Abstract
Objective
Elevated levels of interleukin (IL)-18 have been implicated in the development of atherosclerosis in animals. Data in humans are less clear, and data in women are particularly scarce.
Methods and Results
In a prospective nested case-control study of initially healthy women, we measured baseline plasma IL-18 levels in 253 participants who subsequently developed cardiovascular disease (CVD) and in 253 healthy age- and smoking-matched controls. IL-18 levels were higher at baseline among those who developed CVD (274.1 vs. 233.8 pg/mL, P<0.001), and were associated with future CVD (relative risk (RR) for highest versus lowest quartile 2.53; 95% CI, 1.47–4.35, P<0.001). While that risk was attenuated after adjustment for traditional cardiovascular risk factors (RR 1.60; 95% CI, 0.77–3.34, P=0.13), those with IL-18 levels at or above a threshold of the 90th percentile (442 pg/mL) remained at elevated risk after adjustment (RR 2.40; 95% CI, 1.05–5.56, P=0.04). Levels of IL-18 above this threshold modify the fully adjusted risk of future CVD conferred by elevated levels of total cholesterol (Pinteraction=0.02).
Conclusions
In this population of apparently healthy women, IL-18 levels associate with increased risk of cardiovascular disease, but that risk is attenuated in models adjusting for traditional cardiovascular risk factors. Very high levels of IL-18 interact with hypercholesterolemia to alter CVD risk.
Keywords: Inflammation, Interleukin-18, cholesterol, women, cardiovascular disease
Introduction
Inflammation plays a central role in atherothrombosis.[1] Interleukin-18 (IL-18), also known as interferon-γ (IFN-γ) inducing factor, is a pleiotropic cytokine with wide-ranging effects on human innate and cellular immunity.[2] In mouse models of atherosclerosis, IL-18 overexpression induces atheroma formation and the vulnerable plaque phenotype via an IFN-γ dependent pathway, while overexpression of the endogenous inhibitor of IL-18 slows lesion development.[3–7] Human macrophages, endothelial cells, and smooth muscle cells express the IL-18 receptor, and IL-18 binding induces the production of molecules well-known to have a role in atherogenesis, including interleukin (IL)-6, intracellular adhesion molecule (ICAM)-1, vascular cell adhesion molecule (VCAM)-1, and matrix metalloproteinases (MMP-1/-9/-13). [8, 9] Also in humans, high levels of IL-18 expression have been demonstrated in unstable carotid plaques.[10]
The epidemiologic evidence implicating IL-18 in atherothrombosis has been somewhat less consistent, and data in healthy women are scarce. While IL-18 was independently associated with future coronary heart disease from France and Northern Ireland (the PRIME cohort), this study included only initially healthy men.[10] In the population-based MONICA/KORA Augsburg cohort, IL-18 levels were not associated with future coronary heart disease (CHD) in either women or men, but there were few CHD events among the female participants.[11] In both men and women with preexisting coronary disease, IL-18 was associated with cardiovascular death, and variations in the IL-18 gene were found to influence both levels of IL-18 and clinical outcomes, although this relationship appeared to weaken with a longer duration of follow-up.[12, 13]
We sought to determine if baseline plasma levels of IL-18 were associated with future cardiovascular disease in a population of initially healthy women. We also sought to determine whether the relationship between IL-18 and subsequent cardiovascular risk was modified by other well-established cardiovascular risk factors, including cholesterol levels and chronic inflammation.
Methods
We performed a prospective nested, case-control study of IL-18 as marker of future cardiovascular disease among participants in the Women’s Health Study, a randomized, double-blind, placebo controlled 2×2 factorial design trial of aspirin and vitamin E in the primary prevention of cardiovascular disease and cancer conducted among initially healthy women aged 45 years or older.[14] Participants were enrolled between November 1992 and July 1995 and were followed prospectively over a 6 year period for incident cardiovascular events including nonfatal myocardial infarction, nonfatal stroke, coronary revascularization procedures, and cardiovascular death. All participants in the WHS provided written informed consent and the study protocol was approved by the institutional review board of the Brigham and Women’s Hospital (Boston, Mass).
Among WHS participants, 28,345 women provided blood samples that were stored in liquid nitrogen until the time of analysis. For this analysis, we selected 253 women for whom we had a baseline blood sample who subsequently suffered either a non-fatal myocardial infarction or stroke or died of cardiovascular causes (109 myocardial infarction, 111 stroke, 33 cardiovascular death). The methods of endpoint validation have been described elsewhere in detail.[14] In brief, an endpoints committee comprised of physicians reviewed the medical records of all women who were reported to have suffered a cardiovascular event. A myocardial infarction was confirmed if symptoms met WHO criteria and was accompanied by diagnostic electrocardiographic changes or abnormal elevations in serum cardiac enzymes. Stroke was defined as a new focal neurologic deficit of sudden onset and vascular origin that persisted for more than 24 hours. We also selected 253 control participants who did not develop CVD during follow up. These subjects were then matched with cases for age (within 1 year), smoking status (never, current, or past smoker) and duration of follow-up (± 6 months).
The 253 case-control pairs underwent analysis for plasma IL-18 using an ELISA assay (R & D Systems, Minneapolis, Minnesota) with a sensitivity of 12.5 pg/mL. The day-today variability of the assay at concentrations of 160, 615 and 2621 pg/mL is 6.3, 5.2 and 10.1%, respectively. Blood specimens were analyzed in blinded pairs, and within pairs the position of the case specimen was varied at random to reduce the possibility of systemic bias and to minimize interassay variability. The methods used to evaluate the samples for total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, high-sensitivity C-reactive protein (hsCRP) are described in detail elsewhere.[15, 16]
Statistical Analysis
Population distributions were computed for IL-18 among the cases and controls, and quartiles were defined based on the distribution of the marker within the controls. Because IL-18 levels were skewed, Spearman correlation coefficients were used to test for interrelationships between the marker and each of the lipid subfractions and other novel biomarkers, and the significance of any differences in median values between cases and controls was tested by the Wilcoxon sign-rank test. The significance of any differences in binary categorical variables between cases and controls was tested using McNemar’s test. We used logistic regression, conditioned on the matching variables of age and smoking, to determine an estimate of the relative risk of future cardiovascular events for the second through fourth quartiles relative to lowest quartile of IL-18. Adjusted models of risk controlled for blood pressure (Framingham categories), diabetes, body mass index, hormone therapy use at the time of blood sampling, parental history of myocardial infarction at an age < 60 years, and randomized treatment assignment. We also computed the age and smoking matched and fully adjusted RR of future cardiovascular events for subjects whose IL-18 levels were at or above the 75th, 90th and 95th percentile levels in the control distribution. Using the threshold of the 90th percentile of IL-18 (442.0 pg/mL), tests for interaction with standard cardiovascular risk determinants were also performed. Deviation from an additive model was tested by including a single multiplicative interaction term for the exposures of interest in the logistic regression model. The null hypothesis of no deviation from the additive model was rejected if the P-value for the interaction term was <0.05. Data analysis was conducted using SAS statistical software version 9.1 (SAS Institute Inc., Cary, NC). The authors had full access to the data and take responsibility for its integrity. All authors have read and agree to the manuscript as written.
Results
Baseline characteristics of the study participants are listed in Table 1. As anticipated, a larger number of the cases were diabetic and had a history of hypertension. Cases also had a higher mean body mass index (BMI), and higher baseline levels of total cholesterol, low-density lipoprotein cholesterol (LDL-C), hemoglobin A1C, hsCRP, and homocysteine and lower levels of high-density lipoprotein cholesterol (HDL-C). There were no significant differences in alcohol consumption, exercise frequency, post-menopausal hormone use, or treatment for high cholesterol between the two groups.
Table 1.
Baseline characteristics of the study participants.
| Characteristic | Controls (N=253) | Cases (N=253) | P-value |
|---|---|---|---|
| Age, y* | 60.5 ± 8.7 | 60.5 ± 8.7 | … |
| Smoking status, % | … | ||
| Never | 41.9 | 41.9 | |
| Past | 36.0 | 36.0 | |
| Current | 22.1 | 22.1 | |
| Body Mass Index, kg/m2* | 25.6 ± 4.4 | 27.0 ± 5.2 | 0.001 |
| History of cholesterol > 6.22mmol/L, % | 37.2 | 46.3 | 0.04 |
| History of hypertension, % | 30.8 | 55.3 | <0.0001 |
| Parental history of myocardial infarction at age < 60 years | 11.4 | 15.1 | 0.66 |
| Diabetes, % | 2.0 | 13.0 | <0.0001 |
| Alcohol ≥1 serving/week, % | 35.2 | 34.4 | 0.85 |
| Exercise ≥1 day/week, % | 36.8 | 31.2 | 0.21 |
| Aspirin | 48.6 | 46.6 | 0.66 |
| Post-menopausal hormone use | 37.9 | 39.9 | 0.17 |
| Treatment for high cholesterol, % | 3.6 | 6.7 | 0.12 |
| Total cholesterol, mmol/L †‡ | 5.52 (4.95, 6.22) | 5.85 (5.08, 6.59) | 0.007 |
| HDL cholesterol, mmol/L †‡ | 1.33 (1.08, 1.63) | 1.19 (0.99, 1.45) | <0.0001 |
| LDL cholesterol, mmol/L †‡ | 3.27 (2.70, 3.84) | 3.49 (2.86, 4.07) | 0.03 |
| Lipoprotein (a), μmol/L †§ | 0.45 (0.19, 0.90) | 0.52 (0.19, 1.87) | 0.10 |
| HbA1C, % † | 5.02 (4.89, 5.23) | 5.09 (4.91, 5.45) | 0.0006 |
| Homocysteine, μmol/L † | 10.8 (9.0, 13.5) | 11.2 (9.0, 14.7) | 0.04 |
| hsCRP, mg/L † | 2.3 (0.9, 4.0) | 3.2 (1.6, 6.1) | <0.0001 |
| sICAM-1, ng/mL † | 353.3 (310.2, 398.6) | 378.9 (326.7, 446.1) | 0.001 |
| IL-18, pg/mL † | 233.8 (174.5, 307.5) | 274.1 (191.6, 385.4) | 0.0004 |
Values represent means ± standard deviations.
Values represent medians (interquartile ranges).
To convert total, high density lipoprotein and low density lipoprotein cholesterol to mg/dL, multiply by 38.61.
To convert lipoprotein(a) to mg/dL, multiply by 28.01.
Abbreviations: HDL, high-density lipoprotein; HbA1C, hemoglobin A1C; hsCRP, high-sensitivity C-reactive protein; IL-18, interleukin-18; LDL, low-density lipoprotein; sICAM-1, soluble intracellular adhesion molecule-1.
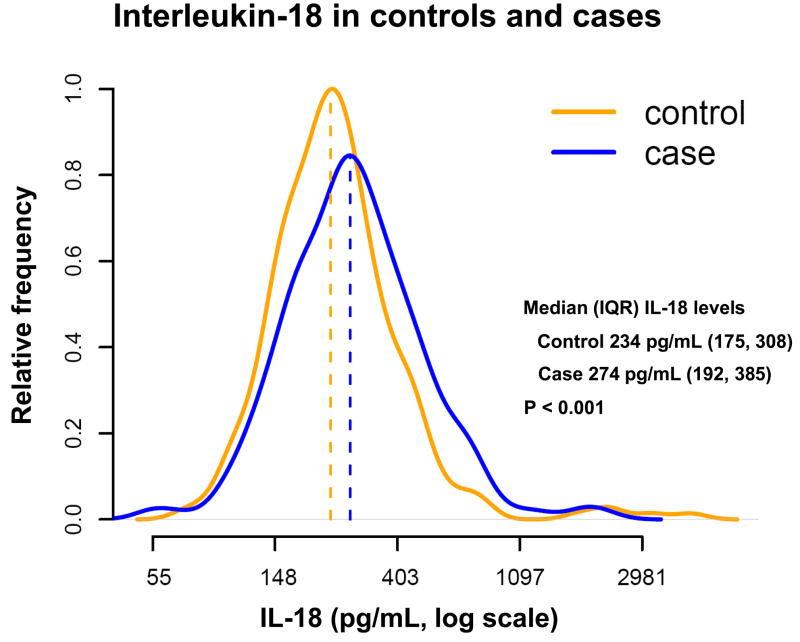
The distribution of IL-18 was right-skewed, and ranged from 25.0 to 2182.3 pg/mL in the cases and 70.2 to 4445.4 pg/mL in the controls (Figure 1). Median levels of IL-18 were higher among cases than controls (274.1 versus 233.8 pg/mL, P<0.001). While IL-18 was not correlated with levels of total cholesterol, LDL-C, creatinine or fibrinogen, there was an association between IL-18 levels and soluble ICAM (sICAM) (r = 0.34), homocysteine (r = 0.28), HDL-C (r = −0.28), hemoglobin A1C (r = 0.20) and hsCRP (r = 0.18) (each P-value < 0.0001).
Figure 1.
Natural logarithm normalized concentrations of interleukin (IL)-18 in picograms per milliliter (pg/mL) for 253 cases and age- and smoking-status matched controls.
The relative risk of future cardiovascular events increased with increasing quartiles of IL-18 such that women in the highest quartile had a relative risk (RR) 2.53 (95% CI, 1.47–4.35, P-trend = 0.0001) times higher than those in the lowest quartile in age- and smoking-matched models (Table 2). However, after adjustment for standard risk factors of cardiovascular disease, including blood pressure, diabetes, body mass index, parental history of myocardial infarction before the age of 60 years, hormone therapy use and randomized treatment assignment, that risk was attenuated such that the 95% CI overlapped 1 and the P-trend was no longer statistically significant. Specifically, the RR for future cardiovascular events for women in the highest quartile of IL-18 compared with those in the lowest quartile was 1.71 (95% CI, 0.87–3.38, P-trend = 0.06). After further adjustments for total and HDL cholesterol, the association with future cardiovascular events was similar (RR 1.60, 95% CI, 0.76–3.31, P-trend = 0.17). Further adjustment for hsCRP or for hemoglobin A1c did not show any further attenuation of the RR. Specifically, the RR for future CVD for extreme quartiles of IL-18 was 1.59 (95% CI, 0.76–3.35, P-trend = 0.15) with hsCRP in the model and 1.51 (95% CI, 071–3.20, P-trend = 0.30) with hemoglobin A1c in the model. Adjustment for sICAM-1 altered the RR estimate for the highest versus the lowest quartile of IL-18, but the 95% confidence intervals overlapped 1 and the P-trend was not significant (2.04, 95% CI 0.77–5.44, P-trend = 0.16, data not shown).
Table 2.
Risk of Future Cardiovascular Events Among Apparently Healthy Women According to Baseline Level of IL-18
| Quartile of IL-18 (pg/mL) |
||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||
| <174.6 | 174.6–233.8 | 233.9–307.5 | >307.5 | P-trend* | ||
| Model 1† | ||||||
| RR | 1.0 | 1.18 | 1.50 | 2.53 | 0.0001 | |
| 95% CI | 0.67–2.08 | 0.85–2.66 | 1.47–4.35 | |||
| P | 0.57 | 0.16 | 0.0008 | |||
| Model 2‡ | ||||||
| RR | 1.0 | 1.01 | 1.15 | 1.71 | 0.06 | |
| 95% CI | 0.49–2.09 | 0.55–2.40 | 0.87–3.38 | |||
| P | 1.01 | 0.70 | 0.12 | |||
| Model 3 || | ||||||
| RR | 1.0 | 1.13 | 1.12 | 1.60 | 0.17 | |
| 95% CI | 0.52–2.45 | 0.52–2.40 | 0.77–3.34 | |||
| P | 0.77 | 0.78 | 0.21 | |||
Abbreviations: RR, relative risk; CI, confidence interval
Tests for trend across quartiles are based on entering a single term based on the median value within that quartile.
Model 1 is matched for age and smoking status.
Model 2 is matched for age and smoking status and is further adjusted for diabetes (yes/no), body mass index (kg/m2), blood pressure (Framingham categories), hormone therapy use at the time of blood sampling, a parental history of myocardial infarction at age < 60 years and randomized treatment assignment.
Model 3 is adjusted for all the factors in Model 2 plus total cholesterol and high-density lipoprotein cholesterol (as continuous variables).
In order to determine whether levels of IL-18 above a certain threshold were associated with CVD, we repeated the analysis and computed the risk of cardiovascular events for subjects whose IL-18 levels were at or above the 75th, 90th and 95th percentiles of the IL-18 distribution among controls. As shown in Table 3, for those subjects in the highest 10% of the IL-18 distribution (above 442.0 pg/mL), the age- and smoking-matched RR of future CVD was 2.20 (1.30–3.73, P = 0.03). After adjustment, the risk of future CVD was actually somewhat higher for these women (RR 2.41, 95% CI, 1.05–5.56, P = 0.04), and remained statistically significant.
Table 3.
Age- and smoking-matched and fully adjusted risk of future cardiovascular disease for three threshold cutpoints of interleukin-18. Cutpoints were established based on the interleukin-18 distribution in the control population. The reference group for this analysis includes all subjects with IL-18 levels below the specified cutpoint.
| Age and Smoking Matched* |
Fully Adjusted† |
||||||
|---|---|---|---|---|---|---|---|
| Cutpoint (percentile) | IL-18 Value (pg/mL) | Number of Controls (%) | Number of Cases (%) | RR | P-value | RR | P-value |
| ≥ 75th | 307.5 | 64 (25) | 104 (41) | 2.00 (1.37–2.92) | 0.003 | 1.46 (0.84–2.53) | 0.18 |
| ≥ 90th | 442.0 | 26 (10) | 50 (20) | 2.20 (1.30–3.73) | 0.004 | 2.41(1.05–5.56) | 0.04 |
| ≥ 95th | 549.3 | 13 (5) | 27 (11) | 2.40 (1.15–5.02) | 0.02 | 2.20 (0.81–5.97) | 0.12 |
Age and smoking matched model is equivalent to Model 1 in Table 2.
The fully adjusted RR is age and smoking matched and further adjusted for diabetes (yes/no), body mass index (kg/m2), blood pressure (Framingham categories), hormone therapy use at the time of blood sampling, a parental history of myocardial infarction at age < 60 years, total cholesterol (mg/dL), high-density lipoprotein cholesterol (mg/dL) and randomized treatment assignment. It is equivalent to Model 3 in Table 2.
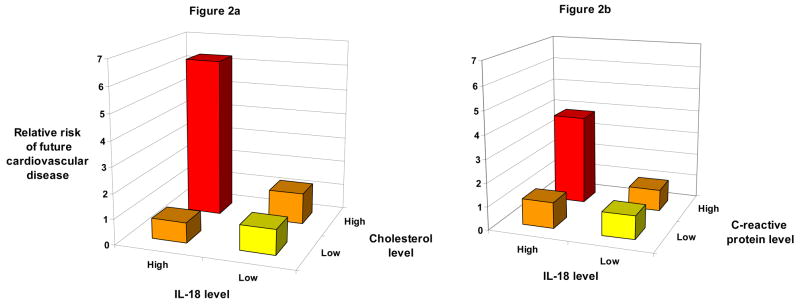
The risk of future CVD for women at or above this threshold of IL-18 was modified by cholesterol and hsCRP levels at, above or below the median (Figures 2a and 2b). Specifically, among women at or above this threshold of IL-18, the fully adjusted RR of future CVD was 6.29 (95% CI, 2.01– 19.7) for those with cholesterol levels at or above the median (5.52 mmol/L), while those with cholesterol levels below the median did not have an increased risk of CVD (RR 0.81; 95% CI, 0.26–2.49). Similarly, among women at or above this threshold of IL-18, those with hsCRP levels at or above the median (2.3 mg/L) had a fully adjusted RR of future CVD of 3.85 (95% CI, 1.12–13.27) while those with hsCRP levels below the median did not have an increased risk of CVD (RR=1.13; 95% CI, 0.38–3.30). Formal tests of interaction were statistically significant in age- and smoking-matched models for total cholesterol (P=0.02) and hsCRP (P=0.01) and for total cholesterol in fully adjusted models (P=0.024). We could detect no significant interaction between IL-18 levels above this threshold and HDL-C levels above or below the median, sICAM-1 levels above or below the median, parental history of MI at age <60 years, diabetes, elevated blood pressure (SBP ≥ 140 and/or DBP ≥ 90 mm Hg), obesity (BMI ≥ 30 kg/m2) or hormone therapy use.
Figure 2.
Fully adjusted relative risk of future cardiovascular disease in initially healthy women according to baseline levels of interleukin (IL)-18 and either (a) total cholesterol or (b) high-sensitivity C-reactive protein (hsCRP). High levels of IL-18 were defined as those falling above the 90th percentile in control subjects (442 pg/mL), while high levels of total cholesterol and hsCRP were those falling above the median in the control population (5.52 mmol/L and 2.3 mg/L, respectively). Formal tests of interaction between total cholesterol and IL-18 were significant in age and smoking adjusted models (Pinteraction =0.02) and in models adjusted for age, current smoking, diabetes (yes/no), blood pressure (Framingham categories), body mass index (kg/m2), parental history of myocardial infarction before the age of 60, hormone therapy, high-density lipoprotein cholesterol (mg/dL), and randomized treatment assignment (Pinteraction =0.024). Formal tests of interaction between hsCRP and IL-18 were significant in age and smoking adjusted models (Pinteraction=0.01), but fell short of statistical significance (Pinteraction =0.125) in models adjusted for age, smoking, diabetes, blood pressure, body mass index, parental history of myocardial infarction before the age of 60, hormone therapy, total cholesterol, high-density lipoprotein cholesterol and treatment assignment.
Finally, to determine if the risk associated with elevated levels of IL-18 diminished with longer durations of follow-up, we calculated the age and smoking adjusted risk per quartile of IL-18 at different points of follow-up. For those women who suffered a cardiovascular event within the first two years of follow-up, the relative risk of cardiovascular disease per increasing quartile of IL-18 was 1.19 (95% CI, 0.9–1.58, P=0.21), while that risk was 1.67 (95% CI, 1.22–2.27, P=0.001) and 1.34 (95% CI, 1.00–1.81, P=0.05) for those with events occurring from 2 to 4 and 4 to 6 years after enrollment, respectively.
Discussion
These data indicate that baseline levels of the inflammatory cytokine IL-18 associate with future cardiovascular disease in a population of apparently healthy women, although the strength of that association is attenuated after controlling for traditional cardiovascular risk factors. In exploratory analyses, levels of IL-18 above a threshold of the 90th percentile (442 pg/mL in this study) are associated with a doubling in risk of future cardiovascular events. We also observed that women who have levels of IL-18 above this threshold and elevated total cholesterol levels are at markedly higher risk for future cardiovascular events than matched controls (RR 6.29; 95% CI, 2.01–19.7, Pinteraction = 0.024), even after adjusting for traditional cardiovascular risk factors. A similar interaction was observed for elevated hsCRP, although it was not significant after adjustment (Pinteraction =0.125).
We believe these data are of interest for several reasons. First, to the best of our knowledge, no data have been published with regard to the association between IL-18 and the risk of incident coronary and cerebrovascular disease in a population of apparently healthy women. Second, while some of our analyses were exploratory and should be interpreted with caution, our data suggest that the fully-adjusted risk of future cardiovascular events may not be linear across all levels of IL-18, but that high levels of IL-18 may be associated with future cardiovascular events, even after adjustment for traditional risk factors, including total and HDL cholesterol. Finally, these data support the hypothesis that inflammation and hyperlipidemia act differently in the pathophysiology of atherothrombosis, and that combination of high levels of inflammatory and lipid biomarkers is associated with a much higher risk of cardiovascular events than elevated levels of either marker in isolation.
Our results differ from those reported in the AtheroGene Study,[12] although the populations studied are quite different. In that study, the association between IL-18 levels and cardiovascular death (n=95) was first noted in subjects with preexisting coronary heart disease. After controlling for a series of potential confounders such as age, diabetes, hypertension, HDL-C, ejection fraction, and hsCRP, IL-18 levels were associated with future cardiovascular death for the first 3.9 years of follow up, with a RR of 3.3 (95% CI, 1.3–8.4, P=0.01) for the highest vs. the lowest quartile of IL-18.[12] This risk is somewhat higher than described here, but the subjects enrolled had preexisting angiographically demonstrated coronary artery disease, and are likely to represent a group at higher risk for cardiovascular events than our population.
Our results are more similar to those described by the same investigators in a nested case-control study within the PRIME cohort in Northern Ireland and France.[10] In that study, men with IL-18 levels in the highest as compared with the lowest tertile had a RR of future CHD of 2.07 (95% CI, 1.40–3.05, P<0.001). After stratifying by country, there was no statistically significant association between IL-18 levels and future risk of CHD in France (RR increase per tertile, 1.29, 95% CI 0.96–1.73, Ptrend = 0.09) while that relationship was still present in Northern Ireland (RR increase per tertile 1.65, 95% CI 1.14–2.40, Ptrend = 0.008).[10] Like the subjects enrolled in PRIME and the MONICA/KORA Augsburg cohort,[11] the women in our study did not have cardiovascular disease at the time of enrollment, and in our study the point estimate of the risk associated with the highest as compared to the lowest quartile of IL-18 (RR 1.60; 95% CI, 0.77–3.34, P-trend = 0.17) was midway between the estimates in the PRIME and MONICA/KORA studies, with 95% CI that overlap both, as well as 1.0.
There are a number of possible explanations for the discrepancy in the point estimates of risk. First, the population enrolled in AtheroGene had preexisting coronary disease and was therefore represented a secondary, rather than primary prevention population.[12] Although the relationship between IL-18 and cardiovascular death was not statistically significant when the follow up was extended beyond 3.9 years, high levels of IL-18 may be a particularly potent marker of elevated risk of death in short-term follow up in this high-risk population with established CHD. These data are consistent with the hypothesis that elevated plasma IL-18 levels may be a systemic as well as a local marker of plaque instability,[3, 6] at least in the short term. In our population of apparently health women, we found that the association between IL-18 and cardiovascular events was essentially the same throughout the duration of follow up.
The PRIME and MONICA/KORA Augsburg cohorts utilized the endpoint of incident coronary events, while we focused on incident cerebrovascular as well as coronary events (109 myocardial infarction, 111 stroke, 33 cardiovascular death). Previous work has suggested that inflammation and hyperlipidemia may play different roles in the development of atherothrombosis in the cerebral and coronary vasculature, and this observation may be true of IL-18 as well.[17] If so, then a hypothetical difference in risk of events in the cerebral and coronary circulation may explain the differences in the point estimates between the three studies. However, we found no differences in the risk associated with elevated IL-18 levels after stratifying by the different types of cardiovascular events, although our power to detect such a difference was limited.
Another possibility is that IL-18 may have a threshold effect, such that levels of IL-18 above 442 pg/mL are associated with a fully-adjusted risk of cardiovascular events that is more than double those with IL-18 levels below this threshold (RR 2.41, 95% CI, 1.05–5.56, P = 0.04). Consistent with this observation, in our data women in the middle two quartiles of the IL-18 distribution had a risk of cardiovascular events that was virtually indistinguishable from those in the lowest quartile after adjustment, suggesting that a threshold of risk occurs at IL-18 levels above the 75th percentile (307.5 pg/mL).
Interestingly, women above the 90th percentile threshold who also had cholesterol levels below the median (5.52 mmol/L) had a similar fully adjusted risk of future cardiovascular events as those who had low levels of IL-18 (RR 0.81, 95% CI 0.26–2.49, Pinteraction =0.024). By contrast, women with high levels of both biomarkers had a fully adjusted risk of future cardiovascular events that was six times higher than those in the lower category for both markers (RR 6.29, 95% CI 2.01–19.7, Pinteraction =0.024). The hypothesis that high levels of inflammation and elevated levels of lipid biomarkers may hasten the development of atherothrombosis has been reported in a number of cohort studies,[18–20] and in randomized trials of lovastatin[21] and atorvastatin and pravastatin.[22] These results support that hypothesis. We also observed an interaction between high levels of IL-18 and hsCRP levels above the median (2.3 mg/L) that was statistically significant before multivariable adjustment, raising the possibility of two different inflammatory pathways interacting to alter atherothrombotic risk.
Limitations of these data should be considered. We relied on a single baseline blood sample for each subject, and thus did not measure any variation that may occur in IL-18 levels with time. Furthermore, our samples were not obtained at a uniform time of day, so any diurnal variations in IL-18 that might exist will not have been captured by our study. Both of these limitations are likely to increase misclassification and would therefore tend to bias our results towards the null. We cannot exclude the possibility that protein degradation may have occurred while these samples were stored at −80°C. However, any protein degradation would have affected both cases and controls equally, as they were handled identically and in a blinded fashion throughout the study. Lastly, if we measured IL-18 levels in more cases and controls, or in the WHS entire cohort, the chances that we would detect a significant difference in cardiovascular risk with elevated IL-18 levels would increase.
While there is strong cellular and molecular evidence of a role for IL-18 in the pathogenesis of atheroma formation,[3, 4, 6] induction of inflammatory cytokines known to associate with cardiovascular disease (IL-6, sICAM-1, MMP-1/-9/-13) [9] and progression to a vulnerable plaque phenotype,[5] the epidemiologic evidence has been somewhat more mixed. These prospective epidemiologic data support the role of IL-18 as an inflammatory marker associated with cardiovascular disease and raise the hypotheses that levels of IL-18 above a threshold of 442 pg/mL are associated with cardiovascular events, and that those high levels of IL-18 interact with hyperlipidemia to alter atherothrombotic risk. While those hypotheses will need to be confirmed in other populations at low risk for cardiovascular disease, our data support a fundamental role for inflammation in the development of cardiovascular disease.
Acknowledgments
We are indebted to the participants and staff of the Women’s Health Study for their dedicated and conscientious collaboration.
Funding Sources: This work was supported through grants from the Leducq Foundation (Paris, France), the Donald W. Reynolds Foundation (Las Vegas, NV), the National Heart, Lung, and Blood Institute (T32 HL007575-21 and HL-43851) and the National Cancer Institute (CA-47988).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Libby P, Ridker PM. Inflammation and atherothrombosis from population biology and bench research to clinical practice. J Am Coll Cardiol. 2006;48:A33–46. [Google Scholar]
- 2.McInnes IB, Gracie JA, Leung BP, Wei XQ, Liew FY. Interleukin 18: a pleiotropic participant in chronic inflammation. Immunol Today. 2000;21:312–5. doi: 10.1016/s0167-5699(00)01648-0. [DOI] [PubMed] [Google Scholar]
- 3.Mallat Z, Corbaz A, Scoazec A, Graber P, Alouani S, Esposito B, et al. Interleukin-18/interleukin-18 binding protein signaling modulates atherosclerotic lesion development and stability. Circ Res. 2001;89:E41–5. doi: 10.1161/hh1901.098735. [DOI] [PubMed] [Google Scholar]
- 4.Whitman SC, Ravisankar P, Daugherty A. Interleukin-18 enhances atherosclerosis in apolipoprotein E(−/−) mice through release of interferon-gamma. Circ Res. 2002;90:E34–8. doi: 10.1161/hh0202.105292. [DOI] [PubMed] [Google Scholar]
- 5.de Nooijer R, von der Thusen JH, Verkleij CJ, Kuiper J, Jukema JW, van der Wall EE, et al. Overexpression of IL-18 decreases intimal collagen content and promotes a vulnerable plaque phenotype in apolipoprotein-E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:2313–9. doi: 10.1161/01.ATV.0000147126.99529.0a. [DOI] [PubMed] [Google Scholar]
- 6.Mallat Z, Corbaz A, Scoazec A, Besnard S, Leseche G, Chvatchko Y, et al. Expression of interleukin-18 in human atherosclerotic plaques and relation to plaque instability. Circulation. 2001;104:1598–603. doi: 10.1161/hc3901.096721. [DOI] [PubMed] [Google Scholar]
- 7.Elhage R, Jawien J, Rudling M, Ljunggren HG, Takeda K, Akira S, et al. Reduced atherosclerosis in interleukin-18 deficient apolipoprotein E-knockout mice. Cardiovasc Res. 2003;59:234–40. doi: 10.1016/s0008-6363(03)00343-2. [DOI] [PubMed] [Google Scholar]
- 8.Vidal-Vanaclocha F, Fantuzzi G, Mendoza L, Fuentes AM, Anasagasti MJ, Martin J, et al. IL-18 regulates IL-1beta-dependent hepatic melanoma metastasis via vascular cell adhesion molecule-1. Proc Natl Acad Sci U S A. 2000;97:734–9. doi: 10.1073/pnas.97.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerdes N, Sukhova GK, Libby P, Reynolds RS, Young JL, Schonbeck U. Expression of interleukin (IL)-18 and functional IL-18 receptor on human vascular endothelial cells, smooth muscle cells, and macrophages: implications for atherogenesis. J Exp Med. 2002;195:245–57. doi: 10.1084/jem.20011022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blankenberg S, Luc G, Ducimetiere P, Arveiler D, Ferrieres J, Amouyel P, et al. Interleukin-18 and the risk of coronary heart disease in European men: the Prospective Epidemiological Study of Myocardial Infarction (PRIME) Circulation. 2003;108:2453–9. doi: 10.1161/01.CIR.0000099509.76044.A2. [DOI] [PubMed] [Google Scholar]
- 11.Koenig W, Khuseyinova N, Baumert J, Thorand B, Loewel H, Chambless L, et al. Increased concentrations of C-reactive protein and IL-6 but not IL-18 are independently associated with incident coronary events in middle-aged men and women: results from the MONICA/KORA Augsburg case-cohort study, 1984–2002. Arterioscler Thromb Vasc Biol. 2006;26:2745–51. doi: 10.1161/01.ATV.0000248096.62495.73. [DOI] [PubMed] [Google Scholar]
- 12.Blankenberg S, Tiret L, Bickel C, Peetz D, Cambien F, Meyer J, et al. Interleukin-18 is a strong predictor of cardiovascular death in stable and unstable angina. Circulation. 2002;106:24–30. doi: 10.1161/01.cir.0000020546.30940.92. [DOI] [PubMed] [Google Scholar]
- 13.Tiret L, Godefroy T, Lubos E, Tregouet NVDA, Barbaux S, et al. Genetic analysis of the interleukin-18 system highlights the role of the interleukin-18 gene in cardiovascular disease. Circulation. 2005;112:643–50. doi: 10.1161/CIRCULATIONAHA.104.519702. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Cook NR, Lee IM, Gordon D, Gaziano JM, Manson JE, et al. A randomized trial of low-dose aspirin in the primary prevention of cardiovascular disease in women. N Engl J Med. 2005;352:1293–304. doi: 10.1056/NEJMoa050613. [DOI] [PubMed] [Google Scholar]
- 15.Pai JK, Curhan GC, Cannuscio CC, Rifai N, Ridker PM, Rimm EB. Stability of novel plasma markers associated with cardiovascular disease: processing within 36 hours of specimen collection. Clin Chem. 2002;48:1781–4. [PubMed] [Google Scholar]
- 16.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. Jama. 2005;294:326–33. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 17.Everett BM, Kurth T, Buring JE, Ridker PM. The Relative Strength of C-Reactive Protein and Lipid Levels as Determinants of Ischemic Stroke Compared With Coronary Heart Disease in Women. Journal of the American College of Cardiology. 2006;48:2235–42. doi: 10.1016/j.jacc.2006.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ridker PM, Cushman M, Stampfer MJ, Tracy RP, Hennekens CH. Inflammation, aspirin, and the risk of cardiovascular disease in apparently healthy men. N Engl J Med. 1997;336:973–9. doi: 10.1056/NEJM199704033361401. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–65. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 20.Koenig W, Lowel H, Baumert J, Meisinger C. C-reactive protein modulates risk prediction based on the Framingham Score: implications for future risk assessment: results from a large cohort study in southern Germany. Circulation. 2004;109:1349–53. doi: 10.1161/01.CIR.0000120707.98922.E3. [DOI] [PubMed] [Google Scholar]
- 21.Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. N Engl J Med. 2001;344:1959–65. doi: 10.1056/NEJM200106283442601. [DOI] [PubMed] [Google Scholar]
- 22.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, et al. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]