Abstract
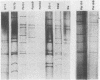
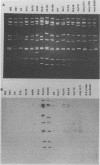
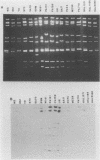
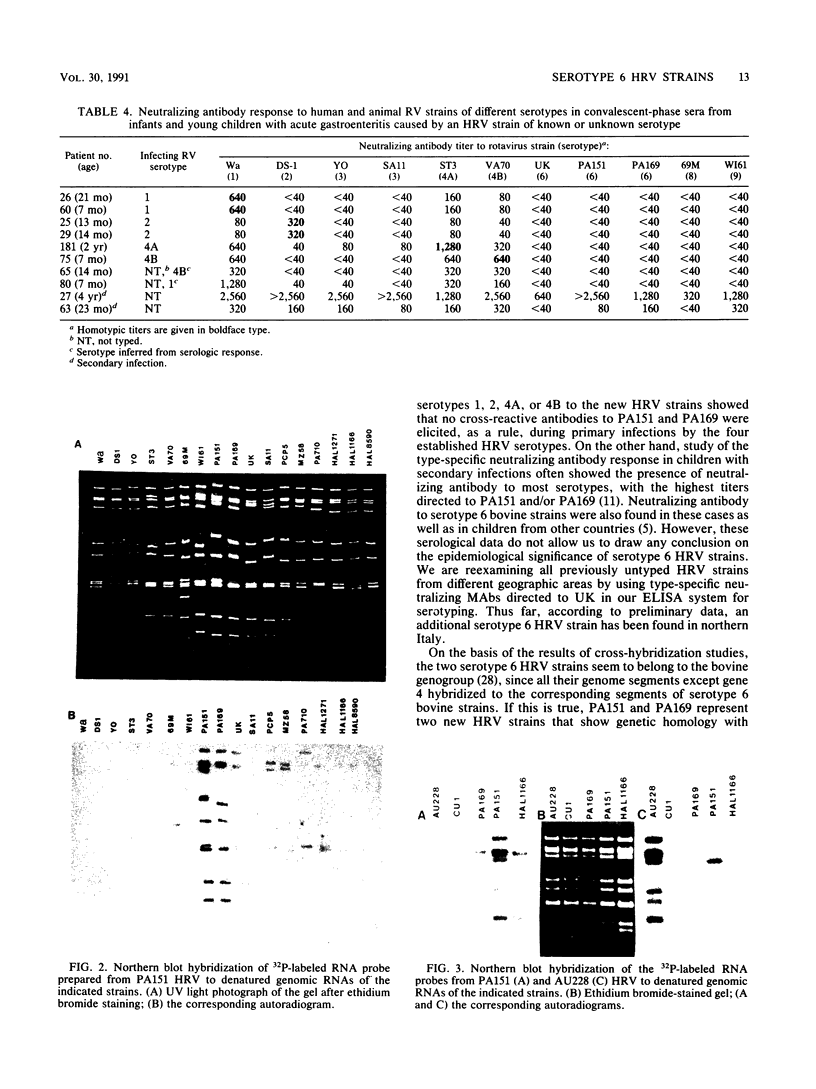
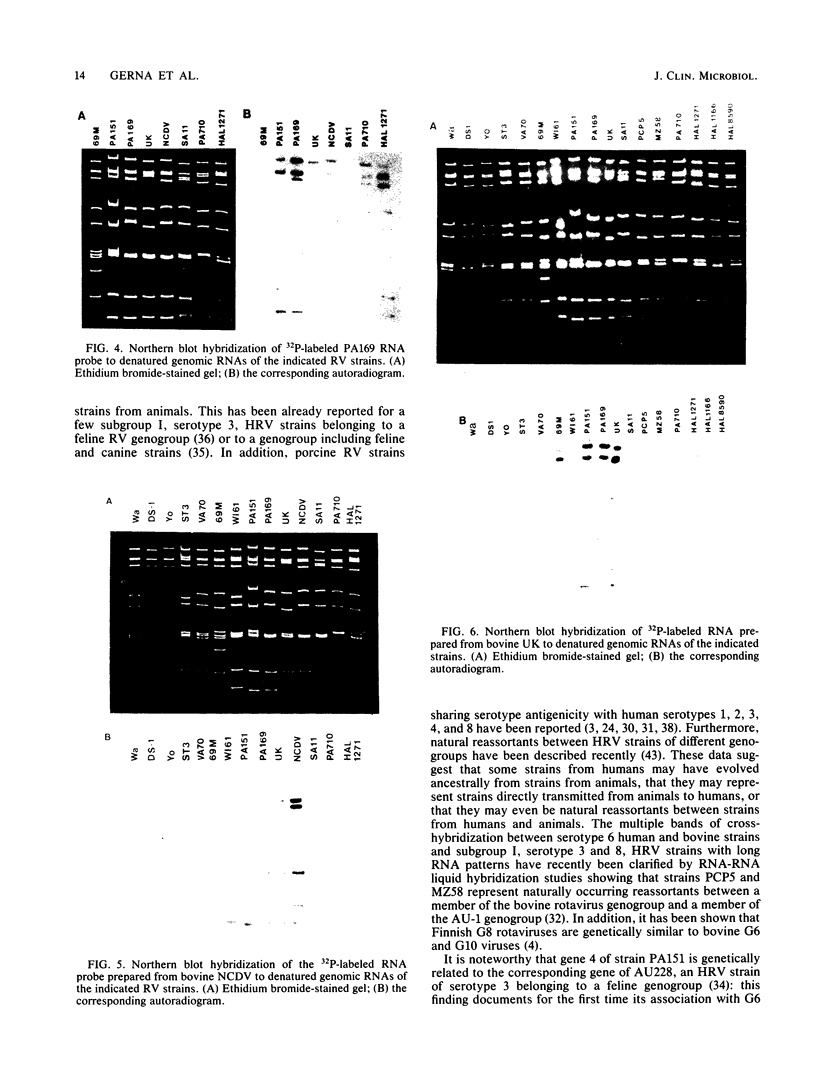
Two new human rotavirus (HRV) strains, PA151 and PA169, with subgroup I specificity and a long RNA pattern, yet with a serotype G (VP7) specificity different from those of any of the six well-established HRV serotypes (G1 to G4, G8, and G9), were isolated 3 months apart from two children with acute gastroenteritis in Sicily, southern Italy, in the winter season of 1987 and 1988. The HRV isolates were adapted to growth in cell cultures and were then characterized by neutralization and RNA-RNA (Northern blot) hybridization. Cross-neutralization studies with type-specific immune sera to RV serotypes 1 to 10 showed the antigenic relatedness of the two strains with serotype 6 bovine strains UK and NCDV. Monoclonal antibodies to VP7 of UK were able to recognize UK and NCDV strains as well as both HRV isolates. Cross-hybridization studies showed a genetic relatedness of PA151 and PA169 to bovine strains for all genes except gene 4. Gene 4 of PA151 appeared to be genetically related to that of AU228 (a human strain of subgroup I and with serotype G3 specificity that belongs to a feline genogroup), whereas gene 4 of PA169 appeared to be unique, yet it was related to gene 4 of two recently reported subgroup I HRV strains, one (PA710) with serotype G3 specificity and the other (HAL1271) with serotype G8 specificity. The new HRV strains must be taken into consideration when deciding strategies for the development of an effective RV vaccine.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arista S., Giovannelli L., Pistoia D., Cascio A., Parea M., Gerna G. Electropherotypes, subgroups and serotypes of human rotavirus strains causing gastroenteritis in infants and young children in Palermo, Italy, from 1985 to 1989. Res Virol. 1990 Jul-Aug;141(4):435–448. doi: 10.1016/0923-2516(90)90044-j. [DOI] [PubMed] [Google Scholar]
- Beards G. M., Desselberger U., Flewett T. H. Temporal and geographical distributions of human rotavirus serotypes, 1983 to 1988. J Clin Microbiol. 1989 Dec;27(12):2827–2833. doi: 10.1128/jcm.27.12.2827-2833.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinzoni R. B., Mattion N. M., Matson D. O., Blackhall J., La Torre J. L., Scodeller E. A., Urasawa S., Taniguchi K., Estes M. K. Porcine rotaviruses antigenically related to human rotavirus serotypes 1 and 2. J Clin Microbiol. 1990 Mar;28(3):633–636. doi: 10.1128/jcm.28.3.633-636.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H., Offit P. A., Gerna G., Bruttin A., Sidoti J. Polypeptide specificity of antiviral serum antibodies in children naturally infected with human rotavirus. J Virol. 1990 Sep;64(9):4130–4136. doi: 10.1128/jvi.64.9.4130-4136.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H., Snodgrass D., Fitzgerald T., Eichhorn W., Gerhards R., Bruttin A. Antigenic and biochemical characterization of bovine rotavirus V1005, a new member of rotavirus serotype 10. J Gen Virol. 1990 Nov;71(Pt 11):2625–2630. doi: 10.1099/0022-1317-71-11-2625. [DOI] [PubMed] [Google Scholar]
- Clark H. F., Hoshino Y., Bell L. M., Groff J., Hess G., Bachman P., Offit P. A. Rotavirus isolate WI61 representing a presumptive new human serotype. J Clin Microbiol. 1987 Sep;25(9):1757–1762. doi: 10.1128/jcm.25.9.1757-1762.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Midthun K., Hoshino Y., Green K., Gorziglia M., Kapikian A. Z., Chanock R. M. Conservation of the fourth gene among rotaviruses recovered from asymptomatic newborn infants and its possible role in attenuation. J Virol. 1986 Dec;60(3):972–979. doi: 10.1128/jvi.60.3.972-979.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores J., Myslinski J., Kalica A. R., Greenberg H. B., Wyatt R. G., Kapikian A. Z., Chanock R. M. In vitro transcription of two human rotaviruses. J Virol. 1982 Sep;43(3):1032–1037. doi: 10.1128/jvi.43.3.1032-1037.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Arista S., Passarani N., Sarasini A., Battaglia M. Electropherotype heterogeneity within serotypes of human rotavirus strains circulating in Italy. Brief report. Arch Virol. 1987;95(1-2):129–135. doi: 10.1007/BF01311340. [DOI] [PubMed] [Google Scholar]
- Gerna G., Battaglia M., Milenesi G., Passarani N., Percivalle E., Cattaneo E. Serotyping of cell culture-adapted subgroup 2 human rotavirus strains by neutralization. Infect Immun. 1984 Feb;43(2):722–729. doi: 10.1128/iai.43.2.722-729.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Passarani N., Uricomb L. E., Parea M., Sarasini A., Battaglia M., Bishop R. F. Solid-phase immune electron microscopy and enzyme-linked immunosorbent assay for typing of human rotavirus strains by using polyclonal and monoclonal antibodies: a comparative study. J Infect Dis. 1989 Feb;159(2):335–339. doi: 10.1093/infdis/159.2.335. [DOI] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Arista S., di Matteo A., Giovannelli L., Parea M., Halonen P. Prevalence of human rotavirus serotypes in some European countries 1981-1988. Scand J Infect Dis. 1990;22(1):5–10. doi: 10.3109/00365549009023112. [DOI] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Di Matteo A., Parea M., Torsellini M., Battaglia M. Rapid detection of human rotavirus strains in stools by single-sandwich enzyme-linked immunosorbent assay systems using monoclonal antibodies. J Virol Methods. 1989 Apr-May;24(1-2):43–56. doi: 10.1016/0166-0934(89)90006-2. [DOI] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Di Matteo A., Zentilin L., Miranda P., Parea M., Baldanti F., Arista S., Milanesi G., Battaglia M. Serotype 3 human rotavirus strains with subgroup I specificity. J Clin Microbiol. 1990 Jun;28(6):1342–1347. doi: 10.1128/jcm.28.6.1342-1347.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Torsellini M., Torre D., Parea M., Battaglia M. Group- and type-specific serologic response in infants and children with primary rotavirus infections and gastroenteritis caused by a strain of known serotype. J Infect Dis. 1990 Jun;161(6):1105–1111. doi: 10.1093/infdis/161.6.1105. [DOI] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Torsellini M., di Matteo A., Baldanti F., Parea M., Battaglia M. Characterization of rotavirus subgroup-specific monoclonal antibodies and use in single-sandwich ELISA systems for rapid subgrouping of human strains. Arch Virol. 1989;107(3-4):315–322. doi: 10.1007/BF01317927. [DOI] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., Zentilin L., Di Matteo A., Miranda P., Parea M., Battaglia M., Milanesi G. Isolation in Europe of 69 M-like (serotype 8) human rotavirus strains with either subgroup I or II specificity and a long RNA electropherotype. Arch Virol. 1990;112(1-2):27–40. doi: 10.1007/BF01348983. [DOI] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., di Matteo A., Parea M., Orsolini P., Battaglia M. Identification of two subtypes of serotype 4 human rotavirus by using VP7-specific neutralizing monoclonal antibodies. J Clin Microbiol. 1988 Jul;26(7):1388–1392. doi: 10.1128/jcm.26.7.1388-1392.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerna G., Sarasini A., di Matteo A., Passarani N., Gagliardi V., Milanesi G., Astaldi Ricotti G. C., Battaglia M. The outer capsid glycoprotein VP7 of simian rotavirus SA11 contains two distinct neutralization epitopes. J Gen Virol. 1988 Apr;69(Pt 4):937–944. doi: 10.1099/0022-1317-69-4-937. [DOI] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanata C. F., Black R. E., del Aguila R., Gil A., Verastegui H., Gerna G., Flores J., Kapikian A. Z., Andre F. E. Protection of Peruvian children against rotavirus diarrhea of specific serotypes by one, two, or three doses of the RIT 4237 attenuated bovine rotavirus vaccine. J Infect Dis. 1989 Mar;159(3):452–459. doi: 10.1093/infdis/159.3.452. [DOI] [PubMed] [Google Scholar]
- Larralde G., Flores J. Identification of gene 4 alleles among human rotaviruses by polymerase chain reaction-derived probes. Virology. 1990 Nov;179(1):469–473. doi: 10.1016/0042-6822(90)90317-k. [DOI] [PubMed] [Google Scholar]
- Matsuno S., Hasegawa A., Mukoyama A., Inouye S. A candidate for a new serotype of human rotavirus. J Virol. 1985 May;54(2):623–624. doi: 10.1128/jvi.54.2.623-624.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattion N. M., Bellinzoni R. C., Blackhall J. O., La Torre J. L., Scodeller E. A. Antigenic characterization of swine rotaviruses in Argentina. J Clin Microbiol. 1989 Apr;27(4):795–798. doi: 10.1128/jcm.27.4.795-798.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesha H. S., Holmes I. H. New porcine rotavirus serotype antigenically related to human rotavirus serotype 3. J Clin Microbiol. 1988 Feb;26(2):171–174. doi: 10.1128/jcm.26.2.171-174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T., Akatani K., Ikegami N. Identification of rotavirus genogroups by RNA-RNA hybridization. Mol Cell Probes. 1989 Sep;3(3):251–261. doi: 10.1016/0890-8508(89)90006-6. [DOI] [PubMed] [Google Scholar]
- Nakagomi O., Nakagomi T., Hoshino Y., Flores J., Kapikian A. Z. Genetic analysis of a human rotavirus that belongs to subgroup I but has an RNA pattern typical of subgroup II human rotaviruses. J Clin Microbiol. 1987 Jul;25(7):1159–1164. doi: 10.1128/jcm.25.7.1159-1164.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi O., Ohshima A., Aboudy Y., Shif I., Mochizuki M., Nakagomi T., Gotlieb-Stematsky T. Molecular identification by RNA-RNA hybridization of a human rotavirus that is closely related to rotaviruses of feline and canine origin. J Clin Microbiol. 1990 Jun;28(6):1198–1203. doi: 10.1128/jcm.28.6.1198-1203.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi T., Nakagomi O. RNA-RNA hybridization identifies a human rotavirus that is genetically related to feline rotavirus. J Virol. 1989 Mar;63(3):1431–1434. doi: 10.1128/jvi.63.3.1431-1434.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeh C. K., Snodgrass D. R., Herring A. J. Evidence for serotypic variation among bovine rotaviruses. Arch Virol. 1984;79(3-4):161–171. doi: 10.1007/BF01310809. [DOI] [PubMed] [Google Scholar]
- Pongsuwanne Y., Taniguchi K., Choonthanom M., Chiwakul M., Susansook T., Saguanwongse S., Jayavasu C., Urasawa S. Subgroup and serotype distributions of human, bovine, and porcine rotavirus in Thailand. J Clin Microbiol. 1989 Sep;27(9):1956–1960. doi: 10.1128/jcm.27.9.1956-1960.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Fitzgerald T., Campbell I., Scott F. M., Browning G. F., Miller D. L., Herring A. J., Greenberg H. B. Rotavirus serotypes 6 and 10 predominate in cattle. J Clin Microbiol. 1990 Mar;28(3):504–507. doi: 10.1128/jcm.28.3.504-507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Nishikawa K., Urasawa T., Urasawa S., Midthun K., Kapikian A. Z., Gorziglia M. Complete nucleotide sequence of the gene encoding VP4 of a human rotavirus (strain K8) which has unique VP4 neutralization epitopes. J Virol. 1989 Sep;63(9):4101–4106. doi: 10.1128/jvi.63.9.4101-4106.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urasawa S., Urasawa T., Wakasugi F., Kobayashi N., Taniguchi K., Lintag I. C., Saniel M. C., Goto H. Presumptive seventh serotype of human rotavirus. Arch Virol. 1990;113(3-4):279–282. doi: 10.1007/BF01316680. [DOI] [PubMed] [Google Scholar]
- Ward R. L., Knowlton D. R., Pierce M. J. Efficiency of human rotavirus propagation in cell culture. J Clin Microbiol. 1984 Jun;19(6):748–753. doi: 10.1128/jcm.19.6.748-753.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward R. L., Nakagomi O., Knowlton D. R., McNeal M. M., Nakagomi T., Clemens J. D., Sack D. A., Schiff G. M. Evidence for natural reassortants of human rotaviruses belonging to different genogroups. J Virol. 1990 Jul;64(7):3219–3225. doi: 10.1128/jvi.64.7.3219-3225.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woode G. N., Kelso N. E., Simpson T. F., Gaul S. K., Evans L. E., Babiuk L. Antigenic relationships among some bovine rotaviruses: serum neutralization and cross-protection in gnotobiotic calves. J Clin Microbiol. 1983 Aug;18(2):358–364. doi: 10.1128/jcm.18.2.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]