Abstract
Aging effects on the Wisconsin Card Sorting Test (WCST) are fairly well established but the mechanisms of the decline are not clearly understood. In this study, we examined the cognitive and neural mechanisms mediating age-related increases in perseveration on the WCST. MRI-based volumetry and measures of selected executive functions in conjunction with the WCST were obtained in a sample of 117 healthy young and older adults. Path analysis indicated that age-related increase in perseveration is completely accounted for by declines in processing speed and temporal processing, deficits in working memory mediated by decreased prefrontal cortical volume, and the indirect influence of prefrontally-mediate declines in inhibition via working memory. We conclude that age-related increase in perseveration is indeed differentially dependent on the integrity of prefrontal cortex and on declines in selected cognitive processes dependent on this region.
Keywords: aging, brain, MRI, working memory, inhibition, processing speed, temporal processing
Cognitive performance depends on the ability to plan, keep track of multiple options, inhibit sub-optimal responses, and switch among tasks while maintaining information in memory. Those skills constitute the broad and somewhat ill-defined construct executive functions (EF; Eslinger, 1996; Miyake et al, 2000). Multiple EF components decline with age (e.g., Zacks & Hasher, 1994; Verhaeghen & Cerella, 2002) and reduced executive control mediates age deficits in other cognitive domains (e.g., Salthouse et al., 2003).
A popular task used in many investigations of age effects on EF is the Wisconsin Card Sorting Test (WCST; see Rhodes, 2004 for review). In WCST, participants sort cards depicting simple geometric designs according to design elements with sorting rules discovered through minimal feedback. After a pattern of correct sorting is established, the rule changes and the participant must adjust to the change. WCST is sensitive (though not specific) to effects of lesions in prefrontal cortex and white matter. The most prominent result is increase in perseverative errors, i.e. tendency to persist in a specific response despite feedback indicating it is no longer correct (see Demakis, 2003 for review). Effects of age on WCST performance resemble those of prefrontal lesions (see Rhodes, 2004 for review), but the cognitive and neural mechanisms underpinning these age-related differences are poorly understood.
Performance on WCST and age differences therein, depend on multiple processes such as working memory, processing speed, inhibition, and set-shifting (e.g., Ashendorf & McCaffrey, 2007; Fristoe et al., 1997; Hartman, Bolton & Fehnel, 2001; Miyake et al., 2000). Structural and functional integrity of prefrontal cortex and adjacent white matter may mediate age effects on perseveration, possibly via association with working memory (Esposito et al., 1999; Gunning-Dixon & Raz, 2003; Head et al., 2002; Raz et al., 1998).
To date, limited neuroanatomical and cognitive mediators were explored as predictors of perseveration on WCST. Thus, the primary goal here was to examine dissociations among multiple brain regions and cognitive processes that may explain age differences in perseveration among healthy adults. We evaluated a series of competing path models and examined contributions of brain regions (prefrontal cortex, prefrontal white matter, caudate, hippocampus), processing speed and four executive mediators – working memory, inhibition, task-switching and temporal processing – to age differences in perseveration. Selection of regions was dictated by their importance in cognitive aging and disproportionate declines in frontal-striatal circuits associated with WCST performance. Visual cortex served as a control region. The hierarchy of variables in the models was predicated on expectations that age would affect regional brain volumes, volumes of frontostriatal regions would be negatively associated with selected executive functions and processing speed, which in turn would lead to increased perseveration.
Method
Participants
Young and older adults were recruited from Memphis, Tennessee and Detroit, Michigan. Participants were from a previous study of neuroanatomical correlates of memory (Head et al., 2008; see Table 1 for demographics), and screened for major medical conditions, gross cognitive status, and depression (Head et al., 2008). All participants provided informed consent and the study was approved by each university.
Cognitive Testing
Wisconsin Card Sorting Test
In a computerized WCST (Neuroscan Corp., Herndon, VA), participants matched a card appearing on the bottom of the screen with one of four key cards displayed at the top using a keypad. The computer provided accuracy feedback. The correct category switched after 10 consecutive correct responses. Participants continued until six categories were achieved or the maximum 128 trials. Indices of performance were perseverative responses and perseverative errors (Head et al., 2002; Raz et al. 1998)
Remaining cognitive tasks are described in detail elsewhere (Head et al., 2008). Four tasks assessed working memory: Computation Span, Size Judgment Span, and verbal and nonverbal n-back tasks. Two Stroop tasks (color and position) assessed inhibition. Two tasks assessed task-switching: switching between indicating the digit appearing on the left versus the right and switching between indicating whether a digit was more or less than five and whether the digit was odd or even. Three measures of temporal processing were verbal and nonverbal temporal order memory and nonverbal recency judgment. Three measures of processing speed were simple reaction time, and Letter and Pattern comparison.
MRI Protocol
Volumetric measures were performed in native space on re-aligned images acquired with a T1-weighted 3-D spoiled gradient recalled (SPGR) sequence (124 contiguous 1.3-mm axial slices, TE=5ms, TR=24ms, FOV=22cm, acquisition matrix 256×192, flip angle=30°) on a 1.5T Signa scanner. There was a mean delay of 98 days (SD=133) between the scan and cognitive testing. Images were re-aligned to correct for undesirable effects of head tilt, pitch and rotation, and re-partitioned in the coronal plane at 1.5-mm thickness. Regions-of-interests - lateral prefrontal cortex, prefrontal white matter, caudate, hippocampus, pericalcarine cortex – were measured with high reliability (intraclass correlation >.90). Because there was no laterality hypothesis, we used sums across hemispheres. The re-alignment, re-partitioning, and manual measurement procedures are described in detail elsewhere (Raz et al., 2004).
Data Conditioning
Data conditioning, including removal of incorrect trials from response time and perseveration data, examination for outliers and normalizing data transformations, is described in a previous publication (Head et al., 2008). Previous analysis (Head et al., 2008) showed that selected tasks represented hypothesized constructs of processing speed, working memory, inhibitory control, temporal processing and task-switching, and in the current analyses, perseverative responses and errors were highly correlated (r=.996; p<.0001). Therefore, composites for each construct were derived by summing scores from the component tasks after variables were standardized to the entire sample. The only site-related difference was greater perseveration observed in the Memphis sub-sample (t(115)=5.86, p<.001; Head et al., 2008).
Data Analyses
We took a classic path analysis approach (Pedhazur, 1997), emphasizing parsimony and theoretical interpretability, implemented in MPLUS software (Muthén & Muthén, 2004) with maximum likelihood estimator. Primary indices of model fit were standardized root mean square (SRMR) and comparative fit index (CFI) with cut-offs of.06 and.95, respectively (Hu & Bentler, 1999). For a more informative estimate of goodness of fit, we calculated the ratio of χ2 to degrees of freedom (df), with a smaller ratio indicating a better fit. We adhered to a relatively conservative ratio of ≤2.00 (Mueller, 1996). The basic structure of models was as following: first tier - age as a continuous variable (within each age group); second tier - brain volumes adjusted for ICV via simple regression; third tier - processing speed, working memory, inhibitory control; fourth tier - temporal processing and task-switching, deemed less basic skills than those included in previous tier; fifth tier - perseveration, the target construct of analysis. Residual of age was set to zero. To maintain recommended 1:10 ratio of variables to observations we did not test verbal versus nonverbal effects. In classic path analysis no reciprocal causation is assumed (Pedhazur, 1997). Therefore, we postulated only unidirectional effects.
Results
Age Differences in Cognitive Performance and Regional Brain Volumes
As previously reported (Head et al., 2008; see Table 3 for descriptive statistics) there were significant age effects on all regional brain volumes (all p’s<.001) except the visual cortex and each of the composite cognitive indices (all p’s<.01). Older adults evidenced greater perseveration than younger adults: M=1.15; SD=1.93; vs. M=−.77; SD=1.65; d=1.09, 95%CI=.68–1.47.
Mediational Analyses
The first reduced model was constructed by imposing restrictions on the baseline model by turning respective paths to zero. The Reduced Model (RM) I was based on the hypothesis that prefrontal-caudate circuits would affect perseveration directly and via their effects on specific executive functions; the hippocampus would not have a direct effect on perseveration; and that the volume of the visual cortex would not affect any cognitive variables. The relations amongst the brain variables, processing speed, and the specific executive functions were based on theoretical considerations and previous modeling (Head et al., 2008). In RM I, age directly affected regional volumes, processing speed and temporal processing; lateral prefrontal cortex affected inhibitory control, working memory and perseveration; caudate and prefrontal white matter affected perseveration; inhibitory control affected working memory, task-switching, and perseveration; processing speed affected inhibitory control, working memory, temporal processing, task-switching and perseveration; working memory affected temporal processing, task-switching, and perseveration; temporal processing affected perseveration; and task-switching affected perseveration. The model fit the data well (χ2=37.127, df=35, p=.37; χ2/df=1.06; CFI=.996; SRMR=.051), but included non-significant paths.
For greater parsimony and theoretical relevance, further restrictions were imposed on RM I to create a nested RM II. RM II tested whether the effects of prefrontal-caudate circuits were mediated by their influence on specific executive functions. In RM II, the paths from lateral prefrontal to perseveration, prefrontal white matter to perseveration, and caudate to perseveration were set to zero. RM II fit the data well: χ2=39.931, df=38, p=.38; χ2/df=1.05; CFI=.997; SRMR=.056. As RM II was hierarchically nested within RM I, we compared them and found no significant difference: χ2 difference=2.804, df=3, ns.
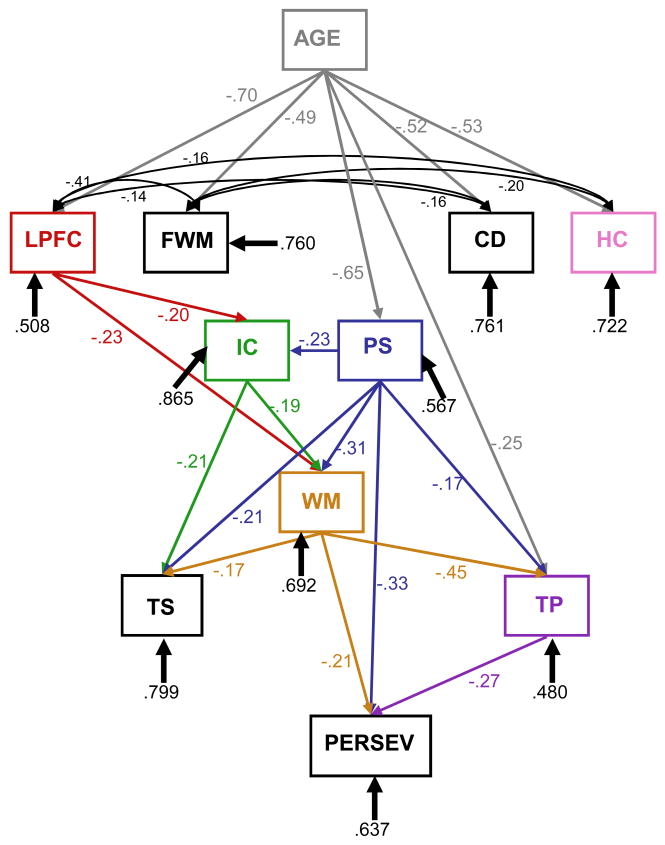
The final model was based on the hypothesis that working memory mediates perseveration and considerations of parsimony, with paths that were essentially zero in RM II set to zero in RM III (see Figure 1). Specifically, the zero paths were task-switching to perseveration and inhibitory control to perseveration. The fit of RM III to the data was: χ2=41.638, df=40, p=.40; χ2/df=1.04; CFI=.997; SRMR=.058, and RM III did not differ from RM III: χ2 difference=1.701, df=2, ns.
Figure 1.
Path model of Reduced Model III with relationships among age, brain volumes, and cognition. Numbers at paths are path coefficients; three-digit numbers at variable boxes are residuals. See text for model fit indices. LPFC=lateral prefrontal cortex; FWM=prefrontal white matter; Cd=caudate nucleus; HC=hippocampus; VC=visual cortex; PS=processing speed; IC=inhibitory control; WM=working memory; TP=temporal processing; TS=task-switching; Persev=perseverative behavior.
Although no significant site differences were observed in the regional brain volumes and most of the cognitive variables, there was a difference in perseveration. The sample size was insufficient for a formal multiple groups analysis, especially considering that the Memphis sample was only 34 individuals. Thus, we examined the fit of RM III only in the Detroit sample. The model fit for the Detroit data was adequate given the reduced sample size: χ2=35.903, df=40, p=.66; χ2/df=.90; CFI=.999; SRMR=.059, indicating stability of the model.
Discussion
We examined neural and cognitive predictors of age differences in perseveration on WCST. Findings replicate age-related increase in perseveration (see Rhodes, 2004 for a review), and association between smaller prefrontal cortical volume and increased perseveration (Gunning-Dixon and Raz, 2003; Raz et al., 1998). The link between differences in prefrontal volume and perseveration was specific, as other examined brain regions affected by age to the same degree evidenced no associations. Although reduced volume of prefrontal white matter did not contribute to perseveration, other indices of white matter integrity may reveal such an association. The volume of white matter, albeit reduced, may nonetheless contain areas of degeneration obscuring effects of declining integrity on cognition. Burden of white matter hyperintensities (an indicator of pathological changes) in prefrontal regions may be more critical in accounting for age-related variation in perseveration (Gunning-Dixon & Raz, 2003).
The influence of prefrontal cortical volume on perseveration was mediated by its effect on working memory and inhibition. In addition, age-related reductions in processing speed and temporal processing contributed to perseverative behavior without mediation by neuroanatomical variables examined here. Mediating effects of executive functions on perseveration were also differential, with significant contributions of working memory, inhibitory control and temporal processing, but not of task-switching.
Slower processing and lower working memory capacity are linked to perseveration (e.g., Fristoe et al., 1997; Hartman, Bolton & Fehnel, 2001; Huzinga, Dolan, & van der Molen, 2006; Huzinga & van der Molen, 2007; but see Miyake et al., 2000). Our findings extend this literature and shed light on the role of inhibition in perseveration. Previous investigations that failed to detect direct contributions of inhibition to perseveration did not examine influences of inhibitory control via its effects on working memory (Huzinga, Dolan, van der Molen, 2006; Miyake et al., 2000). Our model is consistent with theories emphasizing the role of age-related deficits in inhibition on cognition through its impact on working memory (Zacks & Hasher, 1994).
Although temporal processing is a less well-established contributor to cognitive aging, encoding temporal context may be an important component of successful performance on WCST (Hartman, Bolton & Fehnel, 2001). Impaired temporal processing might hinder keeping track of past sorts. Hartman and colleagues (2001) noted providing visual cues about the most recent sort aided older adults in using temporal information leading to reduction of age effects on perseveration. Our results are consistent with that line of reasoning in indicating that temporal processing inefficiencies significantly contribute to age-related increases in perseveration.
While it is intuitive that ability to alternate between sets would be important for WCST performance, task-switching did not emerge as a significant contributor to perseveration. This is in contrast to previous findings (Huzinga & van der Molen, 2007; Miyake et al., 2000; Ashendorf & McCaffrey, 2007), but consistent with other reports of a lack of association between set-shifting and perseveration (Huzinga, Dolan & van der Molen, 2006). Knowledge of changes in sorting rules may induce greater reliance on switching resources (Miyake et al., 2000). In addition, previous studies of WCST and task-switching failed to account for processing speed (Huzinga & van der Molen, 2007; Miyake et al., 2000). Task-switching contribution to higher-order processes may not be unique once processing speed is taken into account (Huzinga, Dolan & van der Molen, 2006; Salthouse et al., 1998). In our models, processing speed and working memory accounted for variance in task-switching and directly predicted perseveration.
Interpretation of our findings is conditioned on several limitations. As all path models driven by theoretical considerations and constrained by limitations of statistical power, ours did not include all potentially relevant cognitive and neural variables. For example, feedback usage may mediate age differences in WCST (Fristoe et al., 1997), and relevant brain sites may include posterior association cortices (see Buchsbaum et al., 2005 for review) Thus, perseveration is clearly not a failure of prefrontal circuits alone, but inclusion of additional variables to describe the whole set of relevant regions necessitates significantly larger samples. Lastly, a cross-sectional study precludes assessment of actual age changes or causality, leaving one with comparative evaluation of several mediational hypotheses.
In summary, age-related increases in perseveration reflect the differential influence of smaller prefrontal cortices. Processes associated with other executive functions and processing speed mediate this effect.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ashendorf L, McCaffrey RJ. Exploring age-related decline on the Wisconsin Card Sorting Test. The Clinical Neuropsychologist. 2007;22:262–272. doi: 10.1080/13854040701218436. [DOI] [PubMed] [Google Scholar]
- Buchsbaum BR, Greer S, Chang W, Berman KF. Meta-analysis of neuroimaging studies of the Wisconsin Card-Sorting Task and component processes. Human Brain Mapping. 2005;25:35–45. doi: 10.1002/hbm.20128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demakis GJ. A meta-analytic review of the sensitivity of the Wisconsin Card Sorting Test to frontal and lateralized frontal brain damage. Neuropsychology. 2003;17:255–264. doi: 10.1037/0894-4105.17.2.255. [DOI] [PubMed] [Google Scholar]
- Eslinger PJ. Conceptualizing, describing, and measuring components of executive function: a summary. In: Lyon GR, Krasnegor NA, editors. Attention, memory, and executive Function. Baltimore: Paul H. Brookes Publishing Co; 1996. pp. 367–395. [Google Scholar]
- Esposito G, Kirby BS, Van Horn JD, Ellmore TM, Berman KF. Context-dependent, neural system-specific neurophysiological concomitants of ageing: mapping PET correlates during cognitive activation. Brain. 1999;122:963–979. doi: 10.1093/brain/122.5.963. [DOI] [PubMed] [Google Scholar]
- Fristoe NM, Salthouse TA, Woodard JL. Examination of age-related deficits on the Wisconsin Card Sorting Test. Neuropsychology. 1997;11:272–285. doi: 10.1037//0894-4105.11.3.428. [DOI] [PubMed] [Google Scholar]
- Gunning-Dixon F, Raz N. Neuroanatomical correlates of selected executive functions in middle-aged and older adults, a prospective MRI study. Neuropsychologia. 2003;41:1929–1941. doi: 10.1016/s0028-3932(03)00129-5. [DOI] [PubMed] [Google Scholar]
- Hartman M, Bolton E, Fehnel SE. Accounting for age differences on the Wisconsin Card Sorting Test: Decreased working memory, not inflexibility. Psychology and Aging. 2001;16:385–399. [PubMed] [Google Scholar]
- Head D, Raz N, Gunning-Dixon F, Williamson A, Acker JD. Age-related differences in the course of cognitive skill acquisition: the role of regional cortical shrinkage and cognitive resources. Psychology and Aging. 2002;17:72–84. doi: 10.1037//0882-7974.17.1.72. [DOI] [PubMed] [Google Scholar]
- Head D, Rodrigue KM, Kennedy KM, Raz N. Neuroanatomical and cognitive mediators of age-related differences in episodic memory. Neuropsychology. 2008;22:491–507. doi: 10.1037/0894-4105.22.4.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis, conventional criteria versus new alternatives. Structural Equation Modeling. 1999;6:1–55. [Google Scholar]
- Huzinga M, Dolan CV, van der Molen MW. Age-related change in executive function: Developmental trends and a latent variable analysis. Neuropsychologia. 2006;44:2017–2036. doi: 10.1016/j.neuropsychologia.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Huzinga M, van der Molen MW. Age-group differences in set-switching and set-maintenance on the Wisconsin Card Sorting Task. Developmental Neuropsychology. 2007;31:193–215. doi: 10.1080/87565640701190817. [DOI] [PubMed] [Google Scholar]
- Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A. The unity and diversity of executive functions and their contributions to complex “frontal lobe” tasks: A latent variable analysis. Cognitive Psychology. 2000;41:49–100. doi: 10.1006/cogp.1999.0734. [DOI] [PubMed] [Google Scholar]
- Mueller R. Basic principles of structural equation modeling. New York: Springer-Verlag; 1996. [Google Scholar]
- Muthén L, Muthén B. Mplus User’s Guide. 3. Los Angeles: Muthén & Muthén; 2004. [Google Scholar]
- Pedhazur EJ. Multiple-regression in behavioral research. 3. Orlando: Harcourt Brace College Publishers; 1997. [Google Scholar]
- Raz N, Gunning-Dixon F, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging, evidence from structural magnetic resonance imaging. Neuropsychology. 1998;12:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- Rhodes MG. Age-related differences in performance on the Wisconsin Card Sorting Test: A meta-analytic review. Psychology and Aging. 2004;19:482–494. doi: 10.1037/0882-7974.19.3.482. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Atkinson TM, Berish DE. Executive functioning as a potential mediator of age-related cognitive decline in normal adults. Journal of Experimental Psychology: General. 2003;132:566–594. doi: 10.1037/0096-3445.132.4.566. [DOI] [PubMed] [Google Scholar]
- Salthouse TA, Fristoe N, McGuthry KE, Hambrick DZ. Relation of task-switching to speed, age, and fluid intelligence. Psychology and Aging. 1998;13:445–461. doi: 10.1037/0882-7974.13.3.445. [DOI] [PubMed] [Google Scholar]
- Verhaeghen P, Cerella J. Aging, executive control, and attention, a review of meta-analyses. Neuroscience and Biobehavioral Review. 2002;26:849–857. doi: 10.1016/s0149-7634(02)00071-4. [DOI] [PubMed] [Google Scholar]
- Zacks RT, Hasher L. Directed ignoring: inhibitory regulation of working memory. In: Dagenbach D, Carr TH, editors. Inhibitory processes in attention, memory and language. San Diego: Academic Press; 1994. pp. 241–264. [Google Scholar]