Abstract
The cross-linking interactions that provide cohesive strength to molluscan adhesive gels were investigated. Metal-based interactions have been shown to play an important role in the glue of the slug Arion subfuscus (Draparnaud), but other types of interactions may also contribute to the glue's strength and their role has not been investigated. This study shows that treatments that normally disrupt hydrophobic or electrostatic interactions have little to no effect on the slug glue. High salt concentrations and non-ionic detergent do not affect the solubility of the proteins in the glue or the ability of the glue proteins to stiffen gels. In contrast, metal chelation markedly disrupts the gel. Experiments with gel filtration chromatography identify a 40 kDa protein that is a central component of the cross-links in the glue. This 40 kDa protein forms robust macromolecular aggregations that are stable even in the presence of high concentrations of salt, non-ionic detergent, urea or metal chelators. Metal chelation during glue secretion, however, may block some of these cross-links. Such robust, non-specific interactions in an aqueous environment are highly unusual for hydrogels and reflect an intriguing cross-linking mechanism.
Keywords: Adhesion, cross-link, electrostatic, gastropod, gel, glue, hydrophobic
Introduction
Many organisms produce glues that differ markedly from man-made glues. One major difference is that biological glues are typically compatible with water. They range from long-term adhesives that may have low water contents to more transient adhesives that often have higher water contents. Despite the presence of water on all the surfaces and in some cases as a major component of the glue itself, these glues create remarkably strong attachments. Determining how they work would help guide a biomimetic approach to developing new glues. Such glues could have a variety of practical applications, especially in the medical field (Graham, in press; Smith and Callow, 2006).
Among the more extreme examples of biological glues with high water contents are the adhesive gels produced by gastropod mollusks. These gels often consist of 97% water yet they can produce attachment forces strong enough that animals such as limpets can be remarkably difficult to detach by hand. Adhesive forces per unit area for limpets are often in the range of a few hundred kilopascals or more (Smith, 2002; 2006). Such adhesive gels are relatively common in the Mollusca, with periwinkle snails, terrestrial snails and terrestrial slugs using them as well as limpets (Smith 2006).
A major goal in the study of these adhesives is to determine the mechanism by which they can resist such strong forces. The typical structure of molluscan mucus consists of a mixture of macromolecules, often dominated by extraordinarily large (1000+ kDa) polymers that tangle up to form a loose network (Smith, 2002, 2006). In the case of molluscan adhesive gels, specific proteins characterize the glue (Smith et al., 1999; Smith and Morin, 2002; Pawlicki et al., 2004) and these appear to control the mechanics of the gel (Pawlicki et al., 2004). These have been referred to as glue proteins. The glue proteins are likely to be involved in cross-linking the gel, thus stiffening it considerably.
The mechanism of cross-linking for these adhesive gels is still unknown though, and it would have to be a mechanism that worked effectively in the presence of water. Pawlicki et al. (2004) found that the glue proteins stiffen gels made of negatively charged polymers. Based on this and the fact that the glue proteins carried a relatively high percentage of charged amino acids, the authors initially suggested that electrostatic interactions may be involved in cross-linking. These interactions are relatively weak underwater, however, due to the high dielectric constant of the medium. Alternatively hydrophobic interactions may play a key role, as they do for barnacle cements (Kamino, 2006). These would work well underwater, and Pawlicki et al. (2004) found that the presence of non-ionic detergents improved their ability to isolate the glue proteins. The importance of these interactions has not been tested, though.
Recently, the possible role of hydrophobic or electrostatic interactions has been overshadowed by the finding that transition metals are present in the glue of the terrestrial slug Arion subfuscus and these metals play a central role in the cross-linking ability of the glue proteins (Werneke et al., 2007). A metal-based cross-linking mechanism would be appealing as it would provide strong cross-links underwater. Transition metals have been shown to be important in other glues and biomaterials (Lichtenegger et al., 2003, 2005; Sever et al., 2004; Waite et al., 2005; Broomell et al., 2006; Sagert et al., 2006). Li and Graham (2007) also found that the trail/defensive mucus of the slug Lehmannia valentiana contains proteins that would likely be able to bind metal cations such as Ca2+ and Mg2+ based on their primary sequence. This is particularly interesting because of the possible similarities between this slug's adhesive mucus and the mucus of A. subfuscus. They are both terrestrial slugs of the order Stylommatophora, and the size of many of the proteins in the adhesive mucus is similar.
The demonstration that metal-based interactions play a central role in the cross-linking of molluscan adhesive gels does not eliminate the possibility that other types of non-covalent interactions assist in cross-linking. These may even play equally important roles. Many commercial gels are cross-linked by hydrogen bonds, electrostatic interactions and hydrophobic interactions (Smith, 2002). These types of bonds also play a large role in microbial adhesion (McEldowney and Fletcher, 1986). Such interactions are common among biomolecules, and they may play a significant role in these glues. To better understand the adhesive gels produced by mollusks, this paper will test whether these non-covalent interactions play a significant role. This information should give us a fuller picture of the mechanism of cross-linking.
Materials and Methods
Animals
Experiments were performed on glue collected from the slug Arion subfuscus (Draparnaud). This animal produces copious quantities of a defensive secretion that rapidly stiffens into a sticky, elastic mass (Werneke et al., 2007). This species has been used because its glue can be collected before or after setting and it is secreted in the quantities necessary to perform many of the experiments. Slugs were collected locally, and glue samples were taken on the day of collection as described by Werneke et al. (2007).
Solubility
To test for hydrophobic interactions, the solubility of A. subfuscus glue was measured in buffers with and without the non-ionic detergent Tween 20. In these trials, large, freshly collected samples of glue from individual slugs were divided into equal parts. Each was placed in a 50 mM Tris-Cl (pH 6.8) buffer with or without 0.5% Tween 20. The final concentration of the glue was 20 mg wet mass per mL of buffer. Eight different trials were performed using different forms of mechanical disruption to dissolve the sample. These typically included one to three cycles of vortexing and heating to 60 or 80°C lasting several minutes each, separated by 40 to 60 minute incubations at room temperature. In addition, sonication or homogenization steps of ten seconds to a minute were often added after one of the heating steps. Though the exact procedure varied slightly between trials, within each trial the control sample and the sample with detergent were treated in the exact same way. Typically, the sample was dissolved over a period of two hours. Samples were then centrifuged at 14,000 rpm for ten minutes and the supernatant with dissolved proteins was collected for analysis. Several additional tests were performed dissolving the samples without heating, in order to be sure that proteins were not aggregating due to the heating.
The protein content of the supernatants was analyzed by discontinuous sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) using the detailed protocols of Hames (1990), as described by Pawlicki et al. (2004). The gels contained 12.5% total acrylamide, and they were stained with Coomassie Blue R-250. Three of the trials using the same dissolution procedure were quantified using a Kodak EDAS 290 gel imaging system. Four primary bands or groups of bands were identified for quantification: these were at 100+, 61, 40 and 15 kDa. The 100+ band refers to several bands above 100 kDa that were considered as a unit because they were close together on the gel and difficult to quantify separately. The 15 kDa protein and possibly the 61 kDa protein are characteristic of the glue, while the others are found in both the glue and the non-adhesive mucus (Pawlicki et al., 2004). The net staining intensity above background for each band or group was measured.
The solubility was also tested with high salt concentration to test for electrostatic interactions. Seven trials were run with a similar protocol, except with or without 1 M sodium chloride rather than Tween-20. Three of the trials using the same dissolution procedure were quantified with the gel imaging system.
Several additional solubility trials were performed using metal chelators. Previous work found that the iron chelator deferoxamine mesylate helped dissolve glue that had not set, but had no effect after the glue had set (Werneke et al., 2007). The effect of chelation on glue that has set was repeated with the more general divalent ion chelator ethylenediaminetetraacetic acid (EDTA) (10 mM). These experiments also differed from those of Werneke et al. (2007) in allowing three to five hours for the chelator to act on the cross-links, and in not involving heating.
Two additional trials were performed testing the combination of 1M sodium chloride and 0.5% Tween-20 versus the control. In addition, the effect of temperature alone was tested qualitatively, as many gels such as agar and gelatin are thermally reversible (Williams and Phillips, 2000). Finally, four trials were performed comparing the solubility of the glue in the neutral Tris buffer to its solubility in SDS-PAGE sample buffer (4% SDS, 10% 2-mercaptoethanol, 10% glycerol in 0.25M Tris, pH 6.8), and several trials tested the effect of different concentrations of urea.
Gel-stiffening assay
The ability of the glue proteins to stiffen gels provides a useful assay for their ability to form cross-links. In the tests for hydrophobic interactions, citrus pectin was used as a test gel. This is a large, negatively charged polysaccharide that is likely to have some similarity to the giant polymers in the natural glue (Pawlicki et al., 2004). The non-ionic detergent octyl β-D-glucopyranoside (octyl glucoside or OGP) was used as it disrupts hydrophobic interactions but is less denaturing than other detergents (Stubbs et al., 1976), and it does not affect pectin directly. Even though pectin does not rely on hydrophobic interactions, Tween is sufficiently strong to have non-specific effects on pectin, which would make the data more difficult to interpret. Samples of A. subfuscus glue were dissolved in 20 mM Tris-Cl (pH = 8) with heating to 80° C, vortexing and sonication. The glue extracts were used to stiffen gels, as described by Werneke et al. (2007). Whole glue extracts containing glue proteins were used instead of purified glue proteins because it greatly simplified the experiment and minimized contamination from reagents that had been used previously to separate the proteins in chromatography. Furthermore, it provided a broader test for other possible interactions aside from just those due to the glue proteins.
Four treatments were set up: 1) a pectin control, 2) pectin with 0.2% octyl glucoside to determine if the detergent directly affected the gel, 3) pectin with dissolved glue to demonstrate the normal stiffening effect of the glue proteins, 4) pectin with dissolved glue and 0.2% octyl glucoside to determine if interfering with hydrophobic interactions blocked the ability of the glue proteins to stiffen gels. The final concentrations were 2% pectin, 10 mM Tris and 25 mM CaCl2 to assist gelling. Samples (0.5 mL) were prepared from concentrated stock solutions, mixed, then their storage moduli were measured immediately on a dynamic rheometer (ARES, TA Instruments, New Castle, DE, USA) as described by Pawlicki et al. (2004). Eight sets of these four treatments were tested.
In addition, the ability of the glue proteins to stiffen gels was tested under conditions that would weaken electrostatic interactions. Agar gels were used with these trials because electrostatic interactions play an important role in the normal mechanics of pectin. Preliminary experiments were performed with the dynamic rheometer, but the rheometer did not appear to be measuring stiffness accurately in some cases, possibly due to the way the agar set in the apparatus. The results were more clear and consistent in qualitative observations. Four treatments were set up: 1) agar alone, 2) agar with 1 M NaCl, 3) agar with dissolved slug glue, and 4) agar with dissolved slug glue and 1 M NaCl. All treatments had a final concentration of 0.4% agar in 10 mM Tris. Eight sets of trials were performed, with some sets of trials including salt concentrations of 0.25 and/or 0.5 M as well as 1 M. Samples (0.5 mL) were mixed from concentrated stock solutions, then cooled in a water bath. They were then gently removed from the tube and observed side by side.
Aggregation of proteins in gel filtration chromatography
The presence of interactions between proteins can be analyzed by their tendency to aggregate in gel filtration chromatography (Beeckmans, 1999). In the present study, slug glue was dissolved and analyzed by gel filtration in the presence of reagents that would interfere with specific types of interactions. Sephacryl S-400 gel was used in a 1.6 cm × 70 cm column. Glue samples were dissolved in 20 mM Tris-Cl (pH 8) with the following added reagents and loaded on a column equilibrated with the same buffer unless otherwise noted: 1) no added reagents, 2) 0.5% Tween 20 (0.1% in the column), 3) 1M sodium chloride, 4) 10 mM EDTA, and 5) 8 M urea with 0.5% Tween (6 M and 0.5% in the column respectively). Samples of glue (50 mg per mL of buffer) were homogenized with a rotor-stator homogenizer, incubated at room temperature for 30 to 60 min, then sonicated. One sample using only Tris buffer was not homogenized but instead sonicated until it was dissolved; note that the amount of sonication that was necessary substantially heated the sample. After dissolving, each sample was centrifuged at 10,000 g and the supernatant (1-1.5 mL) was loaded on the column. A peristaltic pump was used to provide a flow rate of 1.5 mL/min. The relative protein content of the eluate was measured with a UV monitor and fractions of roughly 4 mL were collected. The protein content of selected fractions was analyzed with SDS-PAGE. In most cases, fractions were concentrated by drying in a Speed-vac rotary evaporator before electrophoresis. In some cases this was not necessary or gave bad results due to the amount of buffer solute so the gels were either silver-stained (Plus One silver staining kit, Amersham Pharmacia), or analyzed without either treatment.
The column was calibrated using bovine serum albumin (BSA, 66 kDa), ferritin from horse spleen (440 kDa) and blue dextran (∼ 2000 kDa apparent mass). The positions and widths of the peaks were as expected for the conditions used, based on literature provided by Amersham Pharmacia.
Another set of gel filtration experiments was performed on samples where metals were chelated as the glue set. Glue samples from A. subfuscus were collected directly into 20 mM Tris-Cl (pH 8) with 3 mM deferoxamine and 10 mM EDTA as described by Werneke et al. (2007). In brief, freshly caught slugs were placed in a 15 mL tube containing 2-3 mL of buffer. They were gently vortexed to stimulate secretion and mix the metal-chelating buffer with the glue as soon as it was secreted. The sample was then centrifuged, and the supernatant was sonicated briefly then loaded on the S-400 column with 20 mM Tris-Cl (pH 8).
Results
Solubility
Neither non-ionic detergent nor high salt concentration affected the solubility of slug glue (Fig. 1A, B). In all trials with detergent, there was no clear difference from the control in the staining intensity of any of the bands of dissolved proteins. With salt, some trials gave visibly weaker extractions of all proteins, but this was not consistent. The combination of salt and detergent also did not affect extraction (data not shown). For both treatments, SDS-PAGE results of samples that were not heated were not visibly different from the results for samples that were heated (data not shown). Heating the gels up to 100° C did not visibly change the sample. If samples were homogenized, then left overnight, they formed uniformly viscous gels that did not break down without heat or sonication. Overall, the treatments did not dissolve much of the glue; they left a large pellet and only dissolved 5-20% of the protein based on comparison of the intensity of staining on SDS-PAGE with samples dissolved in concentrated SDS. The SDS-containing buffer fully dissolved the glue, leaving no pellet after centrifugation. 8 M urea similarly dissolved most of the glue, but the staining intensity of the extracted proteins was more variable.
Figure 1.
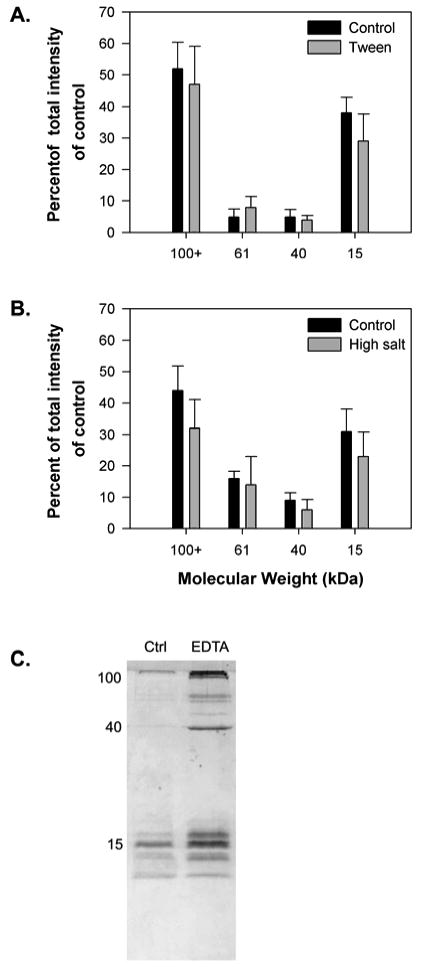
The effect of disrupting hydrophobic interactions, disrupting electrostatic interactions, and removing divalent ions on the solubility of glue from the slug A. subfuscus. A+ B) Quantitative analysis of the effect of the non-ionic detergent Tween-20 (0.5%) and high salt concentration (1 M NaCl) respectively. The bars in A and B show the staining intensity of bands on SDS-PAGE for the four most common proteins in the glue. Band intensities are expressed as a percentage of the total staining intensity of all four bands in the control. Several proteins of greater than 100 kDa are pooled together. The results for each show the mean ± S.D. of three trials dissolved using the same procedure. C) SDS-PAGE showing the effect of divalent ion chelation (10 mM EDTA). The position of three major bands at 15, 40 and 100 kDa is shown on the left.
EDTA had a clear effect on the glue, which became noticeable after extended incubation. After three to five hours of incubation, the gel broke apart upon vortexing and SDS-PAGE showed substantially more dissolved protein (Fig. 1C).
Gel-stiffening assay
The non-ionic detergent octyl glucoside had no effect on the ability of the glue extract to stiffen gels (Fig. 2). The dissolved glue alone more than doubled the stiffness of the pectin (paired Student's t-test, P=0.0002), as was found by Werneke et al. (2007). There was no significant difference, however, between the glue extract with and without detergent (P=0.27). Also, the detergent alone had no significant effect on the gel (P=0.11).
Figure 2.
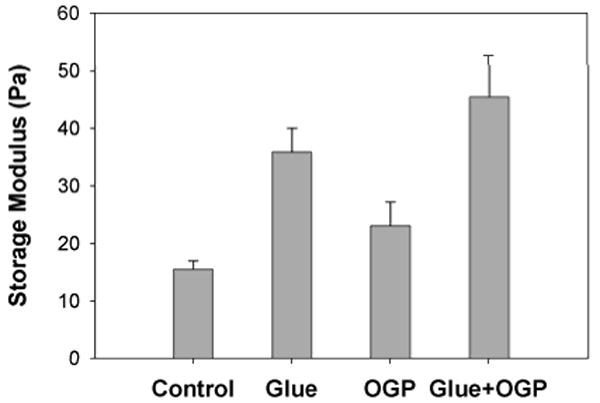
The gel stiffening ability of A. subfuscus glue proteins with or without the use of detergent to block hydrophobic interactions. All gels contained 2% citrus pectin and 10 mM Tris (pH 8). The storage modulus (stiffness) was measured in a dynamic rheometer in the presence of slug glue extract (roughly 0.5 mg/mL total protein), 0.2% octyl glucopyranoside (OGP), or both (Mean ± S.E.M., N = 8).
Similarly, high salt concentration had no visible effect on the ability of the glue to stiffen agar gels (Fig. 3). In all eight trials, the controls were barely gelled while the glue extracts stiffened these gels substantially so that they held the shape of the tube they set in. The presence of salt had no visible effect on this stiffening, and it did not have any clear effect on the controls by itself.
Figure 3.
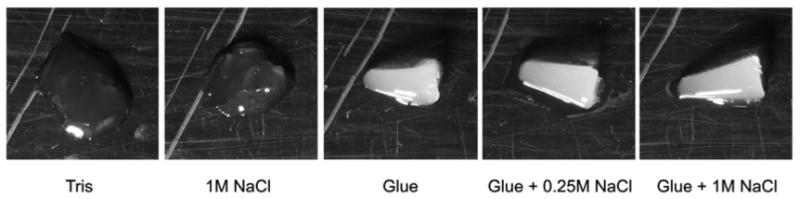
Qualitative results showing the gel-stiffening ability of A. subfuscus glue proteins with or without the use of high salt concentrations to disrupt electrostatic interactions. All gels contained 0.4% agar and 10 mM Tris (pH 8). Samples either had sodium chloride (NaCl), glue extract (roughly 0.5 mg/mL total protein), or both. Note that samples containing the glue appear lighter because of pigment in the glue extract. Samples were mixed in a microcentrifuge tube then gently removed onto a Plexiglas sheet. Firm samples held the shape of the tube.
Aggregation of proteins in gel filtration chromatography
In the chromatography experiments using glue that had set, a significant fraction of the protein eluted in the void volume (41-54 mL) (Figs. 4, 5), indicating that these proteins are in complexes of over 1000 kDa. The primary component of these complexes was a 40 kDa protein, which eluted here rather than its expected elution volume (Fig. 5). The complexes of this protein were not disrupted by salt, Tween, metal chelation or even the combination of 8 M urea and 0.5% Tween. In contrast, the other proteins typically eluted as expected based on their apparent mass in SDS-PAGE. Based on the column calibration, most of the proteins in the glue (roughly 40-200 kDa) should elute between 70-90 mL, with the 15 kDa glue protein eluting after that. It is notable that the 15 kDa protein eluted as expected for its mass and did not appear to be part of the large complex, despite the fact that it has been implicated in gel-stiffening. Finally, there was a large peak between 100 – 120 mL that corresponded to a yellow pigment (Fig. 4). The pigment clearly accounted for most of the absorbance in this peak, as SDS-PAGE often showed less protein in this range than at other volumes, despite the higher UV absorbance. When the samples were sonicated extensively, which also heated the sample, the gel broke down and appeared to dissolve well, but all the proteins aggregated and eluted as a giant complex, not just the 40 kDa protein (Fig 4A, 5A).
Figure 4.
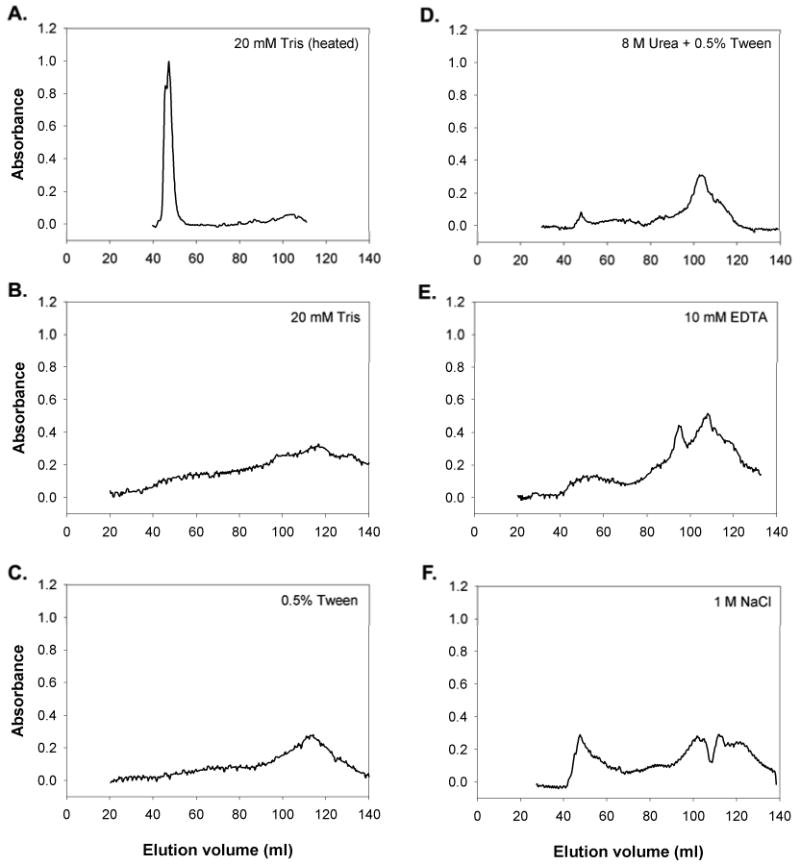
S-400 Gel filtration elution profiles of proteins extracted from the mature glue of the slug A. subfuscus, and the effect of different treatments. The solubilizing buffer was 20 mM Tris-Cl (pH 8) alone (A, B), or with 0.5 % Tween (C), 8 M Urea and 0.5% Tween (D), 10 mM EDTA (E) or 1 M NaCl (F). Sample A was sonicated extensively, which heated the sample. Proteins or macromolecular complexes that are several thousand kDa or larger would elute between 41 and 54 mL, horse ferritin (440 kDa) elutes between 64 and 78 mL, BSA (66 kDa) elutes between 70 and 90 mL, and a yellow pigment in the glue elutes between 100 and 120 mL.
Figure 5.
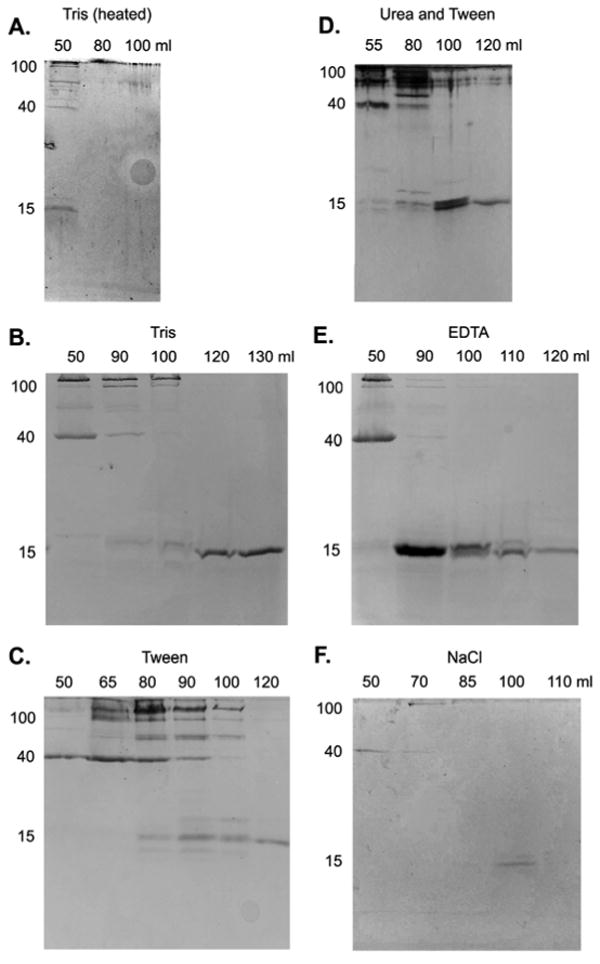
SDS-PAGE of fractions resulting from gel filtration. The solubilizing buffer was 20 mM Tris-Cl (pH 8) alone (A, B), or with 0.5 % Tween (C), 8 M Urea and 0.5% Tween (D), 10 mM EDTA (E) or 1 M NaCl (F). Sample A was sonicated extensively, which heated the sample. The lanes are marked by the elution volume of the fractions they contain. The location of the 15, 40 and 100 kDa proteins are indicated to the left of each. Molecules or complexes eluting between 41 and 54 mL would be larger than 1000 kDa. Horse ferritin (440 kDa) elutes between 64 and 78 mL, BSA (66 kDa) elutes between 70 and 90 mL, and a yellow pigment in the glue elutes between 100 and 120 mL. All gels were stained with Coomassie Blue except D, which was silver stained. B, C and E were concentrated before loading on the gel. The lighter staining in A and F reflects the absence of such treatments.
In trials where the sample was only dissolved in Tris or Tris with Tween, the resolution of the column was poorer. The peaks were much less sharp, with the proteins eluting over a broader range. With Tris alone, the column often became clogged and it was not possible to finish the run. With high salt concentrations the resolution was sharper, and there was a larger peak of 40 kDa proteins in the void volume. It should be noted that samples dissolved in salt tended to have a clearer distinction between dissolved and undissolved material, possibly because the high ion content tended to collapse the gel. Similarly, the EDTA-treated sample gave relatively clear results, and the EDTA broke up the integrity of the gel. There was an additional peak in absorbance at 95 mL for the EDTA sample, and this was consistently present when the sample included a metal chelator, but not in any of the trials without chelation. Note that the decrease in absorbance at 110 mL with the high salt trial was not repeated and may be an artifact.
When samples were collected directly into deferoxamine and EDTA, the protein complex in the void volume was reduced and less consistent (Fig. 6). In the elution profile, the void volume peak was reduced and there was no elevated plateau of absorbance from 50-80 mL as in the other trials. In many of the trials collected into deferoxamine and EDTA, the void volume peak was substantially smaller even than what is seen in Figure 6. In addition, SDS-PAGE demonstrated that the 40 kDa protein was no longer always linked into large complexes. Though it sometimes did elute in the void volume, it was less reliably seen in those fractions. In some cases, it was not detected in the void volume or the fractions expected for proteins of its apparent mass on SDS-PAGE (though it was present in the sample that was loaded onto the column), and in other cases it partially aggregated but also eluted over a range of fractions including those expected for its mass (as seen in Figure 6). It should also be noted that samples collected into the chelators dissolved remarkably rapidly, turning a volume of buffer greater than the volume of the slug yellow and viscous within one or two seconds.
Figure 6.
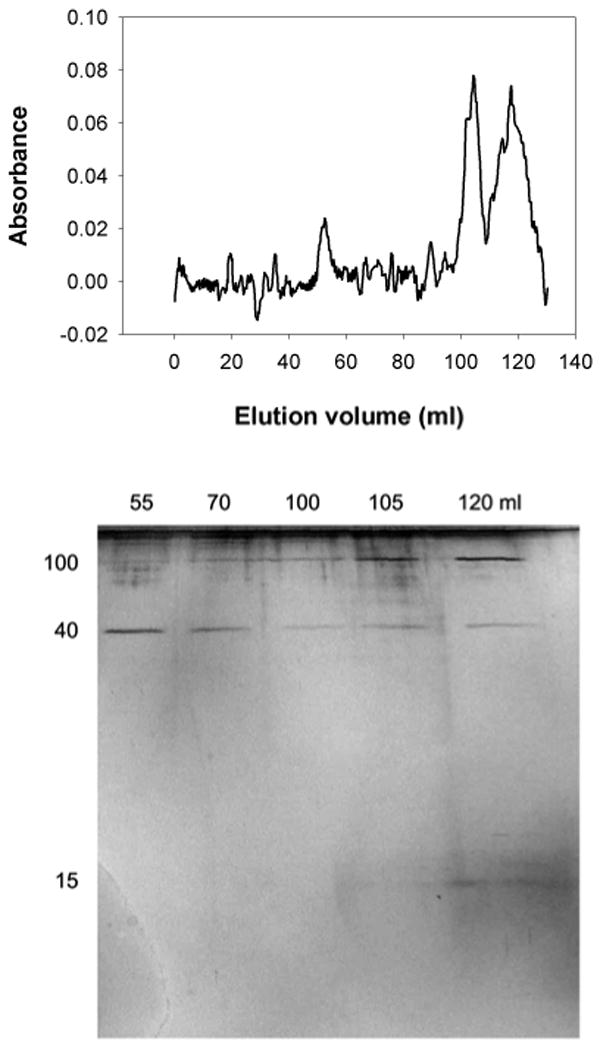
Gel filtration in a Sephacryl S-400 column showing the effect of metal chelation before the glue sets on aggregation of proteins from A. subfuscus glue. The sample was collected into 3 mM deferoxamine and 10 mM EDTA in 20 mM Tris (pH 8). The elution profile is shown above the silver stained SDS-PAGE analysis of the fractions. The lanes are marked by the elution volumes of the fractions they contain. The positions of the 15, 40 and 100 kDa proteins are indicated to the left.
Discussion
Unlike the situation in many well-known gels, electrostatic and hydrophobic interactions do not appear to play a significant role in cross-linking slug glue. High salt or non-ionic detergent concentrations that normally break electrostatic or hydrophobic interactions respectively had no detectable effect on the solubility of proteins in the glue, their ability to stiffen gels or the tendency of specific proteins to aggregate. This was true even at treatment concentrations significantly above what has been reported to break interactions in other systems.
The results also identify a 40 kDa protein as potentially a key component of the cross-linking mechanism. This protein is linked into complexes of more than a megadalton. This suggests that it either links together, or binds to giant polymers such as polysaccharides or proteoglycans. Such giant polymers are common components of biological gels due to the structural framework they provide by tangling (Smith, 2002). Any protein that bound tightly to them could potentially crosslink them into a more rigid, tougher network. The strength of the interactions is surprising, as the 40 kDa protein remained linked in large complexes even in the presence of strongly dissociating conditions. Even 8M urea with non-ionic detergent was insufficient to disrupt these aggregations; only denaturing the proteins in SDS sufficed.
The stability of the interactions that provide structure to the glue distinguishes them from many other non-covalent interactions between polymers. Many commercial gels are stabilized by hydrogen bonding, electrostatic interactions and/or hydrophobic interactions (Williams and Phillips, 2000; Smith, 2002). For example, many gels such as gelatin or agar depend on the formation of helices that aggregate during gelation. Increasing temperature breaks hydrogen bonds that are involved in helix formation and aggregation. Other gels have cross-linking mechanisms that are affected strongly by ionic strength. In carrageenan, salts promote gelation by blocking charge to minimize repulsion and thus promoting helix aggregation. In pectin, divalent ions cross-link the polymers. Altering salt concentration would markedly affect these gels. In methyl cellulose, hydrophobic interactions trigger association of polymers. In all these cases, the gels are clearly different from molluscan adhesive gels in the latter's insensitivity to conditions that disrupt common non-covalent interactions. DNA-ligand interactions are also an interesting comparison, as they also involve large, charged polymers. These interactions are typically electrostatic and they are easily disrupted by increasing salt concentration (Record et al., 1976, 1978; Misra et al., 1994a, b; Fogolari et al., 1997). Even the adhesive interactions between microbes and surfaces often involve hydrophobic and/or electrostatic interactions and may be disrupted by low concentrations of non-ionic detergents (0.1% or less) or salt (0.1 M for example) (Paul and Jeffrey, 1985; McEldowney and Fletcher, 1986).
While salt and detergent solutions typically disrupt electrostatic and hydrophobic interactions, there are situations where these interactions are not easily disrupted. For example, barnacle adhesion depends on hydrophobic interactions. This glue is highly insoluble, and has only recently been effectively dissolved non-proteolytically with the use of a combination of 0.5M dithiothreitol and 7M guanidine hydrochloride at 60° C (Kamino et al., 2000). Since the interactions appear to be primarily hydrophobic, one would expect the strongly chaotropic guanidine hydrochloride to dissolve the cement, but reduction of disulfide bonds was also necessary even though these were not directly involved in cross-linking. Barnacle glue is different, though, in that it is nearly solid rather than a dilute gel. Thus, hydrophobic interactions would be expected to be more extensive. This would add up to greater overall interaction strength, and make it more difficult for reagents to penetrate the glue. In contrast, molluscan adhesive gels are readily homogenized allowing rapid interactions between reagents and gel polymers. Finally, it is unlikely that such strong hydrophobic interactions play a role in molluscan adhesive gels, since simple chelation of metals disrupts the structure of the gel without any effort to disrupt hydrophobic interactions.
Similarly, there is variation in the ability of salt to screen electrostatic interactions. Sodium chloride (1 M) is widely used to break electrostatic interactions in ion-exchange chromatography, and even substantially lower concentrations would disrupt many electrostatic interactions (Dominy et al, 2002; Perez-Jimenez et al., 2004). The type of salt used often matters, however, as the effectiveness of different ions follows the Hofmeister series (von Hippel and Wong, 1964; Curtis and Lue, 2006). Thus, we chose chloride ions, which are intermediate in this series; they are not as chaotropic as some ions, but they should still have a significant effect. Another issue is that salts are better at screening long-range interactions that depend on the net charge on polymers than short-range interactions with specific residues (Dominy et al., 2002). This means that some interactions may be shielded by their binding site. It is relevant that adding detergent with the salt in an attempt to prevent such shielding had no effect. Also high ionic strength not only failed to break existing links where such shielding might occur, it failed to prevent cross-links from forming in the gel-stiffening assay.
In contrast to electrostatic and hydrophobic interactions, transition metals and divalent metal ions play a large role. In fact, the results raise the possibility that the mechanics of the gel rely on more than one type of metal-based cross-linking interaction. The fact that metal chelation disrupts gel structure and improves protein solubility suggests that metal ions play a direct role in holding together the polymers in the gel. Metal removal from the mature glue does not, however, affect the ability of the 40 kDa protein to crosslink into large complexes. Instead, these complexes are stable unless metals are chelated at the time of secretion, before the glue sets. In this case the aggregations do not consistently form. Thus, metals may directly link some polymers together, and may also contribute to the formation of other cross-links. As there are several different metals in the glue, it seems likely that different metals may have different roles. It is worth noting that transition metal specific chelation with deferoxamine affected gel setting, but not the mature glue (Werneke et al., 2007), while the more general divalent ion chelator EDTA did affect the solubility of the mature glue.
The direct effect of metals may involve cross-linking large mucus polymers such as polysaccharides and proteoglycans. This is consistent with the observation that metal chelation in the mature glue disrupts the gel structure but does not affect cross-links between proteins in column chromatography. Changes in interactions between polysaccharides or proteoglycans would not be detected by the chromatography, because the nature of the detection methods and the exclusion limit of the S-400 matrix make these experiments unsuited for their analysis. It is known, however, that divalent ions exert a direct effect on the mechanics of many gels (Smith et al. 1999).
The strong cross-links involving the 40 kDa protein appear to involve a different mechanism. It will be interesting to see if that involves the 15 kDa protein that is characteristic of the glue. Pawlicki et al. (2004) found that there was gel-stiffening activity that resided in fractions containing this 15 kDa glue protein. In their work, samples were solubilized in urea and Tween and dialyzed, thus likely removing any metals that were not tightly bound. The 15 kDa protein, however, has iron bound tightly enough to be present after denaturing electrophoresis (Werneke et al., 2007). As this protein seems to be capable of stiffening gels, but is not strongly enough linked to other proteins to elute as a larger complex in chromatography, it may play a catalytic role rather than being a component of the cross-links. This catalytic role may also depend on the iron that is bound to the protein, and may be responsible for the sensitivity of crosslink formation to metal chelation (Werneke et al., 2007).
Further research is necessary to see if metals play a similar role in other gastropod adhesive gels. While Pawlicki et al. (2004) found that glue proteins from three widely different gastropod species all have similar effects, there are enough differences among the glues that the mechanisms may differ to some extent. For example, disulfide bonds seem to play some role in limpet and periwinkle glue (Smith et al., 1999; Smith and Morin, 2002), though it may be only to maintain intramolecular structure. Also, slug glue is a rapidly setting defensive secretion, and thus may differ qualitatively in some ways from glues used for substrate adhesion. Even other slugs may use a different mechanism, as Li and Graham (2007) found no effect of extended metal chelation with EDTA at room temperature on the glue of another terrestrial slug from the same order, though chelation at 80° C for one hour disrupted the gel structure. Ultimately, it will be important to determine if metals play a central role in other molluscan adhesive gels. It will also be important to determine if they play a role in adhesion in addition to cohesive cross-linking interactions.
In conclusion, the adhesive gels of the slug A. subfuscus depend on robust cross-links. The extent to which these interactions are insensitive to disruptive treatments is surprising, especially compared to other gels (Smith et al., 1999; Smith, 2006). It appears that metals are primarily responsible for the strong cross-links in these glues, and this may involve more than one cross-linking mechanism.
Acknowledgments
This work was supported by Grant Number #1 R15 EB006001-01 from NIH. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of NIH. We thank U. Wiesner for use of the rheometer and H. Waite for helpful discussions.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Beeckmans S. Chromatographic methods to study protein-protein interactions. Methods. 1999;19:278–305. doi: 10.1006/meth.1999.0857. [DOI] [PubMed] [Google Scholar]
- Broomell CC, Mattoni MA, Zok FW, Waite JH. Critical role of zinc in hardening of Nereis jaws. J Exp Biol. 2006;209:3219–3225. doi: 10.1242/jeb.02373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis RA, Lue L. A molecular approach to bioseparations: Protein-protein and protein-salt interactions. Chem Eng Sci. 2006;61:907–923. [Google Scholar]
- Dominy BN, Perl D, Schmid FX, Brooks CL., III The effects of ionic strength on protein stability: the cold shock protein family. J Mol Biol. 2002;319:541–554. doi: 10.1016/S0022-2836(02)00259-0. [DOI] [PubMed] [Google Scholar]
- Fogolari F, Elcock AH, Esposito G, Viglino P, Briggs JM, McCammon JA. Electrostatic effects in homeodomain-DNA interactions. J Mol Biol. 1997;267:368–381. doi: 10.1006/jmbi.1996.0842. [DOI] [PubMed] [Google Scholar]
- Graham LD. Biological adhesives from nature. In: Wnek G, Bowlin GL, editors. Encyclopedia of Biomaterials and Biomedical Engineering. 2nd. Informa Healthcare; New York and London: in press. Also online at http://www.dekker.com/sdek/abstract∼db=enc∼content=a713609104. [Google Scholar]
- Hames BD. One dimensional polyacrylamide gel electrophoresis. In: Hames BD, Rickwood D, editors. Gel Electrophoresis of Proteins: A Practical Approach. IRL Press; Oxford: 1990. pp. 1–147. [Google Scholar]
- Kamino K, Inoue K, Maruyama T, Takamatsu N, Harayama S, Shizuri Y. Barnacle cement proteins. J Biol Chem. 2000;275:27360–27365. doi: 10.1074/jbc.M910363199. [DOI] [PubMed] [Google Scholar]
- Kamino K. Barnacle underwater attachment. In: Smith AM, Callow JA, editors. Biological Adhesives. Springer; Berlin: 2006. pp. 145–166. [Google Scholar]
- Li D, Graham LD. Epidermal secretions of terrestrial flatworms and slugs: Lehmannia valentia mucus contains matrilin-like proteins. Comp Biochem Physiol B. 2007;148:231–244. doi: 10.1016/j.cbpb.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Lichtenegger HC, Schoberl T, Ruokolainen JT, Cross JO, Heald SM, Birkedal H, Waite JH, Stucky GD. Zinc and mechanical prowess in the jaws of Nereis, a marine worm. Proc Natl Acad Sci USA. 2003;100:9144–9149. doi: 10.1073/pnas.1632658100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenegger HC, Birkedal H, Casa DM, Cross JO, Heald SM, Waite JH, Stucky GD. Distribution and role of trace transition metals in Glycera worm jaws studied with synchrotron microbeam techniques. Chem Mater. 2005;17:2927–2931. [Google Scholar]
- McEldowney S, Fletcher M. Variability of the influence of physicochemical factors affecting bacterial adhesion to polystyrene substrata. Appl Environ Microbiol. 1986;52:460–465. doi: 10.1128/aem.52.3.460-465.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misra VK, Sharp KA, Friedman RA, Honig B. Salt effects on ligand-DNA binding: minor groove binding antiobiotics. J Mol Biol. 1994a;238:245–263. doi: 10.1006/jmbi.1994.1285. [DOI] [PubMed] [Google Scholar]
- Misra VK, Hecht JL, Sharp KA, Friedman RA, Honig B. Salt effects on protein-DNA interactions: The λcI repressor and EcoRI endonuclease. J Mol Biol. 1994b;238:264–280. doi: 10.1006/jmbi.1994.1286. [DOI] [PubMed] [Google Scholar]
- Paul JH, Jeffrey WH. Evidence for separate adhesion mechanisms for hydrophilic and hydrophobic surfaces in Vibrio proteolytica. Appl Environ Microbiol. 1985;50:431–437. doi: 10.1128/aem.50.2.431-437.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawlicki JM, Pease LB, Pierce CM, Startz TP, Zhang Y, Smith AM. The effect of molluscan glue proteins on gel mechanics. J Exp Biol. 2004;207:1127–1135. doi: 10.1242/jeb.00859. [DOI] [PubMed] [Google Scholar]
- Perez-Jimenez R, Godoy-Ruiz R, Ibarra-Molero B, Sanchez-Ruiz JM. The efficiency of different salts to screen charge interactions in proteins: A Hofmeister effect? Biophys J. 2004;86:2414–2429. doi: 10.1016/S0006-3495(04)74298-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Record MT, Lohman TM, de Haseth P. Ion effects on ligand-nucleic acid interactions. J Mol Biol. 1976;107:145–158. doi: 10.1016/s0022-2836(76)80023-x. [DOI] [PubMed] [Google Scholar]
- Record MT, Anderson CF, Lohman TM. Thermodynamic analysis of ion effects on the binding and conformational equilibria of proteins and nucleic acids: the roles of ion association or release, screening, and ion effects on water activity. Quart Rev Biophys. 1978;11:103–178. doi: 10.1017/s003358350000202x. [DOI] [PubMed] [Google Scholar]
- Sagert J, Sun C, Waite JH. Chemical subtleties of mussel and polychaete holdfasts. In: Smith AM, Callow JA, editors. Biological Adhesives. Springer; Berlin: 2006. pp. 125–143. [Google Scholar]
- Sever MJ, Weisser JT, Monahan J, Srinivasan S, Wilker JJ. Metal-mediated cross-linking in the generation of a marine-mussel adhesive. Angew Chem Int Ed. 2004;43:448–450. doi: 10.1002/anie.200352759. [DOI] [PubMed] [Google Scholar]
- Smith AM, Quick TJ, St Peter RL. Differences in the composition of adhesive and non-adhesive mucus from the limpet Lottia limatula. Biol Bull. 1999;196:34–44. doi: 10.2307/1543164. [DOI] [PubMed] [Google Scholar]
- Smith AM. The structure and function of adhesive gels from invertebrates. Integr Comp Biol. 2002;42:1164–1171. doi: 10.1093/icb/42.6.1164. [DOI] [PubMed] [Google Scholar]
- Smith AM, Morin MC. Biochemical differences between trail mucus and adhesive mucus from marsh periwinkles. Biol Bull. 2002;203:338–346. doi: 10.2307/1543576. [DOI] [PubMed] [Google Scholar]
- Smith AM. The biochemistry and mechanics of gastropod adhesive gels. In: Smith AM, Callow JA, editors. Biological Adhesives. Springer; Berlin: 2006. pp. 167–182. [Google Scholar]
- Smith AM, Callow JA. Biological Adhesives. Springer; Berlin: 2006. [Google Scholar]
- Stubbs GW, Smith HG, Litman BJ. Alkylglucosides as effective solubilizing agents for bovine rhodopsin: a comparison with several commonly used detergents. Biochim Biophys Acta. 1976;425:46–56. doi: 10.1016/0005-2736(76)90428-4. [DOI] [PubMed] [Google Scholar]
- von Hippel PH, Wong KY. Neutral salts: the generality of their effects on stability of macromolecular conformations. Science. 1964;145:577–580. doi: 10.1126/science.145.3632.577. [DOI] [PubMed] [Google Scholar]
- Waite JH, Andersen NH, Jewhurst S, Sun C. Mussel adhesion: finding the tricks worth mimicking. J Adhesion. 2005;81:1–21. [Google Scholar]
- Werneke SW, Swann C, Farquharson LA, Hamilton KS, Smith AM. The role of metals in molluscan adhesive gels. J Exp Biol. 2007;210:2137–2145. doi: 10.1242/jeb.006098. [DOI] [PubMed] [Google Scholar]
- Williams PA, Phillips GO. Introduction to food hydrocolloids. In: Phillips GO, Williams PA, editors. Handbook of Hydrocolloids. Woodhead Publishing Limited; Cambridge: 2000. pp. 1–20. [Google Scholar]


