Abstract
Sulfation and phosphorylation are post-translational modifications imparting an isobaric 80-Da addition on the side chain of serine, threonine, or tyrosine residues. These two post-translational modifications are often difficult to distinguish because of their similar MS fragmentation patterns. Targeted MS identification of these modifications in specific proteins commonly relies on their prior separation using gel electrophoresis and silver staining. In the present investigation, we report a potential pitfall in the interpretation of these modifications from silver-stained gels due to artifactual sulfation of serine, threonine, and tyrosine residues by sodium thiosulfate, a commonly used reagent that catalyzes the formation of metallic silver deposits onto proteins. Detailed MS analyses of gel-separated protein standards and Escherichia coli cell extracts indicated that several serine, threonine, and tyrosine residues were sulfated using silver staining protocols but not following Coomassie Blue staining. Sodium thiosulfate was identified as the reagent leading to this unexpected side reaction, and the degree of sulfation was correlated with increasing concentrations of thiosulfate up to 0.02%, which is typically used for silver staining. The significance of this artifact is discussed in the broader context of sulfation and phosphorylation site identification from in vivo and in vitro experiments.
Protein phosphorylation represents one of the most important post-translational regulatory mechanisms controlling cell function and cell signaling. Over the years, significant efforts have been devoted to the identification of phosphorylation sites in a wide range of proteins. A number of important studies have identified several thousand sites in large scale phosphoproteome experiments (1–3). More than 500 known eukaryotic kinases phosphorylate proteins on serine, threonine, or tyrosine residues, and it is estimated that one-third of all proteins are phosphorylated at some stage of their life cycle (4–6). Changes in the phosphorylation status of many receptors, transcription factors, protein kinases, and several other substrates regulate a diverse array of biological processes including signal transduction, gene expression, growth, motility, proliferation, apoptosis, and differentiation (6–9).
Sulfation is another type of post-translational modification of serine, threonine, and tyrosine residues. In contrast to protein phosphorylation, the significance of sulfation in modulating protein function and biological processes is poorly understood (10). Since the first report of its occurrence in fibrinogen (11), only 275 tyrosine sulfated proteins have been reported in the UniProt database (12). Tyrosine sulfation is mediated by two Golgi membrane-associated protein tyrosylsulfotransferases, TPST1 and TPST2, which use 3-phosphoadenosine 5′-phosphosulfate as the sulfate group donor (11–13). Consequently many secretory proteins and Golgi membrane-anchored proteins are sulfated on tyrosine residues, including the chemokine receptor CCR5, the adhesion molecule P-selectin glycoprotein ligand 1 (PSGL-1), and coagulation factor VIII (12–14). Generally protein sulfation appears to play a role in mediating protein-protein interaction. Although sulfation was primarily identified on tyrosine residues, a recent report by Medzihradszky et al. (15) indicated that this modification was also found on serine and threonine residues of several eukaryotic proteins, suggesting that its cellular functions and distribution are more significant than originally suspected. However, in contrast to protein phosphorylation, there is no known consensus motif recognized by sulfotransferases, and no dynamic turnover has been described for sulfation (14).
Studies of the functional consequences of protein phosphorylation and sulfation along with the identification of novel substrates are important to refine our knowledge of their potential roles in disease progression. In recent years, MS has become a powerful tool in large scale quantitative proteomics investigations because of its high throughput and high sensitivity capabilities (16, 17). The identification of protein post-translational modifications is typically achieved following either one- or two-dimensional gel electrophoresis prior to MS analysis (18). Gel electrophoresis provides unparalleled separation of intact proteins, thus enabling the resolution of complex cell extracts into bands or spots of minimal complexity. Compared with gel-free analysis of complex protein extracts, gel separation enhances the accuracy of protein assignments and maximizes the dynamic range of protein identification and their modifications. Furthermore many detergents and buffer components that are often necessary to solubilize proteins but are incompatible with MS can be conveniently removed by gel electrophoresis (19). Protein bands or spots separated by gel electrophoresis are commonly visualized by silver staining, which provides relatively high sensitivity (∼1–10 ng) compared with Coomassie Blue staining (∼200–300 ng) (20, 21). However, undesirable side reactions of silver staining reagents that result in modifications such as lysine formylation have been reported recently (21–24). Obviously this artifact can lead to ambiguous interpretation given that formylation can be mistakenly assigned to a dimethylation because of its isobaric mass.
In this study, we report another potential pitfall in the analysis of post-translational modifications in proteins extracted from silver-stained gels. Sulfation of hydroxylated amino acid residues is caused by a side reaction of sodium thiosulfate, a commonly used agent that catalyzes the reduction of AgNO3 into metallic silver as a prerequisite for protein staining. Detailed analyses of both protein standards and Escherichia coli protein extracts separated by gel electrophoresis revealed that several serine, threonine, and tyrosine residues exhibited a +80-Da mass shift when analyzed after staining with silver but not with Coomassie Blue. High precision mass measurements combined with tandem MS analyses were performed using in-gel digests to confirm that the silver staining-specific modification corresponded to sulfation rather protein phosphorylation. The extent of sulfation was correlated with increasing concentrations of the sodium thiosulfate reagent.
EXPERIMENTAL PROCEDURES
Material and Reagents—
Enolase from the yeast Saccharomyces cerevisiae was purchased from Sigma-Aldrich. Bacterial proteins were extracted from BL-21(DE3) E. coli cells purchased from Stratagene. ERK1 samples were prepared from human HEK293 cells transfected with pcDNA3-HAERK1-GST using the calcium phosphate method (12 × 10-cm plates for each condition). The pcDNA3-HAERK1-GST vector expresses the hamster ERK1 cDNA with a hemagglutinin tag epitope at the N terminus and a GST sequence at the C terminus. The day after transfection, the medium was changed to a serum-free medium, and the cells were starved for 24 h. Then the cells were stimulated for 15 min with 10% serum. After stimulation, the cells were washed twice with PBS, frozen in liquid nitrogen, and stored at −80 °C until analysis. Cells were lysed for 30 min at 4 °C in 0.5 ml of lysis buffer (50 mm Tris, pH 7.4, 100 mm NaCl, 5 mm EDTA, 1% Triton, 40 mm β-glycerol phosphate, 50 mm NaF, 1 mm orthovanadate, 0.1 mm PMSF, 1 mm leupeptin, 1 mm pepstatin) per dish. The cellular extracts were cleared by centrifugation for 10 min at 10,000 × g. The supernatants were then incubated with 75 μl of glutathione-Sepharose beads for 1.5 h. The beads were washed four times in TNET buffer (50 mm Tris-HCl, pH 7.4, 100 mm NaCl, 5 mm EDTA, 0.5% Triton X-100) and boiled twice in Laemmli buffer for 5 min at 95 °C to elute the retained ERK1 protein. The concentration of the protein extract was determined using the micro-BCA protein assay kit from Pierce. ACN and methanol (MeOH) were purchased from Fisher Scientific. Acetic acid was purchased from Fluka Biochemika (Oakville, Ontario, Canada). Formic acid (FA)1 was purchased from EMD Chemicals Inc. (Gibbstown, NJ). Sequencing grade modified trypsin was purchased from Promega (Madison, WI). All other chemicals were purchased from Sigma.
Protein Separation by SDS-PAGE—
A total of 2 μg of yeast enolase, 1 μg of ERK1, and 1 μg of E. coli protein extract was subjected to electrophoresis through a 4–12% NuPAGE bis-Tris polyacrylamide gel (Invitrogen) under denaturing conditions. After electrophoresis, proteins were fixed within the polyacrylamide gel by incubating the entire gel in 5% (v/v) acetic acid in a 1:1 (v/v) water:ethanol solution. For silver staining, the gel was first sensitized for 1 min using an aqueous solution of 0.02% sodium thiosulfate (Na2S2O3) unless otherwise indicated. Staining was performed by incubating the gel in 0.1% (v/v) silver nitrate (AgNO3) in water for 25 min at 4 °C. Finally the gel was developed in 3% (w/v) sodium carbonate (Na2CO3, pH 11.4) containing 0.05% formalin (v/v) in water. The staining was then stopped with a solution of 5% (v/v) acetic acid in water. For Coomassie Blue staining, proteins were fixed after gel separation and stained in a one-step procedure by incubating the entire gel in 0.1% (w/v) Coomassie Brilliant Blue R-250 in a 1:8:11 (v/v/v) acetic acid:methanol:water mixture for 1 h at room temperature. The gel was finally rinsed three times in a 1:4:5 (v/v/v) acetic acid:methanol:water solution at room temperature to visualize protein bands.
Destaining and In-gel Digestion—
Protein bands were excised from the gel and destained in 200 μl of destaining solution composed of 30 mm potassium hexacyanoferrate(III) (K3Fe(CN)6) and 100 mm Na2S2O3 in a 1:1 ratio for all silver-stained gel bands. For Coomassie Blue-stained gels, the destaining process was performed by incubating the gel pieces in 200 μl of a 1:1 (v/v) water:ACN solution. Proteins were then reduced with 10 mm DTT in 50 mm ammonium bicarbonate (NH4HCO3), pH 8.5 at 56 °C for 1 h and alkylated using 55 mm iodoacetamide in 50 mm ammonium bicarbonate, pH 8.0 at room temperature for 1 h in the dark. Proteins were digested with trypsin in 50 mm ammonium bicarbonate, pH 8.0 at 37 °C for 4 h. Peptides were extracted with 5% (v/v) TFA in a 1:1 (v/v) water:ACN mixture. Following evaporation to dryness, peptides were resuspended in 30 μl of 0.2% FA in water and analyzed.
β-Elimination—
Dried enolase peptides were dissolved in 20 μl of a freshly prepared saturated barium hydroxide (Ba(OH)2) solution. The mixture was incubated at room temperature for 15 min, and the reaction was terminated by adding 1 μl of 100% FA. The reaction mixture was evaporated in a SpeedVac, and the dried peptides were redissolved in 30 μl of 0.2% FA in water prior to MS analysis.
Mass Spectrometry Analysis—
All MS analyses were performed using an LTQ-Orbitrap hybrid mass spectrometer with a nanoelectrospray ion source (ThermoElectron, San Jose, CA) coupled to a nano-flow LC system (Eksigent, Dublin, Ireland) equipped with a Finnigan AS autosampler (Thermo Electron, San Jose, CA). Protein digests were separated using a 10-cm-long, 150-μm-inner diameter analytical column and a 4-mm-long, 360-μm-inner diameter trap column packed in house with 3-μm C18 particles (Jupiter 300 Å, Phenomenex, Torrance, CA). The mobile phase consisted of 0.2% FA in water (solvent A) and 0.2% FA in ACN (solvent B). The pump flow rate was set to 0.6 μl/min, and peptide elution was achieved using a linear gradient of 5–40% B for the first 53 min followed by a rapid increase to 60% B for the next 3 min. The conventional MS spectra (survey scan) were acquired at high resolution (M/ΔM, 60,000 full-width half-maximum) over the acquisition range of m/z 400–1600. Full scan MS2 and MS3 spectra were analyzed in the linear trap. Peptides were analyzed in data-dependent mode where for each 1-s survey scan the three most intense precursor ions with intensity above 10,000 counts were selected for MS2 sequencing with a total duty cycle of 2.5 s. Product ions arising from a neutral loss of 80 Da from the precursor ion were subsequently targeted for MS3 sequencing. To prevent the reacquisition of product ion spectra from the same precursor ion, a dynamic exclusion window of 0.5 Da was applied for 90 s. Mass calibration used either an internal lock mass (protonated (Si(CH3)2O))6; m/z 445.12057) or external calibration using Calmix (caffeine, MRFA, and Ultramark) and typically provided mass accuracy within 5 ppm for all nano-LC-MS experiments.
Database Searching—
MS data were analyzed using the Xcalibur software (version 2.0 SR1). Peak lists were then generated using the Mascot distiller software (version 2.1.1, Matrix Science, London, UK) where MS processing was done using the LCQ_plus_zoom script. Database searches were performed using the search engine Mascot (version 2.1, Matrix Science). For all protein analyses, searches were conducted using the NCBInr database containing 3,310,354 entries (NCBInr March 3, 2006). The error window for experimental peptide mass values and MS2 fragment ion mass values were set to ±0.02 and 0.5 Da, respectively. The number of allowed missed cleavage sites for trypsin was set to 1, and phosphorylation (STY), oxidation (Met), deamidation (NQ), and carbamidomethylation (Cys) were all selected as variable modifications. No fixed modification was included in the search. Manual inspection of all MS2 and MS3 spectra for modified peptides was performed to validate assignments.
Peptide Detection and Clustering—
Raw data files (.raw) generated from the LTQ-Orbitrap acquisition software were converted into text files representing all ions according to their corresponding m/z values, retention time, peak widths, intensity, and charge state using in-house peptide detection software (25). Intensity values above a user-defined intensity threshold (10,000 counts) were considered for further analysis. Segmentation analyses were performed across different sample sets using hierarchical clustering with criteria based on their respective m/z, charge, and time within user-defined tolerance (±0.05 m/z and ±1 min). Normalization of retention time is then performed on the initial peptide cluster list using a dynamic and nonlinear correction. A moving average time window interpolation scheme is used to compute the time shifts for each peptide across the different data sets. For replicate LC-MS injections, this alignment confines the retention time distribution to less than ±0.1 min (<0.3% relative standard deviation) on average. The generated unique list of peptide clusters allowed the direct comparison of peptide abundance between samples in different conditions to identify those showing reproducible and statistically meaningful changes in abundance.
RESULTS
Evaluation of Staining Protocols on the Occurrence of Protein Sulfation Artifacts—
In a previous study, silver staining was found to introduce chemical modification artifacts on various proteins. More specifically, staining procedures requiring the use of formaldehyde resulted in ɛ-formylation of lysine residues (24). In the course of preliminary gel electrophoresis experiments on protein standards, we encountered another chemical modification artifact associated with the gel staining protocol. To illustrate this problem, we analyzed yeast enolase using electrophoresis through an SDS-polyacrylamide gel, which was then stained with either silver or Coomassie Blue following standard protocols (see “Experimental Procedures”). Bands were excised from the gel and digested with trypsin, and the resulting peptides were analyzed by nano-LC-MSn on an LTQ-Orbitrap mass spectrometer. The acquired data were then searched against a yeast database using the search engine Mascot to identify modified peptides. In total, Mascot identified eight peptides showing an addition of 80 Da in the silver-stained enolase sample: 16GNPTVEVELTTEK28, 33SIVPSGASTGVHEALEMR50, 89AVDDFLISLDGTANK103, 186IGSEVYHNLK195, 244IGLDC(carbamidomethyl)ASSEFFKDGK258 (C(carb) represents carbamidomethylated cysteine), 259YDLDFKNPNSDK270, 313TAGIQIVADDLTVTNPKR330, and 359AAQDSFAAGWGVMVSHR375. This modification potentially can be assigned to either phosphorylation or sulfation. Analyses of all CID tandem mass spectra showed the presence of an abundant fragment ion corresponding to a loss of 80 Da from the precursor ion. However, because no fragment ions generated from cleavages along the peptide backbone were observed after the neutral loss, positive identification of the peptide sequence and the modification site was not possible. This is mainly explained by the fact that phospho- and sulfoester bonds are more labile than peptide bonds upon low energy collisional activation. To circumvent this problem, a subsequent MS3 experiment was performed by isolating and fragmenting the precursor ion [M + 2H − 80]2+. Consequently extensive backbone fragmentation of the modified peptides was achieved, thus enabling sequence assignment of the modified peptides. However, upon inspection the fragmentation pattern of these modified peptides appeared to be similar to that of the unmodified peptide counterpart, making it impossible to confirm the modification site. The MS2 and MS3 spectra of the eight peptides exhibiting a mass shift of 80 Da are presented as supplemental material.
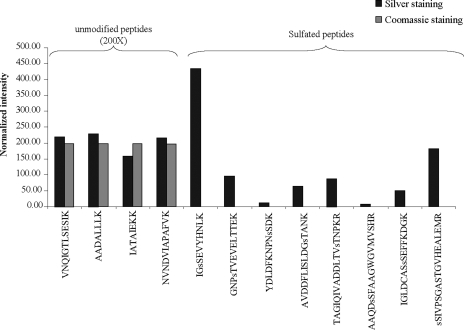
A manual inspection of the LC-MS chromatogram obtained for the Coomassie Blue-stained enolase sample revealed no peptide ion exhibiting an 80-Da shift. Fig. 1 illustrates the comparison of the abundance for all eight modified peptides obtained from the silver- and Coomassie Blue-stained enolase samples. To confirm that the complete absence of these modified peptides in the Coomassie staining-derived samples was not due to variations in gel loading or mass spectrometry performance, the intensity of four of the unmodified yeast enolase peptides (68NVNDVIAPAFVK79, 331IATAIEKK338, 339AADALLLK346, and 347VNQIGTLSESIK358) was compared between the two conditions (Fig. 1). Unmodified enolase peptides were equally detected in both samples. In contrast, peptides bearing a +80-Da mass shift in silver-stained samples were not detected in Coomassie Blue-stained samples. This comparison suggests that the presence of this artifact is specific to the silver staining procedure.
Fig. 1.
Intensity distribution of sulfopeptides and unmodified peptides from silver- and Coomassie Blue-stained yeast enolase. Identity of the peptides was confirmed by MS2 and MS3 product ion spectra and is shown below each bar. The letter “s” preceding the single letter amino acid code indicates the sulfated residue.
Phosphorylation and sulfation of proteins both impart a mass shift of 80 Da on Ser, Thr, and Tyr residues. However, because the mass difference between these two types of modification is only 0.0095 Da, the distinction between sulfation and phosphorylation cannot be achieved using low resolution mass spectrometers. For unambiguous assignment of these modifications, we relied on the LTQ-Orbitrap MS instrument, which provides high resolution (M/ΔM, 60,000) and good mass accuracy (<5 ppm). Mass measurements obtained for all eight modified peptides are listed in Table I. The observed and calculated mass values for each peptide ion were compared for the anticipated phosphopeptide and sulfopeptide counterparts. The mass measurements obtained for the eight modified peptides (ΔM, +80 Da) were in closer agreement with those expected for sulfated peptides. For example, the exact mass value for the peptide 16GNPTVEVELTTEK28 (+80-Da modification) eluting at 31.5 min is 1495.6718 Da. The mass difference between the observed and theoretical values for the sulfated or phosphorylated peptide is +1.1 and −5.2 ppm, respectively. For all eight modified peptides, the average mass difference between the observed and theoretical values was 1.5 ± 0.5 ppm for sulfation compared with −4.3 ± 1.4 ppm for phosphorylation. To provide additional evidence that the +80-Da mass shift on each of these modified peptides is due to sulfation, the difference between the modified peptide and its unmodified counterpart was also calculated. In all cases, the average difference between the modified and unmodified peptides was 79.9549 ± 0.0014 Da confirming the presence of a sulfated moiety (79.9568 Da) and not its phosphorylated counterpart (79.9663 Da).
Table I.
Identification of sulfopeptides from yeast enolase
Sulfated residues are bold. The rows with the dashes correspond to the unmodified peptides. sS, sulfated serine; sY, sulfated tyrosine; sT, sulfated threonine; C(carb), carbamidomethylated cysteine; RT, retention time.
| Peptide sequence | RT | Observed mass | Calculated mass | ΔMassa | Modificationb |
|---|---|---|---|---|---|
| min | Da | Da | ppm | Da | |
| IGsSEVYHNLKc | 22.4 | 1238.5602 | 1238.5590 | 1.0 | 79.9528 |
| IGSEVsYHNLKc | 27.1 | 1238.5600 | 1238.5590 | 0.8 | 79.9526 |
| IGSEVYHNLK | 21.0 | 1158.6074 | 1158.6022 | 4.5 | — |
| GNPsTVEVELTTEKc | 31.5 | 1495.6718 | 1495.6702 | 1.1 | 79.9538 |
| GNPTVEVELTTEK | 28.5 | 1415.7180 | 1415.7134 | 3.2 | — |
| YDLDFKNPNsSDK | 29.1 | 1534.6248 | 1534.6236 | 0.8 | 79.9546 |
| YDLDFKNPNSDK | 27.4 | 1454.6702 | 1454.6666 | 2.5 | — |
| AVDDFLISLDGsTANK | 43.4 | 1657.7514 | 1657.7494 | 1.2 | 79.9560 |
| AVDDFLIsSLDGTANK | 44.0 | 1657.7528 | 1657.7494 | 2.1 | 79.9574 |
| AVDDFLISLDGTANK | 42.7 | 1577.7954 | 1577.7926 | 1.8 | — |
| TAGIQIVADDLTVsTNPKR | 36.4 | 1991.0022 | 1990.9982 | 2.0 | 79.9542 |
| TAGIQIVADDLsTVTNPKR | 40.4 | 1991.0016 | 1990.9982 | 1.7 | 79.9536 |
| TAGIQIVADDLTVTNPKR | 36.7 | 1911.0480 | 1911.0414 | 3.5 | — |
| AAQDsSFAAGWGVMVSHRc | 40.2 | 1868.7964 | 1868.7924 | 2.1 | 79.9563 |
| AAQDSFAAGWGVMVSHR | 38.9 | 1788.8401 | 1788.8356 | 2.5 | — |
| IGLDC(carb)ASsSEFFKDGK | 36.3 | 1752.7354 | 1752.7324 | 1.7 | 79.9548 |
| IGLDC(carb)AsSSEFFKDGKc | 37.7 | 1752.7344 | 1752.7324 | 1.1 | 79.9538 |
| IGLDC(carb)ASSEFFKDGK | 35.3 | 1672.7806 | 1672.7756 | 3.0 | — |
| sSIVPSGASTGVHEALEMRc | 35.8 | 1919.8738 | 1919.8706 | 1.7 | 79.9562 |
| SIVPSGAsSTGVHEALEMR | 36.4 | 1919.8740 | 1919.8706 | 1.8 | 79.9564 |
| SIVPsSGASTGVHEALEMR | 37.0 | 1919.8732 | 1919.8706 | 1.4 | 79.9556 |
| SIVPSGASTGVHEALEMR | 32.9 | 1839.9176 | 1839.9138 | 2.1 | — |
Difference between observed and calculated masses.
Mass difference between modified and unmodified peptide (phosphate, 79.9663; sulfate, 79.9568).
Sulfation site has not been confirmed by β-elimination.
Sulfation Artifacts Occur on Ser, Thr, and Tyr Residues—
Manual inspection of all sulfated peptides identified in the silver-stained enolase sample (Fig. 1) indicated the presence of closely eluting sulfopeptide isomers. For example, the reconstructed ion chromatogram for the sulfopeptide 33SIVPSGASTGVHEALEMR50 at m/z 960.94+2 shows three distinct chromatographic peaks eluting within 2 min from each other (Fig. 2a). Interestingly this peptide contains three Ser residues and a Thr that can be potentially sulfated. This was also observed with other sulfopeptides such as 259YDLDFKNPNSDK270 (one Ser residue) and 244IGLDC(carb)ASSEFFKDGK258 (two Ser residues) where one and two chromatographic peaks appeared, respectively. It is noteworthy that peptides containing other hydroxylated residues, such as threonine and tyrosine, also showed additional peaks. For example, 186IGSEVYHNLK195, 313TAGIQIVADDLTVTNPKR330, and 359AAQDSFAAGWGVMVSHR375 all comprised isomeric elution doublets. On the other hand, not all peptides containing more than one modified residue (Ser, Thr, or Tyr) presented multiple chromatographic peaks. This was the case for peptides 259YDLDFKNPNSDK270 and 16GNPTVEVELTTEK28.
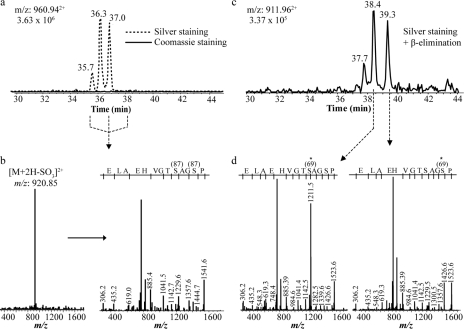
Fig. 2.
Identification of modified peptides from yeast enolase. a, reconstructed ion chromatogram of m/z 960.94 from silver- and Coomassie Blue-stained gel bands. This ion was undetectable in the Coomassie-stained band. b, first generation (MS2) product ion spectrum of m/z 960.94 (left) and second generation (MS3) product ion spectrum of m/z 920.85 (right) obtained from precursor m/z 960.94. c, reconstructed ion chromatogram of m/z 911.96 following β-elimination using Ba(OH)2 of sulfopeptides shown in a. d, MS2 product ion spectrum of two isomeric β-elimination peptides eluting at 38.4 and 39.3 min.
Confirmation of the identity of the sulfopeptide isomers was obtained from detailed examination of MS2 and MS3 spectra. For example, sulfopeptides 33SIVPSGASTGVHEALEMR50 at m/z 960.94+2 eluting at 35.8, 36.4, and 37.0 min are presented in Fig. 2, a and b. The MS2 spectra extracted from the three corresponding peptides all showed a prominent loss of SO3 [M + 2H − 80]2+. Similarly the MS3 spectra of the sulfated fragment ions all showed identical fragmentation patterns confirming the presence of sulfopeptide isomers of 33SIVPSGASTGVHEALEMR50 (Fig. 2).
The prompt dissociation of the sulfate group from the modified peptide during low energy collisional activation precludes the precise localization of the modified residue(s). To identify the sites of modification, we used β-elimination and analysis of the corresponding cleavage products using LC-MS2 as described previously for sulfation (15), phosphorylation (26, 27), and O-glycosylation (28, 29). The alkaline hydrolysis of phosphoester or sulfoester bonds leaves readily traceable β-elimination products that correspond to the dehydrated form of the modified residues (i.e. dehydroalanine and dehydroaminobutyrine for modified Ser and Thr, respectively).
Prior to nano-LC-MS, peptides derived from a silver-stained enolase gel band were subjected to the β-elimination reaction using an aqueous solution of Ba(OH)2. Data obtained for the β-elimination products of the sulfopeptide 33SIVPSGASTGVHEALEMR50 are presented in Fig. 2, c and d. The corresponding peptide ion at m/z 911.96+2 eluted at three different chromatographic retention times: 37.7, 38.4, and 39.3 min (Fig. 2c). Interestingly the elution profile of the β-elimination product peptide is similar to that of its sulfated counterpart (Fig. 2a). However, after β-elimination, all three peptide isomers eluted 2 min later than their sulfated counterparts. This trend is consistent with the notion that loss of the sulfate group caused by β-elimination favors the formation of a more hydrophobic counterpart (30) (e.g. the conversion of a sulfated serine into dehydroalanine). The MS2 spectra of the two main peptides eluting at 38.4 and 39.3 min yielded characteristic y-ions that enabled a straightforward identification of the modified residues (Fig. 2d). Indeed the form of the peptide ion 33SIVPSGASTGVHEALEMR50 eluting at 38.4 min generated a fragment ion at m/z 1211.5 (y11) corresponding to the dehydroalanine conversion of a sulfated Ser8 residue.
In contrast, the peptide ion eluting at 39.3 min did not generate a fragment at m/z 1211.5. Rather a peak at m/z 1229.5, assigned to an unmodified serine at y11, was detected. The exact sulfation site was assigned to Ser5 due to the presence of a fragment ion at m/z 1426.6 corresponding to the dehydroalanine generated after β-elimination. Unfortunately the modified peptide eluting at 37.7 min did not yield an MS2 spectrum of sufficient quality for unambiguous identification of the remaining site, thus leaving either Ser1 or Thr9 as possible modification sites. It is noteworthy that the β-elimination reaction did not provide results for all sulfated peptides. This is because of the fact that β-elimination on phospho-Tyr is inefficient and does not lend itself to the identification of these modified residues (30). In addition, β-elimination of Ser residues flanked by specific amino acids (such as Ser(P)-Pro) is not favored, and Thr(P) is 20 times less susceptible than Ser(P) to undergo this reaction (31, 32). Therefore, the site of modification was inferred on other potential sulfated Ser, Thr, and Tyr residues when MS2 spectra were not available. These results show that the silver staining-induced sulfation artifact affects Ser, Thr, and Tyr residues randomly.
Sodium Thiosulfate Gives Rise to Protein Sulfation Artifacts—
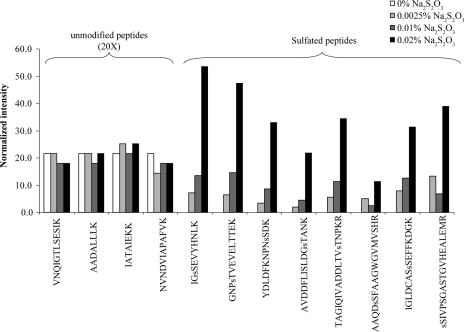
Na2S2O3 is a reducing agent used to sensitize proteins prior to metallic silver deposition on the side chains of amino acids. This pretreatment of the gel greatly improves the silver staining method by increasing the sensitivity and the contrast of the stain (33). Because this chemical is the only potential source of sulfate used in the procedure, we investigated its effect on sulfation of Ser, Thr, and Tyr residues during silver staining. Yeast enolase was subjected to gel electrophoresis and then stained with silver using increasing concentrations of Na2S2O3 (0–0.02%). MS analysis of the tryptic digests of yeast enolase revealed a linear relationship between the abundance of sulfopeptide artifacts and the concentration of Na2S2O3 used during the sensitizing step (Fig. 3). The eight sulfopeptides described previously (Table I) all showed increasing levels of sulfation that were correlated with the Na2S2O3 concentration (Fig. 3). On the other hand, the intensity of four unmodified yeast enolase peptides was not affected by increasing Na2S2O3 concentrations (Fig. 3). In the complete absence of Na2S2O3, no enolase sulfopeptide was detected (Fig. 3). This result indicates that Na2S2O3, which is also used later during the destaining process prior to MS analysis, does not contribute to the formation of the artifact. Taken together, these results clearly demonstrate that the sulfation artifact introduced on Ser, Thr, and Tyr side chains during the silver staining process is caused by sodium thiosulfate. The omission of thiosulfate prevents the formation of this artifact but has a major drawback. Decreasing the amount of Na2S2O3 markedly reduces the sensitivity of protein visualization by silver staining (not shown).
Fig. 3.
Influence of sodium thiosulfate concentration on the abundance of silver staining-induced sulfopeptides. Peptides identified from product ion spectra are shown below each bar. The letter s preceding the single letter amino acid code indicates the sulfated residue.
Sulfation Artifact Caused by Silver Staining of Phosphorylated ERK1—
We found that proteins extracted from silver-stained gels undergo artifactual sulfation that can potentially be mistaken for genuine phosphorylation. To demonstrate the impact of this artifact on the interpretation of real phosphorylation sites and to investigate further how these two types of modifications can be distinguished from each other, we analyzed ERK1 samples. ERK1 is a mitogen-activated protein kinase that is strongly activated in response to various extracellular stimuli and plays a pivotal role in mediating signal transduction (34). Activation of ERK1 is achieved through its phosphorylation on two conserved residues, Thr202 and Tyr204, that are located within its activation loop (35). Therefore, ERK1 provides an adequate model protein to distinguish sulfation artifacts from real phosphorylation events within a single protein. ERK1 samples isolated from HEK293 cells were separated by SDS-PAGE and then stained with either silver or Coomassie Blue. Protein bands were excised, digested with trypsin, and analyzed by MS. We obtained sequence coverage of 59% (23 peptides) and 69% (26 peptides) for silver- and Coomassie Blue-stained ERK1, respectively. Among the 23 peptides detected in the silver-stained band of ERK1, we identified seven peptides bearing a +80-Da modification (Table II). The precise mass of each detected peptide was measured and compared with the calculated mass values for the corresponding phospho- and sulfopeptides. The mass of five peptides (157YIHSANVLHR166, 167DLKPSNLLINTTCDLK182, 213APEIMLNSK221, 311MLTFNPNKR319, and 361LKELIFQETAR371) were in close agreement with the expected mass for sulfopeptides. Interestingly one additional peptide exhibiting a +80-Da mass shift, 192IADPEHTGFLTEYVATR208, eluted at 28.5 and 30.7 min. These two species correspond to phosphorylated and sulfated analogs, respectively (Fig. 4a). As shown in Table II, the peptide eluting at 28.5 min had a measured mass closer to that of the phosphorylated form (−1.7 ppm) than the sulfated form (2.5 ppm). Conversely the peptide eluting at 30.7 min was assigned to a sulfopeptide (−1.4 ppm) and not to the corresponding phosphopeptide because of the larger mass difference (−5.7 ppm). MS/MS analyses of the peptide eluting at 28.5 min showed an abundant fragment ion at m/z 1077.762+ corresponding to the loss of 98 Da (−H3PO4) from the precursor ion together with low intensity fragment ions enabling the sequence assignment and the identification of the phosphorylation site on Thr202 (Fig. 4b). Phosphorylation at this residue represents one of two known phosphorylation sites (Thr202 and Tyr204) that can be independently phosphorylated in ERK1 (36). The observed fragmentation pattern, particularly the loss of 98 Da, is characteristic for phosphopeptides and is generally seen when Ser and Thr residues are phosphorylated. Fig. 4c shows the MS/MS spectrum of the sulfopeptide eluting at 30.7 min where an abundant loss of 80 Da (−SO3) from the precursor ion at m/z 1086.782+ is observed instead of the 98-Da loss (H3PO4) commonly detected for phosphopeptides. In addition, no fragment ion allowing the sequencing of the peptide was detected in striking contrast to the phosphopeptide fragmentation. Hence the sulfopeptide required a subsequent MS3 analysis for proper sequence assignment (Fig. 4d). The five remaining sulfopeptides also produced a neutral loss of 80 Da from the precursor ion. These are presented as supplemental material. We performed β-elimination by alkaline hydrolysis using Ba(OH)2 to identify the exact residues that were sulfated. The assignment of sulfation sites based on these analyses is shown in Table II. Results obtained using the phosphoprotein ERK1 demonstrate that differentiation between the sulfation artifact and real phosphorylation is relatively straightforward using instruments that provide high mass accuracy. Otherwise manual validation of the MS/MS spectra along with the detection of the signature [M + 2H − 98]2+ fragment peak is required for phosphopeptide assignment.
Table II.
Identification of modified peptides from ERK1
Modified residues are bold. The rows with the dashes correspond to the unmodified peptides. sS, sulfated serine; pT, phosphorylated threonine; sT, sulfated threonine; C(carb), carbamidomethylated cysteine; RT, retention time.
| Peptide sequence | RT | Observed mass | Calculated mass | ΔMassa | Modificationb |
|---|---|---|---|---|---|
| min | Da | Da | ppm | Da | |
| APEIMLNsSK | 28.2 | 1081.4799 | 1081.4784 | 1.4 | 79.9563 |
| APEIMLNSK | 26.5 | 1001.5236 | 1001.5215 | 2.1 | — |
| MLsTFNPNKR | 31.6 | 1199.5462 | 1199.5427 | 2.9 | 79.9587 |
| MLTFNPNKR | 27.3 | 1119.5875 | 1119.5859 | 1.4 | — |
| YIHsSANVLHR | 23.8 | 1288.6003 | 1288.5982 | 1.6 | 79.9564 |
| YIHSANVLHR | 20.6 | 1208.6439 | 1208.6414 | 2.1 | — |
| LKELIFQEsTAR | 35.2 | 1426.7165 | 1426.7126 | 2.7 | 79.9587 |
| LKELIFQETAR | 33.2 | 1346.7578 | 1346.7558 | 1.5 | — |
| DLKPSNLLINTsTC(carb)DLK | 42.6 | 1923.9315 | 1923.9281 | 1.8 | 79.9585 |
| DLKPSNLLINTTC(carb)DLK | 35.5 | 1843.9730 | 1843.9713 | 0.9 | — |
| IADPEHDHTGFLsTEYVATR | 30.7 | 2250.9819 | 2250.9851 | −1.4 | 79.9558 |
| IADPEHDHTGFLpTEYVATR | 28.5 | 2250.9907 | 2250.9946 | −1.7 | 79.9646 |
| IADPEHDHTGFLTEYVATR | 30.9 | 2171.0261 | 2171.0283 | −1.0 | — |
Difference between experimental and calculated masses.
Mass difference between modified and unmodified peptide (phosphate, 79.9663; sulfate, 79.9568).
Fig. 4.
Identification of modified peptides derived from ERK1. a, reconstructed ion chromatogram of m/z 1126.50 from silver-stained gel bands. b, first generation product ion spectrum (MS2) of m/z 1126.50 eluting at 28.5 min. c, first generation product ion spectrum of m/z 1126.50 eluting at 30.7 min. d, second generation product ion spectrum (MS3) of m/z 1086.78 obtained from precursor 1126.50 eluting at 30.7 min.
Sulfation Artifacts in Silver-stained Bacterial Proteins—
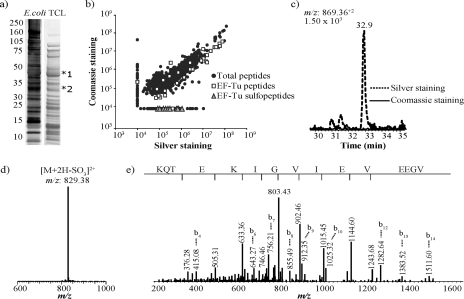
To determine whether the sulfation artifact occurs in a broad range of proteins, protein extracts from E. coli were resolved by SDS-PAGE and stained either with silver or Coomassie Blue. The most prominent bands at 45 (band 1) and 33 kDa (band 2) were excised from silver- and Coomassie Blue-stained gels and digested with trypsin prior to LC-MS/MS analyses (Fig. 5a). Mascot search results provided a list of all the identified proteins in each of the protein gel bands. A script was developed to detect all MS2 spectra containing a neutral loss of 80 Da from assigned and unassigned mass spectra. Sulfopeptides were searched for in the five most abundant proteins in the list. Some of these assignments were based exclusively on peptide mass fingerprints and the presence of a neutral loss of 80 Da in the MS2 spectra. In addition, others were obtained from their MS3 spectra, thus enabling sequencing of the peptide and a more direct confirmation of the identity of the protein of origin (supplemental material). Analysis of the silver-stained band at ∼45-kDa molecular mass revealed the presence of nine sulfopeptides (Fig. 5, b–e). It is noteworthy that no sulfopeptide was detected from the Coomassie-stained gel of the corresponding band. All detected sulfopeptides corresponded to elongation factor Tu (EF-Tu), an abundant 43-kDa protein that plays an essential role in protein biosynthesis (37). However, to our knowledge, no EF-Tu sulfation in bacteria has been reported thus far. To compare EF-Tu sulfation in silver- and Coomassie Blue-stained samples, all unique peptide identifications with Mascot scores above 25 were clustered together. All the observed changes are represented in a scatter plot (Fig. 5b). The comparison of peptides from silver- and Coomassie Blue-stained bands indicated that 84% (1115) of all 1329 ion clusters (including unmodified EF-Tu ions) showed less than a 2-fold change in abundance between the two conditions, whereas 15% (205) of these same ion clusters showed, on average, a change in abundance of 56-fold. This inconsistency in peptide detection for unaffected peptides across the two conditions can be explained by the fact that reproducible cutting of gel bands at equal molecular weights cannot be fully achieved without variations because of manual handling of the gels. Nevertheless because the vast majority of peptides are reproducibly detected in both samples and unmodified EF-Tu peptides are equally present in both conditions, the abundance of EF-Tu sulfopeptides in silver- and Coomassie Blue-stained samples can be efficiently compared. A comparison of the proportion of sulfated and unmodified peptides from EF-Tu indicated that less than 1% (nine) of total peptides was identified as EF-Tu sulfopeptides in the silver-stained sample. For instance, the reconstructed ion chromatogram for the sulfopeptide VGEEVEIVGIKETQK at m/z 869.36+2 that eluted at 32.9 min and displayed a prominent loss of 80 Da in the silver-stained sample was completely absent in the Coomassie-stained condition (Fig. 5, c–e). Accurate mass measurements further confirmed that all modified residues bearing a +80-Da shift corresponded to sulfation artifacts. Finally the analysis of silver and Coomassie Blue staining-derived samples for the gel band at 33 kDa also identified several proteins with sulfopeptides that were specifically present in the silver-stained samples (supplemental material).
Fig. 5.
Identification of sulfopeptide artifacts from silver- and Coomassie Blue-stained gels of E. coli total cell lysate (TCL). a, silver- (left lane) and Coomassie Blue (right lane)-stained gels. b, scatter plot distribution of ion intensities for the peptides identified from silver- and Coomassie band-stained band 1 (shown in a). c, reconstructed ion chromatogram of m/z 869.36 from silver- and Coomassie Blue-stained gel bands. The m/z 869.36 ion was undetectable in the Coomassie Blue-stained sample. d, first generation (MS2) product ion spectrum of m/z 869.36. e, second generation (MS3) product ion spectrum of m/z 829.38 obtained from precursor m/z 869.36.
DISCUSSION
In this study, we report the identification of sulfation as a chemical modification artifact introduced during silver staining of proteins separated by polyacrylamide gel electrophoresis. The analysis of enolase, ERK1, and proteins from bacterial cell lysates derived from silver-stained gels all showed several peptides presenting a +80-Da modification on Ser, Thr, and Tyr residues. Sulfated peptides were not found in samples derived from Coomassie Blue-stained gels. The occurrence of this artifact is of particular concern for studies requiring the identification of protein phosphorylation sites using silver-stained gels, and appropriate staining procedures must be used to avoid ambiguous assignment.
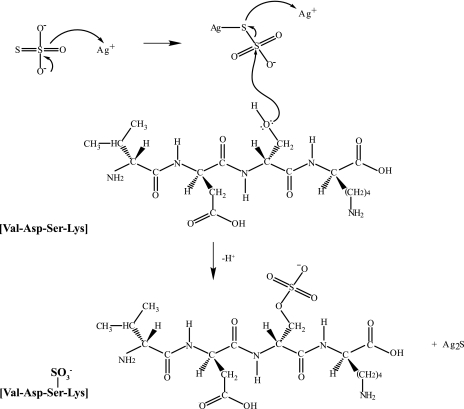
To identify and characterize post-translational modifications of specific proteins, most proteomics laboratories require high resolution separation methods, such as one- or two-dimensional polyacrylamide gel electrophoresis in combination with mass spectrometry. Sensitive gel staining methods enabling the detection of proteins present at low nanogram levels are necessary for most proteomics applications (38). For this purpose, silver staining is a versatile and MS-compatible method providing low nanogram detection sensitivity (21). We have shown that sulfation of specific residues does occur during the silver staining procedure due to sodium thiosulfate, a reducing agent used to sensitize the gel prior to the addition of silver nitrate, thus enhancing the sensitivity and contrast of the staining (39). Formaldehyde, an alternative reagent commonly used in silver staining, generates intramolecular cross-links, leading to low peptide recovery for MS analysis. Formaldehyde also gives rise to formylation artifacts on lysine residues (24). The chemistry of silver staining is well understood and is mediated through the binding of silver ions to amino acid side chains containing sulfhydryl and carboxyl groups with subsequent reduction to free metallic silver under alkaline conditions (39–41). In addition, the terminal sulfur atom of sodium thiosulfate has a high affinity for soft metals such as silver and forms a reactive metallic intermediate (42). Accordingly we propose that, during the staining procedure, the interaction of silver ions with sodium thiosulfate might activate this reducing reagent prior to subsequent nucleophilic reactions with free hydroxyl groups of Ser, Thr, and Tyr residues. A proposed mechanism is presented in Fig. 6. As a result, this reaction would promote the chemical modification of residues through sulfation while forming a stable Ag2S complex necessary for visualizing gel-separated protein bands.
Fig. 6.
Proposed mechanism for sulfation of hydroxylated amino acids in silver-stained gels. Sodium thiosulfate reduces silver ions to form a reactive metallic intermediate that is then subject to nucleophilic attack by the lone electron pairs of hydroxylated residues (shown here for Ser). The thiosulfate intermediate can reduce a second silver ion proximal to acidic residues to produce Ag2S necessary for visualizing the gel-separated protein and liberate a sulfated peptide.
It is noteworthy that sulfation appears to preferentially modify specific hydroxylated amino acids among all available Ser, Thr, and Tyr residues. For example, yeast enolase contains a total of 64 hydroxyamino acids (32 Ser, 19 Thr, and 13 Tyr residues), but only 14 (eight Ser, five Thr, and one Tyr residue) were detectably sulfated following silver staining (Table I). This could be explained in part by the local environment influencing the nucleophilic substituents and hence the reactivity of the hydroxyl group of Ser, Thr, and Tyr side chains such as observed previously for ɛ-methyl-Lys residues of calmodulin (43). Moreover the occurrence of protein sulfation from silver staining is likely influenced by the binding of silver ions to individual amino acids. Indeed previous reports indicated that silver ions bind predominantly to sulfhydryl and carboxylate moieties (39, 40), suggesting that Ser, Thr, and Tyr proximal to Asp, Glu, Met, and Cys residues may have a higher propensity to be sulfated. We examined the distribution of six residues on either side of the sulfated amino acid identified in enolase and ERK1 using WebLogo (44). These analyses revealed that a higher frequency of acidic residues was found proximal to the modified site (supplemental material). Obviously these consensus motif analyses are limited to the linear sequence immediately next to the modified residues, and additional neighboring amino acids might contribute to sulfation depending on the local protein conformation, notwithstanding the fact that silver staining is usually performed after electrophoresis in the presence of SDS. The requirement of proximal sulfhydryl or carboxylate moieties for the sulfation of neighboring hydroxylated residues also suggests that proteins rich in basic amino acids would be less susceptible to undergo protein sulfation.
Sulfation also changes the polarity of hydroxylated residues, rendering them more susceptible to form ionic interactions with nearby ionizable moieties. In most cases that we examined, we noticed that sulfopeptides generally eluted 1–5 min later than their unmodified counterparts (Table I and Table II). This observation suggests that sulfopeptides are generally more hydrophobic than their unmodified peptides under the present elution conditions (aqueous ACN, 0.2% FA, pH 2.5). This could possibly be explained by the formation of salt bridges between the sulfonyl group and adjacent basic moieties (N-terminal NH2; internal His, Lys, or Arg; and/or C-terminal Lys or Arg residues) similar to those observed previously for phosphopeptides (45). The extent of the retention time shift could also be influenced by secondary structural changes on the conformation of the modified peptide.
We also evaluated the degree of modification on yeast enolase by comparing the relative abundance of the sulfopeptides with their corresponding unmodified counterparts. We determined that the population of modified peptides represents ∼2% of the unmodified peptides based on average intensity ratios, suggesting that protein sulfation is a relatively low yield side reaction under the present silver staining conditions (0.02% sodium thiosulfate). Analyses of E. coli extracts also revealed that only the most abundant proteins identified by Mascot showed peptides bearing this modification. Taken together, these results indicate that the extent of sulfation artifacts detectable from silver-stained gels depends not only on the amino acid distribution but also on the relative proportion and abundance of a given protein within a band or spot. Consequently protein sulfation should be taken into consideration when analyzing silver-stained bands or spots derived from partially purified samples obtained by immunoaffinity or conventional protein purification.
Protein sulfation caused by silver staining can also be problematic when trying to identify low abundance phosphopeptides from gel-isolated bands. Indeed the transient nature of protein phosphorylation in vivo can result in purified samples where less than 1–2% of the protein is phosphorylated (46). Consequently protein sulfation caused by silver staining can possibly interfere with the identification of genuine phosphorylation sites. Phosphopeptide isolation methods using IMAC or TiO2 media are also likely to enrich indiscriminately isobaric sulfopeptides and phosphopeptides from in-gel tryptic digests, thus rendering their distinction even more challenging. A case in point is the identification of modified residues from ERK1 where we identified seven peptides bearing the 80-Da modification, but only one (Thr202) was a genuine phosphorylation site located within the activation loop of the kinase. The observation of these low abundance modified peptides could easily have been erroneously assigned to phosphorylation in the absence of further structural validation.
The possibility of obtaining accurate mass measurements together with first and second generation fragment ion spectra within the same LC-MS analysis was essential for the correct assignment of modified peptides. The use of a lock mass on the Orbitrap instrument enabled mass measurements within 5 ppm of the calculated values thus making it possible to distinguish between sulfation and phosphorylation as shown here for two isobaric ERK1 modified peptides. Obviously identification based on mass measurements would not be possible with lower resolution and mass accuracy instruments, and proper differentiation between these two isobaric modifications would rely only on distinct fragmentation patterns.
The fragmentation behavior of phosphopeptides and sulfopeptides has been studied extensively over the past years (15, 47–50). Cleavage of the phosphoester bond leading to a loss of H3PO4 through β-elimination is observed under collisional activation except for phosphorylated Tyr residues that typically retain this modification. Characteristic fragment ions displaying a +80- or a −18-Da shift compared with the unmodified peptide serve as hallmark ions to identify the modification sites of phosphopeptides containing Ser and Thr residues. In contrast, sulfopeptides examined as part of this study all exhibited an abundant loss of SO3 (−80 Da) from the precursor ion with very few sequence-specific fragment ions. Although this fragmentation feature can be used to identify potential sulfopeptides, the sequence of the corresponding peptide could only be revealed following second generation product ion spectra (MS3). However, the prompt loss of SO3 from the precursor ion leaves an intact Ser or Thr residue with fragmentation features identical to that of the unmodified peptide (data not shown). Although collisional activation dissipates sufficient vibrational energy in the product ions to favor dissociation of the labile SO3 group, a recent study indicated that sulfopeptides are also more susceptible to undergo this neutral loss during electron capture dissociation (ECD) compared with their corresponding phosphopeptide analogs (48). This loss was less prominent using electron transfer dissociation (ETD), although several fragments ions were shown to eliminate SO3 (48, 50). Interestingly the replacement of the sulfate proton with an alkali ion such as Na+ appeared to stabilize the prompt loss of SO3, thereby favoring the identification of the modification site in ECD or ETD experiments (48). However, ECD or ETD product ion spectra of modified peptides display c- and z-fragment ions bearing the intact sulfated or phosphorylated moieties and could lead to misassignment of phosphopeptides during the analysis of in-gel digests of silver-stained bands.
Finally the mistaken assignment of artifact sulfopeptides as real phosphorylated peptides is greatly dependent on the type of MS system used to perform the analysis. It is noteworthy that collisional activation of sulfopeptides performed using the LTQ-Orbitrap and Q-TOF instruments generated product ion spectra that greatly differ in their peptide fragmentation patterns. For example, MS2 spectra acquired on the LTQ-Orbitrap are dominated by an abundant neutral loss of SO3 (−80 Da). Sequence assignment of the modified peptide therefore requires an additional step of isolation and fragmentation (MS3). On the other hand, MS2 spectra of sulfopeptides obtained from a Q-TOF instrument display extensive fragmentation along the peptide backbone in addition to the neutral loss of SO3 (supplemental material). Search engines such as Mascot use probability scores to match peptide masses as well as MS2 fragment ion masses to theoretical protein sequences from databases. Consequently sulfopeptides analyzed on the ion trap will have very low scores and will remain unassigned after database searching. The possibility of identifying silver stain-induced sulfopeptide artifacts and misassigning them as phosphopeptides is greatly reduced when analyses are performed on the LTQ-Orbitrap.
In view of the frequent occurrence of sulfation artifacts and the wide use of colloidal silver as a stain compatible with MS identification of gel-separated proteins, users must be aware of this specific problem to avoid misinterpretation of results. This is particularly true for the identification of low abundance phosphopeptides or genuine sulfopeptides that can be isolated from in-gel digests of silver-stained bands where the sulfation artifacts can be easily misidentified as the more common isobaric modification. Alternate stains such as Coomassie Blue or SYPRO dyes that do not require thiosulfate reagents could be used to minimize this artifact when characterizing phosphopeptides or sulfopeptides from in-gel digests. The accessibility of MS equipment providing high accuracy mass measurements (<5 ppm) combined with sensitive multistage fragment ion spectra is critical to obtain unambiguous assignment.
Acknowledgments
The IRIC receives infrastructure support funds from the Fonds de la Recherche en Santé du Québec (FRSQ) and from a Canadian Institutes for Health Research multiresource grant.
Footnotes
Published, MCP Papers in Press, October 20, 2008, DOI 10.1074/mcp.M800327-MCP200
The abbreviations used are: FA, formic acid; ECD, electron capture dissociation; ETD, electron transfer dissociation; bis-Tris, 2-[bis(2-hydroxyethyl)amino]-2-(hydroxymethyl)propane-1,3-diol; EF-Tu, elongation factor Tu.
This work was supported by operating grants from the National Science and Engineering Research Council (to P. T.), the Canada Research Chair program (to P. T., A. V., and S. M.), and the National Cancer Institute of Canada (to S. M.).
The on-line version of this article (available at http://www.mcponline.org) contains supplemental material.
REFERENCES
- 1.Olsen, J. V., Blagoev, B., Gnad, F., Macek, B., Kumar, C., Mortensen, P., and Mann, M. ( 2006) Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell 127, 635–648 [DOI] [PubMed] [Google Scholar]
- 2.Rikova, K., Guo, A., Zeng, Q., Possemato, A., Yu, J., Haack, H., Nardone, J., Lee, K., Reeves, C., Li, Y., Hu, Y., Tan, Z., Stokes, M., Sullivan, L., Mitchell, J., Wetzel, R., Macneill, J., Ren, J. M., Yuan, J., Bakalarski, C. E., Villen, J., Kornhauser, J. M., Smith, B., Li, D., Zhou, X., Gygi, S. P., Gu, T. L., Polakiewicz, R. D., Rush, J., and Comb, M. J. ( 2007) Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 131, 1190–1203 [DOI] [PubMed] [Google Scholar]
- 3.Villen, J., Beausoleil, S. A., Gerber, S. A., and Gygi, S. P. ( 2007) Large-scale phosphorylation analysis of mouse liver. Proc. Natl. Acad. Sci. U. S. A. 104, 1488–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh, C. T. ( 2006) Posttranslational Modification of Proteins: Expanding Nature's Inventory, p. 33, Roberts and Co. Publishers, Greenwood Village, CO
- 5.Zolnierowicz, S., and Bollen, M. ( 2000) Protein phosphorylation and protein phosphatases. De Panne, Belgium, September 19–24, 1999. EMBO J. 19, 483–488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mann, M., Ong, S. E., Gronborg, M., Steen, H., Jensen, O. N., and Pandey, A. ( 2002) Analysis of protein phosphorylation using mass spectrometry: deciphering the phosphoproteome. Trends Biotechnol. 20, 261–268 [DOI] [PubMed] [Google Scholar]
- 7.Bonenfant, D., Schmelzle, T., Jacinto, E., Crespo, J. L., Mini, T., Hall, M. N., and Jenoe, P. ( 2003) Quantitation of changes in protein phosphorylation: a simple method based on stable isotope labeling and mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 100, 880–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McLachlin, D. T., and Chait, B. T. ( 2001) Analysis of phosphorylated proteins and peptides by mass spectrometry. Curr. Opin. Chem. Biol. 5, 591–602 [DOI] [PubMed] [Google Scholar]
- 9.Hunter, T. ( 2000) Signaling—2000 and beyond. Cell 100, 113–127 [DOI] [PubMed] [Google Scholar]
- 10.Strott, C. A. ( 2002) Sulfonation and molecular action. Endocr. Rev. 23, 703–732 [DOI] [PubMed] [Google Scholar]
- 11.Moore, K. L. ( 2003) The biology and enzymology of protein tyrosine O-sulfation. J. Biol. Chem. 278, 24243–24246 [DOI] [PubMed] [Google Scholar]
- 12.Monigatti, F., Hekking, B., and Steen, H. ( 2006) Protein sulfation analysis—a primer. Biochim. Biophys. Acta 1764, 1904–1913 [DOI] [PubMed] [Google Scholar]
- 13.Yu, Y., Hoffhines, A. J., Moore, K. L., and Leary, J. A. ( 2007) Determination of the sites of tyrosine O-sulfation in peptides and proteins. Nat. Methods 4, 583–588 [DOI] [PubMed] [Google Scholar]
- 14.Kehoe, J. W., and Bertozzi, C. R. ( 2000) Tyrosine sulfation: a modulator of extracellular protein-protein interactions. Chem. Biol. 7, R57–R61 [DOI] [PubMed] [Google Scholar]
- 15.Medzihradszky, K. F., Darula, Z., Perlson, E., Fainzilber, M., Chalkley, R. J., Ball, H., Greenbaum, D., Bogyo, M., Tyson, D. R., Bradshaw, R. A., and Burlingame, A. L. ( 2004) O-Sulfonation of serine and threonine: mass spectrometric detection and characterization of a new posttranslational modification in diverse proteins throughout the eukaryotes. Mol. Cell. Proteomics 3, 429–440 [DOI] [PubMed] [Google Scholar]
- 16.Witze, E. S., Old, W. M., Resing, K. A., and Ahn, N. G. ( 2007) Mapping protein post-translational modifications with mass spectrometry. Nat. Methods 4, 798–806 [DOI] [PubMed] [Google Scholar]
- 17.Baldwin, M. A. ( 2004) Protein identification by mass spectrometry: issues to be considered. Mol. Cell. Proteomics 3, 1–9 [DOI] [PubMed] [Google Scholar]
- 18.Mann, M., and Jensen, O. N. ( 2003) Proteomic analysis of post-translational modifications. Nat. Biotechnol. 21, 255–261 [DOI] [PubMed] [Google Scholar]
- 19.Shevchenko, A., Tomas, H., Havlis, J., Olsen, J. V., and Mann, M. ( 2006) In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 1, 2856–2860 [DOI] [PubMed] [Google Scholar]
- 20.Graham, D. R., Elliott, S. T., and Van Eyk, J. E. ( 2005) Broad-based proteomic strategies: a practical guide to proteomics and functional screening. J. Physiol. 563, 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. ( 1996) Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858 [DOI] [PubMed] [Google Scholar]
- 22.Richert, S., Luche, S., Chevallet, M., Van Dorsselaer, A., Leize-Wagner, E., and Rabilloud, T. ( 2004) About the mechanism of interference of silver staining with peptide mass spectrometry. Proteomics 4, 909–916 [DOI] [PubMed] [Google Scholar]
- 23.Metz, B., Kersten, G. F., Baart, G. J., de Jong, A., Meiring, H., ten Hove, J., van Steenbergen, M. J., Hennink, W. E., Crommelin, D. J., and Jiskoot, W. ( 2006) Identification of formaldehyde-induced modifications in proteins: reactions with insulin. Bioconjug. Chem. 17, 815–822 [DOI] [PubMed] [Google Scholar]
- 24.Oses-Prieto, J. A., Zhang, X., and Burlingame, A. L. ( 2007) Formation of ɛ-formyllysine on silver-stained proteins: implications for assignment of isobaric dimethylation sites by tandem mass spectrometry. Mol. Cell. Proteomics 6, 181–192 [DOI] [PubMed] [Google Scholar]
- 25.Jaitly, G., Bonneil, E., Jaitly, N., Eng, K., Pomiès, C., and Thibault, P. ( 2007) Comprehensive profiling of unlabeled peptide ions from large-scale proteomics experiments using one and two dimensional nanoLC-MS/MS, in Proceedings of the 55th ASMS Conference on Mass Spectrometry, Indianapolis, June 3–7, 2007, Indianapolis, IN
- 26.Oda, Y., Nagasu, T., and Chait, B. T. ( 2001) Enrichment analysis of phosphorylated proteins as a tool for probing the phosphoproteome. Nat. Biotechnol. 19, 379–382 [DOI] [PubMed] [Google Scholar]
- 27.Thompson, A. J., Hart, S. R., Franz, C., Barnouin, K., Ridley, A., and Cramer, R. ( 2003) Characterization of protein phosphorylation by mass spectrometry using immobilized metal ion affinity chromatography with on-resin β-elimination and Michael addition. Anal. Chem. 75, 3232–3243 [DOI] [PubMed] [Google Scholar]
- 28.Greis, K. D., Hayes, B. K., Comer, F. I., Kirk, M., Barnes, S., Lowary, T. L., and Hart, G. W. ( 1996) Selective detection and site-analysis of O-GlcNAc-modified glycopeptides by beta-elimination and tandem electrospray mass spectrometry. Anal. Biochem. 234, 38–49 [DOI] [PubMed] [Google Scholar]
- 29.Wells, L., Vosseller, K., Cole, R. N., Cronshaw, J. M., Matunis, M. J., and Hart, G. W. ( 2002) Mapping sites of O-GlcNAc modification using affinity tags for serine and threonine post-translational modifications. Mol. Cell. Proteomics 1, 791–804 [DOI] [PubMed] [Google Scholar]
- 30.Chalmers, M. J., Kolch, W., Emmett, M. R., Marshall, A. G., and Mischak, H. ( 2004) Identification and analysis of phosphopeptides. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 803, 111–120 [DOI] [PubMed] [Google Scholar]
- 31.Byford, M. F. ( 1991). Rapid and selective modification of phosphoserine residues catalysed by Ba2+ ions for their detection during peptide microsequencing. Biochem. J. 280, 261–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li, W., Backlund, P. S., Boykins, R. A., Wang, G., and Chen, H. C. ( 2003) Susceptibility of the hydroxyl groups in serine and threonine to β-elimination/Michael addition under commonly used moderately high-temperature conditions. Anal. Biochem. 323, 94–102 [DOI] [PubMed] [Google Scholar]
- 33.Chevallet, M., Luche, S., and Rabilloud, T. ( 2006) Silver staining of proteins in polyacrylamide gels. Nat. Protoc. 1, 1852–1858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roux, P. P., and Blenis, J. ( 2004) ERK and p38 MAPK-activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol. Mol. Biol. Rev. 68, 320–344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Butch, E. R., and Guan, K. L. ( 1996) Characterization of ERK1 activation site mutants and the effect on recognition by MEK1 and MEK2. J. Biol. Chem. 271, 4230–4235 [DOI] [PubMed] [Google Scholar]
- 36.Ballif, B. A., Roux, P. P., Gerber, S. A., MacKeigan, J. P., Blenis, J., and Gygi, S. P. ( 2005) Quantitative phosphorylation profiling of the ERK/p90 ribosomal S6 kinase-signaling cassette and its targets, the tuberous sclerosis tumor suppressors. Proc. Natl. Acad. Sci. U. S. A. 102, 667–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lippmann, C., Lindschau, C., Vijgenboom, E., Schroder, W., Bosch, L., and Erdmann, V. A. ( 1993) Prokaryotic elongation factor Tu is phosphorylated in vivo. J. Biol. Chem. 268, 601–607 [PubMed] [Google Scholar]
- 38.Gharahdaghi, F., Weinberg, C. R., Meagher, D. A., Imai, B. S., and Mische, S. M. ( 1999) Mass spectrometric identification of proteins from silver-stained polyacrylamide gel: a method for the removal of silver ions to enhance sensitivity. Electrophoresis 20, 601–605 [DOI] [PubMed] [Google Scholar]
- 39.Rabilloud, T. ( 1990) Mechanisms of protein silver staining in polyacrylamide gels: a 10-year synthesis. Electrophoresis 11, 785–794 [DOI] [PubMed] [Google Scholar]
- 40.Granvogl, B., Ploscher, M., and Eichacker, L. A. ( 2007) Sample preparation by in-gel digestion for mass spectrometry-based proteomics. Anal. Bioanal. Chem. 389, 991–1002 [DOI] [PubMed] [Google Scholar]
- 41.Patras, G., Qiao, G. G., and Solomon, D. H. ( 1999) On the mechanism of background silver staining during sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Electrophoresis 20, 2039–2045 [DOI] [PubMed] [Google Scholar]
- 42.Herrmann, W. A. ( 1986). Multiple bonds between transition metals and “bare” main group elements: links between inorganic solid state chemistry and organometallic chemistry. Angew. Chem. Int. Ed. Engl. 25, 56–76 [Google Scholar]
- 43.Zhang, M., and Vogel, H. J. ( 1993) Determination of the side chain pKa values of the lysine residues in calmodulin. J. Biol. Chem. 268, 22420–22428 [PubMed] [Google Scholar]
- 44.Crooks, G. E., Hon, G., Chandonia, J. M., and Brenner, S. E. ( 2004) WebLogo: a sequence logo generator. Genome Res. 14, 1188–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marcantonio, M., Trost, M., Courcelles, M., Desjardins, M., and Thibault, P. ( 2008) Combined enzymatic and data mining approaches for comprehensive phosphoproteome analyses: application to cell signaling events of interferon-γ-stimulated macrophages. Mol. Cell. Proteomics 7, 645–660 [DOI] [PubMed] [Google Scholar]
- 46.Reinders, J., and Sickmann, A. ( 2005) State-of-the-art in phosphoproteomics. Proteomics 5, 4052–4061 [DOI] [PubMed] [Google Scholar]
- 47.DeGnore, J. P., and Qin, J. ( 1998) Fragmentation of phosphopeptides in an ion trap mass spectrometer. J. Am. Soc. Mass Spectrom. 9, 1175–1188 [DOI] [PubMed] [Google Scholar]
- 48.Medzihradszky, K. F., Guan, S., Maltby, D. A., and Burlingame, A. L. ( 2007) Sulfopeptide fragmentation in electron-capture and electron-transfer dissociation. J. Am. Soc. Mass Spectrom. 18, 1617–1624 [DOI] [PubMed] [Google Scholar]
- 49.Nemeth-Cawley, J. F., Karnik, S., and Rouse, J. C. ( 2001) Analysis of sulfated peptides using positive electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 36, 1301–1311 [DOI] [PubMed] [Google Scholar]
- 50.Syka, J. E., Coon, J. J., Schroeder, M. J., Shabanowitz, J., and Hunt, D. F. ( 2004) Peptide and protein sequence analysis by electron transfer dissociation mass spectrometry. Proc. Natl. Acad. Sci. U. S. A. 101, 9528–9533 [DOI] [PMC free article] [PubMed] [Google Scholar]