Abstract
Live-attenuated viruses derived from SIV and SHIV have provided the most consistent protection against challenge with pathogenic viruses, but concerns regarding their long-term safety and efficacy have hampered their clinical usefulness. We report a longitudinal study in which we evaluated the long-term safety and efficacy of ΔvpuSHIVPPC, a live virus vaccine derived from SHIVPPC. Macaques were administered two inoculations of ΔvpuSHIVPPC, three years apart, and followed for eight years. None of the five vaccinated macaques developed an AIDS-like disease from the vaccine. At eight years, macaques were challenged with pathogenic SIV and SHIV. None of the four macaques with detectable cellular-mediated immunity prior to challenge had detectable viral RNA in the plasma. This study demonstrates that multiple inoculations of a live vaccine virus can be used safely and can significantly extend the efficacy of the vaccine, as compared to a single inoculation, which is efficacious for approximately three years.
Keywords: Live-attenuated vaccine, SIV, SHIV, Safety, immune response, correlates of protection
Introduction
The high capacity of human immunodeficiency virus (HIV-1) to mutate and escape immune responses has been a major obstacle to developing an effective vaccine against HIV-1. Despite this challenge, developing a vaccine against HIV-1 is of paramount importance to stem the global pandemic of HIV-1/AIDS. While numerous strategies for vaccines have been designed, none have been fully successful. To date, the most successful vaccines to date in protecting non-human primates against pathogenic SIV and SHIV have been live-attenuated vaccines based on SIV or SHIV (Whitney and Ruprecht, 2004; Robinson, 2007). These viruses were obtained by deleting one or more viral genes. The first such vaccine was SIVmac239 in which nef was deleted. This vaccine was shown to induce protection of immunized macaques against the challenge with the highly pathogenic molecularly cloned SIVmac239 or the uncloned SIVmac251 (Daniel et al., 1992). However, this live-attenuated vaccine virus was found to be pathogenic in infant macaques and some adult macaques (Baba et al., 1995; Baba et al., 1999). The results of these early studies raised a high level of skepticism that a live virus vaccine against HIV-1 could be developed that was safe enough to use in humans.
Since these early studies, several laboratories have investigated other live virus vaccines in order to determine whether an attenuated vaccine could be developed that is both safe and efficacious (Whitney and Ruprecht, 2004). Previously, we derived live-attenuated vaccines from the pathogenic SHIVs to evaluate whether they would protect monkeys from the highly pathogenic SHIVs and SIVs in primates. In earlier studies we described a live-attenuated SHIV-base vaccine ΔvpuΔnefSHIVPPC (Joag et al., 1998b) and showed it to be safe after serial passage (Mackay et al., 2002). However, it replicated poorly in vivo and offered little long-term protection against disease induced by challenge with pathogenic viruses (Joag et al., 1998b; Mackay et al., 2004a).
To improve the immunogenicity of the SHIV-based vaccine, the vaccine was redesigned to generate ΔvpuSHIVPPC (Joag et al., 1998b), a vaccine that replicated much more robustly and was much more successful in conferring protection against disease caused by challenge viruses (Joag et al., 1998b; Silverstein et al., 2000; Mackay et al., 2004a). After challenge with pathogenic virus, vaccinated animals developed plasma virus levels during the acute phase that were at least two orders of magnitude lower than unvaccinated controls (Joag et al., 1998b). Despite the ability of ΔvpuSHIVPPC to reduce viremia, this vaccine did not prevent viremia and many vaccinated animals ultimately succumbed to AIDS. A recent study by Kumar et. al. (2008) supported the finite nature of the protection by showing that the protection induced ΔvpuSHIVPPC vaccine against productive infection by a pathogenic virus only lasted approximately three years after vaccination.
While previous studies established that ΔvpuSHIVPPC has the potential to be a highly effective vaccine, the long-term safety of this vaccine had not been evaluated. In addition, it is unknown whether multiple inoculations with ΔvpuSHIVPPC could extend the protection against disease caused by pathogenic virus. In this report we describe a longitudinal study using adult rhesus macaques immunized twice with ΔvpuSHIVPPC vaccine to evaluate the safety and efficacy of this vaccine virus.
Results
Viral load after immunization with ΔvpuSHIVPPC
Five Indian rhesus macaques (RBq5, RCu5, RTp5, RUr5, and RVq5), were inoculated intradermally with the infectious DNA of the live vaccine virus ΔvpuSHIVPPC, which contains the X4 env gene from the HXB2 strain of HIV-1, and the gag, pol, and nef coding sequences from SIVmac239. This DNA produces replication-competent virus with an average titer between 104 and 105 TCID50 per ml in the culture supernatants of transfected HEK-293 T cells (data not shown). Consistent with previous experiments using ΔvpuSHIVPPC (Joag et al., 1998a), macaques inoculated with this DNA developed a productive infection that lasted four to eight weeks, as indicated by measuring viral RNA in plasma (Table 1). In addition, all animals seroconverted and remained seropositive during the entire study (data not shown).
Table 1.
Viral RNA in plasma (copies/ml) after first inoculation with ΔvpuSHIVPPC a
| RBq | RCu | RTp | RUr | RVq | |
|---|---|---|---|---|---|
| Week 2 | 16,000 | 5,400 | 99,000 | 37,000 | 73,000 |
| Week 4 | 2,100 | 1,200 | ND | 80,000 | 18,000 |
| Week 8 | NDb | ND | ND | ND | 650 |
| Week 133 | ND | ND | ND | ND | ND |
Weeks indicate week after first inoculation with ΔvpuSHIVPPC.
ND = none detected
Circulating CD4+ and CD8+ T cell counts after the first inoculation
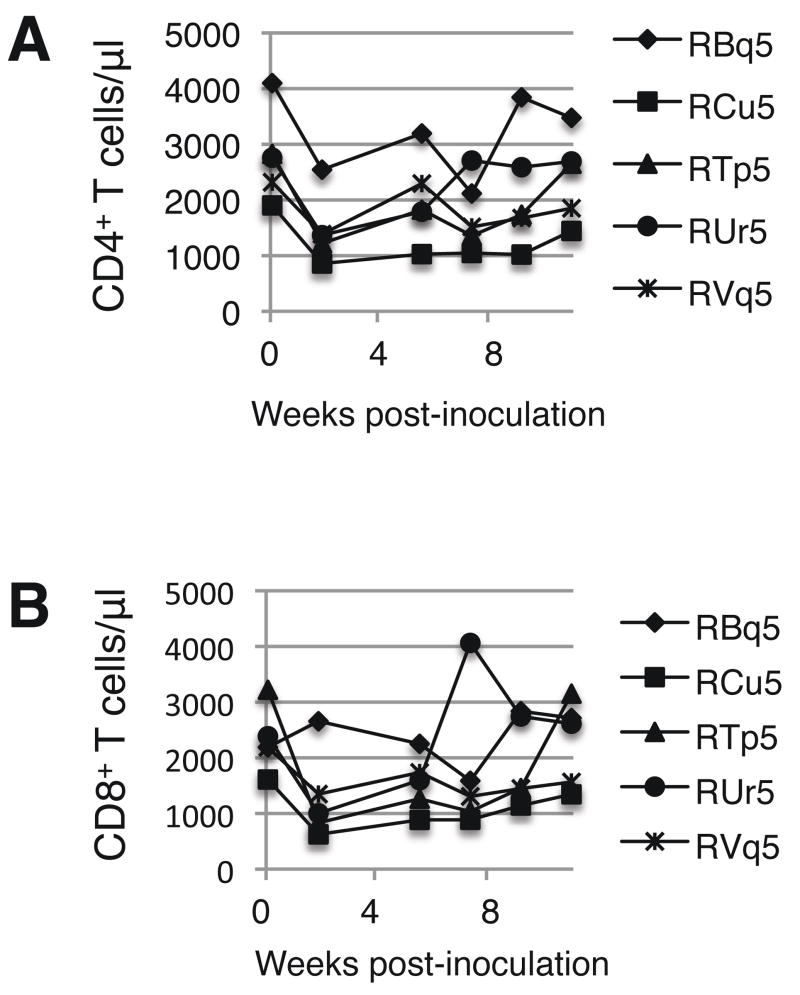
The levels of circulating CD4+ and CD8+ T cells were calculated before and after each inoculation with ΔvpuSHIVPPC. After the first inoculation, the number of circulating CD4+ T cells at two weeks post-inoculation averaged 52% ± 8.6% the number of CD4+ T cells prior to inoculation (Fig. 1A). The number of CD4+ T cells returned to at least 90% of pre-inoculation levels within an average of 12 ± 7.1 weeks after vaccination. The circulating CD8+ T cell levels also declined sharply in four of five macaques in the first weeks post-inoculation (Fig. 1B). The four animals that developed a decline in circulating CD8+ T cell levels had an average of 42% ± 15% the number of CD8+ T cells prior to inoculation. Their CD8+ T cell levels returned to within 90% of pre-inoculation levels within 18 ± 9.5 weeks. Animal RBq did not develop a decline in CD8+ T cells, suggesting that this animal may have had a potent immunologic response against the live virus that protected it from CD8+ T cell loss.
Fig. 1. Circulating CD4+ and CD8+ T cell levels decline after the first inoculation with ΔvpuSHIVPPC.
The absolute numbers of CD4+ (Panel A) and CD8+ (Panel B) T cells were determined by staining whole blood with anti-CD3, anti-CD4, and anti-CD8. The numbers shown represent cells per μl blood.
These data indicated that, while ΔvpuSHIVPPC does not induce an AIDS-like syndrome, the vaccine can cause a transient depletion of CD4+ and CD8+ T cells. By contrast, the pathogenic form of SHIV typically causes severe atrophy of the thymus and ablates the CD4+ T cell compartment in secondary lymphoid tissues within six months of infection (Joag et al., 1996; Joag et al., 1997a; Joag et al., 1997b).
Viral load and T cell levels following re-immunization with ΔvpuSHIVPPC
Three years after the first inoculation (week 161), animals were inoculated orally with 1 ml of stock ΔvpuSHIVPPC virus containing 5×105 TCID50 per ml. Similar to the first inoculation, the second inoculation resulted in a transient productive infection followed by an extended period of latency (Table 2). In four out of the five animals, the peak plasma viral RNA levels after the second inoculation was considerably less than that caused by the first inoculation, suggesting that these animals had developed a degree of immunologic protection against ΔvpuSHIVPPC. Four to eight weeks after the second inoculation, viral RNA was undetectable in the plasma, although an occasional resurgence of viral RNA was detected (Table 2). These spikes suggested that the virus sporadically escaped and produced a low-grade productive infection. These bursts in viral replication were quickly suppressed as the viral RNA levels became undetectable at the next measurement.
Table 2.
Viral RNA in plasma (copies/ml) after second inoculation with ΔvpuSHIVPPC a
| RBq | RCu | RTp | RUr | RVq | |
|---|---|---|---|---|---|
| Week 162 | NDb | 935 | 2,819 | ND | 612 |
| Week 163 | 6,397 | 6,193 | 1,894 | 7,274 | 13,377 |
| Week 164 | ND | ND | 2,348 | 400 | 679 |
| Week 166 | 983 | 1,263 | 2,361 | 5,276 | 514 |
| Week 169 | ND | ND | 414 | ND | ND |
| Week 173 | ND | ND | ND | 1480 | ND |
| Week 182 | 900 | ND | ND | ND | ND |
| Week 186 | ND | ND | ND | ND | 480 |
| Week 195 | ND | ND | ND | ND | ND |
| Week 251 | ND | 468 | 1,572 | 4,344 | ND |
| Week 253 | ND | ND | ND | ND | ND |
Weeks indicate week after first inoculation with ΔvpuSHIVPPC.
ND = none detected
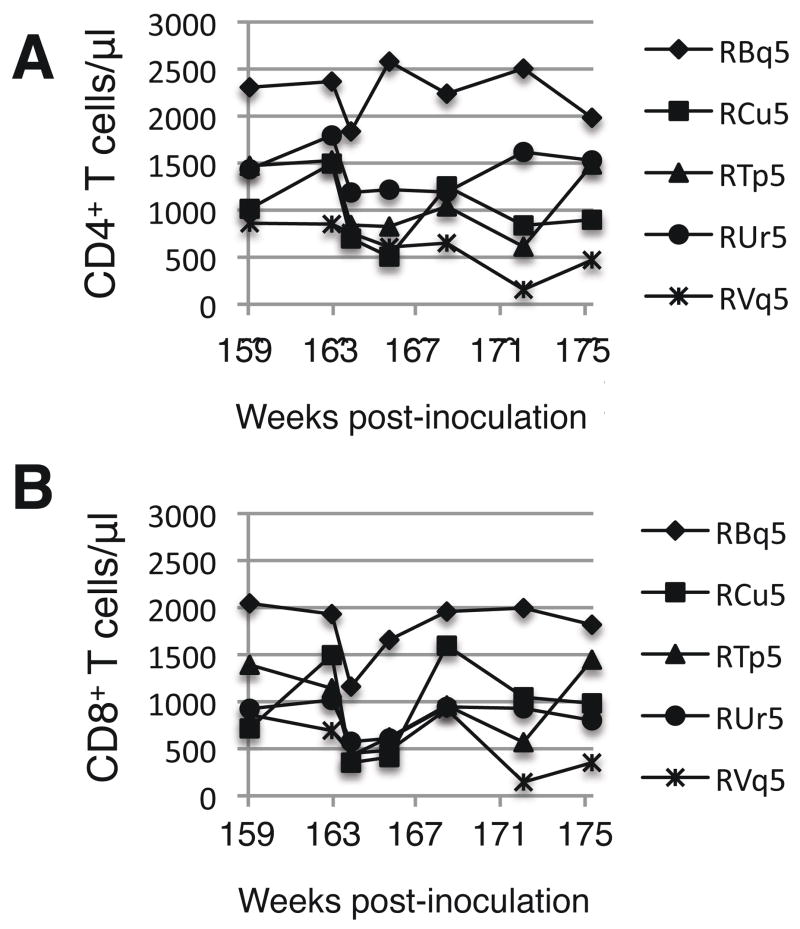
In four of five animals, CD4+ T cells counts dropped declined after the second inoculation with ΔvpuSHIVPPC (Fig. 2). Except for RBq5, the average circulating CD4+ T cell levels five weeks after the second inoculation (week 166) were 65% ± 16% of the number prior to re-inoculation (week 159). Similarly, the number of CD8+ T cells in animals RCu5, RTp5, RUr5, and RVq5 averaged 56% ± 8.8% the level prior to re-infection. RBq5 has no decrease in the number of CD4+ T cells and only a slight reduction in the number of CD8+ T cells after the second inoculation with ΔvpuSHIVPPC, further demonstrating that this animal had an unusual immunologic response to the vaccine.
Fig. 2. Circulating CD4+ and CD8+ T cell levels after the second inoculation with ΔvpuSHIVPPC.
Macaques were re-inoculated at week 161 and the absolute numbers of CD4+ (Panel A) and CD8+ (Panel B) T cells were determined as described in the legend to Fig. 1.
Transient depletion of CD8+ T cells
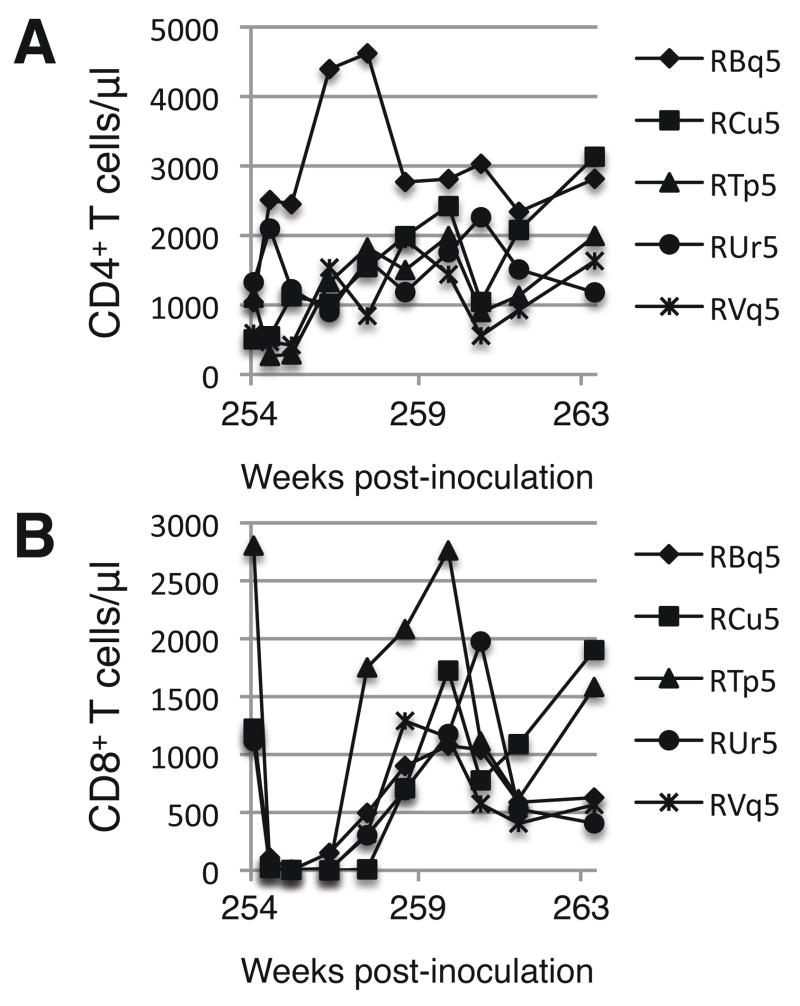
Nearly two years after the second vaccination (week 254), we sought to determine which of the vaccinated animals still harbored the vaccine virus. We administered a course of anti-CD8 antibodies using a procedure previously shown to be highly effective in reducing the level of CD8+ T cells and inducing the re-activation of latent viruses (Jin et al., 1999; Schmitz et al., 1999; Mackay et al., 2004b). In all five animals, the number of CD8+ T cells in the blood decreased to fewer than 10 cells per microliter followed by complete recovery of CD8+ T cells within four weeks (Fig. 3B). Concomitant with the loss of CD8+ T cells was a high level of resurgence in the viral mRNA levels in plasma of all animals except RBq5 (Table 3). These data indicate that all the animals (except RBq5) still harbored the vaccine virus at the time of anti-CD8 treatment. Viral loads returned to undetectable levels upon the recovery of the CD8+ T cell population.
Fig. 3. Circulating CD4+ and CD8+ T cell levels after depletion of CD8+ cells.
Macaques were injected intravenously three times with anti-CD8 antibodies during weeks 254 and 255. The absolute numbers of CD4+ (Panel A) and CD8+ (Panel B) T cells were determined as described in the legend to Fig. 1.
Table 3.
Viral RNA in plasma (copies/ml) after anti-CD8 treatmenta
| RBq | RCu | RTp | RUr | RVq | |
|---|---|---|---|---|---|
| Week 254 Anti-CD8 |
NDb | ND | ND | ND | ND |
| Week 255 | ND | 9,480 | 42,620 | 920 | 85,140 |
| Week 256 | ND | 548,740 | 755,620 | 1,342,640 | 809,700 |
| Week 257 | ND | 37,540 | 18,460 | 57,320 | 11,260 |
| Week 258 | ND | 6,860 | ND | ND | 1,760 |
| Week 259 | ND | 4,120 | 860 | ND | ND |
| Week 263 | ND | ND | ND | ND | ND |
| Week 282 | ND | ND | ND | ND | ND |
| Week 361 | ND | ND | ND | ND | ND |
CD8+ cell immunodepletion led to a robust resurgence in viral load in four of the five animals. Animals were injected three times with anti-CD8 during week 254.
ND = none detected
Two animals (RTp5 and RVq5) had a significant loss of CD4+ T cells following the anti-CD8 treatment, but the levels returned to pre-anti-CD8 levels within one week (Fig. 3A). The other three animals developed an increase in CD4+ T cell counts that subsided upon the return on the CD8+ T cells.
The five animals were followed for an additional three years after the anti-CD8 experiment. No viral RNA was detected in the plasma (Table 3) and the numbers of circulating CD4+ and CD8+ T cells remained stable during this period (data not shown). At week 400, blood cells were isolated to determine whether viral DNA could be detected. Viral DNA could be detected in two of the five animals (RCu5 and RVq5) (data not shown). These data suggested that the other animals may have eliminated the vaccine virus or the virus was present in hematopoietic cells in primary or secondary organs.
Immune responses prior to challenge
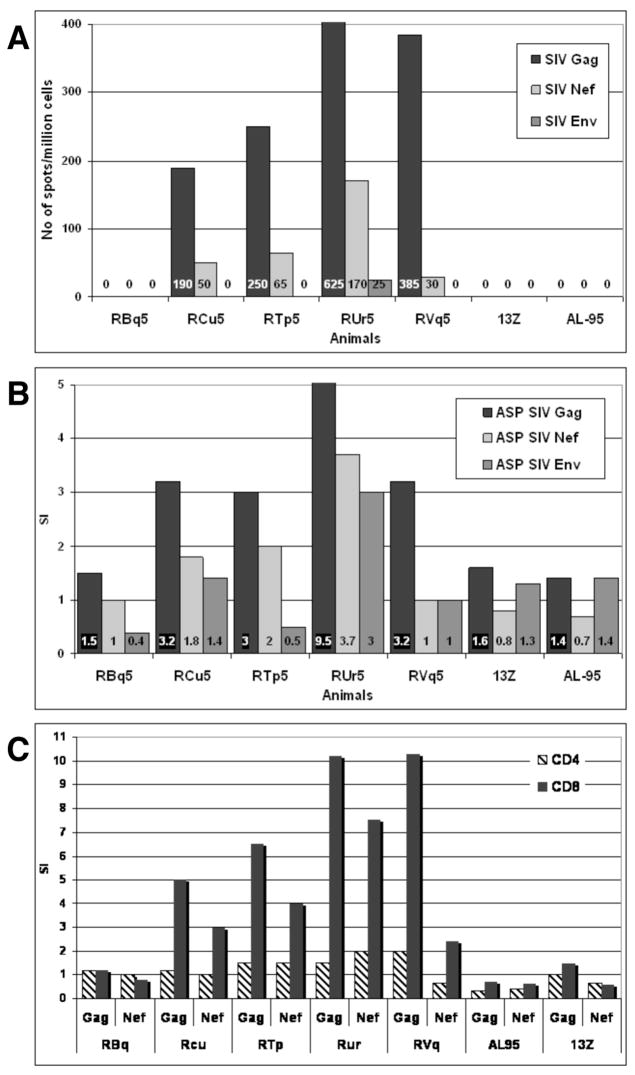
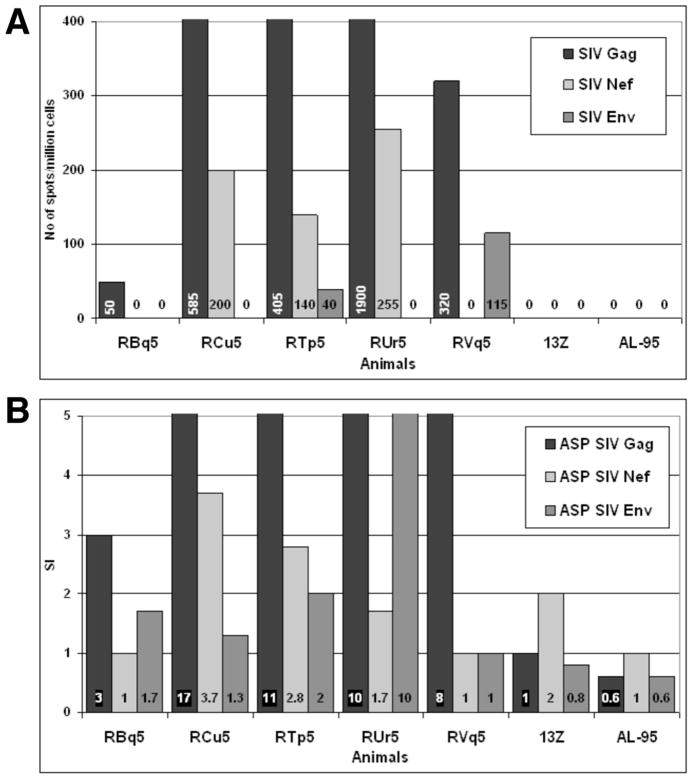
Prior to challenge, Gag-specific, Nef-specific, and Env-specific production of IFNγ by PBMCs was measured by ELISPOT (Fig. 4A) and antigen-specific proliferation (ASP) of PBMCs was measured by [3H] thymidine incorporation (Fig. 4B). The four animals that had resurgence of virus following anti-CD8 treatment (RCu5, RTp5, RUr5, and RVq5) had Gag-specific ELISPOT and ASP responses. In addition, these four animals had Nef-specific ELISPOT responses and one animal (RUr5) had significant Nef-specific ASP. PBMCs from macaque RBq5 did not have any ELISPOT or ASP responses following incubation with peptides of the three viral proteins, SIV Gag, Nef, or Env. Similarly, the two unvaccinated control animals (animals 13Z and AL-95) also lacked ELISPOT and ASP responses against Gag, Nef, or Env.
Fig. 4. Vaccinated animals have Gag-specific and Nef-specific CMI responses, but not Env-specific CMI responses, prior to challenge.
Panel A. PBMCs were stimulated with overlapping peptides from Gag, Nef, or Env and IFNγ production was measured by ELISPOT. The numbers in each bar are the number of IFNγ producing cells/million total PBMCs. Panel B. PBMCs were stimulated with Gag, Nef, or Env peptides and proliferation was measured by [3H]thymidine uptake. The stimulation index (SI) was calculated as the number of cpm induced by peptides divided by the cpm in unstimulated cells. Panel C. PBMCs were loaded with CFSE before stimulation with Gag, Nef, or Env peptides. After four days, cells were harvested labeled with anti-CD3, anti-CD4, and anti-CD8. The SI for CD3+CD4+ and CD3+CD8+ cells was calculated by dividing the percentage of CFSElo cells in the peptide-stimulated samples by the percentage of CFSElo cells in the unstimulated.
While the ASP assay showed that PBMCs proliferated in response to Gag and Nef, this assay did not indicate whether it was the CD4+ or CD8+ T cells that proliferated. To address this question, we measured proliferation by examining the dilution of 5-(and-6)-carboxyfluorescein diacetate, succinimidyl ester (CFSE) by flow cytometry. We electronically gated on CD4+ or CD8+ T cells and then analyzed proliferation. Nearly all of the proliferation detected was a result of proliferation of CD8+ T cells; there was minimal, if any, proliferation of CD4+ T cells (Fig. 4C).
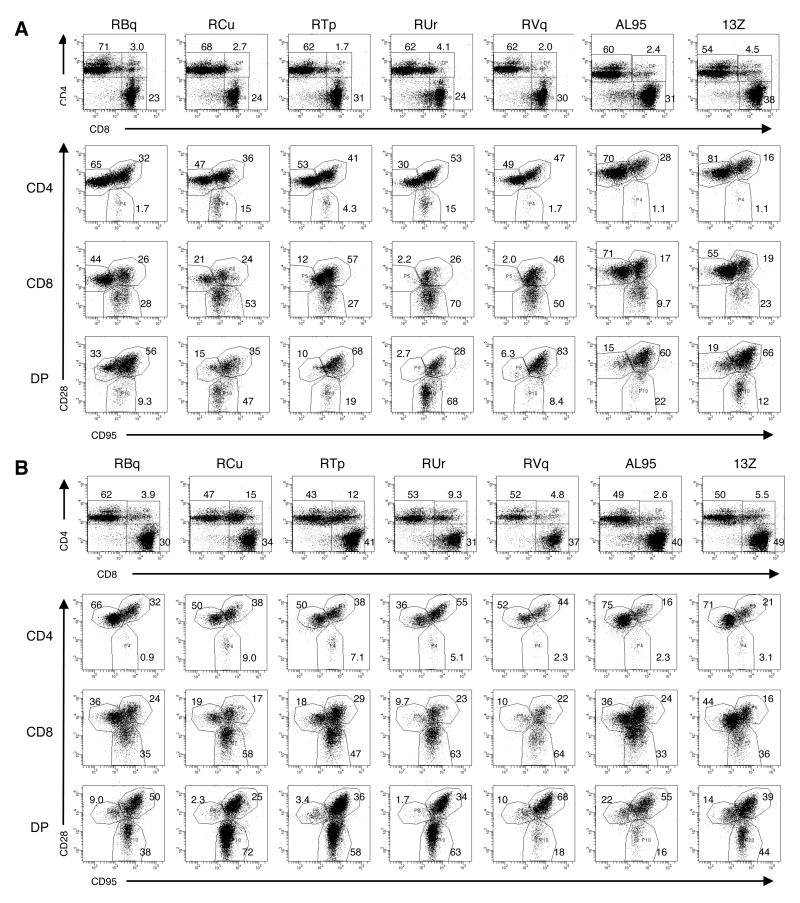
Next, we investigated whether T cells from vaccinated animals had a different surface phenotype than T cells from unvaccinated animals. The percentage of CD3+ PBMCs that were CD4+, CD8+, or CD4+CD8lo was indistinguishable among the seven animals (Fig. 5A). However, the CD8+ T cell population in the four animals with Gag-specific CMI responses had increased percentages of CD95+ cells, compared to RBq5 and the unvaccinated controls (88% ± 9.4% versus 41% ± 14%, p = 0.010) (Fig. 5A). This suggested that the CD8+ T cells in the four successfully vaccinated animals had an activated or memory phenotype. Because these were outbred animals, it was not possible to define the phenotype of the antigen-specific T cells, although the persistence of the proliferation and IFNγ production suggested that the animals developed immunologic memory.
Fig. 5. Macaques that were protected from challenge had an increase in the percentage of activated/memory CD8+ T cells prior to challenge and an expansion of CD4+CD8lo cells after challenge.
Panel A. Prior to challenge, PBMCs were gated on CD3+ cells and analyzed for CD4 and CD8 expression (upper row). Cells were then gated on CD4+, CD8+, or CD4+CD8lo (DP) cells and analyzed for CD28 and CD95 expression (lower three rows). Panel B. Eight weeks after challenge, the experiment in Panel A was repeated.
We also tested whether sera from these animals developed neutralizing antibodies against pathogenic SHIVPPC, the parent virus of the vaccine. The sera were tested nearly eight years after the initial vaccination and prior to challenge. Macaques RBq,5, RCu5, and RTp5 had neutralizing antibody titers less than 1:10 while RUr5 had a titer of 1:80 and RVq5 had a titer of 1:160. These data indicate that only two of the five animals developed and sustained detectable neutralizing antibodies.
Protection against challenge
Nearly eight years after the first inoculation with ΔvpuSHIVPPC, five years after the second inoculation with ΔvpuSHIVPPC, and nearly three years, after the anti-CD8 treatment (week 404), the five vaccinated animals and two unvaccinated control animals were challenged with a mixture of the pathogenic viruses SHIVKU2 and SIVmac239. The four vaccinated animals that had detectable Gag-specific CMI responses prior to challenge had no detectable viremia following challenge with pathogenic virus (Table 4). The two unvaccinated animals and animal RBq5 developed viral RNA loads that exceeded 106 viral copies/ml plasma. Whereas RBq5 ultimately controlled viral replication, the detectable viral mRNA levels persisted in the unvaccinated controls. These data suggested that RBq5 could not control the initial replication of the virus, but had an immune response that protected this animal from chronic viral infections. These data suggested that Gag-specific CMI responses may be predictive of successful vaccination.
Table 4.
Viral RNA in plasma (copies/ml) after challengea
| RBq | RCu | RTp | RUr | RVq | 13Z | AL-95 | |
|---|---|---|---|---|---|---|---|
| Week 404 challenge |
b ND | ND | ND | ND | ND | ND | ND |
| Week 405 | 3,796,200 | ND | ND | ND | ND | 894,144 | 818,988 |
| Week 406 | 11,136 | ND | ND | ND | ND | 2,109,780 | 3,777,240 |
| Week 407 | 86,436 | ND | ND | ND | ND | 258,108 | 88,812 |
| Week 408 | 13,200 | ND | ND | ND | ND | 338,592 | 253,608 |
| Week 412 | ND | ND | ND | ND | ND | 1,784,316 | 140,208 |
Four of five vaccinated animals had no detectable SIV RNA during the eight week period after challenge.
ND = none detected
The four protected animals had greatly augmented Gag-specific and Nef-specific ELISPOT and ASP after challenge, compared to prior to challenge, indicating that infection with pathogenic virus triggered a robust immune response (Fig. 6). Only two animals (RTp5 and RVq5) developed Env-specific ELISPOT and one animal, RUr5, developed Env-specific ASP. These data suggested that Gag-specific and Nef-specific immune responses are more essential than Env-specific immune responses for the protection of animals against challenge with pathogenic SIV and SHIV.
Fig. 6. Vaccinated animals maintain SIV-specific CMI after challenge.
Panel A. IFNγ production by PBMCs was calculated as described in the legend to Fig. 4. Panel B. Peptide-specific proliferation was analyzed by [3H]thymidine uptake and the SI was calculated, as described in the legend to Fig. 4.
Finally, we analyzed the surface phenotype of T cell populations after challenge (Fig. 5B). The four vaccinated macaques that resisted challenge by pathogenic viruses had a four-fold increase in the percentage of CD3+ PBMC that were CD4+CD8lo, compared to their respective pre-challenge phenotypes. On average, 2.6% ± 1.1% of CD3+ PBMCs were CD4+CD8lo prior to challenge and 10% ± 4.3% of CD3+ PBMCs were CD4+CD8lo post-challenge (p = 0.040, paired t test). A statistically significant increase in the CD4+CD8lo population was not observed following challenge with pathogenic viruses in macaque RBq5 or the two control animals. In RBq5 and the control animals, an average of 4.0% ± 1.5% of CD3+ PBMCs were CD4+CD8lo prior to challenge and 3.3% ± 1.1% of CD3+ PBMCs were CD4+CD8lo after challenge (p = 0.11). These data suggested that the increase in CD4+CD8lo cells correlated with immunologic protection.
Discussion
The data presented here represent the longest assessment of the safety and efficacy of a live vaccine virus against SIV in macaques. Animals were inoculated with ΔvpuSHIVPPC twice in order to determine if this vaccine strategy could protect animals from productive infection induced by pathogenic virus. Prior experiments used a single inoculation of ΔvpuSHIVPPC followed three years later by challenge with a cocktail of pathogenic viruses (Kumar et al., 2008). Under these conditions, the vaccine virus was unable to prevent viremia. However, in the current study, animals were vaccinated, re-vaccinated three years later, and then challenged five years after the second inoculation. Four of five animals were aviremic following infection with challenge viruses. These data demonstrate that multiple inoculations with a live virus vaccine can extend the efficacy of the vaccine.
Despite transient declines in circulating CD4+ and CD8+ T cell levels, no sustained loss of CD4+ or CD8+ T cells was observed in any of the five vaccinated animals, indicating that ΔvpuSHIVPPC was safe for use in the context of repeated inoculations over time. A possible explanation for these transient decreases in the number of circulating T cells is that the virus may infect and kill thymocytes. When replication of ΔvpuSHIVPPC is controlled, the thymus may be restored and T cell counts recover. Alternatively, the depletion of circulating T cells could be attributed to re-localization of cells from the blood into lymphoid tissues. Further studies are required to determine the mechanism.
Previous studies demonstrated that the vaccine virus must persist in order for protection against disease induced by pathogenic virus to be sustained (Kumar et al., 2008). In the current study, we treated macaques with anti-CD8 antibodies to transiently deplete the animals of CD8+ T cells to determine if a resurgence in viremia would occur from undetectable reservoirs of the vaccine virus (Table 3). While this treatment demonstrated that four of five vaccinated animals still harbored virus, it also led to a third bolus of vaccine virus. This extra bolus may have boosted the immune responses against Gag and Nef, adding to the efficacy of the vaccine. The animals that had three boluses of viral replication did not develop high viral loads and AIDS, despite having an average of 34-fold increase in viral burdens after the anti-CD8 treatment than after either immunization. These data clearly demonstrate that the virus remained viable, but had not reverted to a pathogenic phenotype.
A striking feature of the protective immunity observed in this study was the complete lack of viremia in successfully vaccinated animals (Table 4). This remarkable suppression of viral replication was likely due to Gag-specific and Nef-specific CD8+ T cells that could recognize and become activated by MHC class I-expressing cells that presented Gag or Nef peptides because no Env-specific immune responses could be detected in most animals. The importance of Gag-specific immune responses was highlighted in a recent paper by Kiepela, et. al. (2007), who showed in HIV-infected individuals that Gag-specific immune responses were associated with lower levels of viremia and Env-specific responses were associated with higher levels of viremia. In addition, it was recently reported that the Gag liberated during the virus-uncoating stage can be processed and presented on MHC class I prior to viral integration (Sacha et al., 2007). In the case of an intravenous injection of challenge virus, it is likely that virus infected the macrophages in the blood and spleen and then Gag-specific CD8+ T cells targeted and killed the infected cells before the virus could complete its life cycle.
Of note, little or no Env-specific cellular-mediated immune responses were detected prior to challenge in any of the animals and only two animals had detectable anti-SHIVPPC neutralizing antibody titers prior to challenge. In addition, the animals were vaccinated with a derivative of SHIV that contains a macaque-adapted HXB2 envelope glycoprotein, which is CXCR4-tropic. The animals were challenged with a mixture of CXCR4-tropic SHIVKU2 and CCR5-tropic SIVmac239. If there were undetectable Env-specific immune responses in the vaccinated animals, these responses would likely be directed against the challenge SHIV, not SIV. However, our data demonstrated that ΔvpuSHIVPPC protected against productive infection by both SHIVKU2 and SIVmac239, suggesting that Env-specific immune responses were not critical for immunological protection of the challenge viruses. This observation is highly significant for vaccine design because Env is not well conserved across HIV-1 strains and subtypes while Gag is more conserved. Thus, it is likely that a single vaccine could be effective against multiple strains of HIV-1 and perhaps multiple subtypes of the virus.
It is unlikely that the mechanism of protection conferred by ΔvpuSHIVPPC is viral interference because ΔvpuSHIVPPC and SIVmac239 have different tropisms. ΔvpuSHIVPPC targets CXCR4-expressing CD4+ T cells, which are primarily naïve peripheral T cells and T cell precursors in the thymus. SIVmac239 targets CCR5-expressing cells, which are primarily memory T cells. Another reason that viral interference is unlikely is that, at the time of challenge, there was no detectable vaccine virus in the plasma. It is difficult to conceive that an undetectable viral load could interfere with a high intravenous dose of challenge virus.
Macaque RBq5 was the outlier in this study. This animal did not develop a resurgence of virus after treatment with the anti-CD8 antibodies (Table 3), suggesting that the animal had eliminated the vaccine virus. The lack of CMI responses against Gag and Nef together with failure of the vaccine virus to persist supported our previous work showing that maintenance of vaccine-induced CMI responses required persistence of the virus (Joag et al., 1998b; Silverstein et al., 2000; Mackay et al., 2004a). More importantly, however, the data from RBq5 reinforced the conclusion from the present study that CMI responses against Gag and Nef correlated with protection from productive infection by the challenge virus. The fact that this animal developed control of the challenge virus, similar to the manner in which it controlled the vaccine virus, suggested that it had mounted a strong innate immune response that did not develop into an adaptive immune response. This conclusion was also supported by the fact that RBq5 did not develop the transient loss of T cells following inoculation with the vaccine see in the other animals.
Coinciding with control of replication of the challenge virus, CD4+CD8lo T cells underwent substantial expansion in the successfully vaccinated animals (Fig. 2). CD4+CD8lo T cells were first described in humans by Blue, et. al. (Blue et al., 1985) and have since been found in healthy individuals as well as in patients with viral infections, tumors, and autoimmune diseases. In healthy individuals, the majority of CD4+CD8lo T cells have a memory phenotype and can be readily activated by virally infected cells in vitro (Nascimbeni et al., 2004; Pahar et al., 2006). This has led to the speculation that peripheral CD4+CD8lo T cells are terminally differentiated memory T cells. In further support of this model, CD4+CD8lo T cells have been shown to be expanded preferentially in an individual infected with HIV for eight years (Weiss et al., 1998). CD4+CD8lo T cells are a target for SIV infection in macaques (Akari et al., 1999). The expansion of this population of cells in the animals resisting productive infection by pathogenic virus suggested that these cells are an important component of the immune response needed to control viral replication.
In conclusion, the results presented here describe a longitudinal study in which macaques were inoculated twice over the course of an eight-year study. Whereas previous studies using a single inoculation demonstrated that ΔvpuSHIVPPC could not protect animals from productive infection by a pathogenic virus beyond three years, this study shows that a second inoculation extended the duration of protection beyond eight years.
Materials and Methods
Animals and vaccination
Indian rhesus macaques were housed individually in the Laboratory Animal Resources building of the University of Kansas Medical Center (Kansas City, KS) under conditions approved by the Association for the Assessment and Accreditation of Laboratory Animal Care. These studies were begun when the animals were five years old. All experiments were performed in compliance with the University of Kansas Medical Center Institutional Animal Care and Use Committee.
Infectious DNA of ΔvpuSHIVKU1 was prepared as described earlier (Joag et al., 1998a). A virus stock was prepared from this DNA by transfecting CEM174 cells with the DNA followed by harvest of supernatant fluid several days later. Animals were inoculated intradermally with 200 μg of the infectious DNA suspended in 2 ml of PBS. The animals were injected at four sites with 0.5 ml of the DNA suspension. Three years later, animals were revaccinated orally with the vaccine virus. Each animal was tranquilized and 1 ml of the viral suspension was placed at the back of the mouth using a 1 ml syringe.
Stocks of SIVmac239 and SHIVKU2 were prepared by inoculating CD8-depleted PBMCs from a rhesus macaque. The supernatant fluid from this culture was collected, aliquoted, and frozen at −80 °C, similar to the vaccine virus. Infectivity of both viruses was assessed in CEM174 cells and showed a titer of approximately 105 TCID/ml.
Processing of blood samples
Peripheral venous blood collected in EDTA was centrifuged to separate plasma and buffy coats. Plasma was frozen for determination of plasma viral RNA concentrations. PBMC were separated from buffy coats by centrifugation through Ficoll-Paque density gradients and portions used for virus isolation, flow cytometry, antigen-specific proliferation (ASP), and ELISPOT assays.
Determining plasma viral RNA using quantitative real-time RT-PCR analysis
Plasma viral RNA loads were measured in RNA extracted from 800–100 μl of EDTA anticoagulated plasma samples as previously described (Mackay et al., 2002; Mackay et al., 2004a). Briefly, RNA samples were subjected to real-time RT-PCR using gag specific primers (forward – GCAGAGGAGGAAATTACCCAGTAC, reverse – CAATTTTACCCAGGCATTTAATGTT), 5′-FAM- and 3′-TAMRA-labeled TaqMan probe (TGTCCACCTGCCATTAAGCCCGA), and the Taqman Universal PCR Mastermix (Applied Biosystems) in duplicate reactions in the ABI PRISM 7700 Sequence Detection System (Hofmann-Lehmann et al., 2000). These primers and probes detect all SHIVs and SIVs in this study. Viral RNA copy numbers were calculated per milliliter of plasma. The minimal level of detection was 360 copies/ml.
Flow cytometry and determination of CD4/CD8 levels
Anti-CD3-FITC, anti-CD4-PE, anti-CD8-APC-Cy7, anti-CD28-APC, and anti-CD95-PE were purchased from BD Bioscience, San Jose, CA. Flow cytometry studies were performed using a BD LSR II (BD Immunocytometry Systems, San Jose, CA). Data were analyzed using BD FACSDiva software (BD Biosciences, San Jose, CA). The absolute numbers of CD4+ and CD8+ T cells were calculated by multiplying the percentage of CD4+CD3+ and CD8+CD3+ cells by the lymphocytes/μl from a complete blood count.
Antigen-Specific Proliferation (ASP) assays
The ASP assays were performed as previously described (Mackay et al., 2004a). Briefly, triplicate cultures of PBMC were incubated with overlapping peptides derived from Gag, Nef, or Env for four days at 37°C. The peptides were 15-mers based on the sequence of the SIVmac239 and spanned the entire length of the proteins. Peptides were kindly provided by the National Institutes of Health AIDS Research and Reference Reagent Program (McKesson Bio-Services, Germantown, MD). [3H]Thymidine was added to the culture 18–24 hours before harvesting. [3H]Thymidine incorporation was measured by liquid scintillation counting (Packard Instrument, Meriden, CT). Stimulation indices (SI) were calculated as mean cpm, divided by the mean cpm in control wells. SI values greater than three were considered significant because SIs greater than two were never obtained with PBMCs from non-immunized animals.
ELISPOT assays
ELSIPOT assays were performed as described previously (Mackay et al., 2004b). Briefly, IFNγ production was measured by incubating PBMC with the same peptides used for ASP analyses. Millipore multiscreen Immobilon-P opaque hydrophobic high-protein binding 96-well plates (0.45μm; Billerica, MA) were coated with anti-monkey IFNγ (Mabtech, Stockholm, Sweden). Cells were incubated with media alone, media containing peptides, or media containing Con A. After incubation and washing, Vectastain AB kit (Vector Laboratories, Burlingame, CA) was added. Color development was performed using Nova-Red. Spots were counted with a stereomicroscope and reported as number of sports/106 PBMC.
CD8+ T cell depletion
On day 0 of treatment, animals were given 10 mg/kg anti-CD8 antibodies subcutaneously. On days 3 and 7, animals were injected intravenously with 5 mg/kg anti-CD8 antibodies. The anti-CD8 antibodies were kindly provided by Dr. Keith Reimann, Beth Israel Deaconess Medical Center, Harvard Medical School. Blood samples were obtained at the indicated time points for analysis.
Neutralizing antibody assay
Two-fold dilutions of sera were mixed with 100 TCID50 and CEMX174 cells. Virus-sera mixtures were incubated at 37 °C for 30 minutes and then inoculated into respective indicator cell cultures. Inoculated cells were examined for development of fusion cytopathic effects everyday for one week.
Acknowledgments
The anti-CD8 mAbs were kindly provided by the National Cell Culture Center (BioVest International, Minneapolis, MN), and produced by Keith Reimann and Joern Schmitz. We especially thank Dr. Reimann for helpful suggestions. We thank Istvan Adany, Zhuang Li, and Ramakrishna Hedge for technical assistance. This publication was made supported by NIH Grant Number P20 RR016443 from the COBRE Program of the National Center for Research Resource, AI051220, AI062340.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akari H, Nam KH, Mori K, Otani I, Shibata H, Adachi A, Terao K, Yoshikawa Y. Effects of SIVmac infection on peripheral blood CD4+CD8+ T lymphocytes in cynomolgus macaques. Clin Immunol. 1999;91:321–329. doi: 10.1006/clim.1999.4700. [DOI] [PubMed] [Google Scholar]
- Baba TW, Jeong YS, Pennick D, Bronson R, Greene MF, Ruprecht RM. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science. 1995;267:1820–1825. doi: 10.1126/science.7892606. [DOI] [PubMed] [Google Scholar]
- Baba TW, Liska V, Khimani AH, Ray NB, Dailey PJ, Penninck D, Bronson R, Greene MF, McClure HM, Martin LN, Ruprecht RM. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat Med. 1999;5:194–203. doi: 10.1038/5557. [DOI] [PubMed] [Google Scholar]
- Blue ML, Daley JF, Levine H, Schlossman SF. Coexpression of T4 and T8 on peripheral blood T cells demonstrated by two-color fluorescence flow cytometry. J Immunol. 1985;134:2281–2286. [PubMed] [Google Scholar]
- Daniel MD, Kirchhoff F, Czajak SC, Sehgal PK, Desrosiers RC. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science. 1992;258:1938–1941. doi: 10.1126/science.1470917. [DOI] [PubMed] [Google Scholar]
- Hofmann-Lehmann R, Swenerton RK, Liska V, Leutenegger CM, Lutz H, McClure HM, Ruprecht RM. Sensitive and robust one-tube real-time reverse transcriptase-polymerase chain reaction to quantify SIV RNA load: comparison of one- versus two-enzyme systems. AIDS Res Hum Retroviruses. 2000;16:1247–1257. doi: 10.1089/08892220050117014. [DOI] [PubMed] [Google Scholar]
- Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, Irwin CE, Safrit JT, Mittler J, Weinberger L, Kostrikis LG, Zhang L, Perelson AS, Ho DD. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joag SV, Adany I, Li Z, Foresman L, Pinson DM, Wang C, Stephens EB, Raghavan R, Narayan O. Animal model of mucosally transmitted human immunodeficiency virus type 1 disease: intravaginal and oral deposition of simian/human immunodeficiency virus in macaques results in systemic infection, elimination of CD4+ T cells, and AIDS. J Virol. 1997a;71:4016–4023. doi: 10.1128/jvi.71.5.4016-4023.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joag SV, Li Z, Foresman L, Pinson DM, Raghavan R, Zhuge W, Adany I, Wang C, Jia F, Sheffer D, Ranchalis J, Watson A, Narayan O. Characterization of the pathogenic KU-SHIV model of acquired immunodeficiency syndrome in macaques. AIDS Res Hum Retroviruses. 1997b;13:635–645. doi: 10.1089/aid.1997.13.635. [DOI] [PubMed] [Google Scholar]
- Joag SV, Li Z, Foresman L, Stephens EB, Zhao LJ, Adany I, Pinson DM, McClure HM, Narayan O. Chimeric simian/human immunodeficiency virus that causes progressive loss of CD4+ T cells and AIDS in pig-tailed macaques. J Virol. 1996;70:3189–3197. doi: 10.1128/jvi.70.5.3189-3197.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joag SV, Liu ZQ, Stephens EB, Smith MS, Kumar A, Li Z, Wang C, Sheffer D, Jia F, Foresman L, Adany I, Lifson J, McClure HM, Narayan O. Oral immunization of macaques with attenuated vaccine virus induces protection against vaginally transmitted AIDS. J Virol. 1998a;72:9069–9078. doi: 10.1128/jvi.72.11.9069-9078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joag SV, Liu ZQ, Stephens EB, Smith MS, Kumar A, Li Z, Wang C, Sheffer D, Jia F, Foresman L, Adany I, Lifson J, McClure HM, Narayan O. Oral immunization of macaques with attenuated vaccine virus induces protection against vaginally transmitted AIDS. J Virol. 1998b;72:9069–9078. doi: 10.1128/jvi.72.11.9069-9078.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiepiela P, Ngumbela K, Thobakgale C, Ramduth D, Honeyborne I, Moodley E, Reddy S, de Pierres C, Mncube Z, Mkhwanazi N, Bishop K, van der Stok M, Nair K, Khan N, Crawford H, Payne R, Leslie A, Prado J, Prendergast A, Frater J, McCarthy N, Brander C, Learn GH, Nickle D, Rousseau C, Coovadia H, Mullins JI, Heckerman D, Walker BD, Goulder P. CD8+ T-cell responses to different HIV proteins have discordant associations with viral load. Nat Med. 2007;13:46–53. doi: 10.1038/nm1520. [DOI] [PubMed] [Google Scholar]
- Kumar A, Liu Z, Sheffer D, Smith M, Singh DK, Buch S, Narayan O. Protection of macaques against AIDS with a live attenuated SHIV vaccine is of finite duration. Virology. 2008;371:238–245. doi: 10.1016/j.virol.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay GA, Liu Z, Singh DK, Smith MS, Mukherjee S, Sheffer D, Jia F, Adany I, Sun KH, Dhillon S, Zhuge W, Narayan O. Protection against late-onset AIDS in macaques prophylactically immunized with a live simian HIV vaccine was dependent on persistence of the vaccine virus. J Immunol. 2004a;173:4100–4107. doi: 10.4049/jimmunol.173.6.4100. [DOI] [PubMed] [Google Scholar]
- Mackay GA, Liu Z, Singh DK, Smith MS, Mukherjee S, Sheffer D, Jia F, Adany I, Sun KH, Dhillon S, Zhuge W, Narayan O. Protection against late-onset AIDS in macaques prophylactically immunized with a live simian HIV vaccine was dependent on persistence of the vaccine virus. J Immunol. 2004b;173:4100–4107. doi: 10.4049/jimmunol.173.6.4100. [DOI] [PubMed] [Google Scholar]
- Mackay GA, Niu Y, Liu ZQ, Mukherjee S, Li Z, Adany I, Buch S, Zhuge W, McClure HM, Narayan O, Smith MS. Presence of Intact vpu and nef genes in nonpathogenic SHIV is essential for acquisition of pathogenicity of this virus by serial passage in macaques. Virology. 2002;295:133–146. doi: 10.1006/viro.2002.1368. [DOI] [PubMed] [Google Scholar]
- Nascimbeni M, Shin EC, Chiriboga L, Kleiner DE, Rehermann B. Peripheral CD4(+)CD8(+) T cells are differentiated effector memory cells with antiviral functions. Blood. 2004;104:478–486. doi: 10.1182/blood-2003-12-4395. [DOI] [PubMed] [Google Scholar]
- Pahar B, Lackner AA, Veazey RS. Intestinal double-positive CD4+CD8+ T cells are highly activated memory cells with an increased capacity to produce cytokines. Eur J Immunol. 2006;36:583–592. doi: 10.1002/eji.200535520. [DOI] [PubMed] [Google Scholar]
- Robinson HL. HIV/AIDS vaccines: 2007. Clin Pharmacol Ther. 2007;82:686–693. doi: 10.1038/sj.clpt.6100408. [DOI] [PubMed] [Google Scholar]
- Sacha JB, Chung C, Rakasz EG, Spencer SP, Jonas AK, Bean AT, Lee W, Burwitz BJ, Stephany JJ, Loffredo JT, Allison DB, Adnan S, Hoji A, Wilson NA, Friedrich TC, Lifson JD, Yang OO, Watkins DI. Gag-Specific CD8+ T Lymphocytes Recognize Infected Cells before AIDS-Virus Integration and Viral Protein Expression. J Immunol. 2007;178:2746–2754. doi: 10.4049/jimmunol.178.5.2746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz JE, Simon MA, Kuroda MJ, Lifton MA, Ollert MW, Vogel CW, Racz P, Tenner-Racz K, Scallon BJ, Dalesandro M, Ghrayeb J, Rieber EP, Sasseville VG, Reimann KA. A nonhuman primate model for the selective elimination of CD8+ lymphocytes using a mouse-human chimeric monoclonal antibody. Am J Pathol. 1999;154:1923–1932. doi: 10.1016/S0002-9440(10)65450-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein PS, Mackay GA, Mukherjee S, Li Z, Piatak M, Jr, Lifson JD, Narayan O, Kumar A. Pathogenic simian/human immunodeficiency virus SHIV(KU) inoculated into immunized macaques caused infection, but virus burdens progressively declined with time. J Virol. 2000;74:10489–10497. doi: 10.1128/jvi.74.22.10489-10497.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss L, Roux A, Garcia S, Demouchy C, Haeffner-Cavaillon N, Kazatchkine MD, Gougeon ML. Persistent expansion, in a human immunodeficiency virus-infected person, of V beta-restricted CD4+CD8+ T lymphocytes that express cytotoxicity-associated molecules and are committed to produce interferon-gamma and tumor necrosis factor-alpha. J Infect Dis. 1998;178:1158–1162. doi: 10.1086/515674. [DOI] [PubMed] [Google Scholar]
- Whitney JB, Ruprecht RM. Live attenuated HIV vaccines: pitfalls and prospects. Curr Opin Infect Dis. 2004;17:17–26. doi: 10.1097/00001432-200402000-00004. [DOI] [PubMed] [Google Scholar]