Abstract
Mixing liposomes with hydrophilic particles will induce fusion of the liposome onto the particle surface. Such supported bilayers have been extensively studied as a model for the cell membrane, while its application in drug delivery has not been pursued. In this communication, we report the use of phospholipids to achieve synergistic loading and encapsulating of a fluorescent dye (calcein) in mesoporous silica nanoparticles, and its delivery into mammalian cells. We found that cationic lipid DOTAP provides the highest calcein loading with the concentration inside silica ∼110× higher than that in the solution under experimental conditions. Compared to some other nanoparticle systems, protocells provide a simple construct for loading, sealing, delivering and releasing, and should serve as a useful system in nanomedicine.
One of the major challenges in nanomedicine is to engineer nanostructures and materials that can efficiently encapsulate drugs at high concentration, cross the cell membrane, and controllably release the cargo at the target site over a prescribed period of time.1 Recently, inorganic nanoparticles, including gold, silica, and carbon nanotubes have emerged as a new generation of drug/therapy delivery vehicles in nanomedicine.2,3 Mesoporous silica nanoparticles are particularly attractive in this regard, because of their biocompatibility and their precisely defined nanoporosity.4-7 With uniform, tunable pore diameters, ranging from ∼2-5-nm and surfaces areas of 700-1500 m2/g, drugs and other components can be loaded by adsorption or capillary filling, and the release profiles adjusted by the combination of pore size and pore surface chemistry.8 Very recently, elegant gating methods, employing coumarin,9 azobenzene,10,11 rotaxane,12 polymers,13,14 or small nanoparticles,15,16 have been established to seal the cargo within the particle and allow its triggered release according to an optical or electrochemical stimulus. Here we describe a synergistic system where liposome fusion on a mesoporous silica particle core simultaneously loads and seals the cargo, creating a ‘protocell’ construct useful for delivery across the cell membrane (Fig. 1B). We observe that fusion of a positively charged liposome on a negatively charged mesoporous silica core serves to load the core with a negatively charged dye (excluded from the mesopores without lipid) to concentrations that can exceed 100x those in solution. Sealed within the protocell, this membrane impermeable dye can be transported across the cell membrane and slowly released within the cell. Compared to other nanoparticle delivery systems, the protocell is simple and takes advantage of the low toxicity and immunogenicity of liposomes along with their ability to be PEGylated or conjugated to extend circulation time and effect targeting. Compared to liposomes, however, the protocell is more stable and takes advantage of the mesoporous core to control both loading and release. As noted in many other relevant papers the mesoporous core can be used additionally to deliver DNA, hydrophobic anti-cancer drugs and proteins.17-19
Figure 1.
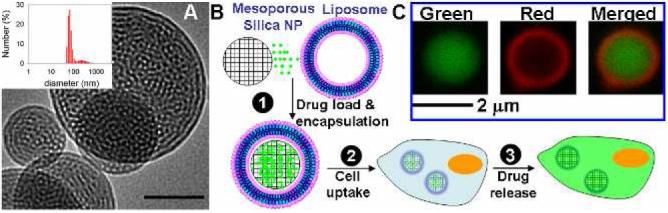
(A) Representative TEM image of mesoporous silica nanoparticles cores. Scale bar = 50 nm. Inset: dynamic light scattering histogram. (B) Simultaneous loading and encapsulation of drugs through liposome fusion on mesoporous silica nanoparticles (1); cellular uptake of loaded protocells (2); and release within cells (3). (C) Confocal fluorescence images of the green-labeled mesoporous core, red-labeled lipid bilayer, and merged image confirming the postulated construct.
Mesoporous silica particle ‘cores’ were prepared by the surfactant templated aerosol-assisted self-assembly method developed in our group.20 After removal of surfactant templates, we obtain hydrophilic nanoparticles characterized by a uniform, ordered, and connected mesoporosity with a specific surface area of 935 m2/g. A representative TEM image of the mesoporous particles is shown in Fig. 1A along with a dynamic light scattering histogram, indicating the average particle size to be ∼100-nm. Liposomes were prepared by extrusion of hydrated lipid films through a filter with pore size of 100 nm using standard protocols and fused with the cores by pipette mixing. Such supported bilayers have been studied extensively as model systems for cell membranes,21,22 whereas their applications in drug delivery have yet to be explored.
Although the sizes of most silica nanoparticles were below the optical resolution of fluorescence microscopy, a small fraction of large nanoparticles were obtained by sedimentation. Fig. 1C shows a confocal image of a porous nanoparticle supported lipid bilayer, where the core was labeled with FITC and the lipid was labeled with Texas Red. A green core and a red shell are clearly observed, confirming liposome fusion on the mesoporous core.
To demonstrate the concept of loading and sealing the silica core through liposome fusion, calcein was chosen as a model drug/probe, because it is membrane impermeable and fluorescent. Incubated with the silica core in the absence of liposomes, the negatively charged dye does not enter the internal mesoporosity due to electrostatic considerations, and the silica cores concentrated at the bottom of the tube are colorless (Fig. 2A, inset). Calcein uptake accompanying liposome fusion was determined by incubation of mesoporous cores in a calcein solution to which liposomes were added. After incubation for 10 minutes, unincorporated dye was removed by multiple rounds of centrifugation, supernatant removal, and washing until no calcein was detectable in the supernatant. The encapsulated calcein was quantified by adding a surfactant (SDS) to release the dye into solution and measuring its absorbance at 500 nm. Fig. 2A shows absorbance measurements as a function of lipid composition conducted with liposomes composed of the zwitterionic lipid 1,2-Dioleoyl-sn-Glycero-3-Phosphocholine (DOPC) plus varying percentages of the negatively charged lipid 1,2-Dioleoyl-sn-Glycero-3-Phosphoserine (DOPS) or positively charged lipid 1,2-Dioleoyl-3-Trimethylammonium-Propane (DOTAP). We observe essentially no calcein loading of the mesoporous core fused with DOPC or liposomes containing the negatively charged DOPS. This is consistent with the fact that, without liposomes, calcein does not enter the pores, and, for the limiting case of 100% DOPS, there is no liposome fusion with the core (see supporting information), emphasizing the role of electrostatic interactions in loading and fusion. In contrast, for protocells formed by fusion of positively charged DOTAP, we observe an exponential increase in calcein loading with percentage DOTAP (Fig. 2A). These results reveal a synergistic system where loading of the negatively charged drug model occurs only by fusion of the positively charged liposome with the negatively charged core. Loading is controlled by the lipid composition, independently of cholesterol, and, as evident in Fig. 2A inset, the core calcein concentration can exceed greatly that in solution (enrichment factor ∼110x for the 100% DOTAP composition, see supporting information). Confocal imaging (Fig. 2B) of representative sub-μm diameter protocells reveals the calcein loading to occur throughout the volume of the core. For cores exceeding several μms in diameter, loading was confined to a ∼0.5-μm thick shell at the core surface. Calcein release profiles determined in buffer for the DOTAP protocell showed 90% release in 18 days (see supporting information). It is important to note that the loading and release for the supported lipid bilayer protocell construct is qualitatively different than that of conventional liposomes, where the cargo concentration is approximately that of the solution in which liposomes are formed (no enrichment) and the release is practically instantaneous.
Figure 2.

(A) Calcein incorporation in mesoporous silica cores after fusion with DOPC/DOTAP or DOPC/DOPS liposomes. Inset: optical image of centrifuged mesoporous silica cores (indicated by the arrows) after incubation with 250 μM calcein solutions with and without DOTAP liposomes. (B) Confocal fluorescence images of calcein loaded protocells formed by fusion of Texas Red-labeled DOTAP liposomes. Green channel, calcein; red channel, Texas Red; merged = red + green.
To study protocell entry into mammalian cells and the feasibility of their use for drug delivery, protocells prepared by fusion of Texas Red-labeled DOTAP liposomes on FITC-labeled mesoporous silica cores (as in Fig. 1C, but sub-μm in diameter) were incubated with Chinese Hamster Ovary cells (CHO) at 37 °C for 4 hrs. The cells were then washed extensively and imaged via confocal fluorescence microscopy. As observed in Fig. 3A, most cells exhibited overlapped green and red fluorescence, suggesting that the core and lipid bilayer were incorporated concurrently as expected for endocytosis. (Additional, ‘smeared-out’ red emission indicates some loss of liposome from the core through either lipid exchange or membrane fusion). To test delivery from the synergistically loaded and sealed protocells, calcein loaded protocells were prepared from DOTAP and incubated with cells as in Fig. 1B. Green fluorescence was observed throughout the cells (Fig. 3C). In contrast, when membrane impermeable, free calcein was incubated with CHO cells, no cellular uptake was observed (Fig. 3B). From a pH-dependent study performed in vitro, we determined calcein release to be much faster at lower pH compared to that at pH 8 (see Supporting Information). Because the loaded protocells most likely reside in endosomal compartments with a localized pH of ∼5.0, release should be facilitated inside the cells as evident from dimmer fluorescence adjoining brighter regions within cells (Fig. 3C), which we postulate to be calcein release into the cytosol.
Figure 3.
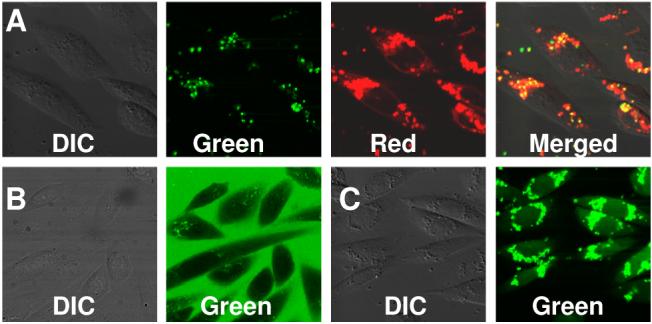
Confocal fluorescence images of protocells with CHO cells. (A) FITC-labeled core and Texas Red-labeled DOTAP shell. (B) CHO incubated with free calcein in media. (C) CHO incubated with calcein encapsulated in supported DOTAP bilayers.
In summary, we have shown that fusion of a positively charged liposome with a negatively charged mesoporous silica core synergistically loads and seals a negatively charged cargo within the core. Adjustment of the liposome composition/charge allows control of the cargo content, whose concentration can exceed greatly that of the surrounding solution. The protocells can be internalized by mammalian cells and, due to enhanced release at lower pH, deliver their contents within the cell. Compared to some other nanoparticle systems, protocells provide a simple construct for loading, sealing, delivering, and releasing, and should serve as a useful vector in nanomedicine.
Supplementary Material
Acknowledgment
This work is funded by Grant Number PHS 2 PN2 EY016570B from the National Institutes of Health through the NIH Roadmap for Medical Research.
Footnotes
Supporting Information Available: Experimental Section and particle characterizations. This material is available free of charge via the Internet at http://pubs.acs.org
References
- 1.Langer R. Nature. 1998;392:5–10. [PubMed] [Google Scholar]
- 2.Sokolova V, Epple M. Angew. Chem., Int. Ed. 2008;47:1382–1395. doi: 10.1002/anie.200703039. [DOI] [PubMed] [Google Scholar]
- 3.Rosi NL, Mirkin CA. Chem. Rev. 2005;105:1547–1562. doi: 10.1021/cr030067f. [DOI] [PubMed] [Google Scholar]
- 4.Vallet-Regi M, Balas F, Arcos D. Angew. Chem., Int. Ed. 2007;46:7548–7558. doi: 10.1002/anie.200604488. [DOI] [PubMed] [Google Scholar]
- 5.Slowing II, Trewyn BG, Giri S, Lin VSY. Adv. Func. Mater. 2007;17:1225–1236. [Google Scholar]
- 6.Julian-Lopez B, Boissiere C, Chaneac C, Grosso D, Vasseur S, Miraux S, Duguet E, Sanchez C. Journal Of Materials Chemistry. 2007;17:1563–1569. [Google Scholar]
- 7.Schlossbauer A, Schaffert D, Kecht J, Wagner E, Bein T. J. Am. Chem. Soc. 2008;130:12558–12559. doi: 10.1021/ja803018w. [DOI] [PubMed] [Google Scholar]
- 8.Jiang XM, Brinker CJ. J. Am. Chem. Soc. 2006;128:4512–4513. doi: 10.1021/ja058260+. [DOI] [PubMed] [Google Scholar]
- 9.Mal NK, Fujiwara M, Tanaka Y. Nature. 2003;421:350–353. doi: 10.1038/nature01362. [DOI] [PubMed] [Google Scholar]
- 10.Liu NG, Dunphy DR, Atanassov P, Bunge SD, Chen Z, Lopez GP, Boyle TJ, Brinker CJ. Nano Letters. 2004;4:551–554. [Google Scholar]
- 11.Angelos S, Choi E, Vogtle F, De Cola L, Zink JI. Journal Of Physical Chemistry C. 2007;111:6589–6592. [Google Scholar]
- 12.Nguyen TD, Tseng HR, Celestre PC, Flood AH, Liu Y, Stoddart JF, Zink JI. Proc. Natl. Acad. Sci. USA. 2005;102:10029–10034. doi: 10.1073/pnas.0504109102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang YJ, Caruso F. Chem. Commum. 2004:1528–1529. doi: 10.1039/b403871a. [DOI] [PubMed] [Google Scholar]
- 14.Radu DR, Lai CY, Wiench JW, Pruski M, Lin VSY. J. Am. Chem. Soc. 2004;126:1640–1641. doi: 10.1021/ja038222v. [DOI] [PubMed] [Google Scholar]
- 15.Lai CY, Trewyn BG, Jeftinija DM, Jeftinija K, Xu S, Jeftinija S, Lin VSY. J. Am. Chem. Soc. 2003;125:4451–4459. doi: 10.1021/ja028650l. [DOI] [PubMed] [Google Scholar]
- 16.Giri S, Trewyn BG, Stellmaker MP, Lin VSY. Angew. Chem., Int. Ed. 2005;44:5038–5044. doi: 10.1002/anie.200501819. [DOI] [PubMed] [Google Scholar]
- 17.Torney F, Trewyn BG, Lin VSY, Wang K. Nature Nanotechnology. 2007;2:295–300. doi: 10.1038/nnano.2007.108. [DOI] [PubMed] [Google Scholar]
- 18.Slowing II, Trewyn BG, Lin VSY. J. Am. Chem. Soc. 2007;129:8845–8849. doi: 10.1021/ja0719780. [DOI] [PubMed] [Google Scholar]
- 19.Lu J, Liong M, Zink JI, Tamanoi F. Small. 2007;3:1341–1346. doi: 10.1002/smll.200700005. [DOI] [PubMed] [Google Scholar]
- 20.Lu YF, Fan HY, Stump A, Ward TL, Rieker T, Brinker CJ. Nature. 1999;398:223–226. [Google Scholar]
- 21.Grakoui A, Bromley SK, Sumen C, Davis MM, Shaw AS, Allen PM, Dustin ML. Science. 1999;285:221–227. [PubMed] [Google Scholar]
- 22.Cornell BA, BraachMaksvytis VLB, King LG, Osman PDJ, Raguse B, Wieczorek L, Pace RJ. Nature. 1997;387:580–583. doi: 10.1038/42432. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


