Abstract
Type-2 diabetes is an adult onset condition that affects millions of people worldwide. The ensuing hyperglycemia renders multiple organs to various complications and increases the risk of learning and memory impairment. The Goto-Kakizaki (GK) rat developed from normoglycemic Wistar-Kyoto (WKY) rat is a model for type-2 diabetes, with insulin resistance developing around 12 weeks of age. We presently analyzed the neural progenitor proliferation and survival of the newly generated cells in the dentate gyrus (DG) and the subventricular zone (SVZ) of 6 and 18 week-old GK and WKY rats. At 6 weeks of age, both GK and WKY cohorts showed similar blood glucose levels (112 ± 14 mg/dL) and similar rates of neural progenitor proliferation. At 18 weeks of age, the GK rats showed significantly increased blood glucose levels (by 92 ± 12%; p<0.05) and higher number of proliferating neural progenitor cells compared to WKY rats (by 183 ± 16% in SVZ and by 36 ± 5% in DG; p<0.05 in both cases). In both the neurogenic areas, 52 ± 9% of the newly formed cells survived to 3 weeks in the 18 weeks old WKY rats, but in the GK rats only 16 ± 7% of the new cells survived to 3 weeks. When cultured from the DG of the 18 week old rats in the presence of FGF2 and IGF1, the GK cohort yielded significantly lower number of neurospheres than the WKY cohort (by 69 ± 7%; p<0.05). These results indicate that hyperglycemic environment induces proliferation of adult neural progenitors, but detrimental to their survival. Impaired neurogenesis might be a promoter of the decreased brain function in type-2 diabetes.
Keywords: Hyperglycemia, Animal model, Neural Progenitors, Proliferation, Survival, Growth factors, Neurospheres
1. INTRODUCTION
Diabetes is a major health concern with approximately 200 million people suffering in the world. This disease occurs in two major forms; while the early-onset type I diabetes is characterized by insulinopenia, commonly caused by the destruction of the insulin producing beta islet cells of the pancreas, the adult-onset type II diabetes results from peripheral insulin resistance. A commonality between the 2 conditions is the hyperglycemia which is detrimental to cognition and other neurological functions (Toth et al., 2007; Kodl and Seaquist, 2008).
One of the major complications of diabetes is decreased learning and memory functions. In mammalian brain, neurogenesis occurs throughout the life in 2 major areas of brain viz., the dentate gyrus (DG) of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles (Gage, 2000; Alvarez Buylla et al. 2002). The new neurons formed by the proliferation of the neural progenitor are thought to replace the dead neurons in the hippocampus and olfactory bulb (Taupin, 2008). In addition, a plethora of growth factors, environmental conditions and diseases have been shown to affect these cells both positively and negatively (Wiltrout et al., 2007).
Recent studies showed that neurogenesis, synaptic plasticity and learning potential will be significantly compromised in the rodent model of type 1 diabetes (Zhang et al, 2008; Stranahan et al, 2008). We currently evaluated the effect of type-2 diabetes on adult brain neural progenitor proliferation and survival using the Goto-Kakizaki (GK) rat which is a spontaneous, non-obese, carrier of the disease created by selective breeding of random Wistar-Kyoto (WKY) rats that showed hyperglycemia (Goto et al, 1975, Ahmad et al, 2008).
2. RESULTS
2.1. Hyperglycemia in GK Rat
At 6 weeks of age, the fasting blood sugar levels were observed to be similar (112.1 ± 14.4 mg/dL) in the GK and WKY rats (n = 30/group) (Fig. 1). At 10 weeks of age, the blood sugar level was not statistically different between the two cohorts or when compared with their respective 6 weeks old levels (Fig. 1). In the WKY rats, the blood glucose level remained unchanged at further ages (Fig. 1). Where as, the GK rats became overtly hyperglycemic by 16 weeks showing significantly higher blood sugar levels than the WKY rats (by 91 ± 15%; p<0.05; n = 18/group) (Fig. 1). The GK rats remained in the hyperglycemic state at 20 weeks of age (Fig. 1). At 6 weeks of age, the body weights were observed to be 154 ± 12 g (GK; n = 30) and 182 ± 14 g (WKY; n = 30). At 18 weeks age, the body weights were observed to be 372 ± 41 g (GK; n = 18) and 464 ± 58 g (WKY; n = 18). Both cohorts of rats were fed on normal rat chow.
Fig. 1.
Blood sugar levels as a function of age in WKY and GK rats. We used 30 WKY and 30 GK rats. As needed by different experiments, at 7 weeks, 9 weeks, 18 weeks, 19 weeks and 21 weeks of age, we killed n = 6 rats/group. Hence, the blood sugar values presented in each group are mean ± SD of n = 30 at 6 weeks, n = 18 at 10 weeks and 16 weeks, and n = 6 at 20 weeks. Statistics: ap<0.05 compared with the respective 6 weeks old value and bp<0.05 compared with the age-matched WKY cohort (one-way ANOVA followed by Tukey-Kramer multiple comparisons test).
2.2. GK rats showed normal progenitor proliferation in the pre-hyperglycemic age
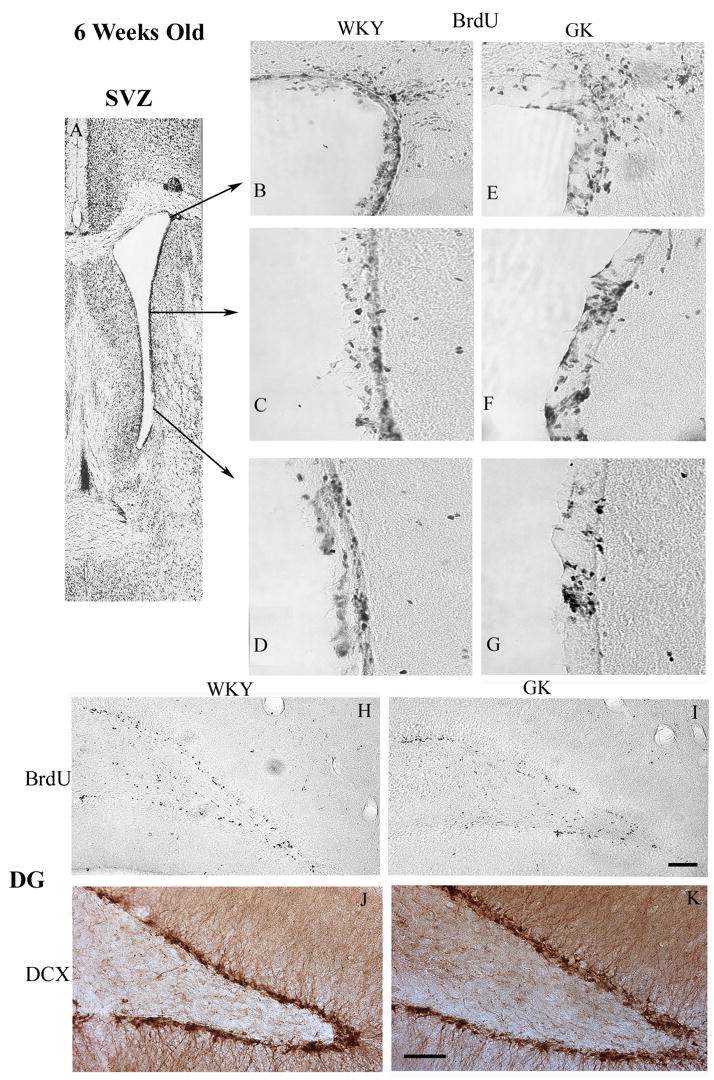
To study the proliferation of neural progenitors, cohorts of 6 weeks and 18 weeks old GK and WKY rats were injected with 5-bromo-2-deoxyuridine (BrdU; 50 mg/Kg; i.p. twice a day) for 5 days and killed on day 6 for immunostaining. When compared between the 6 weeks old WKY and GK rats, the number of proliferating neural progenitors was not significantly different in either SVZ or DG (n = 6/group) (Fig. 2 and Fig. 4). Immunostaining for the immature proliferating neural marker doublecortin (DCX) also showed a similar result (Fig. 2).
Fig. 2.
At 6 weeks of age, WKY and GK rats showed similar pattern of neural progenitor proliferation in the SVZ and DG. Panel A shows the areas from which the images B to G were taken. The BrdU+ proliferating neural progenitors are shown in different areas (indicated with arrows) of SVZ of WKY (B to D) and GK (E to G) cohorts. Panels H and I show the BrdU+ proliferating neural progenitors in the DG of WKY and GK rats, respectively. Panels J and K shows the DCX+ neural progenitors in the DG of WKY and GK rats, respectively. Similar results were observed in 6 rats/group.
Fig. 4.
Number of proliferating neural progenitors in the WKY and GK rats. BrdU was injected twice a day for 5 days and the rats were killed on day 6 for assessing proliferation. Values are mean ± SD of n = 6 rats/group. In case of each rat, the number of BrdU+ cells was counted using 4 brain sections.
2.3. Hyperglycemia increased proliferation, but decreased survival of neural progenitors
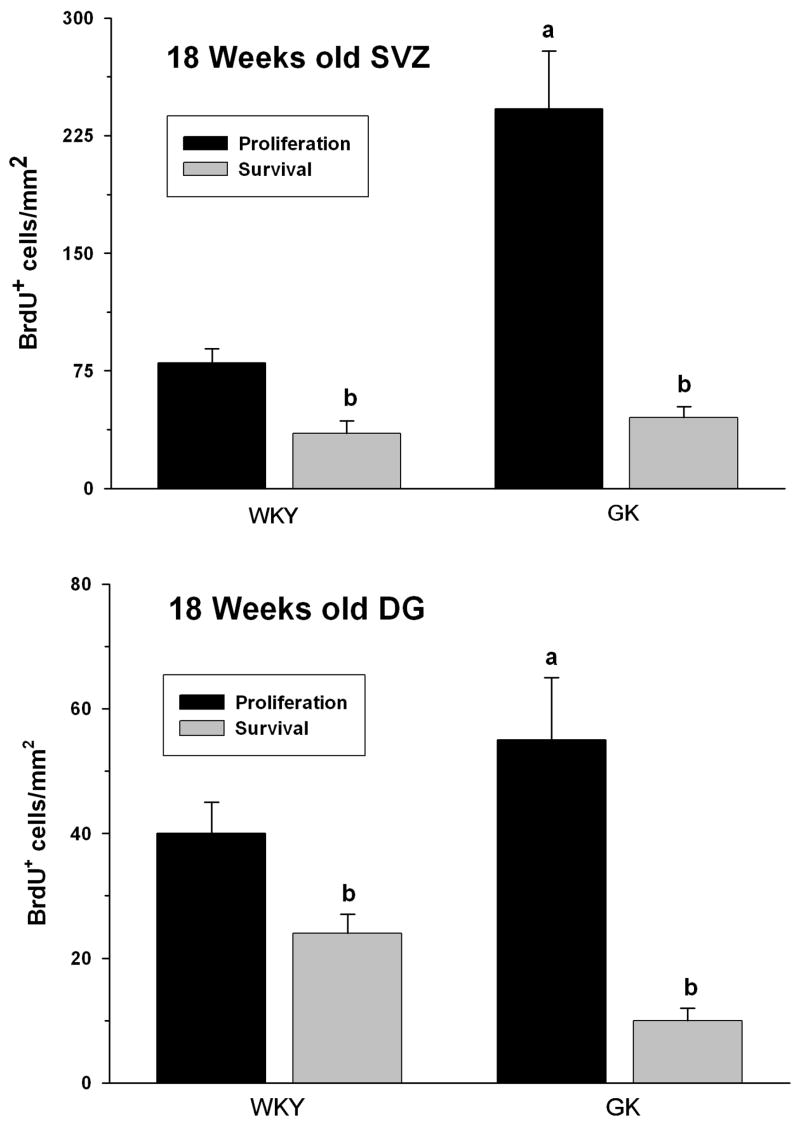
At 18 weeks of age (after they became hyperglycemic), the GK rats showed a significantly higher progenitor proliferation compared to the age-matched WKY rats (n = 6/group) in both SVZ (by 197 ± 21%; p<0.05) and DG (by 44 ± 8%; p<0.05) (Fig. 3 and Fig. 5). We also studied the survival of the newly formed cells by allowing a different cohort of GK and WKY rats (n = 6/group) to survive for 3 weeks after the 5 day BrdU injections. Despite the high level of proliferation, the number of neural progenitors that survived for 3 weeks were significantly lower in the GK compared to WKY rats. The rate of survival was 41 ± 11% in the SVZ and 44 ± 9% in the DG of the WKY rats (Fig. 5). Where as in the GK rats, the survival of the newly proliferated progenitors to 3 weeks was only 12% ± 4% in the SVZ and 15 ± 6% in the DG (n = 6/group) (Fig. 5).
Fig. 3.
At 18 weeks of age, the GK rats showed significantly higher number of proliferating neural progenitors in the SVZ and DG compared to WKY rats. Panel A shows the complete SVZ. The BrdU+ proliferating neural progenitors are shown in different areas of SVZ of WKY (B to D) and GK (E to G) cohorts. Panels H and I shows the BrdU+ proliferating neural progenitors in the DG of WKY and GK rats, respectively. Panels J and K shows the DCX+ neural progenitors in the DG of WKY and GK rats, respectively. Similar results were observed in 6 rats/group.
Fig. 5.
Number of surviving neural progenitors in the WKY and GK rats. BrdU was injected twice a day for 5 days and the rats were killed on day 21 for assessing survival. Values are mean ± SD of n = 6 rats/group. In case of each rat, the number of BrdU+ cells was counted using 4 brain sections. Statistics: ap<0.05 compared with the respective WKY group and bp<0.05 compared with the respective proliferation group (One-way ANOVA followed by Tukey-Kramer multiple comparisons test).
2.4. Impaired responsiveness of GK rat neural progenitors to growth factors
To understand whether the increased proliferation and decreased survival of the neural progenitors in the hyperglycemic rat were due to intrinsic cellular factors or the surrounding environment, we developed neurosphere cultures from the 18 week old GK and WKY rats. We could passage (the cultures were passaged once a week) the neurosphere cultures developed from the SVZ for more than 15 cycles in case of WKY rats; but the GK rat SVZ neurospheres stopped growing after the 3rd passage (data not shown). The neurospheres cultured from the DG of both WKY and GK rats survived for at least 15 passages and the number and size of spheres formed were not significantly different between the 2 cohorts in the absence of exogenously added FGF2 and IGF1 (Fig. 6). When the WKY cultures were grown the presence of growth factors FGF2 and IGF1 (20 ng/ml each), the spheres increased significantly compared to control (by 117 ± 14% in the presence of FGF2 and by 302 ± 41% in the presence of FGF2+IGF1; p<0.05 in both cases; n = 8/group) (Fig. 6). However, the GK spheres showed no such increase in the presence of FGF2 alone or FGF2+IGF1 (Fig. 6).
Fig. 6.
The neural progenitors cultured from the DG of 18 week old WKY rats proliferated rapidly to form neurospheres (A). Addition of FGF2 alone (B) or FGF2 + IGF1 (C), but not IGF1 alone (D), significantly increased the WKY neurosphere proliferation. On the other hand, neural progenitors cultured from the DG of 18 weeks old GK rats proliferated at a similar rate to WKY in the absence of added growth factors (E), but showed a significantly curtailed response to FGF2 alone (F) or FGF2 + IGF1 (G) or IGF1 alone (H). The histogram shows the results of the cell proliferation assay in each of the groups. The bars are mean ± SD of n = 6 per group. Statistics: ap<0.05 compared with the no growth factor added group and bp<0.05 compared with the respective GK group (one-way ANOVA followed by Tukey-Kramer multiple comparisons test).
3. DISCUSSION
In brief, results of the present study shows that adult type-2 diabetic rats show increased proliferation but decreased survival of the neural progenitors. Furthermore, neural progenitors cultured from adult diabetic rats show an impaired responsiveness to growth factors.
Diabetes is known to promote cognitive impairment and vascular dementia in humans (Biessels et al., 2008). Hemoglobin A1c level which is a marker of the long-term hyperglycemia was shown to correlate with cognitive decline in humans (MacLullich et al., 2004). Although the precise mechanisms that mediate this cerebral dysfunction are not known, chronic inflammation and oxidative stress seen in diabetes (MacLullich and Seckl, 2008) are known to promote neuronal death and inhibit neurogenesis (Das and Basu, 2008; Ekdahl et al., 2008; Wiltrout et al., 2007). Neurogenesis in mammals which continues throughout the life helps to replace neurons in the DG of hippocampus that play a role in memory functions. We currently show that neurogenesis is severely impaired in the adult type-2 diabetic GK rats. Interestingly in these animals the proliferation of neural progenitors in both the neurogenic regions increased significantly, but their survival diminished severely. The decreased survival of the newly formed cells might be acting as a feed-forward mechanism to increase the proliferation in a vain attempt to maintain new neuron formation at a constant level.
The precise mechanisms that impair the survival of the newly formed cells are not known at present, but our studies showed that lack of responsiveness to growth factors might be a cause. While the progenitors cultured from the normoglycemic rats formed more and bigger neurospheres when exposed to FGF2 and IGF1, progenitors from the hyperglycemic rats failed to respond to added growth factors. Growth factors are known to play a significant role in proliferation, survival and phenotypic maturation of the neural progenitors under in vivo and in vitro conditions (Palmer et al., 1995; Martens et al., 2002; Nakatomi et al., 2002; Aberg et al., 2003; Dempsey et al., 2003; Naylor et al., 2005; Yan et al., 2006; Kalluri et al., 2007; Wiltrout et al., 2007). Of importance, our laboratory and others showed that infusion of FGF2 and IGF1 into rodent brain significantly increases neural progenitor proliferation under normal and ischemic conditions (Palmer et al., 1999; Tureyen et al., 2005; Kalluri et al., 2005). While FGF2 is thought to be essential for cell division, IGF1 was shown to promote survival of the newly formed cells (Kalluri et al., 2005, 2007). Both these growth factors are known to be essential for maintaining the pluripotency of neurosphere cultures (Richards et al., 1992). In adult neural progenitors, IGF1 induces the phosphorylation of phosphatidylinositol-3-kinase/AKT and glycogen synthase kinase which are known to promote cell survival (Wiltrout et al., 2007). As type-2 diabetic GK rats have a disrupted insulin signaling pathway, the neural progenitors from these animals might not have responded to IGF1 which acts through insulin receptors. However, it is surprising that the hyperglycemic rat progenitors also failed to respond to FGF2 which acts via distinct FGF receptors leading to phosphorylation of the down-stream MAP-kinase ERK. Hence, decreased neurogenesis in type-2 diabetic brain might be due to an inherent defect in the neural progenitors/stem cells to respond to different growth factors. Our previous studies showed that FGF2 enhances progenitor proliferation more robustly in the presence of IGF1 (Kalluri et al., 2007). Increased proliferation in rats infused with FGF2 was shown to be associated with enhanced post-ischemic memory formation and retention (Nakatomi et al., 2002). Hence, disrupted response to FGF2 might contribute to the memory deficits seen in diabetics.
Impaired neurogenesis is not restricted to type-2 diabetes. Recent studies showed that genetically type-1 diabetic mice and STZ treated rats also show decreased neurogenesis (Zhang et al., 2008; Beauquis et al., 2008). Type 1 diabetes induced by streptozotocin injection in rodents was also shown to decrease neural progenitor proliferation (Beauquis et al., 2008; Stranahan et al., 2008). Interestingly, high-fat diet which accelerates diabetes also impairs hippocampal neurogenesis in rodents (Lindqvist et al., 2006). Several studies showed that diabetic humans show declined episodic learning and memory which could be attributed to hippocampal dysfunction (Messier et al., 2005). One of the contributing factors for the impaired hippocampal function might be disruption of neurogenesis mediated by increased glucocorticoids levels known to be present in diabetics. Long-term exposure to high levels of corticosterone is known to disrupt learning in animals (Oitzl et al., 1998) and maintaining physiologic levels of corticosterone was shown to enhance neurogenesis leading to restoration of LTP and reversal of learning deficits in type-2 diabetic mice (Stranahan et al., 2008).
Inflammation and oxidative stress which are commonly seen in diabetic subjects are known inhibitors of neurogenesis (Pluchino et al., 2008; Ekdahl et al., 2008; Kim et al., 2008). Treatment with the anti-oxidant and anti-inflammatory compounds like curcumin and indomethacin was shown to restore the neurogenesis inhibited by inflammation and oxidative stress (Kim et al., 2008; Monje et al., 2003). A recent study from our laboratory showed that the anti-diabetic thiazolidinediones (TZD) rosiglitazone and pioglitazone (PPARγ agonists) significantly decreased stroke-induce brain damage and neurological dysfunction in type-2 diabetic mice by preventing inflammation (Tureyen et al., 2007). Interestingly, PPARs are known to activate the molecular pathways STAT3 and Wnt signaling which control proliferation, differentiation and phenotypic specification of neural progenitor cells (Cimini an Ceru, 2008). Due to their capability to control blood sugar and lipid balance, rosiglitazone and pioglitazone are FDA-approved for type-2 diabetes treatment. As these TZDs control inflammation and oxidative stress, they might provide a better microenvironment for the neural progenitors to proliferate and form mature neurons in the diabetic brain.
In conclusion, our studies for the first time demonstrated a disruption of neurogenesis in adult type-2 diabetic animals which might be a factor responsible for the compromised cognitive and memory functions seen in diabetics. Compounds that could increase neurogenesis might be potentially beneficial in diabetes treatment.
4. EXPERIMENTAL PROCEDURES
4.1. Rats
The WKY and GK rats were obtained at 4 weeks of age from Taconic Animal Farms (Germantown, NY, USA). Animals were housed and cared for in accordance with the Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services Publication number 86–23 (revised in 1986) and all procedures were approved by the animal care committee of the University of Wisconsin-Madison. Blood sugar levels were estimated twice every week using a blood glucose monitoring system (FreeStyle, Abbott Park, IL, USA) using blood obtained from the hind paw. Rats were fasted overnight for blood sugar testing.
4.2. Progenitor proliferation and survival
Neural progenitor cells were estimated with immunohistochemistry in the DG and SVZ of rats as described earlier (Kalluri et al., 2007; Yan et al., 2007). BrdU (Sigma Chemical Co., MO, USA) dissolved in sterile saline was injected i.p. (50 mg/Kg) twice a day for 5 days. The proliferation groups were killed on day 6 and the survival groups were killed on day 21. In brief, the rats were deeply anesthetized with isoflurane, transcardially perfused with buffered paraformaldehyde, the brains were post-fixed, cryoprotected and sectioned serially (coronal; 30 μm thick) covering DG and SVZ. Brain sections were subjected to immunostaining using BrdU antibodies (1:50; biotin-conjugated mouse anti-BrdU; Molecular Probes, Eugene, OR, USA) and DCX antibodies (1:100; rabbit anti-DCX; Santa Cruz Biotechnology, Santa Cruz, CA, USA) essentially as described earlier (Dempsey et al., 2003; Yan et al., 2007). We stereologically counted the BrdU+ proliferating progenitors in the SVZ and DG of each rat with an established procedure that our lab used in several previous studies (Dempsey et al., 2003; Tureyen et al., 2004; Tureyen et al., 2005; Naylor et al., 2005; Park et al., 2007; Yan et al., 2006; Yan et al., 2007). In this method, sections were analyzed microscopically and the images were acquired using a CCD camera (Spot Camera, Diagnostic Instruments Inc., Sterling Heights, MI, USA). The BrdU+ cells reacted with DAB were quantified using 4 sections from each rat covering +1.1 to −0.2 mm from bregma as per the Rat Brain Atlas (Paxinos and Watson, 1998). In each case, the combined area of SGZ and the granular cell layer (GCL) of the DG was traced and measured in mm2 using Image-J software (written by Wayne Rasband, NIH, Bethesda, MD, USA; http://rsb.info.nih.gov/ij). The cells in the selected area were counted and divided by the area to obtain cells per mm2. This method was chosen to compensate for variations in the area of the cell layers between sections. To count the BrdU+ cells in the SVZ, 3 vertical, partial images of the lateral ventricle were microscopically acquired. The images were then compiled to make a complete image of the lateral ventricle using Photoshop (Adobe, San Jose, CA, USA). Using Image-Jsoftware, the rostral blade of the subependymal layer was traced, measured in mm2, the cells in the selected area were counted and divided by the area to obtain cells per mm2. Several previous studies presented the progenitor cell proliferation data as cells/area (Arvidsson et al. 2002; Aberg et al., 2003; Nakatomi et al. 2002). Compared to the total number of cells present in the GCL and SZG, the number of newly formed cells is insignificant. Furthermore, Cameron and McKay (2001) have shown that DG is constantly undergoing proliferation, yet the total number of cells in the GCL does not increase and the volume remains same with proliferation and cell death being in balance.
4.3. Neural Progenitor Culture
Neurospheres were generated from the SVZ and DG of adult GK and WKY rats as described earlier (Kalluri et al., 2007; Yan et al., 2007). In brief, a rat were deeply anesthetized with isoflurane, the brain was removed and washed in ice-cold Dulbecco’s phosphate buffered saline with 4.5 g/L glucose (DPBS/glu). The hippocampus and the lateral ventricle of SVZ were separately dissected under a microscope, and washed in fresh DPBS/glu. Tissues were minced in cold Hank’s balanced salt solution without Mg2+ and Ca2+ (HBSS; Invitrogen) and treated with 0.01% papain (Worthington Biochemical Corporation, Lakewood, NJ, USA), 0.1% dispase II (Boehringer Mannheim, Indianapolis, IN, USA), 0.01% DNase I (Worthington) and 12.4 mmol/L MgSO4 in HBSS at 37°C for 40 min. The cell suspension was triturated every 10 min, centrifuged, and the pellet was rinsed with Dulbecco’s-modified Eagle’s medium (DMEM)/F12 (1:1) (Invitrogen). The cells were suspended in neurobasal medium with B27 (without retinoic acid, Invitrogen), 2 mmol/L glutamine, 0.1 g/mL penicillin/streptomycin (Invitrogen), 2 g/mL heparin (Sigma-Aldrich, St. Louis, MO, USA), 20 ng/mL FGF2 (R&D Systems, Minneapolis, MN, USA), and seeded in T-25 culture flasks. The cultures were maintained at 37°C in an incubator with 5% CO2. Neurospheres were formed by 5 to 7 days of suspension in culture. Neural progenitors were passaged once a week by dissociating neurospheres into single cells with accutase. The neurosphere growth was estimated calorimetrically as described earlier (Kalluri et al., 2007; Yan et al., 2007). The cells were maintained for at least 15 passages. Cells (2 × 104) were suspended in 100 μL neurobasal medium and cultured in the presence and absence of IGF1 (20 ng/mL) and/or FGF2 (20 ng/mL) in a 96-well plate. After 3 days in culture, 20 μL of assay reagent (CellTitre 96 AQueous; Promega Inc., Madison, WI, USA) was added to the cell suspension and incubated at 37°C for 3 h to allow the conversion of tetrazolium salt into a formazan product. The optical density of the formazan product was measured at 490 nm in an ELISA plate reader. The same volume of medium without cells was used as blank.
4.4. Statistical analysis
The data are expressed as mean ± SD. Comparisons among groups were performed by one-way ANOVA followed by Tukey-Kramer multiple comparisons post-hoc test.
Acknowledgments
These studies were partially supported by grants from the United States National Institute of Health (RO1 NS044173 and RO1 NS049448) and the American Heart Association (Grant-in-Aid 0350164N).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aberg MA, Aberg ND, Palmer TD, Alborn AM, Carlsson-Skwirut C, Bang P, Rosengren LE, Olsson T, Gage FH, Eriksson PS. IGF-1 has a direct proliferative effect in adult hippocampal progenitor cells. Mol Cell Neurosci. 2003;24:23–40. doi: 10.1016/s1044-7431(03)00082-4. [DOI] [PubMed] [Google Scholar]
- Ahmad T, Ugarph-Morawski A, Lewitt MS, Li J, Sääf M, Brismar K. Diabetic osteopathy and the IGF system in the Goto-Kakizaki rat. Growth Horm IGF Res. 2008;18:404–411. doi: 10.1016/j.ghir.2008.02.003. [DOI] [PubMed] [Google Scholar]
- Alvarez-Buylla A, Seri B, Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res Bull. 2002;57:751–758. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Beauquis J, Saravia F, Coulaud J, Roig P, Dardenne M, Homo-Delarche F, De Nicola A. Prominently decreased hippocampal neurogenesis in a spontaneous model of type 1 diabetes, the nonobese diabetic mouse. Exp Neurol. 2008;210:359–367. doi: 10.1016/j.expneurol.2007.11.009. [DOI] [PubMed] [Google Scholar]
- Biessels GJ, Deary IJ, Ryan CM. Cognightion and diabetes: a lifespan perspective. Lancet Neurol. 2008;7:184–190. doi: 10.1016/S1474-4422(08)70021-8. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cimini A, Cerù MP. Emerging Roles of Peroxisome Proliferator-Activated Receptors (PPARs) in the Regulation of Neural Stem Cells Proliferation and Differentiation. Stem Cell Rev. 2008 doi: 10.1007/s12015-008-9024-2. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Das S, Basu A. Inflammation: a new candidate in modulating adult neurogenesis. J Neurosci Res. 2008;86:1199–1208. doi: 10.1002/jnr.21585. [DOI] [PubMed] [Google Scholar]
- Dempsey RJ, Sailor KA, Bowen KK, Türeyen K, Vemuganti R. Stroke-induced progenitor cell proliferation in adult spontaneously hypertensive rat brain: effect of exogenous IGF-1 and GDNF. J Neurochem. 2003;87:586–597. doi: 10.1046/j.1471-4159.2003.02022.x. [DOI] [PubMed] [Google Scholar]
- Ekdahl CT, Kokaia Z, Lindvall O. Brain inflammation and adult neurogenesis: The dual role of microglia. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.06.052. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Gage FH. Mammalian neural stem cells. Science. 2000;287:1433–1438. doi: 10.1126/science.287.5457.1433. [DOI] [PubMed] [Google Scholar]
- Goto Y, Kakisaki M, Masaki N. Spontaneous diabetes produced by repeated selective breeding of normal Wistar rats. Proc Jpn Acad. 1975;51:80–85. [Google Scholar]
- Kalluri HS, Vemuganti R, Dempsey RJ. Lack of response to epidermal growth factor in adult neural progenitor cells. Neuroreport. 2005;16:835–838. doi: 10.1097/00001756-200505310-00011. [DOI] [PubMed] [Google Scholar]
- Kalluri HS, Vemuganti R, Dempsey RJ. Mechanism of insulin-like growth factor I-mediated proliferation of adult neural progenitor cells: role of Akt. Eur J Neurosci. 2007;25:1041–1048. doi: 10.1111/j.1460-9568.2007.05336.x. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Son TG, Park HR, Park M, Kim MS, Kim HS, Chung HY, Mattson MP, Lee J. Curcumin stimulates proliferation of embryonic neural progenitor cells and neurogenesis in the adult hippocampus. J Biol Chem. 2008;283:14497–14505. doi: 10.1074/jbc.M708373200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodl CT, Seaquist ER. Cognitive dysfunction and diabetes mellitus. Endocr Rev. 2008;29:494–511. doi: 10.1210/er.2007-0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist A, Mohapel P, Bouter B, Frielingsdorf H, Pizzo D, Brundin P, Erlanson-Albertsson C. High-fat diet impairs hippocampal neurogenesis in male rats. Eur J Neurol. 2006;13:1385–1388. doi: 10.1111/j.1468-1331.2006.01500.x. [DOI] [PubMed] [Google Scholar]
- MacLullich AMJ, Deary IJ, Starr JM, Walker BR, Seckl JR. Glycosylated hemoglobin levels in healthy elderly nondiabetic men and negatively associated with verbal memory. J Am Geriatr Soc. 2004;52:848–849. doi: 10.1111/j.1532-5415.2004.52230_7.x. [DOI] [PubMed] [Google Scholar]
- MacLullich AMJ, Seckl JR. Diabetes and cognitive decline: Are steroids the missing link? Cell Metabolism. 2008;7:286–287. doi: 10.1016/j.cmet.2008.03.012. [DOI] [PubMed] [Google Scholar]
- Martens DJ, Seaberg RM, van der Kooy D. In vivo infusions of exogenous growth factors into the fourth ventricle of the adult mouse brain increase the proliferation of neural progenitors around the fourth ventricle and the central canal of the spinal cord. Eur J Neurosci. 2002;16:1045–1057. doi: 10.1046/j.1460-9568.2002.02181.x. [DOI] [PubMed] [Google Scholar]
- Messier C. Impact of impaired glucose tolerance and type 2 diabetes on cognitive aging. Neurobiol Aging. 2005;26:S26–S30. doi: 10.1016/j.neurobiolaging.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Nakatomi H, Kuriu T, Okabe S, Yamamoto S, Hatano O, Kawahara N, Tamura A, Kirino T, Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- Naylor M, Bowen KK, Sailor KA, Dempsey RJ, Vemuganti R. Preconditioning-induced ischemic tolerance stimulates growth factor expression and neurogenesis in adult rat hippocampus. Neurochem Int. 2005;47:565–572. doi: 10.1016/j.neuint.2005.07.003. [DOI] [PubMed] [Google Scholar]
- Oitzl MS, Fluttert M, Sutanto W, de Kloet ER. Continuous blockade of brain glucocorticoid receptors facilitates spatial learning and memory in rats. Eur J Neurosci. 1998;10:3759–3766. doi: 10.1046/j.1460-9568.1998.00381.x. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Ray J, Gage FH. FGF-2-responsive neuronal progenitors reside in proliferative and quiescent regions of the adult rodent brain. Mol Cell Neurosci. 1995;6:474–486. doi: 10.1006/mcne.1995.1035. [DOI] [PubMed] [Google Scholar]
- Palmer TD, Markakis EA, Willhoite AR, Safar F, Gage FH. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SW, Yan YP, Satriotomo I, Vemuganti R, Dempsey RJ. Substance P is a promoter of adult neural progenitor cell proliferation under normal and ischemic conditions. J Neurosurg. 2007;107:593–599. doi: 10.3171/JNS-07/09/0593. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 4. New York: Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Pluchino S, Muzio L, Imitola J, Deleidi M, Alfaro-Cervello C, Salani G, Porcheri C, Brambilla E, Cavasinni F, Bergamaschi A, Garcia-Verdugo JM, Comi G, Khoury SJ, Martino G. Persistent inflammation alters the function of the endogenous brain stem cell compartment. Brain. 2008 doi: 10.1093/brain/awn198. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci USA. 1992;89:8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Arumugam TV, Cutler RG, Lee K, Egan JM, Mattson MP. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat Neurosci. 2008;11:309–317. doi: 10.1038/nn2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taupin P. Adult neurogenesis, neuroinflammation and therapeutic potential of adult neural stem cells. Int J Med Sci. 2008;5:127–132. doi: 10.7150/ijms.5.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth C, Martinez J, Zochodne DW. RAGE, diabetes, and the nervous system. Curr Mol Med. 2007;7:766–776. doi: 10.2174/156652407783220705. [DOI] [PubMed] [Google Scholar]
- Türeyen K, Vemuganti R, Sailor KA, Bowen KK, Dempsey RJ. Transient focal cerebral ischemia-induced neurogenesis in the dentate gyrus of the adult mouse. J Neurosurg. 2004;101:799–805. doi: 10.3171/jns.2004.101.5.0799. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Vemuganti R, Bowen KK, Sailor KA, Dempsey RJ. EGF and FGF-2 infusion increases post-ischemic neural progenitor cell proliferation in the adult rat brain. Neurosurgery. 2005;57:1254–1263. doi: 10.1227/01.neu.0000186040.96929.8a. [DOI] [PubMed] [Google Scholar]
- Tureyen K, Kapadia R, Bowen KK, Satriotomo I, Liang J, Feinstein DL, Vemuganti R. Peroxisome proliferator-activated receptor-gamma agonists induce neuroprotection following transient focal ischemia in normotensive, normoglycemic as well as hypertensive and type-2 diabetic rodents. J Neurochem. 2007;101:41–56. doi: 10.1111/j.1471-4159.2006.04376.x. [DOI] [PubMed] [Google Scholar]
- Wiltrout C, Lang B, Yan Y, Dempsey RJ, Vemuganti R. Repairing brain after stroke: a review on post-ischemic neurogenesis. Neurochem Int. 2007;50:1028–1041. doi: 10.1016/j.neuint.2007.04.011. [DOI] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Vemuganti R, Dempsey RJ. Insulin-like growth factor-1 is an endogenous mediator of focal ischemia-induced neural progenitor proliferation. Eur J Neurosci. 2006;24:45–54. doi: 10.1111/j.1460-9568.2006.04872.x. [DOI] [PubMed] [Google Scholar]
- Yan YP, Sailor KA, Lang BT, Park SW, Vemuganti R, Dempsey RJ. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow and Metab. 2007;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- Zhang WJ, Tan YF, Yue JT, Vranic M, Wojtowicz JM. Impairment of hippocampal neurogenesis in streptozotocin-treated diabetic rats. Acta Neurol Scand. 2008;117:205–210. doi: 10.1111/j.1600-0404.2007.00928.x. [DOI] [PubMed] [Google Scholar]