Abstract
Background
Polymorphisms in the proinflammatory cytokine interleukin (IL)-1β gene have been associated with systemic atherogenesis, thrombosis and rupture. The aim of this study was to investigate associations between single nucleotide polymorphisms (SNPs) in IL-1β and intracranial hemorrhage (ICH) in the natural course of brain arteriovenous malformation (BAVM) patients.
Method
Two IL-1β promoter SNPs (−511C→T, −31T→C) and 1 synonymous coding SNP in exon 5 at +3953C→T (Phe) were genotyped in 410 BAVM patients. We performed a survival analysis of time to subsequent ICH, censoring cases at first treatment, death or last follow-up. A Cox regression analysis was performed to obtain hazard ratios (HRs) for genotypes adjusted for age, sex, Caucasian race/ethnicity and hemorrhagic presentation.
Results
Subjects with the −31 CC genotype (HR = 2.7; 95% CI 1.1–6.6; p = 0.029) or the −511 TT genotype (HR = 2.6; 95% CI 1.1–6.5; p = 0.039) had a greater risk of subsequent ICH compared with reference genotypes, adjusting for covariates. The +3953C→T SNP was not significantly associated with an increased ICH risk (p = 0.22). The IL-1β promoter polymorphisms were also associated with BAVM susceptibility among a subset of 235 BAVM cases and 255 healthy controls of Caucasian race/ethnicity (p < 0.001).
Conclusion
IL-1β promoter polymorphisms were associated with an increased risk of ICH in BAVM clinical course and with BAVM susceptibility. These results suggest that inflammatory pathways, including the IL-1β cytokine, may play an important role in ICH.
Key Words: Arteriovenous malformations, Cerebrovascular disease, Inflammation, Single nucleotide polymorphisms
Introduction
Brain arteriovenous malformation (BAVM) is a rare but important cause of hemorrhagic stroke in young adults. Most cases are sporadic, but BAVM also frequently occurs in patients with hereditary hemorrhagic telangiectasia [1], an autosomal dominant disorder (OMIM No. 600376) involving loss-of-function mutations in transforming growth factor-β pathway genes. Although the etiology of BAVM is obscure, genetic susceptibility may play a role in both susceptibility and disease progression [2].
Proinflammatory cytokines, including interleukins (ILs), have been reported to be involved in brain ischemic injury; increased availability of ILs in the brain tends to exacerbate damage from ischemic insult [3,4,5]. The effect of IL-1β during the ischemic insult appears to increase, owing to the synergistic effect of other cytokines [6]. Moreover, BAVM tissue specimens express 100- to 1,000-fold higher protein levels of inflammatory markers (myeloperoxidase, matrix metalloproteinase 9 and IL-6) compared with control brain tissue [7, 8].
Recently, common functional polymorphisms in the IL-1β gene have been associated with various cerebrovascular phenotypes, including ischemic stroke [9] and aneurysmal subarachnoid hemorrhage [10]. Therefore, we hypothesized that IL-1β polymorphisms may influence the natural course of BAVM and the risk of subsequent intracranial hemorrhage (ICH) and/or disease susceptibility.
Methods
Study Population
BAVM cases were recruited at the University of California, San Francisco (UCSF) or the Kaiser Permanente Medical Care Plan (KPMCP) of Northern California. Clinical presentation and morphological characteristics were recorded as described previously [11, 12], using standardized definitions [13]. All subjects provided informed consent, and the studies were approved by the institutional review boards of the UCSF and the KPMCP.
Genotyping
Three polymorphic variants in IL-1β (−511C→T, −31T→C and +3953C→T) were selected on the basis of the putative functional effect and previous associations with related phenotypes (table 1) [9, 10]. Genotyping was performed using a template-directed dye-terminator incorporation assay with fluorescence polarization detection [14]. Positive and negative controls were included on all plates to minimize genotyping errors, and any discrepancies were repeated.
Table 1.
Properties of IL-1β polymorphisms selected for genotyping
| Position relative to first base of exon 1 | dbSNP ID | Change | Function |
|---|---|---|---|
| −511 | rs16944 | C→T | promoter polymorphism |
| −31 | rs1143627 | T→C | promoter polymorphism |
| +3953 | rs1143634 | C→T | synonymous polymorphism at an amino acid coding site |
dbSNP ID = SNP database identification.
Statistical Analysis
Linkage disequilibrium, i.e. pairwise correlation, between single nucleotide polymorphisms (SNPs) was determined using both D′ and r2 measures [15]. The χ2 goodness-of-fit test was used to assess whether genotypes were in Hardy-Weinberg equilibrium (HWE) within each and pooling across all race/ethnic groups.
Cohort Analysis
We performed the Kaplan-Meier survival analysis and log-rank test of time to subsequent ICH (primary outcome), defined as a symptomatic event with signs of new intracranial blood on computed tomographic or magnetic resonance imaging, after initial presentation but prior to any intervening treatment. Patients were censored at either date of first treatment, last follow-up or death. Clinical presentation leading to diagnosis was coded dichotomously as hemorrhagic or nonhemorrhagic.
Univariate and multivariate Cox regression analysis was performed to obtain hazard ratios (HRs) and 95% confidence intervals (CIs), adjusting for age, sex, Caucasian race/ethnicity and initial hemorrhagic presentation. The HR describes the relative risk of ICH based on the comparison of ICH rates in the high-risk versus low-risk (reference) genotype groups.
Case-Control Analysis
We also conducted a secondary case-control analysis of 235 BAVM patients and 255 healthy controls of self-reported Caucasian ancestry. Controls were healthy volunteers from the same clinical catchment area without a significant past medical history recruited for a pharmacogenetics study conducted at the UCSF [16]. Logistic regression analysis was performed to obtain odds ratios (ORs) and 95% CIs for each polymorphism, adjusting for age and sex.
Meta-Analysis
As a sensitivity analysis, we conducted a random effects meta-analysis to validate our case-control findings using the pooled IL-1β −31 CC genotype frequency of Caucasian controls from 14 published studies [17,18,19,20,21,22,23,24,25,26,27,28,29,30]. A restricted maximum likelihood method was used to estimate the between-study variance as implemented in the Stata metareg package [31]. All statistical analyses were conducted using Intercooled Stata version 9 (StataCorp LP, College Station, Tex., USA).
Results
Demographic and clinical characteristics of the BAVM cohort are shown in table 2. A total of 27 ICH events occurred during a total of 1,296 person-years at risk, with a mean (standard deviation) follow-up of 3.1 (7.5) years. The mean age of BAVM patients was 35.6 (17.4) years, 55% were of self-reported Caucasian race/ethnicity, 52% were females, and 42% presented with hemorrhage.
Table 2.
Characteristics of 410 BAVM patients by new ICH status during the natural history course
| Characteristic | No new ICH |
New ICH |
Total |
|||
|---|---|---|---|---|---|---|
| n = 383 | % | n = 27 | % | n = 410 | % | |
| Race/ethnicity | ||||||
| African American | 16 | 4.2 | 3 | 11.1 | 19 | 4.6 |
| White | 210 | 54.8 | 15 | 55.6 | 225 | 54.9 |
| Asian/Pacific Islander | 49 | 12.8 | 2 | 7.4 | 51 | 12.4 |
| Hispanic | 97 | 25.3 | 7 | 25.9 | 104 | 25.4 |
| Native American | 5 | 1.3 | 0 | 0.0 | 5 | 1.2 |
| Other/unknown | 6 | 1.6 | 0 | 0.0 | 6 | 1.5 |
| Gender | ||||||
| Male | 182 | 47.5 | 13 | 48.2 | 195 | 47.6 |
| Female | 201 | 52.5 | 14 | 51.9 | 215 | 52.4 |
| Initial presentation | ||||||
| Nonhemorrhagic | 220 | 57.4 | 17 | 63.0 | 237 | 57.8 |
| Hemorrhagic | 163 | 42.6 | 10 | 37.0 | 173 | 42.2 |
| BAVM size | ||||||
| <3 cm | 179 | 46.7 | 10 | 37.0 | 189 | 46.1 |
| ≥3 cm | 143 | 37.3 | 14 | 51.9 | 157 | 38.3 |
| Missing | 61 | 15.9 | 3 | 11.1 | 64 | 15.6 |
| Venous drainage | ||||||
| Superficial | 169 | 44.1 | 13 | 48.2 | 182 | 44.4 |
| Superficial and deep | 107 | 27.9 | 7 | 25.9 | 114 | 27.8 |
| Deep | 59 | 15.4 | 3 | 11.1 | 62 | 15.1 |
| Missing | 48 | 12.5 | 4 | 14.8 | 52 | 12.7 |
| IL-1β-31T→C | ||||||
| TT | 116 | 30.3 | 10 | 37.0 | 126 | 30.7 |
| CT | 178 | 46.5 | 6 | 22.3 | 184 | 44.9 |
| CC | 80 | 20.9 | 10 | 37.0 | 90 | 22.0 |
| Missing | 9 | 2.3 | 1 | 3.7 | 10 | 2.4 |
| IL-1β-511C→T | ||||||
| CC | 116 | 30.3 | 11 | 40.8 | 127 | 31.0 |
| CT | 177 | 46.2 | 5 | 18.5 | 182 | 44.4 |
| TT | 76 | 19.8 | 9 | 33.3 | 85 | 20.7 |
| Missing | 14 | 3.7 | 2 | 7.4 | 16 | 3.9 |
| IL-1β +3953C→T | ||||||
| CC | 268 | 70.0 | 21 | 77.8 | 289 | 70.5 |
| CT | 100 | 26.1 | 3 | 11.1 | 103 | 25.1 |
| TT | 8 | 2.1 | 0 | 0 | 8 | 2.0 |
| Missing | 7 | 1.8 | 3 | 11.1 | 10 | 2.4 |
Genotype frequencies of IL-1β polymorphisms were in HWE among BAVM patients within and across all race/ethnic categories (p > 0.05). The 2 promoter SNPs were strongly correlated and in almost perfect linkage disequilibrium (D′ = 0.98, r2 = 0.96), whereas the exon 5 SNP was more weakly correlated with the promoter SNPs (D′ = 0.74, r2 = 0.08).
Cohort Analysis
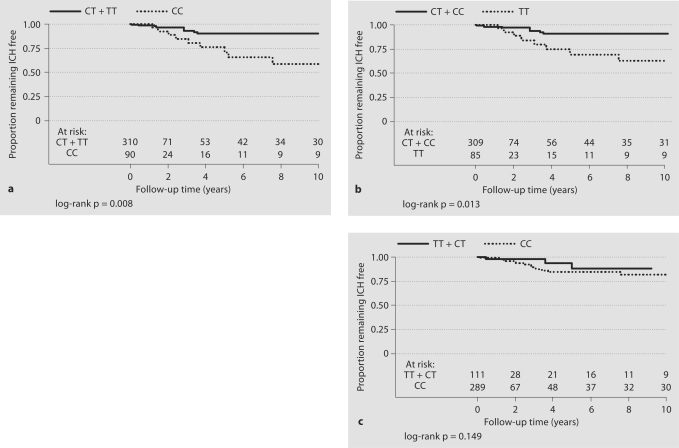
Kaplan-Meier hemorrhage-free survival curves and corresponding log-rank tests by IL-1β genotypes are shown in figure 1, with numbers at risk shown at each 2-year time interval. Patients with the IL-1β −31 CC genotype (p = 0.008) or the −511 TT genotype (p = 0.013) had a greater rate of subsequent ICH compared with reference genotypes (fig. 1a, b). No significant difference in the rate of subsequent ICH was observed for the IL-1β +3953C→T polymorphism (p = 0.145; fig. 1c).
Fig. 1.
Cumulative hemorrhage-free survival probabilities (Kaplan-Meier curves) for the BAVM cohort by IL-1β genotype: IL-1β −31T→C (a), IL1-β −511C→T (b) and IL-1β +3953C→T (c).
The univariate and multivariate HRs for IL-1β polymorphisms are summarized in table 3. Patients with the −31 CC genotype (HR = 2.7; 95% CI 1.1–6.6; p = 0.029) or the −511 TT genotype (HR = 2.6; 95% CI 1.1–6.5; p = 0.039) had a greater risk of subsequent ICH compared with reference genotypes, adjusting for covariates. A nonsignificant increased risk was observed in those with the +3953 CC genotype (HR = 2.2; 95% CI 0.6–7.7; p = 0.218).
Table 3.
Cox regression analysis of time to new ICH and IL-1β polymorphism genotype in BAVM patients
| SNP | Cases | Univariate |
Multivariate1 |
||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | ||
| IL-1β-31CC | 400 | 3.00 | 1.27–7.09 | 0.012 | 2.71 | 1.11–6.60 | 0.029 |
| IL-1β-511TT | 394 | 2.90 | 1.20–7.01 | 0.018 | 2.62 | 1.05–6.53 | 0.039 |
| IL-1β+3953CC | 400 | 2.38 | 0.71–8.33 | 0.158 | 2.17 | 0.63–7.69 | 0.218 |
Adjusted for age at diagnosis, gender, white race and hemorrhagic presentation.
Similar effect sizes for the IL-1β −31 CC genotype were observed when restricting analyses to Caucasians (HR = 2.4; 95% CI 0.7–8.3; p = 0.175) or after further adjustments for deep venous drainage and BAVM size (HR = 2.5; 95% CI 0.9–6.9; p = 0.078). However, these estimates were nonsignificant due to the reduced sample size from examining 1 race/ethnic group or missing angiographic data.
Case-Control Analysis
To determine if IL-1β SNPs were associated with disease susceptibility, we performed a case-control analysis restricted to Caucasians. IL-1β −31 CC or −511 TT genotypes were present at a much higher frequency in BAVM cases than in controls (table 4). The risk of BAVM was higher in subjects with the putative high-risk IL-1β genotypes after adjusting for age and sex: OR of 3.2 (95% CI 1.7–6.1; p < 0.001) for the −31 CC genotype and for the −511 TT genotype, respectively, and 1.8 (95% CI 1.2–2.7; p = 0.004) for the +3953 CC genotype (table 4).
Table 4.
IL-1β genotype frequency and risk of BAVM among Caucasian cases and controls
| SNP | Cases |
Controls |
OR1 | 95% CI | p value | ||
|---|---|---|---|---|---|---|---|
| n | % | n | % | ||||
| IL-1β-31T→C | |||||||
| TT | 91 | 38.7 | 107 | 42.3 | reference | ||
| CT | 103 | 43.8 | 129 | 51.0 | 1.07 | 0.71–1.60 | 0.753 |
| CC | 41 | 17.5 | 17 | 6.7 | 3.35 | 1.71–6.57 | <0.001 |
| Total | 235 | 253 | |||||
| CC versus any T | 3.23 | 1.72–6.10 | <0.001 | ||||
| IL-1β-511C→T | |||||||
| CC | 89 | 38.5 | 110 | 43.1 | reference | ||
| CT | 102 | 44.2 | 128 | 50.2 | 1.10 | 0.73–1.65 | 0.660 |
| TT | 40 | 17.3 | 17 | 6.7 | 3.40 | 1.72–6.71 | <0.001 |
| Total | 231 | 255 | |||||
| TT versus any C | 3.23 | 1.70–6.14 | <0.001 | ||||
| IL-1β +3953C→T | |||||||
| CC | 160 | 68.4 | 143 | 55.8 | reference | ||
| CT | 67 | 28.6 | 99 | 38.7 | 0.58 | 0.39–0.87 | 0.008 |
| TT | 7 | 3.0 | 14 | 5.5 | 0.41 | 0.15–1.11 | 0.080 |
| Total | 234 | 256 | |||||
| CC versus any T | 1.79 | 1.21–2.66 | 0.004 | ||||
Adjusted for age and sex.
However, the observed high-risk genotype frequencies for the 2 promoter SNPs in our Caucasian control population were lower than those previously reported in the published literature and were also out of HWE (p = 0.009). Therefore, we conducted a meta-analysis to validate our IL-1β −31T→C findings using the pooled genotype frequency of controls reported in 14 published studies conducted in Caucasians [17,18,19,20,21,22,23,24,25,26,27,28,29,30]. The IL-1β −31 CC genotype association remained when comparing the BAVM genotype frequency with the published controls. On average, the −31 CC genotype frequency was 5.1% higher in BAVM cases (17%) compared with pooled controls (12%), accounting for variation across studies (p = 0.042).
Discussion
We provide the first report of an association between polymorphic variants in the IL-1β gene with both ICH risk in the natural course of BAVM patients and BAVM susceptibility. Several lines of evidence support our findings. First, all 3 SNPs have previously been implicated in various cerebrovascular phenotypes, including ischemic injury [3], stroke [9, 32] and aneurysmal rupture [10]. For example, the TT genotype of the IL-1β −511C→T polymorphism was significantly associated with an increased risk of subarachnoid hemorrhage [10] and small vessel disease stroke [32] in a Polish population, but with a decreased risk of ischemic stroke in an Italian population [9]. Second, animal studies have shown that IL-1β expression levels are significantly upregulated after intracerebral hemorrhage [33]. Third, all 3 polymorphisms are located in known functional regions of the IL-1β gene (the promoter and an exon), and several studies have demonstrated a genotype-phenotype association. The T allele of the −511 promoter SNP is correlated with enhanced IL-1β production in vivo[34], and the −31 promoter SNP is located in a TATA box transcription initiation site [28]. The +3953C→T (Phe) variant has been shown to influence in vitro IL-1β production in some [35, 36] but not in all studies [37]. Haplotypes containing both −511T and −31C alleles have been shown to have increased transcriptional activity in vitro [38]. Thus, the increased ICH risk in IL-1β high-risk genotype carriers may be due to increased local or peripheral inflammation influencing AVM disease progression.
The IL-1β gene located on chromosome 2q14 is approximately 7 kb long with 7 exons. However, it sits in an IL-1 cytokine cluster spanning approximately 430 kb, containing genes for IL-1α, IL-1β and IL-1 receptor antagonist. Therefore, the association we observed with IL-1β variants could possibly be due to linkage disequilibrium (LD) with variants in these related IL-1 genes or additional variants in the IL-1β gene. As expected, analyses of both IL-1β promoter SNPs yielded similar findings due to the strong LD. Thus, we cannot distinguish whether 1 of the 2 promoter SNPs, or another SNP in the same LD block, is responsible for the observed associations with BAVM susceptibility and ICH.
An increasing number of reports suggest a role of inflammatory signaling in BAVM pathogenesis. Activin-like kinase 1, a transforming growth factor-β superfamily member, is mutated in hereditary hemorrhagic telangiectasia; a common activin-like kinase 1 polymorphism is also strongly associated with sporadic BAVM [39, 40]. The GG genotype of the IL-6 174G→C promoter polymorphism was associated with clinical presentation of ICH in BAVM patients [41] and with highest IL-6 expression levels in BAVM tissue [8]; IL-6 levels correlated strongly with IL-1β mRNA levels [8]. Compared with control brain (structurally normal cortex removed during temporal lobectomy), BAVM tissue had an order of magnitude higher expression of the leukocyte marker myeloperoxidase, which highly correlated and colocalized with matrix metalloprotease 9 and IL-6 expression [7]. Finally, a promoter polymorphism in the tumor necrosis factor-α (−238G→A) gene was associated with new hemorrhage in the natural course of a sample of 280 BAVM cases; the presence of the A allele increases the risk of ICH [12].
Together, these results suggest that inflammatory mechanisms are involved in BAVM susceptibility and progression and warrant replication and further investigation into the precise mechanism of how proinflammatory pathways are involved. A better understanding of BAVM pathogenesis is needed to prevent ICH and potentially help develop novel therapeutics for patient management.
Acknowledgements
The authors thank the UCSF-KPMCP BAVM Study Project members and patients for their participation, and Dr. Kathleen Giacomini for sharing control DNA (U01 GM061390). This work was supported by NIH grants: R01 NS34949, R01 NS41877 and P01NS44155 to W.L.Y. H.K. was supported in part by K23NS058357 and NIH/NCRR UCSF-CTSI grant number UL1 RR024131. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
H.K. and P.G.H. contributed equally to this work.
References
- 1.Fulbright RK, Chaloupka JC, Putman CM, Sze GK, Merriam MM, Lee GK, Fayad PB, Awad IA, White RI., Jr MR of hereditary hemorrhagic telangiectasia: prevalence and spectrum of cerebrovascular malformations. AJNR Am J Neuroradiol. 1998;19:477–484. [PMC free article] [PubMed] [Google Scholar]
- 2.Young WL, Yang GY. Are there genetic influences on sporadic brain arteriovenous malformations? Stroke. 2004;35:2740–2745. doi: 10.1161/01.STR.0000145054.35083.32. [DOI] [PubMed] [Google Scholar]
- 3.Boutin H, LeFeuvre RA, Horai R, Asano M, Iwakura Y, Rothwell NJ. Role of IL-1alpha and IL-1beta in ischemic brain damage. J Neurosci. 2001;21:5528–5534. doi: 10.1523/JNEUROSCI.21-15-05528.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Touzani O, Boutin H, Chuquet J, Rothwell N. Potential mechanisms of interleukin-1 involvement in cerebral ischaemia. J Neuroimmunol. 1999;100:203–215. doi: 10.1016/s0165-5728(99)00202-7. [DOI] [PubMed] [Google Scholar]
- 5.Loddick SA, Rothwell NJ. Neuroprotective effects of human recombinant interleukin-1 receptor antagonist in focal cerebral ischaemia in the rat. J Cereb Blood Flow Metab. 1996;16:932–940. doi: 10.1097/00004647-199609000-00017. [DOI] [PubMed] [Google Scholar]
- 6.Chao CC, Hu S, Ehrlich L, Peterson PK. Interleukin-1 and tumor necrosis factor-alpha synergistically mediate neurotoxicity: involvement of nitric oxide and of N-methyl-D-aspartate receptors. Brain Behav Immun. 1995;9:355–365. doi: 10.1006/brbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- 7.Chen Y, Fan Y, Poon KY, Achrol AS, Lawton MT, Zhu Y, McCulloch CE, Hashimoto T, Lee C, Barbaro NM, Bollen AW, Yang GY, Young WL. MMP-9 expression is associated with leukocytic but not endothelial markers in brain arteriovenous malformations. Front Biosci. 2006;11:3121–3128. doi: 10.2741/2037. [DOI] [PubMed] [Google Scholar]
- 8.Chen Y, Pawlikowska L, Yao JS, Shen F, Zhai W, Achrol AS, Lawton MT, Kwok PY, Yang GY, Young WL. Interleukin-6 involvement in brain arteriovenous malformations. Ann Neurol. 2006;59:72–80. doi: 10.1002/ana.20697. [DOI] [PubMed] [Google Scholar]
- 9.Iacoviello L, Di Castelnuovo A, Gattone M, Pezzini A, Assanelli D, Lorenzet R, Del Zotto E, Colombo M, Napoleone E, Amore C, D'Orazio A, Padovani A, de Gaetano G, Giannuzzi P, Donati MB. Polymorphisms of the interleukin-1beta gene affect the risk of myocardial infarction and ischemic stroke at young age and the response of mononuclear cells to stimulation in vitro. Arterioscler Thromb Vasc Biol. 2005;25:222–227. doi: 10.1161/01.ATV.0000150039.60906.02. [DOI] [PubMed] [Google Scholar]
- 10.Slowik A, Borratynska A, Turaj W, Pera J, Dziedzic T, Wloch D, Szczudlik A, Betlej M, Krzyszkowski T, Czepko R. Interleukin 1beta-511 C/T polymorphism and risk of aneurysmal subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2006;77:279–280. doi: 10.1136/jnnp.2005.075457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim H, Sidney S, McCulloch CE, Poon KY, Singh V, Johnston SC, Ko NU, Achrol AS, Lawton MT, Higashida RT, Young WL. Racial/ethnic differences in longitudinal risk of intracranial hemorrhage in brain arteriovenous malformation patients. Stroke. 2007;38:2430–2437. doi: 10.1161/STROKEAHA.107.485573. [DOI] [PubMed] [Google Scholar]
- 12.Achrol AS, Pawlikowska L, McCulloch CE, Poon KY, Ha C, Zaroff JG, Johnston SC, Lee C, Lawton MT, Sidney S, Marchuk D, Kwok PY, Young WL. Tumor necrosis factor-alpha-238G>A promoter polymorphism is associated with increased risk of new hemorrhage in the natural course of patients with brain arteriovenous malformations. Stroke. 2006;37:231–234. doi: 10.1161/01.STR.0000195133.98378.4b. [DOI] [PubMed] [Google Scholar]
- 13.Joint Writing Group of the Technology Assessment Committee American Society of Interventional and Therapeutic Neuroradiology Joint Section on Cerebrovascular Neurosurgery, a Section of the American Association of Neurological Surgeons and Congress of Neurological Surgeons; Section of Stroke and the Section of Interventional Neurology of the American Academy of Neurology: Reporting terminology for brain arteriovenous malformation clinical and radiographic features for use in clinical trials. Stroke. 2001;32:1430–1442. doi: 10.1161/01.str.32.6.1430. [DOI] [PubMed] [Google Scholar]
- 14.Hsu TM, Kwok PY. Homogeneous primer extension assay with fluorescence polarization detection. Methods Mol Biol. 2003;212:177–187. doi: 10.1385/1-59259-327-5:177. [DOI] [PubMed] [Google Scholar]
- 15.Devlin B, Risch N. A comparison of linkage disequilibrium measures for fine-scale mapping. Genomics. 1995;29:311–322. doi: 10.1006/geno.1995.9003. [DOI] [PubMed] [Google Scholar]
- 16.Shu Y, Brown C, Castro R, Shi R, Lin E, Owen R, Sheardown S, Yue L, Burchard E, Brett C, Giacomini K. Effect of genetic variation in the organic cation transporter 1, OCT1, on metformin pharmacokinetics. Mol Ther. 2008;83:273–280. doi: 10.1038/sj.clpt.6100275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Karasneh J, Hajeer AH, Barrett J, Ollier WE, Thornhill M, Gul A. Association of specific interleukin 1 gene cluster polymorphisms with increased susceptibility for Behcet's disease. Rheumatology (Oxford) 2003;42:860–864. doi: 10.1093/rheumatology/keg232R. [DOI] [PubMed] [Google Scholar]
- 18.Zabaleta J, Camargo MC, Piazuelo MB, Fontham E, Schneider BG, Sicinschi LA, Ferrante W, Balart L, Correa P, Ochoa AC. Association of interleukin-1beta gene polymorphisms with precancerous gastric lesions in African Americans and Caucasians. Am J Gastroenterol. 2006;101:163–171. doi: 10.1111/j.1572-0241.2006.00387.x. [DOI] [PubMed] [Google Scholar]
- 19.Glas J, Torok HP, Schneider A, Brunnler G, Kopp R, Albert ED, Stolte M, Folwaczny C. Allele 2 of the interleukin-1 receptor antagonist gene is associated with early gastric cancer. J Clin Oncol. 2004;22:4746–4752. doi: 10.1200/JCO.2004.03.034. [DOI] [PubMed] [Google Scholar]
- 20.Endler G, Marculescu R, Starkl P, Binder A, Geishofer G, Muller M, Zohrer B, Resch B, Zenz W, Mannhalter C. Polymorphisms in the interleukin-1 gene cluster in children and young adults with systemic meningococcemia. Clin Chem. 2006;52:511–514. doi: 10.1373/clinchem.2005.058537. [DOI] [PubMed] [Google Scholar]
- 21.Seripa D, Matera MG, Dal Forno G, Gravina C, Masullo C, Daniele A, Binetti G, Bonvicini C, Squitti R, Palermo MT, Davis DG, Antuono P, Wekstein DR, Dobrina A, Gennarelli M, Fazio VM. Genotypes and haplotypes in the IL-1 gene cluster: analysis of two genetically and diagnostically distinct groups of Alzheimer patients. Neurobiol Aging. 2005;26:455–464. doi: 10.1016/j.neurobiolaging.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 22.Latkovskis G, Licis N, Kalnins U. C-reactive protein levels and common polymorphisms of the interleukin-1 gene cluster and interleukin-6 gene in patients with coronary heart disease. Eur J Immunogenet. 2004;31:207–213. doi: 10.1111/j.1365-2370.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- 23.Zambon CF, Basso D, Navaglia F, Belluco C, Falda A, Fogar P, Greco E, Gallo N, Rugge M, Di Mario F, Plebani M. Pro- and anti-inflammatory cytokines gene polymorphisms and Helicobacter pylori infection: interactions influence outcome. Cytokine. 2005;29:141–152. doi: 10.1016/j.cyto.2004.10.013. [DOI] [PubMed] [Google Scholar]
- 24.Zee RY, Fernandez-Ortiz A, Macaya C, Pintor E, Fernandez-Cruz A, Lindpaintner K. IL-1 cluster genes and occurrence of post-percutaneous transluminal coronary angioplasty restenosis: a prospective, angiography-based evaluation. Atherosclerosis. 2003;171:259–264. doi: 10.1016/s0021-9150(03)00294-6. [DOI] [PubMed] [Google Scholar]
- 25.Garcia-Gonzalez MA, Lanas A, Savelkoul PH, Santolaria S, Benito R, Crusius JB, Pena AS. Association of interleukin 1 gene family polymorphisms with duodenal ulcer disease. Clin Exp Immunol. 2003;134:525–531. doi: 10.1111/j.1365-2249.2003.02325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Perri F, Piepoli A, Bonvicini C, Gentile A, Quitadamo M, Di Candia M, Cotugno R, Cattaneo F, Zagari MR, Ricciardiello L, Gennarelli M, Bazzoli F, Ranzani GN, Andriulli A. Cytokine gene polymorphisms in gastric cancer patients from two Italian areas at high and low cancer prevalence. Cytokine. 2005;30:293–302. doi: 10.1016/j.cyto.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 27.Starzynska T, Ferenc K, Wex T, Kahne T, Lubinski J, Lawniczak M, Marlicz K, Malfertheiner P. The association between the interleukin-1 polymorphisms and gastric cancer risk depends on the family history of gastric carcinoma in the study population. Am J Gastroenterol. 2006;101:248–254. doi: 10.1111/j.1572-0241.2006.00422.x. [DOI] [PubMed] [Google Scholar]
- 28.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF, Jr, Rabkin CS. Interleukin-1 polymorphisms associated with increased risk of gastric cancer. Nature. 2000;404:398–402. doi: 10.1038/35006081. [DOI] [PubMed] [Google Scholar]
- 29.Palli D, Saieva C, Luzzi I, Masala G, Topa S, Sera F, Gemma S, Zanna I, D'Errico M, Zini E, Guidotti S, Valeri A, Fabbrucci P, Moretti R, Testai E, del Giudice G, Ottini L, Matullo G, Dogliotti E, Gomez-Miguel MJ. Interleukin-1 gene polymorphisms and gastric cancer risk in a high-risk Italian population. Am J Gastroenterol. 2005;100:1941–1948. doi: 10.1111/j.1572-0241.2005.50084.x. [DOI] [PubMed] [Google Scholar]
- 30.Lin RC, Morris BJ. Association analysis of polymorphisms at the interleukin-1 locus in essential hypertension. Am J Med Genet. 2002;107:311–316. doi: 10.1002/ajmg.10177. [DOI] [PubMed] [Google Scholar]
- 31.Thompson SG, Sharp SJ. Explaining heterogeneity in meta-analysis: a comparison of methods. Stat Med. 1999;18:2693–2708. doi: 10.1002/(sici)1097-0258(19991030)18:20<2693::aid-sim235>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 32.Dziedzic T, Slowik A, Pera J, Szczudlik A. Interleukin 1 beta polymorphism (−511) and risk of stroke due to small vessel disease. Cerebrovasc Dis. 2005;20:299–303. doi: 10.1159/000087928. [DOI] [PubMed] [Google Scholar]
- 33.Lu A, Tang Y, Ran R, Ardizzone TL, Wagner KR, Sharp FR. Brain genomics of intracerebral hemorrhage. J Cereb Blood Flow Metab. 2006;26:230–252. doi: 10.1038/sj.jcbfm.9600183. [DOI] [PubMed] [Google Scholar]
- 34.El-Omar EM, Carrington M, Chow WH, McColl KE, Bream JH, Young HA, Herrera J, Lissowska J, Yuan CC, Rothman N, Lanyon G, Martin M, Fraumeni JF, Jr, Rabkin CS. The role of interleukin-1 polymorphisms in the pathogenesis of gastric cancer. Nature. 2001;412:99. doi: 10.1038/35083631. [DOI] [PubMed] [Google Scholar]
- 35.Kimura R, Nishioka T, Soemantri A, Ishida T. Cis-acting effect of the IL1B C-31T polymorphism on IL-1 beta mRNA expression. Genes Immun. 2004;5:572–575. doi: 10.1038/sj.gene.6364128. [DOI] [PubMed] [Google Scholar]
- 36.Pociot F, Molvig J, Wogensen L, Worsaae H, Nerup J. A TaqI polymorphism in the human interleukin-1 beta (IL-1 beta) gene correlates with IL-1 beta secretion in vitro. Eur J Clin Invest. 1992;22:396–402. doi: 10.1111/j.1365-2362.1992.tb01480.x. [DOI] [PubMed] [Google Scholar]
- 37.Dominici R, Malferrari G, Mariani C, Grimaldi L, Biunno I. The interleukin 1-beta exonic (+3953) polymorphism does not alter in vitro protein secretion. Exp Mol Pathol. 2002;73:139–141. doi: 10.1006/exmp.2002.2435. [DOI] [PubMed] [Google Scholar]
- 38.Chen H, Wilkins LM, Aziz N, Cannings C, Wyllie DH, Bingle C, Rogus J, Beck JD, Offenbacher S, Cork MJ, Rafie-Kolpin M, Hsieh CM, Kornman KS, Duff GW. Single nucleotide polymorphisms in the human interleukin-1B gene affect transcription according to haplotype context. Hum Mol Genet. 2006;15:519–529. doi: 10.1093/hmg/ddi469. [DOI] [PubMed] [Google Scholar]
- 39.Pawlikowska L, Tran MN, Achrol AS, Ha C, Burchard EG, Choudhry S, Zaroff J, Lawton MT, Castro RA, McCulloch CE, Marchuk DA, Kwok PY, Young WL. Polymorphisms in transforming growth factor-B-related genes ALK1 and ENG are associated with sporadic brain arteriovenous malformations. Stroke. 2005;36:2278–2280. doi: 10.1161/01.STR.0000182253.91167.fa. [DOI] [PubMed] [Google Scholar]
- 40.Simon M, Franke D, Ludwig M, Aliashkevich AF, Koster G, Oldenburg J, Bostrom A, Ziegler A, Schramm J. Association of a polymorphism of the ACVRL1 gene with sporadic arteriovenous malformations of the central nervous system. J Neurosurg. 2006;104:945–949. doi: 10.3171/jns.2006.104.6.945. [DOI] [PubMed] [Google Scholar]
- 41.Pawlikowska L, Tran MN, Achrol AS, McCulloch CE, Ha C, Lind DL, Hashimoto T, Zaroff J, Lawton MT, Marchuk DA, Kwok PY, Young WL. Polymorphisms in genes involved in inflammatory and angiogenic pathways and the risk of hemorrhagic presentation of brain arteriovenous malformations. Stroke. 2004;35:2294–2300. doi: 10.1161/01.STR.0000141932.44613.b1. [DOI] [PubMed] [Google Scholar]