Abstract
Astrocytes participate in CNS innate immune responses as evident by their ability to produce a wide array of inflammatory mediators upon exposure to diverse stimuli. Although we have established that astrocytes utilize Toll-like receptor 2 (TLR2) to signal inflammatory mediator production in response to S. aureus, a common etiological agent of CNS infections, the signal transduction pathways triggered by this pathogen and how TLR2 expression is regulated remain undefined. Three disparate inhibitors that block distinct steps in the NF-κB pathway, namely SC-514, BAY 11-7082, and CAPE, attenuated nitric oxide (NO), TNF-α, and CXCL2 release from S. aureus-activated astrocytes. Among these proinflammatory mediators, autocrine/paracrine TNF-α was pivotal for augmenting TLR2 expression, since receptor levels were not elevated in astrocytes isolated from TNF-α knockout (KO) mice upon bacterial exposure. Since TLR2 is critical for signaling astrocytic cytokine production in response to S. aureus, we evaluated the effect of TNF-α loss on proinflammatory mediator release. Interestingly, among the molecules assayed, only NO production was significantly attenuated in TNF-α KO astrocytes compared to WT cells. Similar results were obtained following LPS treatment, suggesting that TNF-α is an important regulator of astrocytic TLR2 expression and NO release in response to diverse microbial stimuli. In addition, NF-κB inhibitors attenuated TNF-α-induced TLR2 expression in astrocytes. Overall this study suggests that two important anti-bacterial effector molecules, TLR2 and NO, are regulated, in part, by NF-κB-dependent autocrine/paracrine effects of TNF-α in astrocytes.
Keywords: Toll-like receptor 2 (TLR2), astrocyte, tumor necrosis factor-alpha (TNF-α), nuclear factor-kappa B (NF-κB)
INTRODUCTION
Brain abscess is a serious infectious disease, arising from parencymal seeding of the CNS with pyogenic bacteria such as S. aureus (1, 2). Among the numerous host responses elicited during brain abscess development, astrocyte activation is a hallmark feature of infection (3–5). Reactive astrocytes are localized along the abscess periphery and the immediate activation of these cells likely plays an important role in initiating the host anti-bacterial immune response prior to the influx of professional phagocytes from the peripheral circulation (6).
Toll-like receptors (TLR) are a family of pattern recognition receptors (PRRs) that allow for the recognition of pathogen-associated molecular patterns (PAMPs), motifs conserved across broad subclasses of bacteria, viruses, or fungi (7, 8). To date, thirteen TLRs have been identified, with TLR2 playing a pivotal role in recognizing structural components associated with gram-positive bacteria such as S. aureus (7, 8). Recently, we and others have shown that astrocytes express TLR2, with receptor levels increasing upon exposure to various PAMPs including LPS, PGN, and S. aureus (9–13). In addition, TLR2-deficient astrocytes demonstrated impaired immune responses against S. aureus (9). Despite these observations, the signaling cascades regulating TLR2 expression and subsequent proinflammatory mediator production in activated astrocytes have not yet been defined.
In response to S. aureus, astrocytes, produce a wide array of proinflammatory mediators including NO, TNF-α, interleukin-1β (IL-1β), CXCL2 (macrophage inflammatory protein-2; MIP-2), and CCL2 (macrophage inflammatory protein-1; MCP-1) (9, 10). Among these molecules, TNF-α plays an important role in the generation of a protective anti-bacterial immune response during brain abscess development as well as regulating blood-brain barrier (BBB) permeability (14–17). Since TNF-α is a major product of S. aureus-activated astrocytes and these cells also express TNF-α receptors (18), it is possible that TNF-α released upon bacterial exposure may feedback in autocrine/paracrine manner to further modulate astrocyte anti-bacterial responses. However, the functional importance of TNF-α in influencing downstream effector responses of astrocytes following bacterial stimulation has not yet been described.
Ligand binding of TNF-α to tumor necrosis factor-a receptor 1 (TNFR1) leads to the activation of several signal transduction cascades including NF-κB (19, 20). NF-κB is a key transcription factor for regulating TLR expression as well as proinflammatory mediator production in several immune cell types (21–23). Upon ligation with TNF-α, TNFR1 trimerizes with adjacent TNFR1s leading to the recruitment of several proteins to its cytoplasmic domain including receptor interacting protein (RIP), TNFR1 associated death domain protein (TRADD), and tumor necrosis factor associated factor 2 (TRAF2), ultimately culminating in NF-κB activation (19, 20). In unstimulated cells, NF-κB resides in the cytoplasm in an inactive form associated with inhibitors of κBs (IκBs). In response to cytokines and bacterial products, the inhibitor of κB kinase (IKK) complex is activated, leading to the phosphorylation, ubiquitination, and subsequent degradation of IκBα (24–26). The liberated NF-κB complex then translocates to the nucleus and initiates the transcriptional activation of target genes. Although it is presumed due to the ability of S. aureus to trigger TLR2 activation (27), the relative importance of NF-κB signaling in astrocyte responses to S. aureus has not yet been demonstrated. Here we report that NF-κB is pivotal for regulating astrocyte activation in response to S. aureus since three discrete inhibitors of the NF-κB signaling pathway attenuated proinflammatory mediator production. In addition, S. aureus-induced TLR2 expression was found to be mediated via NF-κB as well as autocrine/paracrine effects of TNF-α. Similar effects on astrocytic TLR2 levels were observed in response to the TLR4 ligand LPS. Collectively, these results suggest that TNF-α plays an important role in amplifying the immune response of astrocytes upon bacterial exposure, in part, by augmenting TLR2 expression.
MATERIALS AND METHODS
Mouse strains
TNF-α knockout (KO) mice on a C57BL/6 background were obtained from Jackson Laboratories (Bar Harbor, ME). C57BL/6 mice were used as a source of wild type (WT) astrocytes and were purchased from Harlan Labs (Indianapolis, IN). The animal use protocol was approved by the UAMS Animal Care and Use Committee and is in accord with NIH guidelines for the use of rodents.
Primary astrocyte cell culture and reagents
Primary astrocytes were derived from C57BL/6 WT or TNF-α KO mice (1–4 days of age) as previously described (9, 10). One concern when working with primary astrocytes relates to the issue of cell purity (28). The summation of several lines of evidence indicates that the results obtained in the current study can be attributed to astrocytes. First, we subjected our mixed glial cultures to a shaking protocol for a minimum of three occurrences, which progressively diminished the number of contaminating microglia over time. Second, upon transitioning mixed glial cultures for astrocyte experiments, the culture medium was supplemented with 0.1 mM L-LME, a microglial cytotoxic agent that has been used extensively as a method for microglial depletion (9, 10, 28, 29). Astrocytes were cultured in L-LME-containing medium for at least 2 weeks prior to use in experiments and were not utilized until the third passage in culture (approximately day 35–42 in vitro). Continued passage of astrocytes also facilitated the elimination of residual microglia that remained firmly attached to the tissue culture flask surface following trypsinization to recover astrocytes. Similar approaches have been cited by other laboratories (30). A final observation to confirm the relative purity of astrocyte cultures prepared in this manner is provided by our extensive experience working with S. aureus and its cell wall product PGN in glia. Our studies have reproducibly demonstrated that these gram-positive microbial stimuli are poor inducers of iNOS expression and subsequent NO production in primary microglia, whereas they are potent activators of iNOS and NO in astrocytes (9, 31, 32). The purity of astrocyte cultures used in these studies as determined by GFAP immunoreactivity and the absence of CD11b staining was routinely ≥ 95%.
Reagents
Recombinant mouse TNF-α was purchased from BD Biosciences (San Diego, CA) in a low endotoxin/no azide form. Caffeic acid phenethyl ester (CAPE), a non-specific inhibitor of the NF-κB pathway (33) and BAY 11-7082 and SC-514, potent inhibitors of IκB-α phosphorylation and IKK-2, respectively (34–36), were purchased from Calbiochem (San Diego, CA). Heat-inactivated S. aureus (strain RN6390, kindly provided by Dr. Ambrose Cheung, Dartmouth Medical School) was prepared as previously described (37) and E. coli O11:B1 LPS was obtained from List Biological Laboratories (Campbell, CA). All non-LPS reagents and culture media were verified to have endotoxin levels < 0.03 EU/ml as determined by Limulus amebocyte lysate assay (LAL; Associates of Cape Cod, Falmouth, MA).
Nitrite assay
Nitrite, a stable end product of resulting from the reaction of NO with molecular oxygen, was used to quantitate NO levels in astrocyte-conditioned supernatants as previously described (10).
Cell viability assays
The effects of NF-κB inhibitors on astrocyte cell viability were evaluated using a standard MTT assay as previously described (9).
Enzyme-linked immunosorbent assay (ELISA)
Quantitation of cytokine and chemokine levels in astrocyte-conditioned medium was performed using standard sandwich ELISA kits according to the manufacturer’s instructions (OptEIA mouse IL-1β and TNF-α, BD Pharmingen; DuoSet mouse CXCL2, R & D Systems, Minneapolis, MN).
Protein extraction and Western blotting
Protein extracts were prepared from primary astrocytes as previously described (38) and quantified using a standard protein assay (bicinchoninic acid protein assay reagent, BCA; Bio-Rad, Hercules, CA). TLR2 and iNOS expression was evaluated by Western blot using goat anti-mouse TLR2 (R & D Systems) or rabbit anti-mouse iNOS (Santa Cruz Biotechnology, Santa Cruz, CA) polyclonal antibodies as previously described (38). For quantitation, non-saturated autoradiographs were scanned and the pixel intensity for each band was determined using the Image/J program (NIH Image) and normalized to the amount of actin. Results are expressed in arbitrary units as the ratio of TLR2 or iNOS to actin.
Statistics
Significant differences between experimental groups were determined using the Student's t test at the 95% confidence interval using Sigma Stat (Chicago, IL).
RESULTS
The NF-κB pathway plays a pivotal role in inducing inflammatory mediator production by astrocytes in response to S. aureus
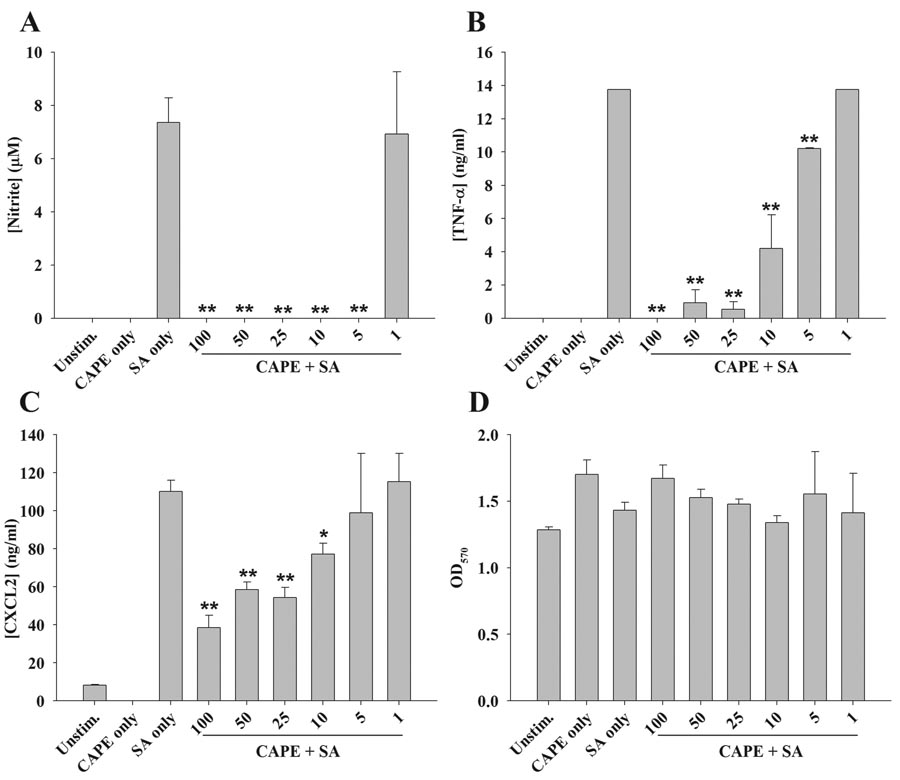
Astrocytes recognize S. aureus via TLR2, which leads to the downstream production of numerous proinflammatory mediators (9). TLR2 engagement leads to the activation of NF-κB and p38 MAPK signaling pathways (22, 23); however, the relative importance of the former has not yet been demonstrated in astrocytes in response to S. aureus. To evaluate the role of NF-κB signaling, astrocytes were treated with CAPE, a broad spectrum NF-κB inhibitor (33). Pre-treatment of cells with various concentrations of CAPE for 1 h prior to S. aureus exposure led to a dose-dependent inhibition of NO, TNF-α, and CXCL2 expression (Figure 1A–C, respectively). Cell viability assays revealed that CAPE was not cytotoxic at any of the concentrations examined, indicating that the anti-inflammatory effects observed were not due to cell death (Figure 1D).
Figure 1. The broad spectrum NF-κB inhibitor CAPE attenuates proinflammatory mediator production by S. aureus-stimulated astrocytes.
Primary astrocytes were seeded in 96-well plates at 1 × 105 cells per well and incubated overnight. The following day, cells were pre-treated for 1 h with the indicated concentrations of CAPE (1–100 µM) followed by stimulation with 107 colony forming units (cfu) of heat-inactivated S. aureus (SA). Cell-free supernatants were collected at 24 h following bacterial exposure and analyzed for nitrite (A), TNF-α (B), and CXCL2 (C) production. Astrocyte viability was assessed using a standard MTT assay and the raw OD570 absorbance values are provided (D). Results are reported as the mean ± SD of three independent wells for each experimental treatment. Significant differences between S. aureus-stimulated astrocytes versus S. aureus + CAPE are denoted with asterisks (*, p < 0.05; **, p < 0.001). Results are representative of four independent experiments.
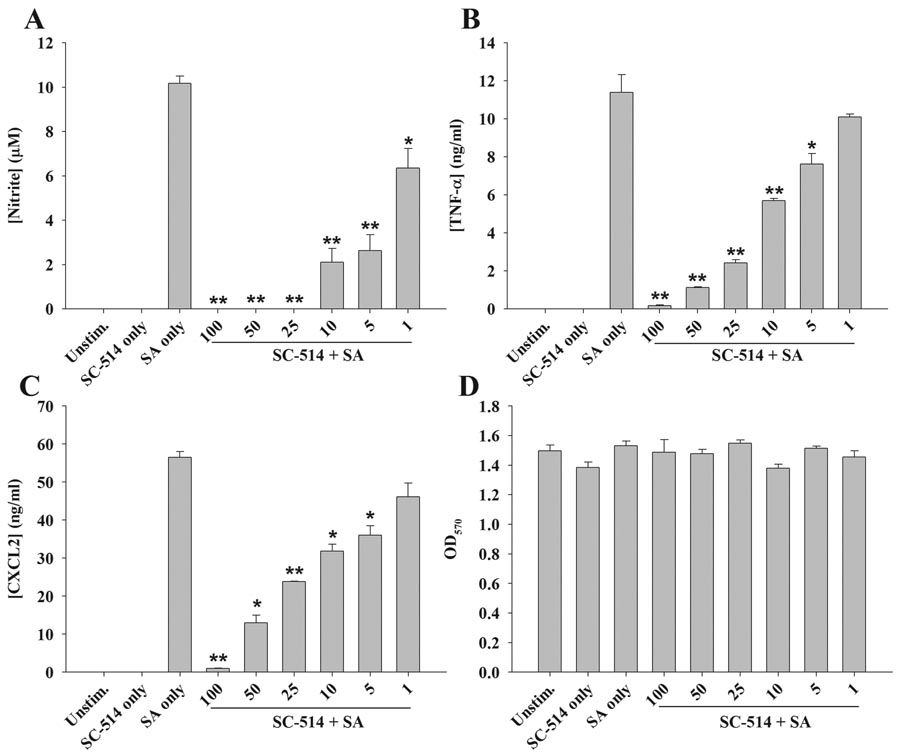
Since CAPE is a rather non-specific inhibitor of NF-κB, the importance of this signaling cascade was confirmed with the use of additional, more specific blockers. Similar to what was observed with CAPE, the IKK complex inhibitor SC-514 was also capable of attenuating NO, TNF-α, and CXCL2 expression in a dose-dependent manner (Figure 2A–C, respectively). Likewise, BAY 11-7082, which affects NF-κB signaling downstream by blocking IκBα phosphorylation, also inhibited proinflammatory mediator release from astrocytes following S. aureus exposure (data not shown). Cell viability assays revealed that neither SC-514 nor BAY 11-7082 were cytotoxic at any of the concentrations examined, indicating that the inhibitory actions of these compounds were not attributable to cell death (Figures 2D and data not shown). Collectively, these findings indicate that NF-κB signaling is a primary pathway for inducing a broad range of astrocytic proinflammatory mediators in response to S. aureus.
Figure 2. The IKK inhibitor SC-514 attenuates astrocytic proinflammatory mediator release in response to S. aureus.
Primary astrocytes were seeded in 96-well plates at 1 × 105 cells per well and incubated overnight. The following day, cells were pre-treated for 1 h with the indicated concentrations of SC-514 (1–100 µM) followed by stimulation with 107 colony forming units (cfu) of heat-inactivated S. aureus (SA). Cell-free supernatants were collected at 24 h following bacterial exposure and analyzed for nitrite (A), TNF-α (B), and CXCL2 (C) production. Astrocyte viability was assessed using a standard MTT assay and the raw OD570 absorbance values are provided (D). Results are reported as the mean ± SD of three independent wells for each experimental treatment. Significant differences between S. aureus-stimulated astrocytes versus S. aureus + SC-514 are denoted with asterisks (*, p < 0.05; **, p < 0.001). Results are representative of two independent experiments.
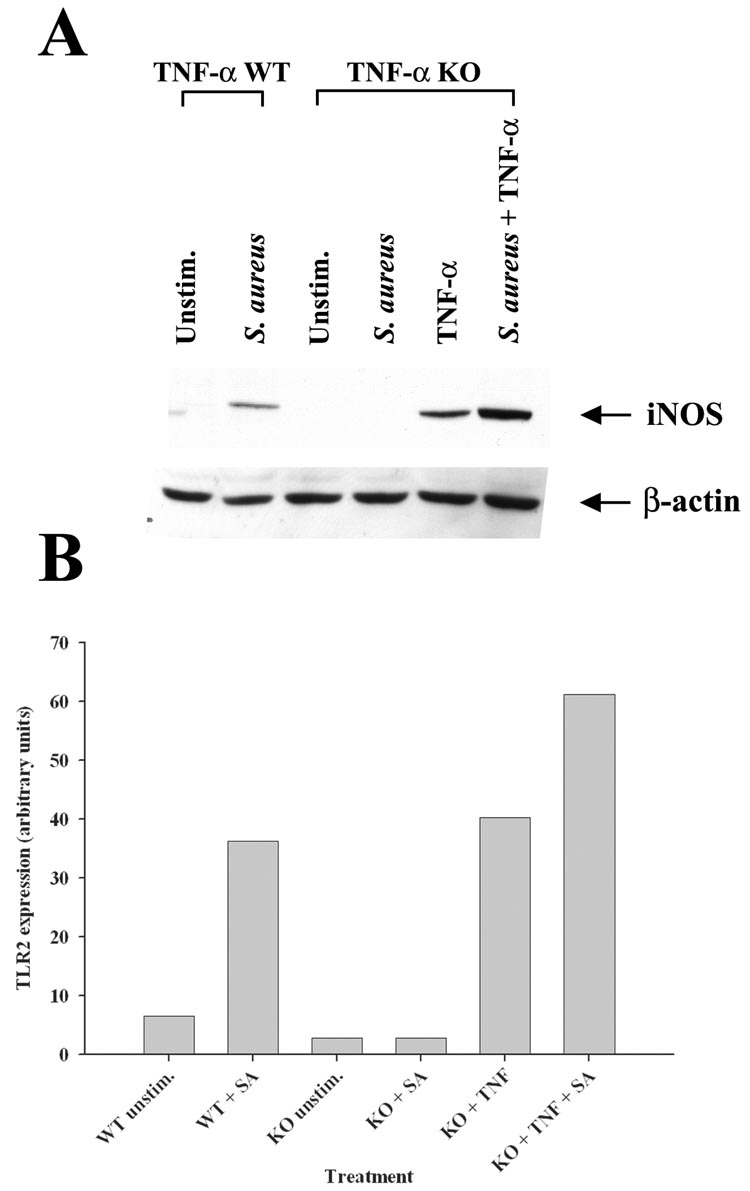
Astrocytes produce TNF-α upon S. aureus stimulation that acts in an autocrine/paracrine manner to augment TLR2 expression
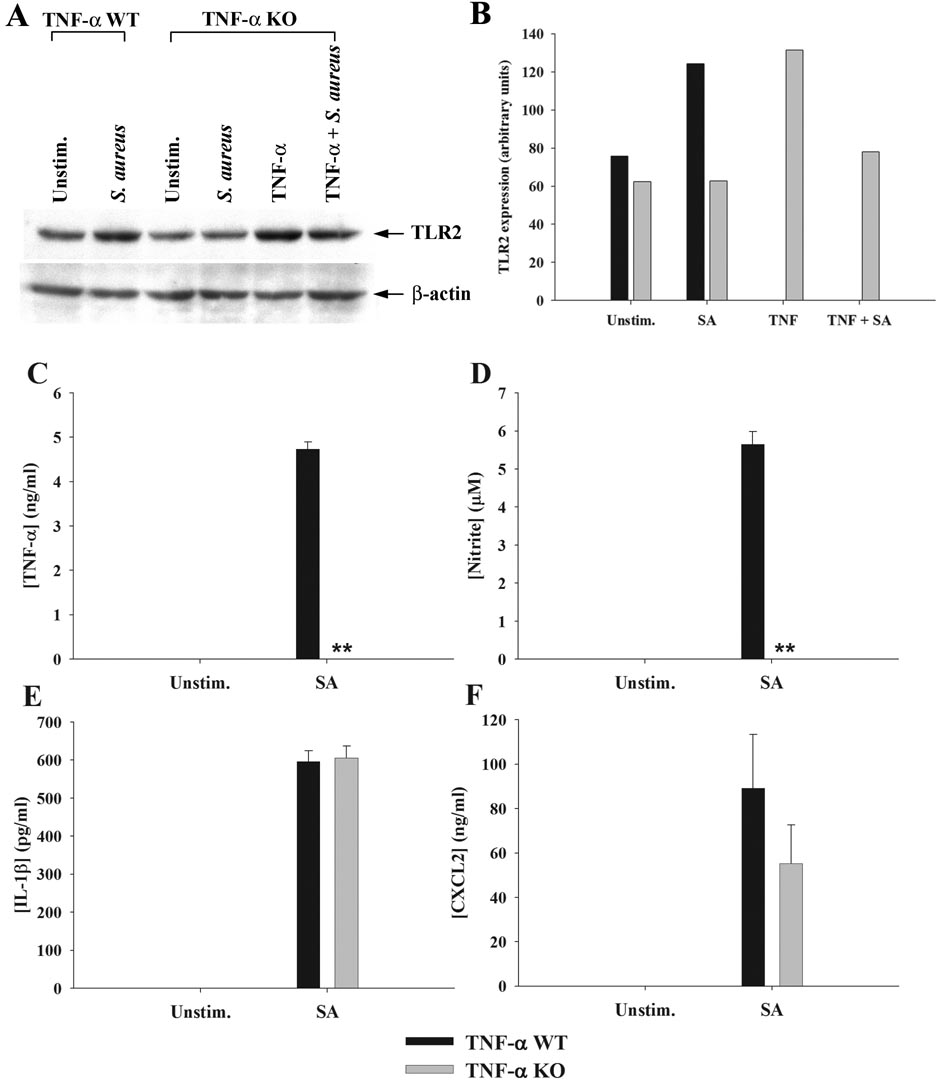
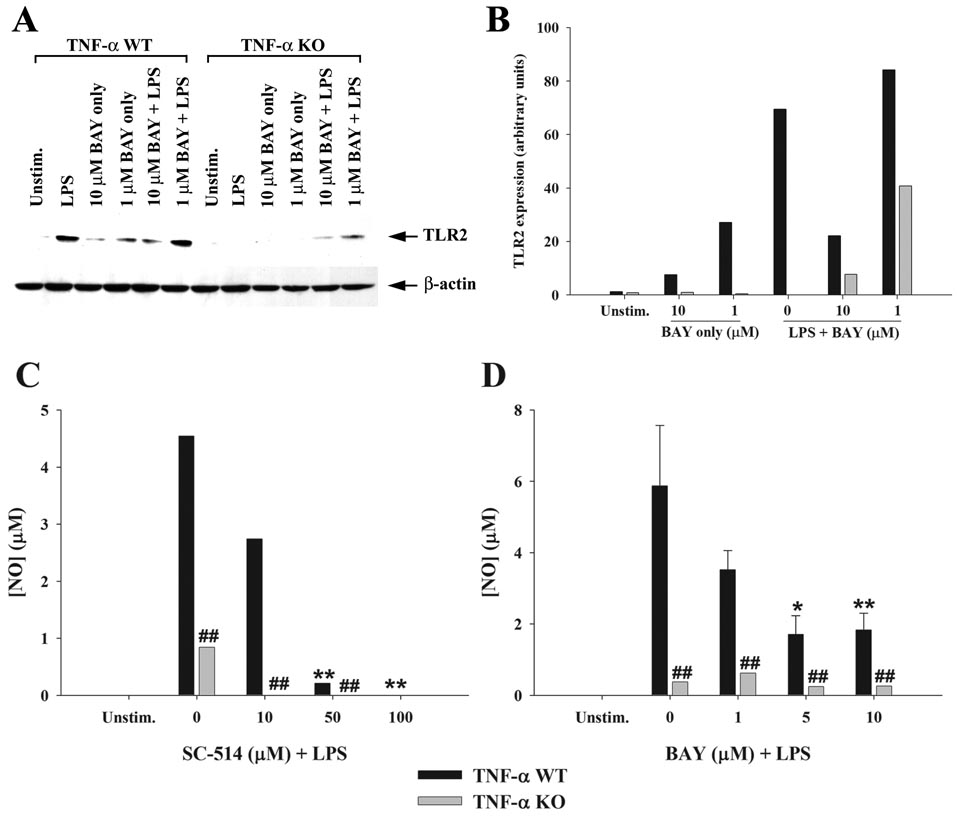
Besides inducing proinflammatory mediator release, another consequence of S. aureus stimulation in astrocytes is an increase in TLR2 expression (9, 10). Since TNF-α is a major product of S. aureus-activated astrocytes, it was plausible that this cytokine may regulate TLR2 expression in an autocrine/paracrine fashion. To investigate this possibility, TLR2 levels were assessed in both TNF-α KO and WT astrocytes in response to S. aureus stimulation. A pivotal role for autocrine/paracrine TNF-α in augmenting TLR2 expression was demonstrated by the fact that receptor levels remained unchanged in TNF-α KO astrocytes following bacterial exposure, whereas TLR2 expression was increased in WT cells in response to S. aureus (Figure 3). Treatment of TNF-α KO astrocytes with recombinant TNF-α was capable of restoring the increase in TLR2 expression, confirming the pivotal autocrine/paracrine actions of this cytokine in response to bacterial activation (Figure 3). Similar effects were observed following LPS stimulation of astrocytes (Figure 4), suggesting that TNF-α is an important regulator of astrocytic TLR2 expression in response to diverse microbial stimuli. Although a slight increase in TLR2 expression was observed in TNF-α KO astrocytes in response to a combination of LPS + BAY 11-7082, this was not a consistent finding and is considered to represent slight biological variation. Although LPS is not a TLR2 ligand, previous studies from other groups have demonstrated that LPS augments TLR2 expression in glia (13, 39–41). The novel finding in this study is that this increase is modulated by the autocrine/paracrine action of TNF-α.
Figure 3. Astrocytes produce TNF-α in response to S. aureus that functions in an autocrine/paracrine manner to increase TLR2 and NO expression.
Primary astrocytes isolated from TNF-α WT or KO mice were stimulated with heat-inactivated S. aureus +/− 100 ng/ml of recombinant mouse TNF-α for 24 h, whereupon whole cell extracts were prepared and analyzed for TLR2 expression by Western blotting (A and B). Results are presented as the raw gel data (A) and quantitative analysis of TLR2 expression by densitometry (B). For quantitation in (B), the pixel intensity of each TLR2 band was normalized to the amount of actin to verify uniformity in gel loading. In addition, cell-free supernatants were collected at 24 h following bacterial exposure and analyzed for TNF-α (C), nitrite (D), IL-1β (E), and CXCL2 (F) production. Results are reported as the mean ± SD of three independent wells for each experimental treatment (C–F). Significant differences between TNF-α WT and KO astrocytes stimulated with S. aureus are denoted with asterisks (**, p < 0.001). Results are representative of two (A–B) or six (C–F) independent experiments.
Figure 4. Autocrine/paracrine TNF-α and NF-κB-dependent signaling augment TLR2 and NO expression in response to LPS stimulation.
Primary astrocytes isolated from TNF-α WT or KO mice were were pre-treated with the indicated concentrations of the IKK or IkBα inhibitors SC-514 or BAY 11-7082, respectively for 1 h followed by LPS (100 ng/ml). Whole cell extracts were prepared 24 h following LPS stimulation and analyzed for TLR2 expression by Western blotting (A and B). Results are presented as the raw gel data (A) and quantitative analysis of TLR2 expression by densitometry (B). For quantitation in (B), the pixel intensity of each TLR2 band was normalized to the amount of actin to verify uniformity in gel loading. In addition, cell-conditioned supernatants were collected 24 h following LPS exposure, whereupon nitrite levels were determined (C and D). Significant differences between WT astrocytes treated with LPS only versus LPS + NF-κB inhibitors are denoted with asterisks (*, p < 0.05; **, p < 0.001), whereas alterations between TNF-α WT versus KO astrocytes are indicated by hatched signs (##, p < 0.001). Results are representative of two independent experiments.
S. aureus-induced NO production is TNF-α-dependent
In addition to investigating the role of TNF-α in regulating TLR2 expression, we also examined whether proinflammatory mediator production was affected upon TNF-α loss. As expected, TNF-α KO astrocytes did not produce TNF-α in response to bacterial challenge, confirming the absence of cytokine in KO cells (Figure 3A). Interestingly, S. aureus-induced NO production was completely ablated in TNF-α KO astrocytes compared to WT cells (Figure 3B), whereas the expression of numerous other proinflammatory mediators (i.e. IL-1β, and CXCL2) was not affected (Figure 3C and D). Cell viability assays revealed similar signals between TNF-α WT and KO astrocytes, indicating that the inability of the latter to produce NO did not originate from different cell seeding densities (data not shown). We also examined iNOS expression in S. aureus-stimulated TNF-α WT and KO astrocytes and similar to our findings with NO release, iNOS was not induced in TNF-α KO cells, whereas TNF-α restoration led to an increase in iNOS expression (Figure 5). A similar TNF-α - and NF-κB-dependence for NO production was observed in response to LPS stimulation (Figure 4). Collectively, these results suggest that TNF-α produced by astrocytes in response to diverse microbial stimuli functions in an autocrine/paracrine manner to induce subsequent NO production.
Figure 5. iNOS expression is regulated by the autocrine/paracrine action of TNF-α in response to S. aureus.
Primary astrocytes isolated from TNF-α WT or KO mice were seeded in 6-well plates at 1 × 106 cells per well and incubated overnight. The following day, cells were stimulated with 108 colony forming units (cfu) of heat-inactivated S. aureus +/− 100 ng/ml of recombinant mouse TNF-α for 24 h, whereupon whole cell extracts were prepared and analyzed for iNOS expression by Western blotting. Results are presented as the raw gel data (A) and quantitative analysis of iNOS expression by densitometry (B). For quantitation in (B), the pixel intensity of each iNOS band was normalized to the amount of actin to verify uniformity in gel loading. Results are expressed in arbitrary units as the ratio of iNOS to actin and are representative of two independent experiments.
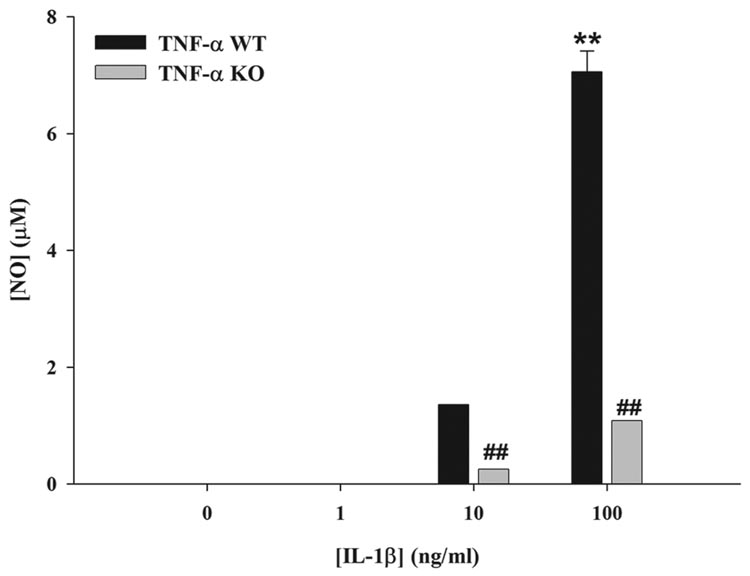
Autocrine/paracrine IL-1β is not sufficient for astrocytic NO production in response to microbial stimuli
Previous studies have reported that IL-1β is a major inducer of NO production in astrocytes, whereas TNF-α had relatively little effect (42–45). These findings contradict our results with TNF-α KO astrocytes, where a strict TNF-α-dependence for NO release was demonstrated. However, our studies differ since a microbial stimulus was applied to astrocytes, which elicits a complex milieu of inflammatory mediators that may rely on the autocrine/paracrine action of TNF-α to induce maximal NO release. To evaluate the effects of IL-1β on NO production, we treated TNF-α KO and WT astrocytes with various concentrations of recombinant mouse IL-1β (i.e. 1–100 ng/ml). The results demonstrated that high doses of IL-1β (i.e. 10 and 100 ng/ml) were capable of inducing NO release from primary astrocytes; however, the lowest concentration of IL-1β tested (i.e. 1 ng/ml) was not sufficient to elicit NO production (Figure 6). This is an important finding since the amount of IL-1 produced by S. aureus-activated astrocytes is significantly less compared to the lowest dose of IL-1β examined (i.e. < 1 ng/ml). Interestingly, NO levels generated by high dose IL-1β in TNF-α KO astrocytes were approximately 7-fold lower compared to WT cells, revealing an important role for TNF-α in regulating NO expression even in response to IL-1β treatment. Collectively, these results indicate that although IL-1β is capable of inducing NO production in primary astrocytes, the level of cytokine required for this response well exceeds that typically produced by astrocytes in response to S. aureus stimulation. Therefore, an autocrine/paracrine effect of IL-1β does not appear to influence NO production by primary astrocytes; rather our data suggests that TNF-α is a major player in dictating NO release.
Figure 6. High dose IL-1β is capable of inducing NO production in primary astrocytes.
TNF-α WT and KO astrocytes were seeded in 96-well plates at 1 × 105 cells per well and incubated overnight. The following day, cells were treated with the indicated concentrations of IL-1β for 24 h, whereupon nitrite levels were determined. Significant differences between unstimulated and IL-1β-treated WT astrocytes are denoted with asterisks (**, p < 0.001), whereas alterations between TNF-α WT versus KO astrocytes are indicated by hatched signs (##, p < 0.001). Results are representative of two independent experiments.
Microbial stimuli augment astrocytic TLR2 expression via a NF-κB-dependent pathway
We have previously reported that S. aureus is a potent inducer of TLR2 expression in astrocytes (9, 10) and other groups have demonstrated that LPS augments TLR2 expression in glia despite the fact that it is not a TLR2 ligand (13, 39–41). To determine the importance of NF-κB-dependent signaling pathways in regulating astrocytic TLR2 expression, Western blots were performed. The IKK and IkBα inhibitors SC-514 and BAY 11-7082, respectively, were capable of attenuating the activation-dependent increase in TLR2 levels in response to either S. aureus or LPS (Figure 4 and data not shown). These results indicate that the NF-κB pathway plays a critical role in enhancing astrocytic TLR2 expression in response to diverse bacterial ligands.
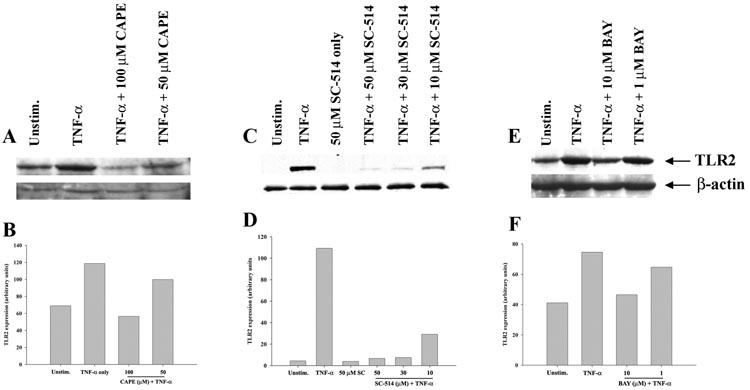
NF-κB-dependent signaling is pivotal for the TNF-α-induced increase in astrocytic TLR2 expression
In several cell types of the innate immune system including macrophages and microglia, TLR2 expression is driven, in part, via NF-κB (46–48). In addition, TNF-α also triggers the NF-κB signaling pathway (19, 20). However, the involvement of NF-κB in regulating astrocytic TLR2 expression in response to TNF-α has not yet been examined. To determine whether TNF-α-induced TLR2 expression in astrocytes is mediated via a NF-κB signaling pathway, we utilized the same series of NF-κB pathway inhibitors previously described. As expected, TNF-α exposure augmented TLR2 expression in astrocytes, which was attenuated upon pre-treatment with either CAPE, SC-514 or BAY 11-7082 in a dose-dependent manner (Figure 7). Collectively, these results suggest that NF-κB represents a major signaling cascade responsible for the TNF-α-mediated induction of TLR2 expression in astrocytes.
Figure 7. Three distinct NF-κB inhibitors attenuate astrocytic TNF-α-induced TLR2 expression.
Primary astrocytes were seeded in 6-well plates at 1 × 106 cells per well and incubated overnight. The following day, cells were pre-treated with various concentrations of CAPE (A and B), SC-514 (C and D), or BAY 11-7082 (E and F) for 1 h prior to TNF-α stimulation (100 ng/ml) for 24 h, whereupon whole cell extracts were prepared and analyzed for TLR2 expression by Western blotting. Results are presented as the raw gel data (A, C, and E) and quantitative analysis of TLR2 expression by densitometry (B, D, and F). For quantitation, the pixel intensity of each TLR2 band was normalized to the amount of actin to verify uniformity in gel loading. Results are expressed in arbitrary units as the ratio of TLR2 to actin and are representative of three independent experiments.
DISCUSSION
Initially thought to act as mere supporting cells, emerging evidence indicates that astrocytes participate in innate immune responses to injurious or infectious stimuli (49–51). Despite the important role TLR2 plays in S. aureus recognition by astrocytes (9), the cell signaling cascades regulating TLR2 expression and subsequent proinflammatory mediator production in activated astrocytes remained to be defined. Based on the known downstream signaling cascades triggered by TLR2 activation (23, 52), NF-κB was envisioned as the most likely pathway involved in regulating S. aureus-induced astrocyte activation. Indeed, other studies have reported that the NF-κB pathway is important for eliciting astrocytic proinflammatory mediator production in response to the TLR4 ligand LPS (53–55). To determine whether the NF-κB pathway participates in regulating astrocytic responses to S. aureus, we performed studies utilizing three distinct pharmacological inhibitors acting against discrete steps along the NF-κB pathway. The results indicated that NF-κB signals are a significant contributor towards inflammatory mediator release in astrocytes following S. aureus exposure.
Subsequent studies revealed that TNF-α is pivotal for regulating S. aureus- and LPS-induced TLR2 expression since TNF-α KO astrocytes failed to upregulate TLR2 upon bacterial exposure. Additional evidence to support this relationship was provided when TLR2 levels were restored upon treatment of TNF-α KO astrocytes with recombinant TNF-α. The effects of TNF-α were observed at concentrations that approximate those detected during brain abscess development (16, 17), although it is difficult to make direct comparisons due to the likelihood that TNF-α concentrations in various tissue microdomains may differ dramatically. To our knowledge, this is the first report to demonstrate the autocrine/paracrine action of any inflammatory mediator on TLR2 expression in astrocytes.
NF-κB is one of the primary signaling cascades triggered by TNF-α binding to its receptor (19, 20). Based on the finding that NF-κB inhibitors attenuated astrocytic inflammatory mediator release and the fact that the mouse TLR2 promoter contains several consensus NF-κB binding motifs (46, 47, 56), we evaluated whether NF-κB influences TLR2 expression in astrocytes following TNF-α exposure. We found that TNF-α-induced TLR2 expression in astrocytes is NF-κB-dependent since all of the NF-κB inhibitors tested blocked the characteristic increase in TLR2 expression observed following cytokine treatment. In addition, NF-κB-dependent signaling was also found to regulate the characteristic increase in astrocytic TLR2 expression observed following both S. aureus and LPS stimulation. Although LPS is not a TLR2 ligand, other studies have also demonstrated that LPS augments TLR2 levels (13, 39–41). Increasing TLR2 expression may facilitate more efficient pathogen recognition since TLR2 also triggers cytokine signaling pathways in response to bacterial lipoproteins that are contained within the outer cell walls of both gram-positive and –negative bacteria. However, this possibility remains speculative. Collectively, these observations are in agreement with previous reports where the NF-κB pathway has been shown to regulate TLR2 expression in various immune cells in response to select stimuli including microglia and macrophages (46–48, 56).
Next we evaluated whether TNF-α loss affected the production of additional immune molecules in astrocytes. Interestingly, we found that TNF-α loss resulted in a selective suppression of NO production in both S. aureus- and LPS-stimulated astrocytes. This observation is in agreement with earlier studies where TNF-α has been shown to induce iNOS expression and subsequent NO production. For example, iNOS expression in response to β-amyloid + IFN-γ stimulation was shown to be mediated, in part, by TNF-α through a TRAF6-, TRAF2-, and NIK-dependent pathway in astrocytes (57). Another recent report has demonstrated that in macrophages, TNF-α is required for iNOS induction in response to IFN-γ via a NF-κB-dependent mechanism (58). However, an important distinction between these reports and our work is the lack of IFN-γ involvement in the current study. In contrast to these reports, others have demonstrated that IL-1β is a major inducer of iNOS in rodent astrocytes, whereas TNF-α is not effective by itself but rather, requires the presence of an additional cytokine such as IL-1β or IFN-γ (42–45). Because of these discrepancies, we directly examined whether IL-1β was capable of inducing NO production in TNF-α WT and KO astrocytes. Only high concentrations of IL-1β (i.e. 10–100 ng/ml) were effective at inducing NO release in primary WT astrocytes, which greatly exceeds levels normally produced by S. aureus-activated astrocytes (i.e. < 1 ng/ml). Collectively, these findings suggest that TNF-α is a major regulator of astrocytic NO production in response to microbial stimuli. The complex interplay between multiple inflammatory mediators in regulating astrocytic NO production is similar to earlier studies by others (30, 45). Specifically, these authors demonstrated that IFN-γ primed astrocytes for maximal TNF-α release in response to LPS stimulation, whereas either stimulus alone was not effective. Likewise, a combination of IFN-γ plus IL-1β was also capable of inducing TNF-α production, revealing synergistic effects of distinct inflammatory mediators (30). This theme of synergy is similar to our findings where endogenous TNF-α is required for NO production in response to microbial stimuli. Surprisingly, we did not observe any consistent differences in the expression of other proinflammatory mediators between TNF-α KO and WT astrocytes. One possibility to explain this finding could stem from the fact that iNOS is a late inducible gene and typically requires priming by alternative mediators such as IFN-γ or IFN-β (30, 44, 58, 59). This is generally not the case for the other inflammatory mediators examined in this study (i.e. IL-1β and CXCL2), which are induced rapidly upon cell activation. However, we cannot exclude the possibility that TNF-α KO astrocytes may produce low levels of NO upon bacterial exposure with extended incubation periods (i.e. 48 h). In addition, IL-1β is unique in that it exists intracellularly in a pre-formed state and requires processing by caspase-1 for maturation into the biologically active cytokine (60, 61). Therefore, inherent differences in the timing of gene expression following cell activation and processing pathways might be responsible, in part, for the selective influence of TNF-α on NO production. It remains possible that TNF-α impacts alternative inflammatory mediators in astrocytes that were not examined in the current study, an issue that remains to be investigated.
It is interesting that a complete blockade of NO production was observed in TNF-α KO astrocytes following S. aureus stimulation since iNOS expression is regulated by several distinct pathways (42). Although we have demonstrated that NF-κB activation is important for regulating iNOS expression, the precise mechanism(s) responsible for this outcome remain to be defined. However, several possibilities may be considered when contemplating the mechanism by which TNF-α dictates iNOS expression in astrocytes. First, other stimuli such as LPS + IFN-γ induce the production of reactive oxygen species (ROS) that have been reported to positively regulate iNOS expression via NF-κB (44, 62). In this instance, the loss of TNF-α may indirectly affect NO production by interfering with ROS release; however, the ability of S. aureus to induce ROS production has not yet been examined. Second, compounds that increase cAMP have been shown to inhibit LPS- and cytokine-induced NO production in several cell types including astrocytes (59, 63, 64); therefore, it is possible that cAMP levels may be elevated in TNF-α KO astrocytes following S. aureus treatment. A similar relationship could occur with regard to phosphoinositol-3 kinase (PI3K), since elevated PI3K levels have been associated with an increase in iNOS expression (65). In addition, NF-κB activation in response to TNF-α requires PI3K activity (66). Since we did not measure cAMP or PI3K levels in response to bacterial exposure, these possibilities remain highly speculative. Finally, a recent study has shown that TNF-α induces the expression of GTP cyclohydrolase, the rate limiting enzyme for tetrahydrobiopterin synthesis, an important co-factor for iNOS activity (67). In the absence of TNF-α, this co-factor may not be induced which could also lead to a reduction in iNOS expression in agreement with our findings.
Previous studies from our laboratory and others have revealed an important role for TNF-α in the host anti-bacterial immune response during brain abscess development (16, 17). By extension, these findings suggest that priming the CNS by TNF-α administration may be a potential method to augment S. aureus recognition and inactivation through elevated TLR2 and NO expression. Particularly, heightened NO production may facilitate efficient pathogen clearance via the formation of peroxynitrite (formed by the reaction between NO and hydrogen peroxide). However, a recent study from our laboratory has demonstrated that although TNF-α is capable of increasing TLR2 expression in microglia, this did not translate into enhanced cytokine production or phagocytosis (48), although bactericidal activity was not examined. It remains possible that enhanced TLR2 expression may significantly impact the host immune response during brain abscess development since the treatment paradigm utilized during our in vitro studies is much less complex compared to what occurs during CNS infections such as brain abscess.
Collectively, these results suggest that S. aureus-induced astrocyte activation is mediated via a NF-κB pathway involving TNF-α as an important effector molecule for amplifying TLR2 expression. The identification of such regulatory pathways and their potent inhibitors might provide effective and safe treatment options for diseases, such as brain abscess, where persistent inflammation contributes to disease pathology.
ACKNOWLEDGEMENTS
The authors would like to thank Gail Wagoner and Paul Malbrough for excellent technical assistance.
Footnotes
This work was supported by the NIH National Institutes of Mental Health (RO1 MH65297) and Neurological Disorders and Stroke (RO1 NS055385) to T.K. and the NINDS supported Core Facility at UAMS (P30 NS047546).
REFERENCES
- 1.Mathisen GE, Johnson JP. Brain abscess. Clin Infect Dis. 1997;25:763–779. doi: 10.1086/515541. quiz 780-761. [DOI] [PubMed] [Google Scholar]
- 2.Townsend GC, Scheld WM. Infections of the central nervous system. Adv Intern Med. 1998;43:403–447. [PubMed] [Google Scholar]
- 3.Baldwin AC, Kielian T. Persistent immune activation associated with a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuroimmunol. 2004;151:24–32. doi: 10.1016/j.jneuroim.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Kielian T, Esen N, Liu S, Phulwani NK, Syed MM, Phillips N, Nishina K, Cheung AL, Schwartzman JD, Ruhe JJ. Minocycline Modulates Neuroinflammation Independently of Its Antimicrobial Activity in Staphylococcus aureus-Induced Brain Abscess. Am J Pathol. 2007;171:1199–1214. doi: 10.2353/ajpath.2007.070231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stenzel W, Soltek S, Schluter D, Deckert M. The intermediate filament GFAP is important for the control of experimental murine Staphylococcus aureus-induced brain abscess and Toxoplasma encephalitis. J Neuropathol Exp Neurol. 2004;63:631–640. doi: 10.1093/jnen/63.6.631. [DOI] [PubMed] [Google Scholar]
- 6.Kielian T. Immunopathogenesis of brain abscess. J Neuroinflammation. 2004;1:16–26. doi: 10.1186/1742-2094-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 8.Kopp E, Medzhitov R. Recognition of microbial infection by Toll-like receptors. Curr Opin Immunol. 2003;15:396–401. doi: 10.1016/s0952-7915(03)00080-3. [DOI] [PubMed] [Google Scholar]
- 9.Esen N, Tanga FY, DeLeo JA, Kielian T. Toll-like receptor 2 (TLR2) mediates astrocyte activation in response to the Gram-positive bacterium Staphylococcus aureus. J Neurochem. 2004;88:746–758. doi: 10.1046/j.1471-4159.2003.02202.x. [DOI] [PubMed] [Google Scholar]
- 10.Phulwani NK, Feinstein DL, Gavrilyuk V, Akar C, Kielian T. 15-deoxy-Delta 12,14-prostaglandin J2 (15d-PGJ2) and ciglitazone modulate Staphylococcus aureus-dependent astrocyte activation primarily through a PPAR-gamma-independent pathway. J Neurochem. 2006;99:1389–1402. doi: 10.1111/j.1471-4159.2006.04183.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carpentier PA, Begolka WS, Olson JK, Elhofy A, Karpus WJ, Miller SD. Differential activation of astrocytes by innate and adaptive immune stimuli. Glia. 2005;49:360–374. doi: 10.1002/glia.20117. [DOI] [PubMed] [Google Scholar]
- 12.Kielian T. Toll-like receptors in central nervous system glial inflammation and homeostasis. J Neurosci Res. 2006;83:711–730. doi: 10.1002/jnr.20767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- 14.Mayhan WG. Cellular mechanisms by which tumor necrosis factor-alpha produces disruption of the blood-brain barrier. Brain Res. 2002;927:144–152. doi: 10.1016/s0006-8993(01)03348-0. [DOI] [PubMed] [Google Scholar]
- 15.Claudio L, Martiney JA, Brosnan CF. Ultrastructural studies of the blood-retina barrier after exposure to interleukin-1 beta or tumor necrosis factor-alpha. Lab Invest. 1994;70:850–861. [PubMed] [Google Scholar]
- 16.Kielian T, Bearden ED, Baldwin AC, Esen N. IL-1 and TNF-alpha play a pivotal role in the host immune response in a mouse model of Staphylococcus aureus-induced experimental brain abscess. J Neuropathol Exp Neurol. 2004;63:381–396. doi: 10.1093/jnen/63.4.381. [DOI] [PubMed] [Google Scholar]
- 17.Stenzel W, Soltek S, Miletic H, Hermann MM, Korner H, Sedgwick JD, Schluter D, Deckert M. An essential role for tumor necrosis factor in the formation of experimental murine Staphylococcus aureus-induced brain abscess and clearance. J Neuropathol Exp Neurol. 2005;64:27–36. doi: 10.1093/jnen/64.1.27. [DOI] [PubMed] [Google Scholar]
- 18.Aranguez I, Torres C, Rubio N. The receptor for tumor necrosis factor on murine astrocytes: characterization, intracellular degradation, and regulation by cytokines and Theiler's murine encephalomyelitis virus. Glia. 1995;13:185–194. doi: 10.1002/glia.440130305. [DOI] [PubMed] [Google Scholar]
- 19.Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- 20.Aggarwal BB. Signalling pathways of the TNF superfamily: a double-edged sword. Nat Rev Immunol. 2003;3:745–756. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 21.Hayden MS, West AP, Ghosh S. NF-kappaB and the immune response. Oncogene. 2006;25:6758–6780. doi: 10.1038/sj.onc.1209943. [DOI] [PubMed] [Google Scholar]
- 22.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 23.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 24.Hacker H, Karin M. Regulation and function of IKK and IKK-related kinases. Sci STKE. 2006;2006 doi: 10.1126/stke.3572006re13. re13. [DOI] [PubMed] [Google Scholar]
- 25.Yamamoto Y, Gaynor RB. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem Sci. 2004;29:72–79. doi: 10.1016/j.tibs.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 26.Perkins ND. Integrating cell-signalling pathways with NF-kappaB and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62. doi: 10.1038/nrm2083. [DOI] [PubMed] [Google Scholar]
- 27.Kawai T, Akira S. Signaling to NF-kappaB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Saura J. Microglial cells in astroglial cultures: a cautionary note. J Neuroinflammation. 2007;4:26–37. doi: 10.1186/1742-2094-4-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hamby ME, Uliasz TF, Hewett SJ, Hewett JA. Characterization of an improved procedure for the removal of microglia from confluent monolayers of primary astrocytes. J Neurosci Methods. 2006;150:128–137. doi: 10.1016/j.jneumeth.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 30.Chung IY, Benveniste EN. Tumor necrosis factor-alpha production by astrocytes. Induction by lipopolysaccharide, IFN-gamma, and IL-1 beta. J Immunol. 1990;144:2999–3007. [PubMed] [Google Scholar]
- 31.Esen N, Kielian T. Central role for MyD88 in the responses of microglia to pathogen-associated molecular patterns. J Immunol. 2006;176:6802–6811. doi: 10.4049/jimmunol.176.11.6802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kielian T, Syed MMd, Liu S, Phillips N, Wagoner G, Drew PD, Esen N. The synthetic PPAR-g agonist ciglitazone attenuates neuroinflammation and accelerates encapsulation in bacterial brain abscesses. Journal of Immunology. 2008;180:5004–5016. doi: 10.4049/jimmunol.180.7.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Natarajan K, Singh S, Burke TR, Jr, Grunberger D, Aggarwal BB. Caffeic acid phenethyl ester is a potent and specific inhibitor of activation of nuclear transcription factor NF-kappa B. Proc Natl Acad Sci U S A. 1996;93:9090–9095. doi: 10.1073/pnas.93.17.9090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kishore N, Sommers C, Mathialagan S, Guzova J, Yao M, Hauser S, Huynh K, Bonar S, Mielke C, Albee L, Weier R, Graneto M, Hanau C, Perry T, Tripp CS. A selective IKK-2 inhibitor blocks NF-kappa B-dependent gene expression in interleukin-1 beta-stimulated synovial fibroblasts. J Biol Chem. 2003;278:32861–32871. doi: 10.1074/jbc.M211439200. [DOI] [PubMed] [Google Scholar]
- 35.Jeong SJ, Pise-Masison CA, Radonovich MF, Park HU, Brady JN. A novel NF-kappaB pathway involving IKKbeta and p65/RelA Ser-536 phosphorylation results in p53 Inhibition in the absence of NF-kappaB transcriptional activity. J Biol Chem. 2005;280:10326–10332. doi: 10.1074/jbc.M412643200. [DOI] [PubMed] [Google Scholar]
- 36.Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cytokine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–21103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
- 37.Kielian T, Mayes P, Kielian M. Characterization of microglial responses to Staphylococcus aureus: effects on cytokine, costimulatory molecule, and Toll-like receptor expression. J Neuroimmunol. 2002;130:86–99. doi: 10.1016/s0165-5728(02)00216-3. [DOI] [PubMed] [Google Scholar]
- 38.Kielian T, Esen N, Bearden ED. Toll-like receptor 2 (TLR2) is pivotal for recognition of S. aureus peptidoglycan but not intact bacteria by microglia. Glia. 2005;49:567–576. doi: 10.1002/glia.20144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu J, Drew PD. Peroxisome proliferator-activated receptor-gamma agonists suppress the production of IL-12 family cytokines by activated glia. J Immunol. 2007;178:1904–1913. doi: 10.4049/jimmunol.178.3.1904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 41.Laflamme N, Soucy G, Rivest S. Circulating cell wall components derived from gram-negative, not gram-positive, bacteria cause a profound induction of the gene-encoding Toll-like receptor 2 in the CNS. J Neurochem. 2001;79:648–657. doi: 10.1046/j.1471-4159.2001.00603.x. [DOI] [PubMed] [Google Scholar]
- 42.Saha RN, Pahan K. Signals for the induction of nitric oxide synthase in astrocytes. Neurochem Int. 2006;49:154–163. doi: 10.1016/j.neuint.2006.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jana M, Anderson JA, Saha RN, Liu X, Pahan K. Regulation of inducible nitric oxide synthase in proinflammatory cytokine-stimulated human primary astrocytes. Free Radic Biol Med. 2005;38:655–664. doi: 10.1016/j.freeradbiomed.2004.11.021. [DOI] [PubMed] [Google Scholar]
- 44.Marcus JS, Karackattu SL, Fleegal MA, Sumners C. Cytokine-stimulated inducible nitric oxide synthase expression in astroglia: role of Erk mitogen-activated protein kinase and NF-kappaB. Glia. 2003;41:152–160. doi: 10.1002/glia.10168. [DOI] [PubMed] [Google Scholar]
- 45.Kozuka N, Itofusa R, Kudo Y, Morita M. Lipopolysaccharide and proinflammatory cytokines require different astrocyte states to induce nitric oxide production. J Neurosci Res. 2005;82:717–728. doi: 10.1002/jnr.20671. [DOI] [PubMed] [Google Scholar]
- 46.Musikacharoen T, Matsuguchi T, Kikuchi T, Yoshikai Y. NF-kappa B and STAT5 play important roles in the regulation of mouse Toll-like receptor 2 gene expression. J Immunol. 2001;166:4516–4524. doi: 10.4049/jimmunol.166.7.4516. [DOI] [PubMed] [Google Scholar]
- 47.Wang T, Lafuse WP, Zwilling BS. NFkappaB and Sp1 elements are necessary for maximal transcription of toll-like receptor 2 induced by Mycobacterium avium. J Immunol. 2001;167:6924–6932. doi: 10.4049/jimmunol.167.12.6924. [DOI] [PubMed] [Google Scholar]
- 48.Syed MM, Phulwani NK, Kielian T. Tumor necrosis factor-alpha (TNF-alpha) regulates Toll-like receptor 2 (TLR2) expression in microglia. J Neurochem. 2007;103:1461–1471. doi: 10.1111/j.1471-4159.2007.04838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dong Y, Benveniste EN. Immune function of astrocytes. Glia. 2001;36:180–190. doi: 10.1002/glia.1107. [DOI] [PubMed] [Google Scholar]
- 50.Farina C, Aloisi F, Meinl E. Astrocytes are active players in cerebral innate immunity. Trends Immunol. 2007;28:138–145. doi: 10.1016/j.it.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 51.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50:427–434. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 52.Akira S. TLR signaling. Curr Top Microbiol Immunol. 2006;311:1–16. doi: 10.1007/3-540-32636-7_1. [DOI] [PubMed] [Google Scholar]
- 53.Zhang-Gandhi CX, Drew PD. Liver X receptor and retinoid X receptor agonists inhibit inflammatory responses of microglia and astrocytes. J Neuroimmunol. 2007;183:50–59. doi: 10.1016/j.jneuroim.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vincent AJ, Choi-Lundberg DL, Harris JA, West AK, Chuah MI. Bacteria and PAMPs activate nuclear factor kappaB and Gro production in a subset of olfactory ensheathing cells and astrocytes but not in Schwann cells. Glia. 2007;55:905–916. doi: 10.1002/glia.20512. [DOI] [PubMed] [Google Scholar]
- 55.Qin L, Li G, Qian X, Liu Y, Wu X, Liu B, Hong JS, Block ML. Interactive role of the toll-like receptor 4 and reactive oxygen species in LPS-induced microglia activation. Glia. 2005;52:78–84. doi: 10.1002/glia.20225. [DOI] [PubMed] [Google Scholar]
- 56.Wang T, Lafuse WP, Takeda K, Akira S, Zwilling BS. Rapid chromatin remodeling of Toll-like receptor 2 promoter during infection of macrophages with Mycobacterium avium. J Immunol. 2002;169:795–801. doi: 10.4049/jimmunol.169.2.795. [DOI] [PubMed] [Google Scholar]
- 57.Akama KT, Van Eldik LJ. Beta-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1beta- and tumor necrosis factor-alpha (TNFalpha)-dependent, and involves a TNFalpha receptor-associated factor- and NFkappaB-inducing kinase-dependent signaling mechanism. J Biol Chem. 2000;275:7918–7924. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- 58.Vila-del Sol V, Diaz-Munoz MD, Fresno M. Requirement of tumor necrosis factor alpha and nuclear factor-kappaB in the induction by IFN-gamma of inducible nitric oxide synthase in macrophages. J Leukoc Biol. 2007;81:272–283. doi: 10.1189/jlb.0905529. [DOI] [PubMed] [Google Scholar]
- 59.Kleinert H, Pautz A, Linker K, Schwarz PM. Regulation of the expression of inducible nitric oxide synthase. Eur J Pharmacol. 2004;500:255–266. doi: 10.1016/j.ejphar.2004.07.030. [DOI] [PubMed] [Google Scholar]
- 60.Burns K, Martinon F, Tschopp J. New insights into the mechanism of IL-1beta maturation. Curr Opin Immunol. 2003;15:26–30. doi: 10.1016/s0952-7915(02)00017-1. [DOI] [PubMed] [Google Scholar]
- 61.Dinarello CA. Interleukin-1 beta, interleukin-18, and the interleukin-1 beta converting enzyme. Ann N Y Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 62.Pahan K, Sheikh FG, Namboodiri AM, Singh I. N-acetyl cysteine inhibits induction of no production by endotoxin or cytokine stimulated rat peritoneal macrophages, C6 glial cells and astrocytes. Free Radic Biol Med. 1998;24:39–48. doi: 10.1016/s0891-5849(97)00137-8. [DOI] [PubMed] [Google Scholar]
- 63.Pahan K, Namboodiri AM, Sheikh FG, Smith BT, Singh I. Increasing cAMP attenuates induction of inducible nitric-oxide synthase in rat primary astrocytes. J Biol Chem. 1997;272:7786–7791. doi: 10.1074/jbc.272.12.7786. [DOI] [PubMed] [Google Scholar]
- 64.Muhl H, Kunz D, Pfeilschifter J. Expression of nitric oxide synthase in rat glomerular mesangial cells mediated by cyclic AMP. Br J Pharmacol. 1994;112:1–8. doi: 10.1111/j.1476-5381.1994.tb13019.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pahan K, Raymond JR, Singh I. Inhibition of phosphatidylinositol 3-kinase induces nitric-oxide synthase in lipopolysaccharide- or cytokine-stimulated C6 glial cells. J Biol Chem. 1999;274:7528–7536. doi: 10.1074/jbc.274.11.7528. [DOI] [PubMed] [Google Scholar]
- 66.Ozes ON, Mayo LD, Gustin JA, Pfeffer SR, Pfeffer LM, Donner DB. NF-kappaB activation by tumour necrosis factor requires the Akt serine-threonine kinase. Nature. 1999;401:82–85. doi: 10.1038/43466. [DOI] [PubMed] [Google Scholar]
- 67.Vann LR, Twitty S, Spiegel S, Milstien S. Divergence in regulation of nitric-oxide synthase and its cofactor tetrahydrobiopterin by tumor necrosis factor-alpha. Ceramide potentiates nitric oxide synthesis without affecting GTP cyclohydrolase I activity. J Biol Chem. 2000;275:13275–13281. doi: 10.1074/jbc.275.18.13275. [DOI] [PubMed] [Google Scholar]