Abstract
Depletion of mitochondrial DNA (mtDNA) and mtDNA-encoded respiratory chain proteins in subcutaneous (SC) fat from patients with HIV lipoatrophy have clearly demonstrated the role of mitochondrial dysfunction in this syndrome. Research in HIV lipoatrophy, however, has been severely hampered by the lack of a suitable surrogate marker in blood or other easily obtained clinical specimens as fat biopsies are invasive and mtDNA levels in peripheral blood mononuclear cells (PBMC) do not consistently correlate with the disease process. We used a simple, rapid, quantitative 2-site dipstick immunoassay to measure OXPHOS enzymes Complex I (CI) and Complex IV (CIV), and rtPCR to measure mtDNA in 26 matched SC fat and PBMC specimens previously banked from individuals on potent antiretroviral (ARV) therapy with HIV lipoatrophy, on similar ARV therapy without lipoatrophy, and in HIV seronegative controls. Significant correlations were found between the respective PBMC and fat levels for both CI (r = 0.442, p = 0.024) and for CIV (r = 0.507, p = 0.008). Both CI and CIV protein levels were also significantly reduced in both PBMCs and fat in lipoatrophic subjects compared to HIV seronegative controls (p ≤ 0.05), while a comparative reduction in mtDNA levels in lipoatrophic subjects was observed only in fat. We conclude that CI and CIV levels in PBMCs correlate to their respective levels in fat and may have utility as surrogate markers of mitochondrial dysfunction in lipoatrophy.
Introduction
HIV lipoatrophy, typically characterized by loss of subcutaneous (sc) fat in the face, buttocks, arms, and legs, is a distressing syndrome seen in HIV-infected subjects following initiation of antiretroviral (ARV) therapy. The syndrome may lead not only to negative self-perception and nonadherence to HIV ARV therapy1–3 but to adverse metabolic abnormalities such as insulin resistance, type 2 diabetes, and hypertriglyceridemia, which may result in increased cardiovascular risk.4–6 Convincing evidence now links development of HIV lipoatrophy to mitochondrial dysfunction in adipose tissue associated with use of certain nucleoside reverse transcriptase inhibitors (NRTIs), in particular with the use of stavudine (d4T) or zidovudine (ZDV).7–9
Research in this field has been severely hampered by the lack of a suitable surrogate marker in blood or other easily obtained clinical specimens.10 NRTIs are known to inhibit mitochondrial DNA (mtDNA) replication through interaction with mitochondrial DNA polymerase-γ.11 mtDNA is the most widely assayed mitochondrial parameter in HIV lipoatrophy. Unfortunately, while mtDNA levels have been consistently demonstrated to be depleted in adipose tissue of lipoatrophic subjects,7,12–14 mtDNA levels in peripheral blood mononuclear cells (PBMCs) do not correlate well with either clinical lipoatrophy or with mtDNA levels in fat.13,15–18 Mitochondrial protein levels, and in particular levels of enzyme complexes of the oxidative phosphorylation system (OXPHOS), may be more informative indicators of mitochondrial function than are measurements of mtDNA. The mitochondrial OXPHOS system is essential for energy metabolism and normally provides more than 95% of ATP used for cellular energy. OXPHOS enzymes complexes I, III, IV, and V contain cores of mtDNA-encoded subunits and so are affected by mtDNA depletion.19,20 Moreover, protein levels can be regulated by other means including both transcriptional and translational control and OXPHOS protein transcripts have been shown to be reduced in the absence of measurable effects on mtDNA levels in HIV-negative individuals exposed briefly (2 weeks) to NRTIs.21 Finally, these complexes are sensitive to oxidative stress and other environmental factors, including the direct action of many therapeutic drugs.22
In this pilot study, we assayed levels of OXPHOS enzymes Complex I (CI) and Complex IV (CIV) in matched sc adipose tissue and PBMC specimens by a novel two-site immunoassay dipstick methodology. We report that CI and CIV enzyme levels correlate well in PBMCs and adipose tissue. Furthermore, compared to specimens from HIV seronegative controls, depletion of both CI and CIV can be demonstrated in both PBMCs and adipose tissue from HIV lipoatrophic subjects on highly active antiretroviral therapy (HAART). These quantitative, simple, and rapid assays performed in PBMCs deserve further study as potential suitable surrogate markers for mitochondrial toxicity in fat.
Materials and Methods
The study was conducted utilizing matched PBMCs and sc adipose tissues available as part of banked specimens from patients who previously participated in a cross-sectional lipoatrophy study conducted by the Hawaii AIDS Clinical Research Program at the University of Hawaii. Consent to utilize banked specimens for future studies related to HIV lipoatrophy was part of the original informed consent document approved by the Committee on Human Studies of the University of Hawaii, Manoa and signed by all participants. Available specimens were from subjects who could be divided into the following cohorts: (1) lipoatrophic cohort: HIV-infected individuals on older NRTI-containing [ZDV, d4T, and/or didanosine (ddI)] potent ARV therapy (three or more drugs) for more than 6 months with self-reported and investigator-confirmed changes of lipoatrophy defined as thinning of the face and/or extremities that temporally began after initiation of such NRTI-containing ARV therapy; (2) nonlipoatrophic cohort: HIV-infected individuals on similar NRTI-containing ARV therapy for more than 6 months without self-reported changes of lipoatrophy; and (3) HIV seronegative controls recruited primarily from friends and partners of HIV-infected patients seen by our program. Subjects were excluded if they had an acute illness within 2 weeks of study entry, persistent or unstable chronic infections or illnesses other than HIV, a history of diabetes mellitus or hypogonadism, or receipt of any anabolic agents, glucocorticoids, appetite stimulants, or lipid-lowering agents within 2 months prior to study entry.
Blood was obtained in each subject in a fasting state defined as nothing by mouth except water for ≥12 h prior to the blood draw. Blood for lactate was collected in sodium fluoride (gray-top) tubes and maintained on ice until the plasma was separated from cells within 30 min of the blood draw. The lactate assays were then performed in real time by a local CLIA and CAP-certified commercial laboratory. For PBMCs, blood was collected in EDTA vacutainer tubes. PBMCs were isolated over a Ficoll-Paque and washed three times with phosphate-buffered saline (PBS). An aliquot of cells was counted using trypan blue and a hemacytometer. Cells were then viably cryopreserved at a concentration of 10 million/1 ml freezing media [10% fresh dimethyl sulfoxide (DMSO)/90% heated fetal bovine serum (FBS)] in 0.5-ml aliquots (5 million cells each) in 1.5–2 ml O-RINGED screw-capped cryovials.
Subcutaneous adipose tissue was obtained by an open biopsy technique at the same visit as the blood draw. Following betadine prep and local 1% lidocaine injection, a 1.5-cm linear incision was made along the gluteal fold in the lateral buttock-thigh area. Approximately 500 mg of fat was then retrieved and the wound closed with two or three resorbable sutures. Twenty to forty milligrams of adipose tissue was used for these studies. This tissue was placed in cryovials and frozen in liquid nitrogen and then stored at −70°C.
OXPHOS CI and CIV protein quantitation dipstick immunoassays were performed as suggested by the manufacturer (MitoSciences, Inc., Eugene, OR). Each vial of viable PBMC was thawed and washed in 0.5 ml of PBS twice before the addition of 0.5 ml of ice-cold extraction buffer [1.5% lauryl maltoside (LM), 25 mM HEPES (pH 7.4), 100 mM NaCl, plus protease inhibitors (Sigma, P-8340)]. Fat samples were thawed on ice in extraction buffer (1:10 wet weight:volume) and homogenized briefly with an ultra-turrax homogenizer. Samples were then mixed gently and kept on ice for 20 min after which they were spun in a microcentrifuge at 16,400 rpm for 20 min at 4°C to remove insoluble cell debris. The supernatant, an extract of detergent-solubilized cellular proteins, was then assayed with the OXPHOS dipstick assays. Protein levels were determined by BCA using bovine serum albumin (BSA) as a reference protein (Pierce Biotechnology, Inc., Rockford, IL) and samples were then loaded on dipsticks at set amounts for each tissue and assay, using amounts previously determined to fall within the linear range of each assay. For CI levels, PBMC proteins were loaded at 6 μg/dipstick (linear range: 1–8 μg/test) and adipose tissue proteins were loaded at 20 μg/dipstick (linear range: 4–60 μg/test). For CIV levels, PBMC proteins were loaded at 1 μg/dipstick (linear range: 0.125–2.0 μg/test) and adipose tissue proteins were loaded at 5 μg/dipstick (linear range: 1.25–20 μg/test). All dipstick tests were run in duplicate.
The CI and CIV dipstick protein quantitation assays utilize a two-antibody sandwich methodology in which the target antigen is “sandwiched” between two capture monoclonal antibodies (mAbs) that bind different epitopes on the same target antigen. One mAb is immobilized in a zone drawn perpendicularly across the narrow width of a 0.5 × 4-cm rectangular nitrocellulose membrane affixed to a ridged plastic backing. When the free long end of the membrane is dipped in a liquid sample (0.025–0.1 ml), the sample is wicked up laterally along the membrane into an adsorbent pad and the entire sample must pass through the narrow zone of immobilized capture mAb. The target antigen, e.g., CI or CIV, if present in the sample, is immunocaptured and concentrated in this zone generating an intense colored line due to the simultaneous capture of the antigen and the labeled detector mAb. The intensity of the line is proportional to the amount of antigen in the sample and can be estimated visually or measured precisely with a densitometric reader. In the present study, dipstick signals were quantitated using an HP Scanjet 3970 flat bed scanner (Hewlett-Packard USA, Houston, TX) and analyses were conducted with NIH Image densitometry software (http://rsb.info.nih.gov/nih-image/). Intraassay CVs (n = 4) were 7.5% for CI assays and 3% for CIV assays. Interassay CVs (n = 3) were 9% for CI assays and 7% for CIV assays. The intraassay and interassay CVs were determined using whole cell protein extracts from cultured human skin fibroblasts extracted as described for PBMCs,
The CI-specific and CIV-specific mAbs that form the basis of the dipstick assays were generated by immunizing mice with biochemically purified bovine CI or CIV, hybridomas prepared, and the resulting mAbs screened for the ability to immunocapture fully assembled CI or CIV. The specificity of each mAb for its respective complex was confirmed by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) analysis of the resulting immunoprecipitate as described previously for CI capture mAbs23 and for CIV mAbs.24 CI (45 subunits) and IV (13 subunits) each has distinct, unique SDS–PAGE subunit protein banding patterns, and the identification of each band can be confirmed by mass spectrometry.23–25 The subunit specificity of the capture mAbs has not been established because they bind native and not denatured antigens, and by design they immunocapture intact, fully assembled enzymes.
Analysis of mtDNA copies/cell in our laboratory is now conducted by absolute quantitative real-time PCR. This technique provides rapid and quantitative analysis of mtDNA copies/cell in cells and tissue. DNA was extracted from frozen PBMCs or adipose tissue at −70°C using a Qiagen DNA kit (Qiagen, Inc). Standardization of real-time PCR was performed using LightCycler FastStart DNA Master SYBR Green I with the Roche LightCycler instrument (Roche, Indianapolis, IN). A dilution series of the control plasmid26 containing the 90 bp mtDNA NADH dehydrogenase, sub-unit 2 and the 98 bp Fas Ligand gene was prepared to set up the standard. Each sample and standard were run in duplicate (20 ml reaction volume) containing SYBR Green Master Mix, 10 pmol of each primer, and an approximately 10 ng sample of DNA. PCR cycling conditions were 95°C for 10 min followed by 40 cycles of 95°C for 3 s, 58°C for 5 s, and 72°C for 5 s. At the conclusion of the PCR, a melt curve analysis was immediately begun with the following conditions: beginning at 65°C, the temperature increased half a degree every 30 s for 60 cycles. The results were then analyzed with Version 4.0 LightCycler software.
Correlations between values were assessed utilizing Pearson correlations after log transformation of the data. Differences between the cohorts were analyzed utilizing the Mann–Whitney U nonparametric test for two independent samples (SPSS Version 15 for Windows).
Results
The analyses were conducted in matched sc fat and PBMC specimens from a total of 26 subjects consisting of 15 HIV-infected lipoatrophic subjects, 4 HIV-infected nonlipoatrophic subjects, and 7 HIV-seronegative controls. The characteristics of these subjects are shown in Table 1. No significant differences were found among the three groups in terms of age, gender, and body mass index. No difference was found between the lipoatrophic and nonlipoatrophic groups in CD4 count, HIV RNA levels, current ARV use, or years of exposure to NRTIs, NNRTs, or protease inhibitors (PIs). All subjects were on three drug combination regimens containing either an NNRTI or PI. One subject each in the nonlipoatrophic and lipoatrophic group was on ddI-containing HAART without the concomitant use of d4T. Evaluation of fasting metabolic parameters showed that fasting triglycerides were higher in the lipoatrophic group compared to the HIV-seronegative control group.
Table 1.
Demographic, HIV Treatment, and Metabolic Characteristics of Subjects by Cohorta
| HIV seronegative | HIV-infected nonlipoatrophic | HIV-infected lipoatrophic | |
|---|---|---|---|
| Number of participants | 7 | 4 | 15 |
| Age (years) | 43 (36–56) | 50 (38–62) | 51 (34–61) |
| Male/female | 7/0 | 4/0 | 14/1 |
| Body mass index (kg/m2) | 26.7 (23.6–29.6) | 22.8 (20.1–32.8) | 24.9 (20.1–34.3) |
| NRTI in use at time of study (on d4T/on ZDV/on ddI) | — | 0/3/1 | 6/8/1 |
| Median exposure time to NRTI (years) | — | 4.5 (1.0–7.0) | 5.0 (1.0–7.0) |
| Patients (number) with Hx NNRTI exposure/median exposure time to NNRTI (years) | — | 4/4.5 (1.0–7.0) | 11/5.0 (1.0–7.0) |
| Patients (number) with Hx PI exposure/median exposure time to PI (years) | — | 1/7 | 5/5 (2.0–7.0) |
| CD4 (cells/mm3) | — | 334 (282–389) | 488 (122–771) |
| HIV RNA (number of patients <50 copies/ml) | — | 4 | 13 |
| ALT (IU/liter) | 26 (24–49) | 25 (10–50) | 42 (12–68) |
| Fasting total cholesterol (mg/dl) | 208 (155–264) | 194 (143–215) | 167 (109–310) |
| Fasting triglycerides (mg/dl) | 72b (51–244) | 146 (65–565) | 176b (53–502) |
| Fasting lactate (mg/dl) | 1.1 (0.6–1.8) | 1.6 (0.7–2.4) | 1.5 (0.6–2.6) |
| Fasting glucose (mg/dl) | 94 (80–100) | 96 (83–111) | 86 (60–107) |
| HOMA IR | 1.53 (0.59–1.90) | 0.91 (0.68–5.95) | 1.69 (0.27–7.43) |
Data are median values unless otherwise specified. The range in values is presented in parentheses.
Values are significantly different by Mann–Whitney test at p < 0.05.
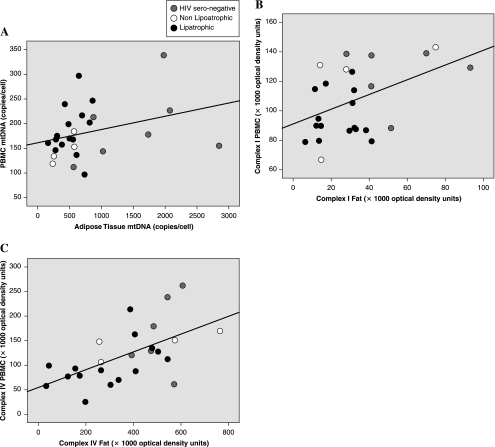
As shown in Fig. 1, statistically significant correlations were demonstrated between fat and PBMCs in CIV levels (r = 0.507, p = 0.008) and in CI levels (r = 0.442, p = 0.024). The correlation between fat and PBMC mtDNA levels failed to reach statistical significance. No significant correlations were found between mtDNA levels in either adipose tissue or PBMCs and the corresponding compartment's CI or CIV values. However, good concordance was found between CI and CIV values in each respective compartment (fat r = 0.404, p = 0.041; PBMC r = 0.723, p < 0.001).
FIG. 1.
Pearson correlations between respective log-transformed mitochondrial DNA, Complex I and Complex IV values in PBMC and sc fat. (A) mtDNA: r = 0.360, p = 0.071; (B) CI: r = 0.442, p = 0.024; (C) CIV: r = 0.507, p = 0.008.
Assessment for correlations between mtDNA and OXPHOS protein levels and various fasting metabolic blood parameters found no significant correlations with serum lactate or total cholesterol. A trend toward significance was seen between HOMA IR and PBMC CI (r = −0.365, p = 0.067). Significant correlations were found between triglyceride and fat CI (r = −0.401, p = 0.038), fat CIV (r = −0.379, p = 0.051), and fat mtDNA (r = −0.466, p = 0.014).
Median values for mtDNA, CI, and CIV levels by HIV and lipoatrophy status are shown in Table 2. The adipose tissue mtDNA levels in HIV-seronegative subjects were higher than in either lipoatrophic or nonlipoatrophic HIV-infected subjects, but no difference among the groups was seen in mtDNA levels performed in PBMCs. In contrast, statistically significant depletions in CI and in CIV levels were noted in both fat and in PBMCs of lipoatrophic subjects compared to seronegative controls. The CI median levels in lipoatrophic subjects were 72% and 70% of respective fat and PBMC levels in HIV-seronegative subjects. The CIV median levels were 56% and 70% of respective fat and PBMC levels in HIV-seronegative subjects. Values in the nonlipoatrophic group showed a significant difference only for CIV when compared to the lipoatrophic group. The results of these analyses were not altered when the two subjects on ddI-containing therapy were excluded.
Table 2.
Mitochondrial DNA, Complex I and Complex IV Values in Subcutaneous Fat and in PBMCs by Cohorta
| |
Subcutaneous adipose tissue |
PBMCs |
||||
|---|---|---|---|---|---|---|
| HIV− | Nonlipoatrophic | Lipoatrophic | HIV− | Nonlipoatrophic | Lipoatrophic | |
| N | 7 | 4 | 15 | 7 | 4 | 15 |
| mtDNA copies/cell (% of value in HIV−) | 1739*,† | 412 | 507* | 178 | 144 | 170 |
| Median | (572–2848) | (236–577) | 165–867) | (112–338) | (119–184) | (97–297) |
| Range | (100%) | (24%) | (29%) | (100%) | (81%) | (96%) |
| Complex I optical density units | ||||||
| Median | 41,300† | 21,400 | 29,600† | 129,200† | 129,400 | 89,800† |
| Range | (28,000–93,000) | (14,300–74,900) | (6,500–41,100) | (88,000–140,000) | (67,000–143,000) | (79,000–126,300) |
| (% of value in HIV−) | (100%) | (52%) | (72%) | (100%) | (100%) | (70%) |
| Complex IV optical density units | ||||||
| Median | 545,000† | 421,600 | 305,800† | 129,200† | 148,900† | 90,100†,‡ |
| Range | (394,000–606,600) | (257,200–765,000) | (34,400–544,500) | (62,000–261,000) | (104,200–169,300) | (25,500–212,400) |
| (% of value in HIV−) | (100%) | (77%) | (56%) | (100%) | (115%) | (70%) |
The range in values is presented in parentheses. Values with the same superscript are significantly different by Mann–Whitney test: *p ≤ 0.001 and †,‡p ≤ 0.05.
Discussion
In this pilot study utilizing convenience samples of banked PBMC and sc fat specimens, good correlations were observed between PBMC and fat values for CI and CIV enzyme levels, with CIV showing the strongest correlation. Lipoatrophic subjects showed significant depletion of both CI and CIV enzyme levels in PBMCs as well as in fat when compared to HIV-seronegative controls. In contrast, the correlation between PBMC and fat mtDNA values was poor and no differences in mtDNA levels could be demonstrated in PBMCs of subjects with HIV lipoatrophy compared to HIV-seronegative control despite clear differences in fat.
Lipoatrophy is a common syndrome in HIV-infected subjects and is estimated to develop in approximately 40–50% of HIV-infected adults treated with ARV therapy.27 The syndrome is strongly associated with mitochondrial toxicity related to the use of d4T and ZDV.9,17 While less clear, the use of ddI has also been reported to be associated with lipoatrophy and with depletion of mtDNA in fat.28,29 In our study, the exclusion of the two patients on ddI (one nonlipoatrophic and one lipoatrophic) did not change the results of any analyses.
Considerable evidence already exists at the fat tissue level to suggest a mitochondrial basis for lipoatrophy. The mitochondrial architecture is disrupted within adipose tissue.12 Consistent with the known ability of NRTIs to inhibit mitochondrial DNA polymerase γ, a key regulatory enzyme of mitochondrial DNA replication, depletion in adipose tissue mtDNA content has been noted in subjects with HIV lipoatrophy.7,17 In addition, depletion in mitochondrial RNA (mtRNA)30 as well as in mtDNA-encoded respiratory chain proteins has been reported.31–33 Interestingly, decreased transcription of mitochondrial RNA without significant depletion of mtDNA has been found in HIV seronegative subjects 2 weeks after initiating dual-NRTI therapy.21
Other factors besides NRTIs may be responsible for alternations in adipose tissue mitochondrial function in the HIV-infected population. A possible role for both protease inhibitors34 and nonnucleoside reverse transcriptase inhibitors (NNRTIs)35 has been suggested. Protease inhibitors may alter expression of mitochondrial genes through nuclear respiratory factor (NRF) 1 and 2, which regulate mitochondrial transcription factor A (mtTFA).21 Substantial alterations of mitochondrial gene expression have been found in adipose tissue of ARV-naive patients suggesting that HIV infection per se may also affect the mitochondria.36
Mitochondrial toxicity is tissue specific, however, and the study of mitochondrial abnormalities and its association with lipoatrophy have been hindered by the need to assay for such abnormalities in fat tissue. Even relatively simple biopsy techniques, such as the retrieval of fat by use of skin punch biopsies, are invasive, carry a small risk of infection, leave residual scars, and are not conducive to large-scale studies of lipoatrophy or for use in clinical care. Thus, a reliable surrogate marker of lipoatrophic tendencies that can be assayed in blood is attractive if it can be validated to closely correspond to mitochondrial assays in fat and to the clinical development of sc fat loss.
Surrogate markers assessed to date include serum lactate measurements, which have proven to have limited utility in guiding HIV / AIDS therapy.37,38 Alternative attempts to utilize surrogate markers in blood have predominantly examined mtDNA levels in PBMCs. However, most published studies, as well as the results in this current study, indicate that mtDNA levels in PBMCs perform poorly in identifying mitochondrial toxicity in fat.13,15–18,39–41 This may be due in part to the difficulty in purifying PBMCs consistently without platelet contamination. Platelets have mitochondria (and mtDNA) but lack nuclei and variable platelet contamination may result in erratic mtDNA:nDNA ratio measurements.13,42
There are few data to date on respiratory chain enzyme or activity levels in PBMCs from subjects with lipoatrophy. Such studies have been hampered by the complexity and labor-intensive nature of conducting traditional OXPHOS enzyme assays and by the need to purify mitochondria. One study reported no difference in the COXII/COXIV ratio in PBMCs between HIV-infected lipoatrophic subjects and HIV-seronegative controls, although differences were noted in fat.32 In another study, activities of OXPHOS complex III and CIV were decreased in mitochondria purified from PBMCs of HIV lipoatrophic subjects.43
In our current study, CI and CIV levels were measured with novel OXPHOS dipstick assays that are simple, rapid, and quantitative and can be done using whole cell and tissue extracts without the need to purify mitochondria. These protein quantitation assays use a two-antibody sandwich methodology and are reproducible with low intraassay and interassay CVs (<10%). Platelet contamination is not an issue for these protein-based assays as both nucleated cells and platelets contain mitochondria and the assay is normalized to total cell protein, not nuclear DNA. Despite the limited sample size of this preliminary study, good correlation was observed between PBMC and fat values for both CI and CIV values suggesting that these assays may be useful as surrogate markers of mitochondrial function in fat. A correlation trend with insulin resistance and some significant correlations with serum triglycerides, both well described in association with lipoatrophy, add strength to the hypothesis that CI and CIV are markers integrally related to this process. Furthermore, analyses by HIV and lipoatrophy status demonstrated that CI and CIV levels were statistically lower in the lipoatrophic cohort compared to the HIV-seronegative control group as can be expected from our understanding of the role of mitochondrial dysfunction in the development of lipoatrophy. The small sample size in our nonlipoatrophic group likely limited our ability to demonstrate differences in enzyme levels between the nonlipoatrophic and lipoatrophic groups. However, while not significant, some depletion of adipose OXPHOS levels was noted in nonlipoatrophic subjects suggesting that this decrease is more likely related to the adverse effects of older NRTIs than to fat loss per se.
The results of this pilot study are limited by its cross-sectional nature, the lack of assessment in HIV+ ARV-naive subjects, and in the overall small sample size, especially in the number of HIV+ nonlipoatrophic subjects. Nevertheless, the significant correlations between CI and CIV enzyme levels in PBMCs and fat and the ability to demonstrate depletions of CI and CIV in PBMCs and in fat of lipoatrophic subjects are encouraging. The study suggests that assessments of CI and CIV in PBMCs may be useful as surrogate markers for mitochondrial toxicity leading to lipoatrophy in HIV-infected subjects. We plan to prospectively study the utility of these dipstick immunoassays in a 150 patient ARV therapy trial of treatment-naive HIV-infected subjects (SEARCH 003) which opened in Thailand in early 2008.
In summary, we report our preliminary observations that CI and CIV enzyme levels in PBMCs correlate to their respective levels in sc fat, and that significant depletion of these mitochondrial-specific enzymes can be demonstrated in both PBMCs and fat from subjects with HIV lipoatrophy compared to HIV-seronegative controls. Further evaluation is warranted to assess the utility of the OXPHOS protein quantitation dipstick immunoassays to monitor lipoatrophic tendencies or other dysfunctions associated with mitochondrial function in HIV-infected subjects.
Acknowledgments
Research was supported by National Institutes of Health Grants AI060409, MD000173, AI068525, AI074554, RR011091, and AI062407.
Disclosure Statement
No competing financial interests exist.
References
- 1.Duran S. Saves M. Spire B, et al. Failure to maintain longterm adherence to highly active antiretroviral therapy: The role of lipodystrophy. AIDS. 2001;15:2441–2244. doi: 10.1097/00002030-200112070-00012. [DOI] [PubMed] [Google Scholar]
- 2.Ammassari A. Antinori A. Cozzi-Lepri A, et al. Relationship between HAART adherence and adipose tissue alterations. J Acquir Immune Defic Syndr. 2002;31(Suppl. 3):S140–144. doi: 10.1097/00126334-200212153-00011. [DOI] [PubMed] [Google Scholar]
- 3.Duran S. Spire B. Raffi F, et al. Self-reported symptoms after initiation of a protease inhibitor in HIV-infected patients and their impact on adherence to HAART. HIV Clin Trials. 2001;2:38–45. doi: 10.1310/R8M7-EQ0M-CNPW-39FC. [DOI] [PubMed] [Google Scholar]
- 4.Saint-Marc T. Partisani M. Poizot-Martin I, et al. Fat distribution evaluated by computed tomography and metabolic abnormalities in patients undergoing antiretroviral therapy: Preliminary results of the LIPOCO study. AIDS. 2000;14:37–49. doi: 10.1097/00002030-200001070-00005. [DOI] [PubMed] [Google Scholar]
- 5.Mynarcik DC. McNurlan MA. Steigbigel RT. Fuhrer J. Gelato MC. Association of severe insulin resistance with both loss of limb fat and elevated serum tumor necrosis factor receptor levels in HIV lipodystrophy. J Acquir Immune Defic Syndr. 2000;25:312–321. doi: 10.1097/00042560-200012010-00004. [DOI] [PubMed] [Google Scholar]
- 6.Vigouroux C. Maachi M. Nguyen TH, et al. Serum adipocytokines are related to lipodystrophy and metabolic disorders in HIV-infected men under antiretroviral therapy. AIDS. 2003;17:1503–1511. doi: 10.1097/00002030-200307040-00011. [DOI] [PubMed] [Google Scholar]
- 7.Shikuma CM. Hu N. Milne C, et al. Mitochondrial DNA decrease in subcutaneous adipose tissue of HIV-infected individuals with peripheral lipoatrophy. AIDS. 2001;15:1801–1809. doi: 10.1097/00002030-200109280-00009. [DOI] [PubMed] [Google Scholar]
- 8.Mallon PW. Miller J. Cooper DA. Carr A. Prospective evaluation of the effects of antiretroviral therapy on body composition in HIV-1-infected men starting therapy. AIDS. 2003;17:971–979. doi: 10.1097/00002030-200305020-00005. [DOI] [PubMed] [Google Scholar]
- 9.Mallal SA. John M. Moore CB. James IR. McKinnon EJ. Contribution of nucleoside analogue reverse transcriptase inhibitors to subcutaneous fat wasting in patients with HIV infection. AIDS. 2000;14:1309–1316. doi: 10.1097/00002030-200007070-00002. [DOI] [PubMed] [Google Scholar]
- 10.Moyle G. Mechanisms of HIV and nucleoside reverse transcriptase inhibitor injury to mitochondria. Antiviral Ther. 2005;10(Suppl. 2):M47–52. [PubMed] [Google Scholar]
- 11.Brinkman K. ter Hofstede HJ. Burger DM. Smeitink JA. Koopmans PP. Adverse effects of reverse transcriptase inhibitors: Mitochondrial toxicity as common pathway. AIDS. 1998;12:1735–1744. doi: 10.1097/00002030-199814000-00004. [DOI] [PubMed] [Google Scholar]
- 12.Nolan D. Hammond E. Martin A, et al. Mitochondrial DNA depletion and morphologic changes in adipocytes associated with nucleoside reverse transcriptase inhibitor therapy. AIDS. 2003;17:1329–1338. doi: 10.1097/00002030-200306130-00007. [DOI] [PubMed] [Google Scholar]
- 13.Gerschenson M. Shiramizu B. LiButti DE. Shikuma CM. Mitochondrial DNA levels of peripheral blood mononuclear cells and subcutaneous adipose tissue from thigh, fat and abdomen of HIV-1 seropositive and negative individuals. Antiviral Ther. 2005;10(Suppl. 2):M83–89. [PubMed] [Google Scholar]
- 14.Walker UA. Bickel M. Lutke Volksbeck SI, et al. Evidence of nucleoside analogue reverse transcriptase inhibitor-associated genetic and structural defects of mitochondria in adipose tissue of HIV-infected patients. J Acquir Immune Defic Syndr. 2002;29:117–121. doi: 10.1097/00042560-200202010-00002. [DOI] [PubMed] [Google Scholar]
- 15.Maagaard A. Holberg-Petersen M. Kvittingen EA. Sandvik L. Bruun JN. Depletion of mitochondrial DNA copies/cell in peripheral blood mononuclear cells in HIV-1-infected treatment-naive patients. HIV Med. 2006;7:53–58. doi: 10.1111/j.1468-1293.2005.00336.x. [DOI] [PubMed] [Google Scholar]
- 16.Cherry CL. Gahan ME. McArthur JC. Lewin SR. Hoy JF. Wesselingh SL. Exposure to dideoxynucleosides is reflected in lowered mitochondrial DNA in subcutaneous fat. J Acquir Immune Defic Syndr. 2002;30:271–277. doi: 10.1097/00126334-200207010-00002. [DOI] [PubMed] [Google Scholar]
- 17.van der Valk M. Casula M. Weverlingz GJ, et al. Prevalence of lipoatrophy and mitochondrial DNA content of blood and subcutaneous fat in HIV-1-infected patients randomly allocated to zidovudine- or stavudine-based therapy. Antiviral Ther. 2004;9:385–393. [PubMed] [Google Scholar]
- 18.Cherry CL. Nolan D. James IR, et al. Tissue-specific associations between mitochondrial DNA levels and current treatment status in HIV-infected individuals. J Acquir Immune Defic Syndr. 2006;42:435–440. doi: 10.1097/01.qai.0000224974.67962.ce. [DOI] [PubMed] [Google Scholar]
- 19.Marusich MF. Robinson BH. Taanman JW, et al. Expression of mtDNA and nDNA encoded respiratory chain proteins in chemically and genetically-derived Rho0 human fibroblasts: A comparison of subunit proteins in normal fibroblasts treated with ethidium bromide and fibroblasts from a patient with mtDNA depletion syndrome. Biochim Biophys Acta. 1997;1362:145–159. doi: 10.1016/s0925-4439(97)00061-6. [DOI] [PubMed] [Google Scholar]
- 20.Janes MS. Hanson BJ. Hill DM, et al. Rapid analysis of mitochondrial DNA depletion by fluorescence in situ hybridization and immunocytochemistry: Potential strategies for HIV therapeutic monitoring. J Histochem Cytochem. 2004;52:1011–1018. doi: 10.1369/jhc.3A6209.2004. [DOI] [PubMed] [Google Scholar]
- 21.Mallon PW. Unemori P. Sedwell R, et al. In vivo, nucleoside reverse-transcriptase inhibitors alter expression of both mitochondrial and lipid metabolism genes in the absence of depletion of mitochondrial DNA. J Infect Dis. 2005;191:1686–1696. doi: 10.1086/429697. [DOI] [PubMed] [Google Scholar]
- 22.Nadanaciva S. Bernal A. Aggeler R. Capaldi R. Will Y. Target identification of drug induced mitochondrial toxicity using immunocapture based OXPHOS activity assays. Toxicol In Vitro. 2007;21:902–911. doi: 10.1016/j.tiv.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 23.Murray J. Zhang B. Taylor SW, et al. The subunit composition of the human NADH dehydrogenase obtained by rapid one-step immunopurification. J Biol Chem. 2003;278:13619–13622. doi: 10.1074/jbc.C300064200. [DOI] [PubMed] [Google Scholar]
- 24.Murray J. Schilling B. Row RH, et al. Small scale immunopurification of cytochrome c oxidase for a high throughput, multiplexing analysis of enzyme activity, and amount. Biotechnol Appl Biochem. 2007;48(Pt. 4):167–178. doi: 10.1042/BA20060223. [DOI] [PubMed] [Google Scholar]
- 25.Murray J. Yonally S. Aggeler R. Marusich MF. Capaldi RA. Focused proteomics: Towards a high throughput monoclonal antibody-based resolution of proteins for diagnosis of mitochondrial diseases. Biochim Biophys Acta. 2004;1659:206–211. doi: 10.1016/j.bbabio.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Shiramizu B. Shikuma KM. Kamemoto L, et al. Placenta and cord blood mitochondrial DNA toxicity in HIV-infected women receiving nucleoside reverse transcriptase inhibitors during pregnancy. J Acquir Immune Defic Syndr. 2003;32:370–374. doi: 10.1097/00126334-200304010-00004. [DOI] [PubMed] [Google Scholar]
- 27.Schambelan M. Benson CA. Carr A, et al. Management of metabolic complications associated with antiretroviral therapy for HIV-1 infection: Recommendations of an International AIDS Society-USA panel. J Acquir Immune Defic Syndr. 2002;31:257–275. doi: 10.1097/00126334-200211010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Tien PC. Barron Y. Justman JE, et al. Antiretroviral therapies associated with lipoatrophy in HIV-infected women. AIDS Patient Care STDS. 2007;21:297–305. doi: 10.1089/apc.2006.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Buffet M. Schwarzinger M. Amellal B, et al. Mitochondrial DNA depletion in adipose tissue of HIV-infected patients with peripheral lipoatrophy. J Clin Virol. 2005;33:60–64. doi: 10.1016/j.jcv.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 30.Galluzzi L. Pinti M. Guaraldi G, et al. Altered mitochondrial RNA production in adipocytes from HIV-infected individuals with lipodystrophy. Antiviral Ther. 2005;10(Suppl. 2):M91–99. [PubMed] [Google Scholar]
- 31.McComsey GA. Paulsen DM. Lonergan JT, et al. Improvements in lipoatrophy, mitochondrial DNA levels and fat apoptosis after replacing stavudine with abacavir or zidovudine. AIDS. 2005;19:15–23. doi: 10.1097/00002030-200501030-00002. [DOI] [PubMed] [Google Scholar]
- 32.Jones SP. Qazi N. Morelese J, et al. Assessment of adipokine expression and mitochondrial toxicity in HIV patients with lipoatrophy on stavudine- and zidovudine-containing regimens. J Acquir Immune Defic Syndr. 2005;40:565–572. doi: 10.1097/01.qai.0000187443.30838.3e. [DOI] [PubMed] [Google Scholar]
- 33.Hammond E. Nolan D. James I. Metcalf C. Mallal S. Reduction of mitochondrial DNA content and respiratory chain activity occurs in adipocytes within 6–12 months of commencing nucleoside reverse transcriptase inhibitor therapy. AIDS. 2004;18:815–817. doi: 10.1097/00002030-200403260-00015. [DOI] [PubMed] [Google Scholar]
- 34.van der Valk M. Gisolf EH. Reiss P, et al. Increased risk of lipodystrophy when nucleoside analogue reverse transcriptase inhibitors are included with protease inhibitors in the treatment of HIV-1 infection. AIDS. 2001;15:847–855. doi: 10.1097/00002030-200105040-00005. [DOI] [PubMed] [Google Scholar]
- 35.Haubrich R. Riddler S. DiRienzo G. Komarow L. Powderly W. Garren K. George T. Rooney J. Mellors J. Havlir D the ACTG 5142 Study Team. Metabolic outcomes of ACTG 5142: A prospective, randomized, phase III trial of NRTI-, PI-, and NNRTI-sparing regimens for initial treatment of HIV-1 infection. Presented at the 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA. Feb 25–28;2007 . [Google Scholar]
- 36.Giralt M. Domingo P. Guallar JP, et al. HIV-1 infection alters gene expression in adipose tissue, which contributes to HIV- 1/HAART-associated lipodystrophy. Antiviral Ther. 2006;11:729–740. [PubMed] [Google Scholar]
- 37.John M. Moore CB. James IR, et al. Chronic hyperlactatemia in HIV-infected patients taking antiretroviral therapy. AIDS. 2001;15:717–723. doi: 10.1097/00002030-200104130-00007. [DOI] [PubMed] [Google Scholar]
- 38.Brinkman K. Editorial response: Hyperlactatemia and hepatic steatosis as features of mitochondrial toxicity of nucleoside analogue reverse transcriptase inhibitors. Clin Infect Dis. 2000;31:167–169. doi: 10.1086/313921. [DOI] [PubMed] [Google Scholar]
- 39.Cossarizza A. Riva A. Pinti M, et al. Increased mitochondrial DNA content in peripheral blood lymphocytes from HIV-infected patients with lipodystrophy. Antiviral Ther. 2003;8:315–321. [PubMed] [Google Scholar]
- 40.McComsey G. Tan DJ. Lederman M. Wilson E. Wong LJ. Analysis of the mitochondrial DNA genome in the peripheral blood leukocytes of HIV-infected patients with or without lipoatrophy. AIDS. 2002;16:513–518. doi: 10.1097/00002030-200203080-00001. [DOI] [PubMed] [Google Scholar]
- 41.Chiappini F. Teicher E. Saffroy R, et al. Prospective evaluation of blood concentration of mitochondrial DNA as a marker of toxicity in 157 consecutively recruited untreated or HAART-treated HIV-positive patients. Lab Invest. 2004;84:908–914. doi: 10.1038/labinvest.3700113. [DOI] [PubMed] [Google Scholar]
- 42.Simpson DM. Kitch D. Evans SR, et al. HIV neuropathy natural history cohort study: Assessment measures and risk factors. Neurology. 2006;66:1679–1687. doi: 10.1212/01.wnl.0000218303.48113.5d. [DOI] [PubMed] [Google Scholar]
- 43.Miro O. Lopez S. Pedrol E, et al. Mitochondrial DNA depletion and respiratory chain enzyme deficiencies are present in peripheral blood mononuclear cells of HIV-infected patients with HAART-related lipodystrophy. Antiviral Ther. 2003;8:333–338. [PubMed] [Google Scholar]