Abstract
In recent decades, protein-based therapeutics have substantially expanded the field of molecular pharmacology due to their outstanding potential for the treatment of disease. Unfortunately, protein pharmaceuticals display a series of intrinsic physical and chemical instability problems during their production, purification, storage, and delivery that can adversely impact their final therapeutic efficacies. This has prompted an intense search for generalized strategies to engineer the long-term stability of proteins during their pharmaceutical employment. Due to the well known effect that glycans have in increasing the overall stability of glycoproteins, rational manipulation of the glycosylation parameters through glycoengineering could become a promising approach to improve both the in vitro and in vivo stability of protein pharmaceuticals. The intent of this review is therefore to further the field of protein glycoengineering by increasing the general understanding of the mechanisms by which glycosylation improves the molecular stability of protein pharmaceuticals. This is achieved by presenting a survey of the different instabilities displayed by protein pharmaceuticals, by addressing which of these instabilities can be improved by glycosylation, and by discussing the possible mechanisms by which glycans induce these stabilization effects.
Keywords: biopharmaceutics, biophysical models, chemical stability, glycosylation, molecular modeling, physical stability, physicochemical properties, proteins, stabilization, thermodynamics
INTRODUCTION
The employment of proteins as pharmaceutical agents has greatly expanded the field of molecular pharmacology as these generally display therapeutically favorable properties, such as, higher target specificity and pharmacological potency when compared to traditional small molecule drugs.1,2 Unfortunately, the structural instability issues generally displayed by this class of molecules still remain one of the biggest challenges to their pharmaceutical employment, as these can negatively impact their final therapeutic efficacies (Tab. 1).2-50 In contrast to traditional small molecule drugs whose physicochemical properties and structural stabilities are often much simpler to predict and control, the structural complexity and diversity arising due to the macromolecular nature of proteins has hampered the development of predictive methods and generalized strategies concerning their chemical as well as their physical stabilizations.51,52 While the protein primary structure is subject to the same chemical instability issues as traditional small molecule therapeutics (e.g. acid-base and redox chemistry, chemical fragmentation, etc), the higher levels of protein structure (e.g., secondary, tertiary) often necessary for therapeutic efficacy can also result in additional physical instability issues (e.g., irreversible conformational changes, local and global unfolding) due to their non-covalent nature.2,15,53-55 The innate propensity of proteins to undergo structural changes coupled with the fact that there is only a marginal difference in thermodynamic stability between their folded and unfolded states provides a significant hurdle for the long-term stabilization of protein pharmaceuticals. This is due to the fact that a thermodynamically stabilized protein could still inactivate kinetically even at the relatively low temperatures used during storage.2,53,55-59 Additionally, as a result of their colloidal nature, proteins are prone to pH, temperature, and concentration dependant precipitation, surface adsorption, and non-native supramolecular aggregation.11,14,20,47,60-65 These instability issues are further compounded by the fact that the various levels of protein structure can become perturbed differently depending on the physicochemical environment to which the protein is exposed.2 This is of special relevance in a pharmaceutical production setting where proteins can be simultaneously exposed to several destabilizing environments during their production, purification, storage, and delivery (Tab. 1).
Table 1.
Chemical and Physical Instabilities Encountered by Protein-based Pharmaceuticals and Typical Countermeasuresa
| Process | Main Stress Factors | Main Degradation Pathways | Typical Countermeasures | Referencesb |
|---|---|---|---|---|
| Purification | Proteases, contaminations,c extremes of pH, high pressures, temperature,f chemical denaturants, high salt and protein concentrations, amphipatic interfaces, hydrophobic surfacesi | Proteolytic and chemical hydrolysis, fragmentations, crosslinking, oxidation, deamidation,e denaturation, adsorption, aggregation,k inactivation | Protease inhibitors, control of pH and temperature, chelating agents,d antioxidants, addition of surface activeg and stabilizing excipientsh | 2, 5, 6, 10, 19-22, 68-72 |
| Liquid storage | Contaminations,c extremes of pH, temperature,f chemical denaturants, high protein concentrations, freeze thawing, amphipatic interfaces, hydrophobic surfacesi | Fragmentations, chemical hydrolysis, oxidation, crosslinking, β-elimination, racemization, deamidation,e denaturation, adsorption, aggregation,k inactivation | Control of pH and temperature, chelating agents,d antioxidants, addition of surface activeg and stabilizing excipientsh | 2, 5-12, 19-22, 47, 49, 50, 68-72 |
| Lyophilization | Ice-water interface, pH changes, dehydration, phase separation | Aggregation,k inactivation | Co-lyophilization with surface activeg and stabilizing excipientsh,j | 4, 18, 23-29, 48, 73 |
| Solid-phase storage | Contaminations,c protein-protein contacts, moisturek | Aggregation,k fragmentation, oxidation, deamidation, inactivation | Similar to lyophilization | 4, 16-18, 30 |
| Spray-drying, Spray-freeze drying | Liquid-air interface, dehydration | Similar to lyophilization | Similar to lyophilization, precipitationl | 31-38, 74 |
| Sustained-release formulationsm | Liquid-organic solvent interface, hydrophobic surfaces,i mechanical stress | Aggregation,k inactivation | Addition of surface activeg and stabilizing excipients,h avoidance of water/organic interfacesn | 39-44, 77 |
Covalent modification as countermeasures are excluded in the table because they are discussed in the paper and in table 2 for glycosylated proteins;
the references cited include many reviews to which the interested reader is referred to for details;
i.e., contaminating (transition) metal ions and proteases can catalyze fragmentations;22
to remove metal ions;2
other prominent chemical instabilities are oxidations and disulfide scrambling;2
control of temperature can be non-trivial when ultrasonication is being used because of local heating events;
mild detergents at low concentration can prevent detrimental interactions of proteins with hydrophobic surfaces/interfaces;42
such excipients include sugars, polyols, and amino acids that stabilize protein structure by so-called preferential exclusion;2,75
the potentially most harmful surfaces are hydrophobic, e.g., Teflon;45
the mechanism of stabilization is believed to be a combination of hydrogen-bond forming propensity and increase in the glass transition temperature in the solid;23
a prominent pathway to aggregation is by so-called sulfide-disulfide interchange;11
precipitation prior to the procedure afforded stabilization;
the sole FDA approved formulation thus far consists in the encapsulation of the protein in microspheres comprised of poly(lactic-co-glycolic) acid;
Due to these stability problems much emphasis has been given to the development of strategies for the effective long-term stabilization of protein pharmaceuticals.2,4,11,61,66-77 These include external stabilization by influencing the properties of the surrounding solvent through the use of stabilizing excipients (e.g., amino acids, sugars, polyols) and internal stabilization by altering the structural characteristics of the protein through chemical modifications (e.g. mutations, glycosylation, pegylation).2,53,58 While many protein pharmaceuticals have been successfully formulated by employing stabilizing mutations, excipients, and pegylation, their use can sometimes be problematic due to limitations, such as, predicting the stabilizing nature of amino acid substitutions, the occurrence of protein and excipient dependant non-generalized stabilization effects, protein / excipient phase separation upon freezing, cross-reactions between some excipients and the multiple chemical functionalities present in proteins, acceleration of certain chemical (e.g. aspartate isomerization) and physical (e.g. aggregation) instabilities by some excipients (e.g., sorbitol, glycerol, sucrose), detection interferences caused by some sugar excipients during various protein analysis methods, and safety concerns regarding the long-term use of pegylated proteins in vivo due to possible PEG induced immunogenecity and chronic accumulation toxicity resulting from its reduced degradation and clearance rates.2,4,33,48,66,78-95
Due to these limitations, there is still a need for further development of additional strategies of protein stabilization.2 Amongst the chemical modification methods, glycosylation represents one of the most promising approaches as it is generally perceived that through manipulation of key glycosylation parameters (e.g. glycosylation degree, glycan size and glycan structural composition) the protein's molecular stability could be engineered as desired.2,66,96-105 In this context, it is important to highlight the fact that glycosylation has been reported to simultaneously stabilize a variety of proteins against almost all of the major physicochemical instabilities encountered during their pharmaceutical employment (Tab. 2), suggesting the generality of these effects.
Table 2.
Protein Instabilities Improved by Glycosylation
| Instability | References | Instability | References |
|---|---|---|---|
| Proteolytic degradation | 96, 121-141 | Heating denaturation | 98, 101-103, 119, 124, 128, 129, 146, 149, 159, 170, 171, 181, 182, 188-195, 202, 204, 205 |
| Oxidation | 145 | Freezing denaturation | 201 |
| Chemical crosslinking | 97, 146, 149 | Precipitation | 159-165 |
| pH denaturation | 124, 137, 171-178 | Kinetic inactivation | 101, 103, 136, 146, 186, 212-218 |
| Chemical denaturation | 136, 164, 171, 172, 181-185, 187, 188 | Aggregation | 97, 101, 103, 130, 218, 222 |
Even though a vast amount of studies have evidenced the fact that glycosylation can lead to enhanced molecular stabilities and therapeutic efficacies for protein pharmaceuticals (Tab. 3), an encompassing perspective on this subject is still missing due to the lack of a comprehensive review of the literature. The intent of this article is therefore to further the field of protein glycoengineering by increasing the general understanding of the mechanisms by which glycosylation improves the molecular stability of protein pharmaceuticals. This is achieved by presenting a survey of the different instabilities displayed by protein pharmaceuticals, by addressing which of these instabilities can be improved by glycosylation, and by discussing the possible mechanisms by which glycans induce these stabilization effects.
Table 3.
Partial List of Approved Protein-based Pharmaceutical Products Stabilized by Glycosylation
| INN* | Brand Name (Company) | Indication | Effects of Glycosylation | Glycan (#) | References |
|---|---|---|---|---|---|
| Agalsidase alfa (galactosidase) | Replagal® (Shire) | Treatment of Fabry disease | Protects against aggregation and precipitation | 3 | 161 |
| Alglucosidase alfa (α-glucosidase) | Myozyme® (Shire) | Treatment of Pompe disease | Protects against thermal denaturation | 6 | 193 |
| Alpha 1-antitrypsin (α1AT) | Prolastin® (Talecris Biotherapeutics) | Treatment of congenital α1AT deficiency with emphysema | Protects against chemical and thermal denaturation | 3 | 181 |
| Bucelipase alfa (cholesterol esterase) | Merispase® (Meristem therapeutics) | Treatment of lipid malabsorption related to exocrine pancreatic insufficiency | Protects against proteolytic degradation | 11 | 126 |
| Chymotrypsin | Wobe Mugos® (Marlyn Nutraceuticals) | Adjunct therapy for multiple myeloma | Protects against thermal, chemical, and kinetic denaturation and aggregation | ** | 101-103, 188 |
| Corifollitropin alfa (FSH) | Gonal-F® (EMD Serono) | Treatment of infertility | Protects against thermal denaturation | 10 | 191 |
| Drotrecogin alfa (CF-XIV, Protein C) | Xigris® (Eli Lilly) | Treatment of severe sepsis | Protects against proteolytic degradation | 4 | 127 |
| Epoetin alfa | Epogen® (Amgen) | Treatment of anemia associated with chronic renal failure (CRF) | Protects against oxidation, thermal, chemical, and pH denaturation, kinetic | 3 | 145, 171, 216, 221 |
| Procrit® (Ortho Biotech) | inactivation, and aggregation | ||||
| IgG-like antibodies | *** | Multiple indications | Protects against proteolysis and thermal denaturation | 2 | 142, 194, 195 |
| Insulin | *** | Treatment of diabetes | Protects against non-disulfide crosslinking and aggregation | ** | 97 |
| Avonex® (Biogen) | |||||
| Interferon beta-1a (rHuInf-β1a) | Rebif® (Pfizer / EMD Serono) | Treatment of multiple sclerosis | Protects against disulfide crosslinking, precipitation, thermal denaturation, and aggregation | 1 | 149, 159, 160 |
| Interferon gamma-1b | Actimmune® (Intermune) | Treatment of chronic granulomatous disease | Protects against proteolytic degradation | 2 | 132 |
| Lenograstim (G-CSF) | Granocyte® (Chugai Pharma) | Treatment of chemotherapy induced neutropenia | Protects against disulfide crosslinking, proteolytic degradation, thermal and pH denaturation, and kinetic inactivation | 1 | 124, 125, 146, 170 |
| Ranpirnase (RNAse) | Onconase® (Alfacell Corp.) | Treatment of malignant mesothelioma | Protects against proteolytic degradation and thermal denaturation | ** | 128, 129, 189, 190 |
| Sargramostin (G-CSF) | Leukin® (Bayer Healthcare) | Treatment after induction chemotherapy with acute myelogenus leukemia | Protects against disulfide crosslinking, proteolytic degradation, thermal and pH denaturation, and kinetic inactivation | 8 | 124, 125, 146, 170 |
| Thyrotropin alfa (TSH) | Thyrogen® (Genzyme) | Detection of thyroid cancer and hypothyroidism | Protects against proteolytic degradation and aggregation | 3 | 130 |
| Urokinase alfa | Abbokinase® (ImaRx Therapeutics) | Treatment of acute massive pulmonary emboli | Protects against proteolytic degradation and thermal denaturation | 2 | 131, 192 |
Information was obtained from the Prescribing Information (PI) for each product.
INN: International nonproprietary name.
Commercially available protein is not glycosylated.
Multiple approved products. Further information available at www.fda.gov and www.biopharma.com.
PROTEIN GLYCOSYLATION
Protein glycosylation is one of the most common structural modifications employed by biological systems to expand proteome diversity.106-108 Evolutionarily, glycosylation is widespread found to occur in proteins through the main domains of life (archaea, eubacteria, bacteria and eukarya).109,110 The prevalence of glycosylation is such that it has been estimated that 50% of all proteins are glycosylated.111 Functionally, glycosylation has been shown to influence a variety of critical biological processes at both the cellular (e.g. intracellular targeting) and protein levels (e.g. protein-protein binding, protein molecular stability).103 It should therefore not come as a surprise that a substantial fraction of the currently approved protein pharmaceuticals need to be properly glycosylated to exhibit optimal therapeutic efficacy.100,112
Structurally, glycosylation is highly complex due to the fact that there can be heterogeneity with respect to the site of glycan attachment (macroheterogeneity) and with respect to the glycan's structure (microheterogeneity). Although many protein residues have been found to be glycosylated with a variety of glycans (for a detailed discussion see review by Sears and Wong), in humans the most prevalent glycosylation sites occur at asparagine residues (N-linked glycosylation through Asn-X-Thr/Ser recognition sequence) and at serine or threonine residues (O-linked glycosylation) with the following monosaccharides: fucose, galactose, mannose (Man), N-acetylglucosamine (GlcNAc), N-acetylgalactosamine, and sialic acid (N-acetylneuraminic acid).109,113-115 Since all of the potential glycosylation sites are not simultaneously occupied this leads to the formation of glycoforms with differences in the number of attached glycans. Further structural complexity can occur due to variability in the glycan's monosaccharide sequence order, branching pattern, and length. In humans N-linked glycan structures are classified in three principal categories according to their monosaccharide content and structure: high mannose type (Man2-6Man3GlcNAc2), mixed type (GlcNAc2Man3GlcNAc2), and hybrid type (Man3GlcNAcMan3GlcNAc2).113 The terminal ends of these glycans are often further functionalized with chemically charged groups (e.g., phosphates, sulfates, carboxylic acids) in human glycoproteins, leading to even greater structural diversity. These charged glycans most probably impact to some degree the overall stability of glycoproteins since they can alter their isoelectric point (pI).116,117 Some of these charged terminal glycans (e.g., sialic acid) have also been found to be critical in regulating the circulatory half-life of glycoproteins. This has led to the development of glycosylation as a novel strategy to improve the therapeutic efficacies of protein pharmaceuticals by engineering their pharmacokinetic profiles (for a detailed discussion see the recent review by Sinclair and Elliot).100
Due to the high degree of structural variability arising from physiological (natural) glycosylation, novel strategies are currently being pursued to create structurally homogeneous pharmaceutical glycoproteins with humanized glycosylation patterns.118 These include engineered glycoprotein expression systems (e.g., yeast, plant, and mammalian cells) as well as enzymatic, chemical, and chemo-enzymatic in vitro glycosylation remodeling methods. Alternatively, to understand the mechanisms by which glycosylation influences protein physicochemical properties researchers have employed comparatively simpler glycosylation strategies. These include enzymatic deglycosylation of natural glycoproteins, chemical glycosylation via the use of structurally simple chemically-activated glycans, and glycation of the lysine residues with reducing sugars via the Maillard reaction. Although some of these glycosylation methods (e.g., glycation) may be undesired for use in protein pharmaceuticals their fundamental scientific value for the understanding the effects of glycosylation on protein stability cannot be ignored.119 This is due to the fact that independently of the method by which the structurally different glycans are attached to the protein surface (e.g. enzymatic and chemical glycosylation, or reductive glycation) they all seem to induce similar stabilization effects.103 In the next sections, we thus focus on discussing which pharmaceutically-relevant chemical and physical protein instabilities have been reported to be ameliorated by glycosylation and discuss possible mechanisms by which glycans achieve such effects.
EFFECTS OF GLYCOSYLATION ON PROTEIN STABILITY CHEMICAL INSTABILITIES PREVENTED BY GLYCOSYLATION
The presence of multiple reactive chemical functionalities in the amino acids side chains of proteins makes them particularly sensitive to several chemical degradation processes. These can include: glutamine (Gln) and asparagine (Asn) deamidation; histidine (His), methionine (Met), cysteine (Cys), tryptophan (Trp), and tyrosine (Tyr) oxidation; serine (Ser), threonine (Thr), phenylalanine (Phe), lysine (Lys), and Cys β-elimination; disulfide fragmentation, exchange, and crosslinking; backbone peptide hydrolysis caused either by proteases or by pH sensitive backbone sequences (e.g., aspartic acid-proline (Asp-X)); transamidation; racemization; and chemically-triggered non-specific crosslinking (Tab. 1).2,6,8,15,54,55,112 For further detailed discussions on the general mechanisms which trigger these chemical instabilities the reader is referred to several excellent reviews on the subject.2,6,8,9,12,55 In the next section, we focus on those chemical instabilities which have been reported to be improved by glycosylation (e.g., proteolytic degradation, oxidation, and chemical crosslinking) (Tab. 2).
Proteolytic Degradation
Protein pharmaceuticals are typically administered intravenously and not via the oral route due to their chemical degradation by the proteases of the digestive system.120 However, the systemic expression of proteases also makes proteins administered by other routes highly susceptibly to proteolytic degradation.120 Therefore, the in vivo molecular stability and therapeutic efficacy of protein pharmaceuticals is intimately related to their stability towards proteolytic degradation.2,6,100,120 In general, glycosylation has been found to protect proteins against proteolytic degradation.96,121-123 Some examples include granulocyte colony stimulating factor (G-CSF) (GRANOCYTE®, Chugai Pharma),124,125 lipase (MERISPASE®; Meristem Therapeutics),126 protein C (XIGRIS®; Eli Lilly),127 ribonuclease (ONCONASE®; Alfacell),128,129 thyroid-stimulating hormone (THYROGEN®; Genzyme),130 urokinase (ABBOKINASE®; ImaRx Therapeutics),131 interferon-γ(ACTIMMUNE®; Intermune),132 streptokinase,133 cellulose,134 ovomucoid,135 amylase,136,137 lysosomal integral membrane proteins Lamp-1 and Lamp-2,138 peroxidase,139 and catalase.140 There is also evidence that this proteolytic stability can be engineered into proteins as was described by Holcenberg et al. upon chemical glycosylation of asparaginase and by Raju and Scallon upon enzymatic glycosylation of IgG-like antibodies.141,142 Particularly, in this last study it was found that altering the end-terminal glycan structures (e.g., N-acetylglucosamine, galactose, and sialic acid) led to increasingly greater in vitro proteolytic stability when subjected to papain digestion.142 Mechanistically, it has been proposed that this proteolytic stability arises due to the fact that the glycan's presence provides a steric hindrance around the peptide backbone of the amino acids adjacent to the glycosylation site.114,115,143 This prevents the contact between the glycoprotein's surface and the cleaving protease's active site.
Oxidation
Protein pharmaceuticals can potentially lose their bioactivity during their manufacture and storage due to the oxidation of several of their amino acid side chains (His, Met, Cys, Trp, and Tyr).2,6,9,22,55,144 These oxidation events have been mainly attributed to the production of active oxygen-based radicals in protein formulations due to the combination of trace amounts of transition metals, atmospheric oxygen, and exposure to ultraviolet light.2,6 Thus far, erythropoietin (EPOGEN®, PROCIT®; Amgen, Ortho) is the sole reported case of a protein whose bioactivity can be impacted by oxidation and where glycosylation has been found to ameliorate this chemical instability.145 The loss of bioactivity for this protein was found to correlate with the levels of tryptophan oxidation when exposed to oxidizing conditions.145 Comparison of the oxidative susceptibility for the naturally glycosylated erythropoietin with that of its deglycosylated form revealed that glycosylation diminished the tryptophan oxidation rates and the inactivation of this protein.145 These results suggest that glycosylation can protect the protein structure from damage by active oxygen radicals although more studies are still needed to shed some light on the mechanisms of this stabilization and to determine the extent to which engineered glycosylation could prevent this type of instability. Also, whether this stabilizing effect is specific to when the glycans are chemically attached to the protein surface or non-specific having to do more with the radical scavenging capabilities of the glycans remains to be established.70
Chemical Crosslinking
Protein therapeutics can form covalent dimers and oligomers due to polymerization triggered by both disulfide and non-disulfide crosslinking pathways.2,6 Preventing the formation of these covalently linked species in protein pharmaceuticals is important as these frequently lead to loss of bioactivity.2,6 Additionally, for many proteins it has been found that this type of instability, in addition to protein unfolding, could trigger the formation of larger soluble and insoluble protein aggregates.2,6,11 There are several reports in the literature were it has been found that glycosylation prevents the formation of these crosslinked species. For example, Oh-eda et al. reported that the presence of the single glycan in human granulocyte colony-stimulating factor (G-CSF) (GRANOCYTE®; Chugai Pharma) prevented the polymerization-induced inactivation of the protein.146 The mechanism by which G-CSF polymerizes was studied by Krishnan et al. and Raso et al. and found to be due to disulfide crosslinking.147,148 Interferon beta (REBIF®, Pfizer / Serono; AVONEX®, Biogen) is another example of a therapeutically relevant protein where glycosylation prevents its inactivation due to disulfide crosslinking.149 Glycosylation has been also reported to prevent non-disulfide protein crosslinking. For example, Baudys et al. reported that engineered chemical glycosylation of insulin, especially at the PheB-1 amino group, suppressed the self-association of the protein into dimers and oligomeric species.97 The formation of these crosslinked insulin species occurs due to a transamidation reaction between AsnA-21 and PheB-1.2 This finding is highly significant since it demonstrates that this type of stabilization can also be engineered into proteins via rationally designed glycosylation. These results additionally suggest that the mechanism by which this type of instability is prevented is due to increased intermolecular steric repulsion between the crosslinking-prone protein species due to the glycan's presence at the protein surface.
PHYSICAL INSTABILITIES PREVENTED BY GLYCOSYLATION
The functional efficacy of proteins critically depends on the conformational stability of their natively folded state.2 Most proteins adopt a tertiary structure by folding as to minimize the exposure of their hydrophobic residues in aqueous solution.56,150-152 This creates a compact native state with a hydrophobic core that is additionally energetically stabilized by the presence of several types of atomic interactions within the protein core (e.g. electrostatic and charge-charge interactions, hydrogen bonds, Van der Waals interactions).151-154 Unfortunately, the resulting thermodynamic and kinetic stability of this state tends to be intrinsically low due to the non-covalent nature of these forces.2,53 Therefore, any physical or chemical phenomena which can disrupt these forces will trigger either small or large scale protein structural changes. These conformationally altered species are more prone to interact either with themselves or with the hydrophobic surfaces and interfaces present during protein manufacturing and storage leading to additionally physical instabilities, such as, adsorption, aggregation, and precipitation.2,42 Examples of pharmaceutically-relevant phenomena that can lead to protein physical instability include exposure to extremes of temperature and pH; exposure to amphipatic interfaces (e.g., aqueous/organic solvent, aqueous/air), hydrophobic surfaces, and chemical denaturants; and formulation at extreme protein concentrations (Tab. 1). For further detailed discussions on the general mechanisms which trigger these physical instabilities the reader is again referred to a series of excellent reviews on the subject.2,6,8,9,11,12,14,42,61,63,64 In the next section, we focus on those physical protein instabilities which have been reported to be improved by glycosylation (e.g., precipitation; pH, chemical, and thermal denaturation; and aggregation) (Tab. 2).
Precipitation
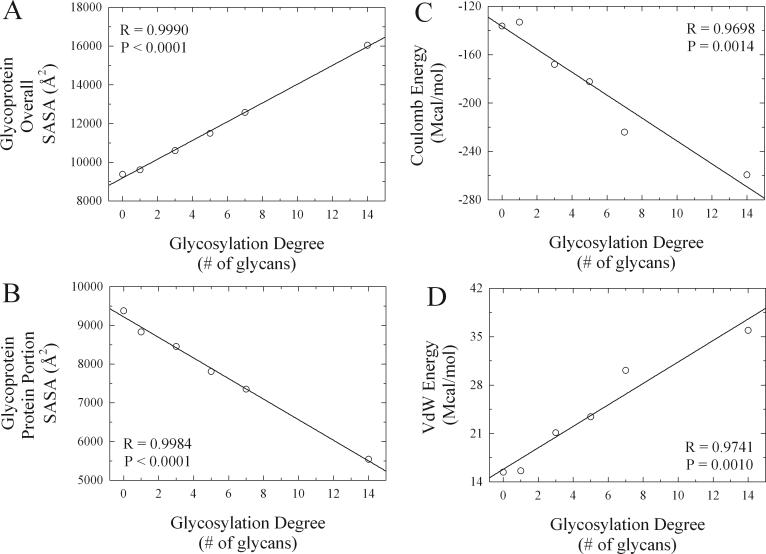
One of the most fundamental challenges when designing a protein-based formulation involves achieving the desired therapeutic protein concentration in solution.2,63 This is due to the fact that protein solubility is not only inversely proportional to the protein concentration but also dependant on the solution's pH, temperature, ionic strength, and excipient concentration.2,52,63,155,156 Therefore, as the target concentration of the formulation is increased (e.g. ≥ 100 mg/mL) protein precipitation becomes a more critical problem.63 Glycosylation has been shown to increase the solubility of many proteins,99,157 although the generality of this effect has been questioned.158 Some examples include interferon beta (REBIF®, Pfizer / Serono; AVONEX®, Biogen),159,160 alpha-galactosidase A (REPLAGAL®, Shire),161 glucose oxidase,162 and invertase.163 While studying the effects of glycosylation on peroxidase, Tams et al. determined that the solubility of the protein showed a linear dependence with the glycosylation degree.164 Although one could logically consider that this increased solubility is due to a greater hydration potential since the glycans have a higher affinity for the aqueous solvent than the polypeptide chain, Bagger et al. recently showed that this is not the case.165 From this study it was concluded that it is unlikely that strengthened interactions with the aqueous solvent are the mechanism for increased protein solubility due to glycosylation.165 Analternative explanation can be provided from a comparative in silico structural and energetic analysis recently performed by Solá and Griebenow on a series of chemically glycosylated α-chymotrypsin conjugates with increasing levels of glycosylation (Fig. 1).103,166 From these computer simulations it was found that the overall molecular solvent accessible surface area (SASA) for the whole glycoprotein increased linearly as the glycosylation degree was increased (Fig. 2A).103,166 The linear dependence of these results are agreement with the solubility findings of Tams et al..164 These results therefore suggest the mechanism by which glycosylation increases protein solubility is due to an increase in the number of possible interactions between the glycoprotein surface and the surrounding solvent molecules due to an overall greater molecular solvent accessible surface area (SASA) caused by the presence of the glycans.
Figure 1.
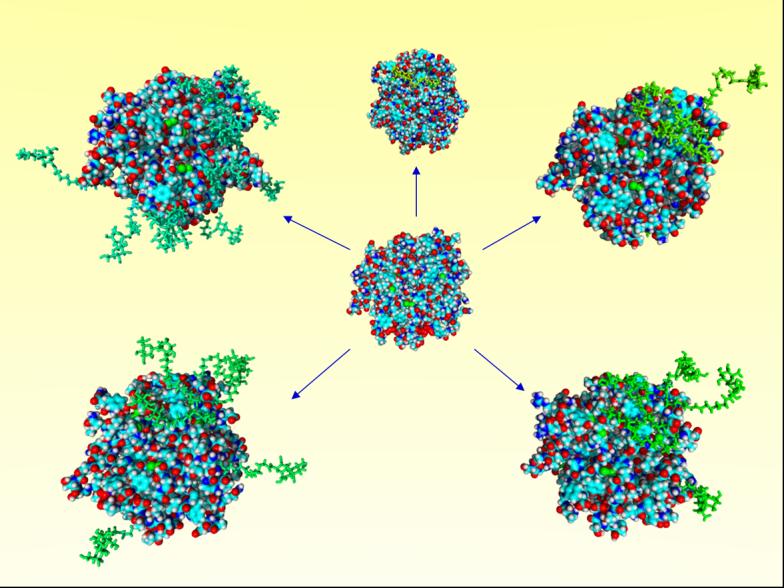
Molecular models for the α-chymotrypsin (α-CT) glycoconjugates engineered through chemical glycosylation. α-CT at center and α-CT glycoconjugates with glycosylation degree increasing clockwise from top (Lacn-α-CT with varied n: 1, 3, 5, 7, and 14). Protein represented in CPK style with standard atom coloring, glycans represented in stick style with green coloring. Reproduced with permission of Springer, from Solá et al.103
Figure 2.
Changes in energetic parameters and in solvent accessible surface area (SASA) for the overall glycoprotein and for the protein portion of the glycoprotein as a function of glycosylation degree. Results were derived from calculations performed on the molecular models constructed for the various α-CT glycoconjugates engineered through chemical glycosylation (see Fig. 1). Glycosylation degree is equal to the number of glycan molecules chemically attached to the protein surface. Adapted with permission of Wiley-Blackwell, from Solá and Griebenow.166
pH Denaturation
Exposure of proteins to extremes of pH can result in loss of structure by disruption of both internal electrostatic forces and charge-charge interactions.2 At extreme pH values, far from the isoelectric point (pI), the unfolding propensity of proteins increases as a result of electrostatic repulsions between similarly charged atoms.2,151,167,168 Additionally, the diminished capability of salt bridge formation between differently charged atoms at extremes of pH can also increase the structural unfolding propensity of proteins.2 This partial unfolding leads to a reduction in local charge density which can further decrease the electrostatic free energy of the protein leading to global unfolding.2,169
There are several reports were glycosylation is essential in maintaining the conformational stability of proteins against pH denaturation. Some examples include GCSF (GRANOCYTE®; Chugai Pharma),124,170 erythropoietin (EPOGEN®, PROCIT®; Amgen, Ortho),171 acid phosphatase,172 amylase,137 bromelain,173 fibronectin,174 cathepsin E,175 glucose oxidase,176 and tripeptidyl peptidase.177 Increased pH stability can be also artificially engineered into proteins as was demonstrated by Masárová through the glycation of penicillin G acylase.178 The half-life for the glycated version of this protein was increased 13-fold at pH 3 and 7-fold at pH 10 when compared to the non-glycated protein.178
Mechanistically this type of stabilization occurs due to an increase in the internal electrostatic interactions of the protein as a result of glycosylation.103 Support for this mechanism was recently provided by the comparative in silico structural and energetic analysis conducted by Solá and Griebenow on a series of chemically glycosylated αchymotrypsin conjugates with increasing levels of glycosylation (Fig. 1).103,166 From these computer simulations it was found that the solvent accessible surface area (SASA) for the protein portion of the glycoconjugates decreased linearly as the number of surface bound glycans was increased (Fig. 2B).103,166 The presence of the glycans thus increases the effective distance between the protein electrostatics and the solvent electrostatics by acting as a molecular spacer. This should lead to an increase in the strength of the internal electrostatic interactions for the protein due to a smaller dielectric screening effect on the protein by the surrounding water molecules.103,166 The observed increase in the coulombic energy parameter (reflected in larger negative values) as the glycosylation degree was increased for the in silico glycoconjugates analyzed by Solá and Griebenow provide support for the occurrence of this phenomena (Fig. 2C). This phenomenon also has the peculiarity that it transforms the overall conformational fluctuations of the protein from being solvent slaved to non-slaved (slaved refers to molecular phenomena influenced by the solvent electric dipole moment fluctuations).103,166,179,180 Physically this transduces into the generally observed decrease in structural dynamics and increase in conformational stability for glycosylated proteins (Fig. 3 and 4).102,103,166
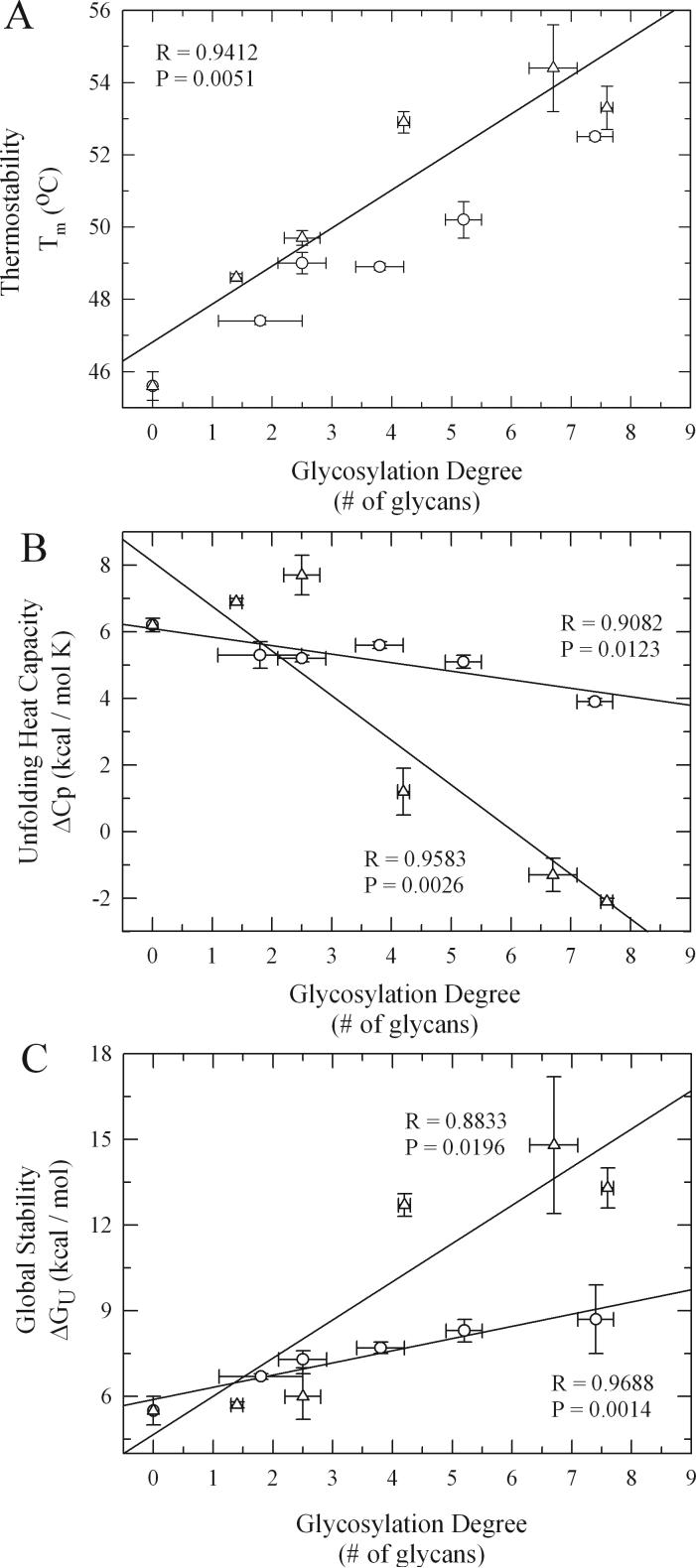
Figure 3.
Changes in thermodynamic unfolding parameters as a function of glycosylation degree and glycan size (lactose (○) and dextran (Δ)) for the various α-CT glycoconjugates engineered through chemical glycosylation. Reproduced with permission of Springer, from Solá et al.103
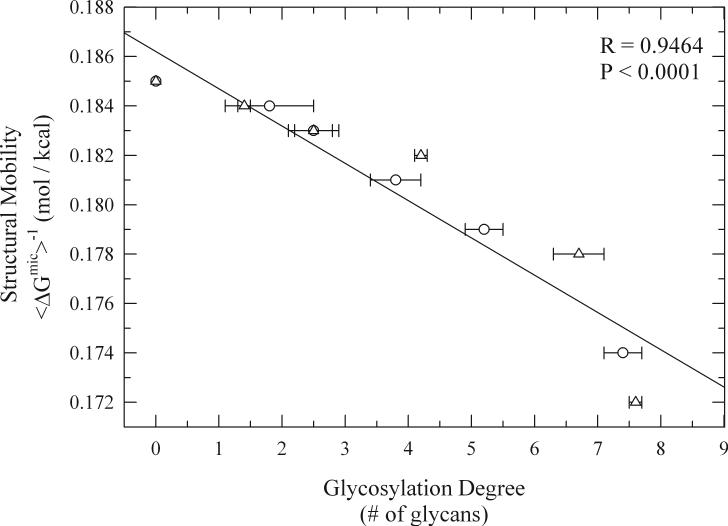
Figure 4.
Changes in protein structural mobility (〈ΔGmic〉−1) as a function of glycosylation degree and glycan size (lactose (○) and dextran (Δ)) for the various α-CT glycoconjugates engineered through chemical glycosylation. Reproduced with permission of Springer, from Solá et al.103
Chemical Denaturation
In addition to electrostatic interactions, the native state of proteins is also conformationally stabilized by other non-covalent forces, such as, hydrophobic interactions and hydrogen bonds. The strength of these forces is often probed indirectly by exposing the protein to chemical denaturants that can selectively disrupt them, such as, guadinidium hydrochloride (GdnHCl), urea, and sodium dodecyl sulfate (SDS).2 Multiple studies have shown that glycosylation can increase the conformational stability of proteins against chemically induced denaturation. Some examples include alpha-1 antitrypsin (PROLASTIN®; Talecris Biotherapeutics),181 erythropoietin (EPOGEN®, PROCIT®; Amgen, Ortho)171 lecithin cholesterol acyltransferase,182 acid phosphatase,172 bromelain,183 lysozyme,184 amylase,185 and peroxidase.186,187 Evidence that this type of stability can also be engineered into proteins was recently provided by Sundaram through the chemical glycosylation of α-chymotrypsin and by Srivastava through the chemical glycosylation of amylase.136,188 In the α-chymotrypsin studies it was found that the protein could be stabilized against both urea and SDS denaturation by glycosylation.188 These results therefore suggest that the mechanism by which glycosylation increases the chemical denaturation stability of proteins must involve an increase in the strength of their hydrogen bonding and hydrophobic interactions. The increase in Van der Waals (VdW) energy as a function of increased glycosylation degree observed by Solá and Griebenow during the in silico structural energetic analysis recently conducted on this protein provides further support to this argument (Fig. 2D).103,166 While increased hydrogen bonding strengths can be explained by the reduced water dielectric screening (H-bonds are treated as pure electrostatic interactions in current protein computational forcefields), increased hydrophobic interaction strengths can be explained by the increased structural compactness and rigidification of the protein core upon glycosylation.103,166
Thermal Denaturation
Proteins can also denature due to exposure to extremes of temperature since all of the forces that stabilize their native-state structure are sensitive to thermal changes.53,56-58 Therefore, it is no surprise that the principal stability indicator used to establish if a formulation strategy stabilizes a protein involves the determination of its thermal denaturation susceptibility.2,6,53,58 Coincidently, this is one of the most fundamental biophysical properties which becomes altered for proteins upon their glycosylation.99,101-103 The number of proteins whose thermal stability has been reported to be increased by glycosylation is extensive. Some pharmaceutically-relevant examples include erythropoietin (EPOGEN®, PROCIT®; Amgen, Ortho),171 alpha 1-antitrypsin (PROLASTIN®; Talecris Biotherapeutics),181 G-CSF (GRANOCYTE®; Chugai Pharma),124,146,170 interferon-beta (REBIF®, Pfizer/EMD Serono; AVONEX®, Biogen),149,159 RNAse (ONCONASE®; Alfacell),129,189,190 follicle-stimulating hormone (GONAL-F®; EMD Serono),191 urokinase (ABBOKINASE®; ImaRx Therapeutics),192 α-glucosidase (MYOZYME®; Shire),193 α-chymotrypsin (MOBE MUGOS®; Marlyn Nutraceuticals),101-103,188 lecithin cholesterol acyltransferase,182 and IgG-like antibodies.194,195 It is important to note that thermodynamic theory also predicts that all proteins will also be susceptible to cold denaturation at ambient pressures.154,196,197 This creates a significant problem during the production of protein-based pharmaceuticals as their handling often requires repeated freeze-thawing cycles.2,10,47,55,198-200 In this context, it was recently reported by Jiang et al. that glycosylation increases the conformational stability of cystatin during freezing.201
Multiple mechanistic studies have been conducted to try to determine the molecular mechanisms involved in protein thermodynamic stabilization by glycosylation. For example, Dwek and coworkers related the increased thermostability of glycosylated RNAse to a decrease in its overall structural dynamics through H/D exchange NMR studies.128,190 Gervais et al. came to the same conclusion upon examination of the structural dynamics of glycosylated G-CSF by NMR.202 It is interesting to note that from the studies conducted by Dwek and coworkers it was found that the reduction in structural mobility due to glycosylation occurred in regions as far as 30Å away from the glycosylation site suggesting that these local effects could be transferred throughout the whole protein structure.203 Additionally, in both of these studies it was found that the glycans interacted weakly with the protein surface suggesting that the glycans extend into the solution, away from the protein surface.128,190,202
Wang et al. performed a systematic study on several natural glycoproteins (invertase, fetuin, glucoamylase, ovotransferrin, and avidin) to determine the generality of these stabilizing effects by glycosylation.98 In this study, the naturally glycosylated proteins were deglycosylated enzymatically and the changes in their stability studied through calorimetric analysis.98 For all these proteins, a decrease in Tm was found after enzymatic deglycosylation with the most glycosylated proteins displaying the greatest changes in Tm. Curiously, the magnitude of this change was found to be independent of the linkage (N- or O-linked) and branching (mono- or multi-branched) of the glycans but dependant on the carbohydrate content of the structurally different glycoproteins.98 Subsequent comparative calorimetric studies between the glycosylated isoform of ovomucoid and its non-glycosylated isoform led DeKoster and Robertson to conclude that the increase in thermodynamic stability of glycoproteins was mainly of an entropic nature due to the lack of change in the enthalpy of unfolding (ΔHm) between these homologous proteins.204 Another study that provided some additional fundamental insights into the increased thermodynamic stability for glycoproteins was performed by Kwon and Yu in 1997 by studying the effects of glycosylation on the unfolding and refolding rates of human alpha 1-antitrypsin (PROLASTIN®; Talecris Biotherapeutics).181 It was found that glycosylation slows the protein unfolding process without affecting the refolding rates significantly. From these results it was proposed that the increase in thermodynamic stability caused by glycosylation could be due to stabilization of the native state and not due to destabilization of the unfolded state.181
Through the use of glycation with small sized glycans (e.g. glucose, fructose) De Jongh and collaborators recently reported that β-lactoglobulin thermostability could be artificially enhanced by increasing the degree of glycosylation, reinforcing the generality of these effects.205 From this work, it was proposed that glycans achieved such effects by lowering the protein's change in heat capacity of unfolding (ΔCp).119 It is important to note that in theory ΔCp can be lowered by both stabilizing the native state as well as by destabilizing the unfolded state (ΔCp = Cp(unfolded) − Cp(native)). To determine the influence that the glycosylation parameters had on increasing the thermodynamic stability of proteins and to further the mechanistic understanding of these effects by glycans, Solá et al. recently performed a detailed experimental thermodynamic analysis on a series of chemically glycosylated α-chymotrypsin conjugates by differential scanning calorimetry (DSC).101-103 In this study, both the amount of surface bound glycans (glycosylation degree) and the size of the attached glycans were systematically varied. It was found that increases in the glycosylation degree shifted the Tm linearly to higher temperature values independently of the glycan's molecular size (Fig. 3A).101-103 It is important to note that although the thermostabilizing effects of both glycation and chemical glycosylation could be caused by a decrease in the protein's isoelectric point (pI) due to alteration of the surface lysine charges, this is not the case. Evidence of this comes from the fact that acetylation of α-chymotrypsin lysine residues which is chemically analogous to glycosylation at the lysine residues and leads to a similar decrease in pI, leads to a decrease in protein stability.206 Interestingly, increasing the pI of proteins by making them more positively-charged through guanidination increases thermostability.206,207 Since the observed increase in thermal stability upon chemical glycosylation occurred only up to a certain maximum temperature and could be statistically correlated with an overall structural rigidification of the protein, from data determined by H/D exchange FTIR experiments (Fig. 4), this suggests that the protein core has reached its maximum compactness.101-103 Therefore the magnitude of thermal stabilization achieved by increasing the glycosylation degree should be specific to each different protein and reflects the maximum amount of native state stabilization that the protein can obtain (it is important to note that additional overall stabilization can be brought about by destabilizing the unfolded state). An additional effect that was observed in this study was that increasing the glycosylation degree led to a decrease in ΔCp although here it was found that increases in glycan size led to a more pronounced lowering of ΔCp, reaching even negative values for the most glycosylated conjugates which is rare for protein unfolding (Fig. 3B).101-103 Since the decrease in ΔCp as a result of increased glycosylation degree could be also related to native-state stabilization through a decrease in protein structural dynamics this result suggests that increasing the glycan's size could possibly destabilize the unfolded state.101,103 This is due to the fact that a negative ΔCp implies a lower Cp for the unfolded state than for the folded state (ΔCp = Cp(unfolded) − Cp(native)). This conclusion is further supported by the fact that the Gibbs free energy of unfolding (ΔGU(25°C)) which is indicative of overall protein stability increased with increases in the glycosylation degree and to an even larger extent with increases in the glycan size (Fig. 3C).101-103 Comparison of the magnitude of maximum gains in overall conformational stability (ΔΔGU(25°C)) induced by chemical glycosylation of αchymotrypsin (ΔΔGU(25°C) ∼ 9 kcal/mol) with those induced by the traditionally employed carbohydrate excipients in liquid formulations (e.g. trehalose, sucrose, fructose) (ΔΔGU(25°C) ∼ 3 kcal/mol) reveals the potentially greater stabilization effect by the covalent attachment of the glycans to the protein surface at a greatly reduced effective molar glycan concentration (∼ 0.1 mM for surface bound glycans vs. 1M for solution free glycans).101,103,208,209 Furthermore, examination of the literature reveals that the average thermodynamic stabilization afforded per glycan unit attached to the protein surface is ∼ 1−2 kcal/mol.103,183,210,211 Mechanistically all of these results suggest that the glycosylation parameters play different roles in the overall thermodynamic stabilization of the protein.103 For example, while the glycosylation degree mainly influences protein thermal stability by stabilizing the native state through increased internal non-covalent forces and decreased structural dynamics, the glycan size can further influence the overall thermodynamic stability of proteins by destabilizing the unfolded state.103
Kinetic Inactivation
The long-term storage times to which protein-based pharmaceuticals are usually exposed provide an additional challenge for the preservation of their structural intactness. This is due to the fact that many of the aforementioned physicochemical instabilities could still occur kinetically for a thermodynamically stabilized protein.2,55,59 Several studies conducted under accelerated degradation conditions suggest that glycosylation can increase the long-term stability of proteins. For example, early reports by Dellacherie et al., Lenders and Crichton, and Srivastava on glycated hemoglobin and amylase evidenced an increase in the functional lifetimes of these proteins when exposed to extremely high temperatures.136,212,213 In subsequent studies, it was found that deglycosylation of catalase, human interleukin 5, erythropoietin, G-CSF, and the chemokine CCL2 led to a decrease in their kinetic stabilities.146,214-217 While studying the effects of the natural glycans of phytase on its overall stability Hoiberg-Nielsen et al. recently found that their presence significantly increased the kinetic stability of the protein by reducing the rate of aggregation while leaving the equilibrium melting temperature relatively unaltered.218 More recently Solá et al. studied the effects of the glycosylation degree and glycan size on the kinetic stability of α-chymotrypsin.101,103 It was found that both the degree of glycosylation and the glycan size increased the protein's inactivation half-lifes but with significantly greater magnitude of kinetic stabilization brought about at increasing glycan size.101,103 In agreement with these results, Tams and Welinder also found a correlation between increased glycosylation amount and increased kinetic stability for peroxidase relating these effects to a dampening of both native and unfolded state backbone fluctuations.186 These results again suggest that both the glycosylation degree and glycan size can play different roles in the kinetic stabilization of proteins with the glycan's size leading to a larger stabilization effect by possibly destabilizing the unfolded state. These results are also intriguing since they highlight the fact that protein samples with similar thermal stabilities (Tm values) will not necessarily display similar kinetic and overall stabilities (ΔGU(25°C)) which is often an assumption during protein stability studies.2
Aggregation
Proteins behave as colloids due to their large molecular sizes coupled with their high intermolecular interaction potentials.20,60,219 This makes the protein structure susceptible to aggregation-prone phase transitions that are dependant on pH, temperature, and protein concentration. Aggregation of protein pharmaceuticals is undesirable due to the potential harmful effects of these on the patient and on the increased production costs due to additional protein recovery and refolding protocols.11,14,20,47,60-65,220,221 There are several reports where glycosylation has been shown to either reduce or prevent protein aggregation. For example, Baudys et al. reported that the physical stability of insulin could be improved by reducing its aggregation kinetics through the chemical attachment of small sized glycans.97 Reduced insulin aggregation was related in this work to prevention of a transamidation crosslinking reaction which suggests a stabilizing mechanism involving steric intermolecular repulsion phenomena.97 Ioannou et al. found that for α-galactosidase A (REPLAGAL®; Shire) glycosylation at Asn215 is required to prevent the exposure of a surface hydrophobic patch that facilitates the aggregation of the protein.161 Weintraub et al. reported that deglycosylation of thyroid-stimulating hormone (THYROGEN®; Genzyme) made the protein more prone to aggregation.130 Similar results were found for erythropoietin (EPOGEN®, PROCIT®; Amgen, Ortho) by Endo et al..222 Hoiberg-Nielsen et al. also reported increased colloidal stability for the glycosylated form of phytase.218 From their studies on this protein it was proposed that the inhibition of aggregation was likely dependant on steric hindrance of the glycans in the unfolded protein state and not on their hydration-related properties.165,218,223 More recently Solá et al. conducted an accelerated aggregation study directed at understanding the mechanisms by which systematic changes in the glycosylation parameters could impact non-specific protein aggregation.101,103 It was found that under extreme conditions (temperature = 60°C and protein concentration = 20 mg/mL), aggregation could not be prevented by the smaller sized glycans irrespective of the amount bound to the protein surface. In contrast, the aggregation process was completely inhibited upon chemical glycosylation with two or more of the larger sized glycans.101,103 All of these results therefore suggest a mechanism in which protein aggregation is prevented due to an increase in steric repulsions between aggregation-prone protein species due to the presence of the glycans on the protein surface.
SUMMARY
Design of successful protein-based therapeutics requires the simultaneous optimization of both in vitro and in vivo molecular stability as well as improved pharmacokinetics and pharmacodynamics. Glycosylation could provide ample opportunities in this respect since in principle all of these could be simultaneously optimized through glycoengineering.100 While the pharmaceutical application of glycosylation still suffers from some technical challenges due to the intrinsically complex nature of glycoprotein structure and the difficulties related to glycoprotein production in host-expression systems (e.g., low glycoprotein expression yields, glycosylation macro- and micro-heterogeneity), further advancements in the understanding of chemical- and enzyme-based glycan remodeling strategies being currently pursued by glycoengineering companies (e.g., Neose Technologies, GlycoFi, GlycArt Biotechnology, GlycoForm), will allow for the rational design of targeted glycoprotein structures.
As discussed in this review, glycosylation has been shown to ameliorate a multitude of pharmaceutically-relevant chemical and physical protein instabilities. Mechanistically, the different glycosylation parameters (e.g., number of glycans attached and glycan molecular size) studied so far can apparently impart different stabilization effects on the protein. While increasing the glycosylation degree apparently stabilizes the protein native state by increasing the internal non-covalent forces and rigidifying the protein structure, increasing the glycan molecular size appears to destabilize the protein unfolded state. The review also points out areas in which a more fundamental knowledge is necessary to further decipher the effects of glycosylation. For example, the impact of glycosylation on the behavior of the unfolded state still needs further investigation. Furthermore, more systematic studies are needed to understand the mechanisms by which glycans prevent chemical instability events. It is important to note the possibility that other instabilities not explored so far (e.g. deamidation, β-elimination, racemization, adsorption to amphipatic interfaces and hydrophobic surfaces) could be also ameliorated or prevented by glycosylation; this therefore remains to be tested. Nevertheless, the significant potential that glycosylation engineering holds towards improving the physicochemical properties of protein pharmaceuticals should lead to further research towards the understanding of the fundamental effects that glycans have on proteins.
ACKNOWLEDGEMENTS
This publication was made possible by a grant (S06 GM08102) from the National Institute of General Medical Sciences (NIGMS) at the National Institutes of Health (NIH) through the Support of Competitive Research (SCORE) program.
REFERENCES
- 1.Andersen DC, Krummen L. Recombinant protein expression for therapeutic applications. Curr Opin Biotechnol. 2002;13(2):117–123. doi: 10.1016/s0958-1669(02)00300-2. [DOI] [PubMed] [Google Scholar]
- 2.Wang W. Instability, stabilization, and formulation of liquid protein pharmaceuticals. Int J Pharm. 1999;185(2):129–188. doi: 10.1016/s0378-5173(99)00152-0. [DOI] [PubMed] [Google Scholar]
- 3.Illanes A. Stability of biocatalysts. Electronic J Biotechnol. 1999;2(11):1–9. [Google Scholar]
- 4.Wang W. Lyophilization and development of solid protein pharmaceuticals. Int J Pharm. 2000;203(1−2):1–60. doi: 10.1016/s0378-5173(00)00423-3. [DOI] [PubMed] [Google Scholar]
- 5.Frokjaer S, Otzen DE. Protein drug stability: a formulation challenge. Nat Rev Drug Discov. 2005;4(4):298–306. doi: 10.1038/nrd1695. [DOI] [PubMed] [Google Scholar]
- 6.Manning MC, Patel K, Borchardt RT. Stability of Protein Pharmaceuticals. Pharm Res. 1989;6(11):903. doi: 10.1023/a:1015929109894. [DOI] [PubMed] [Google Scholar]
- 7.Davis GC. Protein stability: impact upon protein pharmaceuticals. Biologicals. 1993;21(2):105. doi: 10.1006/biol.1993.1057. [DOI] [PubMed] [Google Scholar]
- 8.Krishnamurthy R, Manning MC. The stability factor: importance in formulation development. Curr Pharm Biotechnol. 2002;3(4):361–371. doi: 10.2174/1389201023378229. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JL, Powell MF, Shire SJ. The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Crit Rev Ther Drug Carrier Syst. 1993;10(4):307–377. [PubMed] [Google Scholar]
- 10.Patro SY, Freund E, Chang BS. Protein formulation and fill-finish operations. Biotechnol Annu Rev. 2002;8:55–84. doi: 10.1016/s1387-2656(02)08004-3. [DOI] [PubMed] [Google Scholar]
- 11.Wang W. Protein aggregation and its inhibition in biopharmaceutics. Int J Pharm. 2005;289(1−2):1–30. doi: 10.1016/j.ijpharm.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 12.Wang W, Singh S, Zeng DL, King K, Nema S. Antibody structure, instability, and formulation. J Pharm Sci. 2007;96(1):1–26. doi: 10.1002/jps.20727. [DOI] [PubMed] [Google Scholar]
- 13.Meyer JD, Ho B, Manning MC. Effects of conformation on the chemical stability of pharmaceutically relevant polypeptides. Pharm Biotechnol. 2002;13:85–107. doi: 10.1007/978-1-4615-0557-0_4. [DOI] [PubMed] [Google Scholar]
- 14.Cromwell ME, Hilario E, Jacobson F. Protein aggregation and bioprocessing. AAPS J. 2006;8(3):E572–579. doi: 10.1208/aapsj080366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Volkin DB, Mach H, Middaugh CR. Degradative covalent reactions important to protein stability. Mol Biotechnol. 1997;8(2):105–122. doi: 10.1007/BF02752255. [DOI] [PubMed] [Google Scholar]
- 16.Lai MC, Hageman MJ, Schowen RL, Borchardt RT, Topp EM. Chemical stability of peptides in polymers. 1. Effect of water on peptide deamidation in poly(vinyl alcohol) and poly(vinyl pyrrolidone) matrixes. J Pharm Sci. 1999;88(10):1073–1080. doi: 10.1021/js980227g. [DOI] [PubMed] [Google Scholar]
- 17.Lai MC, Hageman MJ, Schowen RL, Borchardt RT, Laird BB, Topp EM. Chemical stability of peptides in polymers. 2. Discriminating between solvent and plasticizing effects of water on peptide deamidation in poly(vinylpyrrolidone). J Pharm Sci. 1999;88(10):1081–1089. doi: 10.1021/js9802289. [DOI] [PubMed] [Google Scholar]
- 18.Lai MC, Topp EM. Solid-state chemical stability of proteins and peptides. J Pharm Sci. 1999;88(5):489–500. doi: 10.1021/js980374e. [DOI] [PubMed] [Google Scholar]
- 19.Roque AC, Lowe CR, Taipa MA. Antibodies and genetically engineered related molecules: production and purification. Biotechnol Prog. 2004;20(3):639–654. doi: 10.1021/bp030070k. [DOI] [PubMed] [Google Scholar]
- 20.Chi EY, Krishnan S, Kendrick BS, Chang BS, Carpenter JF, Randolph TW. Roles of conformational stability and colloidal stability in the aggregation of recombinant human granulocyte colony-stimulating factor. Protein Sci. 2003;12(5):903–913. doi: 10.1110/ps.0235703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robinson NE. Protein deamidation. Proc Natl Acad Sci USA. 2002;99(8):5283–5288. doi: 10.1073/pnas.082102799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Nguyen TH, Schoneich C, Borchardt RT. Aggregation and precipitation of human relaxin induced by metal-catalyzed oxidation. Biochemistry. 1995;34(17):5762–5772. doi: 10.1021/bi00017a008. [DOI] [PubMed] [Google Scholar]
- 23.Dong A, Prestrelski SJ, Allison SD, Carpenter JF. Infrared spectroscopic studies of lyophilization- and temperature-induced protein aggregation. J Pharm Sci. 1995;84(4):415–424. doi: 10.1002/jps.2600840407. [DOI] [PubMed] [Google Scholar]
- 24.Prestrelski SJ, Arakawa T, Carpenter JF. Separation of freezing- and drying-induced denaturation of lyophilized proteins using stress-specific stabilization. II. Structural studies using infrared spectroscopy. Arch Biochem Biophys. 1993;303(2):465–473. doi: 10.1006/abbi.1993.1310. [DOI] [PubMed] [Google Scholar]
- 25.Prestrelski SJ, Tedeschi N, Arakawa T, Carpenter JF. Dehydration-induced conformational transitions in proteins and their inhibition by stabilizers. Biophys J. 1993;65(2):661–671. doi: 10.1016/S0006-3495(93)81120-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costantino HR, Griebenow K, Mishra P, Langer R, Klibanov A. Fourier-Transform Infrared Spectroscopic Investigation Of Protein Stability In The Lyophilized Form. Biochim Biophys Acta-Protein Struct Molec Enzym. 1995;1253(1):69–74. doi: 10.1016/0167-4838(95)00156-o. [DOI] [PubMed] [Google Scholar]
- 27.Griebenow K, Castellanos I, Carrasquillo KG. Application of FTIR spectroscopy to probe and improve protein structure in sustained release devices. Internet Journal of Vibrational Spectroscopy. 1999;3(5):2. [ wwwijvscom]
- 28.Griebenow K, Klibanov AM. Lyophilization-Induced Reversible Changes In The Secondary Structure Of Proteins. Proc Natl Acad Sci USA. 1995;92(24):10969–10976. doi: 10.1073/pnas.92.24.10969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carrasquillo KG, Sanchez C, Griebenow K. Relationship between conformational stability and lyophilization-induced structural changes in chymotrypsin. Biotechnol Appl Biochem. 2000;31(Pt 1):41–53. doi: 10.1042/ba19990087. [DOI] [PubMed] [Google Scholar]
- 30.Costantino HR, Langer R, Klibanov AM. Moisture-Induced Aggregation Of Lyophilized Insulin. Pharm Res. 1994;11(1):21–29. doi: 10.1023/a:1018981208076. [DOI] [PubMed] [Google Scholar]
- 31.Costantino HR, Firouzabadian L, Hogeland K, Wu C, Beganski C, Carrasquillo KG, Cordova M, Griebenow K, Zale SE, Tracy MA. Protein spray-freeze drying. Effect of atomization conditions on particle size and stability. Pharm Res. 2000;17(11):1374–1383. doi: 10.1023/a:1007570030368. [DOI] [PubMed] [Google Scholar]
- 32.Costantino HR, Firouzabadian L, Wu C, Carrasquillo KG, Griebenow K, Zale SE, Tracy MA. Protein spray freeze drying. 2. Effect of formulation variables on particle size and stability. J Pharm Sci. 2002;91(2):388–395. doi: 10.1002/jps.10059. [DOI] [PubMed] [Google Scholar]
- 33.Tzannis ST, Prestrelski SJ. Moisture effects on protein-excipient interactions in spray-dried powders. Nature of destabilizing effects of sucrose. J Pharm Sci. 1999;88(3):360–370. doi: 10.1021/js9800127. [DOI] [PubMed] [Google Scholar]
- 34.Maa YF, Nguyen PA, Andya JD, Dasovich N, Sweeney TD, Shire SJ, Hsu CC. Effect of spray drying and subsequent processing conditions on residual moisture content and physical/biochemical stability of protein inhalation powders. Pharm Res. 1998;15(5):768–775. doi: 10.1023/a:1011983322594. [DOI] [PubMed] [Google Scholar]
- 35.Abdul-Fattah AM, Kalonia DS, Pikal MJ. The challenge of drying method selection for protein pharmaceuticals: product quality implications. J Pharm Sci. 2007;96(8):1886–1916. doi: 10.1002/jps.20842. [DOI] [PubMed] [Google Scholar]
- 36.Abdul-Fattah AM, Lechuga-Ballesteros D, Kalonia DS, Pikal MJ. The impact of drying method and formulation on the physical properties and stability of methionyl human growth hormone in the amorphous solid state. J Pharm Sci. 2008;97(1):163–184. doi: 10.1002/jps.21085. [DOI] [PubMed] [Google Scholar]
- 37.Abdul-Fattah AM, Truong-Le V, Yee L, Nguyen L, Kalonia DS, Cicerone MT, Pikal MJ. Drying-induced variations in physico-chemical properties of amorphous pharmaceuticals and their impact on stability (I): stability of a monoclonal antibody. J Pharm Sci. 2007;96(8):1983–2008. doi: 10.1002/jps.20859. [DOI] [PubMed] [Google Scholar]
- 38.Abdul-Fattah AM, Truong-Le V, Yee L, Pan E, Ao Y, Kalonia DS, Pikal MJ. Drying-induced variations in physico-chemical properties of amorphous pharmaceuticals and their impact on Stability II: stability of a vaccine. Pharm Res. 2007;24(4):715–727. doi: 10.1007/s11095-006-9191-2. [DOI] [PubMed] [Google Scholar]
- 39.Putney SD, Burke PA. Improving protein therapeutics with sustained-release formulations. Nat Biotechnol. 1998;16(2):153–157. doi: 10.1038/nbt0298-153. [DOI] [PubMed] [Google Scholar]
- 40.Langer R. Drug delivery and targeting. Nature. 1998;392(6679 Suppl):5–10. [PubMed] [Google Scholar]
- 41.van de Weert M, Hennink WE, Jiskoot W. Protein instability in poly(lactic-co-glycolic acid) microparticles. Pharm Res. 2000;17(10):1159–1167. doi: 10.1023/a:1026498209874. [DOI] [PubMed] [Google Scholar]
- 42.Perez C, Castellanos IJ, Costantino HR, Al-Azzam W, Griebenow K. Recent trends in stabilizing protein structure upon encapsulation and release from bioerodible polymers. J Pharm Pharmacol. 2002;54(3):301–313. doi: 10.1211/0022357021778448. [DOI] [PubMed] [Google Scholar]
- 43.Carrasquillo KG, Costantino HR, Cordero RA, Hsu CC, Griebenow K. On the structural preservation of recombinant human growth hormone in a dried film of a synthetic biodegradable polymer. J Pharm Sci. 1999;88(2):166–173. doi: 10.1021/js980272o. [DOI] [PubMed] [Google Scholar]
- 44.Fu K, Griebenow K, Hsieh L, Klibanov AM, Langer R. FTIR characterization of the secondary structure of proteins encapsulated within PLGA microspheres. J Controlled Release. 1999;58(3):357–366. doi: 10.1016/s0168-3659(98)00192-8. [DOI] [PubMed] [Google Scholar]
- 45.Sluzky V, Tamada JA, Klibanov AM, Langer R. Kinetics of insulin aggregation in aqueous solutions upon agitation in the presence of hydrophobic surfaces. Proc Natl Acad Sci USA. 1991;88(21):9377–9381. doi: 10.1073/pnas.88.21.9377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Castellanos IJ, Cruz G, Crespo R, Griebenow K. Encapsulation-induced aggregation and loss in activity of gamma-chymotrypsin and their prevention. J Controlled Release. 2002;81(3):307–319. doi: 10.1016/s0168-3659(02)00073-1. [DOI] [PubMed] [Google Scholar]
- 47.Kueltzo LA, Wang W, Randolph TW, Carpenter JF. Effects of solution conditions, processing parameters, and container materials on aggregation of a monoclonal antibody during freeze-thawing. J Pharm Sci. 2007 doi: 10.1002/jps.21110. In Press. [DOI] [PubMed] [Google Scholar]
- 48.Heller MC, Carpenter JF, Randolph TW. Manipulation of lyophilization-induced phase separation: implications for pharmaceutical proteins. Biotechnol Prog. 1997;13(5):590–596. doi: 10.1021/bp970081b. [DOI] [PubMed] [Google Scholar]
- 49.Chi EY, Krishnan S, Randolph TW, Carpenter JF. Physical stability of proteins in aqueous solution: mechanism and driving forces in nonnative protein aggregation. Pharm Res. 2003;20(9):1325–1336. doi: 10.1023/a:1025771421906. [DOI] [PubMed] [Google Scholar]
- 50.Kerwin BA, Remmele RL., Jr. Protect from light: photodegradation and protein biologics. J Pharm Sci. 2007;96(6):1468–1479. doi: 10.1002/jps.20815. [DOI] [PubMed] [Google Scholar]
- 51.Byrn SR, Xu W, Newman AW. Chemical reactivity in solid-state pharmaceuticals: formulation implications. Adv Drug Deliv Rev. 2001;48(1):115–136. doi: 10.1016/s0169-409x(01)00102-8. [DOI] [PubMed] [Google Scholar]
- 52.Volkin DB, Sanyal G, Burke CJ, Middaugh CR. Preformulation studies as an essential guide to formulation development and manufacture of protein pharmaceuticals. Pharm Biotechnol. 2002;14:1–46. doi: 10.1007/978-1-4615-0549-5_1. [DOI] [PubMed] [Google Scholar]
- 53.Pace CN. Conformational stability of globular proteins. Trends Biochem Sci. 1990;15(1):14–17. doi: 10.1016/0968-0004(90)90124-t. [DOI] [PubMed] [Google Scholar]
- 54.Xie M, Schowen RL. Secondary structure and protein deamidation. J Pharm Sci. 1999;88(1):8–13. doi: 10.1021/js9802493. [DOI] [PubMed] [Google Scholar]
- 55.Arakawa T, Prestrelski SJ, Kenney WC, Carpenter JF. Factors affecting short-term and long-term stabilities of proteins. Adv Drug Deliv Rev. 2001;46(1−3):307–326. doi: 10.1016/s0169-409x(00)00144-7. [DOI] [PubMed] [Google Scholar]
- 56.Privalov PL. Stability of proteins: small globular proteins. Adv Protein Chem. 1979;33:167–241. doi: 10.1016/s0065-3233(08)60460-x. [DOI] [PubMed] [Google Scholar]
- 57.Privalov PL, Tsalkova TN. Micro- and macro-stabilities of globular proteins. Nature. 1979;280(5724):693–696. doi: 10.1038/280693a0. [DOI] [PubMed] [Google Scholar]
- 58.Pace CN. Measuring and increasing protein stability. Trends Biotechnol. 1990;8(4):93–98. doi: 10.1016/0167-7799(90)90146-o. [DOI] [PubMed] [Google Scholar]
- 59.Capelle MAH, Gurny R, Arvinte T. High throughput screening of protein formulation stability: Practical considerations. Eur J Pharm Biopharm. 2007;65(2):131–148. doi: 10.1016/j.ejpb.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 60.Valente JJ, Payne RW, Manning MC, Wilson WW, Henry CS. Colloidal behavior of proteins: effects of the second virial coefficient on solubility, crystallization and aggregation of proteins in aqueous solution. Curr Pharm Biotechnol. 2005;6(6):427–436. doi: 10.2174/138920105775159313. [DOI] [PubMed] [Google Scholar]
- 61.Hawe A, Friess W. Formulation development for hydrophobic therapeutic proteins. Pharm Dev Technol. 2007;12(3):223–237. doi: 10.1080/10837450701247350. [DOI] [PubMed] [Google Scholar]
- 62.Mollmann SH, Jorgensen L, Bukrinsky JT, Elofsson U, Norde W, Frokjaer S. Interfacial adsorption of insulin conformational changes and reversibility of adsorption. Eur J Pharm Sci. 2006;27(2−3):194–204. doi: 10.1016/j.ejps.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 63.Shire SJ, Shahrokh Z, Liu J. Challenges in the development of high protein concentration formulations. J Pharm Sci. 2004;93(6):1390–1402. doi: 10.1002/jps.20079. [DOI] [PubMed] [Google Scholar]
- 64.Roberts CJ. Non-native protein aggregation kinetics. Biotechnol Bioeng. 2007;98(5):927–938. doi: 10.1002/bit.21627. [DOI] [PubMed] [Google Scholar]
- 65.Clark ED. Protein refolding for industrial processes. Curr Opin Biotechnol. 2001;12(2):202–207. doi: 10.1016/s0958-1669(00)00200-7. [DOI] [PubMed] [Google Scholar]
- 66.Marshall SA, Lazar GA, Chirino AJ, Desjarlais JR. Rational design and engineering of therapeutic proteins. Drug Discovery Today. 2003;8(5):212–221. doi: 10.1016/s1359-6446(03)02610-2. [DOI] [PubMed] [Google Scholar]
- 67.Waterman KC, Adami RC, Alsante KM, Hong J, Landis MS, Lombardo F, Roberts CJ. Stabilization of pharmaceuticals to oxidative degradation. Pharm Dev Technol. 2002;7(1):1–32. doi: 10.1081/pdt-120002237. [DOI] [PubMed] [Google Scholar]
- 68.Carpenter JF, Manning MC. Rational Design of Stable Protein Formulations: Theory and Practice. Pharmaceutical Biotechnology. 2002;13 doi: 10.1007/978-1-4615-0557-0_5. [DOI] [PubMed] [Google Scholar]
- 69.Lam XM, Yang JY, Cleland JL. Antioxidants for prevention of methionine oxidation in recombinant monoclonal antibody HER2. J Pharm Sci. 1997;86(11):1250–1255. doi: 10.1021/js970143s. [DOI] [PubMed] [Google Scholar]
- 70.Li S, Patapoff TW, Nguyen TH, Borchardt RT. Inhibitory effect of sugars and polyols on the metal-catalyzed oxidation of human relaxin. J Pharm Sci. 1996;85(8):868–872. doi: 10.1021/js9504550. [DOI] [PubMed] [Google Scholar]
- 71.Arakawa T, Ejima D, Tsumoto K, Obeyama N, Tanaka Y, Kita Y, Timasheff SN. Suppression of protein interactions by arginine: a proposed mechanism of the arginine effects. Biophys Chem. 2007;127(1−2):1–8. doi: 10.1016/j.bpc.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 72.Arakawa T, Tsumoto K, Kita Y, Chang B, Ejima D. Biotechnology applications of amino acids in protein purification and formulations. Amino Acids. 2007;33(4):587–605. doi: 10.1007/s00726-007-0506-3. [DOI] [PubMed] [Google Scholar]
- 73.Costantino HR, Carrasquillo KG, Cordero RA, Mumenthaler M, Hsu CC, Griebenow K. Effect of excipients on the stability and structure of lyophilized recombinant human growth hormone. J Pharm Sci. 1998;87(11):1412–1420. doi: 10.1021/js980069t. [DOI] [PubMed] [Google Scholar]
- 74.Tzannis ST, Prestrelski SJ. Activity-stability considerations of trypsinogen during spray drying: effects of sucrose. J Pharm Sci. 1999;88(3):351–359. doi: 10.1021/js980011e. [DOI] [PubMed] [Google Scholar]
- 75.Timasheff SN. The Control Of Protein Stability And Association By Weak-Interactions With Water - How Do Solvents Affect These Processes. Annual Rev Biophys Biomol Struct. 1993;22:67–97. doi: 10.1146/annurev.bb.22.060193.000435. [DOI] [PubMed] [Google Scholar]
- 76.Perez-Rodriguez C, Montano N, Gonzalez K, Griebenow K. Stabilization of alpha-chymotrypsin at the CH2Cl2/water interface and upon water-in-oil-in-water encapsulation in PLGA microspheres. J Controlled Release. 2003;89(1):71–85. doi: 10.1016/s0168-3659(03)00074-9. [DOI] [PubMed] [Google Scholar]
- 77.Zhu G, Mallery SR, Schwendeman SP. Stabilization of proteins encapsulated in injectable poly (lactide- co-glycolide). Nat Biotechnol. 2000;18(1):52–57. doi: 10.1038/71916. [DOI] [PubMed] [Google Scholar]
- 78.Lee B, Vasmatzis G. Stabilization of protein structures. Current Opinion In Biotechnology. 1997;8(4):423–428. doi: 10.1016/s0958-1669(97)80063-8. [DOI] [PubMed] [Google Scholar]
- 79.Lau KF, Dill KA. Theory for protein mutability and biogenesis. Proc Natl Acad Sci U S A. 1990;87(2):638–642. doi: 10.1073/pnas.87.2.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Capriotti E, Fariselli P, Calabrese R, Casadio R. Predicting protein stability changes from sequences using support vector machines. Bioinformatics. 2005;21(Suppl 2):ii54–58. doi: 10.1093/bioinformatics/bti1109. [DOI] [PubMed] [Google Scholar]
- 81.Capriotti E, Fariselli P, Casadio R. A neural-network-based method for predicting protein stability changes upon single point mutations. Bioinformatics. 2004;20(Suppl 1):i63–68. doi: 10.1093/bioinformatics/bth928. [DOI] [PubMed] [Google Scholar]
- 82.Capriotti E, Fariselli P, Casadio R. I-Mutant2.0: predicting stability changes upon mutation from the protein sequence or structure. Nucleic Acids Res. 2005;33(Web Server issue):W306–310. doi: 10.1093/nar/gki375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Capriotti E, Fariselli P, Rossi I, Casadio R. A three-state prediction of single point mutations on protein stability changes. BMC Bioinformatics. 2008;9(Suppl 2):S6. doi: 10.1186/1471-2105-9-S2-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Randolph TW. Phase separation of excipients during lyophilization: effects on protein stability. J Pharm Sci. 1997;86(11):1198–1203. doi: 10.1021/js970135b. [DOI] [PubMed] [Google Scholar]
- 85.Izutsu K, Aoyagi N, Kojima S. Effect of polymer size and cosolutes on phase separation of poly(vinylpyrrolidone) (PVP) and dextran in frozen solutions. J Pharm Sci. 2005;94(4):709–717. doi: 10.1002/jps.20292. [DOI] [PubMed] [Google Scholar]
- 86.Izutsu K, Yoshioka S, Kojima S, Randolph TW, Carpenter JF. Effects of sugars and polymers on crystallization of poly(ethylene glycol) in frozen solutions: phase separation between incompatible polymers. Pharm Res. 1996;13(9):1393–1400. doi: 10.1023/a:1016086319851. [DOI] [PubMed] [Google Scholar]
- 87.Wakankar AA, Liu J, Vandervelde D, Wang YJ, Shire SJ, Borchardt RT. The effect of cosolutes on the isomerization of aspartic acid residues and conformational stability in a monoclonal antibody. J Pharm Sci. 2007;96(7):1708–1718. doi: 10.1002/jps.20823. [DOI] [PubMed] [Google Scholar]
- 88.Piedmonte DM, Summers C, McAuley A, Karamujic L, Ratnaswamy G. Sorbitol crystallization can lead to protein aggregation in frozen protein formulations. Pharm Res. 2007;24(1):136–146. doi: 10.1007/s11095-006-9131-1. [DOI] [PubMed] [Google Scholar]
- 89.Gabrielson JP, Arthur KK, Kendrick BS, Randolph TW, Stoner MR. Common excipients impair detection of protein aggregates during sedimentation velocity analytical ultracentrifugation. J Pharm Sci. 2008 doi: 10.1002/jps.21403. [DOI] [PubMed] [Google Scholar]
- 90.Gunturi SR, Ghobrial I, Sharma B. Development of a sensitive size exclusion HPLC method with fluorescence detection for the quantitation of recombinant human erythropoietin (r-HuEPO) aggregates. J Pharm Biomed Anal. 2007;43(1):213–221. doi: 10.1016/j.jpba.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 91.Gaberc-Porekar V, Zore I, Podobnik B, Menart V. Obstacles and pitfalls in the PEGylation of therapeutic proteins. Curr Opin Drug Discov Devel. 2008;11(2):242–250. [PubMed] [Google Scholar]
- 92.Ganson NJ, Kelly SJ, Scarlett E, Sundy JS, Hershfield MS. Control of hyperuricemia in subjects with refractory gout, and induction of antibody against poly(ethylene glycol) (PEG), in a phase I trial of subcutaneous PEGylated urate oxidase. Arthritis Res Ther. 2006;8(1):R12. doi: 10.1186/ar1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wang X, Ishida T, Kiwada H. Anti-PEG IgM elicited by injection of liposomes is involved in the enhanced blood clearance of a subsequent dose of PEGylated liposomes. J Control Release. 2007;119(2):236–244. doi: 10.1016/j.jconrel.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 94.Judge A, McClintock K, Phelps JR, Maclachlan I. Hypersensitivity and loss of disease site targeting caused by antibody responses to PEGylated liposomes. Mol Ther. 2006;13(2):328–337. doi: 10.1016/j.ymthe.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 95.Cheng TL, Chen BM, Chern JW, Wu MF, Roffler SR. Efficient clearance of poly(ethylene glycol)-modified immunoenzyme with anti-PEG monoclonal antibody for prodrug cancer therapy. Bioconjug Chem. 2000;11(2):258–266. doi: 10.1021/bc990147j. [DOI] [PubMed] [Google Scholar]
- 96.Vegarud G, Christnsen TB. Glycosylation of Proteins: a new method of enzyme stabilization. Biotechnol Bioeng. 1975;17(9):1391–1397. doi: 10.1002/bit.260170918. [DOI] [PubMed] [Google Scholar]
- 97.Baudys M, Uchio T, Mix D, Wilson D, Kim SW. Physical stabilization of insulin by glycosylation. J Pharm Sci. 1995;84(1):28–33. doi: 10.1002/jps.2600840108. [DOI] [PubMed] [Google Scholar]
- 98.Wang C, Eufemi M, Turano C, Giartosio A. Influence of the carbohydrate moiety on the stability of glycoproteins. Biochemistry. 1996;35(23):7299–7307. doi: 10.1021/bi9517704. [DOI] [PubMed] [Google Scholar]
- 99.Mitra N, Sinha S, Ramya TN, Surolia A. N-linked oligosaccharides as outfitters for glycoprotein folding, form and function. Trends Biochem Sci. 2006;31(3):156–163. doi: 10.1016/j.tibs.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 100.Sinclair AM, Elliott S. Glycoengineering: The effect of glycosylation on the properties of therapeutic proteins. J Pharm Sci. 2005;94(8):1626–1635. doi: 10.1002/jps.20319. [DOI] [PubMed] [Google Scholar]
- 101.Solá RJ, Al-Azzam W, Griebenow K. Engineering of protein thermodynamic, kinetic, and colloidal stability: Chemical glycosylation with monofunctionally activated glycans. Biotechnol Bioeng. 2006;94(6):1072–1079. doi: 10.1002/bit.20933. [DOI] [PubMed] [Google Scholar]
- 102.Solá RJ, Griebenow K. Chemical glycosylation: New insights on the interrelation between protein structural mobility, thermodynamic stability, and catalysis. FEBS Lett. 2006;580(6):1685–1690. doi: 10.1016/j.febslet.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 103.Sola RJ, Rodriguez-Martinez JA, Griebenow K. Modulation of protein biophysical properties by chemical glycosylation: biochemical insights and biomedical implications. Cell Mol Life Sci. 2007;64(16):2133–2152. doi: 10.1007/s00018-007-6551-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhatia PK, Mukhopadhyay A. Protein glycosylation: implications for in vivo functions and therapeutic applications. Adv Biochem Eng Biotechnol. 1999;64:155–201. doi: 10.1007/3-540-49811-7_5. [DOI] [PubMed] [Google Scholar]
- 105.Brown LR. Commercial challenges of protein drug delivery. Expert Opin Drug Deliv. 2005;2(1):29–42. doi: 10.1517/17425247.2.1.29. [DOI] [PubMed] [Google Scholar]
- 106.Weerapana E, Imperiali B. Asparagine-linked protein glycosylation: from eukaryotic to prokaryotic systems. Glycobiology. 2006;16(6):91R–101R. doi: 10.1093/glycob/cwj099. [DOI] [PubMed] [Google Scholar]
- 107.Mann M, Jensen ON. Proteomic analysis of post-translational modifications. Nature Biotechnology. 2003;21(3):255–261. doi: 10.1038/nbt0303-255. [DOI] [PubMed] [Google Scholar]
- 108.Walsh CT, Garneau-Tsodikova S, Gatto GJ. Protein posttranslational modifications: The chemistry of proteome diversifications. Angewandte Chemie-International Edition. 2005;44(45):7342–7372. doi: 10.1002/anie.200501023. [DOI] [PubMed] [Google Scholar]
- 109.Sears P, Wong CH. Enzyme action in glycoprotein synthesis. Cell Mol Life Sci. 1998;54(3):223–252. doi: 10.1007/s000180050146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Lehle L, Strahl S, Tanner W. Protein glycosylation, conserved from yeast to man: a model organism helps elucidate congenital human diseases. Angew Chem Int Ed Engl. 2006;45(41):6802–6818. doi: 10.1002/anie.200601645. [DOI] [PubMed] [Google Scholar]
- 111.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473(1):4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 112.Liu DT. Glycoprotein pharmaceuticals: scientific and regulatory considerations, and the US Orphan Drug Act. Trends Biotechnol. 1992;10(4):114–120. doi: 10.1016/0167-7799(92)90192-x. [DOI] [PubMed] [Google Scholar]
- 113.Hossler P, Mulukutla BC, Hu WS. Systems analysis of N-glycan processing in mammalian cells. PLoS ONE. 2007;2(1):e713. doi: 10.1371/journal.pone.0000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peter-Katalinic J. Methods in enzymology: O-glycosylation of proteins. Methods Enzymol. 2005;405:139–171. doi: 10.1016/S0076-6879(05)05007-X. [DOI] [PubMed] [Google Scholar]
- 115.Medzihradszky KF. Characterization of protein N-glycosylation. Methods Enzymol. 2005;405:116–138. doi: 10.1016/S0076-6879(05)05006-8. [DOI] [PubMed] [Google Scholar]
- 116.Abascal I, Skalaban SR, Grimm KM, Aviles M, Martianez-Menarguez JA, Castells MT, Ballesta J, Alhadeff JA. Alteration of the isoform composition of plasma-membrane-associated rat sperm alpha-L-fucosidase during late epididymal maturation: comparative characterization of the acidic and neutral isoforms. Biochem J. 1998;333(Pt 1):201–207. doi: 10.1042/bj3330201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumar Y, Khachane A, Belwal M, Das S, Somsundaram K, Tatu U. ProteoMod: A new tool to quantitate protein post-translational modifications. Proteomics. 2004;4(6):1672–1683. doi: 10.1002/pmic.200300778. [DOI] [PubMed] [Google Scholar]
- 118.Davis BG. Synthesis of glycoproteins. Chemical Reviews. 2002;102(2):579–601. doi: 10.1021/cr0004310. [DOI] [PubMed] [Google Scholar]
- 119.Van Teeffelen AMM, Broersen K, De Jongh HHJ. Glucosylation of beta-lactoglobulin lowers the heat capacity change of unfolding; a unique way to affect protein thermodynamics. Protein Sci. 2005;14(8):2187–2194. doi: 10.1110/ps.051405005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang L, Persky AM, Hochhaus G, Meibohm B. Pharmacokinetic aspects of biotechnology products. J Pharm Sci. 2004;93(9):2184–2204. doi: 10.1002/jps.20125. [DOI] [PubMed] [Google Scholar]
- 121.Lis H, Sharon N. Protein glycosylation. Structural and functional aspects. Eur J Biochem. 1993;218(1):1–27. doi: 10.1111/j.1432-1033.1993.tb18347.x. [DOI] [PubMed] [Google Scholar]
- 122.Varki A. Biological roles of oligosaccharides: all of the theories are correct. Glycobiology. 1993;3(2):97–130. doi: 10.1093/glycob/3.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Vegarud G, Christensen TB. The resistance of glycoproteins to proteolytic inactivation. Acta Chem Scand B. 1975;29(8):887–888. doi: 10.3891/acta.chem.scand.29b-0887. [DOI] [PubMed] [Google Scholar]
- 124.Nissen C. Glycosylation of recombinant human granulocyte colony stimulating factor: implications for stability and potency. Eur J Cancer. 1994;30A(Suppl 3):S12–14. [PubMed] [Google Scholar]
- 125.Carter CR, Whitmore KM, Thorpe R. The significance of carbohydrates on G-CSF: differential sensitivity of G-CSFs to human neutrophil elastase degradation. J Leukoc Biol. 2004;75(3):515–522. doi: 10.1189/jlb.0803378. [DOI] [PubMed] [Google Scholar]
- 126.Wicker-Planquart C, Canaan S, Riviere M, Dupuis L. Site-directed removal of N-glycosylation sites in human gastric lipase. Eur J Biochem. 1999;262(3):644–651. doi: 10.1046/j.1432-1327.1999.00427.x. [DOI] [PubMed] [Google Scholar]
- 127.Grinnell BW, Walls JD, Gerlitz B. Glycosylation of human protein C affects its secretion, processing, functional activities, and activation by thrombin. J Biol Chem. 1991;266(15):9778–9785. [PubMed] [Google Scholar]
- 128.Rudd PM, Joao HC, Coghill E, Fiten P, Saunders MR, Opdenakker G, Dwek RA. Glycoforms modify the dynamic stability and functional activity of an enzyme. Biochemistry. 1994;33(1):17–22. doi: 10.1021/bi00167a003. [DOI] [PubMed] [Google Scholar]
- 129.Kim BM, Kim H, Raines RT, Lee Y. Glycosylation of onconase increases its conformational stability and toxicity for cancer cells. Biochem Biophys Res Commun. 2004;315(4):976–983. doi: 10.1016/j.bbrc.2004.01.153. [DOI] [PubMed] [Google Scholar]
- 130.Weintraub BD, Stannard BS, Meyers L. Glycosylation of thyroid-stimulating hormone in pituitary tumor cells: influence of high mannose oligosaccharide units on subunit aggregation, combination, and intracellular degradation. Endocrinology. 1983;112(4):1331–1345. doi: 10.1210/endo-112-4-1331. [DOI] [PubMed] [Google Scholar]
- 131.Wang P, Zhang J, Sun Z, Chen Y, Liu JN. Glycosylation of prourokinase produced by Pichia pastoris impairs enzymatic activity but not secretion. Protein Expr Purif. 2000;20(2):179–185. doi: 10.1006/prep.2000.1310. [DOI] [PubMed] [Google Scholar]
- 132.Sareneva T, Pirhonen J, Cantell K, Julkunen I. N-glycosylation of human interferon-gamma: glycans at Asn-25 are critical for protease resistance. Biochem J. 1995;308(Pt 1):9–14. doi: 10.1042/bj3080009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Pratap J, Rajamohan G, Dikshit KL. Characteristics of glycosylated streptokinase secreted from Pichia pastoris: enhanced resistance of SK to proteolysis by glycosylation. Appl Microbiol Biotechnol. 2000;53(4):469–475. doi: 10.1007/s002530051643. [DOI] [PubMed] [Google Scholar]
- 134.Langsford ML, Gilkes NR, Singh B, Moser B, Miller RC, Jr., Warren RA, Kilburn DG. Glycosylation of bacterial cellulases prevents proteolytic cleavage between functional domains. FEBS Lett. 1987;225(1−2):163–167. doi: 10.1016/0014-5793(87)81150-x. [DOI] [PubMed] [Google Scholar]
- 135.Gu JX, Matsuda T, Nakamura R, Ishiguro H, Ohkubo I, Sasaki M, Takahashi N. Chemical deglycosylation of hen ovomucoid: protective effect of carbohydrate moiety on tryptic hydrolysis and heat denaturation. J Biochem (Tokyo) 1989;106(1):66–70. doi: 10.1093/oxfordjournals.jbchem.a122821. [DOI] [PubMed] [Google Scholar]
- 136.Srivastava RAK. Studies on stabilization of amylase by covalent coupling to soluble polysaccharides. Enzyme Microb Technol. 1991;13:164–170. [Google Scholar]
- 137.Jafari-Aghdam J, Khajeh K, Ranjbar B, Nemat-Gorgani M. Deglycosylation of glucoamylase from Aspergillus niger: effects on structure, activity and stability. Biochim Biophys Acta. 2005;1750(1):61–68. doi: 10.1016/j.bbapap.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 138.Kundra R, Kornfeld S. Asparagine-linked oligosaccharides protect Lamp-1 and Lamp-2 from intracellular proteolysis. J Biol Chem. 1999;274(43):31039–31046. doi: 10.1074/jbc.274.43.31039. [DOI] [PubMed] [Google Scholar]
- 139.Duarte-Vazquez MA, Garcia-Almendarez BE, Rojo-Dominguez A, Whitaker JR, Arroyave-Hernandez C, Regalado C. Monosaccharide composition and properties of a deglycosylated turnip peroxidase isozyme. Phytochemistry. 2003;62(1):5–11. doi: 10.1016/s0031-9422(02)00456-9. [DOI] [PubMed] [Google Scholar]
- 140.Wasserman BP, Hultin HO. Effect of deglycosylation on the stability of Aspergillus niger catalase. Arch Biochem Biophys. 1981;212(2):385–392. doi: 10.1016/0003-9861(81)90379-9. [DOI] [PubMed] [Google Scholar]
- 141.Holcenberg JS, Schmer G, Teller DC. Biologic and physical properties of succinylated and glycosylated Acinetobacter glutaminase-asparaginase. J Biol Chem. 1975;250(11):4165–4170. [PubMed] [Google Scholar]
- 142.Raju TS, Scallon B. Fc glycans terminated with N-acetylglucosamine residues increase antibody resistance to papain. Biotechnol Prog. 2007;23(4):964–971. doi: 10.1021/bp070118k. [DOI] [PubMed] [Google Scholar]
- 143.Clowers BH, Dodds ED, Seipert RR, Lebrilla CB. Site determination of protein glycosylation based on digestion with immobilized nonspecific proteases and Fourier transform ion cyclotron resonance mass spectrometry. J Proteome Res. 2007;6(10):4032–4040. doi: 10.1021/pr070317z. [DOI] [PubMed] [Google Scholar]
- 144.Stadtman ER. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu Rev Biochem. 1993;62:797–821. doi: 10.1146/annurev.bi.62.070193.004053. [DOI] [PubMed] [Google Scholar]
- 145.Uchida E, Morimoto K, Kawasaki N, Izaki Y, Abdu Said A, Hayakawa T. Effect of active oxygen radicals on protein and carbohydrate moieties of recombinant human erythropoietin. Free Radic Res. 1997;27(3):311–323. doi: 10.3109/10715769709065769. [DOI] [PubMed] [Google Scholar]
- 146.Oh-eda M, Hasegawa M, Hattori K, Kuboniwa H, Kojima T, Orita T, Tomonou K, Yamazaki T, Ochi N. O-linked sugar chain of human granulocyte colony-stimulating factor protects it against polymerization and denaturation allowing it to retain its biological activity. J Biol Chem. 1990;265(20):11432–11435. [PubMed] [Google Scholar]
- 147.Raso SW, Abel J, Barnes JM, Maloney KM, Pipes G, Treuheit MJ, King J, Brems DN. Aggregation of granulocyte-colony stimulating factor in vitro involves a conformationally altered monomeric state. Protein Sci. 2005;14(9):2246–2257. doi: 10.1110/ps.051489405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Krishnan S, Chi EY, Webb JN, Chang BS, Shan DX, Goldenberg M, Manning MC, Randolph TW, Carpenter JF. Aggregation of granulocyte colony stimulating factor under physiological conditions: Characterization and thermodynamic inhibition. Biochemistry. 2002;41(20):6422–6431. doi: 10.1021/bi012006m. [DOI] [PubMed] [Google Scholar]
- 149.Runkel L, Meier W, Pepinsky RB, Karpusas M, Whitty A, Kimball K, Brickelmaier M, Muldowney C, Jones W, Goelz SE. Structural and functional differences between glycosylated and non-glycosylated forms of human interferon-beta (IFN-beta). Pharm Res. 1998;15(4):641–649. doi: 10.1023/a:1011974512425. [DOI] [PubMed] [Google Scholar]
- 150.Kauzmann W. Some factors in the interpretation of protein denaturation. Adv Protein Chem. 1959;14:1–63. doi: 10.1016/s0065-3233(08)60608-7. [DOI] [PubMed] [Google Scholar]
- 151.Dill KA. Dominant forces in protein folding. Biochemistry. 1990;29(31):7133–7155. doi: 10.1021/bi00483a001. [DOI] [PubMed] [Google Scholar]
- 152.Dill KA. Theory for the folding and stability of globular proteins. Biochemistry. 1985;24(6):1501–1509. doi: 10.1021/bi00327a032. [DOI] [PubMed] [Google Scholar]
- 153.Dill KA. The meaning of hydrophobicity. Science. 1990;250(4978):297–298. doi: 10.1126/science.2218535. [DOI] [PubMed] [Google Scholar]
- 154.Dill KA, Alonso DO, Hutchinson K. Thermal stabilities of globular proteins. Biochemistry. 1989;28(13):5439–5449. doi: 10.1021/bi00439a019. [DOI] [PubMed] [Google Scholar]
- 155.Jenkins WT. Three solutions of the protein solubility problem. Protein Sci. 1998;7(2):376–382. doi: 10.1002/pro.5560070218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Arakawa T, Timasheff SN. Theory of protein solubility. Methods Enzymol. 1985;114:49–77. doi: 10.1016/0076-6879(85)14005-x. [DOI] [PubMed] [Google Scholar]
- 157.Rademacher TW, Parekh RB, Dwek RA. Glycobiology. Annu Rev Biochem. 1988;57:785–838. doi: 10.1146/annurev.bi.57.070188.004033. [DOI] [PubMed] [Google Scholar]
- 158.Lawson EQ, Hedlund BE, Ericson ME, Mood DA, Litman GW, Middaugh R. Effect of carbohydrate on protein solubility. Arch Biochem Biophys. 1983;220(2):572–575. doi: 10.1016/0003-9861(83)90449-6. [DOI] [PubMed] [Google Scholar]
- 159.Karpusas M, Whitty A, Runkel L, Hochman P. The structure of human interferon-beta: implications for activity. Cell Mol Life Sci. 1998;54(11):1203–1216. doi: 10.1007/s000180050248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Conradt HS, Egge H, Peter-Katalinic J, Reiser W, Siklosi T, Schaper K. Structure of the carbohydrate moiety of human interferon-beta secreted by a recombinant Chinese hamster ovary cell line. J Biol Chem. 1987;262(30):14600–14605. [PubMed] [Google Scholar]
- 161.Ioannou YA, Zeidner KM, Grace ME, Desnick RJ. Human alpha-galactosidase A: glycosylation site 3 is essential for enzyme solubility. Biochem J. 1998;332(Pt 3):789–797. doi: 10.1042/bj3320789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Takegawa K, Fujiwara K, Iwahara S, Yamamoto K, Tochikura T. Effect of deglycosylation of N-linked sugar chains on glucose oxidase from Aspergillus niger. Biochem Cell Biol. 1989;67(8):460–464. doi: 10.1139/o89-072. [DOI] [PubMed] [Google Scholar]
- 163.Schulke N, Schmid FX. Effect of glycosylation on the mechanism of renaturation of invertase from yeast. J Biol Chem. 1988;263(18):8832–8837. [PubMed] [Google Scholar]
- 164.Tams JW, Vind J, Welinder KG. Adapting protein solubility by glycosylation. N-glycosylation mutants of Coprinus cinereus peroxidase in salt and organic solutions. Biochim Biophys Acta. 1999;1432(2):214–221. doi: 10.1016/s0167-4838(99)00103-x. [DOI] [PubMed] [Google Scholar]
- 165.Bagger HL, Fuglsang CC, Westh P. Hydration of a glycoprotein: relative water affinity of peptide and glycan moieties. Eur Biophys J. 2006;35(4):367–371. doi: 10.1007/s00249-005-0035-5. [DOI] [PubMed] [Google Scholar]
- 166.Solá RJ, Griebenow K. Influence of modulated structural dynamics on the kinetics of alpha-chymotrypsin catalysis. Insights through chemical glycosylation, molecular dynamics, and domain motion analysis. FEBS J. 2006;273(23):5303–5319. doi: 10.1111/j.1742-4658.2006.05524.x. [DOI] [PubMed] [Google Scholar]
- 167.Goto Y, Fink AL. Conformational states of beta-lactamase: molten-globule states at acidic and alkaline pH with high salt. Biochemistry. 1989;28(3):945–952. doi: 10.1021/bi00429a004. [DOI] [PubMed] [Google Scholar]
- 168.Narhi LO, Kenney WC, Arakawa T. Conformational changes of recombinant human granulocyte-colony stimulating factor induced by pH and guanidine hydrochloride. J Protein Chem. 1991;10(4):359–367. doi: 10.1007/BF01025250. [DOI] [PubMed] [Google Scholar]
- 169.Chan HS, Dill KA. Polymer principles in protein structure and stability. Annu Rev Biophys Biophys Chem. 1991;20:447–490. doi: 10.1146/annurev.bb.20.060191.002311. [DOI] [PubMed] [Google Scholar]
- 170.Ono M. Physicochemical and biochemical characteristics of glycosylated recombinant human granulocyte colony stimulating factor (lenograstim). Eur J Cancer. 1994;30A(Suppl 3):S7–11. [PubMed] [Google Scholar]
- 171.Narhi LO, Arakawa T, Aoki KH, Elmore R, Rohde MF, Boone T, Strickland TW. The effect of carbohydrate on the structure and stability of erythropoietin. J Biol Chem. 1991;266(34):23022–23026. [PubMed] [Google Scholar]
- 172.Barbaric S, Mrsa V, Ries B, Mildner P. Role of the carbohydrate part of yeast acid phosphatase. Arch Biochem Biophys. 1984;234(2):567–575. doi: 10.1016/0003-9861(84)90305-9. [DOI] [PubMed] [Google Scholar]
- 173.Khan RH, Rasheedi S, Haq SK. Effect of pH, temperature and alcohols on the stability of glycosylated and deglycosylated stem bromelain. J Biosci. 2003;28(6):709–714. doi: 10.1007/BF02708431. [DOI] [PubMed] [Google Scholar]
- 174.Ingham KC, Brew SA, Novokhatny VV. Influence of carbohydrate on structure, stability, and function of gelatin-binding fragments of fibronectin. Arch Biochem Biophys. 1995;316(1):235–240. doi: 10.1006/abbi.1995.1033. [DOI] [PubMed] [Google Scholar]
- 175.Yasuda Y, Ikeda S, Sakai H, Tsukuba T, Okamoto K, Nishishita K, Akamine A, Kato Y, Yamamoto K. Role of N-glycosylation in cathepsin E. A comparative study of cathepsin E with distinct N-linked oligosaccharides and its nonglycosylated mutant. Eur J Biochem. 1999;266(2):383–391. doi: 10.1046/j.1432-1327.1999.00863.x. [DOI] [PubMed] [Google Scholar]
- 176.Kalisz HM, Hendle J, Schmid RD. Structural and biochemical properties of glycosylated and deglycosylated glucose oxidase from Penicillium amagasakiense. Appl Microbiol Biotechnol. 1997;47(5):502–507. doi: 10.1007/s002530050963. [DOI] [PubMed] [Google Scholar]
- 177.Wujek P, Kida E, Walus M, Wisniewski KE, Golabek AA. N-glycosylation is crucial for folding, trafficking, and stability of human tripeptidyl-peptidase I. J Biol Chem. 2004;279(13):12827–12839. doi: 10.1074/jbc.M313173200. [DOI] [PubMed] [Google Scholar]
- 178.Masarova J, Mislovicova D, Gemeiner P, Michalkova E. Stability enhancement of Escherichia coli penicillin G acylase by glycosylation with yeast mannan. Biotechnol Appl Biochem. 2001;34(Pt 2):127–133. doi: 10.1042/ba20010037. [DOI] [PubMed] [Google Scholar]
- 179.Fenimore PW, Frauenfelder H, McMahon BH, Parak FG. Slaving: solvent fluctuations dominate protein dynamics and functions. Proc Natl Acad Sci USA. 2002;99(25):16047–16051. doi: 10.1073/pnas.212637899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Fenimore PW, Frauenfelder H, McMahon BH, Young RD. Bulk-solvent and hydration-shell fluctuations, similar to alpha- and beta-fluctuations in glasses, control protein motions and functions. Proc Natl Acad Sci USA. 2004;101(40):14408–14413. doi: 10.1073/pnas.0405573101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kwon KS, Yu MH. Effect of glycosylation on the stability of alpha1-antitrypsin toward urea denaturation and thermal deactivation. Biochim Biophys Acta. 1997;1335(3):265–272. doi: 10.1016/s0304-4165(96)00143-2. [DOI] [PubMed] [Google Scholar]
- 182.Kosman J, Jonas A. Deletion of specific glycan chains affects differentially the stability, local structures, and activity of lecithin-cholesterol acyltransferase. J Biol Chem. 2001;276(40):37230–37236. doi: 10.1074/jbc.M104326200. [DOI] [PubMed] [Google Scholar]
- 183.Rasheedi S, Haq SK, Khan RH. Guanidine hydrochloride denaturation of glycosylated and deglycosylated stem bromelain. Biochemistry (Mosc) 2003;68(10):1097–1100. doi: 10.1023/a:1026354527750. [DOI] [PubMed] [Google Scholar]
- 184.Ueda T, Iwashita H, Hashimoto Y, Imoto T. Stabilization of lysozyme by introducing N-glycosylation signal sequence. J Biochem (Tokyo) 1996;119(1):157–161. doi: 10.1093/oxfordjournals.jbchem.a021201. [DOI] [PubMed] [Google Scholar]
- 185.Williamson G, Belshaw NJ, Noel TR, Ring SG, Williamson MP. O-glycosylation and stability. Unfolding of glucoamylase induced by heat and guanidine hydrochloride. Eur J Biochem. 1992;207(2):661–670. doi: 10.1111/j.1432-1033.1992.tb17093.x. [DOI] [PubMed] [Google Scholar]
- 186.Tams JW, Welinder KG. Kinetic stability of designed glycosylation mutants of Coprinus cinereus peroxidase. Biochem Biophys Res Commun. 2001;286(4):701–706. doi: 10.1006/bbrc.2001.5455. [DOI] [PubMed] [Google Scholar]
- 187.Fatima A, Husain Q. A role of glycosyl moieties in the stabilization of bitter gourd (Momordica charantia) peroxidase. Int J Biol Macromol. 2007;41(1):56–63. doi: 10.1016/j.ijbiomac.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 188.Sundaram PV, Venkatesh R. Retardation of thermal and urea induced inactivation of alpha-chymotrypsin by modification with carbohydrate polymers. Protein Eng. 1998;11(8):699–705. doi: 10.1093/protein/11.8.699. [DOI] [PubMed] [Google Scholar]
- 189.Joao HC, Dwek RA. Effects of glycosylation on protein structure and dynamics in ribonuclease B and some of its individual glycoforms. Eur J Biochem. 1993;218(1):239–244. doi: 10.1111/j.1432-1033.1993.tb18370.x. [DOI] [PubMed] [Google Scholar]
- 190.Joao HC, Scragg IG, Dwek RA. Effects of glycosylation on protein conformation and amide proton exchange rates in RNase B. FEBS Lett. 1992;307(3):343–346. doi: 10.1016/0014-5793(92)80709-p. [DOI] [PubMed] [Google Scholar]
- 191.van Zuylen CW, de Beer T, Leeflang BR, Boelens R, Kaptein R, Kamerling JP, Vliegenthart JF. Mobilities of the inner three core residues and the Man(alpha 1--6) branch of the glycan at Asn78 of the alpha-subunit of human chorionic gonadotropin are restricted by the protein. Biochemistry. 1998;37(7):1933–1940. doi: 10.1021/bi9718548. [DOI] [PubMed] [Google Scholar]
- 192.Yang B, Li TD. Effect of glycosylation at Asn302 of pro-urokinase on its stability in culture supernatant. Chin Med Sci J. 2006;21(2):128–130. [PubMed] [Google Scholar]
- 193.Clark SE, Muslin EH, Henson CA. Effect of adding and removing N-glycosylation recognition sites on the thermostability of barley alpha-glucosidase. Protein Eng Des Sel. 2004;17(3):245–249. doi: 10.1093/protein/gzh028. [DOI] [PubMed] [Google Scholar]
- 194.Liu H, Bulseco GG, Sun J. Effect of posttranslational modifications on the thermal stability of a recombinant monoclonal antibody. Immunol Lett. 2006;106(2):144–153. doi: 10.1016/j.imlet.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 195.Ghirlando R, Lund J, Goodall M, Jefferis R. Glycosylation of human IgGFc: influences on structure revealed by differential scanning micro-calorimetry. Immunol Lett. 1999;68(1):47–52. doi: 10.1016/s0165-2478(99)00029-2. [DOI] [PubMed] [Google Scholar]
- 196.Tang XL, Pikal MJ. Measurement of the kinetics of protein unfolding in viscous systems and implications for protein stability in freeze-drying. Pharm Res. 2005;22(7):1176–1185. doi: 10.1007/s11095-005-6036-3. [DOI] [PubMed] [Google Scholar]
- 197.Privalov PL. Cold denaturation of proteins. Crit Rev Biochem Mol Biol. 1990;25(4):281–305. doi: 10.3109/10409239009090612. [DOI] [PubMed] [Google Scholar]
- 198.Roy I, Gupta MN. Freeze-drying of proteins: some emerging concerns. Biotechnol Appl Biochem. 2004;39(Pt 2):165–177. doi: 10.1042/BA20030133. [DOI] [PubMed] [Google Scholar]
- 199.Cao E, Chen Y, Cui Z, Foster PR. Effect of freezing and thawing rates on denaturation of proteins in aqueous solutions. Biotechnol Bioeng. 2003;82(6):684–690. doi: 10.1002/bit.10612. [DOI] [PubMed] [Google Scholar]
- 200.Pikal-Cleland KA, Rodriguez-Hornedo N, Amidon GL, Carpenter JF. Protein denaturation during freezing and thawing in phosphate buffer systems: monomeric and tetrameric beta-galactosidase. Arch Biochem Biophys. 2000;384(2):398–406. doi: 10.1006/abbi.2000.2088. [DOI] [PubMed] [Google Scholar]
- 201.Jiang ST, Chen GH, Tang SJ, Chen CS. Effect of glycosylation modification (N-Q-(108)I --> N-Q-(108)T) on the freezing stability of recombinant chicken Cystatin overexpressed in Pichia pastoris X-33. J Agric Food Chem. 2002;50(19):5313–5317. doi: 10.1021/jf0200321. [DOI] [PubMed] [Google Scholar]
- 202.Gervais V, Zerial A, Oschkinat H. NMR investigations of the role of the sugar moiety in glycosylated recombinant human granulocyte-colony-stimulating factor. Eur J Biochem. 1997;247(1):386–395. doi: 10.1111/j.1432-1033.1997.00386.x. [DOI] [PubMed] [Google Scholar]
- 203.Wormald MR, Dwek RA. Glycoproteins: glycan presentation and protein-fold stability. Structure Fold Des. 1999;7(7):R155–160. doi: 10.1016/s0969-2126(99)80095-1. [DOI] [PubMed] [Google Scholar]
- 204.DeKoster GT, Robertson AD. Thermodynamics of unfolding for Kazal-type serine protease inhibitors: entropic stabilization of ovomucoid first domain by glycosylation. Biochemistry. 1997;36(8):2323–2331. doi: 10.1021/bi962580b. [DOI] [PubMed] [Google Scholar]
- 205.Broersen K, Voragen AG, Hamer RJ, De Jongh HH. Glycoforms of beta-lactoglobulin with improved thermostability and preserved structural packing. Biotechnol Bioeng. 2004;86(1):78–87. doi: 10.1002/bit.20030. [DOI] [PubMed] [Google Scholar]
- 206.Fojo AT, Whitney PL, Awad WM., Jr. Effects of acetylation and guanidination on alkaline conformations of chymotrypsin. Arch Biochem Biophys. 1983;224(2):636–642. doi: 10.1016/0003-9861(83)90251-5. [DOI] [PubMed] [Google Scholar]
- 207.Cupo P, El-Deiry W, Whitney PL, Awad WM., Jr. Stabilization of proteins by guanidination. J Biol Chem. 1980;255(22):10828–10833. [PubMed] [Google Scholar]
- 208.O'Connor TF, Debenedetti PG, Carbeck JD. Simultaneous determination of structural and thermodynamic effects of carbohydrate solutes on the thermal stability of ribonuclease A. J Am Chem Soc. 2004;126(38):11794–11795. doi: 10.1021/ja0481777. [DOI] [PubMed] [Google Scholar]
- 209.O'Connor TF, Debenedetti PG, Carbeck JD. Stability of proteins in the presence of carbohydrates; experiments and modeling using scaled particle theory. Biophys Chem. 2007;127(1−2):51–63. doi: 10.1016/j.bpc.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 210.Mer G, Hietter H, Lefevre JF. Stabilization of proteins by glycosylation examined by NMR analysis of a fucosylated proteinase inhibitor. Nat Struct Biol. 1996;3(1):45–53. doi: 10.1038/nsb0196-45. [DOI] [PubMed] [Google Scholar]
- 211.Pedrosa C, De Felice FG, Trisciuzzi C, Ferreira ST. Selective neoglycosylation increases the structural stability of vicilin, the 7S storage globulin from pea seeds. Arch Biochem Biophys. 2000;382(2):203–210. doi: 10.1006/abbi.2000.2024. [DOI] [PubMed] [Google Scholar]
- 212.Dellacherie E, Bonneaux F, Labrude P, Vigneron C. Modification of human hemoglobin by covalent association with soluble dextran. Biochim Biophys Acta. 1983;749(1):106–114. doi: 10.1016/0167-4838(83)90157-7. [DOI] [PubMed] [Google Scholar]
- 213.Lenders JP, Chricton RR. Thermal stabilization of amylolytic enzymes by covalent coupling to soluble polysaccharides. Biotechnol Bioeng. 1984;26:1343–1351. doi: 10.1002/bit.260261112. [DOI] [PubMed] [Google Scholar]
- 214.Diaz A, Rangel P, Montes de Oca Y, Lledias F, Hansberg W. Molecular and kinetic study of catalase-1, a durable large catalase of Neurospora crassa. Free Radic Biol Med. 2001;31(11):1323–1333. doi: 10.1016/s0891-5849(01)00637-2. [DOI] [PubMed] [Google Scholar]
- 215.Kodama S, Tsujimoto M, Tsuruoka N, Sugo T, Endo T, Kobata A. Role of sugar chains in the in-vitro activity of recombinant human interleukin 5. Eur J Biochem. 1993;211(3):903–908. doi: 10.1111/j.1432-1033.1993.tb17624.x. [DOI] [PubMed] [Google Scholar]
- 216.Tsuda E, Kawanishi G, Ueda M, Masuda S, Sasaki R. The role of carbohydrate in recombinant human erythropoietin. Eur J Biochem. 1990;188(2):405–411. doi: 10.1111/j.1432-1033.1990.tb15417.x. [DOI] [PubMed] [Google Scholar]
- 217.Ruggiero P, Flati S, Di Cioccio V, Maurizi G, Macchia G, Facchin A, Anacardio R, Maras A, Lucarelli M, Boraschi D. Glycosylation enhances functional stability of the chemotactic cytokine CCL2. Eur Cytokine Netw. 2003;14(2):91–96. [PubMed] [Google Scholar]
- 218.Hoiberg-Nielsen R, Fuglsang CC, Arleth L, Westh P. Interrelationships of glycosylation and aggregation kinetics for Peniophora lycii phytase. Biochemistry. 2006;45(15):5057–5066. doi: 10.1021/bi0522955. [DOI] [PubMed] [Google Scholar]
- 219.Katsonis P, Brandon S, Vekilov PG. Corresponding-states laws for protein solutions. J Phys Chem B Condens Matter Mater Surf Interfaces Biophys. 2006;110(35):17638–17644. doi: 10.1021/jp062698u. [DOI] [PubMed] [Google Scholar]
- 220.Rajan RS, Li T, Aras M, Sloey C, Sutherland W, Arai H, Briddell R, Kinstler O, Lueras AM, Zhang Y, Yeghnazar H, Treuheit M, Brems DN. Modulation of protein aggregation by polyethylene glycol conjugation: GCSF as a case study. Protein Sci. 2006;15(5):1063–1075. doi: 10.1110/ps.052004006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Hermeling S, Crommelin DJ, Schellekens H, Jiskoot W. Structure-immunogenicity relationships of therapeutic proteins. Pharm Res. 2004;21(6):897–903. doi: 10.1023/b:pham.0000029275.41323.a6. [DOI] [PubMed] [Google Scholar]
- 222.Endo Y, Nagai H, Watanabe Y, Ochi K, Takagi T. Heat-induced aggregation of recombinant erythropoietin in the intact and deglycosylated states as monitored by gel permeation chromatography combined with a low-angle laser light scattering technique. J Biochem (Tokyo) 1992;112(5):700–706. doi: 10.1093/oxfordjournals.jbchem.a123961. [DOI] [PubMed] [Google Scholar]
- 223.Bagger HL, Fuglsang CC, Westh P. Preferential binding of two compatible solutes to the glycan moieties of Peniophora lycii phytase. Biochemistry. 2003;42(34):10295–10300. doi: 10.1021/bi034693i. [DOI] [PubMed] [Google Scholar]