Summary
Neuronal AMPA receptors auto-inactivate at high concentrations of glutamate, i.e., the current declines at glutamate concentrations above 10–100 μM. The mechanisms underlying this phenomenon are unclear. Stargazin-like TARPs are AMPA-receptor auxiliary subunits, which modulate receptor trafficking and channel properties. Here we found that neuronal AMPA receptors and recombinant AMPA receptors co-expressed with stargazin auto-inactivate at high concentrations of glutamate, whereas recombinant AMPA receptors expressed alone do not. The reduction of currents at high glutamate concentrations is not associated with a reduction of AMPA receptor number, but rather with the loss of stargazin-associated allosteric modulation of channel gating. We show that receptor desensitization promotes the dissociation of TARP-AMPA receptor complexes in a few milliseconds. This dissociation mechanism contributes to synaptic short-term modulation. The results demonstrate a novel mechanism for dynamic regulation of AMPA receptor activity to tune synaptic strength.
Introduction
Glutamate is the most abundant excitatory neurotransmitter in the brain and acts on three classes of ionotropic glutamate receptors (AMPA, NMDA, and kainate), which function distinctly (Dingledine et al., 1999; Hollmann and Heinemann, 1994; Seeburg, 1993). AMPA receptors mediate fast synaptic transmission, whereas NMDA receptors and kainate receptors are involved in synaptic plasticity (Kandel, 2001; Lerma, 2006; Malenka and Nicoll, 1999; Nicoll and Schmitz, 2005). Together, these three classes of glutamate receptors control and modulate neural circuits in the brain that underlie aspects of cognitive function.
AMPA receptors are hetero-oligomers composed of four subunits GluR1–4 (Hollmann and Heinemann, 1994; Seeburg, 1993), each of which is alternatively spliced to yield two isoforms (flip and flop) (Sommer et al., 1990). Channels composed of different AMPA receptor subunits display quantitative differences in the kinetics of deactivation (channel closure upon glutamate removal) and desensitization (channel closure during continuous exposure to glutamate) (Jonas and Spruston, 1994), and the subunit composition of AMPA receptors plays a major role in controlling the decay of EPSCs. Although the rules that determine the trafficking of heteromeric AMPA receptors remain uncertain, the subunit composition of AMPA receptors also influences the number of synaptic AMPA receptors under basal and activity-dependent conditions (Barry and Ziff, 2002; Malinow and Malenka, 2002; Sheng and Kim, 2002; Song and Huganir, 2002).
The Transmembrane AMPA receptor Regulatory Proteins (TARPs) are auxiliary subunits of AMPA receptors (Nicoll et al., 2006; Ziff, 2007). TARPs consist of four typical isoforms (stargazin/γ-2, γ-3, γ-4, γ-8) and one atypical isoform (γ-7), which each show distinct expression patterns in the brain (Fukaya et al., 2005; Kato et al., 2007; Tomita et al., 2003) and are evolutionally conserved (Walker et al., 2006). TARPs bind to AMPA receptors (Fukata et al., 2005; Nakagawa et al., 2005; Tomita et al., 2003; Vandenberghe et al., 2005) and modulate both their trafficking and channel properties (Chen et al., 2000; Kott et al., 2007; Priel et al., 2005; Tomita et al., 2005a; Tomita et al., 2004; Turetsky et al., 2005; Yamazaki et al., 2004). Mice in which the stargazin/γ-2 gene is disrupted (stargazer) show loss of AMPA receptor activity in cerebellar granule cells (Chen et al., 2000; Hashimoto et al., 1999). In addition, TARP γ-8 knockout mice have altered AMPA receptor trafficking and AMPA receptor activity in the hippocampus (Fukaya et al., 2006; Rouach et al., 2005). TARPs also control EPSC kinetics through their first extracellular loop (Cho et al., 2007; Milstein et al., 2007; Tomita et al., 2005a).
Neuronal AMPA receptors have different properties from recombinant AMPA receptors. For example, neuronal AMPA receptors respond to kainate better than glutamate, whereas recombinant AMPA receptors respond to glutamate better than kainate. This discrepancy has been resolved by co-expressing AMPA receptors with TARPs in recombinant systems, which results in a robust enhancement of kainate efficacy (Kott et al., 2007; Tomita et al., 2005a; Turetsky et al., 2005). Furthermore, glutamate-evoked steady-state current from cells expressing recombinant AMPA receptors have typical sigmoid concentration-response curves (Hollmann and Heinemann, 1994; Robert and Howe, 2003). In contrast, the responses from AMPA receptors in the avian cochlear nucleus and oocytes injected with poly (A) RNA from the rat cerebral cortex show a bell-shaped concentration-response, where the amplitude of the steady-state current declines at glutamate concentrations above 100 μM (Geoffroy et al., 1991; Raman and Trussell, 1992; Vlachova et al., 1987). The mechanisms that give rise to these different concentration-response relationships remain unclear.
In this study, we have examined the molecular mechanisms underlying the auto-inactivation of neuronal AMPA receptors. We found that AMPA receptors in mouse cerebellar granule cells, like the avian cochlear nucleus, also display reduced steady-state currents at glutamate concentrations above 100 μM, demonstrating that this is also a feature of mammalian receptors in neurons. Whereas expression of AMPA receptors alone in Xenopus laevis oocytes did not result in auto-inactivated bell-shaped curves, such curves were obtained upon co-expression of AMPA receptors with stargazin. Although stargazin modulates all AMPA receptor subunits, the magnitude of the stargazin-related reductions in the amplitude of currents evoked by high concentrations of glutamate depended on subunit composition and differed for flip and flop splicing isoforms of AMPA receptors. Our results show that high concentrations of glutamate promote the dissociation of stargazin from AMPA receptors, an effect that occurs within a few milliseconds after receptor desensitization and requires the cytoplasmic domain of AMPA receptors. Furthermore, we found that this auto-inactivation mechanism contributes to short-term modulation of AMPA receptor activity at synapses. The novel mechanism described here could tune synaptic transmission upon neuronal activation or under conditions where ambient levels of glutamate are elevated.
Results
Stargazin modulation of AMPA receptor function depends on glutamate concentration
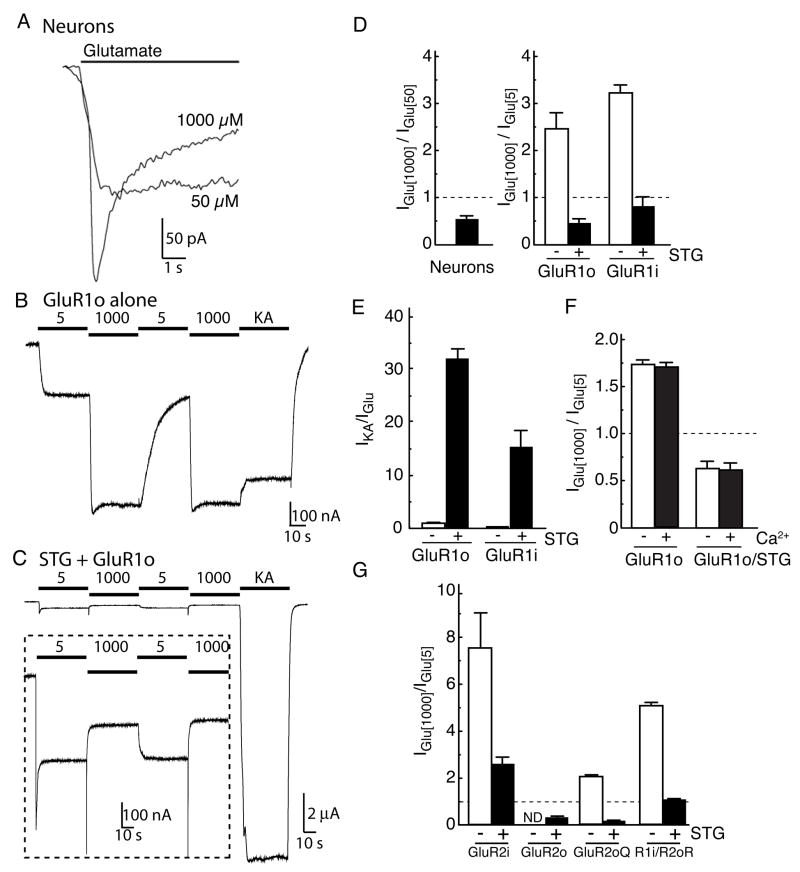
Previous studies have shown that AMPA receptors have a bell-shaped glutamate concentration-response curve in neurons from the avian cochlear nucleus, where the amplitude of the steady-state current declined at glutamate concentrations above 100 μM, so called “auto-inactivation” (Raman and Trussell, 1992; Vlachova et al., 1987). To test whether mammalian AMPA receptors show similar characteristics, we measured glutamate-evoked whole-cell currents in mouse cerebellar granule cells. Glutamate was applied in the continuous presence of cyclothiazide (CTZ), which selectively reduces desensitization of AMPA receptor flip splicing isoforms (and to a lesser extent flop isoforms; (Partin et al., 1994)). Under these conditions, currents were detected at glutamate concentrations of 3 μM. At concentrations of 50 μM and below, the currents did not decline during 5 s applications. At glutamate concentrations of 100 μM and above, the initial amplitude of the response faded (Fig. 1A). Plots of the mean initial amplitude of the currents against glutamate concentration gave typical sigmoid concentration-response relationships and EC50 values of approximately 20μM (data not shown). Whereas the peak amplitudes of whole-cell currents evoked by 1000 μM glutamate were larger than those by 50 μM glutamate (ratio: 1.41 ± 0.07, n=12), steady-state currents were consistently and significantly smaller than the corresponding currents obtained with 50 μM glutamate (Fig. 1A, D).
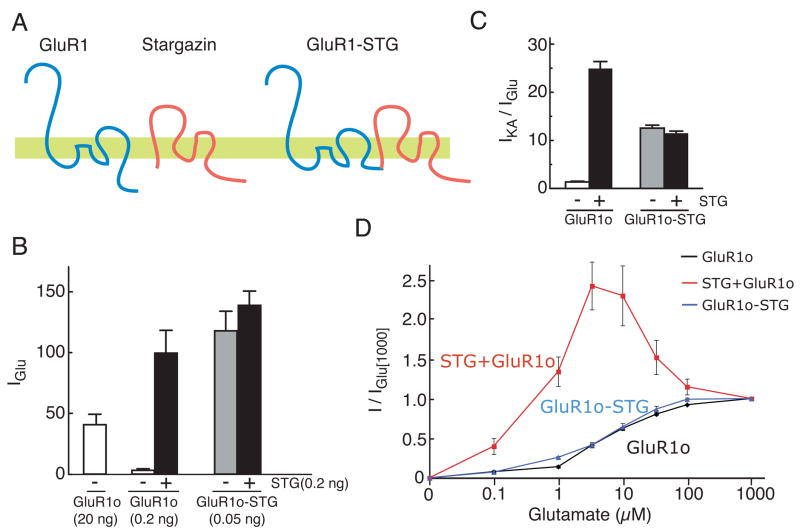
Figure 1. Concentration-dependent modulation of AMPA receptors by stargazin.
(A) Glutamate-evoked whole-cell currents were measured in cultured mouse cerebellar granule cells. The steady-state current evoked by 50 μM glutamate was larger than that of 1000 μM glutamate in the presence of cyclothiazide (100 μM). (B, C) Agonist-evoked steady-state currents were recorded in oocytes injected with GluR1 flop (GluR1o) alone or together with stargazin (STG). Steady-state currents evoked by 1000 μM glutamate were larger than those by 5 μM glutamate or 10 μM kainate (KA) in oocytes expressing GluR1o alone (20 ng) (B). In contrast, stargazin enhances the response to kainate relative to glutamate. Furthermore, steady-state currents evoked by 1000 μM glutamate were smaller than those by 5 μM glutamate in oocytes expressed GluR1o with stargazin (0.2 ng each) (C). (D) Neurons and oocytes co-expressing GluR1 with stargazin have smaller steady-state currents evoked by 1000 μM glutamate than by 50 μM (n=12) and 5 μM glutamate (n=5–7), respectively. (E) Stargazin modulates the KA efficacy of GluR1 (n=6, 7). (F) Stargazin modulates ratio of currents evoked by 1000 μM and 5 μM without calcium in extracellular recording solution. 1.5 mM calcium chloride are replaced with 0.5 mM barium chloride/1.0 mM magnesium chloride (n=5). (G) Stargazin decreases the steady-state current ratio evoked by 1000 and 5 μM glutamate in oocytes expressing the AMPA receptor isoform GluR2 R or Q editing forms, or GluR1/GluR2 heteromers (n=6, 7). ND indicates that agonist-evoked currents were undetectable. Data represent the mean ± s.e.m. from the indicated number of experiments.
To examine the mechanisms that underlie the reduction in the amplitude of steady-state AMPA-receptor currents at near-saturating concentrations of glutamate, we used Xenopus laevis oocytes as a model system (Chen et al., 2003). In oocytes expressing the flop isoform of the GluR1 AMPA-receptor subunit (GluR1o) alone, the steady-state currents evoked by 1000 μM glutamate were larger than the currents evoked by 5 μM glutamate or 10 μM kainate (Fig. 1B, D). Because native AMPA receptors in cerebellar granule cells contain the prototypical TARP stargazin (Chen et al., 2000), we co-expressed stargazin and GluR1o in oocytes to better mimic native receptors. In contrast to GluR1o alone, oocytes co-expressing GluR1o and stargazin gave larger responses to 5 μM glutamate than to 1000 μM glutamate (Fig. 1C, D). Similar effects of stargazin co-expression were seen for the GluR1 flip isoform (GluR1i; Fig. 1D), and stargazin enhanced kainate-evoked currents from oocytes expressing both the flip and flop isoforms of GluR1 (Fig. 1C, E), as reported previously (Kott et al., 2007; Tomita et al., 2005a; Turetsky et al., 2005). We also found that stargazin decreased significantly the steady-state current ratio for 1000 μM and 5 μM glutamate. This effect of stargazin was seen without calcium in the extracellular recording solution (Fig. 1F), and was reproduced for other AMPA receptor isoforms, although the responses to 1000 μM glutamate were still larger than those to 5 μM for the GluR2 flip isoform (Fig. 1G). The results suggest that whether neuronal AMPA receptors show auto-inactivated bell-shaped concentration response curves may depend on the repertoire of AMPA receptor isoforms expressed. However, for all AMPA receptor subunits examined, modulation of gating behavior by stargazin depends on glutamate concentration.
Stargazin-mediated concentration-dependent modulation of AMPA receptors is not associated with a change in receptor number
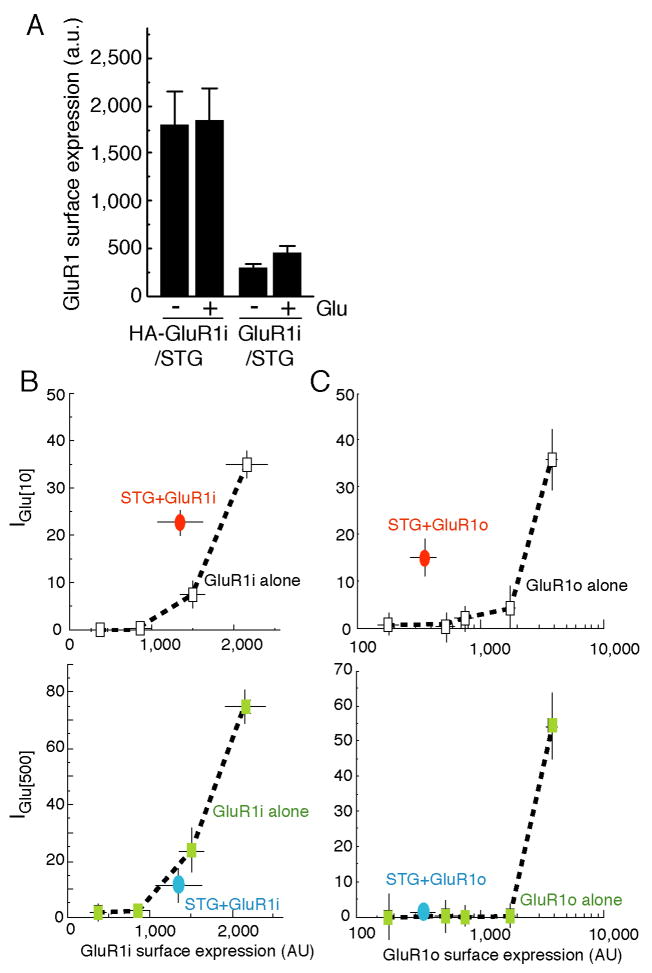
It is well established that stargazin is involved in AMPA receptor trafficking (Nicoll et al., 2006; Ziff, 2007). We therefore asked whether the reduction in current amplitudes at high glutamate concentrations was due to a reduction in the number of AMPA receptors at the cell surface. In oocytes co-expressing extracellularly HA-epitope tagged GluR1 and stargazin, we measured the surface expression of AMPA receptors in the absence or continuous presence of glutamate using chemiluminescence assays (Tomita et al., 2005a). The surface expression of HA-GluR1i was not altered by glutamate application (Fig. 2A). We then correlated the glutamate-evoked current at two different glutamate concentrations (10 μM and 500 μM) to the amount of receptors on the cell surface in the presence or absence of stargazin (Fig. 2B, C). This allowed us to compare the size of glutamate-evoked currents with and without stargazin in oocytes with the same number of surface receptors. For both GluR1 flip and flop receptors, co-expression of stargazin increased the amplitude of currents evoked by 10 μM glutamate but not 500 μM glutamate (Fig. 2B, C).
Figure 2. Concentration-dependent modulation of AMPA receptors by stargazin is not due to changes in surface expression.
(A) Oocytes were injected with extracellular HA epitope-tagged GluR1 flip (HA-GluR1i) and stargazin transcripts, and the surface expression of HA-GluR1i was quantified by chemiluminescence (n=6). The application of 1000 μM glutamate did not alter the surface expression of HA-GluR1i. Signal from oocytes injected with non-tagged GluR1 and stargazin was accounted as background. (B) Surface expression of HA-GluR1i (n=9) and steady-state currents evoked by 10 μM and 500 μM glutamate (n=5) were measured in parallel in oocytes injected with different amounts of HA-GluR1i cRNA and stargazin (STG). Stargazin modulates AMPA receptor function evoked by 10 μM glutamate, but not by 500 μM glutamate. (C) The experiments in (B) were repeated in oocytes expressing GluR1o. Data represent the mean ± s.e.m. from the indicated number of experiments.
Concentration-dependent modulation of AMPA receptors by stargazin requires the cytoplasmic domain of AMPA receptors
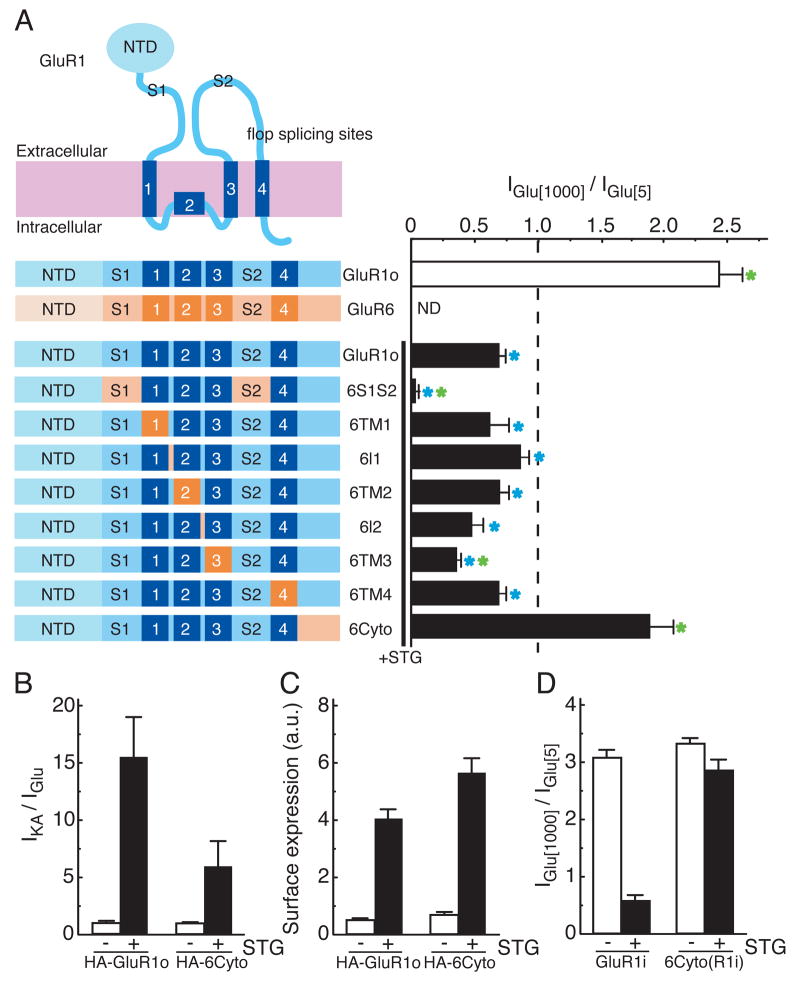
To identify the AMPA receptor domains required for the reduced stargazin-mediated modulation of steady-state currents at high concentrations of glutamate, we constructed chimeric receptors in which regions of the AMPA receptor GluR1o and the kainate receptor GluR6 (which does not interact with stargazin (Chen et al., 2003; Tomita et al., 2007a)) were exchanged (Fig. 3A). We co-expressed the chimeras with stargazin in oocytes and measured the ratio of currents evoked by 1000 μM and 5 μM glutamate. This ratio was less than 1 for each of the chimeras except for receptors in which the cytoplasmic tail of GluR1 was replaced with the corresponding domain from GluR6 (6Cyto). The 6Cyto chimera gave 1000 μM/5 μM ratios that were not significantly different from the corresponding ratios obtained for GluR1o receptors without stargazin co-expression (Fig. 3A). Furthermore, replacing the GluR1 ligand-binding domain with that from GluR6 (6S1S2) resulted in robust reduction of the ratio of currents evoked by 1000 μM and 5 μM glutamate in the presence of stargazin (Fig. 3A), whereas in the absence of stargazin both 6S1S2 and 6Cyto gave larger responses to 1000 μM glutamate than to 5 μM glutamate. Stargazin increased both the kainate efficacy and the surface expression of the 6Cyto chimera, demonstrating that the 6Cyto chimera interacts with stargazin (Fig. 3B, C). Similar effects were observed for a chimeric construct in which the flip isoform of GluR1 was used (6Cyto(R1i); Fig. 3D). In addition, deletion of the N-terminal domain of the AMPA receptors (ΔNTD) did not alter the effects of stargazin on kainate efficacy (Tomita et al., 2007b) nor the concentration-dependent modulation of glutamate responses (Supplementary Fig. 1). The results with the 6Cyto chimeras show that the cytoplasmic tail of GluR1 is required for glutamate concentration dependent modulation of stargazin-mediated AMPA receptor activity, and the results with the 6S1S2 chimera suggest that this modulation is influenced by conformational changes that occur at the level of the ligand-binding domain.
Figure 3. The cytoplasmic domain of AMPA receptors regulates stargazin-mediated modulation of AMPA receptors.
Oocytes were injected with 20 ng GluR1 flop (GluR1o) cRNA or GluR6 cRNA. Stargazin (STG) (0.1 ng) was injected with GluR1o and GluR1o chimeric constructs (0.2 ng) as indicated. Steady-state currents evoked by 5 μM or 1000 μM glutamate were measured by TEVC recording (n=9–11). (A) Replacing the cytoplasmic domain of GluR1o with that of GluR6 (6Cyto) disrupted the concentration-dependent modulation of AMPA receptor function by stargazin, whereas replacing the ligand-binding domain (S1S2) of GluR1 with that of GluR6 (6S1S2) enhanced it. Green and blue asterisks indicate a significant difference compared with GluR1o with STG and GluR1 alone, respectively. Stargazin increases kainate efficacy (B) and the surface expression (C) of both GluR1o and 6Cyto as measured by chemiluminescence (n=9) and TEVC recording (n=9–13). (D) Replacing the cytoplasmic domain of GluR1 flip (GluR1i) with that of GluR6 (6Cyto(R1i)) also disrupted the concentration-dependent modulation of AMPA receptor function by stargazin (n=5, 6). Data represent the mean ± s.e.m. from the indicated number of experiments.
Glutamate promotes the dissociation of stargazin from AMPA receptors
Because the effect of stargazin to potentiate AMPA receptor activity was reduced at high concentrations of glutamate (Fig. 2B, C), we hypothesized that stargazin does not interact with AMPA receptors under these conditions. Indeed, previous co-immunoprecipitation experiments demonstrated that prolonged exposure of purified TARP γ-3/AMPA receptor complexes to 100 μM glutamate reduced the association of the related TARP isoform γ-3 with native AMPA receptors (Tomita et al., 2004).
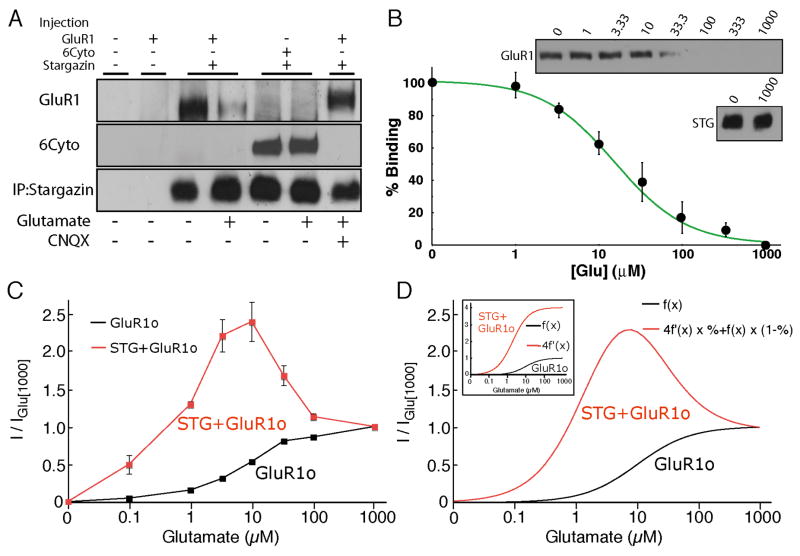
To test the hypothesis that glutamate promotes the dissociation of stargazin, stargazin/GluR1 complexes were immuno-purified with anti-stargazin antibody from oocytes co-expressing GluR1 and stargazin, followed by the addition of glutamate to the immuno-complexes. We found that glutamate caused the dissociation of GluR1 AMPA receptors from stargazin, an effect that was blocked by the competitive AMPA-receptor antagonist CNQX (Fig. 4A). Similar results were obtained from brain homogenates (Supplementary Fig. 2A). Importantly, the same glutamate treatment of immuno-complexes containing stargazin and 6Cyto did not cause dissociation (Fig. 4A), supporting the idea that glutamate-induced dissociation of stargazin is responsible for the reduced steady-state currents seen at high concentrations of glutamate. The glutamate-mediated dissociation of stargazin and GluR1 depended on glutamate concentration, with a mean effective concentration of 16 μM (Fig. 4B). Since AMPA receptor currents evoked by kainate did not show bell-shaped concentration-response curves when AMPA receptors were co-expressed with stargazin (Tomita 2007b), we also examined the effect of kainate in co-immunoprecipitation assays and found that kainate did not result in the dissociation of GluR1 and stargazin (Supplementary Fig. 2B and C).
Figure 4. Glutamate dissociates AMPA receptors from stargazin in a concentration dependent manner.
(A, B) Oocytes were injected with cRNA of GluR1, GluR1 containing the cytoplasmic domain of GluR6 (6Cyto), and stargazin (STG). Solubilized membranes from oocytes were immunoprecipitated with anti-stargazin antibody. Beads were then washed with glutamate at the indicated concentrations and bound proteins were detected by Western blotting. (A) Glutamate (100 μM) dissociates stargazin from GluR1, but not 6Cyto. CNQX (100 μM) prevents the dissociation of GluR1 from stargazin. (B) Glutamate dissociates GluR1 from stargazin in a concentration-dependent manner (n=5). The amount of stargazin was unaltered by glutamate application. (C) The steady-state concentration response curves of GluR1o with (n=7) and without stargazin (n=5). Current amplitudes were normalized to the response evoked by 1000 μM glutamate. (D) Simulated concentration response curves of AMPA receptors with and without stargazin. Stargazin enhances steady-state AMPA-receptor currents (f(x)) 4 fold and decreases the EC50 value by a factor of 5 (4f′(x), small inset). Total simulated evoked currents were calculated as the sum of the percentage of stargazin-bound AMPA receptors and stargazin-dissociated AMPA receptors. For comparison between GluR1 with and without stargazin, currents were normalized to the response evoked by 1000 μM glutamate. Simulated concentration-response curves (D) resembled concentration-response curves obtained experimentally (C). Data in (B) and (C) represent the mean ± s.e.m. from the indicated number of experiments.
The dissociation curve in Fig. 4B allowed us to calculate the fraction of stargazin bound to AMPA receptors at different concentrations of glutamate and to simulate glutamate concentration-response curves for AMPA receptors when they are co-expressed with stargazin (Fig. 4C). Recombinant GluR1o channels showed a typical sigmoid curve, with an EC50 of about 10 μM and a Hill coefficient close to 1 (Fig. 4C and D inset). Stargazin co-expression decreases the glutamate EC50 value for steady-state currents about 5-fold (Kott et al., 2007; Priel et al., 2005; Tomita et al., 2005a; Turetsky et al., 2005; Yamazaki et al., 2004). After normalization for receptor number, the currents evoked by 10 μM glutamate were increased by a factor of about 4 (Fig 2B, C). These results can be used to construct a hypothetical concentration-response curve for GluR1o channels when co-expressed with stargazin (Fig. 4D, inset). The sum of the two curves in Fig. 4D (weighted by the fraction of channels with and without bound stargazin, Fig. 4B) is similar to the curves obtained experimentally from oocytes co-expressing GluR1o and stargazin (Fig. 4C).
Preventing stargazin dissociation enhances steady-state currents evoked by high concentrations of glutamate
If stargazin dissociation gives rise to glutamate-dependent modulation of AMPA receptors, then preventing dissociation of stargazin and AMPA receptors should eliminate it. To test this prediction directly, we generated GluR1-stargazin tandem proteins in which the N-terminus of stargazin was directly fused to the C-terminal end of GluR1 (Fig. 5A). Oocytes injected with the GluR1o-stargazin tandem construct responded to low concentrations of glutamate similarly to oocytes co-injected with GluR1o and stargazin (Fig. 5B). The efficacy of kainate was also enhanced to an extent similar to that when GluR1o and stargazin were co-expressed as separate proteins (Fig. 5C). Co-expression of stargazin with the GluR1o-stargazin tandem protein did not further enhance glutamate-evoked currents or kainate efficacy (Fig. 5B and C). The GluR1o-stargazin tandem therefore forms functional channels that exhibit signature characteristics of stargazin’s effects on AMPA receptor properties. Importantly, steady-state responses of the GluR1o-stargazin tandem receptors did not decline at high glutamate concentrations (Fig. 5D), suggesting that the decline in co-expression experiments is due to the dissociation of stargazin from AMPA receptors.
Figure 5. Blocking the dissociation of stargazin from AMPA receptors prevents concentration-dependent modulation of AMPA receptors.
(A) The topology of GluR1, stargazin and the tandem proteins of GluR1 and stargazin, in which the C-terminus of GluR1 is fused to the N-terminus of stargazin. (B) Stargazin enhanced currents evoked by 5 μM glutamate through GluR1o channels. Oocytes injected with 0.05 ng of the cRNA encoding the GluR1o-stargazin tandem construct responded better than those with GluR1o cRNA (20 ng) alone. Co-expression of stargazin failed to cause a further enhancement of current amplitude. (C) The GluR1o-stargazin tandem protein modulates kainate efficacy to a similar extent as GluR1o and stargazin. The co-expression of STG with the GluR1o-stargazin tandem protein did not produce any additional enhancement of the response. (D) In contrast to receptors expressed in response to the co-expression of GluR1o and stargazin, the GluR1o-stargazin tandem protein did not show a reduction in current amplitudes at high glutamate concentrations. Co-expression of stargazin had no effect on the concentration-response behavior of GluR1o-stargazin receptors. Data represent the mean ± s.e.m. (n=5, 6).
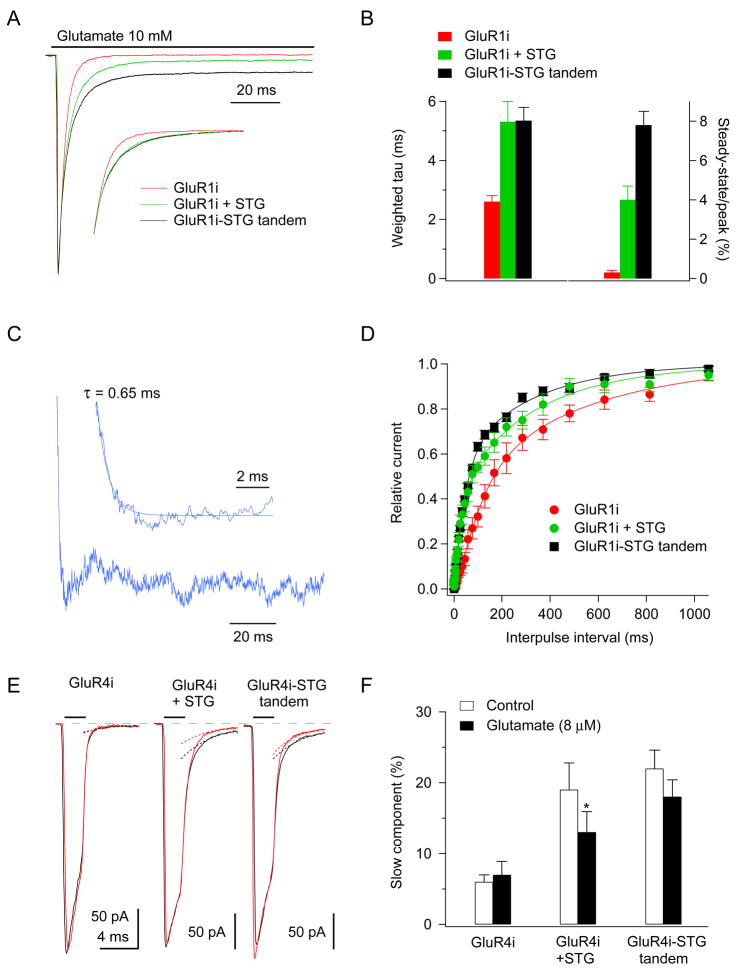
To analyze the kinetics of stargazin dissociation from AMPA receptors, we conducted fast application experiments on outside-out patches from tsA201 cells transfected with GluR1i, GluR1i and stargazin, or a GluR1i-stargazin tandem construct. Typical currents recorded in the three types of patch in response to 100 ms applications of 10 mM glutamate are shown in Figure 6A, where the peak amplitudes have been normalized to allow comparison of the relative steady-state currents. Similar to our results in oocytes (Fig. 5D), steady-state currents were larger for the GluR1i-stargazin tandem (7.8 ± 0.7% of the peak, n = 6) than they were for GluR1i with stargazin (4.0 ± 0.7%, n = 5; P < 0.005) (Fig. 6A and B). In contrast, the effect of stargazin to slow desensitization was similar in the GluR1i-stargazin tandem, as can be seen in the inset to Fig. 6A where the decays of the two scaled currents are virtually identical. Bi-exponential fits to the decays of the two types of responses gave similar values for both the time constants of the fast and slow components (τf and τs = 2.5 ± 0.7 ms and 10.2 ± 1.5 ms, GluR1i + stargazin; 3.0 ± 0.4 ms and 10.7 ± 1.4, GluR1i-stargazin), as well as the relative amplitude of the slow component (As = 37.3 ± 5.7% vs. 32.7 ± 3.3%) (Fig. 6B).
Figure 6. Stargazin dissociates from AMPA receptors within a few milliseconds after receptor desensitization.
(A, B) Responses to 200 ms applications of 10 mM glutamate in outside-out patches from tsA201 cells transfected with GluR1i alone, GluR1i with stargazin, or the GluR1i-stargazin tandem. Each trace is the mean of 10 to 20 trials from individual patches. The currents were scaled so the peak amplitudes were the same (individual peak currents were 1.04 to 1.71 nA). The inset shows the decays of the currents scaled to the same amplitude after subtracting the respective steady-state currents to compare better the respective rates of desensitization. Note that in the inset the record for GluR1i + stargazin (green) and the record for the GluR1i-stargazin tandem (black) are virtually identical. (B) Weighted time constants (left) and steady-state currents (right) obtained from bi-exponential fits to the decays of currents (n = 5–6). (C) Current obtained by subtracting the mean response for GluR1i and stargazin from the mean response for the GluR1i-stargazin tandem (data from all patches were averaged and the peak amplitudes were scaled as in panel A). The inset shows the first 10 ms of the difference current, which decayed mono-exponentially (tau = 0.65 ms). (D) Data for recovery from desensitization obtained from two-pulse protocols (n = 5–6). Smooth curves are double Hodgkin-Huxley fits (Zhang et al., 2006). Stargazin speeds recovery (relative to GluR1i alone) and the rates of recovery for GluR1i with stargazin and the GluR1i-stargazin tandem are similar for inter-pulse intervals less than 40 ms. (E, F) Deactivation protocols (10 mM glutamate for 2 ms) in the absence (black) and continuous presence of 8 μM glutamate (red). The presence of 8 μM glutamate reduced the peak current by about 50% and the pairs of records in each patch have been scaled so that the deactivation decays have the same amplitude. The decays were fitted with bi-exponential functions and the slow component of decay from these fits is shown for each record (dotted curves). (F) The relative amplitude of the slow component of deactivation in the absence (open bars) and presence (filled bars) of 8 μM glutamate. Exposure of the receptors to 8 μM glutamate significantly reduced the amplitude of the slow component for GluR4i with stargazin (P < 0.05), but not for GluR4i alone or GluR4i-stargazin tandem receptors (n = 4–6). Data in (B, D, F) represent the mean ± s.e.m. from the indicated number of experiments.
Comparison of other receptor properties indicated that steady-state currents were selectively enhanced in the GluR1i-stargazin tandem protein. The kinetics of deactivation was not different for GluR1i with stargazin and the GluR1i-stargazin tandem. Hill-type fits to concentration-response data for peak currents gave EC50 values for GluR1i + stargazin (570 μM) and GluR1i-stargazin (607 μM) that were similar to the corresponding value reported for GluR1i alone (717 μM; (Robert and Howe, 2003)); and, unlike steady-state currents, peak currents did not decline at high glutamate concentrations in any of the three conditions. Measurements of peak current amplitudes with and without CTZ (an indication of open probability (Popen) at the peak response; (Cho et al., 2007)) gave similar results for GluR1i with stargazin and the GluR1i-stargazin tandem. The ratio of the peak currents with and without CTZ was 1.28 ± 0.04 (n = 5) for the tandem receptor protein and 1.32 ± 0.05 (n = 5) for GluR1i co-expressed with stargazin. Both values were significantly smaller than the ratio obtained for GluR1i alone (1.54 ± 0.02, n = 4; P < 0.05, one-way ANOVA). The 10–90% rise-times of the currents without CTZ in these experiments were similar for all three receptor types (GluR1i: 282 ± 44 μs; GluR1i + stargazin, 330 ± 44 μs; GluR1i-stargazin, 250 ± 36 μs).
The results show that the primary difference between GluR1i-stargazin tandem receptors and the receptors in the GluR1i with stargazin co-expression experiments is the amplitude of the steady-state currents during sustained applications of glutamate. The results therefore imply that receptor desensitization promotes the dissociation of stargazin/AMPA receptor complexes and that the difference between the currents seen for tandem receptors and the receptors in the GluR1i/stargazin co-expression experiments (Fig. 6A) reflects the rate at which stargazin dissociates. We therefore averaged the glutamate-evoked currents for GluR1i with stargazin and subtracted the mean waveform from the corresponding mean for GluR1i-stargazin receptors (after scaling the currents to have the same peak amplitude). The resultant difference current is shown in Fig. 6C. The difference current develops exponentially with a time constant of 0.65 ms. Similar results were also obtained with GluR4i-stargazin tandem receptors (Supplementary Fig. 3).
The effect of stargazin to speed recovery from desensitization (Priel et al., 2005) contributes to stargazin’s enhancement of steady-state currents. We therefore compared recovery in two-pulse experiments for GluR1i with stargazin and the GluR1i-stargazin tandem (Fig. 6D). At brief intervals the recovery curves were similar. At intervals longer than 40 ms (where recovery was about one-third complete), recovery was slower in the co-expression experiments, suggesting that at these longer intervals some of the receptors that recover from desensitization no longer have stargazin associated with them, Comparison of recovery from desensitization for GluR4i with stargazin and the GluR4i-stargazin tandem supported a similar conclusion.
If it is desensitization that promotes the dissociation of stargazin, then the concentration dependence of stargazin dissociation and desensitization should be similar. One consistent effect of stargazin and other TARPs is to markedly enhance the slow component evident in deactivation decays (Cho et al., 2007; Milstein et al., 2007; Zhang et al., 2006). Therefore if steady-state desensitization promotes the dissociation of stargazin, it should reduce stargazin enhancement of the amplitude of the slow component of deactivation. We therefore measured receptor deactivation in the continuous presence of 8 μM glutamate for GluR4i alone, GluR4i co-expressed with stargazin, and GluR4i-stargazin tandem receptors. As expected, the presence of 8 μM glutamate reduced the size of the peak currents evoked by 2 ms applications of 10 mM glutamate by approximately 50% (Robert and Howe, 2003). Both co-expression of stargazin and the GluR4i-stargazin tandem increased the amplitude of the slow component of deactivation under control conditions, and pre-exposure of the receptors to 8 μM glutamate reduced the relative amplitude of this component in the co-expression experiments but not for GluR4i-stargazin tandem receptors (Fig. 6E, F). The results are consistent with the conclusion that desensitization promotes the dissociation of stargazin from AMPA receptors.
Preventing stargazin dissociation alters short-term modulation of synaptic transmission
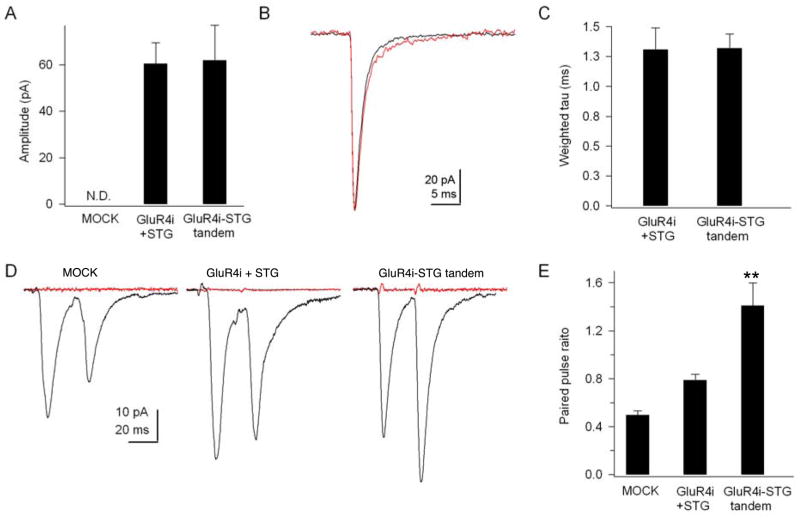
Our fast application results indicate that glutamate-mediated dissociation of stargazin and AMPA receptors occurs within a few milliseconds, suggesting that this mechanism might contribute to basal synaptic transmission and short-term modulation of synaptic transmission. To examine possible roles of stargazin dissociation in synaptic transmission under basal conditions, we examined the effects of stargazin dissociation on basal synaptic transmission using cerebellar granule cell cultures from stargazer mice, which lack functional AMPA receptors (Chen et al., 2000; Cho et al., 2007; Milstein et al., 2007). We overexpressed GFP alone, stargazin/GluR4i/GFP, or GluR4i-stargazin tandem/GFP in stargazer cerebellar granule cells and measured the amplitude and kinetics of spontaneous EPSCs from GFP positive neurons. As previously shown, we did not observe EPSCs in neurons transfected with GFP alone (n=4). On the other hand, we observed frequent EPSCs in neurons expressing stargazin/GluR4i/GFP or GluR4i-stargazin tandem/GFP (n=6), suggesting that both receptors are incorporated into synapses. The decays and amplitude of the EPSCs were similar for stargazin/GluR4i and GluR4i-stargazin tandem-transfected cells, suggesting that all AMPA receptors interact with stargazin under basal conditions in cerebellar granule cells.
Next we examined whether stargazin dissociation plays a role in short-term modulation of synaptic transmission. We first attempted to find paired neurons with GFP transfected cerebellar granule cells. However, the high density of the cultured neurons and the low number of synapses prevented us from finding synaptically connected neurons. We therefore turned to hippocampal cultures, which have been used successfully for paired recordings. We over-expressed GFP with stargazin/GluR4i or the GluR4i-stargazin tandem in hippocampal cultured neurons. Simultaneous whole-cell recordings were made from a GFP-positive and neighboring neurons. In non-transfected neurons and neurons expressing stargazin and GluR4i, we observed paired pulse depression when EPSCs were evoked at an interval of 20 ms (Fig. 7D). The ratio of the peak amplitudes of the second and first EPSC in neurons expressing stargazin and GluR4i was 0.78 ± 0.05 (Fig. 7E). In contrast, we observed paired pulse facilitation in neurons expressing the GluR4i-stargazin tandem receptor (Fig. 7D and E), with a paired pulse ratio of 1.41 ± 0.19. The results suggest that stargazin dissociation contributes to short-term modulation of synaptic transmission.
Figure 7. Stargazin dissociation from AMPA receptors modulates short-term modulation of synaptic transmission.
(A–C) Whole-cell data from cerebellar granule cells. AMPA receptor-mediated spontaneous EPSCs were not observed in cerebellar granule cells from stargazer mice and in stargazer cerebellar granule cells transfected with GFP alone (MOCK) (n=4) (N.D. not detectable). The expression of GluR4i with stargazin (STG) (black) or GluR4i-STG tandem (red) rescued synaptic transmission in neurons from stargazer mice. The amplitude (A, B) and decay (B, C) of spontaneous EPSCs in stargazer granule cells transfected with GluR4i with stargazin or GluR4i-stargazin tandem were not significantly different (n=6). (D, E) Whole-cell paired recordings from primary cultured hippocampal neurons. Neurons were transfected with cDNAs encoding GFP alone, GFP with GluR4i and stargazin, GFP with GluR4i-stargazin tandem. Transfected neurons were identified by GFP expression. Putative pairs of morphologically connected neurons were identified visually and both neurons were patched simultaneously. Once synaptic connectivity was confirmed, pairs of 1 ms jumps −70 mV to + 20 mV were applied to the presynaptic neuron at an interval of 20 ms. Neurons expressing GFP alone (MOCK) and with GluR4i and stargazin showed paired pulse depression, whereas paired pulse facilitation was observed in neurons expressing GFP and the GluR4i-stargazin tandem (n=4–6). (**: P < 0.01, one-way ANOVA). The evoked EPSCs were blocked by the addition of AMPA receptor antagonist CNQX (100μM, red traces in D). All data represent the mean ± s.e.m.
Discussion
This study demonstrates that stargazin modulation of AMPA receptor activity varies with glutamate concentration. The reduced stargazin modulation at high concentrations of glutamate persisted when current amplitudes were normalized for potential differences in receptor number, demonstrating that it does not result from glutamate-induced receptor internalization or modulation of stargazin’s known effects on AMPA receptor trafficking. The glutamate-dependent modulation of current amplitude requires the cytoplasmic domain of AMPA receptors and appears to result from the rapid dissociation of stargazin after receptor desensitization. The dynamic nature of stargazin regulation of AMPA receptor function allows stargazin to play a central role in short-term modulation of synaptic transmission.
Glutamate concentration-dependent responses of AMPA receptors in different types of neurons
We show here that AMPA receptors in mouse cerebellar granule cells and recombinant AMPA receptors co-expressed with stargazin show auto-inactivated concentration-response behavior similar to that described previously for AMPA receptors in avian neurons (Raman and Trussell, 1992; Vlachova et al., 1987). However, it has been reported that AMPA receptors in rat hippocampal pyramidal cells have a sigmoid concentration curve response to glutamate (Patneau and Mayer, 1991). One explanation for these different results is that AMPA receptors in the hippocampus lack TARPs (TARPless AMPA receptors). However, TARP γ-8 knockout mice show a loss of surface AMPA receptor activity in the hippocampus (Rouach et al., 2005), indicating that most surface AMPA receptors in the hippocampus interact with TARPs (TARPin AMPA receptors).
Another possible explanation for the different concentration-response relationships is that AMPA receptors in the hippocampus are composed of different subunits than receptors from other tissues. Stargazin modulated the concentration-dependent response of all AMPA receptors (Fig. 1F), but the modulation was less robust for the GluR2 flip isoform and the currents evoked by 1000 μM glutamate were still larger than those evoked by 5 μM glutamate (Fig. 1F). Interestingly, the size of the reduction in current amplitudes at high glutamate concentrations was larger for flop splice isoforms than for the corresponding flip isoforms for both GluR1 and GluR2 receptors (Fig. 1D, F, Fig. 2B, C). In cerebellar granule cell neurons, where the expression of flip and flop isoforms varies during early postnatal development (Mosbacher et al., 1994), the ratio of steady-state currents evoked by 1000 μM and 50 μM glutamate were smallest in cells in which PEPA (a drug that selectively potentiates glutamate-evoked currents for flop GluR isoforms) resulted in the largest enhancement of glutamate-evoked responses. Importantly, all AMPA receptor subunits and heteromers are modulated by stargazin in a glutamate-dependent and calcium independent manner, albeit to different extents.
The mechanism underlying the bell-shaped concentration response curves
Bell-shaped concentration response curves have also been reported for acetylcholine receptors (Colquhoun and Ogden, 1988; Giniatullin et al., 2005). Both the peak amplitude and the steady state current of acetylcholine receptors decline at millimolar concentrations of acetylcholine, and this ‘fall-off” has been explained as an effect of acetylcholine as an open channel blocker at high concentrations or by preferential occupancy of different desensitization states of the receptor at low and high agonist concentrations (Colquhoun and Ogden, 1988; Giniatullin et al., 2005).
It was suggested that similar reductions in AMPA-receptor peak currents at near-saturating glutamate concentrations might reflect glutamate binding to divalent cations and subsequent open channel block by the chaotropic species (Raman and Trussell, 1992). However, the steady-state current of AMPA receptors declines in the micromolar range (Fig. 4C) (Raman and Trussell, 1992; Vlachova et al., 1987), making it unlikely that the reductions result from open channel block. Bell-shaped concentration-response curves were also observed for steady-state currents of native kainate receptors and were explained by the different concentration dependence of activation and desensitization (Paternain et al., 1998).
Our results suggest that stargazin dissociates from AMPA receptors within milliseconds after receptor desensitization. With the exception of the size of steady-state currents, the GluR1-stargazin tandem protein and GluR1/stargazin complexes showed no difference in receptor properties, including rise time and peak open probability, or deactivation and desensitization kinetics. Because the effect of stargazin to slow the rate at which AMPA receptors desensitize (Chen et al., 2000; Kott et al., 2007; Priel et al., 2005; Tomita et al., 2005a; Tomita et al., 2004; Turetsky et al., 2005; Yamazaki et al., 2004) was unaltered in the tandem receptors, we conclude that dissociation of stargazin from AMPA receptors occurs after desensitization. Further support for this conclusion is provided by our results at low glutamate concentrations for GluR4i (Fig. 6E, F) and from comparisons of the relative amplitudes of the currents evoked by 1000 μM and 5 μM glutamate with and without stargazin for GluR flip and flop isoforms in the absence and presence of cyclothiazide. Cyclothiazide reduced stargazin mediated concentration-dependent modulation of GluR1 flip isoform, but not the GluR1i flop isoform (Supplementary figure 4).
We suggest that in resting and open channel states, the majority of receptors have stargazin associated with them, but the equilibrium between stargazin-coupled and stargazin-uncoupled forms of the receptor shifts toward the stargazin-uncoupled form one the receptors desensitize (Supplementary figure 5). An estimate of the fraction of desensitized receptors still functionally coupled with stargazin can be made from the amplitudes (relative to the peak currents) of the steady-state responses at 10 mM glutamate for GluR1i alone, GluR1i with stargazin, and the GluR1i-stargazin tandem receptor, which were about 0.3%, 4%, and 8%, respectively. These relative amplitudes indicate that roughly half of the receptors contributing to the steady-state currents at near-saturating glutamate have stargazin associated with them. Since stargazin increases the rate of recovery from desensitization, stargazin-coupled receptors will recover more often and contribute more to the steady-state current. Assuming that re-association of stargazin with the receptors is minimal before the receptors once again desensitize, simulations using the simple kinetic mechanism in Supplementary figure 5 suggest that about two-thirds of desensitized receptors are no longer effectively coupled to stargazin. What fraction of the total receptor population have stargazin associated with them will depend on the fraction of the receptors desensitized at steady-state, which will increase with glutamate concentration. As a larger fraction of receptors occupy desensitized states as the concentration of glutamate is increased, a larger fraction of the receptors contributing to the steady-state responses will no longer be effectively coupled with stargazin and stargazin enhancement of receptor gating will be increasingly reduced.
The kinetics of stargazin dissociation from AMPA receptors
Previous studies have demonstrated that the first extracellular loop of stargazin slows desensitization and deactivation of AMPA receptors and controls EPSC decay in neurons (Cho et al., 2007; Milstein et al., 2007; Tomita et al., 2005a). Our results indicate that concentration-dependent modulation of AMPA receptors occurs just after they desensitize. The kinetics of the difference current obtained from GluR1-stargazin tandem protein and GluR1/stargazin complex (Fig. 6C), and the corresponding results for the GluR4-stargazin tandem (Supplementary figure 3C), suggest that stargazin-receptor interactions disengage within milliseconds. Our oocyte studies indicate that stargazin modulation is re-acquired within 1 second (Fig. 1C). It seems unlikely that two large multi-domain membrane proteins would completely dissociate from each other on this time scale. It is possible, however, that a small number of key functional interactions responsible for stargazin modulation of gating are lost initially as a result of conformational changes associated with desensitization. Our biochemical data in oocytes (Fig. 4B) show that prolonged exposure to high concentrations of glutamate completely dissociates stargazin. It is possible that brief applications of glutamate induce rapid and reversible uncoupling of stargazin modulation, whereas prolonged applications of glutamate may result in the complete dissociation and re-distribution of receptors. Indeed, we have previously reported that application of AMPA to neurons for 1 min induces the internalization of AMPA receptors, but not stargazin (Tomita et al., 2004). Recently, a contribution of AMPA receptor desensitization to the distribution of in moving AMPA receptors at synapses was reported (Heine et al., 2008) and our study indicates that AMPA receptor-desensitization dependent trafficking may be influenced by dynamic regulation of TARP/AMPA receptor interactions.
We identified the cytoplasmic domain of the AMPA receptor as a critical region for the dissociation of AMPA receptors from stargazin. It remains unclear, however, how glutamate binding to the extracellular domain of AMPA receptors results in the dissociation of stargazin and AMPA receptors through the cytoplasmic domains of AMPA receptors. Agonist binding to extracellular domains of other receptors, for example receptor tyrosine kinases or G protein coupled receptors, can result in phosphorylation of intracellular residues or the activation of cytoplasmic factors. Whether similar cytoplasmic signaling events contribute to glutamate-induced dissociation of stargazin requires further investigation.
AMPA receptor structure and stargazin dissociation
The structure of the ligand-binding domain (S1–S2) of AMPA receptors in complex with various agonists and drugs has been resolved at the atomic level, and it has been shown that closure of the S1–S2 binding cleft results in the opening of the receptor-channels and that desensitization is associated with rearrangements at the dimer interface (Armstrong et al., 2006; Gouaux, 2004; Mayer and Armstrong, 2004). Previous studies from our laboratory showed that stargazin interacts with the extracellular glutamate-binding domain of AMPA receptors (Tomita et al., 2007b). Interestingly, chimeras in which the ligand-binding domain (S1–S2) of the GluR1 flop AMPA receptor were replaced with the GluR6 kainate receptor showed even greater loss of stargazin modulation of steady-state currents at high glutamate concentrations (Fig. 3A), underscoring the importance of the ligand binding domain for the effects reported here and supporting the idea that structural changes in the ligand-binding domain contribute to stargazin dissociation from AMPA receptors.
The number of stargazin molecules that bind to each receptor, and the number that must dissociate to lose effects on gating, are unknown. The GluR1-stargazin tandem protein forms functional AMPA receptors, suggesting that the stoichiometry is 1:1. However, some stargazin molecules in GluR1-stargazin tandem proteins may not be functionally incorporated into active receptors. Regardless, this GluR1-stargazin tandem protein might be useful for studying the structure of AMPA receptor/stargazin complexes.
Functional implications of receptor auto-inactivation
The dynamic nature of stargazin regulation of AMPA receptor function may allow stargazin to play a central role in short-term modulation of neuronal function. Our results in hippocampal neurons demonstrate that the dissociation of stargazin can contribute to paired pulse depression. The size of this effect would be expected to depend on pre-synaptic firing frequencies and the kinetics of stargazin dissociation and re-association in neurons. Although steady-state currents do not contribute to synaptic transmission, they are responsible for the excitotoxicity that occurs when ambient levels of glutamate are elevated. The dissociation of stargazin that accompanies desensitization of the receptors at glutamate concentrations in the low micromolar range may be a mechanism to protect neurons from excitotoxic damage.
Experimental procedures
Antibodies
The following antibodies were used: rabbit polyclonal antibodies to stargazin and pan-TARP (Tomita et al., 2003), GluR1, GluR2/3, GluR4 and GluR6/7 (Millipore), rat monoclonal antibody to HA epitope (Roche).
Plasmid construction
Using the chimeric primers for GluR1 and GluR6, mutated PCR fragments were subcloned using appropriate restriction enzymes or standard overlap PCR methods (Tomita et al., 2005a). All DNA constructs were verified by sequencing.
Electrophysiology using Xenopus laevis oocytes
Two electrode voltage clamp recordings were performed as described (Tomita et al., 2004). Briefly, cRNAs were transcribed in vitro using the T7 mMessage mMachine kit (Ambion) and injected at the indicated amounts into defolliculated Xenopus laevis oocytes. Two-electrode voltage-clamp analysis (Eh= −70 mV) was carried out 2–3 days post-injection at room temperature. Each agonist was bath applied in recording solution (90 mM NaCl, 1.0 mM KCl, 1.5 mM CaCl2, and 10 mM HEPES (pH = 7.4)).
Surface labeling of oocytes
Surface labeling was performed as described (Tomita et al., 2005a). Briefly, three days after injection, oocytes were incubated for 1 hr with 0.25 μg/ml rat anti-HA antibody (3F10, Roche) followed by a 30 min incubation with HRP conjugated anti-rat Ig. Individual oocytes were then placed into 100 μl SuperSignal ELISA Femto Maximum Sensitivity Substrate (Pierce) and chemiluminescence was quantified using a Veritas Microplate Luminometer (Turner Biosystems). Oocytes were treated with glutamate (1 mM) for 10 min. Glutamate was present in all experimental procedures except for the chemiluminescence reaction.
Immunoprecipitation
For co-immunoprecipitations (Tomita et al., 2003), oocyte membranes were suspended in lysis buffer containing TEEN (25 mM Tris-Cl pH 7.4, 1 mM EDTA, 1 mM EGTA, 150 mM NaCl), 1% Triton X-100, protease inhibitors (1 mM PMSF, 10 μg/ml leupeptin) and centrifuged at 37,000 × g for 40 min. The supernatants were then incubated with 3 μg of affinity-purified antibodies and 20 μl of protein A sepharose beads. The beads were then washed three times with 1% Triton in TEEN. For some experiments, glutamate or CNQX were added for 20 min at 4°C and washed once with 1% Triton X-100 in TEEN. Bound proteins were eluted by heating the resin in 20 μl of 1X SDS-PAGE sample buffer and analyzed by SDS-PAGE.
Patch clamp recording
Whole-cell recordings from cerebellar granule cells and recordings from outside-out patches from transfected tsA201 cells were performed as previously described (Cho et al., 2007; Robert and Howe, 2003). Low-density hippocampal neuron cultures (6 × 104/cm2) were maintained as described (Tomita et al., 2005b) and transfected with cDNA encoding eGFP (0.2 μg), GluR4 + stargazin (1 μg), or GluR4-stargazin tandem (1 μg) at DIV 8 with calcium phosphate. Paired-recording was performed at 10–12 DIV with two EPC 9 amplifiers (HEKA) in external solution containing (in mM): 10 HEPES, 140 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 2.7 MgCl2, and 10 glucose. Patch pipettes (tip resistance 6–8 MΩ) were filled with recording solution that contained (in mM): 130 cesium methanesulfonate, 5 HEPES, 5 Mg-ATP, 0.2 Na-GTP, 20 TEA and 5 EGTA, and all recordings were performed at room temperature. To isolate AMPA receptor-mediated EPSCs, AP-5 (50 μM) and picrotoxin (100 μM) were added to the external solution. To confirm the EPSCs were AMPA-receptor mediated, 100μM CNQX was applied to the bath solution after each recording. To record evoked EPSCs, isolated cell pairs were selected and patched simultaneously in the whole-cell mode. Both cells were voltage clamped at −70 mV. Two voltage jumps (to +20 mV for 1ms) were applied at a 20 ms interval to a neuron (presynaptic cell), and the EPSC in the other neuron (postsynaptic cell) was recorded. Currents were analog low-pass filtered at 3 kHz and digitally sampled at 40 kHz. For each cell pair, 25 events at 0.2 Hz were recorded and averaged for analysis. Mice were obtained from Jackson Laboratory and maintained at the Yale animal facility under the guidelines of the Institutional Animal Care and Use Committee.
Fitting and calculation of concentration response curves
For the results presented in Fig. 5, concentration response curves of AMPA receptors alone was fitted to
The fits to the results gave an EC50 of 10 μM and a Hill coefficient of 1. Because stargazin enhanced AMPA receptor function roughly 4-fold (Tomita et al., 2005a) and decreases the EC50 to 2 μM (Kott et al., 2007; Priel et al., 2005; Tomita et al., 2005a; Turetsky et al., 2005; Yamazaki et al., 2004), the concentration response curve of AMPA receptors with stargazin was fitted to:
The dissociation curve of AMPA receptors from stargazin was fitted to:
The fits to the results gave an IC50 was 16 μM.
The total evoked current from cells expressing AMPA receptors with stargazin was calculated as the sum of curves for AMPA receptors with and without stargazin, where each curve was weighted by the proportion of each channel type estimated from the fit to the dissociation curve:
All concentration response curves were normalized to the size of the response at 1000 μM glutamate.
Supplementary Material
Acknowledgments
The authors thank Fred Sigworth, members of the Tomita lab, and the CNNR program for helpful discussions. We also thank Daniel DiMaio for his Veritas Microplate Luminometer. S.T. is supported by Yale start-up funds, the Esther A. & Joseph Klingenstein Fund and the Edward Mallinckrodt Jr. Foundation. J.R.H. is supported by grants from the NIH (NS047712, NS057725).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Armstrong N, Jasti J, Beich-Frandsen M, Gouaux E. Measurement of conformational changes accompanying desensitization in an ionotropic glutamate receptor. Cell. 2006;127:85–97. doi: 10.1016/j.cell.2006.08.037. [DOI] [PubMed] [Google Scholar]
- Barry MF, Ziff EB. Receptor trafficking and the plasticity of excitatory synapses. Current Opinion in Neurobiology. 2002;12:279–286. doi: 10.1016/s0959-4388(02)00329-x. [DOI] [PubMed] [Google Scholar]
- Chen L, Chetkovich DM, Petralia RS, Sweeney NT, Kawasaki Y, Wenthold RJ, Bredt DS, Nicoll RA. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature. 2000;408:936–943. doi: 10.1038/35050030. [DOI] [PubMed] [Google Scholar]
- Chen L, El-Husseini A, Tomita S, Bredt DS, Nicoll RA. Stargazin differentially controls the trafficking of alpha-amino-3-hydroxyl-5-methyl-4-isoxazolepropionate and kainate receptors. Mol Pharmacol. 2003;64:703–706. doi: 10.1124/mol.64.3.703. [DOI] [PubMed] [Google Scholar]
- Cho CH, St-Gelais F, Zhang W, Tomita S, Howe JR. Two Families of TARP Isoforms that Have Distinct Effects on the Kinetic Properties of AMPA Receptors and Synaptic Currents. Neuron. 2007;55:890–904. doi: 10.1016/j.neuron.2007.08.024. [DOI] [PubMed] [Google Scholar]
- Colquhoun D, Ogden DC. Activation of ion channels in the frog end-plate by high concentrations of acetylcholine. J Physiol. 1988;395:131–159. doi: 10.1113/jphysiol.1988.sp016912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Fukata Y, Tzingounis AV, Trinidad JC, Fukata M, Burlingame AL, Nicoll RA, Bredt DS. Molecular constituents of neuronal AMPA receptors. J Cell Biol. 2005;169:399–404. doi: 10.1083/jcb.200501121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukaya M, Tsujita M, Yamazaki M, Kushiya E, Abe K, Natsume R, Kano M, Kamiya H, Watanabe M, Sakimura K. Abundant distribution of TARP gamma-8 in synaptic and extrasynaptic surface of hippocampal neurons and its major role in AMPA receptor expression on spines and dendrites. Eur J Neurosci. 2006;24:2177–2190. doi: 10.1111/j.1460-9568.2006.05081.x. [DOI] [PubMed] [Google Scholar]
- Fukaya M, Yamazaki M, Sakimura K, Watanabe M. Spatial diversity in gene expression for VDCCgamma subunit family in developing and adult mouse brains. Neurosci Res. 2005;53:376–383. doi: 10.1016/j.neures.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Geoffroy M, Lambolez B, Audinat E, Hamon B, Crepel F, Rossier J, Kado RT. Reduction of desensitization of a glutamate ionotropic receptor by antagonists. Mol Pharmacol. 1991;39:587–591. [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL. Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci. 2005;28:371–378. doi: 10.1016/j.tins.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Gouaux E. Structure and function of AMPA receptors. J Physiol. 2004;554:249–253. doi: 10.1113/jphysiol.2003.054320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, Fukaya M, Qiao X, Sakimura K, Watanabe M, Kano M. Impairment of AMPA receptor function in cerebellar granule cells of ataxic mutant mouse stargazer. J Neurosci. 1999;19:6027–6036. doi: 10.1523/JNEUROSCI.19-14-06027.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine M, Groc L, Frischknecht R, Beique JC, Lounis B, Rumbaugh G, Huganir RL, Cognet L, Choquet D. Surface mobility of postsynaptic AMPARs tunes synaptic transmission. Science. 2008;320:201–205. doi: 10.1126/science.1152089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Jonas P, Spruston N. Mechanisms shaping glutamate-mediated excitatory postsynaptic currents in the CNS. Curr Opin Neurobiol. 1994;4:366–372. doi: 10.1016/0959-4388(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Kandel ER. The molecular biology of memory storage: a dialog between genes and synapses. Biosci Rep. 2001;21:565–611. doi: 10.1023/a:1014775008533. [DOI] [PubMed] [Google Scholar]
- Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, Bredt DS. New transmembrane AMPA receptor regulatory protein isoform, gamma-7, differentially regulates AMPA receptors. J Neurosci. 2007;27:4969–4977. doi: 10.1523/JNEUROSCI.5561-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kott S, Werner M, Korber C, Hollmann M. Electrophysiological properties of AMPA receptors are differentially modulated depending on the associated member of the TARP family. J Neurosci. 2007;27:3780–3789. doi: 10.1523/JNEUROSCI.4185-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerma J. Kainate receptor physiology. Curr Opin Pharmacol. 2006;6:89–97. doi: 10.1016/j.coph.2005.08.004. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation--a decade of progress? Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptor trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- Mayer ML, Armstrong N. Structure and function of glutamate receptor ion channels. Annu Rev Physiol. 2004;66:161–181. doi: 10.1146/annurev.physiol.66.050802.084104. [DOI] [PubMed] [Google Scholar]
- Milstein AD, Zhou W, Karimzadegan S, Bredt DS, Nicoll RA. TARP Subtypes Differentially and Dose-Dependently Control Synaptic AMPA Receptor Gating. Neuron. 2007;55:905–918. doi: 10.1016/j.neuron.2007.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Cheng Y, Ramm E, Sheng M, Walz T. Structure and different conformational states of native AMPA receptor complexes. Nature. 2005;433:545–549. doi: 10.1038/nature03328. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Schmitz D. Synaptic plasticity at hippocampal mossy fibre synapses. Nat Rev Neurosci. 2005;6:863–876. doi: 10.1038/nrn1786. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Tomita S, Bredt DS. Auxiliary subunits assist AMPA-type glutamate receptors. Science. 2006;311:1253–1256. doi: 10.1126/science.1123339. [DOI] [PubMed] [Google Scholar]
- Partin KM, Patneau DK, Mayer ML. Cyclothiazide differentially modulates desensitization of alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptor splice variants. Mol Pharmacol. 1994;46:129–138. [PubMed] [Google Scholar]
- Paternain A, Rodríguez-Moreno A, Villarroel A, Lerma J. Activation and desensitization properties of native and recombinant kainate receptors. Neuropharmacology. 1998;37:1249–1259. doi: 10.1016/s0028-3908(98)00098-7. [DOI] [PubMed] [Google Scholar]
- Patneau DK, Mayer ML. Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron. 1991;6:785–798. doi: 10.1016/0896-6273(91)90175-y. [DOI] [PubMed] [Google Scholar]
- Priel A, Kolleker A, Ayalon G, Gillor M, Osten P, Stern-Bach Y. Stargazin reduces desensitization and slows deactivation of the AMPA-type glutamate receptors. J Neurosci. 2005;25:2682–2686. doi: 10.1523/JNEUROSCI.4834-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raman IM, Trussell LO. The kinetics of the response to glutamate and kainate in neurons of the avian cochlear nucleus. Neuron. 1992;9:173–186. doi: 10.1016/0896-6273(92)90232-3. [DOI] [PubMed] [Google Scholar]
- Robert A, Howe JR. How AMPA receptor desensitization depends on receptor occupancy. J Neurosci. 2003;23:847–858. doi: 10.1523/JNEUROSCI.23-03-00847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouach N, Byrd RK, Petralia RS, Elias GM, Adesnik H, Tomita S, Karimzadegan S, Kealey C, Bredt DS, Nicoll RA. TARP g-8 controls hippocampal AMPA receptor number, distribution and synaptic plasticity. Nature Neuroscience. 2005;8:1525–1533. doi: 10.1038/nn1551. [DOI] [PubMed] [Google Scholar]
- Seeburg PH. The TINS/TiPS Lecture. The molecular biology of mammalian glutamate receptor channels. Trends Neurosci. 1993;16:359–365. doi: 10.1016/0166-2236(93)90093-2. [DOI] [PubMed] [Google Scholar]
- Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298:776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- Sommer B, Keinanen K, Verdoorn TA, Wisden W, Burnashev N, Herb A, Kohler M, Takagi T, Sakmann B, Seeburg PH. Flip and flop: a cell-specific functional switch in glutamate-operated channels of the CNS. Science. 1990;249:1580–1585. doi: 10.1126/science.1699275. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Tomita S, Adesnik H, Sekiguchi M, Zhang W, Wada K, Howe JR, Nicoll RA, Bredt DS. Stargazin modulates AMPA receptor gating and trafficking by distinct domains. Nature. 2005a;435:1052–1058. doi: 10.1038/nature03624. [DOI] [PubMed] [Google Scholar]
- Tomita S, Byrd RK, Rouach N, Bellone C, Venegas A, O’Brien JL, Kim KS, Olsen O, Nicoll RA, Bredt DS. AMPA receptors and stargazin-like transmembrane AMPA receptor-regulatory proteins mediate hippocampal kainate neurotoxicity. Proc Natl Acad Sci U S A. 2007a;104:18784–18788. doi: 10.1073/pnas.0708970104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Chen L, Kawasaki Y, Petralia RS, Wenthold RJ, Nicoll RA, Bredt DS. Functional studies and distribution define a family of transmembrane AMPA receptor regulatory proteins. J Cell Biol. 2003;161:805–816. doi: 10.1083/jcb.200212116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomita S, Fukata M, Nicoll RA, Bredt DS. Dynamic interaction of stargazin-like TARPs with cycling AMPA receptors at synapses. Science. 2004;303:1508–1511. doi: 10.1126/science.1090262. [DOI] [PubMed] [Google Scholar]
- Tomita S, Shenoy A, Fukata Y, Nicoll RA, Bredt DS. Stargazin interacts functionally with the AMPA receptor glutamate-binding module. Neuropharmacology. 2007b;52:87–91. doi: 10.1016/j.neuropharm.2006.07.012. [DOI] [PubMed] [Google Scholar]
- Tomita S, Stein V, Stocker TJ, Nicoll RA, Bredt DS. Bidirectional synaptic plasticity regulated by phosphorylation of stargazin-like TARPs. Neuron. 2005b;45:269–277. doi: 10.1016/j.neuron.2005.01.009. [DOI] [PubMed] [Google Scholar]
- Turetsky D, Garringer E, Patneau DK. Stargazin modulates native AMPA receptor functional properties by two distinct mechanisms. J Neurosci. 2005;25:7438–7448. doi: 10.1523/JNEUROSCI.1108-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberghe W, Nicoll RA, Bredt DS. Stargazin is an AMPA receptor auxiliary subunit. Proc Natl Acad Sci U S A. 2005;102:485–490. doi: 10.1073/pnas.0408269102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlachova V, Vyklicky L, Vyklicky L, Jr, Vyskocil F. The action of excitatory amino acids on chick spinal cord neurones in culture. J Physiol. 1987;386:425–438. doi: 10.1113/jphysiol.1987.sp016542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker CS, Brockie PJ, Madsen DM, Francis MM, Zheng Y, Koduri S, Mellem JE, Strutz-Seebohm N, Maricq AV. Reconstitution of invertebrate glutamate receptor function depends on stargazin-like proteins. Proc Natl Acad Sci U S A. 2006;103:10781–10786. doi: 10.1073/pnas.0604482103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki M, Ohno-Shosaku T, Fukaya M, Kano M, Watanabe M, Sakimura K. A novel action of stargazin as an enhancer of AMPA receptor activity. Neurosci Res. 2004;50:369–374. doi: 10.1016/j.neures.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Zhang W, Robert A, Vogensen SB, Howe JR. The relationship between agonist potency and AMPA receptor kinetics. Biophys J. 2006;91:1336–1346. doi: 10.1529/biophysj.106.084426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziff EB. TARPs and the AMPA receptor trafficking paradox. Neuron. 2007;53:627–633. doi: 10.1016/j.neuron.2007.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.