Abstract
Previous studies using an in vitro model of eyeblink classical conditioning in turtles suggest that increased numbers of synaptic AMPARs supports the acquisition and expression of conditioned responses (CRs). Brain-derived neurotrophic factor (BDNF) and its associated receptor tyrosine kinase, TrkB, is also required for acquisition of CRs. Bath application of BDNF alone induces synaptic delivery of GluR1- and GluR4-containing AMPARs that is blocked by coapplication of the receptor tyrosine kinase inhibitor K252a. The molecular mechanisms involved in BDNF-induced AMPAR trafficking remain largely unknown. The aim of the present study was to determine whether BDNF-induced synaptic AMPAR incorporation utilizes similar cellular mechanisms as AMPAR trafficking that occurs during in vitro classical conditioning. Using pharmacological blockade and confocal imaging, the results show that synaptic delivery of GluR1 subunits during conditioning or BDNF application does not require activity of NMDARs but is mediated by extracellular signal-regulated kinase (ERK). In contrast, synaptic delivery of GluR4-containing AMPARs during both conditioning and BDNF application is NMDAR- as well as ERK-dependent. These findings indicate that BDNF application mimics AMPAR trafficking observed during conditioning by activation of some of the same intracellular signaling pathways and suggest that BDNF is a key signal transduction element in postsynaptic events that mediate conditioning.
Keywords: BDNF, Classical conditioning, AMPAR trafficking, Turtle, ERK
1. Introduction
Previous studies using an in vitro model of eyeblink classical conditioning in turtles suggest that increased numbers of synaptic AMPARs supports the acquisition and expression of conditioned responses (CRs) recorded in the abducens nerve (Keifer, 2001; Mokin & Keifer, 2004; Mokin, Lindahl, & Keifer, 2006; Mokin, Zheng, & Keifer, 2007). In this system, in place of tone and airpuff stimuli normally used in behaving animals, weak electrical stimulation of the auditory nerve (the “tone” conditioned stimulus; CS) is paired with strong stimulation of the trigeminal nerve (the “airpuff” unconditioned stimulus; US) and results in a neural correlate of conditioned eyeblink responses recorded from the abducens nerve which controls blinking in this species (Keifer, 2003). Based on several lines of evidence, conditioning is associated with the synaptic insertion of GluR1- and GluR4-containing AMPARs in abducens motor neurons. Within a few minutes after the onset of paired conditioning stimuli, GluR1-containing AMPARs are trafficked to synapses in order to unsilence them (Mokin et al., 2007). This is followed by the NMDAR-dependent synthesis and synaptic delivery of GluR4-containing AMPARs that is associated with the acquisition of CRs (Keifer, 2001; Mokin & Keifer, 2004; Mokin et al., 2006; Mokin et al., 2007). Conditioning is generated by the mitogen-activated protein kinase (MAPK)-mediated signaling pathways (Keifer, Zheng, & Zhu, 2007). Synaptic incorporation of GluR1 and GluR4 subunits is accomplished by extracellular signal-regulated kinase (ERK) which is activated in the early phase of conditioning during CR acquisition. Recent studies further suggest that protein kinase C (PKC) regulates GluR4 subunit insertion, but not GluR1 subunits (Zheng & Keifer, 2008). Conditioning-related AMPAR trafficking, particularly of GluR4, also involves interactions with the immediate-early gene-encoded protein Arc and the actin cytoskeleton (Keifer, Zheng, & Mokin, 2008; Mokin et al., 2006).
Recently (Li & Keifer, 2008), we determined that brain-derived neurotrophic factor (BDNF) and its associated receptor tyrosine kinase, TrkB, was required for in vitro classical conditioning. Neurotrophic factors generally enhance the survival, growth, and function of neurons. Not only are these involved in cellular proliferation and growth, but they have more recently been implicated in mechanisms of synaptic plasticity (Lu, Pang, & Woo, 2005). For example, hippocampal long-term potentiation (LTP) is impaired when BDNF or TkB function is suppressed by gene knockdown or antibody application (Chen, Kolbeck, Barde, Bonhoeffer, & Kossel, 1999; Xu et al., 2000; Zakharenko et al., 2003). Importantly, the deficits in LTP resulting from BDNF knockdown could be rescued by addition of recombinant BDNF to the medium (Patterson et al., 1996) or adenovirus-mediated BDNF overexpression (Korte et al., 1996). In our preparation, conditioning induced the expression of BDNF and phosphorylation of Trk receptors (Li & Keifer, 2008). Moreover, inhibitors of BDNF such as antibodies to TrkB or bath application of K252a, a protein kinase inhibitor that includes actions on tyrosine kinase receptors, completely suppressed CRs. Bath application of BDNF alone also induced similar molecular changes as observed during conditioning including activation of ERK and synaptic incorporation of GluR1- and GluR4-containing AMPARs. These effects were blocked by coapplication K252a further supporting a role for BDNF in mechanisms of AMPAR trafficking. BDNF-induced AMPAR trafficking has been observed elsewhere (Caldeira et al., 2007; Itami et al., 2003), but the molecular mechanisms involved remain largely unknown. It was reported recently that delivery of AMPARs induced by BDNF in cultured cortical neurons is dependent on Ca2+ influx from IP3-sensitive internal stores (Nakata & Nakamura, 2007). The aim of the present study was to determine whether BDNF-induced synaptic AMPAR incorporation utilizes similar cellular mechanisms as AMPAR trafficking that occurs during in vitro classical conditioning. Using pharmacological manipulation and confocal imaging, the results show that synaptic insertion of GluR1 subunits during conditioning or BDNF application does not require activity of NMDARs but is mediated by ERK. In contrast, synaptic delivery of GluR4-containing AMPARs during both conditioning and BDNF application is NMDAR- as well as ERK-dependent. These findings indicate that BDNF application mimics AMPAR trafficking observed during conditioning by activation of some of the same intracellular signaling pathways and suggest that BDNF is a key signal transduction element in postsynaptic events that mediate conditioning.
2. Methods
2.1. Conditioning procedures
Freshwater pond turtles Pseudemys scripta elegans obtained from commercial suppliers were anesthetized by hypothermia until torpid and decapitated. Protocols involving the use of animals complied with the guidelines of the National Institutes of Health and the Institutional Animal Care and Use Committee. The brain stem was transected at the levels of the trochlear and glossopharyngeal nerves, and the cerebellum was removed as described previously (Anderson & Keifer, 1999). Therefore, this preparation consisted only of the pons, containing the pontine blink circuitry, with the cerebellar circuitry and the red nucleus removed. The brain stem was continuously bathed in physiological saline (2-4 ml/min) containing (in mM): 100 NaCl, 6 KCl, 40 NaHCO3, 2.6 CaCl2, 1.6 MgCl2, and 20 glucose, which was oxygenated with 95% O2/5% CO2 and maintained at room temperature (22-24° C) at pH 7.6 (Anderson & Keifer, 1999). Suction electrodes were used for stimulation and recording of cranial nerves. The US was an approximately two times threshold single shock stimulus applied to the trigeminal nerve; the CS was a 100 Hz, 1 s train stimulus applied to the ipsilateral posterior root of the eighth nerve that was below threshold amplitude required to produce activity in the abducens nerve (Anderson & Keifer, 1999; Keifer, Armstrong, & Houk, 1995). The latter nerve will be referred to as the auditory nerve as it carries predominantly auditory fibers. Neural activity was recorded from the ipsilateral abducens nerve which projects to the extraocular muscles controlling movements of the eye, nictitating membrane, and eyelid. The CS-US interval was 20 ms which is defined here as the time between the offset of the CS and the onset of the US. This brief trace delay interval was found to be optimal for conditioning, however, conditioning is not supported using longer trace intervals (Keifer, 2001). Intertrial interval between the paired stimuli was 30 s. A pairing session consisted of 50 CS-US presentations (25 min in duration) followed by a 30 min rest period during which there was no stimulation. Conditioned responses were defined as abducens nerve activity that occurred during the CS and exceeded the amplitude of double the baseline recording level. Conditioned preparations were those that received paired CS-US stimulation whereas pseudoconditioned preparations received the same number of CS and US exposures that were explicitly unpaired using a CS-US interval randomly selected between 300 ms and 25 s. At the end of the experiments, preparations for immunocytochemistry were immersion fixed in cold 0.5% paraformaldehyde whereas those for protein analysis were frozen in liquid nitrogen and stored at -70° C.
2.2. Pharmacology
BDNF (4554, Santa Cruz Biotechnology, Santa Cruz, CA) stock solution was added immediately before each experiment to physiological saline to a final concentration of 100 ng/ml and applied to preparations for 80 min, the time duration equivalent to two pairing sessions of conditioning. The NMDA receptor antagonist D,L-2-amino-5-phosphonovaleric acid (AP-5; 100 μM; Tocris Cookson, St. Louis, MO), or the MEK inhibitor PD98059 (50 μM; Calbiochem, San Diego, CA), was dissolved in physiological saline and bath applied to preparations 30 min prior to the training procedures or BDNF treatment and continued throughout the experiment.
2.3. Western blot analysis
Brain stem preparations were treated with BDNF, or underwent coapplication of BDNF + AP-5 or BDNF + PD98059 for the equivalent time period of two pairing sessions (80 min). Naive preparations were incubated in physiological saline alone for the same time period as the other groups. Following the physiological experiments, brain stems were homogenized in NP-40 buffer containing protease and phosphatase inhibitors, gently shaken for 2 h, and centrifuged at 10,000g for 20 min. The supernatants were aspirated and protein concentrations determined by the Bradford protein assay. Equal amounts of protein sample were denatured in loading buffer containing 125 mM Tris-HCl (pH 6.8), 20% glycerol, 6% SDS, and 5% β-mercaptoethanol, boiled for 3 min, and subjected to SDS-PAGE. The proteins were transferred to PVDF membranes and blocked with 5% nonfat dry milk in TBST (20 mM Tris at pH 7.6, 150 mM NaCl, and 0.1% Tween-20) for 1 h. Membranes were probed using the following primary antibodies: GluR1 (1:1000, 1504, Chemicon, Temecula, CA), GluR4 (1:1000; 06-308, Upstate, Charlottsville, VA), and synaptophysin (1:500, S5768, Sigma, St. Louis, MO). Following the primary antibodies, the membranes were rinsed with TBST and incubated with HRP-conjugated secondary antibodies for 2 h at room temperature. Proteins were detected using the ECL-Plus chemiluminescence system (Amerisham Pharmacia, Piscataway, NJ) and immunoreactive signals were captured on Kodak X-omatic AR film. Each membrane was reprobed for loading controls with β-actin (1:1,000; Chemicon).
2.4. Glutamate receptor localization, confocal imaging, and data analysis
Tissue sections were cut at 30 μm and preincubated in 10% normal goat serum. Alternate sections were incubated in primary antibodies to GluR1 and synaptophysin, or GluR4 and synaptophysin, overnight at 4° C with gentle shaking. The primary antibodies used were a polyclonal antibody raised in rabbit that recognizes the GluR1 subunit of AMPARs (1:100; 1504, Chemicon), a polyclonal antibody raised in goat that recognizes the GluR4 subunit of AMPARs (1:100; 7614, Santa Cruz), and a monoclonal antibody raised in mouse that recognizes synaptophysin (1:1,000; S5768, Sigma). Specificities of all antibodies were confirmed by Western blot. After the primary antibodies, sections were rinsed and incubated with secondary antibodies for 2 h at room temperature. The secondary antibodies were a Cy3-conjugated goat anti-rabbit IgG (1:100) for GluR1, a Cy3-conjugated rabbit anti-goat IgG (1:100) for GluR4, and a Cy2-conjugated goat anti-mouse IgG (1:50) for synaptophysin (Jackson ImmunoReserach, West Grove, PA). After incubation in the secondaries, sections were rinsed, mounted on slides and coverslipped. Images of labeled neurons in the principal and accessory abducens motor nuclei were obtained using an Olympus Fluoview 500 laser scanning confocal microscope. Tissue samples were scanned using a 60 × 1.4 NA oil immersion objective with dual excitation using a 488 nm argon laser and a 543 nm HeNe laser. Regions of interest of punctate staining from individual neurons were selected from the soma and proximal dendrites where the majority (> 80%) of auditory nerve terminal boutons make synapses on abducens motor neurons (Keifer & Mokin, 2004). Quantification of punctate staining of at least two-fold greater intensity above background was performed using stereological procedures (Mokin & Keifer, 2006) with MetaMorph software (Universal Imaging, Downingtown, PA). Images of two consecutive optical sections were taken using confocal microscopy. Protein puncta were counted in one optical section (sample section) if they were not present in the optical section immediately below the sample section (look-up section) and if they were within the inclusion boundaries of the unbiased counting frame. Colocalized staining indicating the presence of glutamate receptor subunits at synaptic sites was determined when red and green puncta were immediately adjacent to one another or if they were overlapping. All data were analyzed using Statview software (SAS, Cary, NC) by ANOVA and are presented as means ± SEM.
3. Results
3.1. Conditioning experiments and drug treatment
For the immunocytochemistry, six experimental groups (n = 3 preparations/group; 15-30 neurons were analyzed per preparation) were examined after two pairing sessions (or the equivalent duration of 80 minutes): pseudoconditioned preparations (Ps2), conditioned (C2), preparations that underwent the conditioning procedure in the presence of the NMDAR antagonist AP-5 (100 μM; C2+AP-5), BDNF-treated (100 ng/ml; BDNF2), preparations treated by coapplication of BDNF with AP-5 (BDNF2+AP-5), and those treated by coapplication of BDNF with the MEK-ERK antagonist PD98059 (50 μM; BDNF2+PD). The pseudoconditioned group was presented with explicitly unpaired stimuli and showed 0 ± 0% CRs (mean ± SD) after two sessions. Preparations that were conditioned for two pairing sessions demonstrated CR acquisition to 76 ± 14% CRs. The preparations that were presented with conditioning stimuli in the presence of AP-5 for two sessions showed 0 ± 0% CRs. During bath application of BDNF alone, there was no electrical stimulation and no CRs were recorded. However, BDNF application has been shown to induce a number of molecular responses similar to conditioning (Li & Keifer, 2008). Here, BDNF was bath applied for the equivalent time period of two pairing sessions, as was coapplication of BDNF with AP-5 or BDNF with PD98059 in order to examine the effects of these pharmacological blockers on BDNF-induced AMPAR trafficking.
3.2. Synaptic localization of GluR1- and GluR4-containing AMPARs
The primary results of this study are summarized in Fig. 3 and corresponding confocal images of punctate staining for the different experimental groups are shown in Figs. 1 and 2. The overall amount of GluR1 punctate staining did not change after conditioning, BDNF application, or for any other treatment group, compared to pseudoconditioned controls (Fig. 3; F(5,198) = 0.4, p = 0.84). These findings are illustrated in the confocal images of GluR1 shown for all the treatment groups in Figs. 1 and 2. In contrast, the number of GluR4 puncta increased significantly after conditioning for two pairing sessions compared to pseudoconditioned levels (Fig. 3; F(1,63 = 23.4, p < 0.0001), and after treatment with BDNF (F(1,70) = 21.2, p < 0.0001), as observed previously (Li & Keifer, 2008; Mokin et al., 2007). The enhanced number of GluR4 puncta after conditioning and BDNF can be seen in the images in Fig. 1 (compare C2 with Ps2 for GluR4) and Fig. 2 (BDNF2). The conditioning and BDNF-induced increase in GluR4 punctate staining was significantly attenuated by treatment with AP-5 (Fig. 3; F(1,64) = 30.0, p < 0.0001, C2 vs C2+AP-5; F(1,76) = 47.9, p < 0.0001, BDNF2 vs BDNF2+AP-5) and PD98059 (F(1,76) = 53.3, p < 0.0001, BDNF2 vs BDNF2+PD). These findings are also illustrated in Fig. 1 (compare AP-5 with C2 for GluR4) and Fig. 2 (compare BDNF2 with AP-5 and PD). Similar conditioning and BDNF-induced increases in the presynaptic marker synaptophysin were also observed. Synaptophysin staining increased markedly after conditioning for two sessions compared to pseudoconditioned levels (Fig. 3; F(1,118) = 66.5, p < 0.0001) and after BDNF treatment for a comparable amount of time (F(1,130) = 49.0, p < 0.0001), as reported earlier (Li & Keifer, 2008; Mokin et al., 2007). These findings can be seen in Figs. 1 and 2. Treatment of both the conditioned and BDNF-treated preparations with AP-5 significantly attenuated the enhanced levels of synaptophysin (F(1,129) = 13.5, p = 0.0003, C2 vs C2+AP-5; F(1,111) = 7.3, p < 0.01, BDNF2 vs BDNF2+AP-5), but they were not quite lowered to the level of the pseudoconditioned group. The application of PD98059 was more effective in reducing synaptophysin to pseudoconditioned values (F(1,146) = 78.5, p < 0.0001, BDNF2 vs BDNF2+PD). Reduced punctate staining for synaptophysin after AP-5 or PD98059 treatment compared to conditioning or BDNF application is also shown in Figs. 1 and 2.
Fig. 3.
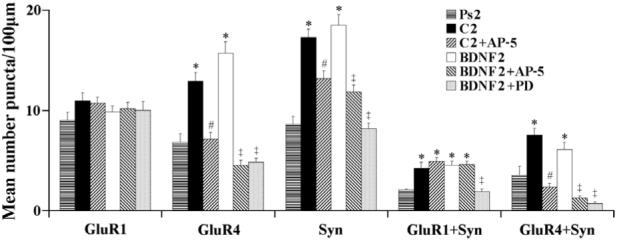
Quantitative analysis of punctate staining of individual puncta for GluR1, GluR4, and synaptophysin (Syn), and for colocalized staining for GluR1 + Syn, and GluR4 + Syn from the different treatment groups. * indicates significant differences from Ps2; # indicates significant differences from C2; ‡ indicates significant differences from BDNF2.
Fig. 1.
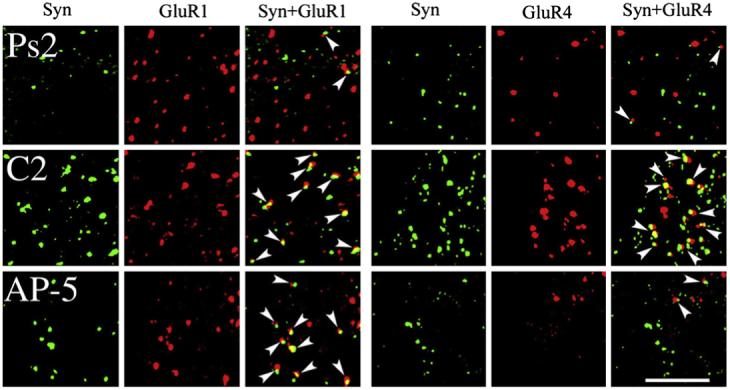
Confocal images of abducens motor neurons showing punctate staining for the presynaptic marker synaptophysin (Syn; green), GluR1 AMPAR subunits (red), or GluR4 AMPAR subunits (red) from each of the conditioned groups examined: pseudoconditioned for two sessions (Ps2), conditioned for two sessions (C2), and conditioned during application of AP-5 for two sessions (AP-5). Colocalization of AMPAR with synaptophysin punctate staining is also shown (GluR1+Syn, GluR4+Syn) and indicated by the arrowheads. Scale bar = 2 μm.
Fig. 2.
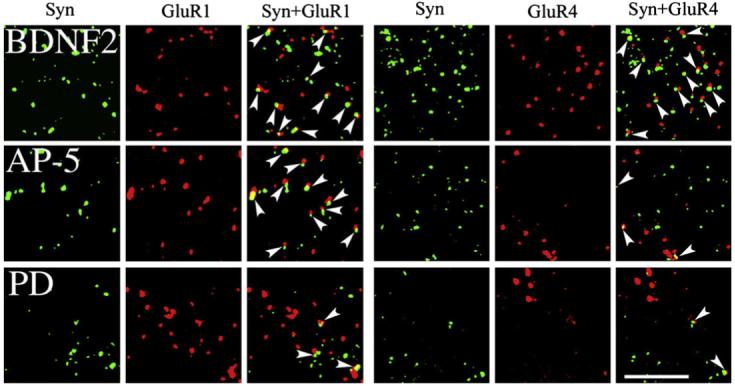
Confocal images of punctate staining of abducens motor neurons for synaptophysin, GluR1, and GluR4 AMPAR subunits from each of the BDNF-treated groups examined: BDNF application for two sessions (BDNF2), coapplication of BDNF with AP-5 for two sessions (AP-5), and coapplication of BDNF with PD98059 for two sessions (PD). Colocalization of AMPAR with synaptophysin punctate staining is also shown (GluR1+Syn, GluR4+Syn) and indicated by the arrowheads. Scale bar =2 μm.
Synaptic localization of GluR1- or GluR4-containing AMPARs was determined by double-label imaging of these subunits (red puncta) with the presynaptic marker synaptophysin (green puncta). Overlapping (yellow puncta) or adjacent puncta indicated colocalization of subunits at synaptic sites. These findings are summarized for GluR1+Syn and GluR4+Syn data in Fig. 3, and are illustrated in Figs. 1 and 2 (arrowheads indicate colocalization). Following conditioning or BDNF application, the synaptic localization of GluR1 subunits was significantly increased above pseudoconditioned levels (Fig. 3; F(1,55) = 18.2, p < 0.0001, C2 vs Ps2; F(1,50) =49.6, p < 0.0001, BDNF2 vs Ps2). This is illustrated by the much greater presence of colocalized puncta as shown in the images for the conditioned (C2) and BDNF-treated (BDNF2) groups compared to the pseudoconditioned (Ps2) cases in Figs. 1 and 2. Bath application of AP-5 for both of the conditioned and BDNF-treated groups failed to significantly alter the colocalization of GluR1 with synaptophysin from these elevated levels (F(1,63) = 0.9, p = 0.35, C2 vs C2+AP5; F(1,63) = 0.0, p = 0.85), BDNF2 vs BDNF2+AP-5). However, treatment with the MEK-ERK inhibitor PD98059 during BDNF application resulted in dramatically reduced colocalization of GluR1 with synaptophysin to pseudoconditioned values (F(1,58) = 33.4, p < 0.0001, BDNF2 vs BDNF2+PD), as is illustrated in Fig. 2. Therefore, the NMDAR antagonist AP-5 failed to attenuate GluR1 synaptic localization whereas the MEK-ERK inhibitor PD98059 was found to be effective. The synaptic localization of GluR4-containing AMPARs was also significantly enhanced after conditioning or BDNF application above pseudoconditioned values (Figs. 1-3; F(1,61) = 12.3, p = 0.0009, C2 vs Ps2; F(1,66) = 4.0, p < 0.05, BDNF2 vs Ps2). However, for GluR4 subunits, AP-5 was a potent inhibitor of colocalization with synaptophysin for both treatment groups (F(1,62) = 48.4, p < 0.0001, C2 vs C2+AP-5; F(1,76) = 44.7, p < 0.0001, BDNF2 vs BDNF2+AP-5). This can be seen in Figs. 1 and 2 in which there are dramatically fewer colocalized puncta for both AP-5-treated groups compared to conditioned or BDNF-treated preparations. Finally, similar to GluR1 subunits, PD98059 was also a strong inhibitor of BDNF-induced GluR4 and synaptophysin colocalization (F(1, 76) = 57.7, p < 0.0001, BDNF2 vs BDNF2+PD). Therefore, unlike GluR1, synaptic localization of GluR4-containing AMPARs was regulated by NMDAR-mediated mechanisms, while similar to GluR1, GluR4 subunits were also sensitive to MEK-ERK signaling pathways.
3.3. Protein analysis of BDNF-treated preparations
In previous studies, Western blot analysis confirmed that after conditioning both GluR4 subunits and synaptophysin increased significantly, while GluR1 subunits did not, and that this increase was attenuated by treatment with AP-5 (Mokin & Keifer, 2004; Mokin et al., 2007). Similar analysis was undertaken here for the BDNF-treated groups to confirm the immunocytochemical findings. As shown in Fig. 4, levels of GluR1 were substantial after treatment with BDNF similar to the naive group and did not decline during coapplication of BDNF with either AP-5 or PD98059. However, as observed for the immunocytochemistry, the BDNF-induced increases in levels of both GluR4 and synaptophysin were attenuated during both AP-5 and PD98059 treatment. Therefore, levels of protein expression for GluR4 subunits and synaptophysin are sensitive to NMDAR and ERK antagonists whereas levels for GluR1 subunits are not.
Fig. 4.
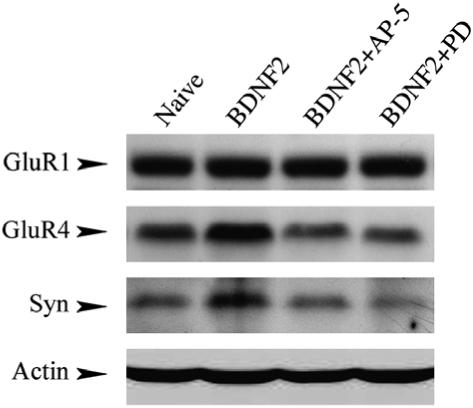
Western blot analysis of protein expression for GluR1 and GluR4 AMPAR subunits and synaptophysin from naive and BDNF-treated groups. Levels of GluR1 were high in naive preparations or after treatment with BDNF and remained high following coapplication of BDNF with AP-5 or PD98059. In contrast, BDNF-induced increases in protein levels for both GluR4 and synaptophysin were reduced after coapplication of BDNF with either AP-5 or PD98059. Actin loading controls are shown in the bottom panel.
4. Discussion
The present study suggests that BDNF-induced trafficking of AMPARs to synaptic sites utilizes some of the same signaling pathways as occurs during in vitro classical conditioning. Namely, during both conditioning and BDNF application, the synaptic incorporation of GluR1 subunits is NMDAR-insensitive but requires MEK-ERK signaling cascades. On the other hand, synaptic incorporation of GluR4 subunits requires both NMDAR- and ERK-mediated mechanisms. These findings begin to reveal the signal transduction pathways utilized during both conditioning and BDNF-induced AMPAR trafficking.
4.1. Conditioning-related and BDNF-induced AMPAR expression and trafficking
The present study confirms that conditioning or BDNF application resulted in a significant increase in colocalization of GluR1- and GluR4-containing AMPARs with the synaptic marker synaptophysin compared to pseudoconditioned preparations. Therefore, BDNF alone may initiate mechanisms that induce synaptic incorporation of AMPARs. BDNF-induced synaptic AMPAR trafficking has also been described for homomeric GFP-GluR1 receptors in CA1 neurons using hippocampal organotypic slices (Caldeira et al., 2007), and for native GluR1 AMPARs in cultured cortical pyramidal neurons (Nakata & Nakamura, 2007). In the present study it was observed that while GluR1 subunits were delivered to synaptic sites during conditioning and BDNF application, the overall number of puncta did not change. This finding suggests that GluR1 does not undergo protein synthesis in this preparation during these manipulations and that existing GluR1-containing AMPARs are delivered to synapses. Similar findings were observed in our previous studies (Li & Keifer, 2008; Mokin et al., 2007). Caldeira et al. (2007) found increased expression of GluR1, GluR2 and GluR3 AMPAR subunits, but not GluR4, after BDNF treatment of organotypic hippocampal cultures that were 7 days in vitro. However, they did not observe this effect after treatment of cultures that were 14 days in vitro. They attributed the lack of effect of BDNF treatment on older cultures to tonic effects of endogenous BDNF on GluR1. In contrast, no change in GluR1 protein expression was observed following BDNF treatment of synaptoneurosome preparations from adult rat (Wu et al., 2004). Interestingly, BDNF application in neo-cortical cultures results in enhanced expression of proteins that comprise the postsynaptic density, particularly PICK1 and GRIP1, that are known to interact with AMPARs (Jourdi et al., 2003). In contrast to GluR1, punctate staining for GluR4 subunits significantly increased with conditioning and BDNF application, as reported previously (Li & Keifer, 2008; Mokin et al., 2007). We have also consistently observed a dramatic increase in the presynaptic protein synaptophysin with conditioning (Mokin & Keifer, 2004; Mokin et al., 2007) and BDNF application (Li & Keifer, 2008) and these findings were also verified here. Therefore, both GluR4 and synaptophysin undergo protein synthesis induced by either conditioning training or BDNF application.
4.2. Signaling pathways in synaptic AMPAR delivery
Previous studies showed that conditioning in this preparation was NMDAR-dependent as CR acquisition and expression is blocked by AP-5 (Keifer, 2001; Keifer & Clark, 2003; Mokin & Keifer, 2004; Mokin et al., 2006). Here, the enhanced expression of GluR4 and synaptophysin punctate staining observed after two sessions of conditioning was also suppressed by AP-5, as was GluR4 colocalization with synaptophysin, as observed previously (Mokin et al., 2006). These findings correspond with the blockade of CR acquisition in AP-5-treated preparations. Suppression of total staining and colocalization of GluR4 and synaptophysin by AP-5 was also observed for BDNF-treated preparations. Therefore, NMDAR-mediated mechanisms support the enhanced expression and colocalization of GluR4 and synaptophysin induced by BDNF application. A different picture emerges for GluR1 subunits. While there is no new synthesis of GluR1 after conditioning or BDNF application, synaptic localization of GluR1-containing AMPARs remains relatively high and is not affected by AP-5 in either treatment group. In hippocampal cultures, BDNF-induced synaptic delivery of GFP-GluR1 was also unaffected by AP-5 (Caldeira et al., 2007), even though it has been reported that GluR1 in BDNF-treated synaptoneurosomes is phosphorylated in an NMDAR-dependent manner (Wu et al., 2004).
In addition to NMDAR-mediated mechanisms, the MAPK signal transduction pathways also have a significant role in AMPAR trafficiking during in vitro classical conditioning (Keifer et al., 2007). That study showed that ERK MAPK was activated early during acquisition of conditioning, followed by p38 MAPK which was activated later during CR expression. Suppression of the acquisition of conditioning by the MEK-ERK antagonist PD98059 resulted in significantly reduced colocalization of both GluR1 and GluR4 with synaptophysin (Zheng, 2008). This finding suggested that ERK drives the synaptic delivery of both GluR1 and GluR4 subunits during the early stages of conditioning. Significantly, BDNF application alone for the equivalent time period of two pairing sessions also results in synaptic insertion of GluR1 and GluR4 subunits as shown in Li and Keifer (2008) and confirmed here, which is blocked by coapplication of K252a. Additionally, BDNF application induces the activation of ERK but not p38 MAPK after the equivalent of two pairing sessions (Li & Keifer, 2008). In the present study, we observed that the ERK antagonist PD98059 significantly attenuated the BDNF-induced colocalization of both GluR1- and GluR4-containing AMPARs with synaptophysin. Therefore, as in conditioning, ERK mediates synaptic delivery of AMPAR subunits when it is driven by BDNF. From the current findings, we can conclude that during both conditioning and BDNF application GluR1-containing AMPARs are delivered to synapses by NMDAR-independent mechanisms whereas GluR4-containing AMPARs utilize NMDAR-mediated signaling pathways. Delivery of both types of AMPARs, however, require ERK. Consistent with these results, Zhu, Qin, Zhao, van Aelst, and Malinow (2002) found that the GTPase Ras, which enhances phosphorylation of ERK, induced synaptic delivery of AMPARs with long cytoplasmic tails, which includes GluR1 and GluR4. BDNF application has been reported to result in phosphorylation of GluR1 at Ser831 but not at Ser845, as determined by phosphorylation site-directed antibodies (Caldeira et al., 2007). Phosphorylation at Ser818 was not examined in that study but is required for synaptic incorporation of GluR1 during hippocampal LTP (Boehm et al., 2006). The exact mechanisms by which BDNF drives synaptic delivery of AMPARs remain to be established.
4.3. Role of BDNF in AMPAR trafficking and acquisition of in vitro classical conditioning
Our current model for in vitro classical conditioning in this preparation is that CR acquisition proceeds in two stages of synaptic AMPAR insertion. The first stage (during pairing session 1) is initiated shortly after the onset of paired CS-US stimulation, prior to CR acquisition, and involves synaptic incorporation of GluR1-containing AMPARs in order to activate NMDARs and unsilence auditory nerve synapses (Mokin et al., 2007). This process occurs quickly and does not involve protein synthesis of GluR1 but is carried out by trafficking of existing GluR1-containing AMPARs (Mokin et al., 2007). In the present study, we show that insertion of GluR1 subunits is NMDAR-independent and requires activation of ERK. The second stage of acquisition (during pairing session 2 when CRs are expressed) involves NMDAR-dependent synthesis and synaptic delivery of GluR4-containing AMPARs (Keifer, 2001; Keifer & Clark, 2003; Mokin & Keifer, 2004). This stage requires activation of PKC as well as ERK (Zheng, 2008). The synaptic localization of GluR4 subunits parallels the expression of CRs and we have therefore hypothesized that synaptic incorporation of GluR4-containing AMPARs during conditioning supports CR acquisition and expression in this preparation (Keifer, 2001; Mokin & Keifer, 2004; Mokin et al., 2006; Mokin et al., 2007). Therefore, paired stimulation induces a rapid, non-NMDAR-mediated synaptic incorporation of GluR1 that (unless blocked by AP-5) activates NMDARs to allow calcium entry and signal transduction mechanisms for synthesis and delivery of GluR4 that supports CR acquisition.
Application of BDNF alone also results in enhanced synaptic incorporation of GluR1 and GluR4 subunits by activation of similar signaling cascades as is observed for paired stimulation during conditioning. Western blot data show that BDNF expression is significantly increased after 25 min of paired stimulation but not after 15 min (Li & Keifer, unpublished data). BDNF expression is preceded by activation of the cAMP response element binding protein (CREB) transcription factor which occurs within at least 15 min of conditioning. Bdnf is a known target gene of CREB (Carlezon, Duman, & Nestler, 2005). Therefore, future studies will be aimed at examining whether BDNF expression is a key element interposed in a signal transduction pathway that initiates the postsynaptic activation of ERK and results in AMPAR trafficking that supports conditioning.
Acknowledgements
We thank Dr. Frances Day for assistance with the confocal microscopy. Supported by National Institutes of Health Grants NS 051187 and P20 RR 015567 which is designated as a Center of Biomedical Research Excellence (COBRE) to J. K.
References
- Anderson CW, Keifer J. Properties of conditioned abducens nerve responses in a highly reduced in vitro brain stem preparation from the turtle. Journal of Neurophysiology. 1999;81:1242–1250. doi: 10.1152/jn.1999.81.3.1242. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho R, Correia SS, Backos DS, et al. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. Journal of Biological Chemistry. 2007;282:12619–12628. doi: 10.1074/jbc.M700607200. [DOI] [PubMed] [Google Scholar]
- Carlezon WA, Jr., Duman RS, Nestler EJ. The many faces of CREB. Trends in Neuroscience. 2005;28:436–445. doi: 10.1016/j.tins.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Chen G, Kolbeck R, Barde YA, Bonhoeffer T, Kossel A. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. Journal of Neuroscience. 1999;19:7983–7990. doi: 10.1523/JNEUROSCI.19-18-07983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itami C, Kimura F, Kohno T, Matsuoka M, Ichikawa M, Tsumoto T, et al. Brain-derived neurotrophic factor-dependent unmasking of “silent” synapses in the developing mouse barrel cortex. Proceedings of the National Academy of Science. 2003;100:13069–13074. doi: 10.1073/pnas.2131948100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdi H, Iwakura Y, Narisawa-Saita M, Ibaraki K, Xiong H, Watanabe M. Brain-derived neurotrophic factor signal enhances and maintains the expression of AMPA receptor-associated PDZ proteins in developing cortical neurons. Developmental Biology. 2003;263:216–230. doi: 10.1016/j.ydbio.2003.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer J. In vitro eye-blink classical conditioning is NMDA receptor dependent and involves redistribution of AMPA receptor subunit GluR4. Journal of Neuroscience. 2001;21:2411–2434. doi: 10.1523/JNEUROSCI.21-07-02434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer J. In vitro classical conditioning of the turtle eyeblink reflex: Approaching cellular mechanisms of acquisition. Cerebellum. 2003;2:55–61. doi: 10.1080/14734220310015610. [DOI] [PubMed] [Google Scholar]
- Keifer J, Armstrong KE, Houk JC. In vitro classical conditioning of abducens nerve discharge in turtles. Journal of Neuroscience. 1995;15:5036–5048. doi: 10.1523/JNEUROSCI.15-07-05036.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer J, Clark TG. Abducens conditioning in in vitro turtle brain stem without cerebellum requires NMDA receptors and involves upregulation of GluR4-containing AMPA receptors. Experimental Brain Research. 2003;151:405–410. doi: 10.1007/s00221-003-1494-5. [DOI] [PubMed] [Google Scholar]
- Keifer J, Mokin M. Distribution of anterogradely labeled trigeminal and auditory nerve boutons on abducens motor neurons in turtles: Implications for in vitro classical conditioning. Journal of Comparative Neurology. 2004;471:144–152. doi: 10.1002/cne.20032. [DOI] [PubMed] [Google Scholar]
- Keifer J, Zheng Z, Mokin M. Synaptic localization of GluR4-containing AMPARs and Arc during acquisition, extinction, and reacquisition of in vitro classical conditioning. Neurobiology of Learning and Memory. 2008;90:301–308. doi: 10.1016/j.nlm.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keifer J, Zheng Z, Zhu D. MAPK signaling pathways mediate AMPA receptor trafficking in an in vitro model of classical conditioning. Journal of Neurophysiology. 2007;97:2067–2074. doi: 10.1152/jn.01154.2006. [DOI] [PubMed] [Google Scholar]
- Korte M, Griesbeck O, Gravel C, Carroll P, Staiger V, Thoenen H, et al. Virus-mediated gene transfer into hippocampal CA1 region restores long-term potentiation in brain-derived neurotrophic factor mutant mice. Proceeding of the National Academy of Science. 1996;93:12547–12552. doi: 10.1073/pnas.93.22.12547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Keifer J. Coordinate action of pre- and postsynaptic BDNF is required for AMPAR trafficking and acquisition of in vitro classical conditioning. Neuroscience. 2008;155:686–697. doi: 10.1016/j.neuroscience.2008.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B, Pang PT, Woo NH. The Yin and Yang of neurotrophin action. Nature Reviews Neuroscience. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- Mokin M, Keifer J. Targeting of GluR4-containing AMPA receptors to synaptic sites during in vitro classical conditioning. Neuroscience. 2004;128:219–228. doi: 10.1016/j.neuroscience.2004.06.009. [DOI] [PubMed] [Google Scholar]
- Mokin M, Keifer J. Quantitative analysis of immunofluorescent punctate staining of synaptically localized proteins using confocal microscopy and stereology. Journal of Neuroscience Methods. 2006;157:218–224. doi: 10.1016/j.jneumeth.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Mokin M, Lindahl JS, Keifer J. Immediate-early gene-encoded protein Arc is associated with synaptic delivery of GluR4-containing AMPA receptors during in vitro classical conditioning. Journal of Neurophysiology. 2006;95:215–224. doi: 10.1152/jn.00737.2005. [DOI] [PubMed] [Google Scholar]
- Mokin M, Zheng Z, Keifer J. Conversion of silent synapses into the active pool by selective GluR1-3 and GluR4 AMPAR trafficking during in vitro classical conditioning. Journal of Neurophysiology. 2007;98:1278–1286. doi: 10.1152/jn.00212.2007. [DOI] [PubMed] [Google Scholar]
- Nakata H, Nakamura S. Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Letters. 2007;58:2047–2054. doi: 10.1016/j.febslet.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Patterson SL, Abel T, Deuel TA, Martin KC, Rose JC, Kandel ER. Recombinant BDNF rescues deficits in basal synaptic transmission and hippocampal LTP in BDNF knockout mice. Neuron. 1996;16:1137–1145. doi: 10.1016/s0896-6273(00)80140-3. [DOI] [PubMed] [Google Scholar]
- Wu K, Len GW, McAuliffe G, Ma C, Tai JP, Xu F, et al. Brain-derived neurotrophic factor acutely enhances tyrosine phosphorylation of the AMPA receptor subunits GluR1 via NMDA receptor-dependent mechanisms. Brain Research. 2004;130:178–186. doi: 10.1016/j.molbrainres.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Xu B, Gottschalk W, Chow A, Wilson RI, Schnell E, Zang K, et al. The role of brain-derived neurotrophic factor receptors in the mature hippocampus: Modulation of long-term potentiation through a presynaptic mechanisms involving TrkB. Journal of Neuroscience. 2000;20:6888–6897. doi: 10.1523/JNEUROSCI.20-18-06888.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakharenko SS, Patterson SL, Dragatsis I, Zeitlin SO, Siegelbaum SA, Kandel ER, et al. Presynaptic BDNF required for a presynaptic but not postsynaptic component of LTP at hippocampal CA1-CA3 synapses. Neuron. 2003;39:975–990. doi: 10.1016/s0896-6273(03)00543-9. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Keifer J. PKC-dependent and independent signaling pathways regulate synaptic GluR1 and GluR4 AMPAR subunits during in vitro classical conditioning. Neuroscience. doi: 10.1016/j.neuroscience.2008.08.042. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, van Aelst L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]


