Abstract
Oocyte maturation invokes complex signaling pathways to achieve cytoplasmic and nuclear competencies for fertilization and development. The Src-family kinases FYN, YES and SRC are expressed in mammalian oocytes but their function during oocyte maturation remains an open question. Using chemical inhibitor, siRNA knockdown, and gene deletion strategies the function of Src-family kinases was evaluated in mouse oocytes during maturation under in vivo and in vitro conditions. Suppression of Src-family as a group with SKI606 greatly reduced meiotic cell cycle progression to metaphase-II. Knockdown of FYN kinase expression after injection of FYN siRNA resulted in an approximately 50% reduction in progression to metaphase-II similar to what was observed in oocytes isolated from FYN (−/−) mice matured in vitro. Meiotic cell cycle impairment due to a Fyn kinase deficiency was also evident during oocyte maturation in vivo since ovulated cumulus oocyte complexes collected from FYN (−/−) mice included immature metaphase-I oocytes (18%). Commonalities in meiotic spindle and chromosome alignment defects under these experimental conditions demonstrate a significant role for Fyn kinase activity in meiotic maturation.
Keywords: Meiosis, maturation, mouse, oocyte, phosphotyrosine, Src, Fyn, protein kinase
Introduction
Mammalian oocytes acquire essential properties for fertilization and early development as they mature coincident with the process of ovulation. Meiotic maturation is elicited in vivo by the periovulatory LH surge and encompasses a series of events that occur over a time span of hours in rodents to days in the case of humans and other mammalian species (Edwards, 1965). While it has long been recognized that mammalian oocytes proceed from prophase-I arrest (GV) through to metaphase-II (MII) when removed from the inhibitory environment of the ovarian follicle (Cho et al., 1974), there is an emerging notion that a complex signaling dialogue between companion granulosa cells and oocytes modulates the reinitiation, progression, and arrest of meiosis at metaphase-II. Thus, original studies invoking cAMP and protein kinase A (PKA) signaling and communication between the oocyte and cumulus granulosa cells (Anderson and Albertini, 1976; Cho et al., 1974; Eppig et al., 1983; Lindner et al., 1974; Schultz et al., 1983) appear as oversimplifications since recent studies have identified a complex multifactorial sequence of signaling effectors to be operative (Zhang et al., 2007). Specifically, reception of LH in mural granulosa cells, at least in rodents, is followed by de novo production of EGF family ligands that drive the reinitiation of meiosis. At the level of the oocyte, mechanisms involving selective inactivation of oocyte phosphodiesterases leading to decreased cAMP and PKA activities within the oocyte appear linked to a rise in Cdk1/cyclinB and p44/42 MAPK supporting meiotic maturation from the GV to metaphase-II stages (Eppig et al., 1983; Liang et al., 2007; Mehlmann et al., 2006; Panigone et al., 2008).
Of the pathways recently implicated in oocyte maturation and egg activation in mammals are those involving Src-family protein tyrosine kinases (SFKs) (McGinnis et al., 2007; Mehlmann and Jaffe, 2005; Meng et al., 2006; Talmor-Cohen et al., 2004a; Zheng et al., 2007). Src-family protein tyrosine kinases are a family of nine closely related protein tyrosine kinases of which three (FYN, YES and sometimes SRC) have been identified in mammalian oocytes (Mehlmann and Jaffe, 2005; Meng et al., 2006; Talmor-Cohen et al., 2004a; Talmor et al., 1998). The involvement of Src-family signaling pathways in mammalian oocyte maturation was shown by in vitro maturation studies in mouse oocytes where the SFK inhibitor PP2 blocked germinal vesicle breakdown (GVBD) (Zheng et al., 2007). Src-family kinases localize to region of the meiotic metaphase-II spindle and Fyn kinase co-precipitates with tubulin from egg cytoplasm (McGinnis et al., 2007; Meng et al., 2006; Talmor-Cohen et al., 2004b; Talmor et al., 1998) further implicating participation of SFKs in meiotic spindle function. An idea supported by the fact that aged rat oocytes undergo meiotic spindle disruption in the presence of SFK inhibitors (PP2 or SU6656) (Talmor-Cohen et al., 2004b). Moreover, while chemical inhibition of SFKs during fertilization of mouse oocytes permitted resumption of meiosis, the completion of meiosis-II and the first mitotic cell cycle were inhibited in association with severe abnormalities in spindle microtubule organization and chromosome alignment (McGinnis et al., 2007). Since injection of a constitutively active form of Fyn into MII rat oocytes caused egg activation, metaphase-anaphase transition and extrusion of the second polar body (Talmor-Cohen et al., 2004b) these studies collectively implicate SFKs s in microtubule dynamics and cell cycle progression in the mature mammalian oocyte and fertilized egg. The rather limited evidence implicating SFKs in earlier stages of meiotic maturation of mammalian oocytes prompted the present investigation into the role of SFKs in meiotic cell cycle progression in mouse oocytes with reference to meiotic spindle morphogenesis and functionality.
MATERIALS AND METHODS
Oocyte collection
Cumulus-Oocyte-Complexes (COC) were collected from 6–7 week old female mice. Most experiments used CF1 female mice (Harlan Sprague-Dawley, Indianapolis IN or Charles River Laboratories, Wilmington MA). FYN knock-out mice (B6/129S7-Fyntm1Sor/J; FYN (−/−) (Stein et al., 1992)) and the recommended control (B6/129SF2/J) mice were purchased from Jackson Laboratories (Bar Harbor, ME) and a homozygous knock-out colony was maintained at the University of Kansas Medical Center. Mice were housed in a temperature and light-controlled room on a 14L:10D light cycle and experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (National Academy of Sciences 1996). Mice were euthanized by isofluorothane inhalation anesthesia followed by cervical dislocation. Females were stimulated with 5 IU equine chorionic gonadotropin (eCG; Calbiochem, San Diego CA). Ovaries were collected at 42–46 hours (h) post-eCG. COC were released from large antral follicles into HEPES-buffered KSOM (FHM, Chemicon-Millipore, Billerica MA) with 4 mg/ml BSA (mFHM). For experiments in which ovulated oocytes were used, female mice were stimulated with 5 IU eCG followed 48h later with 5 IU human chorionic gondadotropin (hCG) and ovulated oocytes were collected from the oviducts 15–16 h post-hCG. For experiments examining the effects of chemical inhibitors of Src kinases, COC were released from the ovary directly into mFHM containing the final concentration of the inhibitor treatment, then transferred directly into maturation medium supplemented with the same concentration of the chemical inhibitors. Prior to release of COCs, each ovary was cut into 2 pieces and the 4 ovarian pieces from each donor female were allocated to different treatments. In other experiments, COC were released into media without Src inhibitor, with or without 300 μM cAMP according to the individual experimental protocol (see below). While most experiments were conducted with KSOM_MAT (see below) as a semi-defined culture medium, in vitro maturation media for the FYN −/− and their control COC were supplemented with 5% fetal bovine serum to provide optimum conditions for cumulus expansion. In experiments that used oocytes without cumulus cells, COC were collected as stated then cumulus cells manually removed by repeated pipetting with a pulled glass pipette.
Pharmacological treatment of oocytes
To test the effects of Src-family PTK chemical inhibitor on meiosis, oocytes with or without their companion cumulus cells were cultured for 16–17 h in KSOM-MAT (KSOMAA (Chemicon-Millipore, Billerica MA (Biggers et al., 2000)) supplemented with 5 mM glucose (Biggers and McGinnis, 2001), 1 mM glycyl-glutamine, 0.23 mM pyruvate, 4 mg/ml BSA, 0.6 mM L-cysteine, 0.5 mg/ml D-glucosamine, 0.02 μM ascorbate, 1% insulin-transferrin-selenium (ITS), 0.2 IU/ml recombinant human FSH (Serono Reproductive Biological Institute) and 10 ng/ml EGF Calbiochem, San Diego CA). SKI606 (Calbiochem, San Diego CA) was prepared as 10 mM stock solution in DMSO and stored −20°C. Media were prepared fresh on the day of oocyte collection and pre-equilibrated in a humidified (6%CO2, 5%O2 and 89%N2) incubator (Sanyo) for at least 2 h before oocyte culture. Denuded oocytes were matured in groups of <10 per well in 60-well NUNC Terasaki plates with 10.5 μl medium over layered with 3 μl oil (0.22 μm sterile filtered Sigma Embryo Tested Mineral Oil stored in the dark; cat. M8410). Intact COC were cultured in 30–50 μl drops covered with oil in NUNC 4-well plates. After 16–17 h in vitro maturation, oocytes were fixed for labeling and confocal image analysis.
Fixation and immunohistochemical staining
Methods for fixation and immunohistochemistry were similar to those previously reported (McGinnis et al 2007). Briefly, oocytes and COC were fixed for 10 min at room temperature in FHM medium with 3% paraformaldehyde followed by 30 min at 35°C in 2% formaldehyde microtubule stabilization buffer (MTSB-XF (Messinger and Albertini, 1991)). After fixation, COC were transferred into wash solution (McGinnis et al 2007) and held overnight at 4°C. All fixatives and wash solutions were supplemented with 40 μM phenylarsine oxide, 100 μM sodium orthovanadate and 10 μM okadaic acid to inhibit phosphatase activity. Antibodies used included Clone 28 antibody (Biosource International, Camarillo CA USA) to localize activated forms of Src-family PTKs, anti-phosphotyrosine antibody (clone 4G10, Upstate, Lake Placid NY, USA), α and β tubulin (Sigma). All of these were mouse monoclonal antibodies. YOL 1/34 rat monoclonal α tubulin (Abcam, Cambridge MA). Secondary antibodies were Alexa 488 or Alexa 568 (goat anti-mouse or goat anti-rat depending on the source of the primary antibody; Molecular Probes, Eugene OR). Oocytes were labeled with primary antibodies at 35°C for 1h or overnight at 4°C followed by secondary antibody for 1h. After secondary labeling, oocytes were transferred to a wash solution containing 1 μg/ml Hoechst 33258 with or without 1:100 Alexa 568-phalloidin and stored in the dark overnight at 4°C. Oocytes and COC were mounted the following morning and imaged (mounting medium consisted of 1:1 glycerol: PBS supplemented with 5 mg/ml sodium azide and 1 μg/ml Hoechst 33258). All chemicals, hormones and reagents were purchased from Sigma Chemical Company, St. Louis, MO unless otherwise stated.
siRNA Knock-down of FYN PTK
COC were collected in mFHM supplemented with 300 μM cAMP to prevent GVBD. Most of the cumulus cells were removed by brief exposure to 0.3 mg/ml hyaluronidase and gentle pipetting with a fine glass pipet. FYN siRNA (Santa Cruz #sc-35425) and a 20–25 nt non-targeting scrambled control siRNA (Santa Cruz #sc-37007 & sc-36869) were prepared with supplied diluent at a concentration of 100 μM. Immediately before injections, siRNA was thawed and centrifuged at 16,000g for 10 min at 4°C then back loaded into 0.3 μm Egg-Jek needles (MicroJek, Kansas City, KS). Injections were performed on an inverted Nikon Eclipse TE2000-S with an Eppendorf FemtoJet injection system. Preliminary studies were conducted to determine the optimum siRNA concentration. Three concentrations were tested including, 0.7, 1.40 and 2.8 μM. At 2.8 μM, control siRNA caused a reduction in the percentage of oocytes maturing to MII therefore this concentration was considered too high. The lower two concentrations of the control siRNA produced no measurable inhibition of maturation therefore 1.4 μM was selected for use in these studies. Following completion of siRNA microinjections, oocytes were transferred to Terasaki plates and culture for 4–5 h in KSOM-MAT supplemented with 300 μM cAMP to maintain GV arrest and to allow for siRNA inhibition of endogenous mRNA. Oocytes were examined at the beginning and end of this culture and graded for presence of a visible GV. Following this culture, oocytes were washed without cAMP and matured 17h in KSOM-MAT. The selective knock-down of Fyn was examined by semi-quantitative RT-PCR for both Fyn and Yes kinases.
Semi-quantitative RT-PCR
Groups of 10 oocytes (Fyn siRNA, control siRNA and non-injected) were dissolved into 500 μl of TRIzol-Reagent (Invitrogen) and stored at −80C. A known concentration of Rabbit αGlobin mRNA was added to each tube of oocytes (1.0 pg/oocyte) in TriReagent. The methods for cDNA preparation and RT-PCR were as previously published (Barrett and Albertini, 2007). Primers for Fyn and Yes kinases were generated using Primer Express 2.0 from Applied Biosystems (Fyn5′AGT GCC ATA CCC AGG CAT GA; Fyn3′GTG GGC AGG GCA TCC TAT AG; Yes5′GCT TCC ACA GCT GGT TGA TAT G; Yes3′AGA TCT CGG TGA ATA TAG TTC ATT CTT TC; Integrated DNA Technologies, Coralville IA). Relative levels of mRNA as determine by RT-PCR were statistically compare by t-test. P-value of less than 0.05 was considered significant.
Imaging and data analysis
Oocytes were imaged by serial z-sections (1–2 μm depth) on a Zeiss LSM510 confocal microscope. Serial z-sections were used to establish 3-dimensional relationships between the oolema, chromatin, meiotic spindle, first polar body and companion cumulus cells. Statistical analysis of meiotic maturation was performed using SPSS software (SPSS Inc, Chicago IL). Data were analyzed by ANOVA followed by Bonferonni post-hoc comparisons for experiments with 3 or more treatments. P-value of less than 0.05 was considered significant.
RESULTS
Activated Src-family kinases localize to microtubules in mouse oocytes throughout meiotic maturation
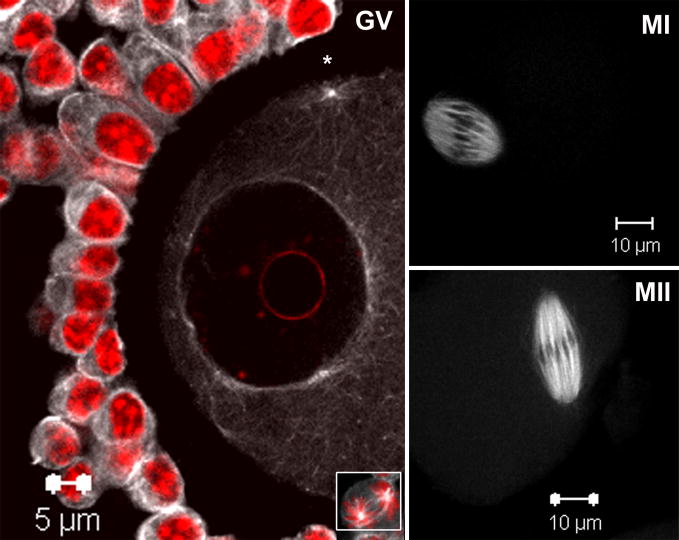
Previous work had demonstrated that activated SFKs were concentrated in close association with microtubules of meiotic spindles in MII oocytes (McGinnis et al., 2007). While Fyn kinase has been shown to localize in the region of premetaphase spindle (Talmor-Cohen et al., 2004b), the specific association of activated SFKs to microtubules at the germinal vesicle stage oocyte has not been demonstrated (Zheng et al., 2007). Therefore, as an initial step we sought to determine the localization of activated SFKs during the early stages of meiotic maturation. To accomplish this, we used a phosphorylation site-specific antibody (clone 28) that recognizes the activated (dephosphorylated) Y527 in the carboxyl tail region of Src-family proteins (Kawakatsu et al., 1996). Epitope distribution was determined by confocal fluorescence microscopy in oocytes fixed following 0, 8 and 16 hours of in vitro maturation as seen in fig. 1. In germinal vesicle stage immature oocytes, clone 28 staining was observed in a pattern that has been associated with cytoplasmic microtubules that surround the GV (Messinger and Albertini, 1991) as well as those emanating from cortical microtubule organizing centers (* MTOCs; fig. 1 GV). Clone 28 also labeled cumulus cells associated with the zona pellucida with staining being disposed along the cumulus cell surface and throughout the cytoplasm. Mitotic figures are commonly seen in the cumulus and in dividing cells, a prominent spindle labeling was readily apparent (fig. 1, insert). In oocytes at metaphase of meiosis-I or II (fig. 1 MI and MII), activated Src-family PTKs were distributed throughout the meiotic spindles in a pattern not unlike that seen in our previous study of MII oocytes (McGinnis et al., 2007). These findings clearly indicate that the clone 28 epitope is specifically expressed in a microtubule-associated pattern throughout all stages of oocyte maturation in mouse oocytes.
Fig. 1. Activated SFKs distribute in microtubule-like patterns at the GV, MI and MII stages of meiosis.
Germinal vesicle stage oocytes were collected from PMSG primed mouse ovaries and matured in vitro for 0, 8 or 16 hours followed by fixation. Oocytes were labeled with a monoclonal antibody against activated Src-family PTKs (clone 28) and detected with Alexa-488-goat anti-mouse IgG (white) and co-labeled with the DNA dye Hoechst 33258 (red). Active SFKs are distributed in microtubule-like patterns consistent within oocytes and companion cumulus cells; see mitotic spindle microtubule labeling in cumulus cell (GV inset). Within GV stage oocytes, SFKs localized to cytoplasmic microtubule arrays surrounding the nuclear envelope as well as cortical microtubule organizing centers (*). At MI and MII, SFKs localize primarily to microtubules of the metaphase spindle.
Chemical inhibition of Src-family kinases s during meiotic maturation causes a dose dependant failure of progression through metaphase of MI
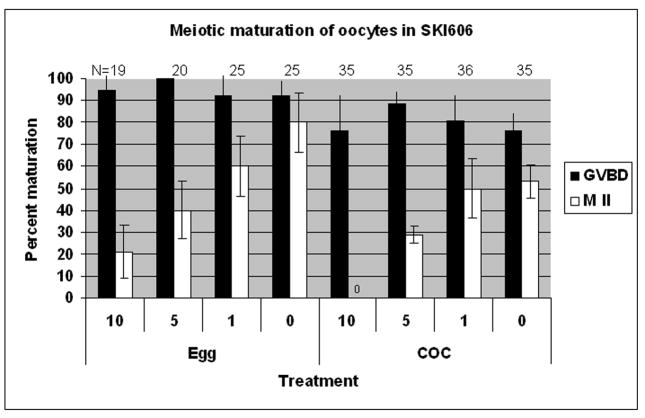
To determine whether meiotic progression requires SFK activity, cumulus enclosed or denuded oocytes were matured in the presence of the inhibitor, SKI606. COC collected from ovaries of eCG primed mature CF1 female mice were released directly into media containing 1, 5 or 10 μM SKI606 or the solvent DMSO as a control. SKI606 is a new and highly selective inhibitor of Src-family kinases and the closely related Abl kinases (Boschelli et al., 2004; Boschelli et al., 2001) and has been used previously in studies of SFK activities in mouse zygotes (McGinnis et al., 2007). The three concentrations of SKI606 were selected on the basis of preliminary dose response experiments (not shown) and our previously published studies with pronuclear stage zygotes (McGinnis et al., 2007). SKI606 had no effect on the ability of oocytes to resume meiosis (GVBD 75–100% in all groups), however, maturation through metaphase-I was significantly reduced as seen in fig. 2A. Exposure of either denuded oocytes (Egg) or cumulus enclosed oocytes (COC) to concentrations of SKI606 at 5μM or greater caused approximately 50% maturation failure. Oocytes that were enclosed by cumulus exhibited a similar response to SKI606. Oocytes matured in the continuous presence of the SFK inhibitor and progressed to metaphase-II exhibited marked defects in spindle and chromatin organization (fig. 2B). Notably at 5 and 10 μM concentrations, all of the oocytes that matured to MII in the presence of SKI606 were distinctly abnormal as detailed below. The effect of SKI606 was reversible (table 1) since oocytes exposed to the inhibitor for 5 h followed by wash-out and 17h of culture progressed to MII at a frequency that was 87% of that observed in controls (P>0.05).
Fig. 2. Chemical inhibition of SFKs during maturation reduces meiotic potential and induces metaphase chromosome alignment and spindle errors.
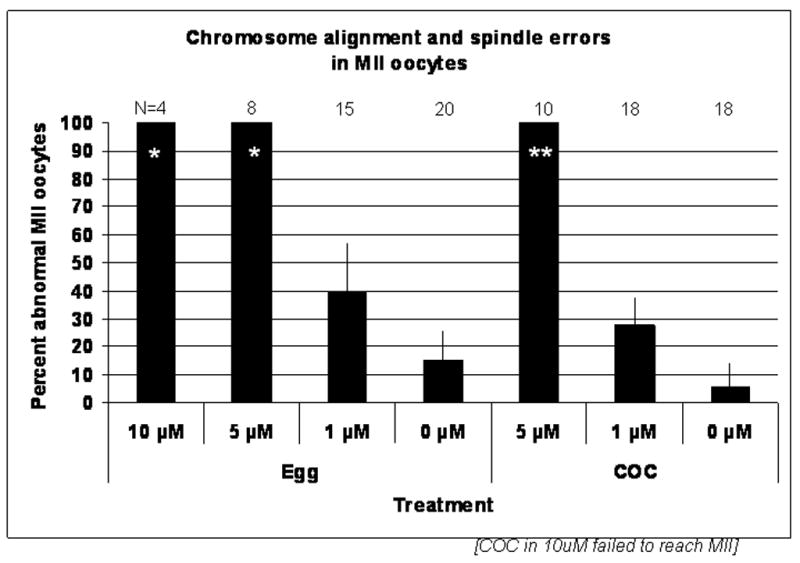
COC were released from the ovaries of PMSG primed female CF1 mice at 40h post-hCG then cultured in KSOM-MAT medium supplemented with SFK inhibitor SKI606 for 17h. The concentration range of 0–10 μM chosen was based on previous studies (McGinnis et al., 2007). (A) Inhibition of SFKs blocked meiosis at metaphase I in a dose dependant manner in both cumulus intact (COC) and denuded oocytes (Egg). No significant effect on the number of oocytes that underwent GVBD (black bars) was observed. However, maturation to metaphase-II (white bars) was inhibited. Cumulus-enclosed oocytes were more sensitive to inhibitor, with none maturing to MII in the 10μM dose and maturation in 5 μM being significantly lower than 1 μM or 0 μM (P<0.05). The ability for denuded oocytes to mature was also dose dependant with significantly fewer oocytes maturing to meiosis II in medium with 10 μM as compared to 0 or 1 μM. (B) SKI606 treated oocytes that progressed to metaphase II exhibited chromosome and spindle abnormalities. Of those oocytes that progressed to metaphase II in 5 or 10 μM (*) SKI606, 100% were abnormal as compared to the 1 and 0 μM concentrations.
Table 1.
The short-term exposure inhibitory effects of SKI606 are reversible.
| Exposure Time | N | %GVBD | %MII |
|---|---|---|---|
| 5h DMSO | 12 | 100a | 100c |
| 5h SKI | 35 | 80a | 87c |
Treated oocytes matured through GVBD to metaphase-II at rates similar to controls (P>0.05).
Inhibition of Src-family kinases during meiosis results in spindle and chromosomal abnormalities
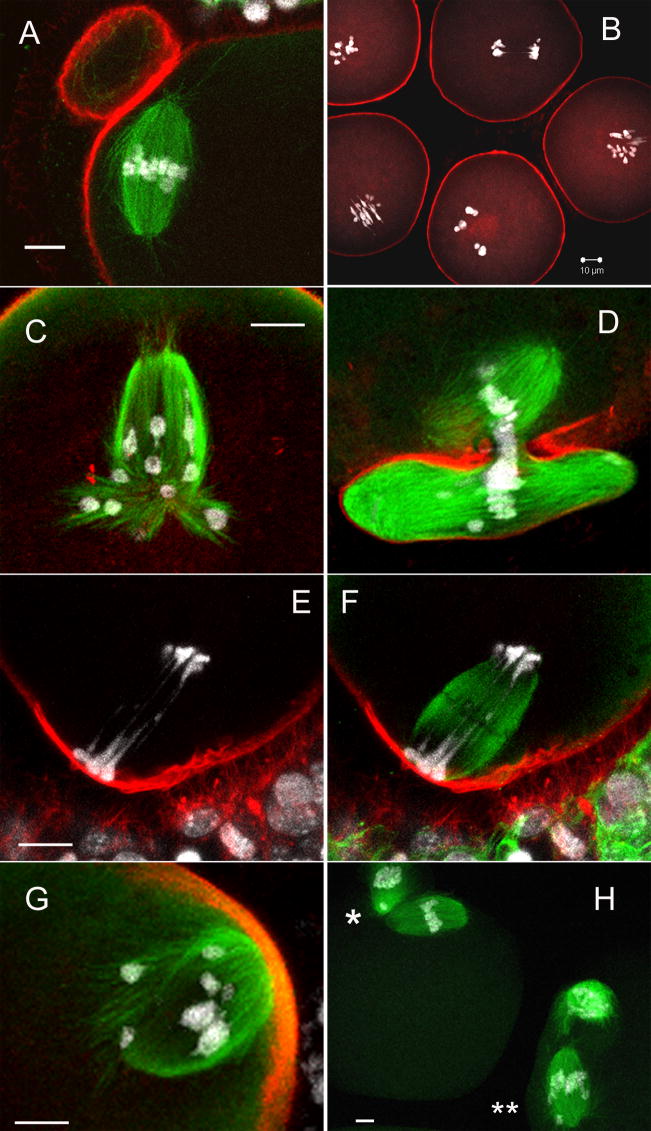
To ascertain how SFK inhibition was influencing meiotic progression, confocal microscopy was performed on control and treated oocytes matured under the conditions described above. While phalloidin (f-actin) staining failed to reveal major changes in actin organization within oocytes, tubulin and DNA labeling revealed a range of defects in chromatin and microtubule disposition (fig. 3). Control oocytes matured to metaphase-II exhibited extruded polar bodies and chromosomes aligned on a bipolar meiotic spindle (fig. 3A). SKI606 induced abnormalities that appeared to reflect disruption of the interactions between chromosomes and spindle microtubules. For example, displaced chromosomes were found on MI (fig. 3B, 3G) or MII spindles (fig. 3H**) and in many cases bivalent segregation failed to occur at anaphase or telophase of MI (fig. 3B, 3E–F). Thus, MI stage oocytes entered anaphase without disengaging homologues as evidenced by the presence of elongated anaphase spindles contained unresolved bivalents along nearly the entire length of the spindle (fig. 3E, 3F). Telophase oocytes also exhibited lagging chromosomes trapped within the contractile ring of forming polar bodies (fig. 3D, 3H*). In addition to the positioning of chromosomes, SKI606 induced a variety of severe spindle aberrations during meiosis. Many spindles were loosely organized and irregular in shaped, extending microtubule bundles towards displaced chromosomes (fig. 3G). Some oocytes exhibited segregated chromosomes in anaphase, but failed to extrude a polar body (fig. 3C). In such cases, oocytes with two spindles were formed, one of which was monopolar and the other being bipolar. In the example shown here (fig. 3C), the monopolar spindle was attached to a single pole of the bipolar spindle. However, this characteristic was not consistent for all oocytes that failed to complete cytokinesis. In most cases, bipolar and monopolar spindles were completely separate entities. Monopolar spindles displayed microtubule bundles that extended up to and along the oolema (not shown). Cortical actin localization appeared normal: As has commonly been seen, chromatin subjacent to the oolema was associated with cortical f-actin caps (fig. 3E–H) and f-actin density was increased in cortical regions involved in cytokinesis and the polar body extrusion (fig. 3D). The abnormalities observed after continuous exposure to SKI606 indicate that while spindle morphogenesis following GVBD appeared to occur on schedule relative to controls, treated oocytes are impaired in their ability to support chromosome segregation during the metaphase-anaphase transition of meiosis-I.
Fig. 3. Inhibition of Src-related kinases with SKI606 induced abnormal spindles and misalignment of chromosomes during meiosis.
Denuded oocytes (eggs) and intact COC matured 17h in 0, 1, 5 or 10 μM SKI606 (fig. 2) were fixed and co-labeled with monoclonal mouse anti-αβ tubulin (microtubules; green) and goat anti-mouse Alexa-488, phalloidin-Alexa-568 (f-actin; red) and Hoechst 33258 (DNA; white) and oocytes were confocal imaged and examined as described previously. Three-dimensional reconstructions were used to examine oocyte meiotic status and the associations of tubulin, actin and DNA. The majority of control oocytes (A = 0 μM SKI606) matured and arrested at metaphase of meiosis II with normal spindle morphology, chromatin aligned on the metaphase plate and an extruded polar body. Oocytes exposed to SKI606 arrested at various stages of meiosis, many with displaced chromosomes and spindle microtubules that branch abnormally in seeming attempts to enclose these aberrant chromosomes (B–H). Fewer abnormal oocytes occurred in the lower doses of SKI606 as compared to the higher doses, however the general types of abnormalities were the same across all concentrations of the inhibitor (malformed spindles and wayward chromosomes: B, C, G = 10 μM; D, E, F = 5 μM; H = 1 μM SKI606, respectively). Panels E (tubulin label turned-off) and F (with tubulin (green)) are the same oocyte. Both images are shown to demonstrate the stretched chromosomes that failed to separate (E) even though the spindle microtubules have attempted to elongate and undergo anaphase (F). Panel H demonstrates two oocytes cultured in low (1 μM) SKI606 which extruded polar bodies but have displaced chromosomes. One oocyte has a chromosome that failed to segregate and was trapped in the constriction site between the polar body and the oocyte (*) while the second oocyte has multiple chromosomes displaced along the spindle (**). [scale bar = 10 μm]
Src-family kinases are required for maintenance of meiotic arrest
In the above inhibitor study, it was noted that oocytes exposed to SKI606 tended to undergo GVBD earlier than controls suggesting that meiotic resumption might be accelerated by this treatment. To further test this with better meiotic synchrony, we cultured immature oocytes with 300 μM dbcAMP which has been shown to prevent GVBD for up to 6 hours even in the presence of the maturation stimulators FSH and EGF (Downs and Chen, 2008). Thus, to establish whether SFKs participate in maintenance of meiotic arrest, we cultured oocytes in KSOM-MAT (FSH and EGF supplemented media) with 300 μM dbcAMP with or without 10 μM SKI606 for 5 hrs, washed samples in drug free medium, and allowed maturation to proceed for17h (in absence of dbcAMP). Following wash-out and IVM, both control and SKI606-treated oocytes matured to normal metaphase-II (93% with SKI606 vs 100% controls, table 2) confirming the reversibility of SKI606. However, inhibition of SFKs in the presence of dbcAMP either permitted or induced the resumption of meiotic maturation within 5 hr of treatment (60% GVBD with SKI606 vs 0% controls) suggesting that SKI606 sensitive kinases may participate in GV arrest.
Table 2.
Precocious meiotic resumption of oocytes cultured with Src inhibitor.
| Exposure Time | N | %GVBD | %MII |
|---|---|---|---|
| 5h cAMP | 10 | 0a | 100c |
| 5h SKI+cAMP | 43 | 60b | 93c |
Oocytes cultured with the Src inhibitor and dbcAMP resumed meiosis and initiated GVBD while control oocytes remained arrested at GV (a,b) P>0.05. Following wash-out from the dbcAMP both control and SKI606 treated oocytes matured to MII(c) P>0.05.
Injection of FYN siRNA into GV stage oocytes demonstrates a specific requirement for Fyn kinase during meiotic maturation
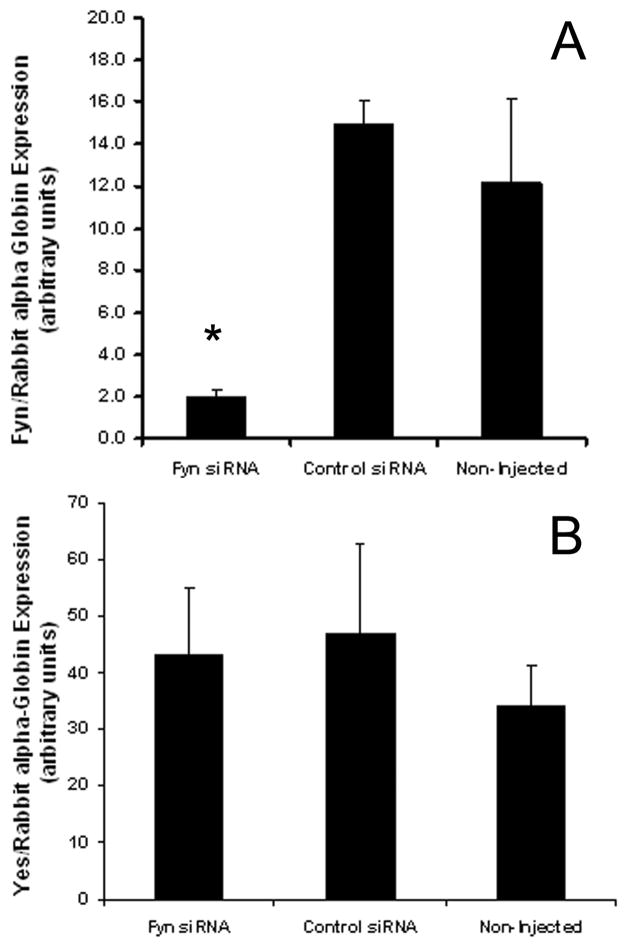
One drawback of the pharmacological approach described above is that SKI606 inhibits the activity of all members of the Src-family and Alb-family kinases. Since FYN kinase is the most highly expressed of the Src-family members in mouse oocytes (Novartis Mouse Gene Atlas; https://biogps.gnf.org), we sought to more specifically test the role of Fyn kinase in oocyte maturation. Mouse oocytes arrested at the GV stage using dbcAMP were injected with either FYN siRNA or a 20–25 nt non-targeting control siRNA. Following injections, oocytes were cultured for 4–5h in complete maturation medium supplemented with 300 μM dbcAMP to prevent resumption of meiosis and to allow time for depletion of FYN mRNA. GV stage oocytes were then washed free of dbcAMP and matured in vitro for 17h. After 17h of culture, oocytes were fixed and labeled for tubulin, actin and DNA for confocal microscopy as above. To confirm the knock-down and specificity of the Fyn siRNA, sets of 10 oocytes were pooled after 17h of culture and tested for mRNA levels of Fyn and Yes kinases. Yes kinase is a Src-family member closely related to Fyn and is the second highest expressed of the SFKs in mouse oocytes (Novartis Mouse Gene Atlas; https://biogps.gnf.org). Kinase mRNA levels were normalized against a known concentration of rabbit alpha-globin mRNA. The Fyn siRNA caused a significant (~80%, P<0.05) decrease in Fyn mRNA as compared to scrambled control siRNA injected or non-injected oocytes (n=4, 2 and 4 replicates, respectively; fig. 4A). There was no difference in the level of Fyn mRNA between control siRNA injected versus non-injected oocytes and no effect of Fyn siRNA on the levels of Yes kinase demonstrating the specificity of the Fyn knock-down (fig. 4B).
Fig. 4. Microinjection of Fyn siRNA significantly reduced levels of Fyn mRNA.
Oocytes injected with Fyn siRNA were matured 17h then pooled in sets of 10 and tested by semi-quanititative RT-PCR for levels of Fyn mRNA and the closely related Yes mRNA. Injection of Fyn siRNA resulted in a significant (~80%; *P<0.05) knock-down of Fyn mRNA as compared to scrambled control siRNA injected and non-injected oocytes (A). Fyn siRNA had no effect on the levels of Yes kinase mRNA (B). (n = 4, 2, 4 replicates, respectively)
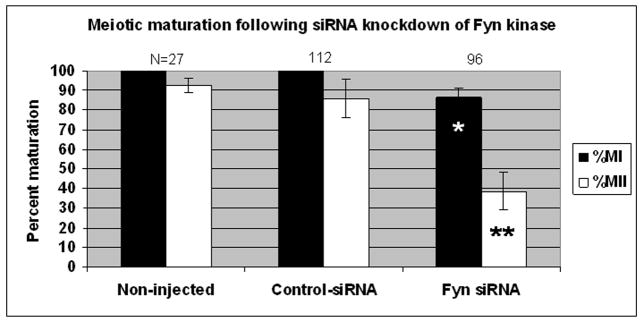
Oocytes injected with FYN siRNA undergo GVBD but exhibited a reduced capacity to progress to metaphase-I (83% vs 100% of controls; P<0.05, fig. 5). Few oocytes were able to mature to metaphase-II (39% vs 86%; P<0.05). Injection of FYN siRNA did not induce premature resumption of meiosis in the presence of dbcAMP nor were overt chromatin or spindle organizational defects observed (not shown). The selectivity of FYN siRNA relative to controls implies a direct role for FYN in meiotic progression of mouse oocytes. To further test this, we next examined the phenotypes of oocytes collected from FYN knockout animals.
Fig. 5. Fyn PTK activity is required for meiotic maturation of mouse oocytes.
Oocytes injected with FYN siRNA were held arrested at the GV stage in 300 μM cAMP for 4–5 h, washed in cAMP-free media, and cultured in maturation media for 16–17 h. Controls included injection with an equal concentration of scrambled control siRNA or processing in the above solutions without injection. Seven replicates with siRNA were conducted using both types of controls. Oocytes were labeled for microtubules and DNA as described above (fig. 3) and scored to determine meiotic stage. Injection of FYN siRNA into GV stage oocytes significantly decreased meiotic maturation since fewer FYN siRNA oocytes reached metaphase of MI compared to control siRNA injected or non-injected oocytes (83% versus 100% and 100%, respectively; *P<0.05). Maturation to MII was also impaired with only 39% of FYN siRNA injected oocytes reaching metaphase-II as compared to control siRNA and non-injected oocytes (86% and 93%, respectively; **P<0.05).
FYN (−/−) mice ovulate oocytes exhibiting defects in meiotic progression and spindle organization
To further examine the role of Fyn kinase during oocyte meiotic progression, we compared oocyte maturation of normal and FYN (−/−) mice both in vivo and in vitro. Mature females were superovulated and oocytes were collected from oviducts 16 h post-hCG. The total number of oocytes ovulated by FYN (−/−) females were not different from control females (17 and 22, respectively; table 3). While all ovulated oocytes isolated from both FYN (−/−) and control animals had undergone GVBD (68 and 111 oocytes, respectively), only 85% (58/68) of oocytes from FYN (−/−) animals had matured to metaphase-II compared to 99% (110/111) of controls (P<0.05%). Moreover, ovulated FYN (−/−) oocytes exhibited a slightly higher percentage of spindle and chromosome alignment abnormalities relative to controls (19% (11/58) versus 4% (4/110), respectively) although this difference was not statistically significant. The types of abnormalities detected in oocytes from Fyn (−/−) animals were associated with chromosome alignment and meiotic spindles (fig. 6). Since ovulated FYN (−/−) oocytes exhibited reduced maturation, we next tested maturation using our in vitro culture system. This provided a more consistent controlled environment under which the maturation competence of FYN (−/−) oocytes could be ascertained without in vivo variability due to endocrine and/or ovarian factors.
Table 3.
FYN (−/−) mice ovulate oocytes with meiotic progression defects
| Strain | # Donors | # Oocytes | %GVBD | %MII | % abnormal MII |
|---|---|---|---|---|---|
| B6/129 | 5 | 111 | 100 | 99a | 4c |
| Fyn KO | 4 | 68 | 100 | 85b | 19c |
All ovulated oocytes initiated GVBD. A significant percentage of ovulated FYN (−/−) oocytes failed to reach metaphase-II and were blocked at metaphase-I (a,b) P<0.05. There was a tendency for FYN (−/−) oocytes to exhibit abnormal spindle and chromatin alignment however this difference was not statistically significant (c) P>0.05.
Fig. 6. Superovulated FYN (−/−) oocytes exhibit misaligned chromosomes.
Oocytes were collected from superovulated FYN (−/−) and wildtype control mice 16h post-hCG. Control mice ovulated metaphase-II stage oocytes, 110/111, 99%, whereas only 85% (58/68) of FYN (−/−) had reached metaphase-II. Analysis of cytoskeleton and chromatin organization showed normal chromosome alignment in 99% of control MII oocytes (B6/129), however 19% of the MII and 6% of MI stage FYN (−/−) oocytes exhibited misaligned chromosomes. Differential interference contrast (DIC) images of ovulated oocytes B6/129 (MII) and FYN KO (MI) labeled with DNA dye Hoechst 33258 (white).
FYN knock-out mice exhibit a meiosis I defect
Fully grown oocytes (with or without cumulus cells) were collected from the ovaries of mature females at 44 h post-eCG similar to previous experiments. More oocytes from antral follicles were obtained from FYN (−/−) mice (n=3) as compared to normal control females (n=4; 53 oocytes versus 26 oocytes per donor, respectively). COC from FYN (−/−) mice matured in vitro for 16 h exhibited cumulus expansion similar to wildtype controls indicating that cumulus cell expansion is not influenced by the loss of Fyn.
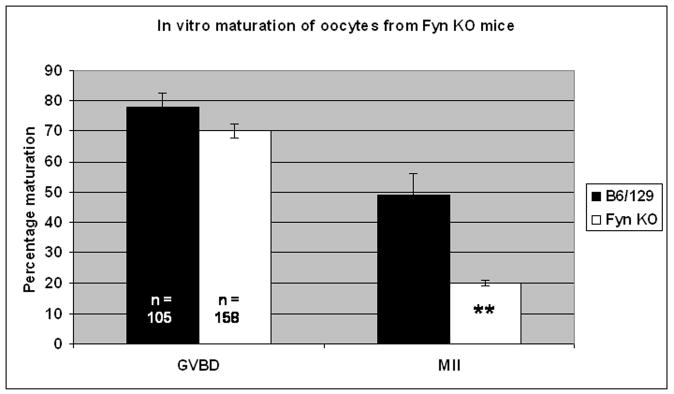
The ability of FYN (−/−) oocytes to mature in vitro was significantly reduced compared to controls. FYN (−/−) oocytes progressed through GVBD but arrested at various stages between GVBD and metaphase-I. Only 20% (32/158) of FYN (−/−) oocytes matured to metaphase-II as compared to 49% (51/105) of controls (fig. 7). Of those oocytes from FYN (−/−) females that matured to metaphase-II, 22% of these (7/32) exhibited abnormal spindle formation and/or chromosome misalignment compared to only 4% (2/51) of controls although this difference was not statistically significant. FYN (−/−) oocytes that blocked at metaphase-I also demonstrated spindle and chromosome alignment errors but at a low incidence (5/78, 6%). No cytoskeletal defects were observed in metaphase-I arrested oocytes from control animals (0/31). The errors observed in FYN (−/−) oocytes resembled those identified in our earlier chemical inhibitor studies with misaligned chromosomes and malformed spindles. Thus, three distinct experimental strategies including biochemical inhibition of Src-related kinases, as well as siRNA knock-down, and gene knockout of Fyn kinase have yielded results consistent with a role for SFKs in meiotic progression, chromosome segregation and spindle function in mouse oocytes.
Fig. 7. Oocytes from FYN (−/−) mice exhibit reduced meiotic potential.
Oocytes and COC from FYN (−/−) and wildtype controls collected at the GV stage were matured in vitro for 16hrs and subjected to fluorescence analysis as before. FYN (−/−) oocytes exhibited reduced meiotic potential as indicated by the fact that while FYN (−/−) oocytes underwent GVBD to comparable levels as wildtype controls (70 % versus 76%, respectively), significantly fewer FYN (−/−) oocytes achieved MII (20% versus 49%; **P<0.05).
DISCUSSION
Oocytes prepare for fertilization and later development by undergoing a programmed series of maturational events in response to an ovulation-inducing surge of LH. At the level of the ovarian follicle, prominent changes in several signaling pathways occur during ovulation that ensure the coordinate induction of ovum release, cumulus expansion and oocyte maturation. Amongst these signaling pathways, SRC, RAS, and EGF have all been implicated in the terminal differentiation of granulosa cells (Fan et al., 2008; Shiota et al., 2003; Wayne et al., 2007) and preliminary reports have invoked a role for Src within the oocyte itself with reference to meiotic maturation and egg activation (McGinnis et al., 2007; Mehlmann and Jaffe, 2005; Meng et al., 2006; Talmor-Cohen et al., 2004a; Zheng et al., 2007).
The present studies aimed to better define the activities of SFKs during meiotic maturation in mouse oocytes by taking advantage of the experimental tractability of this model system. A series of in vitro maturation experiments showed that SFKs function in meiotic progression through the metaphase-anaphase transition of meiosis-I with the most prominent defects being a failure to segregate homologous chromosomes. From pharmacological inhibition, FYN siRNA knockdown, and the use of FYN knockout mice, resolution of bivalents and progress to metaphase-II appears to require the activity of SFKs, and in particular FYN kinase. The fact that genetic depletion of FYN kinase activity caused a similar series of meiosis defects both in vivo and in vitro, involving failure of chromosome disjunction during anaphase and abnormal organization of spindle microtubules, suggests that FYN assists in the coordination of karyokinesis and cytokinesis during meiotic maturation and that additional effectors such as other SFKs are likely to integrate meiotic cell cycle progression in this system. Fyn (−/−) mice are viable but females produce only 2–3 litters after which reproduction fails (Kinsey lab records). Interestingly, histological examination of ovaries following superovulation found antral follicles in Fyn (−/−) females that failed to ovulate (not shown). This may explain the disparity in the number of oocytes obtained between our in vitro maturation studies where oocytes are manually extracted from all antral follicles as compared to ovulated oocytes retrieved from the oviducts. Fyn (−/−) ovaries yielded far more oocytes than wildtype ovaries and a major proportion of the Fyn (−/−) oocytes were incapable of normal oocyte maturation, while the superovulated oocytes retrieved from the oviducts were more similar between the Fyn (−/−) and control animals. Studies are currently ongoing in our laboratory to better define these issues in the knock-out mouse.
The mechanism of Fyn action in chromosome segregation and spindle function may involve its close association with spindle microtubules. Previous studies on rat oocytes demonstrated localization of Fyn kinase to microtubule-containing structures (Talmor-Cohen et al., 2004b; Talmor et al., 1998). The fact that FYN is the most highly expressed SFK in mouse oocytes (Genomics Institute of the Novartis Research Foundation (“GNF”), https://biogps.gnf.org (Su et al., 2002)), together with our earlier finding that activated SFKs were associated with spindle structures after fertilization (McGinnis et al., 2007) led us to further define the functional significance of this association during oocyte maturation. The results presented here indicated that this spindle-associated Fyn is highly active since it was detected with the Clone 28 antibody. This association with microtubules continued during spindle morphogenesis following GVBD and persisted within spindles throughout meiotic maturation. Activated SFK localization was also maintained with MTOCs at all stages consistent with previous studies using pericentrin and MPM2 which demonstrated Ser/Thr phospho-proteins associated with MTOCs throughout meiotic maturation (Messinger and Albertini, 1991). Talmor, Kinsey and Shalgi (1998) first demonstrated the co-localization of Fyn kinase to the meiotic spindle in mammalian oocytes and later proved the association of Fyn with tubulin in co-immunoprecipitation assays (Talmor-Cohen et al., 2004b). We have now demonstrated activated Src-family kinases associate with meiotic spindles and cytoplasmic microtubules at all stages of meiosis.
SFKs in general and Fyn in particular are known to be involved in somatic cell mitotic cell cycle progression (Roche et al., 1995; Thomas and Brugge, 1997). Direct interactions of Fyn with dynein (Campbell et al., 1998) and γ-tubulin (Kukharskyy et al., 2004; Sulimenko et al., 2006) have been proposed as responsible for mitotic arrest and microtubule stability in a variety of mammalian cell types (Moasser et al., 1999). In the mammalian oocytes, γTubulin is involved in spindle positioning and size (Barrett and Albertini, 2007). Together with our current results this suggests a possible relationship between Fyn kinase signaling and γ-tubulin in the regulation of the meiotic spindle. Moreover, the presence of monopolar spindles and displaced chromosomes further implies that as yet unresolved forces exerted during bivalent attachment to the spindle may be under the local control Src-related kinases integrated into the meiotic spindle.
We have also shown that inhibition of Src-related kinases with the chemical inhibitor SKI606 and siRNA of Fyn kinase prevented the progression of meiosis from MI to metaphase-II. This finding contrasts those reported by (Zheng et al., 2007) in which mouse oocytes matured in the presence of the SFK inhibitor (PP2) were unable to undergo GVBD. Our studies using the inhibitor SKI606 did not reveal a significant inhibitory effect on GVBD. In fact, we found that under meiosis arresting conditions, this drug induced the resumption of meiosis (Table 2). The variance in the results of Zheng et al. (2007) and this work may be due in part to differences in the specificity or action of the inhibitors. For example, SKI606 represents a new generation of quinolinecarbonitrile derivatives with high specificity for SFKs (Boschelli et al., 2004). This compound inhibits Src in vitro with an IC50 of 1.2 nM, while having low affinity for other kinases such as receptor protein tyrosine kinase ErbB-2 (IC50 of 2.6 μM) and Ser/Thr kinase Cdk4 (IC50 of 19 μM) (Boschelli et al., 2001). The affinity of SKI606 is similar between Src and other family members such as Fyn (Boschelli et al., 2001). The Src inhibitor PP2 has an in vitro IC50 of 5 nM for Fyn kinase (Hanke et al., 1996). Like PP2 (Clark and Peterson, 2003), SKI606 also inhibits the Src-related Abl kinase with an in vitro IC50 of 1.0 nM. While the general activities of SKI606 and PP2 are similar, slight differences in affinity and selectivity may account for the varied effects reported here and elsewhere. More likely, variations in chemical inhibitor results may take their origins in IVM culture protocols and/or differences in mouse strains used since mouse strain variations have been reported for both in vivo and in vitro matured oocytes (Ibanez et al., 2005a; Ibanez et al., 2005b). Additionally, Zheng and colleagues (2007) cultured naked oocytes in basal M2 medium without supplementation. While basal salt media allow for spontaneous meiotic resumption of cultured oocytes from many mammalian species (Edwards, 1965), these conditions are inadequate for production of oocytes of high developmental potential (Eppig et al., 2000). In our studies we used a modified KSOMAA medium supplemented with factors designed to induce meiotic maturation and cumulus expansion (FSH, EGF, cysteine, glucosamine and ascorbic acid; see methods section). FSH and EGF are known activators of Src-family kinases in somatic and granulosa cells (Cottom et al., 2003; Wayne et al., 2007), therefore media containing these hormones may activate signaling pathways during meiotic maturation that are not operative in a basal salt medium.
In summary, the suppression of Fyn activity caused defects in spindle organization and chromosome segregation that in some cases brought meiotic maturation to a halt whereas in other cases permitted advancement of the cell cycle to metaphase-II albeit with significant distortions in the spindle. The lack of complete penetrance as seen in oocytes from FYN (−/−) animals, implies some level of redundancy in function for ovarian SFKs. Taken together, these results suggests that the primary active Src-family kinase associated with the spindle of the mammalian oocyte is Fyn and that disruption of Fyn kinase leads to failure of meiotic maturation by disrupting the cell cycle dynamics of spindle and chromosome organization.
Acknowledgments
Supported by NICHD 42076, the Hall Family Foundation and the ESHE Fund (D.F.A.) and NICHD 14846 to (W.H.K.). We would like to thank Dr Lane Christenson and the members of his laboratory for assistance with the RT-PCR and Dr Jinping Luo for assistance with the Fyn (−/−) mice.
Abbreviations
- COC
cumulus oocyte complex
- dbcAMP
dibutyryl cAMP
- GVBD
germinal vesicle breakdown
- IVM
in vitro maturation
- MI
meiosis-I
- MII
meiosis-II
- MTOCs
microtubule organizing centers
- SFK
Src-family protein tyrosine kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Anderson E, Albertini DF. Gap junctions between the oocyte and companion follicle cells in the mammalian ovary. J Cell Biol. 1976;71:680–686. doi: 10.1083/jcb.71.2.680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett SL, Albertini DF. Allocation of gamma-tubulin between oocyte cortex and meiotic spindle influences asymmetric cytokinesis in the mouse oocyte. Biol Reprod. 2007;76:949–57. doi: 10.1095/biolreprod.106.057141. [DOI] [PubMed] [Google Scholar]
- Biggers JD, McGinnis LK. Evidence that glucose is not always an inhibitor of mouse preimplantation development in vitro. Hum Reprod. 2001;16:153–163. doi: 10.1093/humrep/16.1.153. [DOI] [PubMed] [Google Scholar]
- Biggers JD, McGinnis LK, Raffin M. Amino acids and preimplantation development of the mouse in protein-free potassium simplex optimized medium. Biol Reprod. 2000;63:281–93. doi: 10.1095/biolreprod63.1.281. [DOI] [PubMed] [Google Scholar]
- Boschelli DH, Wang YD, Johnson S, Wu B, Ye F, Barrios Sosa AC, Golas JM, Boschelli F. 7-Alkoxy-4-phenylamino-3-quinolinecar-bonitriles as dual inhibitors of Src and Abl kinases. J Med Chem. 2004;47:1599–601. doi: 10.1021/jm0499458. [DOI] [PubMed] [Google Scholar]
- Boschelli DH, Ye F, Wang YD, Dutia M, Johnson SL, Wu B, Miller K, Powell DW, Yaczko D, Young M, Tischler M, Arndt K, Discafani C, Etienne C, Gibbons J, Grod J, Lucas J, Weber JM, Boschelli F. Optimization of 4-phenylamino-3-quinolinecarbonitriles as potent inhibitors of Src kinase activity. J Med Chem. 2001;44:3965–77. doi: 10.1021/jm0102250. [DOI] [PubMed] [Google Scholar]
- Campbell KS, Cooper S, Dessing M, Yates S, Buder A. Interaction of p59fyn kinase with the dynein light chain, TCTEX-1, and colocalization during cytokinesis. J Immunol. 1998;161:1728–1737. [PubMed] [Google Scholar]
- Cho WK, Stern S, Biggers JD. Inhibitory effect of dibutyryl cAMP on mouse oocyte maturation in vitro. J Exp Zool. 1974;187:383–6. doi: 10.1002/jez.1401870307. [DOI] [PubMed] [Google Scholar]
- Clark DD, Peterson BR. Analysis of protein tyrosine kinase inhibitors in recombinant yeast lacking the ERG6 gene. Chembiochem. 2003;4:101–7. doi: 10.1002/cbic.200390001. [DOI] [PubMed] [Google Scholar]
- Cottom J, Salvador LM, Maizels ET, Reierstad S, Park Y, Carr DW, Davare MA, Hell JW, Palmer SS, Dent P, Kawakatsu H, Ogata M, Hunzicker-Dunn M. Follicle-stimulating hormone activates extracellular signal-regulated kinase but not extracellular signal-regulated kinase kinase through a 100-kDa phosphotyrosine phosphatase. J Biol Chem. 2003;278:7167–7179. doi: 10.1074/jbc.M203901200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs SM, Chen J. EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol Reprod Dev. 2008;75:105–114. doi: 10.1002/mrd.20781. [DOI] [PubMed] [Google Scholar]
- Edwards RG. Maturation in vitro of mouse, sheep, cow, pig, rhesus monkey and human ovarian oocytes. Nature. 1965;208:349–51. doi: 10.1038/208349a0. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Freter RR, Ward-Bailey PF, Schultz RM. Inhibition of oocyte maturation in the mouse: Participation of cAMP, steroid hormones, and a putative maturation-inhibitory factor. Dev Biol. 1983;100:39–49. doi: 10.1016/0012-1606(83)90198-7. [DOI] [PubMed] [Google Scholar]
- Eppig JJ, Hosoe M, O’Brien MJ, Pendola FM, Requena A, Watanabe S. Conditions that affect acquisition of developmental competence by mouse oocytes in vitro: FSH, insulin, glucose and ascorbic acid. Mol Cell Endocrinol. 2000;163:109. doi: 10.1016/s0303-7207(99)00247-6. [DOI] [PubMed] [Google Scholar]
- Fan HY, Shimada M, Liu Z, Cahill N, Noma N, Wu Y, Gossen J, Richards JS. Selective expression of KrasG12D in granulosa cells of the mouse ovary causes defects in follicle development and ovulation. Development. 2008;135:2127–2137. doi: 10.1242/dev.020560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Ibanez E, Albertini DF, Overstrom EW. Effect of genetic background and activating stimulus on the timing of meiotic cell cycle progression in parthenogenetically activated mouse oocytes. Reproduction. 2005a;129:27–38. doi: 10.1530/rep.1.00452. [DOI] [PubMed] [Google Scholar]
- Ibanez E, Sanfins A, Combelles CMH, Overstrom EW, Albertini DF. Genetic strain variations in the metaphase-II phenotype of mouse oocytes matured in vivo or in vitro. Reproduction. 2005b;130:845–855. doi: 10.1530/rep.1.00558. [DOI] [PubMed] [Google Scholar]
- Kawakatsu H, Sakai T, Takagaki Y, Shinoda Y, Saito M, Owada MK, Yano J. A new monoclonal antibody which selectively recognizes the active form of Src tyrosine kinase. J Biol Chem. 1996;271:5680–5685. doi: 10.1074/jbc.271.10.5680. [DOI] [PubMed] [Google Scholar]
- Kukharskyy V, Sulimenko V, Macurek L, Sulimenko T, Draberova E, Draber P. Complexes of γ-tubulin with nonreceptor protein tyrosine kinases Src and Fyn in differentiating P19 embryonal carcinoma cells. Exp Cell Res. 2004;298:218–228. doi: 10.1016/j.yexcr.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Liang CG, Su YQ, Fan HY, Schatten H, Sun QY. Mechanisms regulating oocyte meiotic rsumption: Roles of mitogen-activated protein kinase. Mol Endocrinol. 2007;21:2037–2055. doi: 10.1210/me.2006-0408. [DOI] [PubMed] [Google Scholar]
- Lindner HR, Tsafriri A, Lieberman ME, Zor U, Koch Y, Bauminger S, Barnea A. Gonadotropin action on cultured Graafian follicles: induction of maturation division of the mammalian oocyte and differentiation of the luteal cell. Recent Prog Horm Res. 1974;30:79–138. doi: 10.1016/b978-0-12-571130-2.50007-9. [DOI] [PubMed] [Google Scholar]
- McGinnis LK, Albertini DF, Kinsey WH. Localized activation of Src-family protein kinases in the mouse egg. Dev Biol. 2007;306:241–254. doi: 10.1016/j.ydbio.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlmann LM, Jaffe LA. SH2 domain-mediated activation of an SRC family kinase is not required to initiate Ca2+ release at fertilization in mouse eggs. Reproduction. 2005;129:557–564. doi: 10.1530/rep.1.00638. [DOI] [PubMed] [Google Scholar]
- Mehlmann LM, Kalinowski RR, Ross LF, Parlow AF, Hewlett EL, Jaffe LA. Meiotic resumption in response to luteinizing hormone is independent of a Gi family G protein or calcium in the mouse oocyte. Dev Biol. 2006;299:345–55. doi: 10.1016/j.ydbio.2006.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng L, Luo J, Li C, Kinsey WH. Role of Src homology 2 domain-mediated PTK signaling in mouse zygotic development. Reproduction. 2006;132:413–421. doi: 10.1530/rep.1.01151. [DOI] [PubMed] [Google Scholar]
- Messinger SM, Albertini DF. Centrosome and microtubule dymanics during meiotic progression in the mouse oocyte. J Cell Sci. 1991;100:289–98. doi: 10.1242/jcs.100.2.289. [DOI] [PubMed] [Google Scholar]
- Moasser MM, Srethapakdi M, Sachar KS, Kraker AJ, Rosen N. Inhibition of Src kinases by a selective tyrosine kinase inhibitor causes mitotic arrest. Cancer Res. 1999;59:6145–52. [PubMed] [Google Scholar]
- Panigone S, Hsieh M, Fu M, Persani L, Conti M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22:924–936. doi: 10.1210/me.2007-0246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roche S, Fumagalli S, Courtneidge SA. Requirement for Src family protein tyrosine kinases in G2 for fibroblast cell division. Science. 1995;269:1567–9. doi: 10.1126/science.7545311. [DOI] [PubMed] [Google Scholar]
- Schultz RM, Montgomery RR, Belanoff JR. Regulation of mouse oocyte meiotic maturation: Implication of a decrease in oocyte cAMP and protein dephosphorylation in commitment to resume meiosis. Dev Biol. 1983;97:264–273. doi: 10.1016/0012-1606(83)90085-4. [DOI] [PubMed] [Google Scholar]
- Shiota M, Tanihiro T, Nakagawa Y, Aoki N, Ishida N, Miyazaki K, Ullrich A, Miyazaki H. Protein Tyrosine Phosphatase PTP20 Induces Actin Cytoskeleton Reorganization by Dephosphorylating p190 RhoGAP in Rat Ovarian Granulosa Cells Stimulated with Follicle-Stimulating Hormone. Mol Endocrinol. 2003;17:534–549. doi: 10.1210/me.2002-0187. [DOI] [PubMed] [Google Scholar]
- Stein PL, Lee HM, Rich S, Soriano P. pp59fyn mutant mice display differential signaling in thymocytes and peripheral T cells. Cell. 1992;70:741. doi: 10.1016/0092-8674(92)90308-y. [DOI] [PubMed] [Google Scholar]
- Su AI, Cooke MP, Ching KA, Hakak Y, Walker JR, Wiltshire T, Orth AP, Vega RG, Sapinoso LM, Moqrich A, Patapoutian A, Hampton GM, Schultz PG, Hogenesch JB. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci U S A. 2002;99:4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulimenko V, Draberova E, Sulimenko T, Macurek L, Richterova V, Draber P, Draber P. Regulation of microtubule formation in activated mast cells by complexes of {gamma}-tubulin with Fyn and Syk kinases. J Immunol. 2006;176:7243–7253. doi: 10.4049/jimmunol.176.12.7243. [DOI] [PubMed] [Google Scholar]
- Talmor-Cohen A, Tomashov-Matar R, Eliyahu E, Shapiro R, Shalgi R. Are Src family kinases involved in cell cycle resumption in rat eggs? Reproduction. 2004a;127:455–63. doi: 10.1530/rep.1.00104. [DOI] [PubMed] [Google Scholar]
- Talmor-Cohen A, Tomashov-Matar R, Tsai WB, Kinsey WH, Shalgi R. Fyn kinase-tubulin interaction during meiosis of rat eggs. Reproduction. 2004b;128:387–393. doi: 10.1530/rep.1.00266. [DOI] [PubMed] [Google Scholar]
- Talmor A, Kinsey WH, Shalgi R. Expression and immunolocalization of p59c-fyn tyrosine kinase in rat eggs. Dev Biol. 1998;194:38–46. doi: 10.1006/dbio.1997.8816. [DOI] [PubMed] [Google Scholar]
- Thomas SM, Brugge JS. Cellular functions regulated by Src family kinases. Annu Rev Cell Dev Biol. 1997;13:513–609. doi: 10.1146/annurev.cellbio.13.1.513. [DOI] [PubMed] [Google Scholar]
- Wayne CM, Fan HY, Cheng X, Richards JS. FSH-induces multiple signaling cascades: evidence that activation of SRC, RAS and the EGF receptor are critical for granulosa cell differentiation. Mol Endocrinol. 2007;21:1940–57. doi: 10.1210/me.2007-0020. [DOI] [PubMed] [Google Scholar]
- Zhang M, Xia G, Zhou B, Wang C. Gonadotropin-controlled mammal oocyte meiotic resumption. Front Biosci. 2007;12:282–96. doi: 10.2741/2064. [DOI] [PubMed] [Google Scholar]
- Zheng KG, Meng XQ, Yang Y, Yu YS, Liu DC, Li YL. Requirements of Src family kinase during meiotic maturation in mouse oocyte. Mol Reprod Dev. 2007;74:126–131. doi: 10.1002/mrd.20613. [DOI] [PubMed] [Google Scholar]