Abstract
The medial temporal lobe cortex (MTLC) occupies a pivotal position at the interface between neocortical association areas and the hippocampus. It has been suggested that the MTLC contains functionally distinct regions, with perirhinal cortex (PRc) preferentially supporting object processing and posterior parahippocampal cortex (PHc) preferentially supporting encoding of spatial information. Measuring differential BOLD responsiveness to objects, scenes and other stimulus categories, we find a double dissociation between an anterior PRc response to objects and a posterior PHc response to scene stimuli. Furthermore, an anatomical ROI based approach was undertaken in an effort to understand the response profile underlying this double dissociation. We did not see any evidence for a sharp border between putatively distinct scene-preferential and object-preferential MTLC regions. Instead, scene-preferential responsiveness was noted to drop off in a graded, linear fashion in successively anterior MTLC regions until object-preferential responsiveness emerged in anterior PRc, although objects produced above baseline responses across the anterior-posterior extent of the parahippocampal gyrus. Other stimulus categories, such as faces and words, led to above baseline activation in either a few confined regions (faces) or none at all (words). Thus, what differentiated regions along the parahippocampal gryus was the relative response to objects and scenes, not simply above baseline responses to either category. This pattern raises the possibility that posterior PHc, and anterior PRc are situated at the ends of a single organizational continuum supported by the entire length of MTLC.
Introduction
Much of human functional imaging research is focused on revealing functional specialization within the human brain. This work is predicated on the notion that there exist computationally discrete and spatially bounded processing modules that selectively represent and/or process particular kinds of stimuli (Fodor, 1983, 2000). This idea has motivated research on putative cortical modules within the ventral visual processing stream, and the study of circumscribed regions exhibiting response specificity for faces, places, objects, body parts and word forms (Epstein and Kanwisher, 1998; Downing et al., 2006; but see Haxby et al., 2001).
Another tenet of the modularity hypothesis is that category-specific modules need not exist in higher level, integrative or central processing arenas (Fodor, 1983). In the context of the ventral stream, this view would predict that functional modules should be less common as one moves away from unimodal visual cortex. Hence, a fundamental question is whether category-specificity exists in central processing systems, such as the medial temporal lobe (MTL), a region known to be critical for integrating multi-modal representations in the service of long-term episodic memory.
The MTL consists of the hippocampal formation strongly innervated by underlying MTL neocortical regions including the perirhinal (PRc) and posterior parahippocampal (PHc) cortices that together occupy what we will refer to as medial temporal lobe cortex (MTLC). While there is strong evidence that the hippocampus supports domain-general relational encoding processes (Cohen and Eichenbaum, 1993; Davachi, 2006), it remains to be seen to what extent MTLC may or may not exhibit well-circumscribed category-specific responses.
One strong piece of evidence in favor of category-specificity within MTLC is the existence of the ‘parahippocampal place area’, or PPA (Epstein and Kanwisher, 1998), that occupies the most posterior portion of MTLC and parts of the retrosplenial and fusiform cortices. The PPA has been reported to manifest two critical properties in favor of modularity: response specificity and a well-circumscribed localization (Epstein and Kanwisher, 1998; Spiridon et al., 2006). In particular, the PPA responds to scene stimuli more than to other stimulus categories such as faces, objects and houses (Epstein and Kanwisher, 1998; Epstein et al., 1999) and it has been reported that activation to scenes in the PPA drops off dramatically at the borders of this region (Spiridon et al., 2006). However, much of the extant work on the PPA does not consider (but see Bar and Aminoff, 2003) that the vast majority of this region occupies the posterior MTLC, a component of the MTL that is thought to integrate information from multiple posterior cortical processing streams (Suzuki and Amaral, 1994; Insausti et al., 1998; Pruessner et al., 2002; Lavenex et al., 2002). Hence, one fundamental question is how the functional characteristics of the PPA relate to the rest of the MTLC.
Hints about the specific contributions of PRc and PHc to memory processing have been gleaned from neuroanatomical tract-tracing studies. PRc receives the majority of its input from temporal lobe structures in the ‘ventral visual stream’ known to be critical for object recognition (Mishkin et al., 1983: Ungerleider and Haxby, 1994). While the PHc also receives input from ventral visual areas, it is the only MTL cortical region to receive direct input from the posterior parietal cortex (Suzuki and Amaral, 1994) or the ‘dorsal visual stream’ thought to be important in representation of and guiding movement through space (Mishkin et al., 1983; Ungerleider and Haxby, 1994). Echoing the proposed distinctions between the functions of the ventral and dorsal visual processing streams, it has been proposed that PRc and PHc may be the mnemonic counterparts of these posterior processing streams, providing a substrate for refining and integrating perceptual information about space and objects into mnemonic representations.
Consistent with this notion, neurophysiological and lesion studies in animals have also provided support for a division of labor between the PRc and the PHc. PRc neurons have been reported to show increased c-fos expression in response to object novelty compared to object-location novelty, while postrhinal cortical neurons, thought to be the rodent counterpart of the human PHc, exhibit the opposite effect (Wan et al., 2001). Furthermore, recent lesion studies have revealed a double dissociation between the effects of lesions to PRc and PHc on object and spatial memory (Meunier et al., 1993; Eacott et al., 1994; Nemanic et al., 2004; Alvarado and Bachevalier, 2005; for review Malkova and Mishkin, 2003). Finally, human fMRI studies have also provided evidence for this dissociation. As described above, activation in posterior MTLC has been reported when subjects view (Epstein and Kanwisher, 1998) or imagine (Epstein et al., 1999; Davachi et al., 2003) scene stimuli and a number of studies have also reported dissociations along the MTLC depending on whether object or scene representations are evoked (Bar and Aminoff 2003; Pihlajamaki et al., 2004; Aminoff et al., 2006; Buffalo et al., 2006). The results of these studies are mostly consistent with the hypothesis outlined above in that spatial tasks generally engage more posterior regions of the MTLC and object tasks activate MTLC regions that are more anterior. We will directly examine the responses to objects and scenes as well as to other stimulus categories in an effort to gain a more complete understanding of category-specificity within human MTLC.
Based on previous work, we expect to reveal a double dissociation between object and spatial responses across PHc and PRc. However, in addition, we aim to better understand the nature of the underlying functional organization that supports these reported dissociations. At least two distinct organizational scenarios are possible. One possibility is that there are two functionally and anatomically distinct MTLC module-like regions, PRc and PHc, that selectively support object and spatial encoding, respectively, and are separated by a sharp, clear boundary. (By selective, we mean that the response to a category is greater than the response to all other categories as well as significantly above baseline. This term additionally implies that there are no significant differences between activation to other categories. For example, for a region to be selective to scenes, this region should show scenes > all other categories and, in addition, there should be no additional differences in activation between the other categories. If other differences exist, the activation is defined as being ‘differential’ as opposed to ‘selective’ here.) This view, common in current work, motivates study of how one region - say PRc - contributes to memory independent of other regions- such as PHc.
Another possibility, however, is that the MTLC may not be organized into clear, highly selective category-specific modules with strict boundaries. Instead, it is possible that reported double dissociations emerge at the ends of a single underlying representational gradient. According to this view, most of the MTLC may be responsive to processing both spatial and object stimuli but the relative degree of preferential activation might change gradually across successive MTLC regions giving rise to significant differences only at the extremes. In fact, some recent work focusing primarily on PHc has reported at least two distinct adjacent subregions within PHc: a posterior PHc region preferentially responsive to spatial associations and an adjacent anterior PHc region preferentially responsive to non-spatial associations (Bar and Aminoff, 2003; Aminoff et al., 2007). These findings are less consistent with the notion that PRc and PHc are homogenous processing modules in the medial temporal lobe and begs the question of what is the response along the entire parahippocampal gyrus, including both PRc and PHc.
To understand the organization of the MTLC, and the responses underlying reported dissociations (Bar and Aminoff, 2003; Pihlajamaki et al., 2004; Aminoff et al., 2006; Buffalo et al., 2006), it is necessary to examine multiple adjacent anatomically-defined regions of interest (aROIs) within the MTLC. To this end, in the present study, we divided the MTLC into seven, adjacent, equally-sized anatomical regions, or aROIs and measured the response in each region to object and scene stimuli, as well as other comparison stimulus categories. Subjects underwent fMRI scanning while performing a one-back task with pictures from five different stimulus categories: objects, scenes, faces, words, and pseudowords (Supplemental Figure 1 and Methods). Using this aROI approach we (1) computed the extent to which MTLC regions exhibited response selectivity to particular categories and (2) whether any specificity exhibited well-circumscribed borders, by examining how differential stimulus patterns change across adjacent regions of the MTLC.
We found a significant double-dissociation between scene- and object-responsive portions of the human MTLC, providing support for two distinct processing modules in the PRc and PHc. However, our further anatomical ROI analyses do not necessarily support a modularity interpretation, since analysis of responses along the entire extent of the MTLC revealed a gradient. Specifically, our data show a gradual, linear transition between the responsiveness of MTLC cortical areas to object and scene stimuli. Implications for this organization remain to be studied and are discussed further.
RESULTS
Directed Contrasts
As expected, a contrast comparing the BOLD response to novel scenes to all other stimulus categories revealed a cluster of voxels in bilateral posterior PHc (see Supplementary Table 1 for all other activation clusters), or the PPA (Epstein and Kanwisher, 1998) in all subjects (average y = − 48). In order to assess selectivity, the BOLD response to all five stimulus categories, separately for novel and repeating blocks, was extracted from each subject's functionally defined PPA. An omnibus repeated-measures ANOVA confirmed significant activation differences between the five stimuli in both hemispheres [right: F(5,35) = 30.2, P = 3.9−11; left: F(5,35) = 24.7, P = 1.4−10]. Planned pairwise contrasts showed that activation during novel scene blocks was greater than all other stimulus categories, including novel objects [right: all t's(7) > 5, all Ps < 0.001, Figure 1A; left: all t's(7) > 7, all Ps < 0.0001].
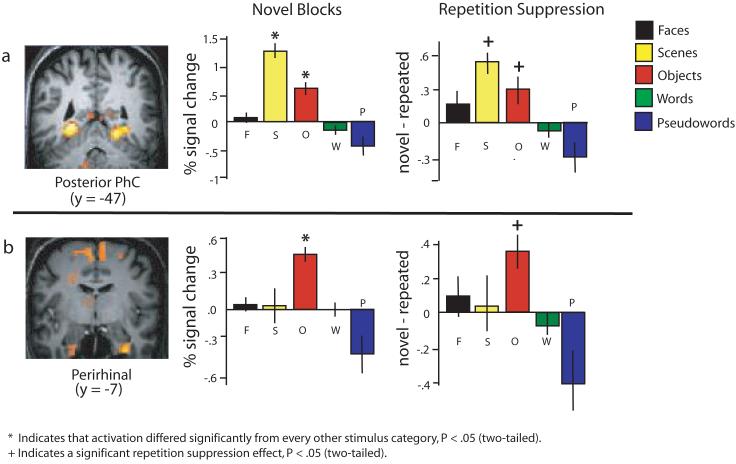
Figure 1. Scene and object differential responsiveness in MTLC.
(a) A contrast comparing the BOLD response to scenes to that of all other stimulus categories revealed an area of activation in bilateral PHc displayed here on a high resolution anatomical of one subject. A plot of percent signal change for each condition (± s.e.m.) shows that scenes activate the posterior PHc (shown for right hemisphere) significantly more than all other stimulus categories and, furthermore, that objects also activate this region significantly more than all other stimulus categories, other than scenes. Repetition suppression effects were found to be significant in the PHc for both scenes and objects. (b) A contrast comparing the BOLD response to objects to that of all other stimulus categories revealed activation in PRc displayed here on the same subject's high-resolution anatomical image. Objects activate the PRc (data shown for right) more than all other stimulus categories in novel blocks, and significant repetition suppressions effects are only found for object stimuli.
Importantly, as noted in previous reports (Epstein and Kanwisher, 1998; Ewbank et al., 2005; Diana et al., 2008), a significant above-baseline response was also elicited by novel objects in the PPA [right: t(7) = 8, P = 0.00008; left: t(7) = 4.7, P = 0.002] and objects also elicited more activation than all other stimulus categories including faces [right: t(7) = 3.8, P = 0.007; left t(7) = 32.9, P = 0.022], words [right t(7) = 8, P = 0.00009; left, t(7) = 5.3, P = 0.001] and pseudo-words [right: t(7) = 5.9, P = 0.001; t(7) = 3.9, P = 0.006]. In turn, none of these other stimulus categories elicited significant above baseline activation [all t's(7) < 1.3, all Ps > .05.]
In addition to the response to novel stimuli, the extent to which activation decreases upon stimulus repetition, or repetition suppression, has often been used to reveal underlying representations (Koustaal et al., 2001; Ewbank et al., 2005; Kourtzi and Huberle, 2005; Turk-Brown et al., 2006; Schacter et al., 2007). Accordingly, significant repetition suppression was noted in the PPA both for scene [right: t(7) = 7, P = .0002; left, t(7) = 5.5, P = .001] and object stimuli [right, t(7) = 3.6, P = .009, Figure 1A; left, t(7) = 3.6, P = .03], but not for faces, words or pseudowords [all t's(7) < 1.7, all Ps > .17]. Thus, in sum, we find that activation in the PPA is differentially responsive to scenes, but does not appear to be selective, as it also responds to novel objects and shows suppression to repeated objects.
We next examined whether PRc exhibits preferential responsiveness to object stimuli. A whole-brain contrast comparing the BOLD response to novel objects to all other categories of stimuli revealed significant activation in right PRc, albeit at different anterior-posterior levels, in all eight subjects (see Supplementary Table 1 for all other activation clusters). Six of the eight subjects exhibited activation in anterior right PRc (average y = −3) while two exhibited activation in a more posterior region of right PRc (average y = −17) (see Figure 1B). Furthermore, significant activation in left PRc was also evident in six out of eight subjects. Because the right PRc activation was evident in all eight subjects, we focus the follow-up statistical analyses on the right PRc only. Omnibus repeated-measures ANOVAs confirmed that there were significant differences between the five stimulus categories [F(5, 35) = 6.8, P = .0001]. Planned pairwise comparisons further showed that BOLD activation during novel object blocks was significantly greater than during each of the other stimulus categories including faces [t(7) = 7.8, P = .0001], scenes [t(7) = 7.7, P = 0.032], words [t(7) = 6.5, P = .0003] and pseudo-words [t(7) = 4.3, P = .004]. Furthermore, significant repetition suppression effects in this region were only seen for objects [t(7) = 3.5, P = .01] and not for scenes [t(7) = .003, P = .99] or any of the other stimulus categories [all t's(7) < .8, Ps > .4, Figure 1B]. Hence, the PRc response appears to be more selective than responses in PPA.
Thus, a dissociation between the response to objects and scenes was evident between these two MTLC regions. In order to statistically examine the dissociation, we tested for and found a significant Region (PRc, PPA) * Stimulus (object, scene) interaction [F(1,7) = 26, P = .03; Figure 2]. Furthermore, when the magnitude of repetition suppression in these regions was included in the analysis, a significant triple interaction Region * Stimulus * Novelty; [F(1,7) = 8.4, P = .02] was revealed. Namely, repetition suppression is greater for scenes in PPA and for objects in PRc, further strengthening the notion of a differential involvement of PHc and PRc in scene and object specific encoding processes, respectively.
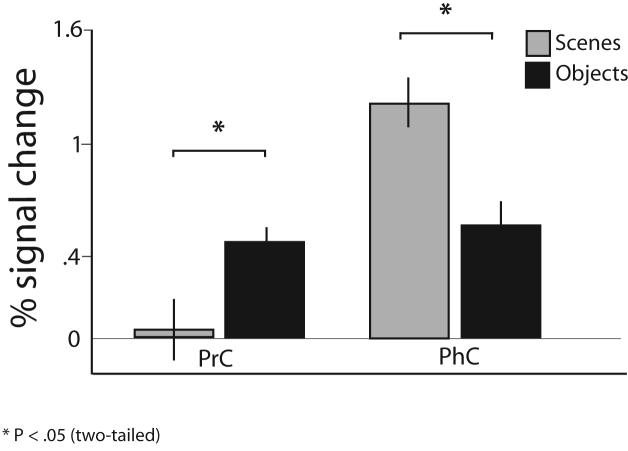
Figure 2. Interaction between scene and object responsiveness in PHc and PRc.
A significant two-way interaction between objects and scenes in the PHc and the PRc. The average percent signal change collapsed across timepoints 3 - 12 (± s.e.m.) in PRc and PHc regions is displayed for novel object and scene blocks. A within-subjects ANOVA showed a significant Stimulus * Region interaction (P = .02) and a significant Stimulus * Region * Novelty triple interaction (P = .03; not shown).
Anatomical ROI Analyses
One interpretation of these results is that the PRc and posterior regions of the PHc are distinct modules whose underlying properties confer upon them differential involvement in the processing and encoding of object and scene stimuli. However, this conclusion falls short of providing a complete account of MTL cortical organization as it leaves unspecified the role of a large portion of MTLC that lies between the PRc regions revealed in the whole-brain contrasts and the PPA. Specifically, the anterior portion of the PHc did not emerge in any of the directed contrasts described above nor in any other directed contrasts we employed (e.g. faces > all, words > all) and the posterior PRc only appeared in two of eight subjects.
In order to gain a more complete understanding of the response profile along the entire MTLC, we examined the response profiles of the regions that lie between the scene responsive posterior PHc (or PPA) and the object responsive anterior PRc by drawing a series of equally spaced anatomical regions of interest (aROIs) along the entire extent of the MTLC. Anatomical ROIs were hand drawn for each subject individually along the entire extent of the MTLC starting at y = +3 and ending at y = −53 in both the right and left hemispheres. Tracing of the MTLC closely followed the guidelines outlined by Insausti et al., (1998) (See Figure 3A,B and Methods for details). From each of these aROIs, BOLD activation was extracted for novel object and scene blocks as well as all other stimulus categories.
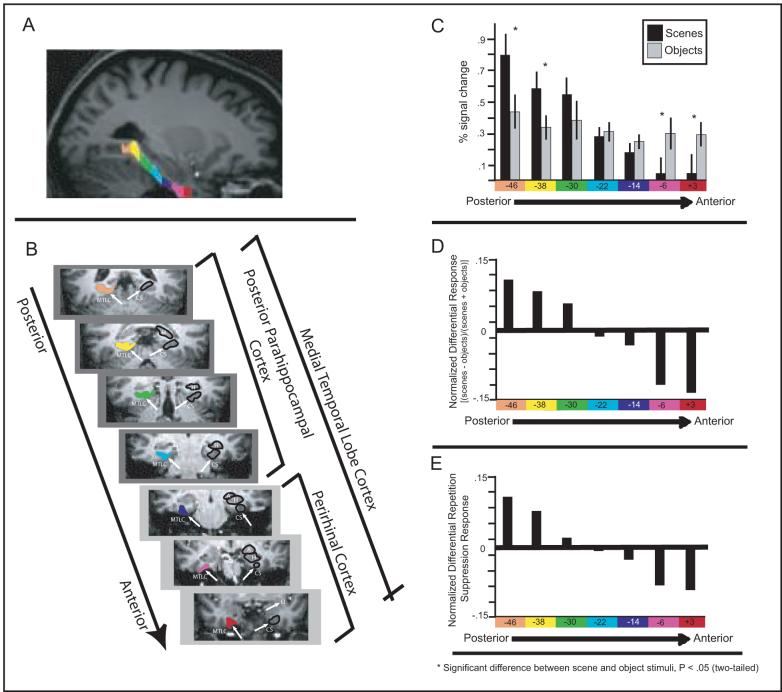
Figure 3. Pattern of activation across seven adjacent anatomical regions of interest (aROIs) in the right medial temporal lobe cortex (MTLC).
(a) Representative coronal slices through medial temporal lobe cortex with anatomical ROIs. Coronal sections through the antero-posterior extent of the medial temporal lobe of one participant starting at the limen insula (LI) anteriorly. Medial temporal lobe cortical aROIs are represented as color-coded regions in the left hemisphere. The hippocampus (H), amygdala (A) and collateral suclus (CS) are outlined in the right hemisphere. The three most anterior aROIs (presented on light gray background) are in the perihinal cortex (PRc). The four most posterior aROIs (presented on dark gray background) are in the parahippocampal cortex (PHc). The gyrus intralimbicus (not shown) marks the PRc/PHc border. All boundaries were modeled directly from Insausti et al., (1998). (b) Seven aROIs, each 8 mm in the anterior-posterior dimension, are color coded and displayed on a high-resolution sagittal section from a representative subject. (c) BOLD % signal change during novel object (gray) and scene (black) blocks are plotted for each aROI, color-coded to correspond to the regions shown in (a and b). Activation to scenes declines in a linear fashion across the seven aROIs while activation to objects appears flat across these same aROIs. (d) To alleviate across-region comparisons, the response to scenes and objects was normalized by computing the difference between scenes and objects as a function of their sum. Linear contrast analyses performed on the normalized responses revealed that a linear (P = .001), but not any other polynomial function (all Ps > .15), significantly fits these data. These data demonstrate that preferential responsiveness gradually, and linearly, shifts from scenes to objects along the anterior-posterior extent of the MTLC. (e) The repetition suppression effects for objects and scenes was normalized by computing the difference between the scene repetition suppression effect (novel scenes - repeated scenes) and the object repetition suppression effect (novel objects - repeated objects ), as a function of their sum. Linear contrast analyses performed on the normalized responses revealed that a linear (p < .05), but not any other polynomial function (all ps > .7), significantly fits these data.
The two most posterior aROIs (spanning from y = − 53 to y = − 41) most closely correspond to reported coordinates of the PPA as defined both by previous studies (e.g. Epstein and Kanwisher, 1998) and in the present functional data (y = −48 in both hemispheres). Consistent with previous work, in these two aROIS, it was found that novel scenes elicited a significantly stronger response than novel objects in both the right [t's(7) > 4, Ps < .005; Figure 3C] and left [t's(7) > 3.3, Ps < .01; Supplementary Figure 2B] hemispheres. On the other hand, BOLD activation in anterior regions of MTLC, in PRc, showed the opposite effect and was greater for novel objects compared to novel scenes. Specifically, in the right hemisphere, this pattern was seen in the two most anterior aROIs [(y = +3 to −5) (t(7) = 3, P < .05) (y = −5 to −12) (t(7) = 2.9, P < .05); Figure 3C)]. In the left hemisphere, this effect was significant in the second most anterior aROI [t(7) = 3, P < .05] and marginal in the most anterior aROI [t(7) = 2, P = 0.078; Supplementary Figure 2B].
Critically, however, upon examination of activation across all MTLC aROIs, a striking overarching pattern was revealed. Starting with the most posterior aROI and moving anteriorly across the extent of the MTLC, BOLD responses to scenes declined in a linear, graded fashion [Figure 3C and, for left hemisphere, Supplementary Figure 2B]. Indeed, a linear contrast revealed that activation to scenes is significantly linear in both hemispheres [left: F(1,7) = 24, P < .005; right F(1,7) = 20.1, P = .005] and that no other polynomial trend analysis significantly fits the data (all Ps > .2).
Unlike the response to scenes that showed a linear decrease moving anteriorly along the MTLC, the response to objects appears to be of similar magnitude across all seven aROIs (Figure 4B). This suggests that object encoding may be significant along the entire extent of the MTLC. In support of this notion, the BOLD response to objects compared to baseline was significant in all seven aROIs in the right hemisphere and in five aROIs in the left hemisphere (with effects being marginal in the remaining two aROIs). Importantly, this was not a global effect, as other perceptually salient stimuli such as faces did not elicit above-baseline activation along the majority of the MTLC, and words and pseudowords did not elicit above baseline activation in any of the aROIs (Figure 4A,B).
Figure 4. Response in MTLC aROIs to all stimulus categories.
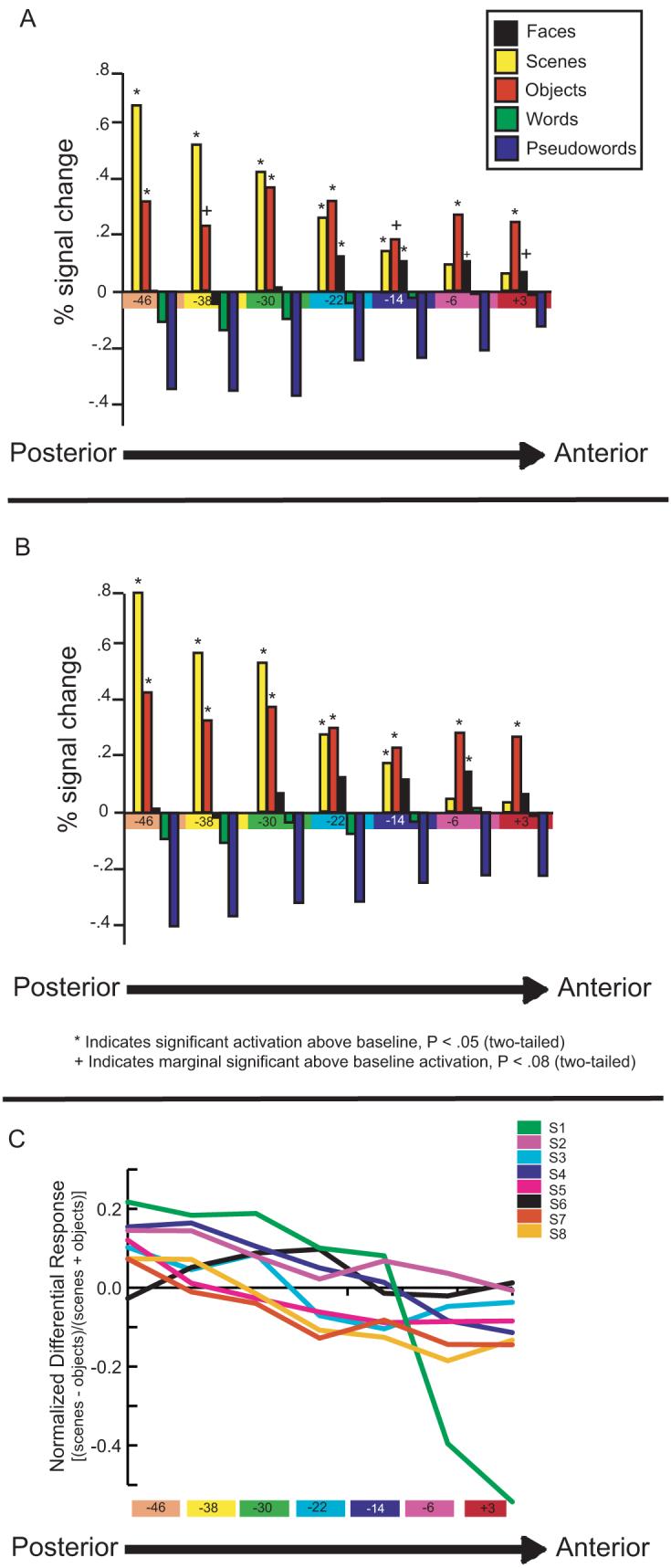
BOLD response to novel objects, scenes, faces, words and pseudowords in all MTLC aROIs (± s.e.m.) is plotted in the right (a) and left (b) hemispheres. In both hemispheres, objects elicited above baseline activation across the entire MTLC with the only exception being two aROIs in the left hemisphere that were marginally significant. Scenes elicited above baseline activation in all aROIs except for the two most anterior aROIs, bilaterally. Faces elicited significant or marginally significant above-baseline activation in the four most anterior aROIs in the left hemisphere and in the two most anterior aROIs in the right hemisphere. The activation for words and pseudowords was not significantly above baseline in any aROI. (c) The normalized difference between scene and object activation is plotted for each subject individually across the seven aROIs in the right hemisphere. Linear regression analyses carried out for each participant separately revealed that the R2 value was highly significant in seven out of eight subjects (average R2 value = .7).
Abbreviations: S1 = Subject 1, S2 = Subject 2 etc.
However, caution is warranted when comparing the percent signal change across different brain regions, or adjacent ROIs within the same region, as the dynamic range of the BOLD response may differ significantly across brain regions. This is especially true in the most anterior portions of the MTL known to suffer from signal to noise reductions as a result of signal drop out (Olman, Davachi and Inati, submitted; Ojemann et al., 1997) Thus, in order to account for regional variations in overall BOLD responsiveness, the BOLD response was normalized by computing the activation difference between scenes and objects as a function of the total response to both scenes and objects (Scene − Object/Scene + Object) in each of the seven aROIs bilaterally.
Examination of the normalized difference response, an indication of the relative response to scenes and objects, revealed a linear crossover from posterior to anterior portions of the MTLC. Specifically, the relative activation of scenes compared to objects systematically diminishes in a linear fashion as one moves anteriorly along the MTLC until object stimuli gradually produce greater percent signal change than scenes (see Figure 3D for the right hemisphere). A repeated-measures ANOVA and a linear contrast analysis revealed that the relative activation of scenes to objects does indeed change linearly in both hemispheres [left: F(1,7) = 13.8, P < .01; right F(1,7) = 40.1, P = .001]. Importantly, no other polynomial trend significantly fits the data (all Ps > .15).
Since it is possible that a significant linear trend may emerge in group-averaged data but may not necessarily be evidenced in individual subject responses, we used linear regression to assess the extent to which each subject's data showed a linear trend across the extent of the MTLC. Seven out of eight subjects had significant regression scores in the right hemisphere (R2 values > .55, all Ps < .001) and the average R2 value across all eight subjects was .7 (Figure 4C). Interestingly, in the left hemisphere, more individual subject variance was observed. Five out of eight subjects showed a strong linear effect with R2 values greater than .7. Of the other three subjects, the R2 for two subjects failed to reach significance. This pattern of results is consistent with previous studies that have also reported laterality effects in the MTLC (Epstein and Kanwisher, 1998).
Another methodological concern is that a linear trend may emerge from procedures such as data smoothing. To alleviate this concern, we performed the same procedures on subject's unsmoothed and unnormalized functional images. Importantly, visual inspection revealed the same pattern even with no data smoothing (Supplementary Fugure 3B,C). A significant linear trend was still evident in both the right and the left hemispheres [Fs (6, 42) > 3, Ps < .05] while no other polynomial trend fit the data (all Ps > .55). We further examined the linear trends across the seven ROIs separately for each subject. The average R2 value across all eight subjects was .52 on the right and .5 on the left. These data confirm that the linear trend observed with the standard 6 mm smoothing kernel is not an artifact of data smoothing or normalization.
We also tested whether there was evidence for a repetition suppression gradient. A similar effect for the right hemisphere was observed when looking for changes across regions in the magnitude of the repetition suppression effects. A repeated-measures ANOVA revealed that the relative change in activation with repetition, or repetition suppression, for scenes and objects also changed linearly in the right hemispheres (Figure 3E), F(1,7) = 6.4, P < .05, with no other polynomial trend being significant (all Ps > .7). The repetition suppression linear trend, however, fell short of significance in the left hemisphere F(1,7) = 2.3, P = .13.
Importantly, objects were not the only stimulus category to elicit above baseline activation in PRc. Activation to faces in the right PRc was found to be enhanced compared to baseline in the second most anterior aROI [y = −14 to −21: t(7) = 7, P < .05]. In the left hemisphere, a marginal effect was seen in the two most anterior aROIs [y = +3 to −5: t(7) = 7, P = .053; y = −6 to −15: t(7) = 7, P = .07] and a significant effect in the next two aROIs [y = −14 to −21: t(7) = 7, P < .05; y = −22 to −29: t(7) = 7, P < .05; Figure 4A,B]. However, while faces, like objects, showed above baseline activation in anterior PRc, they did not elicit above baseline activation in posterior regions of the PHc. In addition, no difference was found between faces and scenes in the PRc [left: F(1,7) = .8, P > .7; right F(1,7) = .3, P > .7]. Thus, there does not appear to be the same reciprocal relationship between responsiveness to scenes and faces as there is for scenes and objects. This will be discussed further below.
Discussion
The results of the present experiment reveal two elements of functional organization within the human MTLC. First, a double dissociation was revealed between activation in PRc and posterior PHc during object and scene processing. This dissociation in humans complements known differences in non-human primate anatomical connectivity (Suzuki and Amaral, 1994), emerging results from animal lesion, human neuropsychological (Lee et al., 2005) and fMRI studies (Norman and Eacott, 2005; Wan et al., 2001), and immediate early gene expression in animal models (Wan et al., 2001). Importantly, our data also highlight that scene differential responsiveness in posterior PHc is more consistently localized across participants than object-selective responsiveness in anterior PRc. Specifically, while all participants did show object preferential responses in PRc globally, the precise localization of this response within PRc varied across participants.
In addition, the results of the aROI analysis reveal a novel element of the functional organization of the MTLC. Specifically, it was found that the double dissociation between the response to objects and scenes evident in portions of PRc and PHc, respectively, does not exhibit a well-defined boundary, but, instead, emerges at the extremes of an underlying linear crossover in the relative response to objects and scenes along the entire anterior-posterior extent of the MTLC. Our anatomically-based ROI analyses revealed that while activation in the most posterior portions of the PHc was strongest for scene stimuli relative to object and other stimulus categories, this preferential response for scenes declined in a linear fashion along the MTLC axis until the most anterior PRc, where the inverse was observed. Importantly, these results replicate previous work reporting double dissociations along the anterior-posterior MTLC axis (Meunier et al., 1993; Bar and Aminoff, 2003; Pihlajamaki et al., 2004; Alvarado and Bachevalier, 2005; Buffalo et al., 2006; Aminoff et al., 2007) but, critically, reveal hints about the organizational structure underlying these dissociations.
Furthermore, the graded nature of representation along the MTLC may explain why aROI analyses in the MTLC have not always yielded double dissociations between PRc and PHc. For example Aminoff et al., (2007) examined activation in three anatomical ROIs, two of which divided the PHc into anterior and posterior regions, and one including the entire PRc. Consistent with our findings, they reported that the posterior PHc responded more strongly to spatial information while anterior PHc responded more to abstract objects than to spatial stimuli. In that same analysis, no differences were reported between spatial and abstract object stimuli within the PRc aROI. The results from the present aROI analysis suggest that PRc may be heterogenous in its relative responsiveness to object and spatial stimuli, thus the lack of a differentiation in PRc in the Aminoff et al., (2007) analysis could have been because that analysis collapses across heterogenous regions of PRc. Likely for a similar reason, another recent study (Diana et al., 2008) that examined PRc and PHc aROIs, did not find differences in the PRc between objects and scenes. Combining our results with these apparent negative findings suggests that pooling across the entire PRc may decrease the sensitivity of the analysis since the greatest differences between objects and scenes are observed in the most anterior regions of PRc. Thus the present findings add nicely to the results of Aminoff et al., (2007), which presented evidence of a hierarchical organization in the PHc, in showing that the entire MTLC, including PRc, is organized along a gradient, rather than consisting of two discrete functional regions.
Unlike objects and scenes, activation for words and pseudo words was below baseline throughout the MTLC. Many previous studies that used verbal stimuli (Davachi et al 2003; Ranganath et al., 2004; Staresina and Davachi, 2006, 2008) have shown that the perirhinal cortical activation supports item encoding. Specifically, activation in the PrC at encoding was greater in these studies for words that were later remembered compared to those that were later forgotten. Importantly, however, these fMRI studies typically require subjects to answer encoding questions based on the representations of the presented word stimuli. What is driving the perirhinal response is likely the meaning, concept and/or visual image of the referent of the words, not simply the symbols themselves. Thus, the low activation for letter strings in our study was likely the result of the relatively minimal demand on creating mental representations. These findings suggest that MTLC activation for verbal stimuli should be a function of the extent to which participants use presented words to create internal representations of real items.
Activation for faces was likewise not significantly above baseline across the MTLC, with the exception of anterior PrC. Consistent with the lack of above baseline activation for faces in the posterior MTLC, previous studies found that faces do not elicit above baseline activation in the PPA (Epstein and Kanwisher, 1998). On the other hand, the above baseline activation for faces in the anterior PrC is complementary with a number of studies showing that memory for faces is impaired in patients with PrC damage (Taylor et al., 2007; Bird et al., 2007), and may correspond to the processing and binding of item features (Lee et al, 2005).
The suggestion that the functional organization along MTLC may be organized along a continuum presents an alternative viewpoint on theories and empirical approaches that prescribe discrete functional roles to regions situated within the MTLC. All of the empirical work focused on understanding the role of MTLC in episodic memory has adopted an ‘either-or’ approach, contrasting the effects of inputs and lesions to (Meunier et al., 1993; Eacott et al., 1994; Nemanic et al., 2004; Alvarado and Bachevalier, 2005; Malkova and Mishkin, 2003) or activation in (Pihlajamaki et al., 2004; Janzen et al., 2004; Buffalo et al., 2006) purportedly discrete regions, rather than seeking to reveal dimensions that might be similar across adjacent, interconnected, cortical areas (but see Bar and Aminoff, 2003; Aminoff et al., 2007). By not systematically examining the effects of experimental manipulations across the entire MTLC, one cannot examine the underlying response patterns that provide the foundations of the observed dissociations.
That said, there are a few findings that challenge a discrete differentiation between PHc and PRc and are consistent with the present findings. Most notably, a recent lesion study (Nemanic et al., 2004) showed that acquisition, but not steady-state performance, of an object memory task is equally impaired by both PHc and PRc lesions suggesting that PHc contributes in some form, albeit temporarily, to object memory. These data are consistent with our findings of above-baseline activation for object stimuli along the entire MTLC axis, including the posterior PHc, providing evidence in favor of a preferential, but not for a selective, activation profile in posterior PHc. Similar evidence was obtained in recent fMRI studies. For example, Janzen and van Turennout (2004) reported posterior PHc activation to navigationally-relevant object stimuli and a series of experiments by Aminoff and colleagues, discussed above, (Bar and Aminoff, 2003 and Aminoff et al., 2007) report differential PHc activation depending on whether objects are associated with a spatial or non-spatial context. Indeed, our finding that mid-PHc responds similarly to both object and scene stimuli is consistent with these findings suggesting that mid-PHc thus, perhaps, representing a convergence zone of object and spatial input.
Of course, a critical remaining question is what might be the basis for this underlying gradient? What the present data highlight is that objects and scenes may do a good job of capturing some property of what the underlying representational and organizational structure within the MTLC actually may be. What the present experiment does not reveal, however, is what fundamental dimension do object and scene stimuli capture? One speculation is that the regions along the MTLC may exhibit differential receptive field properties, just as inferotemporal and posterior parietal neurons, the input structures to MTLC, have been shown to have differing receptive field sizes and properties (Tanaka, 1996; Andersen et al., 1997). For example, PRc may contain a larger proportion of neurons with large receptive fields sensitive to stimulus identity while PHc may contain a larger proportion of neurons with smaller and more peripheral receptive fields ideal for coding the spatial layout of a scene or stimulus. Consistent with this, a recent electrophysiological investigation in non-human primates reported that, while overall both PRc and PHc contained neurons that respond to stimuli in both the fovea and periphery, a larger proportion of PRc neurons responded to simple visual stimuli (bars of light) in the fovea compared to neurons in PHc, whereas a greater proportion of PHc neurons exhibit both foveal and peripheral receptive fields (Sato et al., 2003). Indeed, receptive field properties have previously been suggested to be at least one component that may confer preferential stimulus processing across cortical regions such as PPA and FFA (Levy, 2001; Epstein et al., 2003). However, many other possibilities exist and further work is needed to test these hypotheses.
In conclusion, the present results suggest that while there are significant dissociations between object- and scene- responsiveness in MTLC, the underlying response pattern from whence the dissociations arise may best be described as a gradient. Consideration of organizing principles both of the regions that provide input to the MTLC as well as those germinated locally, holds promise for illuminating the role of these regions in memory formation. Furthermore, the broader implications of these results can be applied to the understanding of other cortical regions in the brain. Namely, in addition to the pursuit of functional specialization, examination of graded changes as they occur across or within brain regions will undoubtedly lead to a richer understanding of brain organization and, hence, function. Indeed, recent studies have begun to reveal gradients in the hippocampus Kjelstrup et al. (2008) and along various higher level processing regions, including the inferior frontal gyrus (Dobbins et al., 2002), the parietal cortex (Levy, 2007) and across prefrontal cortical regions more generally (Badre and D'Esposito, 2007).
Method
Participants
Five female and three male right-handed native English speakers participated in this study. All subjects had normal or corrected-to-normal vision. Informed consent was obtained in a manner approved by the Institutional Review Board at New York University, and subjects were paid for their participation. Mean ± SD age of the subjects was 24.5 ± 3.5.
Stimuli
Stimuli consisted of images drawn from five different categories: faces, scenes, objects, words and pseudowords. In addition, both pictures of objects and drawings of objects were used. Stimuli were obtained from various online sources including IMSI MasterClips© and MasterPhotos™, IMSI, San Rafael, CA. Object stimuli were obtained from a picture database used previously by Rossion and Pourtois (2004). All word and pseudoword stimuli were between five and eight letters in length and either two or three syllables. All words were non-concrete nouns. Forty-five exemplars from each of the six stimulus categories were used (Supplemental Figure 1).
Design
Over four functional MR runs, subjects were visually presented with 20-second blocks of stimuli interleaved with 20-second baseline fixation periods. Each of the four functional runs consisted of 15 blocks, each of which contained 10 stimuli from one of the categories described above. ‘Novel’ blocks contained primarily novel stimuli (see below) while the ‘Repeating’ blocks contained two stimuli that repeated throughout the block. Each subject viewed a total of eight novel and two repeating blocks for each stimulus category resulting in 60 blocks total per subject. Each stimulus was presented on a black background for 1500 msec followed by 500 msec of fixation.
In order to ensure that subjects were attending to and processing each stimulus, they performed a one-back task during scanning. They were instructed to press a button whenever any stimulus was presented twice in a row. ‘Novel’ blocks contained mostly novel stimuli except that either one or two stimuli (counterbalanced across blocks) were presented twice, one immediately following the other. ‘Repeating’ blocks contained two stimuli only that alternated except for one or two immediate repetitions requiring a response from the subject. Thus, within both novel and repeating blocks, subjects were required to respond either once or twice. Performance on the one-back task was near ceiling for all subjects (Mean = 97.3% correct). Performance did not differ for the novel (97.5% correct) and repeating (97.1% correct) blocks [F(1,4) = .12, P = .73].
Imaging parameters
Data were acquired with a 3T Siemens Allegra MRI system using a whole-head coil. Functional data were acquired using a gradient-echo echo-planar pulse sequence, (TR = 2 sec; TE = 30 ms; 36 slices oriented perpendicular to the hippocampal axis; 3 × 3 × 3 mm voxel size; 0.6 mm interslice gap; 480 volume acquisitions per run). High-resolution T1-weighted (magnetization-prepared rapid-acquisition gradient echo) images were collected for anatomical visualization. A vacuum pillow minimized head motion. Visual stimuli were projected onto a screen that was viewed through a mirror, and responses were collected with a magnet-compatible button box.
Data were analyzed using SPM2 (Wellcome Department of Cognitive Neurology, University College London, London, UK). During preprocessing, images were corrected for differences in slice acquisition timing, followed by motion correction across all runs. Structural and functional data were spatially normalized to an echo planar imaging template, and voxels were spatially smoothed with a 6 mm full-width half-maximum isotropic Gaussian kernel. Additional analyses were conducted on the pre-smoothed data to test whether the gradient pattern revealed initially was an artifact of the larger smoothing kernel.
General statistical analyses
Data analysis was performed using the general linear model implemented in SPM2 (Wellcome Department of Cognitive Neurology, University College London, London, UK). Stimulus blocks for each category were modeled using a box-car canonical hemodynamic response function and its temporal derivative. The four runs were concatenated and modeled as one continuous run to improve parameter estimability. Accordingly, mean signal and drift per run were separately modeled as confounds. Subject-specific estimates for task-related activation were entered into a second-level random-effects analysis (one-sample T-test). Regions consisting of at least five contiguous voxels that exceeded an uncorrected threshold of P < 0.005 were considered reliable.
In order to reveal brain regions that responded differentially to objects and scenes, a two-step approach was taken. First, a contrast of scene > all other categories (objects, faces, words, pseudowords) was conducted to reveal voxels whose activation was greater for scenes compared to the mean of all other categories combined. Regions consisting of at least five contiguous voxels that exceeded an uncorrected threshold of P < 0.001 were considered reliable. Because this one contrast cannot reveal whether differences exist between all pairwise comparisons of conditions, the deconvolved blood oxygenation level-dependent (BOLD) time course response for each category within each ROI was extracted using the MarsBaR toolbox (Brett, Anton, Valabregue & Poline, 2002). All time course data was then analyzed using Analyses of Variance (ANOVA). The choice of sample size was determined based on previous functional localizer experiments that employed block designs (Epstein and Kanwisher, 1998; Epstien et al., 1999) that use similar sample sizes and consistently report large effect sizes. To make sure that the homogeneity of variance assumption were not violated, Mauchly's test of Sphericity was used. All ANOVAs that are reported below met the homogeneity of variance assumption (all Ps > .2).
The time-course data were then submitted to a within subjects ANOVA in order to examine the differences between all stimulus categories. The dependent measure of ‘response’ that was used in the ANOVA's was the mean BOLD response, collapsed across time points (TRs) 3 though 12. This conformed to the peak of the response across each block. All follow-up statistical analyses were performed using this same average response across these TRs. This same process was again used to reveal regions showing greater activation for object stimuli compared to all other stimulus classes.
Repetition suppression within these regions was also examined by subtracting the average BOLD response (collapsing across TRs 3 - 12) for blocks with repeating stimuli from that of novel blocks within each stimulus category. The difference (novel - repeated) BOLD response was then compared to a test value of zero with a one-sample t-test in order to test the significance of the repetition suppression effects. All statistical analyses were carried out separately for the two hemispheres.
Finally, we found that BOLD activation to pictures of objects elicited significantly stronger responses than to drawings of objects in all areas of the brain examined, perhaps, indicating a global, non-specific response. This same effect has been noted in previous reports (Halgren et al., 2000). For this reason and because our scene stimuli are also pictures, not drawings, we limited all scene and object comparison analyses to object pictures versus scene pictures.
Anatomical ROIs
Anatomical regions of interest (aROIs) were drawn on each subject's anatomical images using MRcro software (Rorden and Brett, 2000). Seven ROIs were drawn along the anterior-posterior extent of both the left and right MTLC spanning from an anterior MNI coordinate of y = 3 to a posterior coordinate of y = −53. Each ROI was 8 mm long (with the exception of the most anterior ROI which was 9 mm) in the anterior/posterior direction while width varied with the width of the parahippocampal gyrus itself. The medio-lateral MTLC borders were drawn according to the guidelines outlined by Insausti et al., (1998).
A threefold approach was used to examine the response profiles within aROIs with respect to objects and scenes. First, the BOLD time courses for object and scene blocks were extracted from each aROI using the MarsBaR toolbox. This allowed us to examine the response profiles of the regions lying between the PRc and the posterior PHc, and also to examine the PRc and the PHc with more anatomical precision than can be attained using functional ROIs. As with the functional ROIs, the BOLD response was averaged across TRs 3-12. Within-subject t-tests were then carried out to examine any differences in object/scene responsiveness. Within each aROI, we also examined whether the BOLD signal was significantly different from baseline with a one-sample t-test, which compared the average BOLD response across TRs 3-12 to a test value of zero.
In the second part of the analysis we were interested in examining the pattern of activity along the entire MTLC. BOLD responses extracted from each of the seven aROIs (again using the average BOLD response across TRs 3-12) were entered into a one-way ANOVA. A set of linear contrast weights [−3, −2, −1, 0 1, 2, 3] was assigned to each level (aROI) in the ANOVA to examine whether the response to scenes changes linearly across the MTLC. Quadratic, cubic and other polynomial trends analyses (up to the 6th order) were also examined to determine whether the pattern of activation along MTLC can be described by other functions. All trends were evaluated at a P level of .05.
In order to provide a measure of the extent to which each aROI contributes to object and scene processing, independent of the size of the overall signal within the aROI, we normalized the responses by calculating the proportion of the response to each stimulus category relative to the overall response to both stimulus categories in each aROI. The following formula was used to obtain the proportion scores: (Scenes−Objects)/(Scenes+ Objects) (see Grill-Spector et al., 2006). Prior to obtaining the proportions, all scores were shifted by a constant of 1 to get rid of outlier scores and negative values (see Simmons et al., 2007). To examine whether preferential responsiveness to scenes increases linearly in the posterior direction, each subject's proportion data were subjected to a linear regression analysis. The resulting R2 values were then averaged to get a measure of the size of the linear effect across all subjects.
Supplementary Material
Acknowledgements
The authors would like to acknowledge Lin Wang for initial data collection, Kevin Ochsner and Liz Phelps for helpful comments on initial versions of the manuscript and Bernhard Staresina for helpful discussions. Grant support from RO1 MH074692.
NIH grant # 074692
References
- Alvarado MC, Bachevalier J. Comparison of the effects of damage to the perirhinal and parahippocampal cortex on transverse patterning and location memory in rhesus macaques. J. Neurosci. 2005;25:1599–1609. doi: 10.1523/JNEUROSCI.4457-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aminoff E, Gronau N, Bar M. The parahippocampal cortex mediates spatial and nonspatial associations. Cereb. Cortex. 2007;17:1493–1503. doi: 10.1093/cercor/bhl078. [DOI] [PubMed] [Google Scholar]
- Andersen RA, Snyder LH, Bradley DC, Xing J. Multimodal representation of space in the posterior parietal cortex and its use in planning movements. Annu. Rev. Neurosci. 1997;20:303–330. doi: 10.1146/annurev.neuro.20.1.303. [DOI] [PubMed] [Google Scholar]
- Badre D, D'Esposito M. Functional Magnetic Resonance Imaging evidence for a hierarchical organization of the prefrontal cortex. J. of Cog. Neurosci. 2007;19:1–18. doi: 10.1162/jocn.2007.19.12.2082. [DOI] [PubMed] [Google Scholar]
- Bar M, Aminoff E. Cortical analysis of visual context. Neuron. 2003;38:347–358. doi: 10.1016/s0896-6273(03)00167-3. [DOI] [PubMed] [Google Scholar]
- Buffalo EA, Bellgowan PSF, Martin A. Distinct roles for medial temporal lobe structures in memory for objects and their locations. Learn. Mem. 2006;13:638–643. doi: 10.1101/lm.251906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen NJ, Eichenbaum H. Memory, amnesia, and the hippocampal system. MIT Press; Cambridge, MA: 1993. [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr. Opin. Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Davachi L, Mitchell JP, Wagner AD. Multiple routes to memory: distinct medial temporal processes build item and source memories. Proc. Natl. Acad. Sci. USA. 2003;100:2157–2162. doi: 10.1073/pnas.0337195100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downing PE, Chan AW, Peelen MV, Dodds CM, Kanwisher N. Domain specificity in visual cortex. Cereb. Cortex. 2006;16:1453–1461. doi: 10.1093/cercor/bhj086. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Diana RA, Yonelinas AP, Ranganath C. High-resolution multi-voxel pattern analysis of category selectivity in the medial temporal lobes. Hippocampus. 2008;18(6):536–541. doi: 10.1002/hipo.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eacott MJ, Gaffan D, Murray EA. Preserved recognition memory for small sets, and impaired stimulus identification for large sets, following rhinal cortex ablations in monkeys. Eur. J. Neurosci. 1994;6:1466–78. doi: 10.1111/j.1460-9568.1994.tb01008.x. [DOI] [PubMed] [Google Scholar]
- Epstein R, Graham KS, Downing PE. Viewpoint-Specific Scene Representations in Human Parahippocampal Cortex. Neuron. 2003;37:865–876. doi: 10.1016/s0896-6273(03)00117-x. [DOI] [PubMed] [Google Scholar]
- Epstein R, Kanwisher N. A cortical representation of the local visual environment. Nature. 1998;392:598–601. doi: 10.1038/33402. [DOI] [PubMed] [Google Scholar]
- Ewbank MP, Schluppeck D, Andrews TJ. fMR-adaptation reveals a distributed representation of inanimate objects and places in human visual cortex. NeuroImage. 2005;28:268–279. doi: 10.1016/j.neuroimage.2005.06.036. [DOI] [PubMed] [Google Scholar]
- Fodor J. The Modularity of Mind. MIT Press; Cambridge, MA: 1983. [Google Scholar]
- Fodor J. The Mind Doesn't Work that Way. MIT Press; Cambridge, MA: 2000. [Google Scholar]
- Grill-Spector K, Sayres R, Ress D. High-resolution imaging reveals highly selective nonface clusters in the fusiform face area. Nature Neuroscience. 2006;9:1177–1185. doi: 10.1038/nn1745. [DOI] [PubMed] [Google Scholar]
- Halgren E, Raij T, Marinkovic K, Jousmäki V, Hari R. Cognitive response profile of the human fusiform face frea as fetermined by MEG. Cereb. Cortex. 2000;10:69–81. doi: 10.1093/cercor/10.1.69. [DOI] [PubMed] [Google Scholar]
- Haxby JV, Gobbini MI, Furey ML, Ishai A, Schouten JL, Pietrini P. Distributed and overlapping representations of faces and objects in ventral temporal cortex. Science. 2001;293:2425–30. doi: 10.1126/science.1063736. [DOI] [PubMed] [Google Scholar]
- Insausti R, Juottonen K, Soininen H, Insausti AM, Partanen K, Vainio P, Laakso MP, Pitkanen A. MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. Am. J. Neuroradiol. 1998;19:659–671. [PMC free article] [PubMed] [Google Scholar]
- Janzen G, van Turennout M. Selective neural representation of objects relevant for navigation. Nat. Neurosci. 2004;7:673–677. doi: 10.1038/nn1257. [DOI] [PubMed] [Google Scholar]
- Kanwisher N, McDermott J, Chun MM. The fusiform face area: a module in human extrastriate cortex specialized for face perception. J. Neurosci. 1997;17:4302–4311. doi: 10.1523/JNEUROSCI.17-11-04302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjelstrup KB, Solstad T, Brun VH, Hafting T, Leutgeb S, Witter MP, Moser EI, Moser M. Finite Scale of Spatial Representation in the Hippocampus. Science. 2008;5885(321):140–143. doi: 10.1126/science.1157086. [DOI] [PubMed] [Google Scholar]
- Koutstaal W, Wagner AD, Rotte M, Maril A, Buckner RL, Schacter DL. Perceptual specificity in visual object priming: functional magnetic resonance imaging evidence for a laterality difference in fusiform cortex. Neuropsychologia. 2001;39:184–199. doi: 10.1016/s0028-3932(00)00087-7. [DOI] [PubMed] [Google Scholar]
- Kourtzi Z, Huberle E. Spatiotemporal characteristics of form analysis in the human visual cortex revealed by rapid event-related fMRI adaptation. NeurImage. 2005;28:440–452. doi: 10.1016/j.neuroimage.2005.06.017. [DOI] [PubMed] [Google Scholar]
- Lavenex P, Suzuki WA, Amaral DG. Perirhinal and parahippocampal cortices of the macaque monkey: Projections to the neocortex. J. Comp. Neurol. 2002;4:394–420. doi: 10.1002/cne.10243. [DOI] [PubMed] [Google Scholar]
- Lee AC, Buckley MJ, Pegman SJ, Spiers H, Scahill VL, Gaffan D, Bussey TJ, Davies RR, Kapur N, Hodges JR, Graham KS. Hippocampus. 2005;15:782–797. doi: 10.1002/hipo.20101. [DOI] [PubMed] [Google Scholar]
- Levy I, Hasson U, Avidan G, Hendler T, Malach R. Center-periphery organization of human object areas. Nat. Neurosci. 2001;4:533–539. doi: 10.1038/87490. [DOI] [PubMed] [Google Scholar]
- Levy I, Schluppeck D, Heeger DJ, Glimcher PW. Specificity of human cortical areas for reaches and saccades. J. Neurosci. 2007;27:4687–4696. doi: 10.1523/JNEUROSCI.0459-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: Two cortical pathways. Trends Neurosci. 1983;6:414–417. [Google Scholar]
- Malkova L, Mishkin M. One-trial memory for object-place associations after separate lesions of hippocampus and posterior parahippocampal region in the monkey. J. Neurosci. 2003;23:1956–1965. doi: 10.1523/JNEUROSCI.23-05-01956.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Mishkin M, Murray EA. Effects on visual recognition of combined and separate ablations of the entorhinal and perirhinal cortex in rhesus monkeys. J. Neurosci. 1993;13:5418–5432. doi: 10.1523/JNEUROSCI.13-12-05418.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The Hippocampal/Parahippocampal Regions and Recognition Memory: Insights from Visual Paired Comparison versus Object-Delayed Nonmatching in Monkeys. J. Neurosci. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman G, Eacott MJ. Dissociable effects of lesions to the perirhinal cortex and the postrhinal cortex on memory for context and objects in rats. Behav. Neurosci. 2005;119:557–566. doi: 10.1037/0735-7044.119.2.557. [DOI] [PubMed] [Google Scholar]
- Ojemann JG, Akbudak E, Snyder AZ, McKinstry RC, Raichle ME, Conturo TE. Anatomic localization and quantitative analysis of gradient refocused echo-planar fMRI susceptibility analysis. Neuroimage. 1997;6:156–167. doi: 10.1006/nimg.1997.0289. [DOI] [PubMed] [Google Scholar]
- Ranganath C, Yonelinas AP, Cohen MX, Dy CJ, Tom SM, D'Esposito M. Dissociable correlates of recollection and familiarity within the medial temporal lobes. Neuropsychologia. 2004;42:2–13. doi: 10.1016/j.neuropsychologia.2003.07.006. [DOI] [PubMed] [Google Scholar]
- Rossion B, Pourtois G. Revisiting Snodgrass and Vanderwart's object pictorial set: the role of surface detail in basic-level object recognition. Perception. 2004;33:217–36. doi: 10.1068/p5117. [DOI] [PubMed] [Google Scholar]
- Pihlajamaki M, Tanila H, Kononen M, Hanninen T, Hamalainen A, Soininen H, Aronen HJ. Visual presentation of novel objects and new spatial arrangements of objects differentially activates the medial temporal lobe subareas in humans. Eur. J. Neurosci. 2004;19:1939–1949. doi: 10.1111/j.1460-9568.2004.03282.x. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Köhler S, Crane J, Pruessner M, Lord C, Andrea B, Kabani N, Collins L, Evans AC. Volumetry of temporopolar, perirhinal, entorhinal and parahippocampal cortex from high-resolution MR images: considering the variability of the collateral sulcus. Cereb. Cortex. 2002;12:1342–1353. doi: 10.1093/cercor/12.12.1342. [DOI] [PubMed] [Google Scholar]
- Rorden C, Brett M. Stereotaxic display of brain lesions Behav. Neurol. 2000;12:191–200. doi: 10.1155/2000/421719. [DOI] [PubMed] [Google Scholar]
- Sato N, Nakamura K. Visual response properties of neurons in the parahippocampal cortex of monkeys. J. Neurophysio. 2003;90:876–886. doi: 10.1152/jn.01089.2002. [DOI] [PubMed] [Google Scholar]
- Schacter DL, Wig GS, Stevens WD. Reductions in cortical activity during priming. Curr. Opin. Neurobiol. 2007;17:171–176. doi: 10.1016/j.conb.2007.02.001. [DOI] [PubMed] [Google Scholar]
- Simmons KW, Bellgowan PSF, Martin A. Measuring selectivity in fMRI data. Nat. Neurosci. 2007;1:4–5. doi: 10.1038/nn0107-4. [DOI] [PubMed] [Google Scholar]
- Spiridon M, Fischl B, Kanwisher N. Location and spatial profile of category-specific regions in human extrastriate cortex. Hum. Brain Mapp. 2006;27:77–89. doi: 10.1002/hbm.20169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina BP, Davachi L. Differential encoding mechanisms for subsequent associative recognition and free recall. The Journal of Neuroscience. 2006;26(36):9162–9172. doi: 10.1523/JNEUROSCI.2877-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staresina B, Davachi L. Selective and Shared Functional Contributions of Medial Temporal Lobe Subregions to Encoding Items, their Features and their Contexts. Journal of Cognitive Neuroscience. 2008;20(8):1–12. doi: 10.1162/jocn.2008.20104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki WA, Amaral DG. Topographic organization of the reciprocal connections between the monkey entorhinal cortex and the perirhinal and parahippocampal cortices. J. Neurosci. 1994;14:1856–1877. doi: 10.1523/JNEUROSCI.14-03-01856.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K. Inferotemporal cortex and object vision. Annu. Rev. Neurosci. 1996;19:109–139. doi: 10.1146/annurev.ne.19.030196.000545. [DOI] [PubMed] [Google Scholar]
- Turk-Browne NB, Yi DJ, Chun MM. Linking implicit and explicit memory: common encoding factors and shared representations. Neuron. 2006;49:917–927. doi: 10.1016/j.neuron.2006.01.030. [DOI] [PubMed] [Google Scholar]
- Ungerleider LG, Haxby JV. ‘What’ and ‘where’ in the human brain. Curr Opin Neurobiol. 1994;4:157–165. doi: 10.1016/0959-4388(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Wan HE, Warburton C, KusÂmierek P, Aggleton JP, Kowalska DM, Brown MW. Fos imaging reveals differential neuronal activation of areas of rat temporal cortex by novel and familiar sounds. Eur. J. Neurosci. 2001;14:118–124. doi: 10.1046/j.0953-816x.2001.01625.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.