Abstract
Objective
Studies of young relatives at elevated risk for schizophrenia have pointed to the importance of a variety of neurobiological, cognitive, and clinical risk factors for the disorder; yet few have employed integrated models to estimate the joint contribution of these factors to heightened schizophrenic risk. We tested the predictive power of an integrated psychobiological model of schizophrenia risk to subsequent psychopathology development among young relatives at risk for the disorder.
Methods
Young first (n = 66) and second (n = 20) degree relatives of schizophrenia probands were followed for an average of 3 (SD = 1.13) years to examine their trajectories toward psychopathology development. Neurobiologic, cognitive, and clinical measures were employed in an integrated structural equation model to estimate their contribution to the prospective emergence of psychopathology.
Results
Results indicated that neurobiological, neurocognitive, and psychosis proneness factors at baseline were all uniquely predictive of subsequent psychopathology development, and that an integrated model of psychopathology development that took into account these factors provided an excellent fit to the observed data. Subsequent classification analyses of model accuracy using likelihood ratios adjusting for the base-rate of psychopathology development in this sample revealed that individuals identified by this model had a 71% chance of developing psychopathology in the future.
Conclusions
An integrated model of biobehavioral risk factors may provide a powerful method for predicting psychopathology and schizophrenia risk in at-risk samples. If validated, this model may be useful for early detection and intervention programs. Future research will need to focus particularly on predicting schizophrenia development and refining models to further enhance sensitivity.
Keywords: genetics, high risk, schizophrenia, structural equation modeling
1. Introduction
The prediction of psychosis and related psychopathology has become a critical focus in schizophrenia research. Long-term premorbid studies of relatives at risk for schizophrenia are critical to closing in on the etiopathology of the disease and providing promising directions for the earliest of prevention and intervention strategies. Investigations of high risk samples have frequently observed cognitive, clinical, and neurobiologic deficits (Keshavan et al., 2005). Studies of individuals a clinical high risk for schizophrenia have demonstrated greater decreases in brain volume, poorer neurocognitive performance on neuropsychological tests, and increased psychopathology in individuals who will eventually develop psychosis (Pantelis et al., 2003; Pukrop et al., 2007; Yung, Phillips, Yuen, & McGorry, 2004). Unfortunately, few prospective studies have examined the contribution of such deficits toward heightened schizophrenia risk or the development of major psychopathology in young, at-risk relatives to elucidate the earliest risk markers for the disorder. Existing prospective investigations have frequently found early levels of psychopathology and psychosis proneness, brain abnormalities, and neurocognitive dysfunction to be longitudinal predictors of schizophrenia signs and symptoms (Johnstone et al., 2005; Lawrie et al., 2001; Sorensen et al., 2006). However, to our knowledge, none have provided an integrated examination of the contribution of these related deficits.
It is unlikely that a single etiological factor is responsible for the development of schizophrenia, and interactions between variations in multiple genetic and environmental factors seem more plausible. Further, the predictive power of individual factors by themselves are not large (e.g., Sorensen et al., 2006). An integrative model that takes into account relevant relations between neurobiological, cognitive, and clinical endophenotypes for the disorder may provide a more powerful method for detecting risk for schizophrenia at the earliest phases of the illness. This research makes use of structural equation modeling to conduct a preliminary prospective investigation of the predictive utility of such a model to the emergence of major psychopathology in young at-risk relatives of patients with schizophrenia.
2. Method
2.1. Participants
Participants included 86 individuals with a first (n = 66) or second-degree relative (n = 20) diagnosed with schizophrenia or schizoaffective disorder by the Structured Clinical Interview for DSM-IV (SCID; First et al., 2002). Individuals were excluded if they had mental retardation, any lifetime evidence of a psychotic disorder, prior exposure to antipsychotic medications, recent history of substance use, or significant neurological or medical conditions. Participants were young (mean age = 15.22 [SD = 3.50] years), about half (n = 47) were female, and half were Caucasian (n = 43). Participants had on average 8.76 (SD = 3.38) years of education. The majority of participants (n = 54) were offspring, 13 were nieces/nephews, 12 were siblings, 6 were grandchildren, and 1 was an aunt/uncle of those with schizophrenia or schizoaffective disorder. A total of 48 relatives shared the same affected family member (median 2 relatives/affected family member) diagnosed with schizophrenia or schizoaffective disorder.
2.2. Neuropsychological and Psychopathological Measures
Measures of neurocognitive function were collected from the Revised Wechsler Adult Intelligence Scale (Wechsler, 1981); Wisconsin Card Sorting Test (Heaton et al. 1993); Spatial Working Memory Test (Cogtest, 2002); and a category/letter fluency task (Benton & Hamscher, 1978) to assess IQ, executive functioning, working memory, and verbal fluency, respectively. Psychosis proneness was assessed using total scores from the Perceptual Aberration, Magical Ideation, and Social Anhedonia scales developed by Chapman and colleagues (Chapman et al., 1978; Eckblad & Chapman, 1983; Eckblad et al., 1982). The development of clinical psychopathology meeting DSM-IV criteria was assessed using the SCID. In addition, the behavioral disorders section of the Schedule for Affective Disorders and Schizophrenia-Child Version (K-SADS; Ambrosini et al., 1989) was used to supplement SCID information. Participants were considered to have developed significant psychopathology if they either (1) were diagnosis free at baseline and met SCID/K-SADS criteria for an Axis I disorder at follow-up, or (2) developed a more severe disorder between baseline and follow-up (e.g., an adjustment disorder that later developed into a psychotic disorder).
2.3. Image Acquisition and Processing
Brain morphology data were collected using structural magnetic resonance imaging with a 1.5-T Signa whole body scanner and head coil (GE Medical Systems, Milwaukee, WI). Whole brain volume was acquired in 124 1.5mm-thick contiguous coronal slices with spoiled gradient recalled acquisition in steady state pulse sequence (TE = 5ms, TR = 25ms, acquisition matrix = 256 × 192, FOV = 24cm). Structural images were checked manually for motion and quality independently by trained research associates, normalized to standard MNI space and segmented using SPM5 (Wellcome Department of Cognitive Neurology, Institute of Neurology, London). Inhomogeneity artifacts were corrected during post-processing using a bias correction algorithm built into the segmentation procedure, and segmented images were smoothed using a 12mm Gaussian kernel. Volumetric measurements of total brain volume were extracted using image masks provided by the Wake Forest University PickAtlas toolbox for SPM5 (Maldjian et al., 2003), with regional definitions from Tzourio-Mazoyer and colleagues (2002).
2.4. Procedures
Participants were recruited from affected family members receiving services at Western Psychiatric Institute and Clinic, Pittsburgh. Upon recruitment, family members were assessed for a schizophrenia/schizoaffective disorder diagnosis using the SCID and screened for exclusion criteria. Eligible participants received the aforementioned clinical, neuropsychological, and neuroimaging assessments at baseline. Participants were then assessed for the development of significant clinical psychopathology, in most cases yearly, for an average of 3 (SD = 1.13) years using the SCID/K-SADS. This research was approved and reviewed annually by the University of Pittsburgh Medical Center Institutional Review Board. All participants and/or their guardians provided written informed consent prior to participation.
2.5. Data Analysis
Structural equation modeling was used to test an integrated predictive model of psychopathology development from baseline cognitive, clinical, and neurobiological factors. This analytic technique is based upon traditional path analytic approaches that make use of multivariate linear or logistic regression (Lewis-Beck, 1974), but extends these methods by incorporating factor analysis to distill the common latent (unobserved) variables represented by multiple measured variables (Kline, 2005). Each structural equation model therefore consists of both measurement and structural components to first define the latent factors of interest (measurement component) and then test various hypothesized relations among these factors (structural component). As in multiple regression, relations can be tested in the presence of potentially confounding variables by entering such variables as correlated predictors in structural equation models, and then estimating the regression coefficient of the relationship of interest in the presence of the potential confounder.
This approach is particularly useful when there are many different imperfectly measured variables (e.g., IQ scores, perseverative errors during the Wisconsin Card Sorting Test, spatial working memory scores) that putatively represent a single higher-order domain (e.g., cognition). In such a case, a single multiple regression model with all related variables predicting an outcome would be difficult to interpret due to multicollinearity among the measured variables. In addition, each measured variable is inevitably associated with some degree of measurement error. By defining a measurement component through confirmatory factor analysis, structural equation modeling provides a single representation (i.e., factor) of the domain of interest based on all measured variables. This substantially reduces the amount of repeated inference testing involved when multiple measures are present, and provides the investigator with a parsimonious method of building predictive models of relations between general constructs of interest that can be measured by many different variables. Further, due to the nature confirmatory factor analysis, structural equation modeling also provides a more sensitive test of relations between variables, as latent factors do not contain the measurement error inherent in imperfectly measured variables.
While structural equation modeling has a number of advantages, as discussed above, it depends on moderate to large sample sizes, especially as the parameters estimated within a model increase (Kim, 2005). In addition, despite the use of the term “confirmatory” structural equation modeling and confirmatory factor analysis are not definitive techniques, as equally compelling models may exist that are mathematically equivalent in accuracy as the one an investigator chooses (Tomarken & Waller, 2003). Support for models are based not only on statistical accuracy, but also must be grounded in previous evidence and/or theory. Further, the same assumptions regarding causal inference apply to structural equation modeling as correlation and regression analyses, in that associations between variables do not necessarily indicate a causal, unidirectional relationship.
Most structural equation models are estimated using the maximum-likelihood (ML) approach (Fisher, 1925), however in this research, because the primary dependent variable was binary (did or did not develop psychopathology), weighted least squares mean and variance adjusted (WLSMV) estimation was used (Muthén & Muthén, 2001). Unlike maximum-likelihood approaches that assume normality, the WLSMV does not make this assumption and uses a latent probit function to describe outcome among dichotomous variables. Tests of the accuracy of structural equation models, regardless of the method of estimation, depend on inspection of the overall model fit to the observed data, as well as the individuals relationships among the variables and factors specified in the model. A number of different statistics have been developed to provide information on model fit, and it is usually recommended that several statistics be employed when using structural equation modeling (Kline, 2005). The χ2 statistic is a common measure of model fit, with significant values indicating poor model accuracy. However, since the χ2 statistic is based partially on sample size, it tends to be biased in larger samples. Less biased estimates of model fit that are commonly used include the Comparative Fit Index (CFI) and the Root Mean Square Error of Approximation (RMSEA). The CFI is an incremental fit index that measures the degree of improvement in model fit over a comparison model where no relationship exists among the variables. Values of .90 and above indicate well-fitting models (Bentler, 1990). The RMSEA is a residual fit index that measures the amount of error in the overall structural equation model, with values of .08 and below indicative of well-fitting models. The CFI, RMSEA, and an adjusted χ2 statistic for categorical data were used as indexes of model fit in this research.
Missing data were handled using expectation-maximization (EM) estimates (Dempster, Laird, & Rubin, 1977). This approach uses complex mathematical modeling based on ML estimation to provide an estimated covariance matrix based on an observed covariance matrix with data missing at random. Monte-Carlo examinations have repeatedly found that the EM approach to handling missing data provides a substantial reduction in the bias of covariance matrices estimated with other common missing data techniques, such as list-wise and pair-wise deletion, as well as mean and regression imputation (Schafer & Graham, 2002)
3. Results
Of the 86 individuals followed over the course of the study, approximately half (n = 42, 49%) had a baseline Axis I diagnosis, and 24 (28%) experienced an initial development (n = 13, 15%) or worsening (n = 11, 13%) of psychopathology. Most individuals who developed subsequent psychopathology were diagnosed with mood (n = 9, 11% of the entire sample) or attention/behavioral (n = 6, 7% of the entire sample) disorders, 3 (4%) were diagnosed with a psychotic disorder not otherwise specified, 2 (2%) with schizophrenia, 2 (2%) with anxiety disorders, and 2 (2%) with other psychiatric disorders.
In order to examine the different contributors to the development of major psychopathology among this sample, an integrated structural equation model of baseline neurobiologic, cognitive, and clinical factors was constructed and used to predict subsequent psychopathology development, after adjusting for age, gender, and baseline diagnostic status. Bivariate relations among the variables used to construct this model are presented in Table 1. As can be seen in Figure 1, results from this structural equation model indicated that neurobiologic, psychosis proneness, and neurocognitive factors at baseline were all significant independent predictors of subsequent psychopathology development. In particular, at-risk relatives who had less total brain volume, and experienced greater neurocognitive dysfunction and psychosis proneness at baseline were significantly more likely to develop psychopathology. Surprisingly, there were no significant relations between these three exogenous factors, as suggested by the general lack of consistent between-domain bivariate associations shown in Table 1. Estimates of model fit indicated that this integrated model provided an excellent representation of the observed data, χ2(26, N = 86) = 30.63, p =.24, CFI =.95, RMSEA =.05.
Table 1.
Descriptive Statistics and Intercorrelations Among Neurobiological, Cognitive, and Clinical Study Variables (N = 86)
| Variable | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||||||
| 1. Age | - | ||||||||||||
| 2. Sex (1 = Male; 2 = Female) | .02 | - | |||||||||||
| Neurobiology | |||||||||||||
| 3. Total brain volume | .25* | −.52* | - | ||||||||||
| Cognition | |||||||||||||
| 4. IQ | .14 | −.06 | .08 | - | |||||||||
| 5. Verbal fluency | .51* | −.08 | −.00 | .47* | - | ||||||||
| 6. Spatial working memory | −.35* | .12 | −.04 | −.47* | −.50* | - | |||||||
| 7. WCST: Perseverative errors | −.32* | .05 | −.11 | −.39* | −.24* | .30* | - | ||||||
| 8. WCST: Non-perseverative errors | −.27* | .10 | −.23* | −.35* | −.41* | .60* | .59* | - | |||||
| Clinical | |||||||||||||
| 9. Chapman: Social anhedonia | −.37* | −.10 | −.13 | −.37* | −.16 | .32* | .33* | .37* | - | ||||
| 10. Chapman: Magical ideation | −.22* | −.11 | −.06 | −.24* | −.08 | .23* | .14 | −.00 | .51* | - | |||
| 11. Chapman: Perceptual aberration | −.25* | .05 | −.02 | −.31* | −.15 | .15 | .03 | .04 | .53* | .66* | - | ||
| 12. Axis 1 diagnosis at baseline (0 = No; 1 = Yes) | .06 | −.14 | .05 | −.31* | −.10 | .27* | .14 | .16 | .40* | .21† | .31* | - | |
| 13. Development of psychopathology (0 = No; 1 = Yes) | −.11 | −.06 | −.19† (-.23*) | −.23* (−.22*) | −.05 (.00) | .10 (.07) | .14 (.11) | .28* (.27*) | .15 (.11) | .18† (.16) | .30* (.29*) | −.04 (−.04) | - |
|
| |||||||||||||
| Mean | 15.22 | 1.55 | 1100.69 | 104.03 | 13.92 | 82.49 | 11.80 | 12.46 | 6.74 | 4.88 | 2.86 | .49 | .28 |
| Standard Deviation | 3.48 | .50 | 99.45 | 14.92 | 3.93 | 43.92 | 8.81 | 11.63 | 4.61 | 3.21 | 3.40 | .50 | .45 |
Note. Relationships in parentheses with psychopathology development are based on partial correlation analyses adjusting for age and sex. Within-domain relations appear in boldface.
p <.10,
p <.05
Figure 1. Structural Equation Model Predicting the Emergence of Psychopathology in Individuals At Risk for Schizophrenia From Clinical, Neurobiological, Cognitive Characteristics.a.
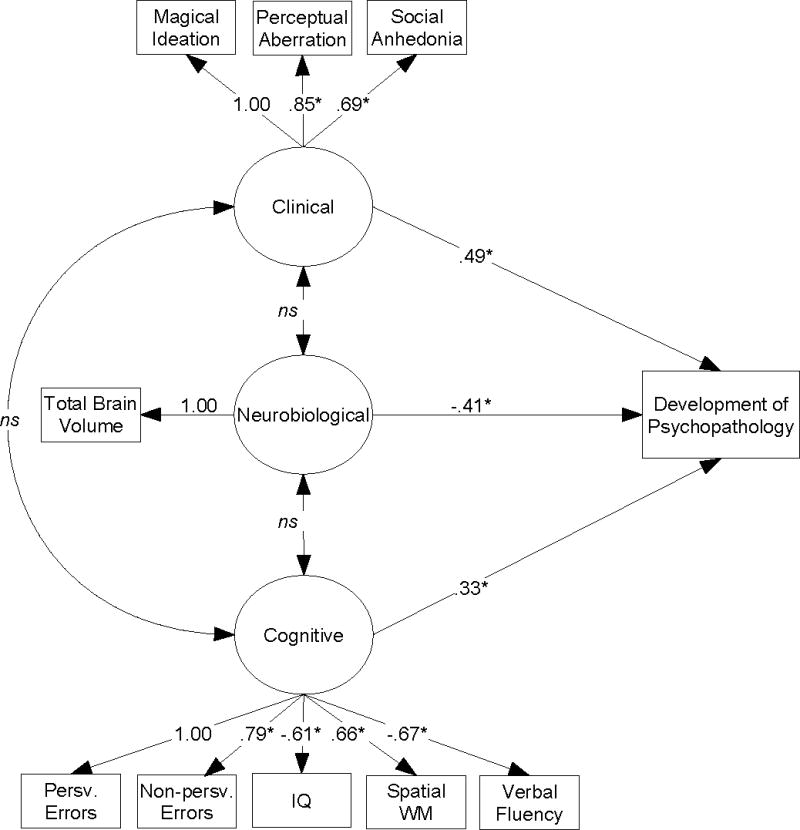
aχ2(26, N = 86) = 30.63, p =.24, CFI =.95, RMSEA =.05
Path parameters are presented as standardized regression coefficients. Model estimates are adjusted for age, gender, and baseline diagnostic status.
WM = Working memory
*p ≤.05
Subsequent classification analyses conducted to determine the accuracy with which this psychobiological model predicted future psychopathology development showed that 80% of participants were correctly classified by this model. However, as can be seen in Table 2, while model specificity was excellent (specificity = .92), sensitivity was low (sensitivity = .50), indicating that the model was better at identifying individuals who would remain healthy than those would would eventually develop psychopathology. Given the low base-rate of psychopathology development in the sample (28%), a lower sensitivity might be expected. As such, likelihood ratios were computed to provide estimates of model accuracy accounting for prevalence. The likelihood ratio of positive test results based on this model was 6.25, and the likelihood ratio of negative test results was .54. Based on a 28% prevalence (pre-test OR = .39), individuals had a 71% chance (post-test OR = 2.44) of developing psychopathology if they were identified as developing psychopathology by this psychobiological model, whereas those identified as not developing psychopathology by the model only had a 17% chance of developing psychopathology (post-test OR = .21).
Table 2.
Prediction of Psychopathology Development Based on an Integrated Psychobiological Structural Equation Model.
| Model-Based Prediction |
|||
|---|---|---|---|
| Actual | n | Predicted Developing Psychopathology | Predicted Not Developing Psychopathology |
| Developed Psychopathology | 24 | 12 (50%) | 12 (50%) |
| Did Not Develop Psychopathology | 62 | 5 (8%) | 57 (92%) |
Note. Efficiency (95% CI) =.80 (.73–.88), Sensitivity (95% CI) =.50 (.30–.71), Specificity (95% CI) =.92 (.85–.99). Confidence intervals are based on 1000 bootstrapped sample replications.
4. Discussion
Identifying risk factors for schizophrenia has become an important undertaking for informing early intervention and prevention programs, yet little is known about the earliest factors that may contribute to the development of psychosis and related psychopathology. In particular, although previous studies have suggested a multiplicity of potential risk factors (Keshavan et al., 2005), prospective studies that simultaneously examine multiple individual and biological factors within an integrative framework have been noticeably absent. Further, although research has increasingly indicated that a broad range of psychopathology may precede psychosis development (Keshavan et al., in press), few prospective investigations have examined predictors of general psychopathology development in young relatives at risk for schizophrenia. We examined, through structural equation modeling, the relative contribution of neurobiologic, cognitive, and clinical risk factors for schizophrenia to the prospective development of major psychopathology among relatives at risk for the disorder. Results supported findings from studies of individuals risk factors (Johnstone et al., 2005; Lawrie et al., 2001; Sorensen et al., 2006), and showed that reductions in total brain volume, increases in psychosis proneness, and the presence of deficits in neurocognitive function at baseline were all independent and significant predictors of subsequent psychopathology development in at-risk relatives. Further, this model accurately classified 80% of those who did versus did not develop psychopathology.
Despite the efficiency of our psychobiological model of psychopathology development, future work is clearly needed to increase model sensitivity and ensure that early intervention and prevention strategies are available to all individuals for whom these methods are indicated. While we found that our model was highly specific (specificity = .92), its sensitivity was low (sensitivity = .50). Likelihood ratio tests accounting for the low prevalence of psychopathology development were more positive, indicating that individuals identified by this model had a 71% probability of develop subsequent psychopathology. Nonetheless, refinements to this model may increase sensitivity and prove quite helpful to case detection. Specific refinements that may be particularly promising include adding additional neurobiologic and cognitive markers, such as abnormalities in brain metabolism from magnetic resonance spectroscopy measures and deficits in social cognition, both of which have been documented in high risk samples (Kee, Horan, Mintz, & Green, 2004; Keshavan, Stanley, Montrose, Minshew, & Pettegrew, 2003).
It is also important to note that little to no cross-sectional relations existed between exogenous neurobiological, clinical, and cognitive factors at baseline. Few studies have examined the interrelations among these factors in high risk samples, although some do suggest that if present, the overlap between neurobiologic, neurocognitive, and clinical dysfunction may not be substantial in non-clinical high risk samples (Byrne et al., 2003; Conklin, Curtis, Calkins, & Iacono, 2005; Keshavan et al., 2002), compared to the more pervasive relations seen in clinical populations (Antonova, Sharma, Morris, & Kumari, 2004; Bilder et al., 2000). While the modest sample size employed in this research may have precluded the detection of significant, albeit small relations between these factors, and restricted range on clinical and neurocognitive tests in this non-clinical high risk sample may have further impeded the detection of significant relations, it is also possible that gross brain structure, neurocognitive function, and psychosis proneness represent largely independent risk factors many years prior to the onset of psychotic illness. As schizophrenia and related psychotic disorders progress and early neurodevelopmental insults come to bear on programmed brain development, neurobiologic and cognitive functions decline, and psychopathology emerges (Keshavan & Hogarty, 1999). At such a time, associations between these factors may become much stronger as they orchestrate the disease progression toward overt illness manifestations. Subsequent investigations will need to examine the longitudinal convergence of these factors over time to more clearly understand their possible shift from early orthogonality to close interconnectedness as psychosis develops.
Although these results are limited by a modest sample size, they suggest that a tripartite assessment of neurobiological, cognitive, and clinical abnormalities may be particularly powerful for identifying at-risk relatives who will eventually develop major clinical psychopathology. Such individuals may be particularly at risk for transitioning to schizophrenia and related psychotic disorders, given the cascade of affective and behavioral psychopathology that frequently seems to precede the development of psychosis (Yung & McGorry, 1996). Subsequent studies will need to replicate these findings in larger samples, particularly those that contain sufficient numbers of individuals with schizophrenia, so that the predictive accuracy of the model employed in this research to the development of schizophrenia can be examined. If validated, this integrated psychobiological model of schizophrenia risk could help target early intervention and detection programs to those most in need.
5. Conclusion
A psychobiological model encompassing neurobiologic, cognitive, and clinical risk factors for schizophrenia was significantly predictive of longitudinal psychopathology development among young relatives at risk for psychosis. Models integrating these psychobiological factors may hold significant utility for the early detection of schizophrenia and related disorders.
Acknowledgments
This work was supported in part by NIMH grants MH 64023, 01180 (MSK), a NARSAD independent Investigator award (MSK), NARSAD and GCRC grant M01 RR00056. We wish to thank Vaibhav Diwadkar for his help with various aspects of this study.
Abbreviations
- SCID
Structured Clinical Interview for DSM-IV
- K-SADS
Schedule for Affective Disorders and Schizophrenia-Child Version
- CFI
Comparative Fit Index
- EM
Expectation Maximization
- ML
Maximum Likelihood
- RMSEA
Root Mean Square Error of Approximation
- WLSMV
Weighted Least Squares Mean and Variance adjusted
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ambrosini PJ, Metz C, Prabucki K, Lee J. Videotape reliability of the third revised edition of the K-SADS. J Am Acad Child Adolesc Psychiatry. 1989;28:723–728. doi: 10.1097/00004583-198909000-00013. [DOI] [PubMed] [Google Scholar]
- Antonova E, Sharma T, Morris R, Kumari V. The relationship between brain structure and neurocognition in schizophrenia: a selective review. Schizophr Res. 2004;70:117–145. doi: 10.1016/j.schres.2003.12.002. [DOI] [PubMed] [Google Scholar]
- Bentler PM. Comparative fit indexes in structural models. Psychol Bull. 1990;107:238–346. doi: 10.1037/0033-2909.107.2.238. [DOI] [PubMed] [Google Scholar]
- Benton AL, Hamscher K. Multilingual Aphasia Examination Manual (revised) Iowa City, IA: University of Iowa; 1978. [Google Scholar]
- Bilder RM, Goldman RS, Robinson D, Reiter G, Bell L, Bates JA, Pappadopulos E, Willson DF, Alvir JMJ, Woerner MG, et al. Neuropsychology of first-episode schizophrenia: Initial characterization and clinical correlates. Am J Psychiatry. 2000;157:549–559. doi: 10.1176/appi.ajp.157.4.549. [DOI] [PubMed] [Google Scholar]
- Byrne M, Clafferty BA, Cosway R, Grant E, Hodges A, Whalley HC, Lawrie SM, Owens DG, Johnstone EC. Neuropsychology, genetic liability, and psychotic symptoms in those at high risk of schizophrenia. J Abnorm Psychol. 2003;112:38–48. [PubMed] [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Body-image aberration in schizophrenia. J Abnorm Psychol. 1978;87:399–407. doi: 10.1037//0021-843x.87.4.399. [DOI] [PubMed] [Google Scholar]
- Cogtest. Cogtest: Computerised Cognitive Battery for Clinical Trials. 2002 Available at http://www.cogtest.com.
- Conklin HM, Curtis CE, Calkins ME, Iacono WG. Working memory functioning in schizophrenia patients and their first-degree relatives: cognitive functioning shedding light on etiology. Neuropsychologia. 2005;43:930–942. doi: 10.1016/j.neuropsychologia.2004.09.013. [DOI] [PubMed] [Google Scholar]
- Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data using the EM algorithm. Journal of the Royal Statistical Society. Series B (Methodological) 1977;39:1–38. [Google Scholar]
- Eckblad M, Chapman LJ. Magical ideation as an indicator of schizotypy. J Consult Clin Psychol. 1983;51:215–225. doi: 10.1037//0022-006x.51.2.215. [DOI] [PubMed] [Google Scholar]
- Eckblad M, Chapman LJ, Chapman JP, Mishlove M. The Revised Social Anhedonia Scale. Unpublished manuscript 1982 [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview For DSM-IV-TR Axis I Disorders, Research Version. Patient. New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Fisher RA. Theory of statistical estimation. Proceedings of the Cambridge Philosophical Society. 1925;22:700–725. [Google Scholar]
- Heaton RK, Chelune GJ, Talley JL, Kay GG, Curtiss G. Wisconsin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources Inc; 1993. [Google Scholar]
- Johnstone EC, Ebmeier KP, Miller P, Owens DGC, Lawrie SM. Predicting schizophrenia: findings from the Edinburgh High-Risk Study. Br J Psychiatry. 2005;186:18–25. doi: 10.1192/bjp.186.1.18. [DOI] [PubMed] [Google Scholar]
- Kee KS, Horan WP, Mintz J, Green MF. Do the siblings of schizophrenia patients demonstrate affect perception deficits? Schizophr Res. 2004;67:87–94. doi: 10.1016/s0920-9964(03)00217-2. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Hogarty GE. Brain maturational processes and delayed onset in schizophrenia. Dev Psychopathol. 1999;11:525–543. doi: 10.1017/s0954579499002199. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Dick E, Mankowski I, Harenski K, Montrose DM, Diwadkar V, DeBellis M. Decreased left amygdala and hippocampal volumes in young offspring at risk for schizophrenia. Schizophr Res. 2002;58:173–183. doi: 10.1016/s0920-9964(01)00404-2. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Diwadkar VA, Montrose DM, Rajarethinam R, Sweeney JA. Premorbid indicators and risk for schizophrenia: A selective review and update. Schizophr Res. 2005;79:45–57. doi: 10.1016/j.schres.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Keshavan MS, Montrose DM, Rajarethinam R, Diwadkar V, Prasad K, Sweeney JA. Psychopathology among offspring of parents with schizophrenia: Relationship to premorbid impairments. Schizophr Res. doi: 10.1016/j.schres.2008.03.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keshavan MS, Stanley JA, Montrose DM, Minshew NJ, Pettegrew JW. Prefrontal membrane phospholipid metabolism of child and adolescent offspring at risk for schizophrenia or schizoaffective disorder: an in vivo 31 P MRS study. Mol Psychiatry. 2003;8:316–323. doi: 10.1038/sj.mp.4001325. [DOI] [PubMed] [Google Scholar]
- Kim KH. The Relation Among Fit Indexes, Power, and Sample Size in Structural Equation Modeling. Structural Equation Modeling. 2005;12:368–390. [Google Scholar]
- Kline RB. Principles and Practice of Structural Equation Modeling. 2. New York: Guilford; 2005. [Google Scholar]
- Lawrie SM, Whalley HC, Abukmeil SS, Kestelman JN, Donnelly L, Miller P, Best JJK, Owens DGC, Johnstone EC. Brain structure, genetic liability, and psychotic symptoms in subjects at high risk of developing schizophrenia. Biol Psychiatry. 2001;49:811–823. doi: 10.1016/s0006-3223(00)01117-3. [DOI] [PubMed] [Google Scholar]
- Lewis-Beck MS. Determining the importance of an independent variable: A path analytic solution. Soc Sci Res. 1974;3:95–107. [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fmri data sets. NeuroImage. 2003;19:1233–1239. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- Muthén LK, Muthén BO. Mplus User’s guide. Los Angles, CA: Muthén & Muthén; 2001. [Google Scholar]
- Pantelis C, Velakoulis D, McGorry PD, Wood SJ, Suckling J, Phillips LJ, Yung AR, Bullmore ET, Brewer W, Soulsby B, et al. Neuroanatomical abnormalities before and after onset of psychosis: a cross-sectional and longitudinal MRI comparison. Lancet. 2003;361:281–288. doi: 10.1016/S0140-6736(03)12323-9. [DOI] [PubMed] [Google Scholar]
- Pukrop R, Ruhrmann S, Schultze-Lutter F, Bechdolf A, Brockhaus-Dumke A, Klosterkötter J. Neurocognitive indicators for a conversion to psychosis: Comparison of patients in a potentially initial prodromal state who did or did not convert to a psychosis. Schizophr Res. 2007;92:116–125. doi: 10.1016/j.schres.2007.01.020. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Graham JW. Missing data: Our view of the state of the art. Psychol Methods. 2002;7:147–177. [PubMed] [Google Scholar]
- Sorensen HJ, Mortensen EL, Parnas J, Mednick SA. Premorbid Neurocognitive Functioning in Schizophrenia Spectrum Disorder. Schizophr Bull. 2006;32:578–583. doi: 10.1093/schbul/sbj040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomarken AJ, Waller NG. Potential problems with well fitting models. J Abnorm Psychol. 2003;112:578–598. doi: 10.1037/0021-843X.112.4.578. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papthanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Adult Intelligence Scale-Revised. New York: Psychological Corp; 1981. [Google Scholar]
- Yung AR, McGorry PD. The prodromal phase of first-episode psychosis: past and current conceptualizations. Schizophr Bull. 1996;22:353–370. doi: 10.1093/schbul/22.2.353. [DOI] [PubMed] [Google Scholar]
- Yung AR, Phillips LJ, Yuen HP, McGorry PD. Risk factors for psychosis in an ultra high-risk group: psychopathology and clinical features. Schizophr Res. 2004;67:131–142. doi: 10.1016/S0920-9964(03)00192-0. [DOI] [PubMed] [Google Scholar]


