Abstract
Chronic neuropathic pain remains an unmet clinical problem because it is often resistant to conventional analgesics. Metabotropic glutamate receptors (mGluRs) are involved in nociceptive processing at the spinal level, but their functions in neuropathic pain are not fully known. In this study, we investigated the role of group III mGluRs in the control of spinal excitatory and inhibitory synaptic transmission in a rat model of neuropathic pain induced by L5/L6 spinal nerve ligation. Whole-cell recording of lamina II neurons was performed in spinal cord slices from control and nerve-ligated rats. The baseline amplitude of glutamatergic EPSCs evoked from primary afferents was significantly larger in nerve-injured rats than in control rats. However, the baseline frequency of GABAergic and glycinergic IPSCs was much lower in nerve-injured rats than in control rats. The group III mGluR agonist L(+)-2-amino-4-phosphonbutyric acid (L-AP4) produced a greater inhibition of the amplitude of monosynaptic and polysynaptic evoked EPSCs in nerve-injured rats than in control rats. L-AP4 inhibited the frequency of miniature EPSCs in 66.7% of neurons in control rats but its inhibitory effect was observed in all neurons tested in nerve-injured rats. Furthermore, L-AP4 similarly inhibited the frequency of GABAergic and glycinergic IPSCs in control and nerve-injured rats. Our study suggests that spinal nerve injury augments glutamatergic input from primary afferents but decreases GABAergic and glycinergic input to spinal dorsal horn neurons. Activation of group III mGluRs attenuates glutamatergic input from primary afferents in nerve-injured rats, which could explain the antinociceptive effect of group III mGluR agonists on neuropathic pain.
Introduction
Nerve injury caused by trauma or surgery can produce neuropathic pain, a chronic condition that is often resistant to conventional analgesics (Sindrup and Jensen, 1999, Woolf and Mannion, 1999). Allodynia and hyperalgesia are not only the result of peripheral sensitization at the site of injury but also a consequence of hyperactivity of the spinal dorsal horn neurons (central sensitization) (Matzner and Devor, 1994, Woolf and Mannion, 1999). Several mechanisms have been proposed to explain central sensitization in neuropathic pain. These posited mechanisms include upregulation of spinal ionotropic glutamate receptors, a decrease in spinal inhibitory GABAergic neurons (disinhibition), and the triggering, by peripheral nerve injury, of the central sprouting of myelinated afferent fibers beyond their original sites of innervation (Woolf et al., 1992, Harris et al., 1996, Moore et al., 2002). However, the molecular mechanisms regulating spinal synaptic transmission in neuropathic pain remain to be defined.
Glutamate is a major excitatory neurotransmitter that conveys sensory information from primary afferents to spinal dorsal horn neurons. Glutamate acts through two broad classes of glutamate receptors: ionotropic receptors and metabotropic receptors (mGluRs). Ionotropic glutamate receptors are primarily involved in fast synaptic transmission, and mGluRs play a role in synaptic plasticity at various levels of the nervous system (Anwyl, 1999). Eight mGluRs have been cloned, and they have been classified into groups I (mGluR 1 and 5), II (mGluR 2 and 3) and III (mGluR4, 6, 7, and 8) according to their sequence homologous characteristics, signal transduction mechanisms, and pharmacological profile (Anwyl, 1999, Schoepp et al., 1999). Group I mGluRs are coupled to Gq/11 proteins leading to activation of phospholipase C (Conn and Pin, 1997, Anwyl, 1999). Groups II and III mGluRs are typically coupled to inhibitory Gi/o proteins, thereby inhibiting cAMP formation and voltage-gated Ca2+ channels (Pin and Duvoisin, 1995, Stefani et al., 1998, Anwyl, 1999, Capogna, 2004). Two subtypes of group III mGluRs have been identified in the rat spinal cord. In this regard, the immunoreactivity for mGluR4 and mGluR7 has been found in the superficial dorsal horn, especially on the primary afferent terminals (Ohishi et al., 1995, Li et al., 1997, Azkue et al., 2001). However, at the present time, little is known about the functional changes of these receptors and their cellular actions in the spinal dorsal horn in neuropathic pain.
We and others have shown that intrathecal administration of the group III mGluR agonist L(+)-2-amino-4-phosphonbutyric acid (L-AP4) attenuates allodynia in nerve-injured rats (Fisher et al., 2002, Chen and Pan, 2005). Topical spinal application of L-AP4 also significantly inhibits the evoked response of ascending dorsal horn neurons in nerve-ligated, but not in sham control, rats (Chen and Pan, 2005). However, the underlying cellular and molecular mechanisms of the potentiated spinal effect of group III mGluR agonists on neuropathic pain are not clear. Therefore, in the present study, we determined the functional changes of group III mGluRs in the control of excitatory and inhibitory synaptic transmission in the spinal dorsal horn in a rat model of neuropathic pain.
Methods and Materials
Animals
Male Sprague-Dawley rats (8 weeks old; Harlan, Indianapolis, IN) were used in this study. All the surgical preparation and experimental protocols were approved by the Animal Care and Use Committee of the University of Texas M. D. Anderson Cancer Center and conformed to the NIH guidelines on the ethical use of animals. All efforts were made to minimize the number of animals used and their suffering.
Neuropathic pain model
L5 and L6 spinal nerve ligation was used as an experimental model of neuropathic pain in our study. We first induced anesthesia with 2–3% isoflurane. Next, we isolated the left L5 and L6 spinal nerves and ligated them tightly with 5-0 silk suture, according to the method described by Kim and Chung (Kim and Chung, 1992). Sham animals were used as controls, and they underwent similar surgical procedures except nerve ligation. Final electrophysiological recording was done 2–3 weeks after surgery. Among rats that had undergone spinal nerve ligation, only those with confirmed tactile allodynia were used for the recording. The presence of tactile allodynia was evaluated 2 weeks after surgery by applying von Frey filament to the plantar surface of rats’ hindpaw on the surgery side, as described previously (Chaplan et al., 1997, Chen and Pan, 2005).
Spinal cord slice preparation
Rats were anesthetized with 2% isoflurane in O2, and the lumbar segment of the spinal cord at L5 and L6 levels was rapidly removed through laminectomy. The rats were then killed with 5% isoflurane. The lumbar spinal cord segments were immediately placed in ice-cold sucrose artificial cerebrospinal fluid presaturated with 95%O2 and 5% CO2. The sucrose artificial cerebrospinal fluid contained (mM) sucrose, 234; KCl, 3.6; MgCl2, 1.2; CaCl2, 2.5; NaH2PO4, 1.2; glucose, 12.0; and NaHCO3, 25.0. The tissue was then placed in a shallow groove formed in a gelatin block and glued onto the stage of a vibratome (Technical Product International, St. Louis, MO). Transverse spinal cord slices were cut to 400 μm in the ice-cold sucrose artificial cerebrospinal fluid and then preincubated in Krebs solution oxygenated with 95% O2 and 5% CO2 at 34°C for at least 1 h before being transferred to the recording chamber. The Krebs solution contained (mM) NaCl, 117.0; KCl, 3.6; MgCl2, 1.2; CaCl2, 2.5; NaH2PO4, 1.2; glucose, 11.0; and NaHCO3, 25.0.
Electrophysiological recording
Recordings of postsynaptic currents were performed using the whole-cell voltage-clamp method, as we described previously (Zhang et al., 2005). The spinal lamina II has a distinct translucent appearance and can be easily distinguished under a microscope. Lamina II neurons in the spinal slice were identified under a fixed-stage microscope (BX51WI, Olympus, Tokyo, Japan) with differential interference contrast/infrared illumination. The electrode for whole-cell recordings was pulled from borosilicate glass capillaries with a puller (P-97, Sutter Instrument, Novato, CA). The impedance of the pipette was 4–7 MΩ when filled with the internal solution. The internal pipette solution for recording inhibitory postsynaptic currents (IPSCs) contained (in mM) Cs2SO4, 110; KCl, 5; MgCl2, 2.0; CaCl2, 0.5; HEPES, 5.0; EGTA, 5.0; ATP-Mg, 5.0; Na-GTP, 0.5; guanosine 5′-O-(2-thiodiphosphate) (GDP-β-S), 1; and QX314 10 and was adjusted to pH 7.2–7.4 with 1 M CsOH (290–320 mOsm). The internal pipette solution for recording excitatory postsynaptic currents (EPSCs) contained (in mM) K-gluconate 135; KCl, 5; MgCl2, 2.0; CaCl2, 0.5; HEPES, 5.0; EGTA, 5.0; ATP-Mg, 5.0; Na-GTP, 0.5; and guanosine 5′-O-(2-thiodiphosphate) (GDP-β-S), 1; QX314 10; adjusted to pH 7.2–7.4 with 1 M KOH (290–320 mOsm). GDP-β-S was added to the internal solution to block the possible postsynaptic effect mediated by group III mGluR agonists through G proteins (Zhang et al., 2005). QX314 was added to the internal solution to suppress the action potential generation from the recorded cells. The slice was placed in a glass-bottomed chamber (Warner Instruments, Hamden, CT) and fixed with parallel nylon threads supported by a U-shaped stainless steel weight. The slice was continuously perfused with Krebs solution at 5.0 ml/min at 34°C maintained by an inline solution heater and a temperature controller (TC-324; Warner Instruments).
Recordings of postsynaptic currents began 5–6 min after whole-cell access was established and the current reached a steady state. The input resistance was monitored and the recording was abandoned if it changed by more than 15%. Postsynaptic currents were recorded using an amplifier (MultiClamp700A, Axon Instruments, Foster City, CA) at a holding potential of 0 mV for IPSCs and −70 mV for EPSCs. Signals were filtered at 1–2 kHz, digitized at 10 kHz, and stored in a computer with pCLAMP 9.0 (Axon Instruments). The miniature excitatory postsynaptic currents (mEPSCs) were recorded in the presence of 2 μM strychnine, 10 μM bicuculline, and 1 μM TTX. The evoked EPSCs (eEPSCs) of lamina II neurons were induced by electrical stimulation with a fixed intensity (0.2 ms, 0.5 mA, and 0.2 Hz) through a bipolar tungsten electrode placed on the dorsal root entry zone. Recordings of eEPSCs were similar to mEPSCs except that TTX was not used. GABAergic inhibitory postsynaptic currents (IPSCs) were recorded in the presence of 20 μM 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) and 2 μM strychnine. Glycinergic sIPSCs were recorded in the presence of 20 μM CNQX and 10 μM bicuculline. To record the miniature IPSCs (mIPSCs), 1 μM TTX was added to the perfusion solution.
L-AP4 and (RS)-α-cyclopropyl-4-phosphonophenylglycine (CPPG) were purchased from Tocris (Ellisville, MO). GDP-β-S, strychnine, CNQX, and bicuculline were obtained from Sigma-Aldrich. TTX and QX314 were obtained from Alomone Labs (Jerusalem, Israel). Drugs were dissolved in Krebs solution and perfused into the slice chamber using syringe pumps.
Data analysis
Data are presented as the means ± S.E.M. The sIPSCs, mIPSCs, and mEPSCs were analyzed off-line with a peak detection program (MiniAnalysis, Synaptosoft, Decatur, GA). Measurements of the amplitude and frequency of sIPSCs, mIPSCs, and mEPSCs were performed over a period of at least 1 min during control, drug application, and recovery. The sIPSCs and mIPSCs were detected by the fast rise time of the signal over an amplitude threshold above the background noise. We manually excluded the event when the noise was erroneously identified as the sIPSCs, mIPSCs, or mEPSCs by the program. The background noise level was typically constant throughout the recording of a single neuron. The cumulative probability of the amplitude and inter-event interval of sIPSCs, mIPSCs, and mEPSCs was compared using the Komogorov-Smirnov test, which estimates the probability that two cumulative distributions are similar. Analyses of the effect of L-AP4 on the amplitude of eEPSCs were performed using Clampfit (Axon Instruments). The EC50 value of the L-AP4 effect was determined by nonlinear regression analysis of the concentration–response data using Prism (GraphPad Software, San Diego, CA). Differences were determined by repeated-measures or two-way analysis of variance. P < 0.05 was considered to be statistically significant.
Results
The tactile threshold was measured by applying von Frey filaments to the plantar surface of the rats’ hindpaw on the surgery side. The mechanical threshold significantly decreased from 20.3 ± 0.43 to 2.72 ± 0.13 g in 27 nerve-ligated rats two weeks after nerve ligation. The mechanical threshold was not altered significantly by sham surgery in 22 control rats (from 21.33 ± 0.32 to 19.98 ± 0.37 g).
Effect of L-AP4 on monosynaptic and polysynaptic EPSCs in nerve-injured rats
To determine the role of group III mGluRs in the control of glutamatergic input from primary afferents to dorsal horn neurons in control and nerve-injured rats, we assessed the effect of the group III mGluR agonist L-AP4 on identified monosynaptic and polysynaptic evoked eEPSCs elicited from the dorsal root or dorsal root entry zone. All recordings were done on lamina II neurons at the L5 spinal level ipsilateral to the surgery side (nerve ligation or sham). The eEPSCs were considered monosynaptic if the latency was constant after electrical stimulation (0.2 Hz) and if no conduction failure or increased latency occurred when stimulation frequency was increased to 20 Hz (although the amplitude of eEPSCs was markedly attenuated). In contrast, the latency of polysynaptic eEPSCs varied and conduction failure occurred when the stimulation frequency was increased to 20 Hz (Wang et al., 2007, Zhang et al., 2007). Bath application of 20 μM CNQX, a non-NMDA receptor antagonist, abolished eEPSCs at the end of the recording (data not shown).
In nerve-injured rats, the baseline amplitude of monosynaptic eEPSCs was 333.97 ± 30.42 pA (n = 10), which was significantly larger than that (211.17 ± 18.30 pA, n = 17) in control rats. L-AP4 is the most widely used group III mGluR agonist (Conn and Pin, 1997, Thomsen, 1997, Schoepp et al., 1999, Acuna-Goycolea et al., 2004). L-AP4 was chosen for our study because it has at least 500-fold selectivity for group III mGluRs over other mGluRs, and it represents the best group III mGluR agonist currently available in terms of potency and specificity (Conn and Pin, 1997, Schoepp et al., 1999). L-AP4 (5–100 μM, each concentration was applied for 3 min in ascending order) significantly reduced the peak amplitude of monosynaptic eEPSCs in a concentration-dependent manner in both the control and nerve-injured rats (Fig. 1, A and C). However, the inhibitory effect of L-AP4 on the amplitude of monosynaptic eEPSCs was significantly greater in the nerve-injured group than in the control group (Fig. 1E). The EC50 values for the effect of L-AP4 on monosynaptic eEPSCs were 8.31 ± 0.05 and 4.02 ± 0.06 μM (P < 0.05) in control and nerved-injured rats, respectively.
Figure 1.
Concentration-dependent effect of L-AP4 on the amplitude of monosynaptic and polysynaptic EPSCs of lamina II neurons evoked from primary afferents in control and nerve-injured rats. A and B, original traces of monosynaptic eEPSCs of lamina II neurons during control and application of different concentrations of L-AP4 in one control and one nerve-injured rat. These traces are the mean of 10 consecutive responses. C and D, summary data showing the effect of L-AP4 on the peak amplitude of monosynaptic and polysynaptic eEPSCs in control and nerve-injured rats. E and F, summary data comparing the percent inhibition of the amplitude of eEPSCs by L-AP4 in control and nerve-injured rats. Data are presented as means ± S.E.M. * P < 0.05 compared with the baseline control. # P < 0.05 compared with the corresponding value in the control group.
The baseline amplitude of polysynaptic eEPSCs was also significantly larger in the nerve-injured group than in the control group (Fig. 1, B and D). Bath application of 5–100 μM of L-AP4 inhibited the amplitude of polysynaptic eEPSCs in a dose-dependent manner in both the control and nerve-ligated rats (Fig. 1, B and D). When the effect of L-AP4 was normalized with the baseline amplitude of eEPSCs, its inhibitory effect on the amplitude of polysynaptic eEPSCs was significantly greater in the nerve-injured rats than in the sham control rats (Fig. 1F). The EC50 values for the effect of L-AP4 on polysynaptic eEPSCs were 8.27 ± 0.05 and 4.25 ± 0.06 μM (P < 0.05) in control and nerved-injured groups, respectively. These results suggest that the glutamatergic input from primary afferents to spinal dorsal horn neurons is augmented after nerve injury. Furthermore, group III mGluRs are implicated in the control of primary afferent input in the spinal dorsal horn after nerve injury.
Effect of L-AP4 on mEPSCs in control and nerve-injured rats
To further determine the role of presynaptic group III mGluRs in the control of glutamatergic input to spinal dorsal horn neurons, we examined the effect of L-AP4 on mEPSCs in lamina II neurons of control and nerve-injured rats. The effect of 50 μM L-AP4 on the frequency and amplitude of mEPSCs was studied in 24 neurons from control rats and 18 neurons from nerve-injured rats. In control rats, 50 μM L-AP4 significantly decreased the frequency of mEPSCs without affecting the amplitude (from 18.48 ± 2.26 to 18.25 ± 2.53 pA, P > 0.05) in 16 of 24 neurons (66.7%, Fig. 2, A, C, and E). L-AP4 had no significant effect on the frequency and amplitude (from 20.35 ± 3.25 to 20.05 ± 3.24 pA, P > 0.05) of mEPSCs in another 8 (33.3%) cells recorded from control rats (Fig. 2C). In nerve-injured rats, 50 μM L-AP4 significantly decreased the frequency, but not the amplitude, of mEPSCs in all 22 neurons tested (Fig. 2, D and F). There were no significant differences in the baseline frequency of mEPSCs and the inhibitory effect of L-AP4 on the mEPSC frequency between the control and nerve-injured rats (Fig. 2, E and F). In both control and nerve-injured groups, bath application of 200 μM CPPG, a highly specific group III mGluR antagonist, for 3 min alone had no significant effect on mEPSCs. CPPG has a much higher potency and greater selectivity for group III mGluRs than does MAP4 or MSOP (Schoepp et al., 1999). CPPG completely blocked the inhibitory effect of L-AP4 on the frequency of mEPSCs in both groups (n = 5 cells in each group, Fig. 2, A and B). These data suggest that nerve injury may increase the number of group III mGluR-expressing glutamatergic terminals in the spinal dorsal horn.
Figure 2.
Effect of L-AP4 on glutamatergic mEPSCs of lamina II neurons in control and nerve-injured rats. A and B, original traces of mEPSCs during control, application of 50 μM of L-AP4, 200 μM CPPG, and CPPG plus L-AP4 in one control and one nerve-injured rat. C and D, cumulative probability plots of mEPSCs of the same neurons in A and B showing the distribution of the amplitude and interevent interval during control, application of 50 μM of L-AP4, and washout. E, summary data showing that 50 μM of L-AP4 inhibited the frequency of mEPSCs in 16 neurons but failed to reduce the frequency of mEPSCs in another 8 neurons in control rats. F, summary data showing that 50 μM of L-AP4 decreased the frequency of mEPSCs in all 22 neurons in nerve-injured rats. Data are presented as means ± S.E.M. * P < 0.05 compared with the baseline control.
Effect of L-AP4 on GABAergic sIPSCs and mIPSCs of lamina II neurons in control and nerve-injured rats
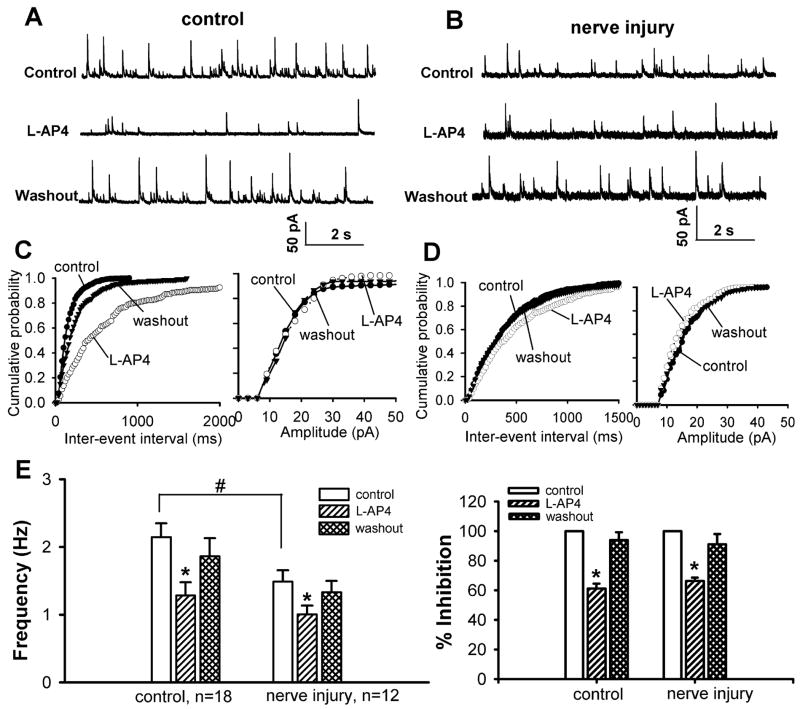
To record GABAergic sIPSCs, 2 μM strychnine was continuously perfused to the bath to block glycine-mediated IPSCs in the spinal cord (Wang et al., 2007). The basal frequency of GABAergic sIPSCs of lamina II neurons was significantly reduced in nerve-injured rats compared with that in control rats (Fig. 3, A-E). However, there was no significant difference in the amplitude (15.10 ± 1.12 vs. 14.34 ± 1.14 pA) of GABAergic sIPSCs between the control and nerve-injured groups. Bath application of 50 μM L-AP4 for 3 min significantly decreased the frequency, but not the amplitude, of GABAergic sIPSCs in 18 cells from the control group and 12 cells from the nerve-ligated group (Fig. 3). There was no significant difference in the inhibitory effect of L-AP4 on the frequency of GABAergic sIPSCs between the two groups.
Figure 3.
Effect of L-AP4 on GABAergic sIPSCs of lamina II neurons in control and nerve-injured rats. A and B, original traces of GABAergic sIPSCs during control, application of 50 μM of L-AP4 and washout in one control and one nerve-injured rat. C and D, cumulative probability plots of GABAergic sIPSCs of the same neuron in A and B showing the distribution of the amplitude and inter-event interval during control, application of L-AP4, and washout. E, summary data comparing the baseline frequency and the effect of 50 μM of L-AP4 on the frequency of GABAergic sIPSCs in control and nerve-injured rats. Data presented are the means ± S.E.M. * P < 0.05 compared with the pre-drug control. # P < 0.05 compared with the corresponding value in the control group.
In addition, the baseline frequency of GABAergic mIPSCs was significantly decreased in nerve-injured rats compared with that in control rats (Fig. 4, A–E). There was no significant difference in the amplitude (13.35 ± 1.28 vs. 12.22 ± 0.75 pA) of GABAergic mIPSCs between the control and nerve-injured groups. Bath application of 50 μM L-AP4 significantly decreased the frequency, but not the amplitude, of mIPSCs in 11 cells from the control group and 13 cells from the nerve-ligated group (Fig. 4). There was no significant difference in the inhibitory effect of L-AP4 on the frequency of GABAergic mIPSCs between the two groups. These data suggest that peripheral nerve injury attenuates GABAergic input to spinal dorsal horn neurons. However, it is less likely that the function of presynaptic group III mGluRs expressed on GABAergic neurons and their terminals in the spinal cord is altered in this animal model of neuropathic pain.
Figure 4.
Effect of L-AP4 on GABAergic mIPSCs of lamina II neurons in control and nerve-injured rats. A and B, original traces of GABAergic mIPSCs during control, application of 50 μM of L-AP4, and washout in one control and one nerve-injured rat. C and D, cumulative probability plots of GABAergic mIPSCs of the same neuron in A and B showing the distribution of the amplitude and inter-event interval during control, application of L-AP4, and washout. E, summary data comparing the baseline frequency of GABAergic mIPSCs and the effect of 50 μM of L-AP4 on the frequency of GABAergic mIPSCs in control and nerve-injured rats. Data are presented as means ± S.E.M. * P < 0.05 compared with the baseline control. # P < 0.05 compared with the corresponding value in the control group.
Effect of L-AP4 on glycinergic sIPSCs and mIPSCs of lamina II neurons in control and nerve-injured rats
To study glycinergic sIPSCs, 10 μM bicuculline was continuously perfused to the bath to block GABA-mediated fast IPSCs in the spinal cord. The basal frequency of glycinergic sIPSCs of lamina II neurons was significantly lower in nerve-injured rats than in control rats (Fig. 5). There was no significant difference in the amplitude (32.01 ± 3.22 vs. 29.72 ± 2.17 pA) of glycinergic sIPSCs between the control and nerve-injured groups. Bath application of 50 μM L-AP4 for 3 min significantly decreased the frequency, but not the amplitude, of glycinergic sIPSCs in 23 cells from the control group and 14 cells from the nerve-ligated group (Fig. 5). However, there was no significant difference in the inhibitory effect of L-AP4 on the frequency of glycinergic sIPSCs between the control and nerve-ligated rats.
Figure 5.
Effect of L-AP4 on glycinergic sIPSCs of lamina II neurons in control and nerve-injured rats. A and B, original traces of glycinergic sIPSCs during control, application of 50 μM of L-AP4, and washout in one control and one nerve-injured rat. C and D, cumulative probability plots of glycinergic sIPSCs of the same neuron in A and B showing the distribution of the amplitude and inter-event interval during control, application of L-AP4, and washout. E, summary data comparing the baseline frequency of glycinergic sIPSCs and the effect of 50 μM of L-AP4 on the frequency of glycinergic sIPSCs in control and nerve-injured rats. Data are presented as means ± S.E.M. * P < 0.05 compared with the pre-drug control. # P < 0.05 compared with the corresponding value in the control group.
Furthermore, the baseline frequency of glycinergic mIPSCs was significantly reduced in nerve-injured rats compared with that in control rats (Fig. 6). However, there was no significant difference in the amplitude (28.81 ± 3.02 vs. 28.02 ± 1.84 pA) of glycinergic mIPSCs between the control and nerve-injured groups. Bath application of 50 μM L-AP4 significantly decreased the frequency, but not the amplitude, of mIPSCs in 16 cells from the control group and 13 cells from the nerve-ligated group (Fig. 6). There was no significant difference in the inhibitory effect of L-AP4 on the frequency of glycinergic mIPSCs between the two groups. These findings suggest that nerve injury reduces glycinergic input to spinal dorsal horn neurons but may not alter the function of presynaptic group III mGluRs expressed on glycinergic neurons and their terminals in the spinal cord.
Figure 6.
Effect of L-AP4 on glycinergic mIPSCs of lamina II neurons in control rats and nerve-injured rats. A and B, original traces of glycinergic mIPSCs during control, application of 50 μM of L-AP4, and washout in control and nerve-injured rats. C and D, cumulative probability plots of glycinergic mIPSCs of the same neuron in A and B showing the distribution of the amplitude and interevent interval during control, application of 50 μM of L-AP4, and washout. E, summary data comparing the baseline frequency of glycinergic mIPSCs and the effect of L-AP4 on the frequency of glycinergic mIPSCs in control and nerve-injured rats. Data presented are the means ± S.E.M. * P < 0.05 compared with the baseline control. # P < 0.05 compared with the corresponding value in the control group.
Discussion
To our knowledge, this is the first study of the role of group III mGluRs in the regulation of spinal synaptic transmission in neuropathic pain conditions. In the present study, we specifically investigated the functional changes in group III mGluRs in the control of spinal excitatory and inhibitory synaptic transmission in a rat model of neuropathic pain. We found that the baseline amplitude of monosynaptic and polysynaptic eEPSCs was significantly larger in the nerve injury group than in the sham control group. In contrast, the baseline frequency of GABAergic and glycinergic of sIPSCs and mIPSCs was significantly lower in nerve-injured rats than in control rats. We also found that the group III mGluR agonist L-AP4 had a greater inhibitory effect on the amplitude of monosynaptic and polysynaptic eEPSCs in nerve-injured rats than in control rats. However, the inhibitory effect of L-AP4 on GABAergic and glycinergic IPSCs was not significantly different between the two groups. These findings provide new evidence about the plasticity of group III mGluRs in the differential regulation of glutamatergic, GABAergic and glycinergic transmission in the spinal dorsal horn in neuropathic pain.
Altered GABAergic and glycinergic synaptic input to spinal dorsal horn neurons has not been specifically evaluated in rats subjected to spinal nerve ligation. GABA and glycine are the most important inhibitory neurotransmitters in the spinal cord. Blocking spinal GABAA receptors with bicuculline produces tactile allodynia in rats (Sorkin et al., 1998). Also, blockade of glycine receptors in the spinal cord leads to hypersensitivity of dorsal horn neurons and allodynia in rats (Yaksh, 1989, Cronin et al., 2004). Sciatic nerve injury can lead to a reduction in GABAergic IPSCs evoked from primary afferents and a loss of GABAergic interneurons in the spinal cord (Moore et al., 2002). Apoptosis of GABAergic neurons or downregulation of spinal GABA synthesis may account for reduced GABAergic input in the spinal dorsal horn after nerve injury (Castro-Lopes et al., 1993, Ibuki et al., 1997, Moore et al., 2002). Consistent with these results, we found that the baseline frequency, but not the amplitude, of GABAergic sIPSCs and mIPSCs was significantly lower in spinal nerve-injured rats than in control rats, suggesting that presynaptic GABA release is decreased in the spinal cord in this rat model of neuropathic pain. Interestingly, we found that the basal frequency, but not the amplitude, of glycinergic sIPSCs and mIPSCs was also significantly lower in nerve-injured rats than in control rats. These data suggest that presynaptic glycine release is decreased in the spinal dorsal horn after spinal nerve ligation. Glycine-like immunoreactive axons, dendrites, and cell bodies are present in the spinal superficial dorsal horn (Todd and Lochhead, 1990, Todd and Sullivan, 1990). It has been suggested that glycine receptor immunoreactivity in the spinal cord is reduced after sciatic nerve injury in rats (Simpson and Huang, 1998). However, because the amplitude of glycinergic sIPSCs and mIPSCs was not significantly different between the control and nerve-injured groups, it is less likely that reduced glycinergic input in nerve-injured rats is due to a decrease in glycine receptors after nerve injury. Collectively, our results provide important new information that suggests that spinal nerve injury causes a reduction in both GABAergic and glycinergic input to spinal dorsal horn neurons, which may contribute to central sensitization in this rat model of neuropathic pain.
In the present study, we found that the basal amplitude of monosynaptic and polysynaptic eEPSCs elicited from primary afferents was significantly larger in nerve-injured rats than in control rats. This finding suggests that spinal nerve injury induces an increase in the glutamatergic input from primary afferents to spinal dorsal horn neurons. Our results are consistent with those of previous studies that found augmented glutamatergic EPSCs from primary afferents in other animal models of neuropathic pain (Kohno et al., 2003, Wang et al., 2007). Augmented glutamatergic input after nerve injury could result from increased excitability of primary afferent nerves and ectopic discharges from injured afferent nerves or DRG neurons (Matzner and Devor, 1994, Liu et al., 2001). Furthermore, the reciprocal interaction between primary afferents and GABAergic interneurons in the spinal cord is important for nociceptive processing in the spinal cord. Thus, reduced GABAergic input, caused by spinal nerve ligation, may also contribute to reduced presynaptic inhibition of primary afferents (Lomeli et al., 1998, Willis, 1999) and increased glutamatergic input to spinal dorsal horn neurons.
The underlying physiological function of the potentiated effect of group III mGluR agonists on neuropathic pain are unknown. Although activation of group III mGluRs with L-AP4 inhibits excitatory and inhibitory synaptic transmission in the spinal cord in normal rats (Gerber et al., 2000), spinally administered L-AP4 has no effect on the nociceptive threshold in these animals (Chen and Pan, 2005). In addition to their presence on primary afferent terminals (Ohishi et al., 1995, Li et al., 1997, Azkue et al., 2001), group III mGluRs may be present on the GABAergic interneurons in the spinal cord (Zhou et al., 2007). The concurrent effect of the group III mGluR agonists on both excitatory and inhibitory input to spinal dorsal horn neurons may explain the lack of the net effect of L-AP4 on nociception in normal animals (Chen and Pan, 2005). Nevertheless, intrathecal administration of L-AP4 reduces acute antinociceptive responses induced by formalin injection in rats (Fisher and Coderre, 1996), dose-dependently inhibits the evoked response of ascending dorsal horn neurons, and attenuates allodynia in rats subjected to spinal nerve ligation (Chen and Pan, 2005). In the present study, we found that L-AP4 had a significantly greater inhibitory effect on glutamatergic EPSCs evoked from primary afferents in nerve-injured rats than in control rats. Furthermore, L-AP4 inhibited the frequency of mEPSCs in all lamina II neurons tested from nerve-injured rats, but this effect occurred in only approximately 67% of neurons from sham control rats. The latter finding suggests that nerve injury may promote the sprouting of group III mGluR-expressing afferent terminals in lamina II. This possibility is supported by the finding that peripheral nerve injury promotes sprouting of myelinated primary afferent terminals into the spinal lamina II region (Woolf et al., 1992). Nevertheless, more recent studies have shown that nerve-injury-induced sprouting is much less pronounced than previously reported by the Woolf’s group (Bao et al., 2002, Pan et al., 2003, Woodbury et al., 2008), and others have found no evidence of sprouting of Aβ-afferent fibers into the superficial dorsal horn (Hughes et al., 2003, Shehab et al., 2004). Because increased glutamatergic input from primary afferents plays an important role in neuropathic pain caused by nerve injury, our findings could explain the significant inhibitory effect of group III mGluR agonists on spinal dorsal horn neurons and allodynia in nerve-injured rats (Chen and Pan, 2005). Our study provides novel information that suggests that group III mGluRs may have a more important function in the control of primary afferent input to spinal dorsal horn neurons in neuropathic pain state than in control condition, which is likely the functional basis for the potent antinociceptive effect of group III mGluR agonists on neuropathic pain.
We found that L-AP4 had a similar inhibitory effect on GABAergic and sIPSCs and mIPSCs in control and nerve-injured rats. These data suggest that group III mGluRs can function as heteroreceptors to regulate synaptic GABA release and that group III mGluRs expressed on GABAergic neurons are not affected by nerve injury. Furthermore, we observed that L-AP4 caused a similar degree of inhibition of the frequency of glycinergic sIPSCs and mIPSCs in control and nerve-injured rats, suggesting that the group III mGluRs expressed on glycinergic neurons and terminals are not altered by nerve injury. It is not clear whether and how the inhibitory effect of L-AP4 on synaptic GABA and glycine release contribute to its antinociceptive effect in neuropathic pain. GABA can inhibit primary afferent input by presynaptic inhibition (via primary afferent depolarization) through GABAA and GABAB receptors (Rudomin and Schmidt, 1999, Willis, 1999, Li et al., 2002). It has been shown that GABA-mediated primary afferent depolarization is enhanced to the point that depolarization can generate spike activity in the afferent terminals after nerve injury (Cervero and Laird, 1996). Thus, reducing GABA release and GABAA activation at the primary afferent terminals by a group III mGluR agonist may attenuate hyperexcitability of spinal dorsal horn neurons in neuropathic pain (Garcia-Nicas et al., 2006).
In summary, the data from our study suggest that nerve injury increases glutamatergic input from primary afferents but reduces GABAergic and glycinergic input to spinal dorsal horn neurons. Furthermore, the group III mGluRs on primary afferent terminals are upregulated, whereas those present on GABAergic and glycinergic neurons and terminals are not significantly altered in this animal model of neuropathic pain. These findings provide important new information about the plasticity of group III mGluRs in neuropathic pain and the molecular basis for the antiallodynic effect of spinally administered group III mGluR agonists.
Acknowledgments
This study was supported by National Institutes of Health grants GM64830 and NS45602.
List of abbreviations
- sIPSCs
spontaneous inhibitory postsynaptic currents
- mIPSCs
miniature inhibitory postsynaptic currents
- eEPSCs
evoked excitatory postsynaptic currents
- mEPSCs
miniature excitatory postsynaptic currents
- L-AP4
L(+)-2-amino-4-phosphonbutyric acid
- CPPG
(RS)-α-cyclopropyl-4-phosphonophenylglycine
- GDP-β-S
guanosine 5′-O-(2-thiodiphosphate)
- mGluRs
metabotropic glutamate receptors
- CNQX
6-cyano-7-nitroquinoxaline-2,3-dione
- GABA
γ-aminobutyric acid
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acuna-Goycolea C, Li Y, Van Den Pol AN. Group III metabotropic glutamate receptors maintain tonic inhibition of excitatory synaptic input to hypocretin/orexin neurons. J Neurosci. 2004;24:3013–3022. doi: 10.1523/JNEUROSCI.5416-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anwyl R. Metabotropic glutamate receptors: electrophysiological properties and role in plasticity. Brain Res Brain Res Rev. 1999;29:83–120. doi: 10.1016/s0165-0173(98)00050-2. [DOI] [PubMed] [Google Scholar]
- Azkue JJ, Murga M, Fernandez-Capetillo O, Mateos JM, Elezgarai I, Benitez R, Osorio A, Diez J, Puente N, Bilbao A, Bidaurrazaga A, Kuhn R, Grandes P. Immunoreactivity for the group III metabotropic glutamate receptor subtype mGluR4a in the superficial laminae of the rat spinal dorsal horn. J Comp Neurol. 2001;430:448–457. doi: 10.1002/1096-9861(20010219)430:4<448::aid-cne1042>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Bao L, Wang HF, Cai HJ, Tong YG, Jin SX, Lu YJ, Grant G, Hokfelt T, Zhang X. Peripheral axotomy induces only very limited sprouting of coarse myelinated afferents into inner lamina II of rat spinal cord. Eur J Neurosci. 2002;16:175–185. doi: 10.1046/j.1460-9568.2002.02080.x. [DOI] [PubMed] [Google Scholar]
- Capogna M. Distinct properties of presynaptic group II and III metabotropic glutamate receptor-mediated inhibition of perforant pathway-CA1 EPSCs. Eur J Neurosci. 2004;19:2847–2858. doi: 10.1111/j.1460-9568.2004.03378.x. [DOI] [PubMed] [Google Scholar]
- Castro-Lopes JM, Tavares I, Coimbra A. GABA decreases in the spinal cord dorsal horn after peripheral neurectomy. Brain Res. 1993;620:287–291. doi: 10.1016/0006-8993(93)90167-l. [DOI] [PubMed] [Google Scholar]
- Cervero F, Laird JM. Mechanisms of touch-evoked pain (allodynia): a new model. Pain. 1996;68:13–23. doi: 10.1016/S0304-3959(96)03165-X. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Malmberg AB, Yaksh TL. Efficacy of spinal NMDA receptor antagonism in formalin hyperalgesia and nerve injury evoked allodynia in the rat. J Pharmacol Exp Ther. 1997;280:829–838. [PubMed] [Google Scholar]
- Chen SR, Pan HL. Distinct roles of group III metabotropic glutamate receptors in control of nociception and dorsal horn neurons in normal and nerve-injured Rats. J Pharmacol Exp Ther. 2005;312:120–126. doi: 10.1124/jpet.104.073817. [DOI] [PubMed] [Google Scholar]
- Conn PJ, Pin JP. Pharmacology and functions of metabotropic glutamate receptors. Annu Rev Pharmacol Toxicol. 1997;37:205–237. doi: 10.1146/annurev.pharmtox.37.1.205. [DOI] [PubMed] [Google Scholar]
- Cronin JN, Bradbury EJ, Lidierth M. Laminar distribution of GABAA- and glycine-receptor mediated tonic inhibition in the dorsal horn of the rat lumbar spinal cord: effects of picrotoxin and strychnine on expression of Fos-like immunoreactivity. Pain. 2004;112:156–163. doi: 10.1016/j.pain.2004.08.010. [DOI] [PubMed] [Google Scholar]
- Fisher K, Coderre TJ. The contribution of metabotropic glutamate receptors (mGluRs) to formalin-induced nociception. Pain. 1996;68:255–263. doi: 10.1016/s0304-3959(96)03212-5. [DOI] [PubMed] [Google Scholar]
- Fisher K, Lefebvre C, Coderre TJ. Antinociceptive effects following intrathecal pretreatment with selective metabotropic glutamate receptor compounds in a rat model of neuropathic pain. Pharmacol Biochem Behav. 2002;73:411–418. doi: 10.1016/s0091-3057(02)00832-8. [DOI] [PubMed] [Google Scholar]
- Garcia-Nicas E, Laird JM, Cervero F. GABAA-Receptor blockade reverses the injury-induced sensitization of nociceptor-specific (NS) neurons in the spinal dorsal horn of the rat. J Neurophysiol. 2006;96:661–670. doi: 10.1152/jn.00377.2006. [DOI] [PubMed] [Google Scholar]
- Gerber G, Zhong J, Youn D, Randic M. Group II and group III metabotropic glutamate receptor agonists depress synaptic transmission in the rat spinal cord dorsal horn. Neuroscience. 2000;100:393–406. doi: 10.1016/s0306-4522(00)00269-4. [DOI] [PubMed] [Google Scholar]
- Harris JA, Corsi M, Quartaroli M, Arban R, Bentivoglio M. Upregulation of spinal glutamate receptors in chronic pain. Neuroscience. 1996;74:7–12. doi: 10.1016/0306-4522(96)00196-0. [DOI] [PubMed] [Google Scholar]
- Hughes DI, Scott DT, Todd AJ, Riddell JS. Lack of evidence for sprouting of Abeta afferents into the superficial laminas of the spinal cord dorsal horn after nerve section. J Neurosci. 2003;23:9491–9499. doi: 10.1523/JNEUROSCI.23-29-09491.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibuki T, Hama AT, Wang XT, Pappas GD, Sagen J. Loss of GABA-immunoreactivity in the spinal dorsal horn of rats with peripheral nerve injury and promotion of recovery by adrenal medullary grafts. Neuroscience. 1997;76:845–858. doi: 10.1016/s0306-4522(96)00341-7. [DOI] [PubMed] [Google Scholar]
- Kim SH, Chung JM. An experimental model for peripheral neuropathy produced by segmental spinal nerve ligation in the rat. Pain. 1992;50:355–363. doi: 10.1016/0304-3959(92)90041-9. [DOI] [PubMed] [Google Scholar]
- Kohno T, Moore KA, Baba H, Woolf CJ. Peripheral nerve injury alters excitatory synaptic transmission in lamina II of the rat dorsal horn. J Physiol. 2003;548:131–138. doi: 10.1113/jphysiol.2002.036186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DP, Chen SR, Pan YZ, Levey AI, Pan HL. Role of presynaptic muscarinic and GABA(B) receptors in spinal glutamate release and cholinergic analgesia in rats. J Physiol. 2002;543:807–818. doi: 10.1113/jphysiol.2002.020644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ohishi H, Kinoshita A, Shigemoto R, Nomura S, Mizuno N. Localization of a metabotropic glutamate receptor, mGluR7, in axon terminals of presumed nociceptive, primary afferent fibers in the superficial layers of the spinal dorsal horn: an electron microscope study in the rat. Neurosci Lett. 1997;223:153–156. doi: 10.1016/s0304-3940(97)13429-2. [DOI] [PubMed] [Google Scholar]
- Liu CN, Raber P, Ziv-Sefer S, Devor M. Hyperexcitability in sensory neurons of rats selected for high versus low neuropathic pain phenotype. Neuroscience. 2001;105:265–275. doi: 10.1016/s0306-4522(01)00161-0. [DOI] [PubMed] [Google Scholar]
- Lomeli J, Quevedo J, Linares P, Rudomin P. Local control of information flow in segmental and ascending collaterals of single afferents. Nature. 1998;395:600–604. doi: 10.1038/26975. [DOI] [PubMed] [Google Scholar]
- Matzner O, Devor M. Hyperexcitability at sites of nerve injury depends on voltage-sensitive Na+ channels. J Neurophysiol. 1994;72:349–359. doi: 10.1152/jn.1994.72.1.349. [DOI] [PubMed] [Google Scholar]
- Moore KA, Kohno T, Karchewski LA, Scholz J, Baba H, Woolf CJ. Partial peripheral nerve injury promotes a selective loss of GABAergic inhibition in the superficial dorsal horn of the spinal cord. J Neurosci. 2002;22:6724–6731. doi: 10.1523/JNEUROSCI.22-15-06724.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohishi H, Nomura S, Ding YQ, Shigemoto R, Wada E, Kinoshita A, Li JL, Neki A, Nakanishi S, Mizuno N. Presynaptic localization of a metabotropic glutamate receptor, mGluR7, in the primary afferent neurons: an immunohistochemical study in the rat. Neurosci Lett. 1995;202:85–88. doi: 10.1016/0304-3940(95)12207-9. [DOI] [PubMed] [Google Scholar]
- Pan HL, Khan GM, Alloway KD, Chen SR. Resiniferatoxin induces paradoxical changes in thermal and mechanical sensitivities in rats: mechanism of action. J Neurosci. 2003;23:2911–2919. doi: 10.1523/JNEUROSCI.23-07-02911.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pin JP, Duvoisin R. The metabotropic glutamate receptors: structure and functions. Neuropharmacology. 1995;34:1–26. doi: 10.1016/0028-3908(94)00129-g. [DOI] [PubMed] [Google Scholar]
- Rudomin P, Schmidt RF. Presynaptic inhibition in the vertebrate spinal cord revisited. Exp Brain Res. 1999;129:1–37. doi: 10.1007/s002210050933. [DOI] [PubMed] [Google Scholar]
- Schoepp DD, Jane DE, Monn JA. Pharmacological agents acting at subtypes of metabotropic glutamate receptors. Neuropharmacology. 1999;38:1431–1476. doi: 10.1016/s0028-3908(99)00092-1. [DOI] [PubMed] [Google Scholar]
- Shehab SA, Spike RC, Todd AJ. Do central terminals of intact myelinated primary afferents sprout into the superficial dorsal horn of rat spinal cord after injury to a neighboring peripheral nerve? J Comp Neurol. 2004;474:427–437. doi: 10.1002/cne.20147. [DOI] [PubMed] [Google Scholar]
- Simpson RK, Jr, Huang W. Glycine receptor reduction within segmental gray matter in a rat model in neuropathic pain. Neurol Res. 1998;20:161–168. doi: 10.1080/01616412.1998.11740500. [DOI] [PubMed] [Google Scholar]
- Sindrup SH, Jensen TS. Efficacy of pharmacological treatments of neuropathic pain: an update and effect related to mechanism of drug action. Pain. 1999;83:389–400. doi: 10.1016/S0304-3959(99)00154-2. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Puig S, Jones DL. Spinal bicuculline produces hypersensitivity of dorsal horn neurons: effects of excitatory amino acid antagonists. Pain. 1998;77:181–190. doi: 10.1016/S0304-3959(98)00094-3. [DOI] [PubMed] [Google Scholar]
- Stefani A, Spadoni F, Bernardi G. Group III metabotropic glutamate receptor agonists modulate high voltage-activated Ca2+ currents in pyramidal neurons of the adult rat. Exp Brain Res. 1998;119:237–244. doi: 10.1007/s002210050337. [DOI] [PubMed] [Google Scholar]
- Thomsen C. The L-AP4 receptor. Gen Pharmacol. 1997;29:151–158. doi: 10.1016/s0306-3623(96)00417-x. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Lochhead V. GABA-like immunoreactivity in type I glomeruli of rat substantia gelatinosa. Brain Res. 1990;514:171–174. doi: 10.1016/0006-8993(90)90454-j. [DOI] [PubMed] [Google Scholar]
- Todd AJ, Sullivan AC. Light microscope study of the coexistence of GABA-like and glycine-like immunoreactivities in the spinal cord of the rat. J Comp Neurol. 1990;296:496–505. doi: 10.1002/cne.902960312. [DOI] [PubMed] [Google Scholar]
- Wang XL, Zhang HM, Chen SR, Pan HL. Altered synaptic input and GABAB receptor function in spinal superficial dorsal horn neurons in rats with diabetic neuropathy. J Physiol. 2007;579:849–861. doi: 10.1113/jphysiol.2006.126102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis WD., Jr Dorsal root potentials and dorsal root reflexes: a double-edged sword. Exp Brain Res. 1999;124:395–421. doi: 10.1007/s002210050637. [DOI] [PubMed] [Google Scholar]
- Woodbury CJ, Kullmann FA, McIlwrath SL, Koerber HR. Identity of myelinated cutaneous sensory neurons projecting to nocireceptive laminae following nerve injury in adult mice. J Comp Neurol. 2008;508:500–509. doi: 10.1002/cne.21693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- Woolf CJ, Shortland P, Coggeshall RE. Peripheral nerve injury triggers central sprouting of myelinated afferents. Nature. 1992;355:75–78. doi: 10.1038/355075a0. [DOI] [PubMed] [Google Scholar]
- Yaksh TL. Behavioral and autonomic correlates of the tactile evoked allodynia produced by spinal glycine inhibition: effects of modulatory receptor systems and excitatory amino acid antagonists. Pain. 1989;37:111–123. doi: 10.1016/0304-3959(89)90160-7. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Chen SR, Pan HL. Regulation of glutamate release from primary afferents and interneurons in the spinal cord by muscarinic receptor subtypes. J Neurophysiol. 2007;97:102–109. doi: 10.1152/jn.00586.2006. [DOI] [PubMed] [Google Scholar]
- Zhang HM, Li DP, Chen SR, Pan HL. M2, M3, and M4 receptor subtypes contribute to muscarinic potentiation of GABAergic inputs to spinal dorsal horn neurons. J Pharmacol Exp Ther. 2005;313:697–704. doi: 10.1124/jpet.104.079939. [DOI] [PubMed] [Google Scholar]
- Zhou HY, Zhang HM, Chen SR, Pan HL. Increased nociceptive input rapidly modulates spinal GABAergic transmission through endogenously released glutamate. J Neurophysiol. 2007;97:871–882. doi: 10.1152/jn.00964.2006. [DOI] [PubMed] [Google Scholar]