Abstract
Mechanisms of action as well as cellular targets of glatiramer acetate (GA) in multiple sclerosis (MS) are still not entirely understood. IL-1β is present in CNS-infiltrating macrophages and microglial cells and is an important mediator of inflammation in experimental autoimmune encephalitis (EAE), the MS animal model. A natural inhibitor of IL-1β, the secreted form of IL-1 receptor antagonist (sIL-1Ra) improves EAE disease course. In this study we examined the effects of GA on the IL-1 system. In vivo, GA treatment enhanced sIL-1Ra blood levels in both EAE mice and patients with MS, whereas IL-1β levels remained undetectable. In vitro, GA per se induced the transcription and production of sIL-1Ra in isolated human monocytes. Furthermore, in T cell contact-activated monocytes, a mechanism relevant to chronic inflammation, GA strongly diminished the expression of IL-1β and enhanced that of sIL-1Ra. This contrasts with the effect of GA in monocytes activated upon acute inflammatory conditions. Indeed, in LPS-activated monocytes, IL-1β and sIL-1Ra production were increased in the presence of GA. These results demonstrate that, in chronic inflammatory conditions, GA enhances circulating sIL-1Ra levels and directly affects monocytes by triggering a bias toward a less inflammatory profile, increasing sIL-1Ra while diminishing IL-1β production. This study sheds light on a mechanism that is likely to participate in the therapeutic effects of GA in MS.
Keywords: experimental autoimmune encephalitis, cellular contact, inflammation, autoimmune disease
Glatiramer acetate (GA; copolymer-1; Copaxone) is composed of a mixture of synthetic peptides of 50 to 90 aa randomly composed of L-glutamic acid (E), L-lysine (K), L-alanine (A), and L-tyrosine (Y). Initially developed to mimic the myelin basic protein, a major component of the myelin sheath, and to induce experimental autoimmune encephalitis (EAE), GA unexpectedly inhibited EAE in both rodents and monkeys (1). In subsequent clinical trials, GA reduced relapse rate and progression of disability in patients with relapsing–remitting multiple sclerosis (MS; RRMS) leading to its approval in 1995 (2).
A number of investigations in MS and EAE addressed the immunological basis of GA clinical effects. However, the mechanisms of GA action are still elusive. Initial investigations attributed most GA activity to a preferential Th2-polarization of myelin-specific T cells, thus focusing on its effects on the adaptive immune response (3). However, recent reports indicated that GA treatment also exerts immunomodulatory activity on cells of the monocytic lineage, i.e., monocytes/macrophages and dendritic cells (4–9). For instance, monocytes from GA-treated patients with MS secrete less IL-12 and TNF in response to LPS stimulation compared with monocytes from healthy controls and untreated patients with MS (4). Accordingly, dendritic cells and monocytes isolated from GA-treated patients produce more anti-inflammatory IL-10 and less pro-inflammatory IL-12 (5, 9). Furthermore, GA promotes the development of anti-inflammatory type II monocytes in EAE, accompanied by induction of regulatory T cells and increased secretion of both IL-10 and TGF-β (10).
IL-1β is a pleiotropic pro-inflammatory cytokine whose production is tightly controlled at several levels (11). Indeed, as recently reviewed, there are several roadblocks to the release of IL-1β beginning with the transcription of the IL1B gene and ending with the exit of the active cytokine from the cell. In the extracellular space, IL-1β activity is mainly ruled by the secreted IL-1 receptor antagonist (sIL-1Ra), which binds type I IL-1 receptor without triggering signals (12). As it potently inhibits the various effects of IL-1, sIL-1Ra is considered an important regulator of the inflammatory and overall immune response mediated by IL-1 (13). Because of its extreme efficacy as a pro-inflammatory mediator, if these intracellular and extracellular roadblocks are not enough to limit IL-1β activity, it may also be reduced by the preferential binding to the cell surface or soluble form of type II IL-1 receptor, preventing it from triggering the signal-transducing type I receptor (11). Finally, the facilitation of IL-1β processing by the caspase 1 inflammasome through ATP activation of the P2X7 receptor can also be viewed as a potential roadblock to the activity of IL-1β (11).
IL-1β is mainly produced upon activation of cells of the monocytic lineage. In chronic/sterile immuno-inflammatory diseases, the factors triggering pro-inflammatory cytokine production are still elusive. T cells may exert a pathological effect through direct cellular contact with monocytes/macrophages, inducing massive up-regulation of IL-1β and TNF (14). Besides triggering pro-inflammatory cytokine production, contact-mediated activation of monocytes induces the production and/or shedding of cytokine inhibitors such as sIL-1Ra and soluble receptors of IL-1 and TNF (15). The importance of T cell contact-mediated activation of monocytic cells in MS was further demonstrated in vitro in co-cultures of T cells and microglial cells (16, 17).
In MS, IL-1β is mainly expressed by microglial cells and infiltrating monocyte/macrophages throughout the white matter and in acute lesions (18). This assertion was further confirmed in EAE studies. Indeed, dark agouti rats treated with sIL-1Ra during the induction of EAE, or after adoptive transfer with myelin antigen-primed lymph node cells, develop milder signs of the disease (19). sIL-1Ra delivered by non-replicative HSV-1 vectors in EAE C57BL/6 mice delays disease onset and decreases disease severity (20). In addition, IL-1α/β double deficient (IL-1−/−) mice exhibit significant resistance to EAE induction with reduction in disease severity, whereas IL-1Ra−/− mice are highly susceptible to EAE induction in the absence of pertussis toxin administration (21). These observations demonstrate that the IL-1/IL-1Ra system is crucial for autoantigen-specific T cell induction in mice and that sIL-1Ra efficiently blocks IL-1β effects and ameliorates EAE disease course (19–22).
In this study we addressed the question of the effects of GA on IL-1 system in vivo and in vitro. The results show that GA-treatment increases the circulating levels of sIL-1Ra in both EAE mice and patients with MS. This is reflected in vitro by the direct effect of GA on human blood monocytes. Indeed, GA induces the production of the cytokine inhibitor sIL-1Ra and diminishes the production of IL-1β in conditions related to chronic inflammation, i.e., in monocytes activated by direct contact with stimulated T cells.
Results
sIL-1Ra Serum Levels Are Elevated in GA-Treated EAE Mice.
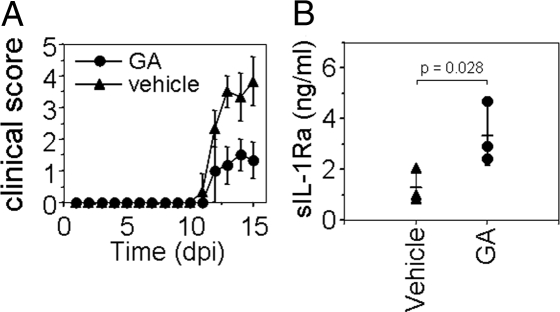
To assess whether GA-treatment affected sIL-1Ra levels in the MS animal model, EAE was induced in mice treated either with GA or PBS solution (i.e., vehicle). As shown in Fig. 1A, EAE severity was reduced in GA-treated mice, as previously demonstrated (10). At peak disease, mouse sera were analyzed for levels of sIL-1Ra and IL-1β. IL-1β was not detectable in any of the sera (not shown). However, sIL-1Ra was significantly elevated in mice treated with GA (3,336 ± 1,190 pg/mL sIL-1Ra, mean ± SD) compared with animals that received vehicle as a control (1,296 ± 657 pg/mL sIL-1Ra; Fig. 1B). This demonstrates that GA-treatment enhanced sIL-1Ra concentration in EAE mouse serum.
Fig. 1.
sIL-1Ra levels are elevated in sera of EAE mice treated with GA. (A) GA ameliorates EAE. C57BL/6 mice were injected s.c. daily with GA (150 μg) or vehicle (PBS solution) 7 d before immunization with 10 μg myelin oligodendrocyte glycoprotein 35–55 peptide (dpi, day post-immunization). (B) EAE mice treated (GA) or not (vehicle) were killed at disease peak and their serum analyzed for IL-1β and sIL-1Ra content. IL-1β was not detected (not shown).
sIL-1Ra Levels Are Elevated in Sera of Patients with MS Treated with GA.
sIL-1Ra circulating levels in MS have been shown to vary as a function of clinical status and treatment, so we examined whether GA-treatment would affect sIL-1Ra levels in patients with MS. IL-1β and sIL-1Ra levels were measured in sera of patients with RRMS treated with GA or untreated, and in healthy controls (Table 1). IL-1β was not detectable in any of the sera. As shown in Fig. 2, sIL-1Ra was significantly increased in serum of patients treated with GA (434 ± 265 pg/mL sIL-1Ra) whereas there was no significant difference between untreated patients (218 ± 60 pg/mL sIL-1Ra) and healthy controls (188 ± 65 pg/ml sIL-1Ra). This demonstrates that GA treatment enhances sIL-1Ra in the serum of patients with MS.
Table 1.
Clinical characteristics of patients with MS and healthy controls
| Clinical category | Sex | Age (y) | Disease duration (y) | EDSS | GA treatment duration (mo) |
|---|---|---|---|---|---|
| Healthy controls | |||||
| 1 | F | 31 | — | — | — |
| 2 | F | 34 | — | — | — |
| 3 | F | 36 | — | — | — |
| 4 | M | 29 | — | — | — |
| 5 | M | 24 | — | — | — |
| 6 | F | 24 | — | — | — |
| 7 | F | 30 | — | — | — |
| 8 | F | 35 | — | — | — |
| 9 | F | 44 | — | — | — |
| 10 | M | 40 | — | — | — |
| Mean ± SD | — | 32.7 ± 6.4 | — | — | — |
| Untreated RRMS | |||||
| 1 | F | 45 | 17 | 3.0 | — |
| 2 | F | 31 | 10 | 1.5 | — |
| 3 | M | 15 | 1 | 1.5 | — |
| 4 | F | 23 | 1 | 2.0 | — |
| 5 | F | 41 | 6 | 7.0 | — |
| 6 | F | 36 | 3 | 2.0 | — |
| 7 | F | 34 | 5 | 2.0 | — |
| 8 | M | 27 | 3 | 1.5 | — |
| 9 | F | 31 | 10 | 1.0 | — |
| 10 | F | 46 | 6 | 2.5 | — |
| 11 | M | 41 | 6 | 4.0 | — |
| Mean ± SD | — | 33.6 ± 9.6 | 6.2 ± 4.7 | 2.5 ± 1.7 | — |
| GA-treated RRMS | |||||
| 1 | M | 39 | 6 | 2.5 | 56 |
| 2 | F | 33 | 7 | 2.0 | 18 |
| 3 | M | 26 | 8 | 1.5 | 60 |
| 4 | F | 45 | 15 | 1.5 | 27 |
| 5 | F | 32 | 11 | 2.0 | 13 |
| 6 | F | 27 | 4 | 0 | 44 |
| 7 | M | 23 | 2 | 1.0 | 29 |
| 8 | M | 39 | 9 | 2.0 | 36 |
| 9 | F | 25 | 5 | 1.0 | 19 |
| Mean ± SD | — | 32.1 ± 7.6 | 7.4 ± 3.9 | 1.5 ± 0.8 | 33.6 ± 16.8 |
EDSS: Expanded Disability Status Score; RRMS: relapsing-remitting multiple sclerosis; GA: glatiramer acetate. Data expressed at time of sampling.
Fig. 2.
sIL-1Ra levels are elevated in sera of patients with MS treated with GA. The levels of sIL-1Ra and IL-1β were measured in sera of patients with RRMS treated with GA or not treated, and age-matched healthy controls, as described in Table 1. IL-1β was under the detection limit (15 pg/mL) in all individuals. There was no significant difference between healthy controls and untreated patients with RRMS. Results are presented as a box plot (GraphPad Prism 4).
GA Differentially Regulates IL-1β and sIL-1Ra Production in Human Monocytes.
To assess whether GA per se would affect the IL-1 system in human monocytes, freshly isolated human monocytes were activated by increasing doses of GA. The production of sIL-1Ra was enhanced by GA in a dose-dependent manner, reaching a plateau at 25 μg/mL (Fig. 3A). The latter dose was used for the in vitro experiments described later. Noticeably, GA did not induce IL-1β production, demonstrating that GA triggers an anti-inflammatory bias in human monocyte cytokine production.
Fig. 3.
GA differentially regulates IL-1β and sIL-1Ra production in human monocytes. (A) Monocytes (5 × 104 cells/200 μL/well; 96-well plates) were activated with the indicated dose of GA. After 48 h, supernatants were harvested and the production of IL-1β (open circles) and sIL-1Ra (filled circles) were measured in triplicate wells and represented as mean ± SD. Results are representative of 3 different experiments. (B) Monocytes (5 × 104 cells/200 μL/well; 96-well plates) were preincubated for 1 h with or without 25 μg/mL GA and then cultured for 48 h in the presence or absence of CEsHUT (1 μg/mL) or LPS (100 ng/mL). sIL-1Ra was measured in culture supernatants (mean ± SD, n = 3 different experiments). (C) Monocytes (5 × 104 cells/200 μL/well; 96-well plates) were preincubated for 1 h with or without 25 μg/mL GA and then cultured for 48 h in the presence or absence of CEsHUT (6 μg/mL) or LPS (100 ng/mL). IL-1β was measured in culture supernatants (mean ± SD, n = 3 different experiments, i.e., monocytes prepared from 3 different blood donors).
To confirm that GA affected the IL-1 system, we assessed its effect on human monocytes activated upon chronic/sterile and acute/infectious inflammatory conditions as mimicked by direct cellular contact with stimulated T cells and LPS, respectively. Studies of cell-cell interactions such as those occurring in T cell contact activation of human monocytes are usually complicated by the simultaneous presence of at least 2 viable cell types. To obviate this problem, and possible interferences caused by the fact that target cells are potentially phagocytic, isolated membranes from stimulated HUT-78 cells were solubilized with CHAPS and used as a stimulus, referred to as CEsHUT (23). As shown in Fig. 3B, GA enhanced the production of sIL-1Ra in monocytes activated by CEsHUT and LPS in a similar manner, and GA-induced sIL-1Ra production was additive to that triggered by CEsHUT or LPS. In contrast, the production of IL-1β induced by CEsHUT was inhibited by GA, whereas LPS-induced production of IL-1β was enhanced in the presence of GA (Fig. 3C). These observations suggest that GA displays opposite effects on signaling events downstream of LPS and CEsHUT.
GA Affects the Expression of Cytokine Transcripts.
To assess whether GA affected the production of cytokines at the transcriptional level, monocytes were incubated for 1 h with increasing doses of GA and then activated by CEsHUT or not activated. As shown in Fig. 4A, GA, in the absence of other stimuli, induced the expression of sIL-1Ra transcript in a dose-dependent manner, whereas that of IL-1β was not induced. When monocytes were activated by CEsHUT, GA diminished IL-1β mRNA expression by 30% whereas it enhanced sIL-1Ra mRNA expression by 25% in monocytes activated by CEsHUT (Fig. 4B), corroborating the effects of GA on protein production (Fig. 3). Together, these data suggest that GA displays opposite activity toward IL-1 system members in monocytes/macrophages by directly inducing sIL-1Ra expression and production and by modulating both IL-1β and sIL-1Ra expression/production induced by CEsHUT.
Fig. 4.
GA affects sIL-1Ra and IL-1β mRNA in both CEsHUT-activated and resting monocytes. (A) Monocytes (2 × 106 cells/well/3 mL) were preincubated for 1 h with the indicated dose of GA and then cultured for 3 h in the presence of CEsHUT (6 μg/mL). Total mRNA was isolated and analyzed by duplex real-time quantitative PCR (see Materials and Methods) for the presence of IL-1β (open circles) and sIL-1Ra (filled circles) transcripts. Results are presented as percentage of mRNA expression level, 100% being transcript expression measured after 3 h of monocyte activation by CEsHUT in the absence of GA; mean ± SD of 3 different experiments. (B) Monocytes (2 × 106 cells/well/3 mL) were activated for 18 h with the indicated dose of GA. Total mRNA was isolated and analyzed by duplex real-time quantitative PCR (see Materials and Methods) for the presence of IL-1β (open circles) and sIL-1Ra (filled circles) transcripts. Results are presented as percentage of mRNA expression level, 100% being the transcript level at 50 μg/mL GA; mean ± SD of 3 different experiments, i.e., monocytes prepared from 3 different blood donors.
Discussion
The present study sheds light on mechanisms by which GA might exert beneficial effects in MS. GA treatment enhances sIL-1Ra blood levels in patients with MS and in EAE mice. This is likely to be the consequence of the direct triggering effect of GA on monocytic production of sIL-1Ra. In addition, GA diminishes monocytic IL-1β production induced by direct contact with stimulated T cells. Thus, through different mechanisms, GA dampens IL-1β activity, which correlates with disease severity.
Recent insights derived from studies on the mechanism of action of GA show a pivotal role of monocytes in the modulation of the immune system and highlight the importance of these cells as a target for pharmacologic intervention in autoimmune diseases (4–10, 24). These results suggest that GA might be useful in autoimmune diseases other than MS, as suggested by its beneficial effect in animal models of autoimmune diseases such as uveoretinitis (25) and inflammatory bowel disease (26), and graft rejection (27), whereas its efficacy has not been demonstrated in animal models of systemic lupus erythematosus (28) and collagen-induced arthritis (29).
The premise that GA enhances sIL-1Ra levels in treated patients with MS is reminiscent of observations made with another immunomodulator used in MS. Indeed, IFNβ also increases circulating serum levels of sIL-1Ra in patients with MS (30). Interestingly, with both immunomodulators, the circulating levels of sIL-1Ra are doubled in treated patients compared with untreated individuals. Together these observations suggest that the enhancement of sIL-1Ra might be relevant to therapeutic effects of both GA and IFNβ. Indeed, sIL-1Ra is transported and expressed into the CNS, where it could inhibit the pro-inflammatory activities of IL-1β, whose expression is increased in MS lesions (18, 31). The efficiency of sIL-1Ra treatment was demonstrated in EAE animals, in which it results in delayed and milder disease (19, 22). Besides, polymorphisms encoded within the IL-1 gene cluster were associated with MS (32). In particular, mild/moderate disease has been correlated to allele 2 of the IL-1Ra gene (IL1RN) variable number of tandem repeats genotype, which favors the production of sIL-1Ra (12, 33). In addition, families displaying high innate IL-1β/sIL-1Ra ratio are at increased risk to have a relative who develops MS (34). Together, these studies reinforce the potential clinical benefit of GA to selectively induce sIL-1Ra secretion by monocytes in MS, as demonstrated in the present study. Of note, direct treatment with the commercially available form of sIL-1Ra (Anakinra) may represent an alternative treatment for MS, although its short lifespan once injected in humans may limit its efficacy (13). Nevertheless, as demonstrated here, the enhancement of intrinsic production of sIL-1Ra might be a mediator of the beneficial effects of GA in MS.
Most studies have focused on the effects of GA treatment on the adaptive immune system, in particular on T cells. Recent data favor the view that primary immune modulation of APC directs T cell immune modulation as a secondary step. Indeed, in mice lacking mature B and T cells, the GA treatment effect on monocytes is unbowed as indicated by an anti-inflammatory “type II” cytokine shift (10). This indicates that GA does not require T cells or T cell products to alter monocytic cytokine production. Furthermore, adoptive transfer of highly purified, GA-induced type II monocytes into mice with EAE triggers T cell immune modulation and ameliorates the disease course of recipient mice. However, MHC II-deficient type II monocytes were unable to mediate this effect on T cells or disease severity (10). Taken together, these observations indicate that in vivo GA treatment exerts a direct effect on APC, which rules T cell immune modulation as the effector arm of GA clinical benefit in CNS autoimmune disease. The present study confirm the direct effect of GA on cells of the monocytic lineage by demonstrating that it down-regulates T cell contact-induced IL-1β production and directly triggers the production of sIL-1Ra. Thus, GA directly affects both the antigen presentation and cytokine production of monocytic cells.
Direct cellular contact with stimulated T cells is a major pathway for the production of IL-1β and TNF in monocytes/macrophages under sterile conditions (17, 35, 36). Indeed, contact-mediated activation of monocytes/macrophages by stimulated T lymphocytes is as potent as optimal doses of LPS to inducing IL-1β and TNF production in monocytes (37, 38). This model was recently used to assess the potency of kinase inhibitors in acute and chronic inflammatory conditions (39). It is thus likely that this mechanism is highly relevant to the pathogenesis and persistence of chronic/sterile inflammation in diseases such as MS. The effect of GA on cytokine production induced by contact with stimulated T cells in human microglial cells was previously demonstrated (16). Stimulated T cells that were pretreated with GA induced lower levels of TNF, IL-1β, and IL-6 in human microglial cells and phorbol 12-myristate 13-acetate (PMA)/IFNγ-treated U937 monocytic cells. However, in the latter study, GA was absent during microglial cell activation, implying that GA rather inhibited the ability of T cells to activate cytokine production by cells of the monocytic lineage. The present study demonstrates that GA influences cytokine production by acting directly on human monocytes. Together, these studies suggest that GA affects the activation stage of both T cells and monocytes/macrophages to diminish contact-induced pro-inflammatory cytokine production.
GA displays opposite effects on monocytes activated by LPS and T cell contact. Indeed, in contrast with CEsHUT-activated monocytes, the production of the pro-inflammatory cytokine IL-1β was up-regulated when cells were activated by LPS. This result is in agreement with a previous study showing that GA enhances the production of IL-1β in the human monocytic cell line THP-1 when activated by LPS (40). Thus, GA displays opposite effects on cytokine production by monocytes activated upon acute/infectious (i.e., LPS) and chronic/sterile (i.e., CEsHUT) inflammatory conditions. Therefore, the use of LPS as an in vitro stimulus should be used with caution to mimic inflammatory conditions when chronic/sterile inflammatory diseases are investigated (39).
In conclusion, this study demonstrates that GA directly affects monocytes/macrophages by triggering the production of the anti-inflammatory cytokine sIL-1Ra. As sIL-1Ra can be both transported through the blood–brain barrier and induced within the CNS, it might exert immunomodulatory effects in both systemic and CNS compartments. In the latter, GA may also dampen the production and activity of IL-1β. Finally, the present data strengthen recent demonstrations that, in addition to the modulation of the adaptive immune system, GA significantly affects the innate immune system.
Materials and Methods
Patients.
Patients and healthy volunteers were recruited at the University Hospital of Geneva in accordance with institutional guidelines, and approval of the local ethical committee was obtained. Blood was drawn from 10 healthy controls, 11 untreated patients with RRMS, and 9 GA-treated patients with RRMS (Table 1). Sex, age, clinical score, and disease duration were matched between groups. All enrolled patients had definite RRMS according to revised McDonald criteria (41). At the time of blood sampling, GA-treated patients received 20 mg of GA s.c. daily for at least 1 year, with mean treatment duration of 33.6 ± 16.8 months (Table 1). None of the patients were receiving an immunomodulatory or immunosuppressive drug in addition to GA. Patients from the untreated group did not receive any immunosuppressive or immunomodulatory drug for at least 6 months preceding the study.
EAE Induction and GA Treatment.
EAE was induced in 6 C57BL/6 mice using 10 μg myelin oligodendrocyte glycoprotein 35–55 peptide in complete Freund adjuvant. After immunization and 48 h later, mice received an i.v. injection of 200 ng pertussis toxin. Mice were scored as follows: 0, no symptoms; 1, decreased tail tone; 2, mild monoparesis or paraparesis; 3, severe paraparesis; 4, paraplegia and/or quadriparesis; and 5, moribund condition or death. Mice received daily s.c. injections of 150 μg GA suspended in PBS solution (n = 3) or PBS solution alone (n = 3) starting 7 days before EAE induction as described elsewhere (42). All experiments were carried out in accordance with guidelines prescribed by the Institutional Animal Care and Use Committee at the University of California, San Francisco.
Materials.
FCS, streptomycin, penicillin, L-glutamine, RPMI-1640, and PBS solution free of Ca2+ and Mg2+ were purchased from Gibco; purified phytohemagglutinin from EY Laboratories; Lymphoprep from Axis-Shield; PMA, polymyxin B sulfate, and mouse anti-β-tubulin antibody from Sigma; and GA from Sanofi-Aventis. Other reagents were of analytical grade or better.
Monocytes.
Peripheral blood monocytes were isolated from buffy coats of blood of healthy volunteers as previously described (43). To avoid activation by endotoxin, polymyxin B sulfate (2 μg/mL) was added in all solutions during isolation procedure.
T Cell Stimulation and Membrane Isolation.
HUT-78, a human T cell line, was obtained from the American Type Culture Collection. Cells were maintained in RPMI-1640 medium supplemented with 10% heat-inactivated FCS, 50 μg/mL streptomycin, 50 IU/mL penicillin, and 2 mM L-glutamine in 5% CO2-air humidified atmosphere at 37 °C. HUT-78 cells (2 × 106 cells/mL) were stimulated for 6 h by phytohemagglutinin (1 μg/mL) and PMA (5 ng/mL). Plasma membranes of stimulated HUT-78 cells were prepared as previously described and solubilized in 8 mM CHAPS (23, 44). CHAPS extract of membranes of stimulated HUT-78 cells was referred as to CEsHUT. Previous studies demonstrated that fixed, stimulated HUT-78 cells, plasma membranes of the latter cells, and CEsHUT display similar ability to induce cytokine production in human monocytes (15). To activate monocytes, CEsHUT was used at either 1 μg/mL or 6 μg/mL proteins as previously determined (38, 44).
Cytokine Production.
Monocytes (5 × 104 cells/well/200 μL) were activated with the indicated stimulus in RPMI medium 1640 supplemented with 10% heat-inactivated FCS, 50 μg/mL streptomycin, 50 U/mL penicillin, 2 mM L-glutamine, and 5 μg/mL polymyxin B sulfate in 96-well plates and cultured for 48 h. The production of sIL-1Ra and IL-1β was measured in culture supernatants and patients' serum by commercially available enzyme immunoassay: IL-1β (Immunotech) and sIL-1Ra (Quantikine; R&D Systems). IL-1β and sIL-1Ra concentrations in serum of patients with RRMS and healthy controls were determined by triplicate measurements of the same sample.
mRNA Quantification.
Monocytes (2 × 106 cells/well/3 mL) were cultured in 6-well plates with the indicated stimulus for 3 h or 18 h. Total RNA was isolated and analyzed by quantitative real-time PCR as previously described (23).
Statistics.
When required, significance of differences between groups was evaluated using the Student t test.
Acknowledgments.
This work was supported by Swiss National Science Foundation Grants 320000–116259 (to D.B.) and 310000–113653 (to P.H.L.); Swiss Society for Multiple Sclerosis grants (to D.B. and P.H.L.); a Hans Wilsdorf Foundation grant (to D.B.); Swiss National Science Foundation Advanced Researcher Fellowship PA00A-119532 (N.M.); National Multiple Sclerosis Society Fellowship (M.S.W.); National Institutes of Health Grants RO1 AI509709 and RO1 AI073737 (to S.S.Z.); National Multiple Sclerosis Society Grants RG 4124 and RG 3913 (to S.S.Z.); Maisin Foundation (S.S.Z.); and the Dana Foundation (S.S.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Sela M, Teitelbaum D. Glatiramer acetate in the treatment of multiple sclerosis. Expert Opin Pharmacother. 2001;2:1149–1165. doi: 10.1517/14656566.2.7.1149. [DOI] [PubMed] [Google Scholar]
- 2.Johnson KP, et al. Copolymer 1 reduces relapse rate and improves disability in relapsing-remitting multiple sclerosis: results of a phase III multicenter, double-blind placebo-controlled trial. The Copolymer 1 Multiple Sclerosis Study Group. Neurology. 1995;45:1268–1276. doi: 10.1212/wnl.45.7.1268. [DOI] [PubMed] [Google Scholar]
- 3.Neuhaus O, Farina C, Wekerle H, Hohlfeld R. Mechanisms of action of glatiramer acetate in multiple sclerosis. Neurology. 2001;56:702–708. doi: 10.1212/wnl.56.6.702. [DOI] [PubMed] [Google Scholar]
- 4.Weber MS, et al. Multiple sclerosis: glatiramer acetate inhibits monocyte reactivity in vitro and in vivo. Brain. 2004;127:1370–1378. doi: 10.1093/brain/awh163. [DOI] [PubMed] [Google Scholar]
- 5.Kim HJ, et al. Type 2 monocyte and microglia differentiation mediated by glatiramer acetate therapy in patients with multiple sclerosis. J Immunol. 2004;172:7144–7153. doi: 10.4049/jimmunol.172.11.7144. [DOI] [PubMed] [Google Scholar]
- 6.Vieira PL, Heystek HC, Wormmeester J, Wierenga EA, Kapsenberg ML. Glatiramer acetate (copolymer-1, copaxone) promotes Th2 cell development and increased IL-10 production through modulation of dendritic cells. J Immunol. 2003;170:4483–4488. doi: 10.4049/jimmunol.170.9.4483. [DOI] [PubMed] [Google Scholar]
- 7.Jung S, et al. Induction of IL-10 in rat peritoneal macrophages and dendritic cells by glatiramer acetate. J Neuroimmunol. 2004;148:63–73. doi: 10.1016/j.jneuroim.2003.11.014. [DOI] [PubMed] [Google Scholar]
- 8.Stasiolek M, et al. Impaired maturation and altered regulatory function of plasmacytoid dendritic cells in multiple sclerosis. Brain. 2006;129:1293–1305. doi: 10.1093/brain/awl043. [DOI] [PubMed] [Google Scholar]
- 9.Hussien Y, Sanna A, Soderstrom M, Link H, Huang YM. Glatiramer acetate and IFN-beta act on dendritic cells in multiple sclerosis. J Neuroimmunol. 2001;121:102–110. doi: 10.1016/s0165-5728(01)00432-5. [DOI] [PubMed] [Google Scholar]
- 10.Weber MS, et al. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- 11.Dinarello CA. Mutations in cryopyrin: bypassing roadblocks in the caspase 1 inflammasome for interleukin-1beta secretion and disease activity. Arthritis Rheum. 2007;56:2817–2822. doi: 10.1002/art.22841. [DOI] [PubMed] [Google Scholar]
- 12.Burger D, Dayer JM. IL-1Ra. In: Oppenheim JJ, Feldmann M, editors. Cytokine Reference. London: Academic; 2000. pp. 319–336. [Google Scholar]
- 13.Burger D, Dayer JM, Palmer G, Gabay C. Is IL-1 a good therapeutic target in the treatment of arthritis? Best Pract Res Clin Rheumatol. 2006;20:879–896. doi: 10.1016/j.berh.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Burger D, Dayer JM. The role of human T lymphocyte-monocyte contact in inflammation and tissue destruction. Arthritis Res. 2002;4(suppl 3):S169–S176. doi: 10.1186/ar558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Burger D, Dayer JM, Molnarfi N. Cell contact dependence of inflammatory events. In: Smolen JS, Lipsky PE, editors. Contemporary Targeted Therapies in Rheumatology. Abingdon, UK: Taylor & Francis Books; 2007. pp. 85–103. [Google Scholar]
- 16.Chabot S, et al. Cytokine production in T lymphocyte-microglia interaction is attenuated by glatiramer acetate: a mechanism for therapeutic efficacy in multiple sclerosis. Mult Scler. 2002;8:299–306. doi: 10.1191/1352458502ms810oa. [DOI] [PubMed] [Google Scholar]
- 17.Dasgupta S, Jana M, Liu X, Pahan K. Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells. J Biol Chem. 2003;278:22424–22431. doi: 10.1074/jbc.M301789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cannella B, Raine CS. The adhesion molecule and cytokine profile of multiple sclerosis lesions. Ann Neurol. 1995;37:424–435. doi: 10.1002/ana.410370404. [DOI] [PubMed] [Google Scholar]
- 19.Badovinac V, Mostarica-Stojkovic M, Dinarello CA, Stosic-Grujicic S. Interleukin-1 receptor antagonist suppresses experimental autoimmune encephalomyelitis (EAE) in rats by influencing the activation and proliferation of encephalitogenic cells. J Neuroimmunol. 1998;85:87–95. doi: 10.1016/s0165-5728(98)00020-4. [DOI] [PubMed] [Google Scholar]
- 20.Furlan R, et al. HSV-1-mediated IL-1 receptor antagonist gene therapy ameliorates MOG(35–55)-induced experimental autoimmune encephalomyelitis in C57BL/6 mice. Gene Ther. 2007;14:93–98. doi: 10.1038/sj.gt.3302805. [DOI] [PubMed] [Google Scholar]
- 21.Matsuki T, Nakae S, Sudo K, Horai R, Iwakura Y. Abnormal T cell activation caused by the imbalance of the IL-1/IL-1R antagonist system is responsible for the development of experimental autoimmune encephalomyelitis. Int Immunol. 2006;18:399–407. doi: 10.1093/intimm/dxh379. [DOI] [PubMed] [Google Scholar]
- 22.Martin D, Near SL. Protective effect of the interleukin-1 receptor antagonist (IL-1ra) on experimental allergic encephalomyelitis in rats. J Neuroimmunol. 1995;61:241–245. doi: 10.1016/0165-5728(95)00108-e. [DOI] [PubMed] [Google Scholar]
- 23.Molnarfi N, Gruaz L, Dayer JM, Burger D. Opposite regulation of IL-1beta and secreted IL-1 receptor antagonist production by phosphatidylinositide-3 kinases in human monocytes activated by lipopolysaccharides or contact with T cells. J Immunol. 2007;178:446–454. doi: 10.4049/jimmunol.178.1.446. [DOI] [PubMed] [Google Scholar]
- 24.Kantengwa S, et al. Inhibition of naive Th1 CD4+ T cells by glatiramer acetate in multiple sclerosis. J Neuroimmunol. 2007;185:123–129. doi: 10.1016/j.jneuroim.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 25.Zhang M, et al. Copolymer 1 inhibits experimental autoimmune uveoretinitis. J Neuroimmunol. 2000;103:189–194. doi: 10.1016/s0165-5728(99)00239-8. [DOI] [PubMed] [Google Scholar]
- 26.Aharoni R, Sonego H, Brenner O, Eilam R, Arnon R. The therapeutic effect of glatiramer acetate in a murine model of inflammatory bowel disease is mediated by anti-inflammatory T-cells. Immunol Lett. 2007;112:110–119. doi: 10.1016/j.imlet.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Arnon R, Aharoni R. Mechanism of action of glatiramer acetate in multiple sclerosis and its potential for the development of new applications. Proc Natl Acad Sci USA. 2004;101(suppl 2):14593–14598. doi: 10.1073/pnas.0404887101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borel P, et al. Glatiramer acetate treatment does not modify the clinical course of (NZB x BXSB)F1 lupus murine model. Int Immunol. 2008;20:1313–1319. doi: 10.1093/intimm/dxn086. [DOI] [PubMed] [Google Scholar]
- 29.Zheng B, Switzer K, Marinova E, Zhang J, Han S. Exacerbation of autoimmune arthritis by copolymer-I through promoting type 1 immune response and autoantibody production. Autoimmunity. 2008;41:363–371. doi: 10.1080/08916930801931001. [DOI] [PubMed] [Google Scholar]
- 30.Nicoletti F, et al. Circulating serum levels of IL-1ra in patients with relapsing remitting multiple sclerosis are normal during remission phases but significantly increased either during exacerbations or in response to IFN-beta treatment. Cytokine. 1996;8:395–400. doi: 10.1006/cyto.1996.0054. [DOI] [PubMed] [Google Scholar]
- 31.Gutierrez EG, Banks WA, Kastin AJ. Blood-borne interleukin-1 receptor antagonist crosses the blood-brain barrier. J Neuroimmunol. 1994;55:153–160. doi: 10.1016/0165-5728(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 32.Kantarci OH, de Andrade M, Weinshenker BG. Identifying disease modifying genes in multiple sclerosis. J Neuroimmunol. 2002;123:144–159. doi: 10.1016/s0165-5728(01)00481-7. [DOI] [PubMed] [Google Scholar]
- 33.Mann CL, et al. Interleukin 1 genotypes in multiple sclerosis and relationship to disease severity. J Neuroimmunol. 2002;129:197–204. doi: 10.1016/s0165-5728(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 34.de Jong BA, et al. Production of IL-1beta and IL-1Ra as risk factors for susceptibility and progression of relapse-onset multiple sclerosis. J Neuroimmunol. 2002;126:172–179. doi: 10.1016/s0165-5728(02)00056-5. [DOI] [PubMed] [Google Scholar]
- 35.McInnes IB, Leung BP, Sturrock RD, Field M, Liew FY. Interleukin-15 mediates T cell-dependent regulation of tumor necrosis factor-alpha production in rheumatoid arthritis. Nat Med. 1997;3:189–195. doi: 10.1038/nm0297-189. [DOI] [PubMed] [Google Scholar]
- 36.Avice MN, Sarfati M, Triebel F, Delespesse G, Demeure CE. Lymphocyte activation gene-3, a MHC class II ligand expressed on activated T cells, stimulates TNF-alpha and IL-alpha production by monocytes and dendritic cells. J Immunol. 1999;162:2748–2753. [PubMed] [Google Scholar]
- 37.Burger D. Cell contact-mediated signaling of monocytes by stimulated T cells: a major pathway for cytokine induction. Eur Cytokine Netw. 2000;11:346–353. [PubMed] [Google Scholar]
- 38.Molnarfi N, Gruaz L, Dayer JM, Burger D. Opposite effects of IFNbeta on cytokine homeostasis in LPS- and T cell contact-activated human monocytes. J Neuroimmunol. 2004;146:76–83. doi: 10.1016/j.jneuroim.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 39.Li YY, et al. The identification of a small molecule inhibitor that specifically reduces T cell-mediated adaptive but not LPS-mediated innate immunity by T cell membrane-monocyte contact bioassay. Immunol Lett. 2008;117:114–118. doi: 10.1016/j.imlet.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 40.Li Q, Milo R, Panitch H, Swoveland P, Bever CT., Jr Glatiramer acetate blocks the activation of THP-1 cells by interferon-gamma. Eur J Pharmacol. 1998;342:303–310. doi: 10.1016/s0014-2999(97)01509-4. [DOI] [PubMed] [Google Scholar]
- 41.Polman CH, et al. Diagnostic criteria for multiple sclerosis: 2005 revisions to the “McDonald criteria”. Ann Neurol. 2005;58:840–846. doi: 10.1002/ana.20703. [DOI] [PubMed] [Google Scholar]
- 42.Weber MS, Hohlfeld R, Zamvil SS. Mechanism of action of glatiramer acetate in treatment of multiple sclerosis. Neurotherapeutics. 2007;4:647–653. doi: 10.1016/j.nurt.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hyka N, et al. Apolipoprotein A-I inhibits the production of interleukin-1beta and tumor necrosis factor-alpha by blocking contact-mediated activation of monocytes by T lymphocytes. Blood. 2001;97:2381–2389. doi: 10.1182/blood.v97.8.2381. [DOI] [PubMed] [Google Scholar]
- 44.Burger D, Molnarfi N, Gruaz L, Dayer JM. Differential induction of IL-1beta and TNF by CD40 ligand or cellular contact with stimulated T cells depends on the maturation stage of human monocytes. J Immunol. 2004;173:1292–1297. doi: 10.4049/jimmunol.173.2.1292. [DOI] [PubMed] [Google Scholar]