Abstract
Background
Patients with newly acquired methicillin-resistant Staphylococcus aureus (MRSA) have significant risks of short-term morbidity and mortality due to this pathogen. We were interested in assessing whether long-term carriers have persistent risks of disease and whether all carriers, regardless of the duration of carriage, should be considered to be reasonable candidates for interventions to reduce the risk of infection.
Methods
We conducted a single-center retrospective cohort study to evaluate the risk of subsequent MRSA infection and death among patients known to have harbored MRSA for at least 1 year (i.e., prevalent carriers).
Results
Among 281 prevalent carriers, 65 (23%) developed a total of 96 discrete and unrelated MRSA infections in the year after their identification as prevalent carriers. The most common infections were pneumonia (accounting for 39% of MRSA infections), soft-tissue infection (14%), and central venous catheter infection (14%). Twenty-four percent of all infections involved bacteremia. Thirty-eight MRSA infections occurred during a new hospitalization, and 32 (84%) of these infections were the reason for admission to the hospital. MRSA contributed to 14 deaths, with 6 of these deaths deemed to be attributable to MRSA. Harboring MRSA for <2 years and MRSA colonization at the time of detection as a prevalent carrier were predictive of subsequent infection with MRSA.
Conclusions
Individuals who are known to have harbored MRSA for >1 year are at high risk for subsequent MRSA morbidity and mortality and should be considered to be targets for intervention, in addition to individuals who have newly acquired this pathogen.
Methicillin-resistant Staphylococcus aureus (MRSA) is an antibiotic-resistant pathogen that causes a variety of serious infections. Although community-acquired MRSA is becoming increasingly common [1–3], the bulk of severe morbidity and mortality attributable to MRSA continues to occur among hospitalized patients [4–9]. More than 10% of inpatients who are newly colonized with MRSA develop subsequent infection during the same hospital stay [10], and among newly colonized patients in the intensive care unit, the risk of short-term bacteremia approaches 40% [11].
This high risk of infection among new MRSA carriers has increased the need for preventative interventions in this population. However, MRSA-positive populations predominantly consist of patients whose MRSA status has been established for some time. The risk of subsequent infection in this group remains unknown.
We previously found that 33% of new carriers in a tertiary care center developed invasive sequelae in the year following acquisition of MRSA [7]. These infections were severe, with 26% of infections involving bacteremia and no reduction in the risk of bacteremia after hospital discharge. It is reasonable to hypothesize that the risk of subsequent infection extends beyond 1 year, because MRSA colonization often persists for prolonged periods after hospital discharge [12]. One study has suggested that MRSA may be carried in the nares for a mean of 42 months [13]. We sought to assess whether patients who were known to harbor MRSA for >1 year continued to be at increased risk for MRSA infection.
PATIENTS AND METHODS
We conducted a retrospective cohort study to evaluate the frequency of subsequent MRSA infection among patients who were already known to have harbored MRSA for at least 1 year (i.e., prevalent carriers). We identified a cohort of adult patients who had an MRSA-positive culture from 1 January 2002 through 31 December 2005 at the Brigham and Women’s Hospital (BWH), an 800-bed tertiary care hospital in Boston, Massachusetts. Patients were retained in the cohort if they had evidence of MRSA positivity at least 1 year earlier based on microbiology and infection-control records. There were no exclusion criteria for MRSA events occurring during this 1-year period. We assessed the further risk of MRSA infection in the year after identification as a prevalent carrier when evaluating all available BWH inpatient and outpatient medical records.
We obtained demographic and comorbidity information for all prevalent carriers. Comorbidities were identified using diagnoses of diabetes mellitus, end-stage renal disease, end-stage liver disease, hematologic malignancies, solid tumor malignancies, and other noncancer immunocompromised states based on BWH International Classification of Diseases, Ninth Revision, codes from the prior year. Identified comorbidities were confirmed through medical record review. Malignancies were recorded only if there was evidence of treatment within the preceding year. We also identified known risk factors for subsequent MRSA infection, including active wounds, intensive care unit admission, and intubation at the time of detection as a prevalent carrier. In addition, we assessed whether prevalent carriers had undergone a surgical procedure in the previous 6 months or had been admitted to BWH in the previous year. For prevalent carriers who were identified during an inpatient stay, we also determined their preadmission location, hospital discharge disposition, and length of hospital stay.
Medical records were reviewed to identify the source of the MRSA-positive culture at the time of detection as a prevalent carrier and to determine whether the positive culture result represented colonization or infection based upon National Nosocomial Infections Surveillance criteria [14]. All subsequent MRSA isolates within a 1-year period were evaluated for evidence of distinct MRSA infection. Subsequent MRSA infections were described according to (1) the number of days between detection as a prevalent carrier and the onset of MRSA infection, (2) the type of infection, (3) whether the infection was associated with MRSA bacteremia, and (4) the antibiotic susceptibility pattern of the MRSA isolate. Infection type was assigned according to the primary source of infection on the basis of National Nosocomial Infections Surveillance criteria.
We provided patient characteristics as the percentage of total patients with the specified attribute. We determined the proportion of patients who developed any subsequent MRSA infection and the proportion of patients who died within 1 year after the time of detection as a prevalent carrier. We also assessed whether deaths were associated with MRSA infection at the time of detection or during the year following detection as a prevalent carrier and, if associated, whether deaths were attributable to MRSA. Death was deemed to be associated with MRSA if MRSA bacteremia was found within 7 days of death or if there was active MRSA infection at the time of death [6]. Death was additionally deemed to be attributable to MRSA if the above criteria were met and there was no other cause of death.
Potential predictors of subsequent MRSA infection among prevalent carriers were assessed using χ2 tests. Variables significant in bivariate testing at a level of α < .2 were entered into a logistic regression model (SAS, version 9.1; SAS). Final model variables were retained at α = .05.
RESULTS
We identified 281 patients with an MRSA-positive culture who had evidence of MRSA positivity at least 1 year earlier. Seventy percent of patients had an MRSA-positive culture between the time of first evidence of MRSA positivity and detection as a prevalent carrier. Patient characteristics are summarized in table 1. The mean age of patients at the time of detection as a prevalent carrier was 61.5 years (median age, 62.9 years), and the mean time since first evidence of MRSA positivity was 2.4 years (median period, 1.6 years). Most individuals were inpatients at the time that they were determined to be prevalent carriers, and the majority (162 [73%] of 221) had an MRSA-positive culture within 2 calendar days of admission to BWH. The mean length of hospital stay among inpatients was 12.7 days (median duration, 7.0 days).
Table 1.
Characteristics of patients at time of identification as a methicillin-resistant Staphylococcus aureus (MRSA) prevalent carrier.
| Characteristic | No. (%) of patients (n = 281) |
|---|---|
| Sex | |
| Male | 151 (54) |
| Female | 130 (46) |
| Race | |
| White | 237 (84) |
| Black | 36 (13) |
| Other | 8 (3) |
| Age | |
| 18–44 years | 48 (17) |
| 45–54 years | 50 (18) |
| 55–64 years | 58 (21) |
| 65–74 years | 60 (21) |
| 75–84 years | 47 (17) |
| ≥85 years | 18 (6) |
| Comorbidity | |
| Diabetes mellitus | 100 (36) |
| End-stage renal disease | 29 (10) |
| End-stage liver disease | 13 (5) |
| Solid-organ cancer | 41 (15) |
| Hematologic malignancy | 14 (5) |
| Immunocompromised, noncancer | 26 (9) |
| Health care location | |
| Inpatient | 221 (79) |
| Outpatient | 60 (21) |
| Potential risk factors for MRSA infection | |
| Wound | 180 (64) |
| Intubation | 54 (19) |
| Intensive care unit admission | 39 (14) |
| Hospital admission in previous year | 191 (68) |
| Surgical procedure in previous 6 months | 110 (39) |
| Preadmission locationa,b | |
| Home | 165 (75) |
| Hospital transfer | 33 (15) |
| Rehabilitation | 9 (4) |
| Skilled nursing facility | 14 (6) |
| Discharge dispositiona,b | |
| Home | 126 (57) |
| Hospital transfer | 4 (2) |
| Rehabilitation | 60 (27) |
| Skilled nursing facility | 17 (8) |
| Deceasedc | 14 (6) |
| Time since first known evidence of MRSA positivity | |
| 1 to <2 years | 175 (62) |
| 2 to <3 years | 49 (17) |
| 3 to <4 years | 22 (8) |
| ≥4 years | 35 (16) |
n = 221.
Evaluation for admission where patient identified as prevalent carrier a with known history of MRSA ≥1 year.
Deaths refer to all-cause mortality during the enrollment hospitalization.
Of the 281 patients who were found to harbor MRSA for at least 1 year, 52% were infected and 48% were colonized at the time of entry into the study as a prevalent carrier. Among those patients who were colonized, common sources of MRSA colonization included nares (63% of patients), sputum (18%), and wounds (10%). Sixty-five patients (23%) developed a total of 96 additional discrete and unrelated MRSA infections in the year after their detection as prevalent carriers (table 2). These MRSA infections occurred a mean of 3.8 months (a median of 2.4 months) after the time of detection as a prevalent carrier. Most patients experienced pulmonary, soft-tissue, and central venous catheter infections. Among the 14 infections categorized as “other,” there were 4 urinary tract infections, 3 surgical site infections, 3 sinusitis infections, 2 gastrointestinal system infections, 1 parotitis infection, and 1 oral cavity infection. Of all infections, 24% were associated with MRSA bacteremia. Ninety-one percent of infections were caused by strains with drug susceptibility patterns that were identical to those for the first positive MRSA isolate when evaluating the following antibiotics: erythromycin, clindamycin, gentamicin, levofloxacin, trimethoprim-sulfamethoxazole, tetracycline, rifampin, and vancomycin.
Table 2.
Sources of subsequent infection with methicillin-resistant Staphylococcus aureus (MRSA).
| Infection | No. of patients (n = 65)a | No. (%) of MRSA infections | ||
|---|---|---|---|---|
| Overall (n = 96) | ≤6 Months after identification as prevalent case patient (n = 69) | >6 Months after identification as prevalent case patient (n = 27) | ||
| Pneumonia | 32 | 37 (39) | 31 (45) | 6 (22) |
| Soft tissue | 11 | 13 (14) | 5 (7) | 8 (30) |
| Central venous catheter | 8 | 13 (14) | 11 (16) | 2 (8) |
| Primary bloodstream | 9 | 11 (11) | 9 (13) | 2 (8) |
| Bone/joint | 6 | 8 (8) | 5 (7) | 3 (11) |
| Other | 12 | 14 (15) | 8 (12) | 6 (22) |
| Associated bacteremia | 15 | 23 (24)b | 19 (28)b | 4 (15)b |
Some patients experienced multiple infections.
Percentage of total infections associated with MRSA bacteremia.
The risk of subsequent infection according to the time since first known evidence of MRSA positivity is shown in table 3. Risk of subsequent infection was significantly higher among patients whose time since first evidence of MRSA positivity was 1–2 years (48 [27%] of 175), compared with patients whose time since first evidence of positivity was ≥2 years (17 [16%] of 106; P = .03).
Table 3.
Risk of subsequent infection and death in the year after identification as a methicillin-resistant Staphylococcus aureus (MRSA) prevalent carrier.
| Time since first known evidence of MRSA positivity | No. of patients | No. (%) of patients with subsequent MRSA infectiona | No. (%) of deathsb | ||
|---|---|---|---|---|---|
| Attributable to MRSA | Associated with MRSA | All causes | |||
| 1 to <2 years | 175 | 48 (27) | 4 (2) | 9 (5) | 24 (14) |
| 2 to <3 years | 49 | 10 (20) | 1 (2) | 2 (4) | 7 (14) |
| 3 to <4 years | 22 | 2 (9) | 0 (0) | 0 (0) | 4 (18) |
| ≥4 years | 35 | 5 (14) | 1 (3) | 3 (9) | 6 (17) |
| Overall | 281 | 65 (23) | 6 (2) | 14 (5) | 41 (15) |
Patients with subsequent discrete MRSA infection within 1 year after the time of identification as a prevalent carrier.
Deaths occurring within 1 year after the time of detection as a prevalent carrier. Deaths attributable to MRSA are included within deaths associated with MRSA. Deaths associated with MRSA include deaths associated with MRSA infections present at enrollment and subsequent MRSA infections.
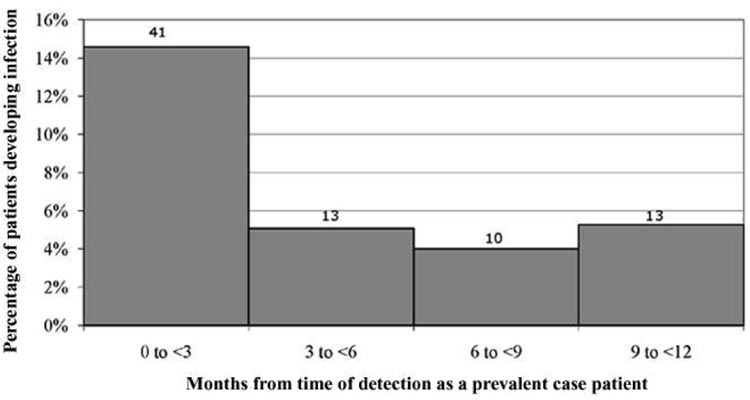
Figure 1 displays the percentage of patients who developed subsequent infection in the year after detection as a prevalent carrier. Risk of infection was significantly higher in the first quarter than it was in any other quarter in the subsequent year. Subsequent MRSA infections also occurred more frequently among patients who were colonized with MRSA (39 [29%] of 136) than among patients who were infected with MRSA (26 [18%] of 145) at the time of detection as a prevalent carrier (P = .03).
Figure 1.
The percentage of patients who developed subsequent methicillin-resistant Staphylococcus aureus infection in quarterly intervals following the time of detection as a prevalent carrier. The number of patients experiencing infection in each interval is shown. Some patients experienced multiple infections. Denominators (not shown) have been corrected for patients who died before the analyzed interval. Risk of infection was significantly greater in the first quarter than in any other quarter (P < .001).
Death occurred in 41 patients (15%). When assessing deaths associated with MRSA (14 deaths; 34%), one-half were associated with MRSA infections present at enrollment, and one-half were associated with subsequent MRSA infections. When assessing deaths attributable to MRSA, 3 deaths were attributable to MRSA infections that were present at enrollment, and 3 were attributable to subsequent MRSA infections. The majority of deaths associated with MRSA occurred during the hospitalization in which the patients were determined to be prevalent carriers (9 [64%] of 14). There was no significant difference in the number of deaths from all causes or in the number of deaths associated with MRSA between patients who were known to harbor MRSA for 1–2 years and patients who were known to harbor MRSA for ≥2 years. Among those who died, the mean time to death from all causes from the time of detection as a prevalent carrier was 3.4 months (median time to death, 1.3 months).
Subsequent hospitalizations with MRSA were common. Thirty-eight MRSA infections (40%) were detected during later hospitalizations, and 32 (84%) of those 38 infections were responsible for the patient being hospitalized. The most common MRSA infections resulting in hospital admission were pneumonia (11 [34%] of 32 cases), soft-tissue infection (7 [22%] of 32), and primary bloodstream infection (5 [16%] of 32).
Harboring MRSA for <2 years and MRSA colonization at the time of detection as a prevalent carriers were significantly associated with subsequent infection due to MRSA. There was also a trend for male sex to be predictive of MRSA infection (table 4). Other demographic, comorbidity, and hospital information were not predictive of subsequent MRSA infection.
Table 4.
Predictors of subsequent infection due to methicillin-resistant Staphylococcus aureus (MRSA) at the time of identification as a prevalent carrier.
| Predictor | OR (95% CI) | P |
|---|---|---|
| Harboring MRSA for 1–2 years | 2.2 (1.2–4.1) | .02 |
| MRSA colonization vs. infection | 1.9 (1.1–3.4) | .03 |
| Male sex | 1.7 (0.9–3.0) | .07 |
DISCUSSION
The increasing morbidity and mortality attributable to MRSA have heightened the need for preventative interventions targeting patients who are at risk for MRSA infection. These risks, together with recent legislation permitting Medicare to withhold payments for certain hospital-acquired infections [15], have increased the attractiveness of eradicating MRSA carriage in hopes of preventing a portion of hospital-acquired infections among patients who are at risk for hospitalization. Prior reports have focused on the risks of MRSA-associated morbidity and mortality in the immediate period following new acquisition of MRSA. Our data suggest that patients who continue to harbor MRSA remain at continued risk of MRSA infection and death, regardless of time since the initial detection of MRSA positivity.
Current preventative strategies for decolonizing MRSA carriers have targeted all prevalent carriers with MRSA, despite the fact that risks for subsequent infection have only been studied among individuals with recent MRSA acquisition [16–18]. This study provides an estimate of the risk among the larger group of prevalent carriers and may be helpful in predicting the overall impact that successful decolonization can have on MRSA infection. At our hospital, there are 2–3 times as many hospital admissions involving patients previously known to harbor MRSA than there are hospital admissions of individuals who are newly detected as MRSA carriers each year; all known MRSA carriers account for a total ~3% of annual hospital admissions. Our results support the application of decolonization or other preventative interventions to all known carriers of MRSA with recent exposure to the health care system or with substantial risks of future hospitalization.
We recognize that the risk of infection in the first year after initial MRSA detection may exceed the risk of infection in subsequent years. Earlier work involving the same hospital population suggests that 33% of patients with newly acquired MRSA develop invasive disease in the ensuing 12 months [7], whereas our results suggest a 27% risk in the second year of carriage and a 16% risk thereafter. Although the latter 2 estimates are likely to be underestimated by our study, because patients may not return to the same institution for all future care [7], it is worth noting that substantial risks persist. In addition, it is likely that there is some misclassification of newly detected cases, because MRSA detection is often reliant on indications for clinical cultures. It is possible that some of the 33% risk noted in our patients actually reflects the risk in patients who have harbored MRSA for some time.
In fact, we propose that much of the risk of subsequent MRSA infection may not be attributable to the time since the initial acquisition of MRSA but may instead be attributable to the risks associated with hospitalization or undergoing a surgical procedure. We submit that these high risks of MRSA infection among culture-positive prevalent carriers are not only preferentially detected because of hospitalization but may, in fact, be incurred because of the device-related, wound-related, and immunologic declines associated with a current illness. This is supported by our finding of a higher risk of MRSA infection during the 3 months following the time of detection as a prevalent carrier. After this 3-month perihospitalization period, patients appeared to incur infections at a lower rate. Although additional studies are needed to confirm these findings, long-term carriers may experience an out-of-hospital risk of MRSA infection of ~5% per quarter that becomes elevated 3-fold or more at the time of hospitalization.
We were surprised to find that colonized patients were at greater risk of subsequent MRSA infection. We believe that this may result from the protective effect that MRSA-infected patients receive from active therapy, which may extend for several weeks beyond infection detection. During this treatment period, these patients are unlikely to be at risk for additional MRSA disease.
There are several limitations to this study. First, our definition of prevalent carriers assumes that patients continuously harbored MRSA. It is possible that these patients instead experienced serial acquisition of MRSA strains and that the high risk of infection found in this study was because of recurrent recent acquisition. We did not collect or strain-type isolates to confirm persistent carriage. However, other studies suggest that 40%–60% of patients persistently carry the same strains over time [19–22], and we have previously shown that repeat MRSA infection following initial acquisition is primarily caused by the same strain [23]. Furthermore, in this study, nearly all infections were caused by strains with antibiotic susceptibility profiles that were identical to those for the patient’s first positive isolate.
Second, as mentioned above, our reported risk of MRSA infection is likely to be an underestimate, because we could not account for infections that occurred at other medical facilities. Third, we note that active surveillance nares cultures for MRSA were instituted in September 2003 for intensive care unit patients. Inclusion of patients whose first detection of MRSA was the result of surveillance may change the reported risk of subsequent infection because of surveillance bias. However, we found no difference in the risk of infection between patients whose acquisition cultures were nares cultures and patients whose initial cultures were not nares cultures (data not shown). Fourth, observation bias may have contributed to the high number of hospital admissions attributable to MRSA. Fifth, deaths associated with MRSA may have been underestimated. Of the 41 patients who died in the subsequent year, 5 had an MRSA-positive culture at the time of death, but details of the cause of death were unavailable. Lastly, our results reflect the infection and mortality rates for a patient population at a single tertiary care center and may not be generalizable to other hospitals or patient groups.
In summary, we found that MRSA carriers remain at considerable risk for subsequent MRSA infection, regardless of the time since the initial detection of MRSA carriage. These risks not only involve serious infection, such as MRSA-associated pneumonia and bacteremia, but also include substantial MRSA-associated mortality. Many of these infections resulted in subsequent hospital admissions. Interventions aimed at preventing post-acquisition MRSA morbidity and mortality should include both incident and prevalent carriers, and predictions of impact should account for the large risks of infections seen in both groups.
Acknowledgments
We thank Dr. Richard Platt for his important contributions to the study and manuscript preparation.
Financial support. Centers for Disease Control and Prevention (Prevention Epicenters Program, UR8/CCU115079) and the National Institutes of Health (K23AI64161).
Footnotes
Potential conflicts of interest. R.D. and S.S.H.: no conflicts.
References
- 1.Moran GJ, Krishnadasan A, Gorwitz RJ, et al. Methicillin-resistant S. aureus infections among patients in the emergency department. N Engl J Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 2.Fridkin SK, Hageman JC, Morrison M, et al. Methicillin-resistant Staphylococcus aureus disease in three communities. N Engl J Med. 2005;352:1436–1444. doi: 10.1056/NEJMoa043252. Erratum in: N Engl J Med 2005; 352:2362. [DOI] [PubMed] [Google Scholar]
- 3.Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. JAMA. 1998;279:593–598. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- 4.Cosgrove SE, Sakoulas G, Perencevich EN, Schwaber MJ, Karchmer AW, Carmeli Y. Comparison of mortality associated with methicillin-resistant and methicillin-susceptible Staphylococcus aureus bacteremia: a meta-analysis. Clin Infect Dis. 2003;36:53–59. doi: 10.1086/345476. [DOI] [PubMed] [Google Scholar]
- 5.Blot SI, Vandewoude KH, Hoste EA, Colardyn FA. Outcome and attributable mortality in critically ill patients with bacteremia involving methicillin-susceptible and methicillin-resistant Staphylococcus aureus. Arch Intern Med. 2002;162:2229–2235. doi: 10.1001/archinte.162.19.2229. [DOI] [PubMed] [Google Scholar]
- 6.Pujol M, Pena C, Pallares R, Ayats J, Ariza J, Gudiol F. Risk factors for nosocomial bacteremia due to methicillin-resistant Staphylococcus aureus. Eur J Clin Microbiol Infect Dis. 1994;13:96–102. doi: 10.1007/BF02026134. [DOI] [PubMed] [Google Scholar]
- 7.Huang SS, Hinrichsen VL, Stulgis L, et al. Program and abstracts of the Society of Healthcare Epidemiology of America Annual Meeting (Chicago) Arlington, VA: Society for Healthcare Epidemiology of America; 2006. Methicillin-resistant Staphylococcus aureus infection in the year following detection of carriage. [abstract 157] [Google Scholar]
- 8.Huang SS, Platt R. Risk of methicillin-resistant Staphylococcus aureus infection after previous infection or colonization. Clin Infect Dis. 2003;36:281–285. doi: 10.1086/345955. [DOI] [PubMed] [Google Scholar]
- 9.Klevens RM, Morrison MA, Nadle J, et al. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 10.Coello R, Glynn JR, Gaspar C, Picazzo JJ, Fereres J. Risk factors for developing clinical infection with methicillin-resistant Staphylococcus aureus (MRSA) amongst hospital patients initially only colonized with MRSA. J Hosp Infect. 1997;37:39–46. doi: 10.1016/s0195-6701(97)90071-2. [DOI] [PubMed] [Google Scholar]
- 11.Pujol M, Pena C, Pallares R, et al. Nosocomial Staphylococcus aureus bacteremia among nasal carriers of methicillin-resistant and methicillin-susceptible strain. Am J Med. 1996;100:509–516. doi: 10.1016/s0002-9343(96)00014-9. [DOI] [PubMed] [Google Scholar]
- 12.Scanvic A, Denic L, Gaillon S, Giry P, Andremont A, Lucet JC. Duration of colonization by methicillin-resistant Staphylococcus aureus after hospital discharge and risk factors for prolonged carriage. Clin Infect Dis. 2001;32:1393–1398. doi: 10.1086/320151. [DOI] [PubMed] [Google Scholar]
- 13.Sanford MD, Widmer AF, Bale MJ, Jones RN, Wenzel RP. Efficient detection and long-term persistence of the carriage of methicillin-resistant Staphyloccocus aureus. Clin Infect Dis. 1994;19:1123–1128. doi: 10.1093/clinids/19.6.1123. [DOI] [PubMed] [Google Scholar]
- 14.Horan TC, Gaynes RP. Surveillance of nosocomial infections. In: Mayhall CG, editor. Hospital epidemiology and infection control. 3rd ed. Philadelphia: Lippincott Williams & Wilkins; 2004. pp. 1659–1702. [Google Scholar]
- 15. [Accessed 20 November 2007];Centers for Medicare and Medicaid Web site. Available at: http://www.cms.hhs.gov/QuarterlyProviderUpdates/
- 16.Ridenour G, Lampen R, Federspiel J, Kritchevsky S, Wong E, Climo M. Selective use of intranasal mupirocin and chlorhexidine bathing and the incidence of methicillin-resistant Staphylococcus aureus colonization and infection among intensive care unit patients. Infect Control Hosp Epidemiol. 2007;28:1155–1161. doi: 10.1086/520102. [DOI] [PubMed] [Google Scholar]
- 17.Simor AE, Phillips E, McGeer A, Konvalinka A, Loeb M, Devlin HR, Kiss A. Randomized controlled trial of chlorhexidine gluconate for washing, intranasal mupirocin, and rifampin and doxycycline versus no treatment for the eradication of methicillin-resistant Staphylococcus aureus colonization. Clin Infect Dis. 2007;44:178–185. doi: 10.1086/510392. [DOI] [PubMed] [Google Scholar]
- 18.Sandri AM, Dalarosa MG, Ruschel de Alcantara L, da Silva Elias L, Zavascki AP. Reduction in incidence of nosocomial methicillin-resistant Staphylococcus aureus (MRSA) infection in an intensive care unit: role of treatment with mupirocin ointment and chlorhexidine baths for nasal carriers of MRSA. Infect Control Hosp Epidemiol. 2006;27:185–187. doi: 10.1086/500625. [DOI] [PubMed] [Google Scholar]
- 19.Lim MSC, Marshall CL, Spelman D. Carriage of multiple subtypes of methicillin-resistant Staphylococcus aureus by intensive care unit patients. Infect Control Hosp Epidemiol. 2006;27:1063–1067. doi: 10.1086/507959. [DOI] [PubMed] [Google Scholar]
- 20.Maslow JN, Brecher S, Gunn J, Durbin A, Barlow MA, Arbeit RD. Variation and persistence of methicillin-resistant Staphylococcus aureus strains among individual patients over extended periods of time. Eur J Clin Microbiol Infect Dis. 1995;14:282–290. doi: 10.1007/BF02116520. [DOI] [PubMed] [Google Scholar]
- 21.Fattom A, Fuller S, Propst M, et al. Safety and immunogenicity of a booster dose of Staphylococcus aureus types 5 and 8 capsular polysaccharide conjugate vaccine (StaphVAX) in hemodialysis patients. Vaccine. 2004;23:656–663. doi: 10.1016/j.vaccine.2004.06.043. [DOI] [PubMed] [Google Scholar]
- 22.Herwaldt LA, Pottinger JM, Coffman S, Tjaden J. Molecular epidemiology of methicillin-resistant Staphylococcus aureus in a Veterans Administration Medical Center. Infect Control Hosp Epidemiol. 2002;23:502–505. doi: 10.1086/502096. [DOI] [PubMed] [Google Scholar]
- 23.Huang SS, Diekema D, Warren DK, et al. Strain-relatedness of isolates from patients with serial MRSA infection. Clin Infect Dis. 2008 doi: 10.1086/529381. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]