalcoholic fatty liver disease (NAFLD)) intrahepatic triglyceride (IHTG) content. The data are expressed as (a) total palmitate rate of appearance (Ra) into plasma, (b) palmitate Ra per kg fat mass (FM), which represents adipose tissue lipolytic activity in relationship to endogenous fat stores, and (c) palmitate rate of disappearance (Rd) from plasma per kg fat-free mass (FFM), which represents FFA availability to oxidative tissues.*Value significantly different from corresponding value in the normal IHTG group, P ≤ 0.01. †Value significantly different from corresponding basal value, P < 0.001.
DISCUSSION
Circulating insulin is the major regulator of adipose tissue lipolytic activity during both basal and postprandial conditions (25,26). It has been hypothesized that impaired insulin-mediated suppression of lipolysis is involved in the pathogenesis and pathophysiology of NAFLD (5,27). Excessive release of fatty acids from adipose tissue and their increased delivery to liver and skeletal muscle can cause steatosis and contribute to muscle and liver insulin resistance (12,14,15,28) commonly observed in NAFLD (5,11). In this study, we evaluated FFA metabolism in obese adolescents who had normal or increased IHTG content to test the hypothesis that NAFLD is associated with adipose tissue insulin resistance and adverse alterations in FFA metabolism. The hyperinsulinemic-euglycemic clamp procedure, in conjunction with stable isotopically labeled tracer infusion, was used to evaluate FFA metabolism during basal (postabsorptive state) conditions and physiological hyperinsulinemia (postprandial condition) (21). Subjects with normal and increased IHTG content were carefully matched on age, sex, Tanner stage, BMI, and percent body fat to eliminate potential confounding factors that might influence FFA metabolism and insulin action.
The findings from this study demonstrate that NAFLD is associated with marked alterations in fatty acid metabolism during both basal conditions and physiological hyperinsulinemia. During the basal state, fatty acid kinetics, expressed as total FFA Ra (index of FFA released into the circulation per person), FFA released per unit of fat mass (index of adipose tissue lipolytic activity), and the rate of FFA uptake per unit of FFM (index of FFA available for use by the major oxidative tissues), were higher in subjects with increased IHTG content than those with normal IHTG. Although lipolytic rates decreased in both groups during insulin infusion, the rate of FFA release into plasma, expressed as total, per unit of fat mass, or per unit of FFM, was twofold greater in subjects with NAFLD than those with normal IHTG content. Therefore, adipose tissue lipolytic activity and the rate of FFA release into the circulation are constantly greater in obese adolescents with NAFLD than in those with normal IHTG throughout the day.
The results from our study suggest that the failure of insulin to adequately suppress lipolysis and the subsequent increased delivery of FFA to liver and muscle is involved in the development of both steatosis and insulin resistance in obese adolescents who have NAFLD. Intrahepatic fatty acids that are not oxidized by the liver for fuel are reesterified to triglyceride and will accumulate in the liver if triglyceride production exceeds triglyceride export within very low-density lipoprotein particles. Therefore, excessive hepatic fatty acid uptake will increase IHTG content (29). In addition, increased plasma FFA availability impairs the ability of insulin to suppress hepatic glucose production and stimulate muscle glucose uptake (30-34). Intracellular fatty acids and fatty acid metabolites can interfere with the insulin signaling cascade directly (28,35) or indirectly, by increasing inflammatory mediators (36).
In adults, NAFLD is usually associated with other metabolic complications of abdominal obesity, and it has been proposed that NAFLD should be considered a component of the metabolic syndrome (10). The data from this study, in conjunction with our recent findings that NAFLD is associated with insulin resistance in both liver and muscle (11), suggest that increased IHTG content in obese nondiabetic adolescents is part of a systemic derangement in insulin action that involves liver, skeletal muscle, and adipose tissue. Moreover, the relationship between NAFLD and insulin resistance was independent of age, sex, Tanner stage, BMI, and percent body fat. Therefore, IHTG content could be a useful clinical marker that can help identify obese adolescents who are at increased risk of developing obesity-related metabolic diseases.
In summary, the results from this study support the notion that adipose tissue insulin resistance is involved in the pathogenesis of steatosis and contributes to the metabolic complications associated with NAFLD. The rate of FFA release into plasma was much greater in obese adolescents with NAFLD during both postabsorptive conditions and physiological hyperinsulinemia suggesting that liver and muscle are continually exposed to an excessive FFA load, which can result in an accumulation of intracellular triglycerides and impair insulin action. These data help explain why increased IHTG content in obese adolescents is associated with a systemic derangement in insulin action that involves liver, skeletal muscle, and adipose tissue.
ACKNOWLEDGMENTS
We thank the nursing staff of the Center for Applied Research Sciences for their help in performing the studies, Freida Custodio, Jennifer Shew, Adewole Okunade, and Gary Skolnick for their technical assistance, and the study subjects for their participation. This study was supported by National Institutes of Health grants DK 37948, DK 56341 (Clinical Nutrition Research Unit), RR024992 (Clinical and Translational Science Award), and RR-00954 (Biomedical Mass Spectrometry Resource). No conflicts of interest exist.
Footnotes
DISCLOSURE
The authors declared no conflict of interest.
REFERENCES
- 1.Sagi R, Reif S, Neuman G, et al. Nonalcoholic fatty liver disease in overweight children and adolescents. Acta Paediatr. 2007;96:1209–1213. doi: 10.1111/j.1651-2227.2007.00399.x. [DOI] [PubMed] [Google Scholar]
- 2.Lerret SM, Skelton JA. Pediatric nonalcoholic fatty liver disease. Gastroenterol Nurs. 2008;31:115–119. doi: 10.1097/01.SGA.0000316530.31366.6e. [DOI] [PubMed] [Google Scholar]
- 3.Tominaga K, Kurata JH, Chen YK, et al. Prevalence of fatty liver in Japanese children and relationship to obesity. An epidemiological ultrasonographic survey. Dig Dis Sci. 1995;40:2002–2009. doi: 10.1007/BF02208670. [DOI] [PubMed] [Google Scholar]
- 4.Franzese A, Vajro P, Argenziano A, et al. Liver involvement in obese children. Ultrasonography and liver enzyme levels at diagnosis and during follow-up in an Italian population. Dig Dis Sci. 1997;42:1428–1432. doi: 10.1023/a:1018850223495. [DOI] [PubMed] [Google Scholar]
- 5.Korenblat KM, Fabbrini E, Mohammed BS, Klein S. Liver, muscle, and adipose tissue insulin action is directly related to intrahepatic triglyceride content in obese subjects. Gastroenterology. 2008;134:1369–1375. doi: 10.1053/j.gastro.2008.01.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seppala-Lindroos A, Vehkavaara S, Hakkinen AM, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab. 2002;87:3023–3028. doi: 10.1210/jcem.87.7.8638. [DOI] [PubMed] [Google Scholar]
- 7.Gastaldelli A, Cusi K, Pettiti M, et al. Relationship between hepatic/visceral fat and hepatic insulin resistance in nondiabetic and type 2 diabetic subjects. Gastroenterology. 2007;133:496–506. doi: 10.1053/j.gastro.2007.04.068. [DOI] [PubMed] [Google Scholar]
- 8.Sanyal AJ, Campbell-Sargent C, Mirshahi F, et al. Nonalcoholic steatohepatitis: association of insulin resistance and mitochondrial abnormalities. Gastroenterology. 2001;120:1183–1192. doi: 10.1053/gast.2001.23256. [DOI] [PubMed] [Google Scholar]
- 9.Bugianesi E, Gastaldelli A, Vanni E, et al. Insulin resistance in non-diabetic patients with non-alcoholic fatty liver disease: sites and mechanisms. Diabetologia. 2005;48:634–642. doi: 10.1007/s00125-005-1682-x. [DOI] [PubMed] [Google Scholar]
- 10.Marchesini G, Brizi M, Bianchi G, et al. Nonalcoholic fatty liver disease: a feature of the metabolic syndrome. Diabetes. 2001;50:1844–1850. doi: 10.2337/diabetes.50.8.1844. [DOI] [PubMed] [Google Scholar]
- 11.Deivanayagam S, Mohammed BS, Vitola BE, et al. Nonalcoholic fatty liver disease is associated with hepatic and skeletal muscle insulin resistance in overweight adolescents. Am J Clin Nutr. 2008;88:257–262. doi: 10.1093/ajcn/88.2.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelley DE, Mandarino LJ. Fuel selection in human skeletal muscle in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 13.Boden G. Interaction between free fatty acids and glucose metabolism. Curr Opin Clin Nutr Metab Care. 2002;5:545–549. doi: 10.1097/00075197-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 14.Boden G. Role of fatty acids in the pathogenesis of insulin resistance and NIDDM. Diabetes. 1997;46:3–10. [PubMed] [Google Scholar]
- 15.Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983;72:1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Szczepaniak LS, Nurenberg P, Leonard D, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–e468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 17.Genton L, Hans D, Kyle UG, Pichard C. Dual-energy X-ray absorptiometry and body composition: differences between devices and comparison with reference methods. Nutrition. 2002;18:66–70. doi: 10.1016/s0899-9007(01)00700-6. [DOI] [PubMed] [Google Scholar]
- 18.Abate N, Burns D, Peshock RM, Garg A, Grundy SM. Estimation of adipose tissue mass by magnetic resonance imaging: validation against dissection in human cadavers. J Lipid Res. 1994;35:1490–1496. [PubMed] [Google Scholar]
- 19.Frimel TN, Deivanayagam S, Bashir A, O’Connor R, Klein S. Assessment of intrahepatic triglyceride content using magnetic resonance spectroscopy. J Cardiometab Syndr. 2007;2:136–138. doi: 10.1111/j.1559-4564.2007.07168.x. [DOI] [PubMed] [Google Scholar]
- 20.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am J Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 21.Maffeis C, Bonadonna RC, Consolaro A, et al. Ghrelin, insulin sensitivity and postprandial glucose disposal in overweight and obese children. Eur J Endocrinol. 2006;154:61–68. doi: 10.1530/eje.1.02055. [DOI] [PubMed] [Google Scholar]
- 22.Patterson BW, Zhao G, Elias N, Hachey DL, Klein S. Validation of a new procedure to determine plasma fatty acid concentration and isotopic enrichment. J Lipid Res. 1999;40:2118–2124. [PubMed] [Google Scholar]
- 23.Steele R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- 24.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 25.Horowitz JF, Klein S. Whole body and abdominal lipolytic sensitivity to epinephrine is suppressed in upper body obese women. Am J Physiol Endocrinol Metab. 2000;278:E1144–E1152. doi: 10.1152/ajpendo.2000.278.6.E1144. [DOI] [PubMed] [Google Scholar]
- 26.Stumvoll M, Jacob S, Wahl HG, et al. Suppression of systemic, intramuscular, and subcutaneous adipose tissue lipolysis by insulin in humans. J Clin Endocrinol Metab. 2000;85:3740–3745. doi: 10.1210/jcem.85.10.6898. [DOI] [PubMed] [Google Scholar]
- 27.Fabbrini E, Mohammed BS, Magkos F, et al. Alterations in adipose tissue and hepatic lipid kinetics in obese men and women with nonalcoholic fatty liver disease. Gastroenterology. 2008;134:424–431. doi: 10.1053/j.gastro.2007.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 29.Goldberg IJ, Ginsberg HN. Ins and outs modulating hepatic triglyceride and development of nonalcoholic fatty liver disease. Gastroenterology. 2006;130:1343–1346. doi: 10.1053/j.gastro.2006.02.040. [DOI] [PubMed] [Google Scholar]
- 30.Mittelman SD, Bergman RN. Inhibition of lipolysis causes suppression of endogenous glucose production independent of changes in insulin. Am J Physiol Endocrinol Metab. 2000;279:E630–E637. doi: 10.1152/ajpendo.2000.279.3.E630. [DOI] [PubMed] [Google Scholar]
- 31.Boden G, Jadali F, White J, et al. Effects of fat on insulin-stimulated carbohydrate metabolism in normal men. J Clin Invest. 1991;88:960–966. doi: 10.1172/JCI115399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Boden G, Chen X. Effects of fat on glucose uptake and utilization in patients with non-insulin-dependent diabetes. J Clin Invest. 1995;96:1261–1268. doi: 10.1172/JCI118160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 35.Yu C, Chen Y, Cline GW, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 36.Tripathy D, Mohanty P, Dhindsa S, et al. Elevation of free fatty acids induces inflammation and impairs vascular reactivity in healthy subjects. Diabetes. 2003;52:2882–2887. doi: 10.2337/diabetes.52.12.2882. [DOI] [PubMed] [Google Scholar]


