Abstract
Signaling from Gi/o-coupled G protein–coupled receptors (GPCRs), such as the serotonin 1B, cannabinoid 1, and dopamine D2 receptors, inhibits cAMP production by adenylyl cyclases and activates protein kinases, such as Src, mitogen-activated protein kinases 1 and 2, and Akt. Activation of these protein kinases results in stimulation of neurite outgrowth in the central nervous system (CNS) and in neuronal cell lines. This Connections Map traces downstream signaling pathways from Gi/o-coupled GPCRs to key protein kinases and key transcription factors involved in neuronal differentiation. Components in the Science Signaling Connections Map are linked to Nature Molecule Pages. This interoperability provides ready access to detail that includes information about specific states for the nodes.
Overview
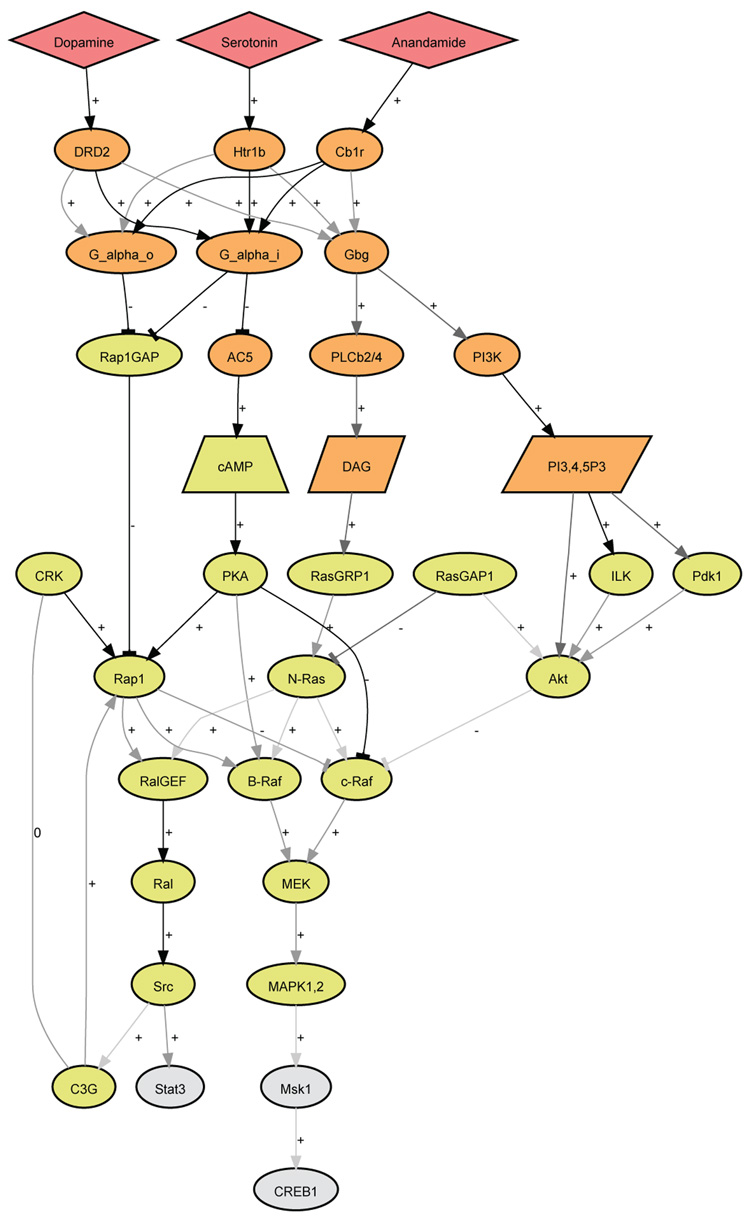
This Connections Map describes signaling from three G protein–coupled receptors (GPCRs): the serotonin 1B (Htr1b), the cannabinoid 1 (CB1R), and the dopamine D2 (Drd2) receptors, which are coupled to Gi/o-type G proteins (Fig. 1). Signaling by these three GPCRs through Gi/o inhibits cAMP production and also regulates other downstream effectors through Gα and Gβγ subunits. Through the heterotrimeric G protein subunits, these receptors activate key protein kinases, such as Src, mitogen-activated protein kinases 1 and 2 (MAPK1,2), and Akt. Activation of these protein kinases in turn stimulates the two transcription factor regulators cAMP response element–binding protein (CREB) and Stat3. This network is known to stimulate neurite outgrowth in the central nervous system (CNS) and in neuronal cell lines. Stimulation of these receptors initiates the transcriptional program required for neuronal differentiation, which starts with neurite outgrowth. This network illustrates downstream signaling pathways that are common to these receptors, and activation of any one of these receptors can stimulate neurite outgrowth. The primary literature associated with each relation and node comes from studies of CNS neurons in rodents, as well as cultured neurons from rodents or neuronally derived mammalian cell lines, such as the mouse neuroblastoma cell line Neuro2A. The pathway is based on network analysis that assembled interactions from sparse functional studies. It is not certain that all these connections function together to form pathways and networks in all neuronal cell lines, for example, Neuro2A cells. However, the individual links themselves are well established to be present and functional in different but similar settings.
Fig. 1.
Pathway image captured from the dynamic graphical display of the information in the Database of Cell Signaling, available 23 December 2008. For a key to the colors and symbols and to access the underlying data, please visit the pathway (http://stke.sciencemag.org/cgi/cm/stkecm;CMP_20329).
Neurite Outgrowth
During development, neurons make connections with other neurons, and such connections are assembled into functional networks by growing axons and dendrites. Axons and dendrites originating from differentiating neuronal progenitor cells are collectively called neurites. Neurite out-growth is regulated by an array of extracellular cues that signal to cells instructions that are translated into phenotypic changes in cell shape and function. The molecular mechanisms underlying this phenomenon are of interest in neuroscience. However, although many components and interactions have been discovered in this well-studied system, much is still unknown. Neuronal differentiation is a complex process whereby neuronal precursors undergo morphological changes, such as the development of dendrites and an axon, as well as develop the capacity to send and receive electrical signals. These alterations are initiated through changes in the expression of genes that encode a range of proteins, including synaptic proteins involved in neurotransmitter synthesis and release. Neurites develop in differentiating neurons initially as processes arising from cytoskeletal rearrangement; subsequently, the neurites differentiate into dendrites and axons. Neurite outgrowth is commonly used in the laboratory for monitoring early neuronal differentiation of cultured neuronal cell lines. PC12 cells, a rat pheochromocytoma cell line, and Neuro2A cells, a mouse neuroblastoma cell line, are commonly used model systems to study the pathways involved in early neuronal differentiation and the interactions between cell signaling pathways (1, 2). The Differentiation Pathway in PC12 Cells (http://stke.sciencemag.org/cgi/cm/stkecm;CMP_8038) illustrates how input from a receptor tyrosine kinase (RTK), TrkA, and a Gs-coupled G protein–coupled receptor (GPCR), the pituitary adenylate cyclase–activating peptide (PACAP) receptor PAC1R, stimulate complementary pathways leading to neuronal differentiation of PC12 cells. PC12 and Neuro2A cells can be driven to differentiate by specific ligands. For example, nerve growth factor (NGF), a neurotrophin and an agonist of TrkA, triggers PC12 cells to differentiate (3–5), and HU-210, a cannabinoid receptor agonist, triggers Neuro2A to differentiate (6).
G Protein–Coupled Receptors and Heterotrimeric G Proteins
The receptor commonly associated with stimulation of neurite outgrowth and neuronal differentiation is TrkA, the receptor for NGF. GPCRs also stimulate neurite outgrowth by distinct mechanisms (7). Activation of several Gi/o-coupled receptors drives CNS neuronal cells to differentiate. For example, neuronal outgrowth was induced in cultured cortical neurons by activation of the endogenous Drd2 dopamine D2 receptors (8). Stimulation of the endogenous CB1R cannabinoid receptor, a Gi/o-coupled receptor in Neuro2A cells, can drive the neuronal differentiation of these cells (6). Whereas tetrahydrocannabinol is a well-known molecule found in cannabis that activates CB1R receptors, anandamide and 2-arachidonoylglycerol are two endogenous CB1R agonists. Two other Gi/o-coupled GPCRs have also been implicated in stimulation of neurite outgrowth. Activation of 5-HT1A serotonin receptors transfected into Neuro2A cells stimulated neurite outgrowth (9), and activation of transfected serotonin 1B (5-HT1B) receptors enhanced neurite outgrowth of thalamic neurons (10).
Small GTPases
The balance of activity among the small guanosine triphosphatases (GTPases), such as Rac, Rho, Rap, Ral, and Ras, seems to be critical for the switch from signaling that drives proliferation toward signaling that promotes differentiation and neurite outgrowth. This Connections Map includes Ras, Ral, and Rap1, as well as the GTPase-activating proteins (GAPs) and guanine nucleotide exchange factors (GEFs) that affect their activity. Ras GEFs called Ras guanine nucleotide–releasing proteins (RasGRPs) were identified as diacylglycerol (DAG)– and calcium-activated Ras-stimulating proteins and are thought to be involved in the neurite outgrowth (11, 12). This links phospholipase C–β (PLC-β) to the Ras pathway. Gβγ subunits activate PLC-β (13), which provides a mechanism for GPCRs to activate the Ras–mitogen-activated protein kinase (MAPK) pathway.RasGAP1 affects the balance between Akt activity and MAPK1,2 activity. On one hand, through its GAP activity, RasGAP1 inhibits Ras (14), which reduces MAPK activity. It was also shown that RasGAP1 can bind directly to Akt, independently from the MAPK pathways, and this interaction contributes to Akt phosphorylation and enzyme activation (15). Akt is a well-established downstream effector of the phosphoinositide 3-kinase (PI3K) pathway, which is also stimulated by GPCR activation or RTK activation, and is required for neurite outgrowth (16, 17). PI3K is a lipid kinase that increases the production of PIP3 (18); this results in the activation of the kinases phosphoinositide-dependent kinase 1 (PDK1) (19) and integrin-linked kinase (ILK) (20), which are upstream activators of Akt.
In PC12 cells stimulated with NGF, the GTPase Rap1 forms a complex with B-Raf, which results in the activation of MAPK (3, 21). Rap1 is activated by the C3G exchange factor and the adaptor protein Crk (3), which form a complex (22, 23). Both Ras and Rap1 activate B-Raf in an additive manner (24), whereas Rap1 inhibits c-Raf (25). Rap1 activity may also be regulated by Gαi signaling through the cAMP-regulated GEFs, such as Epac, although currently there is no evidence for this connection. Gαi is a well-established inhibitor of adenylyl cyclases (ACs) (26), which results in reduced synthesis of cAMP and inhibition of protein kinase A (PKA). In the Connections Map, we have used AC5 because this Gs-stimulated isoform is abundant in the brain (27). Several competing GEFs and GAPs play an important role in the pathway. For example, Gαo is abundant in the brain and contributes to neurite outgrowth (28). Jordan et al. (29) found that Gαo directly binds to Rap1GAP and targets this protein for degradation by the proteasome. The reduction in Rap1GAP activity resulted in enhanced Rap1 activation and neurite outgrowth. Besides its canonical effect on MAPK signaling through B-Raf, Rap1 can activate Src by direct activation of RalGEF, which stimulates the GTPase Ral, resulting in Src activation (6). Src tyrosine phosphorylates C3G (30), a GEF for Rap1, setting up a putative positive-feedback loop. Src also phosphorylates and activates Stat3, which stimulates the expression of genes important for neurite outgrowth in Neuro2A cells.
The MAPK Cascade
B-Raf can be activated by Rap1 or Ras. B-Raf is an upstream kinase in the MAPK cascade. MAPK signaling plays a major role in neuronal differentiation (31, 32), and a relatively long duration of MAPK activation (33) is one trigger for neurite outgrowth. Within the MAPK cascade, B-Raf and c-Raf phosphorylate and activate the MAPK kinase (MAPKK) mitogen-activated or extracellular signal–regulated protein kinase (MEK) (34–36). The active MEKs then phosphorylate and activate MAPK1,2 (36). Downstream of MAPK, there are many transcription factor effectors, such as cAMP-response element binding protein (CREB1), that are important for turning on genes necessary for forming neurites. CREB is directly activated by an intermediary kinase, MSK1, which is activated by MAPK1,2 (37). CREB1 is a well-established transcription factor in early initiation of neurite outgrowth (17). It is also known that activation of PKA through the cAMP pathway antagonizes c-Raf activation, because there is a reduction in cAMP production and, as a result, a reduction in PKA activity in this pathway; this is consistent with sustained c-Raf and MAPK activation (38).
It is noteworthy that this pathway contains only a portion of the signaling proteins that are involved in the neurite outgrowth process. Less canonical mechanisms such as Wnt and arrestin signaling are likely to also be engaged.
Pathway Details
Scope: Specific
Organism: vertebrates: mammals: Rodentia: mice
Canonical Pathway: G alpha i Pathway (http://stke.sciencemag.org/cgi/cm/stkecm;CMP_7430)
References and Notes
- 1.Adler EM, Gough NR, Blundon JA. Differentiation of PC12 cells. Sci. STKE. 2006;2006:tr9. doi: 10.1126/stke.3512006tr9. [DOI] [PubMed] [Google Scholar]
- 2.Adler EM. Cell culture as a model system for teaching: Using PC12 cells. Sci. STKE. 2006;2006:tr5. doi: 10.1126/stke.3342006tr5. [DOI] [PubMed] [Google Scholar]
- 3.York RD, Yao H, Dillon T, Ellig CL, Eckert SP, McCleskey EW, Stork PJS. Rap1 mediates sustained MAP kinase activation induced by nerve growth factor. Nature. 1998;392:622–626. doi: 10.1038/33451. [DOI] [PubMed] [Google Scholar]
- 4.Lange-Carter CA, Johnson GL. Ras-dependent growth factor regulation of MEK kinase in PC12 cells. Science. 1994;265:1458–1461. doi: 10.1126/science.8073291. [DOI] [PubMed] [Google Scholar]
- 5.Huff K, End D, Guroff G. Nerve growth factor-induced alteration in the response of PC12 pheochromocytoma cells to epidermal growth factor. J. Cell Biol. 1981;88:189–198. doi: 10.1083/jcb.88.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He JC, Gomes I, Nguyen T, Jayaram G, Ram PT, Devi LA, Iyengar R. The Gαo/i-coupled cannabinoid receptor-mediated neurite outgrowth involves Rap regulation of Src and Stat3. J. Biol. Chem. 2005;280:33426–33434. doi: 10.1074/jbc.M502812200. [DOI] [PubMed] [Google Scholar]
- 7.He JC, Neves SR, Jordan JD, Iyengar R. Role of the Go/i signaling network in the regulation of neurite outgrowth. Can. J. Physiol. Pharmacol. 2006;84:687–694. doi: 10.1139/y06-025. [DOI] [PubMed] [Google Scholar]
- 8.Reinoso BS, Undie AS, Levitt P. Dopamine receptors mediate differential morphological effects on cerebral cortical neurons in vitro. J. Neurosci. Res. 1996;43:439–453. doi: 10.1002/(SICI)1097-4547(19960215)43:4<439::AID-JNR5>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 9.Fricker AD, Rios C, Devi LA, Gomes I. Serotonin receptor activation leads to neurite outgrowth and neuronal survival. Brain Res. Mol. Brain Res. 2005;138:228–235. doi: 10.1016/j.molbrainres.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 10.Lotto B, Upton L, Price DJ, Gaspar P. Serotonin receptor activation enhances neurite outgrowth of thalamic neurones in rodents. Neurosci. Lett. 1999;269:87–90. doi: 10.1016/s0304-3940(99)00422-x. [DOI] [PubMed] [Google Scholar]
- 11.Borasio GD, John J, Wittinghofer A, Barde YA, Sendtner M, Heumann R. ras p21 protein promotes survival and fiber outgrowth of cultured embryonic neurons. Neuron. 1989;2:1087–1096. doi: 10.1016/0896-6273(89)90233-x. [DOI] [PubMed] [Google Scholar]
- 12.Ebinu JO, Bottorff DA, Chan EYW, Stang SL, Dunn RJ, Stone JC. RasGRP, a Ras guanyl nucleotide-releasing protein with calcium-and diacylglycerol-binding motifs. Science. 1998;280:1082–1086. doi: 10.1126/science.280.5366.1082. [DOI] [PubMed] [Google Scholar]
- 13.Camps M, Carozzi A, Schnabel P, Scheer A, Parker PJ, Gierschik P. Isozyme-selective stimulation of phospholipase C-β2 by G protein βγ-subunits. Nature. 1992;360:684–686. doi: 10.1038/360684a0. [DOI] [PubMed] [Google Scholar]
- 14.Bollag G, McCormick F. Differential regulation of rasGAP and neurofibromatosis gene product activities. Nature. 1991;351:576–579. doi: 10.1038/351576a0. [DOI] [PubMed] [Google Scholar]
- 15.Yue Y, Lypowy J, Hedhli N, Abdellatif M. Ras GTPase-activating protein binds to Akt and is required for its activation. J. Biol. Chem. 2004;279:12883–12889. doi: 10.1074/jbc.M312308200. [DOI] [PubMed] [Google Scholar]
- 16.Kimura K, Hattori S, Kabuyama Y, Shizawa Y, Takayanagi J, Nakamura S, Toki S, Matsuda Y, Onodera K, Fukui Y. Neurite outgrowth of PC12 cells is suppressed by wortmannin, a specific inhibitor of phosphatidylinositol 3-kinase. J. Biol. Chem. 1994;269:18961–18967. [PubMed] [Google Scholar]
- 17.Bromberg KD, Ma’ayan A, Neves SR, Iyengar R. Design logic of a cannabinoid receptor signaling network that triggers neurite outgrowth. Science. 2008;320:903–909. doi: 10.1126/science.1152662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Whitman M, Downes CP, Keeler M, Keller T, Cantley L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature. 1988;332:644–646. doi: 10.1038/332644a0. [DOI] [PubMed] [Google Scholar]
- 19.Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PRJ, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 1997;7:261–269. doi: 10.1016/s0960-9822(06)00122-9. [DOI] [PubMed] [Google Scholar]
- 20.Delcommenne M, Tan C, Gray V, Rue L, Woodgett J, Dedhar S. Phosphoinositide-3-OH kinase-dependent regulation of glycogen synthase kinase 3 and protein kinase B/AKT by the integrin-linked kinase. Proc. Natl. Acad. Sci. U.S.A. 1998;95:11211–11216. doi: 10.1073/pnas.95.19.11211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oshima M, Sithanandam G, Rapp UR, Guroff G. The phosphorylation and activation of B-raf in PC12 cells stimulated by nerve growth factor. J. Biol. Chem. 1991;266:23753–23760. [PubMed] [Google Scholar]
- 22.Knudsen BS, Feller SM, Hanafusa H. Four proline-rich sequences of the guanine-nucleotide exchange factor C3G bind with unique specificity to the first Src homology 3 domain of Crk. J. Biol. Chem. 1994;269:32781–32787. [PubMed] [Google Scholar]
- 23.Tanaka S, Morishita T, Hashimoto Y, Hattori S, Nakamura S, Shibuya M, Matuoka K, Takenawa T, Kurata T, Nagashima K. C3G, a guanine nucleotide-releasing protein expressed ubiquitously, binds to the Src homology 3 domains of CRK and GRB2/ASH proteins. Proc. Natl. Acad. Sci. U.S.A. 1994;91:3443–3447. doi: 10.1073/pnas.91.8.3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohtsuka T, Shimizu K, Yamamori B, Kuroda S, Takai Y. Activation of brain B-Raf protein kinase by Rap1B small GTP-binding protein. J. Biol. Chem. 1996;271:1258–1261. doi: 10.1074/jbc.271.3.1258. [DOI] [PubMed] [Google Scholar]
- 25.Hu C-D, Kariya K-i, Okada T, Qi X, Song C, Kataoka T. Effect of phosphorylation on activities of Rap1A to interact with Raf-1 and to suppress Ras-dependent Raf-1 activation. J. Biol. Chem. 1999;274:48–51. doi: 10.1074/jbc.274.1.48. [DOI] [PubMed] [Google Scholar]
- 26.Taussig R, Iñiguez-Lluhi JA, Gilman AG. Inhibition of adenylyl cyclase by Gi alpha. Science. 1993;261:218–221. doi: 10.1126/science.8327893. [DOI] [PubMed] [Google Scholar]
- 27.Glatt CE, Snyder SH. Cloning and expression of an adenylyl cyclase localized to the corpus striatum. Nature. 1993;361:536–538. doi: 10.1038/361536a0. [DOI] [PubMed] [Google Scholar]
- 28.Strittmatter SM, Fishman MC, Zhu XP. Activated mutants of the alpha subunit of Go promote an increased number of neurites per cell. J. Neurosci. 1994;14:2327–2338. doi: 10.1523/JNEUROSCI.14-04-02327.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jordan JD, He JC, Eungdamrong NJ, Gomes I, Ali W, Nguyen T, Bivona TG, Philips MR, Devi LA, Iyengar R. Cannabinoid receptor-induced neurite outgrowth is mediated by Rap1 Activation through Gαo/i-triggered proteasomal degradation of Rap1GAPII. J. Biol. Chem. 2005;280:11413–11421. doi: 10.1074/jbc.M411521200. [DOI] [PubMed] [Google Scholar]
- 30.Radha V, Rajanna A, Swarup G. Phosphorylated guanine nucleotide exchange factor C3G, induced by pervanadate and Src family kinases localizes to the Golgi and subcortical actin cytoskeleton. BMC Cell Biol. 2004;5:31. doi: 10.1186/1471-2121-5-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas D, Bradshaw RA. Differential utilization of ShcA tyrosine residues and functional domains in the transduction of epidermal growth factor-induced mitogen-activated protein kinase activation in 293T cells and nerve growth factor-induced neurite outgrowth in PC12 cells: Identification of a new Grb2.Sos1 binding site. J. Biol. Chem. 1997;272:22293–22299. doi: 10.1074/jbc.272.35.22293. [DOI] [PubMed] [Google Scholar]
- 32.Gatti A. Divergence in the upstream signaling of nerve growth factor (NGF) and epidermal growth factor (EGF) Neuroreport. 2003;14:1031–1035. doi: 10.1097/01.wnr.0000071765.92388.c6. [DOI] [PubMed] [Google Scholar]
- 33.Kao S-c, Jaiswal RK, Kolch W, Landreth GE. Identification of the mechanisms regulating the differential activation of the MAPK cascade by epidermal growth factor and nerve growth factor in PC12 cells. J. Biol. Chem. 2001;276:18169–18177. doi: 10.1074/jbc.M008870200. [DOI] [PubMed] [Google Scholar]
- 34.Papin C, Eychène A, Brunet A, Pagès G, Pouyssègur J, Calothy G, Barnier JV. B-Raf protein isoforms interact with and phosphorylate Mek-1 on serine residues 218 and 222. Oncogene. 1995;10:1647–1651. [PubMed] [Google Scholar]
- 35.Gardner AM, Vaillancourt RR, Lange-Carter CA, Johnson GL. MEK-1 phosphorylation by MEK kinase, Raf, and mitogen-activated protein kinase: Analysis of phosphopeptides and regulation of activity. Mol. Biol. Cell. 1994;5:193–201. doi: 10.1091/mbc.5.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xu S, Robbins D, Frost J, Dang A, Lange-Carter C, Cobb MH. MEKK1 phosphorylates MEK1 and MEK2 but does not cause activation of mitogen-activated protein kinase. Proc. Natl. Acad. Sci. U.S.A. 1995;92:6808–6812. doi: 10.1073/pnas.92.15.6808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Deak M, Clifton AD, Lucocq JM, Alessi DR. Mitogen- and stress-activated protein kinase-1 (MSK1) is directly activated by MAPK and SAPK2/p38, and may mediate activation of CREB. EMBO J. 1998;17:4426–4441. doi: 10.1093/emboj/17.15.4426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vaillancourt RR, Gardner AM, Johnson GL. B-Raf-dependent regulation of the MEK-1/mitogen-activated protein kinase pathway in PC12 cells and regulation by cyclic AMP. Mol. Cell. Biol. 1994;14:6522–6530. doi: 10.1128/mcb.14.10.6522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.This work is a part of the activities conducted by the Systems Biology Center New York (SBCNY) supported by NIH grant no. P50-GM071558.