Abstract
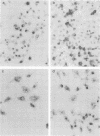
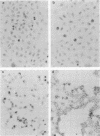
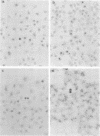
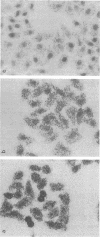
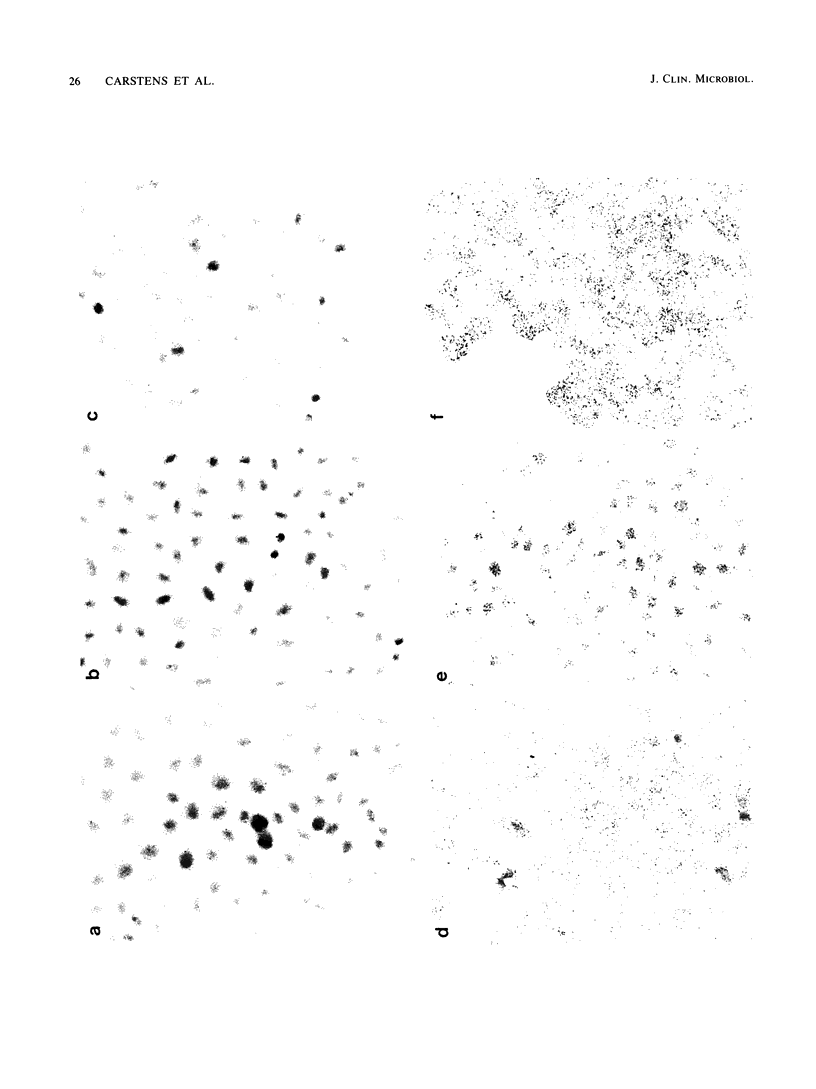
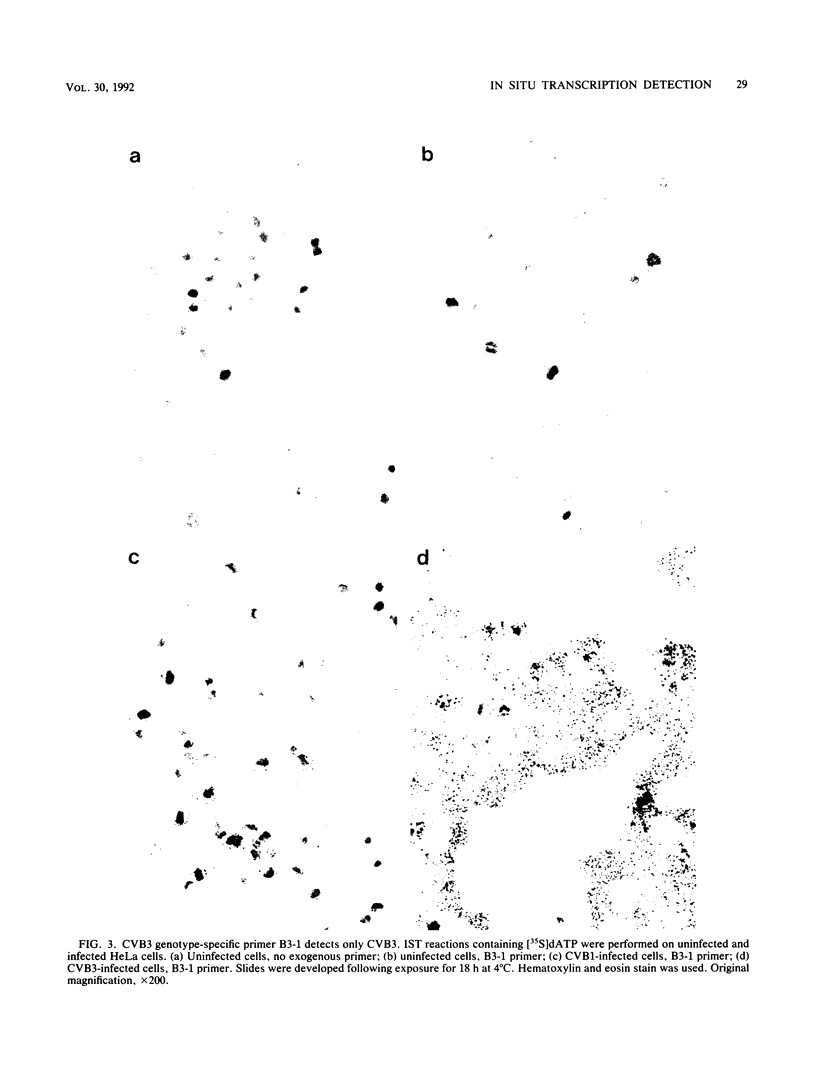
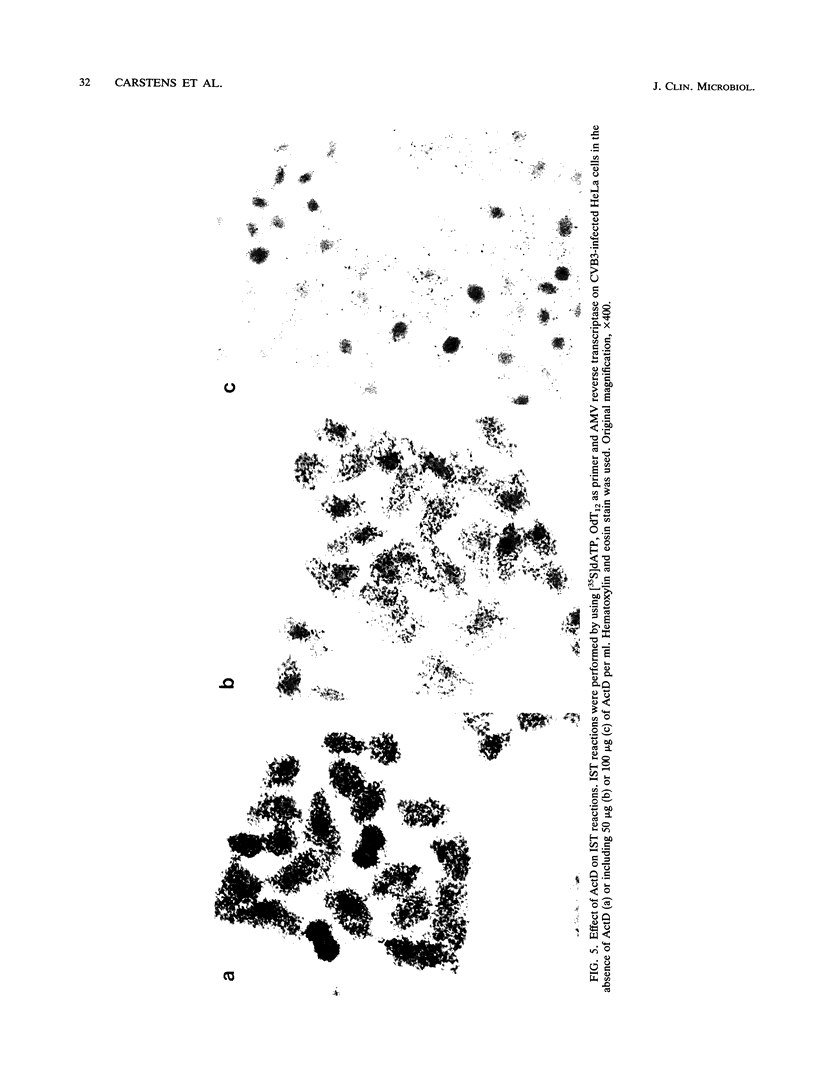
In situ transcription (IST) was shown to be useful for the detection of human enteroviral RNA in cultured cells. A primer to detect a wide variety of enteroviral genomes and a coxsackievirus type B3 genome-specific primer were demonstrated to be efficient in IST assays. Transcription times greater than 10 to 30 min did not significantly improve the acquisition of a specific signal, whereas the signal-to-noise ratio decreased with time. Inclusion of actinomycin D to suppress DNA-dependent DNA polymerase activity in reverse transcriptase decreased the signal that was obtained without improving the signal-to-noise ratio. Use of RNase H-free murine leukemia virus reverse transcriptase in the IST reaction increased the signal versus that obtained by use of the avian myeloblastosis virus enzyme, which contains endogenous RNase H activity. Exogenous RNase H added to the transcription reaction ablated the signal. Background transcription because of poorly hybridized (mismatched) primers was reduced after primer hybridization and prior to the transcription reaction by rinsing fixed cells with 3 M tetramethylammonium chloride at temperatures which dissociate mismatched primer-template duplexes. The rapid detection time and the simplicity of application suggest that IST can be performed with a high specificity for the detection of enteroviral genomic sequences in cultured cells and may be more useful than in situ hybridization for the detection of enteroviral genomes.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman N. M., Tracy S., Gauntt C. J., Fortmueller U. Molecular detection and identification of enteroviruses using enzymatic amplification and nucleic acid hybridization. J Clin Microbiol. 1990 May;28(5):843–850. doi: 10.1128/jcm.28.5.843-850.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller B. A., Chapman N. M., Beck M. A., Pallansch M. A., Gauntt C. J., Tracy S. M. Echovirus 22 is an atypical enterovirus. J Virol. 1990 Jun;64(6):2692–2701. doi: 10.1128/jvi.64.6.2692-2701.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiLella A. G., Woo S. L. Hybridization of genomic DNA to oligonucleotide probes in the presence of tetramethylammonium chloride. Methods Enzymol. 1987;152:447–451. doi: 10.1016/0076-6879(87)52052-3. [DOI] [PubMed] [Google Scholar]
- Easton A. J., Eglin R. P. The detection of coxsackievirus RNA in cardiac tissue by in situ hybridization. J Gen Virol. 1988 Feb;69(Pt 2):285–291. doi: 10.1099/0022-1317-69-2-285. [DOI] [PubMed] [Google Scholar]
- Gurgo C., Ray R. K., Thiry L., Green M. Inhibitors of the RNA and DNA dependent polymerase activities of RNA tumour viruses. Nat New Biol. 1971 Jan 27;229(4):111–114. doi: 10.1038/newbio229111a0. [DOI] [PubMed] [Google Scholar]
- Hayashi S., Gillam I. C., Delaney A. D., Tener G. M. Acetylation of chromosome squashes of Drosophila melanogaster decreases the background in autoradiographs from hybridization with [125I]-labeled RNA. J Histochem Cytochem. 1978 Aug;26(8):677–679. doi: 10.1177/26.8.99471. [DOI] [PubMed] [Google Scholar]
- Hyypiä T., Auvinen P., Maaronen M. Polymerase chain reaction for human picornaviruses. J Gen Virol. 1989 Dec;70(Pt 12):3261–3268. doi: 10.1099/0022-1317-70-12-3261. [DOI] [PubMed] [Google Scholar]
- Kacian D. L., Myers J. C. Synthesis of extensive, possibly complete, DNA copies of poliovirus RNA in high yields and at high specific activities. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2191–2195. doi: 10.1073/pnas.73.7.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandolf R., Ameis D., Kirschner P., Canu A., Hofschneider P. H. In situ detection of enteroviral genomes in myocardial cells by nucleic acid hybridization: an approach to the diagnosis of viral heart disease. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6272–6276. doi: 10.1073/pnas.84.17.6272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotewicz M. L., Sampson C. M., D'Alessio J. M., Gerard G. F. Isolation of cloned Moloney murine leukemia virus reverse transcriptase lacking ribonuclease H activity. Nucleic Acids Res. 1988 Jan 11;16(1):265–277. doi: 10.1093/nar/16.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laird-Meeter K., van Domburg R., Bos E., Hugenholtz P. G. Survival at 5 to 10 years after aorto-coronary bypass operations in 1041 consecutive patients. Eur Heart J. 1987 May;8(5):449–456. doi: 10.1093/oxfordjournals.eurheartj.a062304. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucleic Acids Res. 1985 Mar 11;13(5):1777–1799. doi: 10.1093/nar/13.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada I., Matsumori A., Kawai C., Yodoi J., Tracy S. The viral genome in experimental murine Coxsackievirus B3 myocarditis: a Northern blotting analysis. J Mol Cell Cardiol. 1990 Sep;22(9):999–1008. doi: 10.1016/0022-2828(90)91039-a. [DOI] [PubMed] [Google Scholar]
- Petitjean J., Quibriac M., Freymuth F., Fuchs F., Laconche N., Aymard M., Kopecka H. Specific detection of enteroviruses in clinical samples by molecular hybridization using poliovirus subgenomic riboprobes. J Clin Microbiol. 1990 Feb;28(2):307–311. doi: 10.1128/jcm.28.2.307-311.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reich E., Goldberg I. H. Actinomycin and nucleic acid function. Prog Nucleic Acid Res Mol Biol. 1964;3:183–234. doi: 10.1016/s0079-6603(08)60742-4. [DOI] [PubMed] [Google Scholar]
- Rotbart H. A., Abzug M. J., Murray R. S., Murphy N. L., Levin M. J. Intracellular detection of sense and antisense enteroviral RNA by in situ hybridization. J Virol Methods. 1988 Dec;22(2-3):295–301. doi: 10.1016/0166-0934(88)90111-5. [DOI] [PubMed] [Google Scholar]
- Rotbart H. A. Enzymatic RNA amplification of the enteroviruses. J Clin Microbiol. 1990 Mar;28(3):438–442. doi: 10.1128/jcm.28.3.438-442.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotbart H. A., Levin M. J., Villarreal L. P., Tracy S. M., Semler B. L., Wimmer E. Factors affecting the detection of enteroviruses in cerebrospinal fluid with coxsackievirus B3 and poliovirus 1 cDNA probes. J Clin Microbiol. 1985 Aug;22(2):220–224. doi: 10.1128/jcm.22.2.220-224.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotbart H. A., Levin M. J., Villarreal L. P. Use of subgenomic poliovirus DNA hybridization probes to detect the major subgroups of enteroviruses. J Clin Microbiol. 1984 Dec;20(6):1105–1108. doi: 10.1128/jcm.20.6.1105-1108.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Summers J., Mason W. S. Replication of the genome of a hepatitis B--like virus by reverse transcription of an RNA intermediate. Cell. 1982 Jun;29(2):403–415. doi: 10.1016/0092-8674(82)90157-x. [DOI] [PubMed] [Google Scholar]
- Tecott L. H., Barchas J. D., Eberwine J. H. In situ transcription: specific synthesis of complementary DNA in fixed tissue sections. Science. 1988 Jun 17;240(4859):1661–1664. doi: 10.1126/science.2454508. [DOI] [PubMed] [Google Scholar]
- Temin H. M., Baltimore D. RNA-directed DNA synthesis and RNA tumor viruses. Adv Virus Res. 1972;17:129–186. doi: 10.1016/s0065-3527(08)60749-6. [DOI] [PubMed] [Google Scholar]
- Tracy S. A comparison of genomic homologies among the coxsackievirus B group: use of fragments of the cloned coxsackievirus B3 genome as probes. J Gen Virol. 1984 Dec;65(Pt 12):2167–2172. doi: 10.1099/0022-1317-65-12-2167. [DOI] [PubMed] [Google Scholar]
- Tracy S., Chapman N. M., McManus B. M., Pallansch M. A., Beck M. A., Carstens J. A molecular and serologic evaluation of enteroviral involvement in human myocarditis. J Mol Cell Cardiol. 1990 Apr;22(4):403–414. doi: 10.1016/0022-2828(90)91476-n. [DOI] [PubMed] [Google Scholar]
- Tracy S. Comparison of genomic homologies in the coxsackievirus B group by use of cDNA:RNA dot-blot hybridization. J Clin Microbiol. 1985 Mar;21(3):371–374. doi: 10.1128/jcm.21.3.371-374.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tracy S., Smith R. A. A comparison of the genomes of polioviruses by cDNA:RNA hybridization. J Gen Virol. 1981 Jul;55(Pt 1):193–199. doi: 10.1099/0022-1317-55-1-193. [DOI] [PubMed] [Google Scholar]
- Tracy S., Wiegand V., McManus B., Gauntt C., Pallansch M., Beck M., Chapman N. Molecular approaches to enteroviral diagnosis in idiopathic cardiomyopathy and myocarditis. J Am Coll Cardiol. 1990 Jun;15(7):1688–1694. doi: 10.1016/0735-1097(90)92846-t. [DOI] [PubMed] [Google Scholar]
- Wiegand V., Tracy S., Chapman N., Wucherpfennig C. Enteroviral infection in end stage dilated cardiomyopathy. Klin Wochenschr. 1990 Sep 14;68(18):914–920. doi: 10.1007/BF01649038. [DOI] [PubMed] [Google Scholar]