Abstract
With the development of an insulin autoantibody (IAA) assay performed in 96-well filtration plates, we have evaluated prospectively the development of IAA in NOD mice (from 4 weeks of age) and children (from 7 to 10 months of age) at genetic risk for the development of type 1 diabetes. NOD mice had heterogeneous expression of IAA despite being inbred. IAA reached a peak between 8 and 16 weeks and then declined. IAA expression by NOD mice at 8 weeks of age was strongly associated with early development of diabetes, which occurred at 16–18 weeks of age (NOD mice IAA+ at 8 weeks: 83% (5/6) diabetic by 18 weeks versus 11% (1/9) of IAA negative at 8 weeks; P < .01). In man, IAA was frequently present as early as 9 months of age, the first sampling time. Of five children found to have persistent IAA before 1 year of age, four have progressed to diabetes (all before 3.5 years of age) and the fifth is currently less than age 2. Of the 929 children not expressing persistent IAA before age 1, only one has progressed to diabetes to date (age onset 3), and this child expressed IAA at his second visit (age 1.1). In new onset patients, the highest levels of IAA correlated with an earlier age of diabetes onset. Our data suggest that the program for developing diabetes of NOD mice and humans is relatively “fixed” early in life and, for NOD mice, a high risk of early development of diabetes is often determined by 8 weeks of age. With such early determination of high risk of progression to diabetes, immunologic therapies in humans may need to be tested in children before the development of IAA for maximal efficacy.
Keywords: insulin autoantibodies, radioassay, type 1 diabetes
Type 1A diabetes mellitus, as defined by an expert panel of the American Diabetes Association, is characterized by the presence of antiislet autoantibodies (1). There has been tremendous progress in defining islet autoantigens and developing antiislet autoantibody assays (2–4). In the most recent Immunology of Diabetes Workshop, a series of antiislet autoantibody assays were compared (2). Although the GAD65 autoantibody (GAA) and ICA512 (IA-2) autoantibody (ICA512AA) assays showed good concordance between laboratories, the insulin autoantibody (IAA) assays were divergent, with marked differences between laboratories in sensitivity and specificity. In this workshop, many of the IAA assays utilizing <600 μl of sera had sensitivities ≈1/2 of those utilizing a larger volume of serum (2). It is likely that such marked differences in interlaboratory measurement of IAA and the technical difficulty of current assays contributes to differences in the reported importance of IAA for disease prediction.
Despite the difficulties of IAA determination, a number of laboratories have routinely measured IAA with large sera volume assays for large series of patients followed to the development of diabetes. Studies from multiple countries have reported that IAA has an important role in diabetes prediction (5–8). Antiinsulin autoantibodies appear to be unique in that their levels are dramatically and inversely correlated with the age at which type 1 diabetes develops. We hypothesize that this inverse relationship results from a correlation of higher levels of IAA with the rate at which autoantibody-positive individuals progress to type 1 diabetes [“dual parameter model”(9)]. This past year, Williams et al. (10) and Naserke et al. (8) described IAA assays that utilize either Protein A-Sepharose or Protein A/Protein G-Sepharose. These assays utilize smaller serum volumes and correlate well with large volume IAA assays. The assays utilize centrifugation with aspiration to separate antibody-bound insulin from free insulin and counting of individual tubes in a γ counter. Having utilized these microassays, we reasoned that it might be possible to perform the IAA assay with 125I insulin in our standard 96-well filtration plates and quantitate precipitated 125I-insulin with a multichannel 96-well β counter. Scintillation fluid is directly added to the 96-well plate (Top Counter scintillation counter). We have developed such an assay and have evaluated a series of samples from individuals with new onset diabetes, individuals at risk for type 1 diabetes, control subjects, and NOD mice. We were surprised to find in NOD mice high levels of IAA early in life. These autoantibodies at 8 weeks of age strongly correlated with early development of diabetes, and, in a similar manner, four of five children persistently expressing IAA since 9 months of age progressed to diabetes before 3.5 years of age.
Research Design and Methods
Subjects.
The subjects of the current study consisted of 934 young children from the DAISY study (Diabetes Autoimmunity Study in the Young) who have been followed since 7–10 months of age with average initial test at 9 months of age. The range of follow-up was from 0 to 5.25 years, with average years of follow-up 1.86 and median 1.46. Of these children, 131 were a sibling or offspring of a patient with type 1 diabetes, and 803 were children from the general population with HLA typing at birth from cord blood as previously described (11).
In addition, 105 new onset patients with type 1A diabetes (within 7 days of diagnosis, at least one autoantibody positive, age from 1 to 45 years with median age of 11) and 106 healthy controls without a family history of type 1A diabetes (age range of 5–50 years, median age of 14 years) were studied to establish the upper limit of normal controls for the current IAA assay. Subjects or their parents gave informed consent to be studied, and the protocol was approved by the Institutional Review Boards of the University of Colorado.
NOD Mice.
We studied in a “cross-sectional” manner (one sample per mouse) 54 NOD serum samples from 38 female and 16 male mice at age 5–25 weeks (mean age 12 weeks and median age 12.5 weeks). All of the NOD mice studied in this group were not diabetic at the time when the blood was taken. In addition, 15 NOD female mice were evaluated prospectively beginning at 4 weeks of age until the development of diabetes or until 36 weeks of age. We also analyzed serum samples from 15 BALB/c mice (11 female and 4 male) at ages 7–24 weeks (mean age 13 weeks and median age 14 weeks) and 8 male and 5 female C57/B6 mice (5 at 8 weeks of age, 4 at 14 weeks of age, and 4 at 16 weeks of age).
Standard IAA Assay.
IAA were measured with a standard IAA radioassay (12) utilizing competition with unlabeled insulin with 600 μl of sera per determination (150 μl duplicates with and without unlabeled insulin). After a 7-day incubation at 4°C, antibody–antigen complexes were precipitated with polyethylene glycol 8000, and the results were calculated as the difference (Δ cpm) between the tube without cold insulin and the tube with cold insulin and were expressed as nU/ml (calculation formula: sample Δ cpm × 10,000/total cpm). In proficiency testing, our assay gave 100% sensitivity and specificity. In the Immunology of Diabetes Society's Combined Autoantibody Workshop of 1995, sensitivity of our IAA assay was one of the highest (56%) with a specificity of 98% (2). The interassay coefficient of variation is 10.3% (n = 7) (12). The upper limit of normals (42 nU/ml) was established as 99th percentile of 198 control subjects.
Ninety-Six-Well Filtration Plate Micro-IAA Assay.
125I-insulin (Amersham) of 20,000 cpm was incubated with 5 μl of serum with and without cold human insulin, respectively, at a 1:5 dilution of serum for 3 days at 4°C in buffer A [20 mM Tris⋅HCl buffer (pH 7.4) containing 150 mM NaCl, 1% BSA, 0.15% Tween-20, and 0.1% sodium azide). Fifty microliters of 50% Protein A/8% Protein G-Sepharose (Pharmacia) were added to the incubation in a MultiScreen-NOB 96-well filtration plate (Millipore), which was precoated with buffer A overnight at room temperature. The plate then was placed on a plate-shaker and was shaken at a low speed for 45 minutes at 4°C followed by two cycles of four washes per each cycle with cold buffer B (the same buffer as buffer A except for 0.1% BSA) by using the Millipore vacuum-operated 96-well plate washer. After washing, 40 μl of scintillation liquid (Microscint-20; Packard) was added to each well, and radioactivity was determined directly in the 96-well plate with a TopCount (96-well plate β-counter; Packard) scintillation counter. Within the limit of the highest positive we studied (9,000 cpm), radioactivity in one well did not influence counts in neighboring wells without added 125I-insulin. The result was calculated based on the difference in counts per minute (Δ cpm) between the well without cold insulin and the well with cold insulin and was expressed as an index: index = (sample Δ cpm − negative control Δ cpm)/(positive control Δ cpm − negative control Δ cpm). The interassay coefficient of variation was 11% (n = 8). The limit of normal (0.010) was chosen as the 99th percentile from receiver operating characteristic curves in 106 healthy control subjects and 105 patients with recent onset diabetes.
GAA and ICA512AA Assay.
GAA and ICA512AA assays were performed with methods previously described (13). In brief, labeled recombinant GAD65 and ICA512bdc were produced by in vitro transcription/translation with differential labeling (3H-GAD65 and 35S-ICA512). The combined GAA and ICA512AA radioassay was performed on a 96-well filtration plate (Millipore) and radioactivity was counted on a TopCount 96-well plate β-counter (Packard). The levels of both antibodies were expressed as an index. The interassay coefficients of variation are 6% and 9.6% (n = 10) for GAA and ICA512AA, respectively. The upper limits of normals (0.032 for GAA; 0.071 for ICA512AA) were established as the 99th percentile for GAA and as the 100th percentile for ICA512AA in 198 healthy controls. In the Immunology of Diabetes Society's Combined Autoantibody Workshop of 1995, sensitivity (for diabetes less than age 30) for the GAA assay was 82% and specificity was 99% (2). Sensitivity for the ICA512AA assay was 73% and specificity was 100%.
Statistics.
Statistical analysis was performed using regression analysis and Fisher exact test with epistat (Round Rock, Richardson, TX) software. The survival curve analysis was performed with prizm software (GraphPad, San Diego).
Results
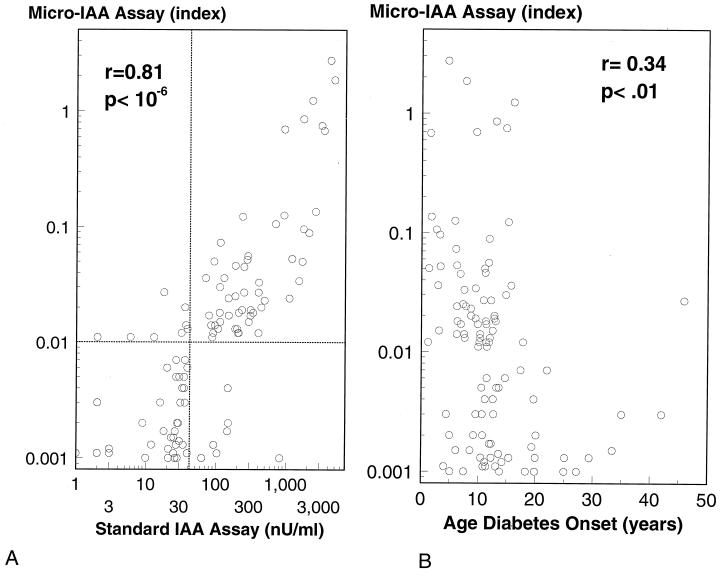
With the 96-well filtration plate micro-IAA assay we analyzed 105 serum samples from new onset patients with type 1A diabetes (age from 1 to 45 years, the median age of 11) and 106 serum samples from healthy control subjects. Using the 99th percentile of the 106 normal controls, 69/105 (66%) of the new onset patients with type 1A diabetes were positive for IAA (Fig. 1). Fig. 2A compares the levels of this new micro-IAA assay with our standard large volume assay in the same 105 patients. Positivity was set at the 99th percentile of normal subjects for both assays (>42 nU/ml for the standard assay and an index >0.01 for the microassay). Sensitivity of the microassay (58%) was similar to our standard assay (56%). It has been reported (14) that hemolyzed sera create false positives with standard polyethylene glycol precipitation IAA assays. The serum strongly positive for IAA by the standard assay (800 nU/ml) but negative with the microassay was hemolyzed (Fig. 2A). Similar to prior reports (9), with the current IAA assay, the level of IAA of the 105 new onset patients was inversely correlated with the age of type 1 diabetes (Fig. 2B) onset.
Figure 1.
Samples from 106 healthy normal control subjects and 105 new onset patients with type 1 diabetes were analyzed with the 96-well filtration plate IAA assay. Sixty-six percent (69/105) of new onset patients were above the 99th percentile of 106 normal controls.
Figure 2.
(A) Serum samples from 105 new onset patients of type 1 diabetes were analyzed with our standard IAA assay and new 96-well filtration plate IAA assay. The levels of IAA determined with these two methods were very well correlated. One sample with the highest IAA level by standard IAA assay but negative by new micro-IAA assay was hemolyzed. (B) Inverse correlation between level of insulin autoantibodies at diagnosis versus age of diabetes onset.
From the DAISY study, 934 young children have had IAA determined before 1 year of age, including 131 sibling or offspring of a patient with type 1 diabetes and 803 children from the general population. Among these children, eight were found IAA-positive at 9 months on their initial screening samples. For three of the children, IAA did not persist and were negative at 1.2 years of age on their second visit. None of these children have developed diabetes. Five children expressed IAA persistently since their first visit at 9 months of age and four have developed type 1 diabetes at ages 1, 2, 2.6, and 3.5, respectively (Fig. 3). The fifth is not yet diabetic but is less than age 2. Only one child among the remaining 926 children who were IAA-negative before 1 year of age has developed diabetes (onset at 3 years of age, and this child became IAA-positive at 13 months of age). All five children who have been followed to diabetes had IAA as their first detectable autoantibody, with one child expressing IAA and GAD65 autoantibodies simultaneously at his first visit at 9 months of age. This child developed diabetes at 12 months of age. For one patient, a peak of antiinsulin autoantibodies was seen between 1 and 1.7 years of age, with diabetes onset at 2.6 years (Fig. 3B).
Figure 3.
Levels of insulin autoantibodies versus age for children of the DAISY study followed to development of diabetes. (A) Relatives of patients with type 1 diabetes. (B) Individuals from the general population without a diabetic relative identified by HLA typing of cord blood and followed to onset of diabetes.
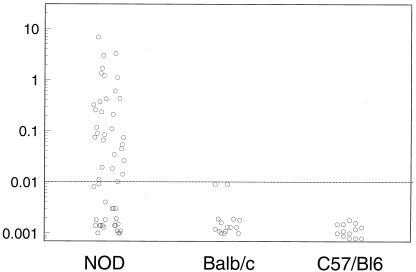
It has been difficult to measure IAA of NOD mice with standard fluid phase assays. Our prior studies demonstrated low levels of such autoantibodies when we utilized polyethylene glycol precipitation (15). Applying the microassay to NOD sera, we were surprised to find IAA equivalent to that detected in patients with new onset diabetes. We analyzed samples from 54 nondiabetic NOD mice (age 5–25 weeks) and 28 age-matched BALB/c and C57/B6 mice. For NOD mice, 31/54 (57%) were positive, with 19/25 (75%) positive between 10 and 20 weeks of age versus 0 of 15 BALB/c and 0 of 13 C57/B6 mice. Noticeably, the normal ranges and levels of IAA in mice were very similar to that of humans (see Fig. 4). Prospective evaluation of 15 female NOD mice revealed the appearance of IAA at 8 weeks of age for six mice and after 8 weeks for seven mice, and two mice were negative for IAA (Table 1). Of note, of the six mice expressing insulin autoantibodies at 8 weeks of age, five became diabetic between 16 and 18 weeks (Table 1). The survival curve analysis showed significant earlier development diabetes for the mice expressing IAA at 8 weeks of age (P < .01) compared with the mice expressing IAA after 8 weeks. Fig. 5 shows the levels of IAA (A) and corresponding blood glucose levels (B) for the mice expressing IAA at 8 weeks of age or at 20 weeks or later and linear regression for all 15 mice (age of first detection of IAA versus age of diabetes) (C).
Figure 4.
Serum samples from 54 NOD mice and 23 BALB/c and C57/B6 mice were analyzed with the 96-well filtration plate IAA assay. The normal ranges and levels of IAA in mice were very similar to that in humans.
Table 1.
Prospective follow-up of NOD mice for insulin autoantibodies with the microassay and onset of diabetes
| Age of developing IAA, weeks | Age of diabetes onset, weeks |
|---|---|
| 8 (n = 6) | 16, 16, 16, 16, 18, 26* |
| 12 (n = 1) | 20 |
| 16 (n = 3) | 16, 24, 24 |
| 20 (n = 2) | 24, 26 |
| 24 (n = 1) | >36 |
| Negative (n = 2) | 24, >36 |
This mouse showed a diabetic blood glucose level at 16 weeks of age (251 mg/dl), which reverted to normal for 10 weeks (see Fig. 5).
Figure 5.
Insulin autoantibodies and blood glucose for the NOD mice expressing IAA at 8 weeks of age and at 20 weeks or later followed from 4 weeks of age until diabetes or 36 weeks. (A) The levels of insulin autoantibodies. (B) The level of blood glucose. (C) Age of first detection of insulin autoantibodies plotted versus age of diabetes onset for all 15 prospectively evaluated mice. Two mice with open symbols did not have IAA detected.
Discussion
An increasing body of evidence indicates that detection of insulin autoantibodies (IAA) is important for the prediction and diagnosis of type 1A diabetes. Prior workshops indicated that IAA measured by ELISA techniques did not detect IAA associated with diabetes risk, and, thus, most investigators have utilized fluid phase radioassays (16). The volume of sera necessary for high sensitivity radioassays, with polyethylene glycol precipitation and centrifugation followed by γ counting, have limited the determination of IAA (2). The assay described by Williams and coworkers using Protein A-Sepharose allowed a major reduction in the amount of sera utilized and has improved assay specificity but still relies on centrifugation and γ counting (8, 10). Our current assay builds on their assay and eliminates centrifugation and allows semiautomated handling, including counting, directly in 96-well plates. With our current assay, IAA can be measured in a manner similar to the determination of GAD65 autoantibodies (GAA) and ICA512 (IA2) autoantibodies (ICA512AA).
IAA are frequently found in young children developing diabetes (17, 18), and IAA are among the first detectable autoantibodies in children (19). In present study, 5/5 children who have been followed up to diabetes had IAA as their first detectable autoantibody, with only 1 child found to express IAA and GAA simultaneously on his first visit sample at 9 months of age. With sensitive IAA assays, older children and adults with or developing type 1 diabetes are also frequently antibody-positive (6). Thus, the current microassay with sensitivity equivalent to large volume standard assays should aid in the diagnosis of type 1A diabetes of both young and older individuals.
Standard assays for IAA have relied on polyethylene glycol for precipitation of autoantibodies. Polyethylene glycol precipitation is not specific for immunoglobulins, and this probably relates to reported false positive results for cord blood samples (20) and for hemolyzed blood samples (14).
Multiple antiislet autoantibodies indicate a higher risk for progression to type 1A diabetes in contrast to individuals expressing only a single antibody (IAA, GAA, ICA512AA, or cytoplasmic ICA autoantibodies) (4, 6, 21, 22). Of five children who progressed to diabetes in the present study, three expressed two autoantibodies (IAA and GAA), one three autoantibodies (IAA, GAA, and ICA512AA), and one a single autoantibody (IAA) before the onset of diabetes. We routinely determine GAA and ICA512AA simultaneously with differential in vitro labeling (35S-ICA512 and 3H-GAD65) of the respective antigens. It may be possible to add 125I-insulin to such simultaneous determination, but this will likely require reducing the background counts (in the absence of autoantibodies) of the GAA/ICA512AA assay. At present, we test samples for GAA, ICA512AA, and IAA in two separate assays (one for GAA and ICA512AA and the other for IAA). In man, a number of reports, and our present data, indicate that IAA is among the first autoantibodies to appear, and the dominant autoantibodies are IgG1 (23). It has been reported that there is a peak of IAA early in life of prediabetic infants. IgG1 autoantibodies comprised the bulk of the IAA. One of the five children we followed from birth to diabetes had such a peak.
Of five children in the DAISY study who expressed IAA persistently since 9 months of age, four progressed to diabetes with an average follow-up of 1.1 years, and the child not yet diabetic is less than 2 years of age. Only one child of remaining 929 children who were IAA-negative before 1 year of age developed diabetes at 3 years of age. We hypothesize that immunologic therapies such as “immunologic vaccination” may be most effective if introduced before the development of such autoantibodies. The design of trials in such a young group may be particularly difficult, but recent studies indicate a risk as high as 40% of developing antiislet autoantibodies for DR3/4 relatives of patients with type 1 diabetes (24).
Antiinsulin autoantibodies of NOD mice show a dramatic peak between 8 and 16 weeks of age. Autoantibody positivity at 8 weeks of age was strongly associated with early development of diabetes by 18 weeks of age (5/6 vs. 1/9, P < 0.01). It is remarkable that this early phenotypic variation is present for inbred NOD mice raised in a similar pathogen free environment. The apparent program for the development of type 1 diabetes at 16 weeks of age correlates with a single immunologic measurement at 8 weeks of age. It has been hypothesized that NOD mice acutely develop type 1 diabetes with an acute switch from a “benign” to a malignant insulitis (25–27), although direct histologic evaluation of NOD mice suggests a more chronic process of β-cell destruction (28). To date, we have been unable, similar to most investigators, to detect GAD65 autoantibodies in NOD mice, and thus we cannot rule out a similar relationship with other autoantibodies (29, 30). The inverse relationship between age of onset of type 1 diabetes and insulin autoantibodies seen in humans is not present for GAD65 autoantibodies (9). Thus, we hypothesize that a program related to an immune response to insulin is in place early in life in the majority of NOD mice and children developing type 1 diabetes. The ability to identify high- risk humans and NOD mice by early expression of insulin autoantibodies should aid in deciphering the relevant components of this program and suggest the potential importance of early intervention for optimal disease prevention.
Acknowledgments
This research was supported by grants from the National Institutes of Health (DK32083, 5R01DK32493-14, and 1R01DK55969-01), the American Diabetes Association, and the Children's Diabetes Foundation, and by Grant M01 RR00069 from the General Clinical Research Program, National Centers for Research Resources, National Institutes of Health. N.A. is a recipient of a Mentor-Based Fellowship from the American Diabetes Association.
Abbreviations
- GAA
GAD65 autoantibody
- IAA
insulin autoantibody
Footnotes
Article published online before print: Proc. Natl. Acad. Sci. USA, 10.1073/pnas.040556697.
Article and publication date are at www.pnas.org/cgi/doi/10.1073/pnas.040556697
References
- 1.American Diabetes Association. Diabetes Care. 1997;20:1183–1197. [Google Scholar]
- 2.Verge C F, Stenger D, Bonifacio E, Colman P G, Pilcher C, Bingley P J, Eisenbarth G S. Diabetes. 1998;47:1857–1866. doi: 10.2337/diabetes.47.12.1857. [DOI] [PubMed] [Google Scholar]
- 3.Atkinson M A, Maclaren N K. J Clin Invest. 1993;92:1608–1616. doi: 10.1172/JCI116745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bingley P J, Bonifacio E, Williams A J K, Genovese S, Bottazzo G F, Gale E A M. Diabetes. 1997;46:1701–1710. doi: 10.2337/diab.46.11.1701. [DOI] [PubMed] [Google Scholar]
- 5.Ziegler A G, Ziegler R, Vardi P, Jackson R A, Soeldner J S, Eisenbarth G S. Diabetes. 1989;38:1320–1325. doi: 10.2337/diab.38.10.1320. [DOI] [PubMed] [Google Scholar]
- 6.Verge C F, Gianani R, Kawasaki E, Yu L, Pietropaolo M, Jackson R A, Chase H P, Eisenbarth G S. Diabetes. 1996;45:926–933. doi: 10.2337/diab.45.7.926. [DOI] [PubMed] [Google Scholar]
- 7.Colman P G, McNair P D, Caudwell J P, Howard N J, Colaguri S, Harrison L C. Autoimmunity. 1993;15,Suppl.:31. (abstr.). [Google Scholar]
- 8.Naserke H E, Dozio N, Ziegler A-G, Bonifacio E. Diabetologia. 1998;41:681–683. doi: 10.1007/s001250050968. [DOI] [PubMed] [Google Scholar]
- 9.Eisenbarth G S, Gianani R, Yu L, Pietropaolo M, Verge C F, Chase H P, Redondo M J, Colman P, Harrison L, Jackson R. Proc Assoc Am Physicians. 1998;110:126–135. [PubMed] [Google Scholar]
- 10.Williams A J K, Bingley P J, Bonifacio E, Palmer J P, Gale E A M. J Autoimmun. 1997;10:473–478. doi: 10.1006/jaut.1997.0154. [DOI] [PubMed] [Google Scholar]
- 11.Rewers M, Bugawan T L, Norris J M, Blair A, Beaty B, Hoffman M, McDuffie R S, Jr, Hamman R F, Klingensmith G, Eisenbarth G S, Erlich H A. Diabetologia. 1996;39:807–812. doi: 10.1007/s001250050514. [DOI] [PubMed] [Google Scholar]
- 12.Vardi P, Dib S A, Tuttleman M, Connelly J E, Grinbergs M, Rabizadeh A, Riley W J, Maclaren N K, Eisenbarth G S, Soeldner J S. Diabetes. 1987;36:1286–1291. doi: 10.2337/diab.36.11.1286. [DOI] [PubMed] [Google Scholar]
- 13.Yu L, Rewers M, Gianani R, Kawasaki E, Zhang Y, Verge C, Chase P, Klingensmith G, Erlich H, Norris J, Eisenbarth G S. J Clin Endocrinol Metab. 1996;81:4264–4267. doi: 10.1210/jcem.81.12.8954025. [DOI] [PubMed] [Google Scholar]
- 14.Naserke H E, Bonifacio E, Ziegler A-G. J Clin Endocrinol Metab. 1999;84:1239–1243. doi: 10.1210/jcem.84.4.5597. [DOI] [PubMed] [Google Scholar]
- 15.Ziegler A G, Vardi P, Ricker A T, Hattori M, Soeldner J S, Eisenbarth G S. Diabetes. 1989;38:358–363. doi: 10.2337/diab.38.3.358. [DOI] [PubMed] [Google Scholar]
- 16.Greenbaum C, Palmer J P, Kuglin B, Kolb H. J Clin Endocrinol Metab. 1992;74:1040–1044. doi: 10.1210/jcem.74.5.1569152. [DOI] [PubMed] [Google Scholar]
- 17.Dean B M, Becker F, McNally J M, Tarn A C, Schwartz B, Gale E A, Bottazzo G F. Diabetologia. 1986;29:339–342. doi: 10.1007/BF00452073. [DOI] [PubMed] [Google Scholar]
- 18.Vardi P, Ziegler A G, Matthews J H, Dib S, Keller R J, Ricker A T, Wolfsdorf J I, Herskowitz R D, Rabizadeh A, Eisenbarth G S, Soeldner J S. Diabetes Care. 1988;11:736–739. doi: 10.2337/diacare.11.9.736. [DOI] [PubMed] [Google Scholar]
- 19.Roll U, Christie M R, Fuchtenbusch M, Ziegler A. Diabetes. 1995;44:77. (abstr.). [Google Scholar]
- 20.Bilbao J R, Calvo B, Urrutia I, Linares A, Castano L. Diabetes. 1997;46:713–716. doi: 10.2337/diab.46.4.713. [DOI] [PubMed] [Google Scholar]
- 21.Bonifacio E, Genovese S, Braghi S, Bazzigaluppi E, Lampasona V, Bingley P J, Rogge L, Pastore M R, Bognetti E, Bottazzo G F, et al. Diabetologia. 1995;38:816–822. doi: 10.1007/s001250050358. [DOI] [PubMed] [Google Scholar]
- 22.Bingley P J, Christie M R, Bonifacio E, Bonfanti R, Shattock M, Fonte M-T, Bottazzo G-F, Gale E A M. Diabetes. 1994;43:1304–1310. doi: 10.2337/diab.43.11.1304. [DOI] [PubMed] [Google Scholar]
- 23.Bonifacio E, Scirpoli M, Kredel K, Fuchtenbusch M, Ziegler A G. J Immunol. 1999;163:525–532. [PubMed] [Google Scholar]
- 24.Eisenbarth G S, Elsey C, Yu L, Rewers M. Diabetes. 1998;47:210. doi: 10.2337/diabetes.47.5.733. (abstr.). [DOI] [PubMed] [Google Scholar]
- 25.Gazda L S, Charlton B, Lafferty K J. J Autoimmun. 1997;10:261–270. doi: 10.1006/jaut.1997.0138. [DOI] [PubMed] [Google Scholar]
- 26.Dilts S M, Lafferty K J. J Autoimmun. 1999;12:229–232. doi: 10.1006/jaut.1999.0284. [DOI] [PubMed] [Google Scholar]
- 27.Shimada A, Charlton B, Taylor-Edwards C, Fathman C G. Diabetes. 1996;45:1063–1067. doi: 10.2337/diab.45.8.1063. [DOI] [PubMed] [Google Scholar]
- 28.Sreenan S, Pick A J, Levisetti M, Baldwin A C, Pugh W, Polonsky K S. Diabetes. 1999;48:989–996. doi: 10.2337/diabetes.48.5.989. [DOI] [PubMed] [Google Scholar]
- 29.Bieg S, Seissler J, Herberg L, Northemann W, Scherbaum W A. Autoimmunity. 1994;17:189–194. doi: 10.3109/08916939409010653. [DOI] [PubMed] [Google Scholar]
- 30.Ziegler B, Augstein P, Luhder F, Northemann W, Hamann J, Schlosser M, Kloting I, Michaelis D, Ziegler M. Diabetes Res. 1994;25:47–64. [PubMed] [Google Scholar]