Abstract
Members of the tumor necrosis factor (TNF)-receptor (R) family may be involved in the tissue remodeling that occurs in the primate corpus luteum (CL) during development and regression. As a first step towards addressing this issue, studies assessed TNF ligand-R expression and regulation in CL collected from monkeys during the early (ECL, d3–5), mid (MCL, d7–8), mid-late (MLCL, d10–11), late (LCL, d14–16), and very late (VLCL, menses) luteal phase of the menstrual cycle. CL were also collected after gonadotropin and/or steroid ablation and replacement (with hLH and the progestin R5020) for 3 days at mid-late luteal phase. TNF-α, -β, FAS ligand (FASL), and TNF-R1 mRNA levels were 2- to 6-fold greater (p<0.05) at the MLCL or LCL phase as compared to earlier (ECL, MCL). In contrast, TNF-R2 and FAS mRNA levels did not change during the luteal phase. Immunohistochemical staining for TNF-β, TNF-R1, TNF-R2, FAS, and FASL was observed in luteal cells, whereas only TNF-β staining was observed in endothelial cells. Several TNF-R components were influenced by LH and/or steroid ablation; notably, steroid ablation reduced (p<0.05) luteal TNF-α, but not TNF-β, mRNA levels, which was prevented by progestin treatment. In contrast, steroid ablation increased (p<0.05) luteal cell immunostaining for FAS and FASL, which was reduced by progestin treatment. Thus, several members of the TNF R-ligand family are expressed in the primate CL in an LH- and/or progestin-dependent manner. Peak expression in the late luteal phase may signify a role for the TNF-R system in death receptor-mediated apoptosis during luteolysis.
Keywords: FAS, apoptosis, luteolysis, luteinizing hormone, progesterone
Introduction
Following ovulation, the somatic cells remaining in the collapsed follicle differentiate to form the cells of the corpus luteum (CL). This unique and transient endocrine gland functions through secretion of progesterone (P) to permit both initiation and maintenance of intrauterine pregnancy. If fertilization and implantation do not occur after ovulation, the CL regresses, by a process termed luteolysis (Stouffer, 2003). Luteinizing hormone (LH) secretion during the luteal phase plays a critical luteotropic role in regulating the development, function and lifespan of the primate CL (Stouffer, 2003). Recent evidence suggests that LH actions are mediated at least in part, via locally produced factors, including the steroid hormone P (Stouffer, 2003). Both the formation and regression of the CL requires extensive tissue remodeling and the molecular mechanisms that regulate this activity in the primate CL during the menstrual cycle are, for the most part, unknown. Robker et al (Robker et al., 2000) recently identified several proteases, whose expression in the ovulatory, luteinizing follicle or corpus luteum is regulated by P. Moreover, our laboratory group reported that the expression of various proteases in the monkey CL, including members of the caspase (Peluffo et al., 2005) and matrix metalloproteinase (Chaffin and Stouffer, 1999;Young and Stouffer, 2004) families, are regulated by P or other locally produced steroids. Also, Okuda et al (Okuda et al., 2004), observed that intraluteal P4 suppresses apoptosis in bovine luteal cells through the inhibition of caspase-3 mRNA expression and inhibition of caspase-3 activation.
The concept that regression of the primate CL involves apoptosis is not without controversy. There is some evidence that apoptosis occurs during luteolysis (Fraser et al., 1995;Gaytan et al., 1998;Shikone et al., 1996;Sugino et al., 2000;Vaskivuo et al., 2002a;Yuan and Giudice, 1997), but other studies suggest that autophagocytosis is involved in luteal cell death (Fraser et al., 1999;Morales et al., 2000;Quatacker, 1971). Apoptosis can be triggered by multiple different stimuli originating from within or outside the cell. The death receptor pathway is one of the most important regulatory mechanisms of apoptosis in mammalian cells (Zapata et al., 2001). Members of the tumor necrosis factor (TNF)-receptor (R) super family, including FAS, are transmembrane proteins that mediate tissue remodeling, including apoptosis, in various tissues. These receptors are defined by a variable number of cysteine-rich domains (n=2 to 6 CRDs) in the extracellular region, and these CRDs form tertiary structures that are responsible for ligand binding. The receptors are activated by either soluble or membrane-bound cytokines, such as TNF-α, TNF-β and Fas-ligand (FASL). Their activation leads to oligomerization of the receptor and subsequent recruitment of death domain adaptor molecules (FADD, TRADD) (Hueber et al., 1997). Initiator caspases are reportedly activated following direct association with death domain proteins. Notably, cytokines and resident immune cells are proposed to play a role in regulating luteal functions in rodents, domestic animals and women (Brannstrom and Friden, 1997;Friedman et al., 2000;Quirk et al., 1995;Terranova and Rice, 1997;Vaskivuo et al., 2002b). Moreover, during luteolysis, macrophages are attracted to and infiltrate the CL, increasing the local production of cytokines (Abdo et al., 2003;Brannstrom et al., 1994).
Since TNF-Rs and their ligands are present in the ovary and/or CL of many species (Chen et al., 1993;Kondo et al., 1996;Morrison and Marcinkiewicz, 2002;Roughton et al., 1999;Sakamaki et al., 1997;Sakumoto et al., 2000;Taniguchi et al., 2002;Wang et al., 1992), we hypothesized that this death R-ligand system is involved in the tissue remodeling associated with development and regression of the primate CL. As a first step towards addressing this issue, studies were designed to determine if members of the TNF R-ligand family (TNF-α and -β, TNF-RI and -RII, FASL and FAS) are expressed in the rhesus macaque CL during the menstrual cycle. Also, to determine if their expression in the developed CL is regulated by luteotropic factors (LH, P), we studied the same parameters after gonadotropin and/or steroid hormone ablation and replacement at mid-to-late luteal phase of the cycle.
Results
CL at Specific Stages of the Menstrual Cycle: mRNA Expression
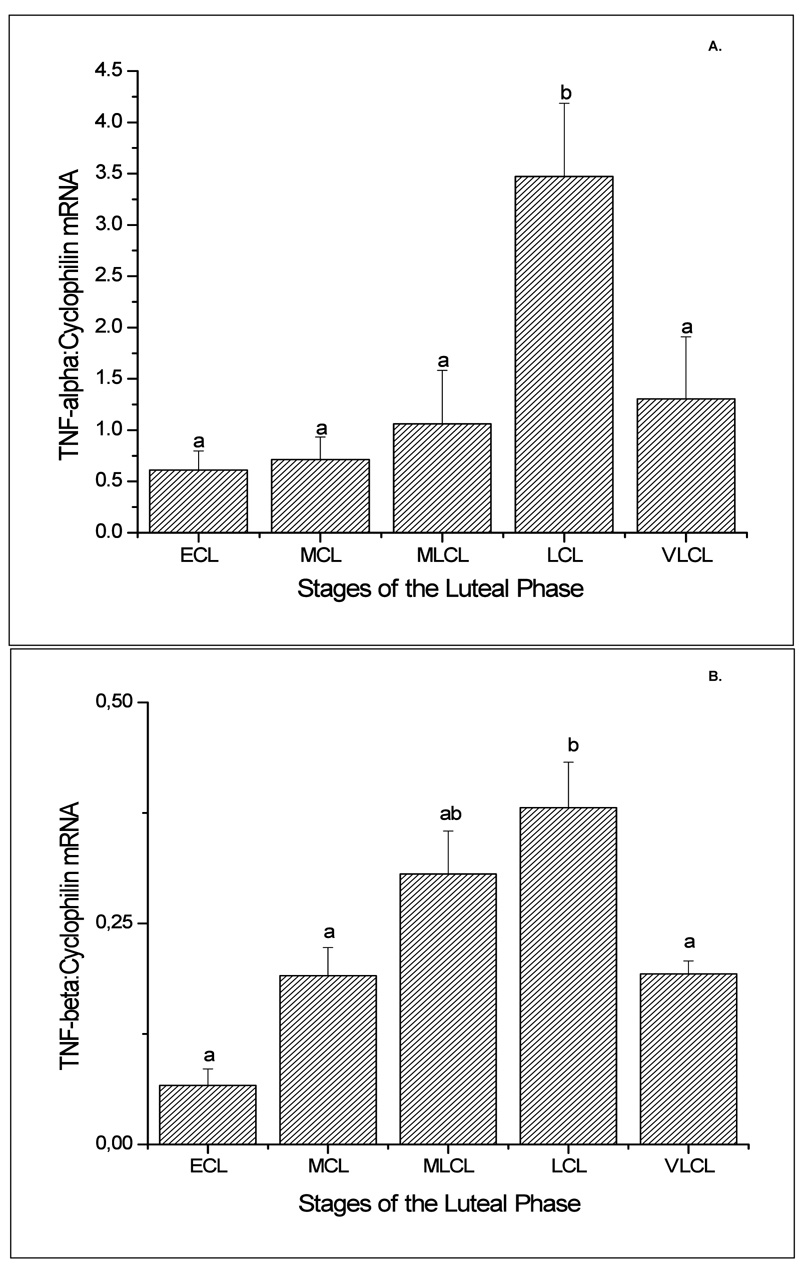
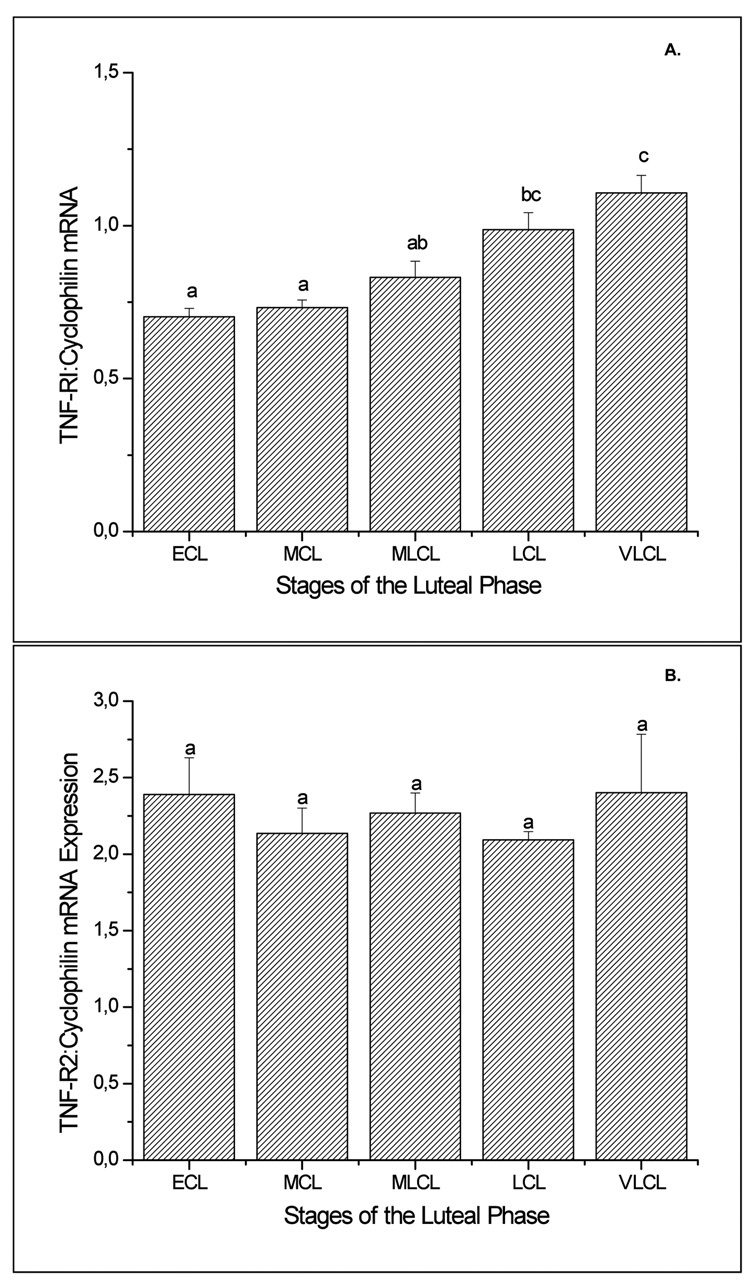
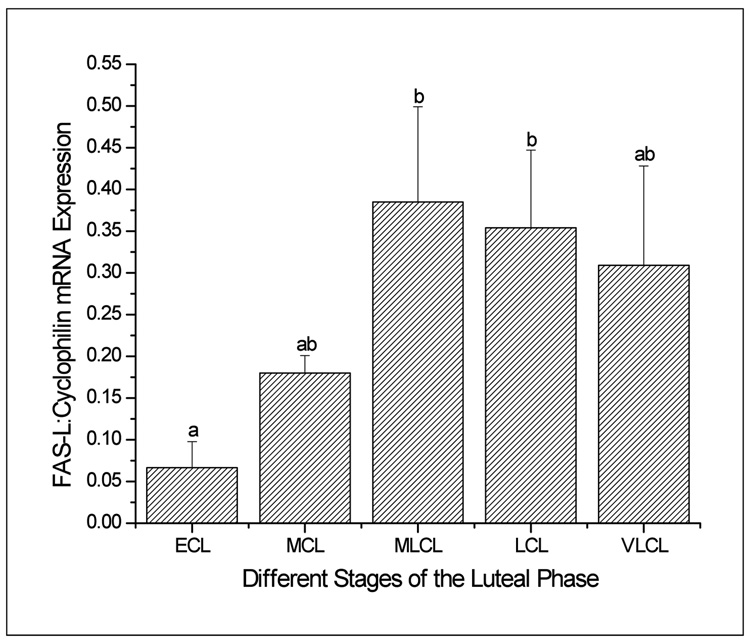
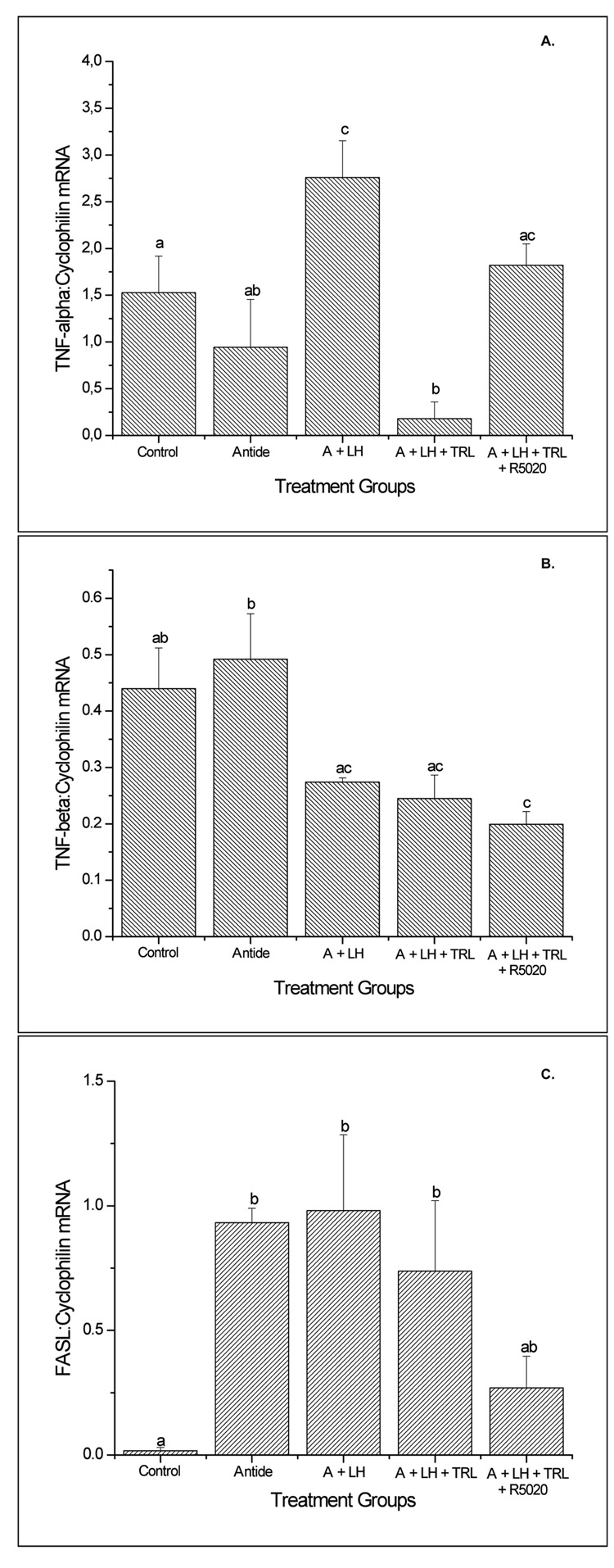
Luteal TNF-α mRNA levels, as determined by sqRT-PCR, displayed an abrupt increase (2- to 6-fold greater; p<0.05) at the late luteal (LCL) stage as compared to other stages (Figure 1, panel A). TNF-β mRNA levels progressively increased to peak at the LCL (6-fold greater than at early luteal (ECL) stage; p<0.05) and declined (2-fold) by the very late luteal (VLCL) stage (Figure 1, panel B). TNF-R1 mRNA levels were modestly but significantly (p<0.05) higher in CL at LCL compared to earlier stages, and remained elevated at the VLCL stage (Figure 2, panel A). In contrast, TNF-R2 (Figure 2, panel B) and FAS (data not shown) mRNA levels in CL did not change (p>0.05) throughout the luteal lifespan in the menstrual cycle. In the case of FASL, despite variability among the samples, especially at the late stages, mRNA levels were 5-6-fold greater in mid-late luteal (MLCL) or LCL, compared to ECL (Figure 3; p<0.05). The macaque cDNA sequences were very similar to the corresponding human sequences for TNF-α (93%), TNF-β (90%), TNF-RI (93%), TNF-RII (97%), and FASL (100%).
Figure 1.
Semi-quantitative (sq) RT-PCR results (mean ± SEM; n=3–4 per group) for TNF-α (Panel A) and TNF- β (Panel B) mRNA in the macaque CL during the early (ECL, day 3–5), mid (MCL, day 7–8), mid-late (MLCL, day 10–12), late (LCL, day 14–16) and very-late (VLCL, day 17–19) luteal phase. Values were standardized to their respective cyclophilin control values. Different letters a,b represent significant differences (p<0.05) in TNF mRNA levels between stages of the luteal phase.
Figure 2.
Levels of TNF-R1 (Panel A) and TNF-R2 (Panel B) mRNA, as determined by sq RT-PCR, in the macaque CL at specific stages the natural menstrual cycle (ECL, MCL, MLCL, LCL, VLCL). Values are presented as the mean ± SEM (n=3–4 per group) relative to cyclophilin mRNA. Different letters a,b,c represent significant differences (p<0.05) in TNF-R1 mRNA levels between stages of the luteal phase.
Figure 3.
Levels of FASL mRNA, as determined by sq RT-PCR in the macaque CL at specific stages (ECL to VLCL) of the menstrual cycle. Values are presented as the mean ± SEM (n=3–4/group) relative to cyclophilin mRNA. Different letters a,b represent significant differences (p<0.05) between groups.
CL at Specific Stages of the Menstrual Cycle: Protein Localization
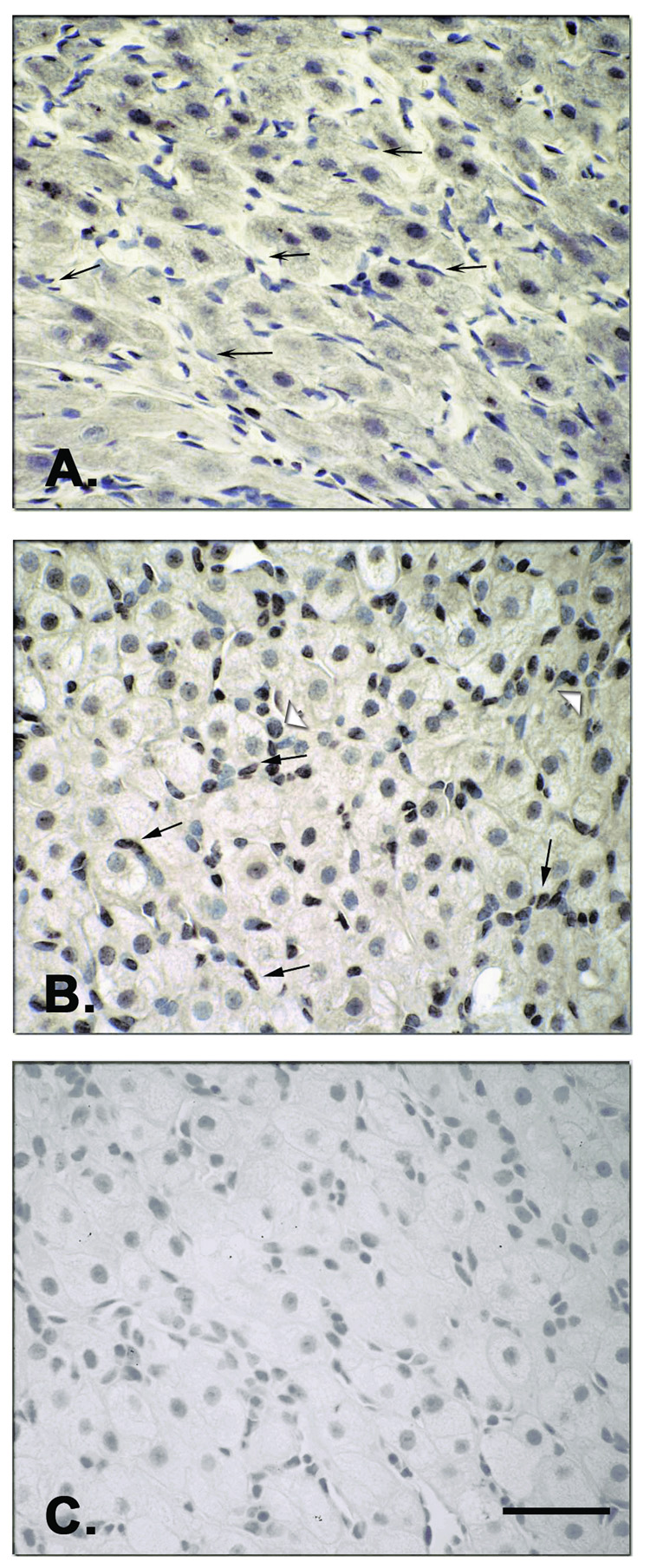
Immunostaining for TNF-β in the macaque CL was localized to granulosa and theca luteal cells, as well as in immune cells (leukocytes) during the natural luteal phase of the cycle (Figure 4A, B). Moreover, transient staining of TNF-β was evident in luteal endothelial cells (arrows), but only at the MLCL stage (Figure 4, panel B). Specific (compared to preabsorption with antigen) staining was detected in the cytoplasm and, in some cases, in the cell nucleus. Specific staining in the ovarian stroma (not shown), as well as preabsorbed Ab staining in the CL (Fig. 4C), was minimal.
Figure 4.
Immunohistochemistry for TNF-β in the CL at selected stages (MCL, Panel A; MLCL, Panel B) during the natural menstrual cycle. Specific immunostaining (as compared to controls-preabsorbed Ab; e.g., Panel C) was found throughout the luteal phase in the granulosa luteal cells and in the theca luteal cells (not shown), but not in the stroma. Notably, luteal endothelial cells (arrows) only stained in the MLCL and not in earlier (MCL) or later stages. Immune cells (leukocytes) appeared to be stained as well (open arrowheads). Specific staining was detected in the cytoplasm and, in some cells, in the nucleus. Scale bar 50 µm.
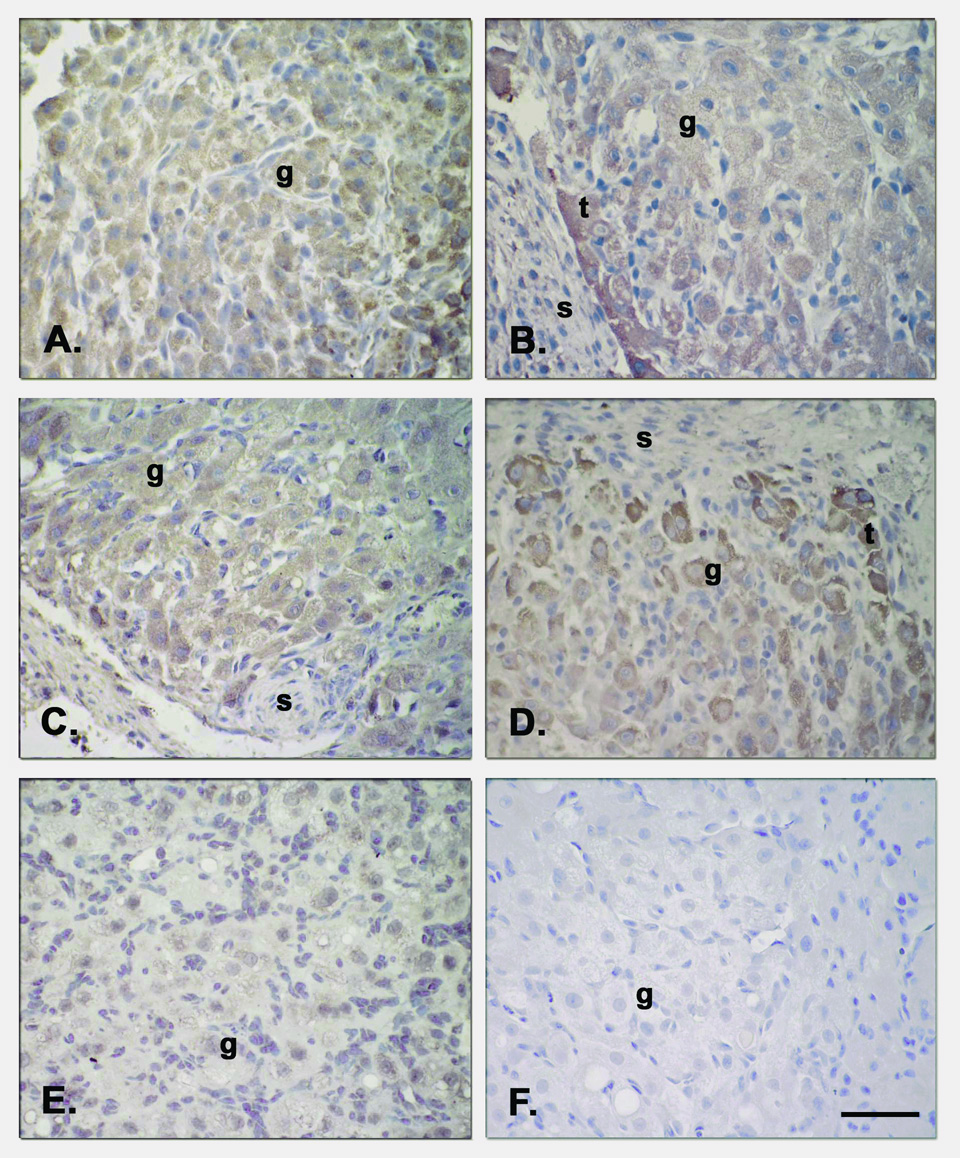
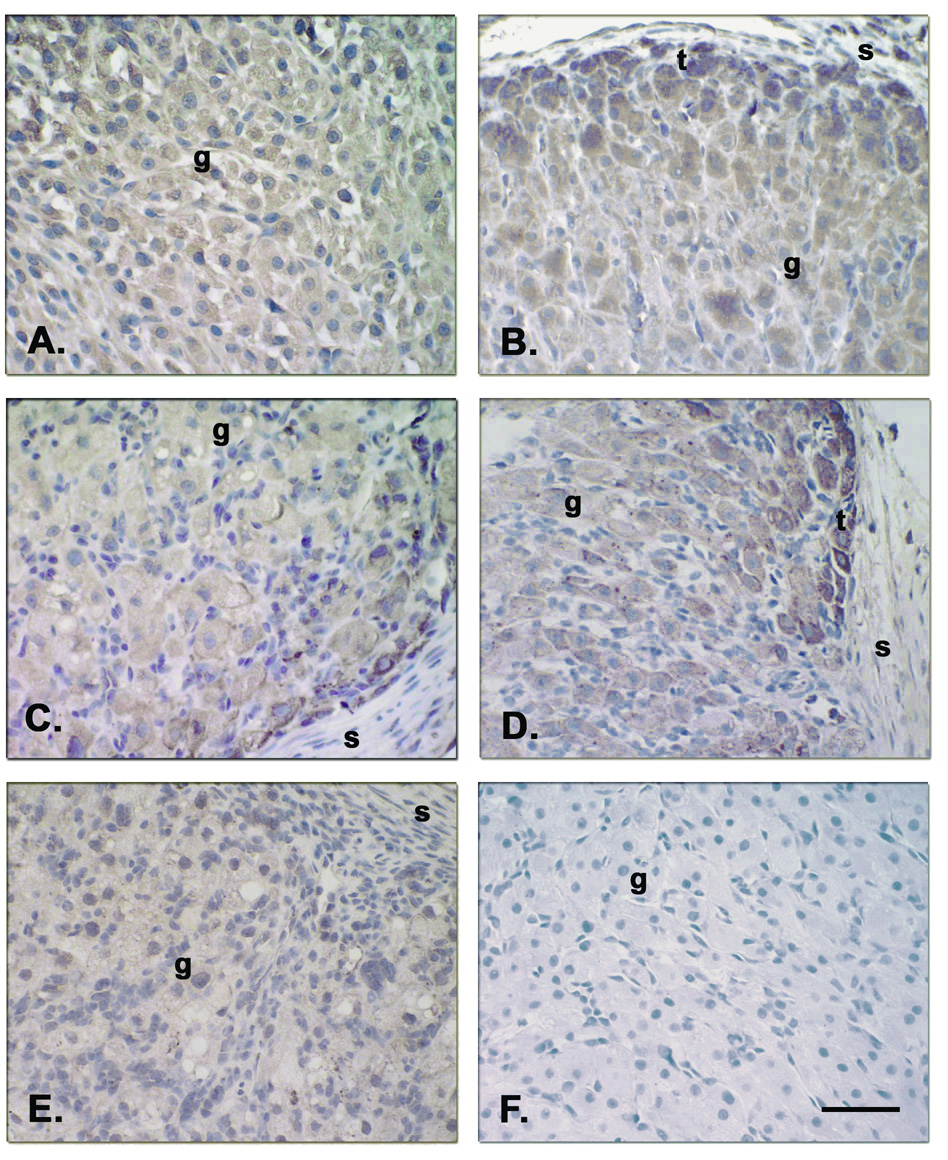
Intense staining for TNF-R1 and TNF-R2 (Figure 5), FAS and FASL (Figure 6) was detected in the granulosa (g) and theca (t) luteal cells of CL at all stages of the luteal phase, but not in endothelial cells or the surrounding stroma, nor in the negative control (preabsorbed Ab). Moreover, staining for these four proteins appeared more intense in the theca luteal cells along the vessels and around the periphery of the CL, particularly at the MCL to LCL stages (Fig. 5, panels B and D; Fig. 6, panels B and D).
Figure 5.
Immunolabeling for TNF-R1 and TNF-R2 in macaque CL throughout the luteal phase during the natural menstrual cycle [TNF-R1 at LCL (Panel C), and TNF-R2 at ECL (Panel A), MCL (Panel B), LCL (Panel D), and VLCL (Panel E)]. Specific staining for TNF-R1 and TNF-R2 was found in granulosa (g) and theca (t) luteal cells during all stages of the luteal phase, but not in endothelial cells, the surrounding stroma (s) or in the control TNF-R2 Ab preabsorption, Panel F). Staining for TNF-R appeared more intense in the theca luteal cells along vessels or around the periphery, by MCL to LCL stages, than in the granulosa luteal cells. Scale bar 50 µm.
Figure 6.
Immunostaining for FASL and FAS in CL at various stages of the luteal lifespan during the natural menstrual cycle [FASL at ECL (Panel A), MCL (Panel B), LCL (Panel D) and VLCL(Panel E), and FAS at LCL (Panel C)]. Both FASL and its receptor followed a similar pattern of specific staining where theca (t) luteal cells appeared more intensely stained than the granulosa (g) cells by the MCL to LCL stages. Staining was not found in the negative control (FASL Ab preabsorption, Panel F) nor in the endothelial cells or stroma cells (s). Scale bar 50 µm.
CL Following Gonadotropin and/or Steroid Ablation and Replacement: mRNA Expression
TNF-α mRNA levels (Figure 7, panel A) were detectable in CL from control and antide-treated groups, and levels increased (p<0.05) after LH administration compared to antide alone. Trilostane treatment reversed this effect and decreased (p<0.05) mRNA to lower levels than those in control tissues. Notably, replacement of progesterone with R5020 restored TNF- α mRNA expression to control levels. TNF- β mRNA levels (Figure 7, panel B) decreased modestly with LH treatment compared to antide tissues. However, trilostane and R5020 treatments did not restore TNF- β mRNA expression. TNF-RI mRNA expression was detectable in all groups. The only treatment effect occurred with R5020 exposure, which decreased (p<0.05) TNF-R1 mRNA by 50% compared to controls (not shown). TNF-R2 and FAS mRNA levels in CL did not vary between treatment groups (data not shown). However, FASL mRNA levels increased markedly (p<0.05, Figure 7C) after antide treatment, only to be reduced to near control levels by selective replacement of progestin (R5020), not by LH or LH + trilostane.
Figure 7.
The sq RT-PCR results (mean ± SEM; n=3–4 per group) for TNF-α (Panel A), TNF-β (Panel B) and FAS-L (Panel C) mRNA in the macaque CL following gonadotropin and/or steroid ablation and replacement. The various treatment groups include control (no treatment, MLCL) , Antide (A), Antide + LH (A + LH), Antide + LH + trilostane (A + LH + TRL) and Antide + LH + trilostane + R5020 (A + LH + TRL + R5020). Different letters a,b represent significant differences (p<0.05) between groups.
CL after Gonadotropin and/or Steroid Ablation and Replacement: Protein Immunostaining
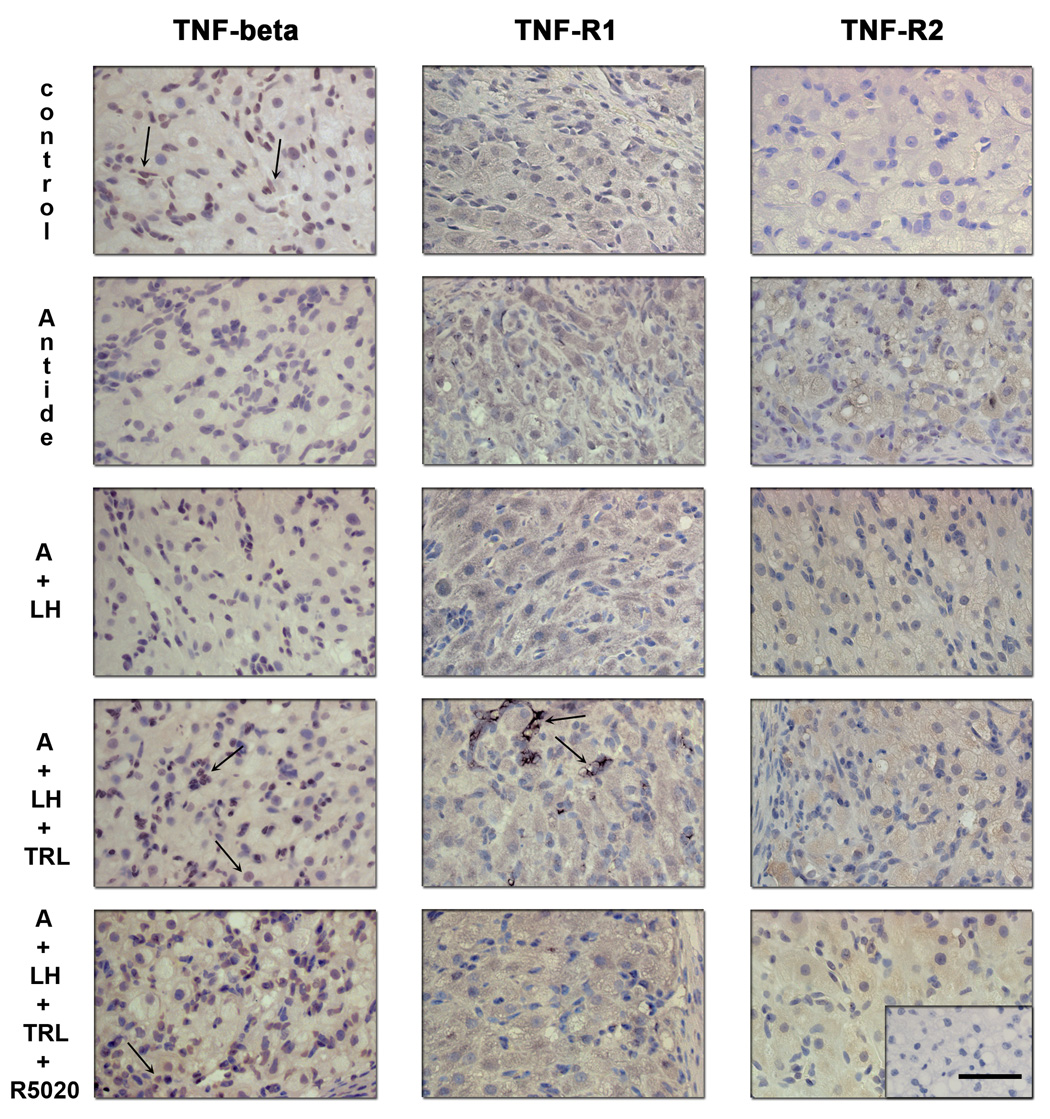
Compared to control CL from MLCL stage, immunostaining for TNF-β (Figure 8; left panels) in the luteal steroidogenic and endothelial cells appeared to decrease after antide treatment. LH failed to restore its expression in endothelial cells, but staining in luteal cells was evident. However, trilostane treatment appeared to restore TNF-β staining in endothelial cells (arrows), and R5020 administration did not alter staining. Negative control showed no staining (not shown). Immunostaining for TNF-R1 (Figure 8; center panels) displayed little change between groups, except for intense staining of discrete regions of luteal cells (arrows) after trilostane treatment. Such staining was absent following addition of R5020. TNF-R2 protein expression (Figure 8; right panels) appeared to increase after antide treatment and LH prevented this increase. Trilostane treatment with/without R5020 mimicked the antide with/without LH effects.
Figure 8.
Immunohistochemistry for TNF-β, TNF-R1 and TNF-R2 following gonadotropin and/or steroid ablation and replacement. Controls (untreated, MLCL) exhibited staining comparable to that described in detail in previous Figure 4 and Figure 5. Specific staining for TNF-β (left panels) was detected in the cytoplasm and, in some cases, in the cell nucleus of the granulosa, theca luteal cells and endothelial cells (arrows). Immunostaining in the luteal endothelial cells appeared to decrease after antide (A) treatment and LH failed to restore its expression. Trilostane and R5020 treatments appeared to restore its expression. Immunostaining for TNF-R1 (center panels) was found in the granulosa and theca luteal cells, but not in endothelial cells and displayed little change in expression, except for an increase in discrete groups of luteal cells after trilostane treatment. TNF-R2 protein expression (right panels) was also found in the granulosa and theca luteal cells and appeared to increase after antide treatment. LH prevented this increase and Trilostane treatment was able to mimic in part the antide effect. Scale bar 50 µm.
Specific staining for FAS (Figure 9; right panels) was appreciable in control MLCL tissue and did not appear to change with antide treatment or addition of LH. In contrast, trilostane treatment markedly increased FAS staining and R5020 addition reduced the staining. FASL immunolabeling (Figure 9; left panels) displayed a similar pattern as FAS across treatment groups. Trilostane treatment greatly increased FASL staining and R5020 reduced this intensity. Specific staining for these proteins was found in the granulosa and theca luteal cells, but not in endothelial cells or the surrounding stroma nor in the negative control (Ab preabsorbed sections; bottom panel insert).
Figure 9.
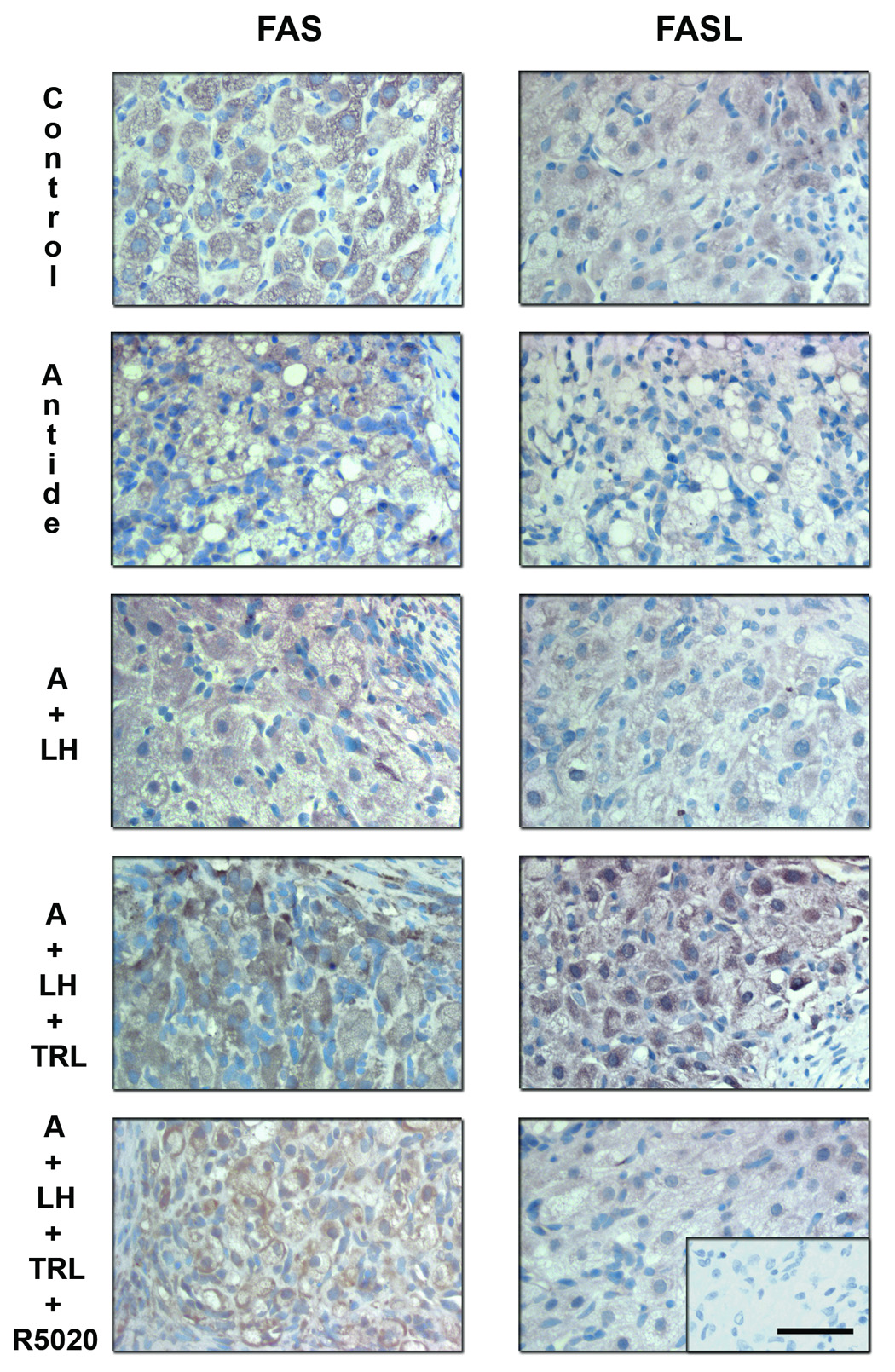
Immunohistochemistry for FAS and FASL following gonadotropin and/or steroid ablation and replacement. Specific staining for these proteins was observed in the granulosa and theca luteal cells, but not in endothelial cells or the surrounding stroma (see previous Figure 5). Immunostaining for FAS (left panels) was evident in control tissue (MLCL), as well as after the antide (A) treatment and LH replacement. However, trilostane treatment increased FAS expression and R5020 was able to reduce to some extent its expression. Trilostane treatment also greatly increased FASL (right panels) immunostaining and R5020 prevented this increase. Scale bar 50 µm.
Discussion
This study provides the first evidence regarding both mRNA and protein expression of multiple members of the TNF-R system in the primate CL during luteinization and luteolysis in the menstrual cycle. Moreover, it analyzed their expression after gonadotropin and/or steroid hormone ablation and replacement at mid to late luteal phase (day 9–11 post-LH surge), just prior to spontaneous luteolysis in naturally cycling rhesus monkeys.
RT-PCR analyses, combined with sequence verification of partial cDNAs, confirmed the mRNA expression of TNF-α and TNF-β in the macaque CL throughout the luteal phase of the menstrual cycle. There was an increase in TNF-α and β mRNA levels in the CL at late luteal phase, when regression is occurring and an increase in apoptotic signals is expected (Shikone et al., 1996;Sugino et al., 2000). Others reported unchanged mRNA levels, but an increase in TNF-α protein, in the bovine CL during the estrous cycle (Petroff et al., 1999;Sakumoto et al., 2000). Unfortunately, the available TNF-α antibodies could not be validated on paraffin sections of the macaque CL, so we could not study TNF-α at the protein level. However, the highest levels of TNF-α in whole blood were reported in the women during the luteal phase of ovulatory cycles (Amory et al., 2004). TNF-α is primarily expressed in the monocytes or macrophages, but it is also found in the endothelial cells of the CL (Petroff et al., 1999;Zhao et al., 1998). Interestingly, immune cells, as well as cytokines, play a role in regulating luteal function in rats, mice, cows and women (Brannstrom and Friden, 1997;Friedman et al., 2000;Terranova and Rice, 1997). Moreover, during luteolysis, macrophages are attracted to and infiltrate the CL, increasing the local production of cytokines (Brannstrom et al., 1994). It is thought that endothelial cells of the CL are the first to undergo apoptosis and TNF-α can induce the death of this type of cell (Friedman et al., 2000;Pru et al., 2003). Our data are consistent with this hypothesis.
Protein and mRNA analyses also support the expression of TNF-R1 and -R2, both of which can bind TNF-α and -β, in the macaque CL. Whereas, TNF-R2 mRNA levels did not change during the luteal phase, TNF-R1 mRNA levels increased modestly in the CL in the late luteal phase during luteolysis. These results on TNF-R1 mRNA expression in the primate CL are comparable to previous reports on the bovine CL (Friedman et al., 2000); reports to date have not focused on TNF-R2. Immunohistochemical analyses for TNF-R1 and -R2 detected high expression of these proteins in both granulosa and theca luteal cells in the macaque CL throughout the natural luteal phase. In contrast to reports on the bovine CL (Friedman et al., 2000), endothelial cells in monkey CL displayed no staining for TNF-R1 or -R2; this may be due to species differences. Notably, more intense staining was found in the theca luteal cells (especially in the mid-late to late luteal phase), suggesting a differential regulation of theca-versus granulosa- derived cells during luteolysis in the primate CL.
There are reports that TNF-α can initiate luteotropic or luteolytic effects depending on the stage of the cycle, species or local milieu in the CL (Davis and Rueda, 2002). The dynamic and increased expression of components of the TNF R-ligand system (TNF-α and -β, TNF-R1) in the CL during the late luteal phase may signify a role for death receptor-mediated processes (e.g., apoptosis) during luteolysis in the natural menstrual cycle of the rhesus monkey. Nevertheless, appreciable expression of these proteins was found during all stages of the luteal phase. It is important to consider that the binding of TNF-α to its receptor can trigger the survival of the cells through the activation of the nuclear factor-kappa B (NF-κB) (Beg and Baltimore, 1996;Wang et al., 1996;Wu et al., 1966), so the involvement of TNF-R pathways in various pro- or anti-apoptotic mechanisms throughout the CL lifespan warrants consideration. Also, the TNF R-ligand system may be regulated by the balance between ligand and receptor levels.
The current study also provides mRNA and protein data supporting the expression of the FAS-FASL system in the macaque CL throughout the menstrual cycle. The expression and/or action of the FAS-FASL system was reported in the CL of different species (Quirk et al., 1995;Quirk et al., 2000;Roughton et al., 1999;Taniguchi et al., 2002); mouse and human luteal cells are sensitive to FAS-mediated apoptosis (Quirk et al., 1995;Quirk et al., 2000). Rougton et al (Roughton et al., 1999) observed an increase in FAS and FASL expression during CL regression in pregnant rats, and Taniguchi et al (Taniguchi et al., 2002) determined that the FAS-FASL system can mediate luteal cell death in the bovine CL. Notably, the FAS-FASL system was highly expressed (mRNA and protein levels) in the monkey CL. The increased (6-fold) expression of FASL in the late luteal phase is consistent with a role for the FAS-FASL system in primate CL, and its apparent regulation by progestin (see below), during luteolysis.
Several components of the TNF-R family appear regulated by LH either directly or indirectly via progestin action in the primate CL. The only component that was stimulated by LH and progestin (R5020) was TNF-α mRNA levels (as noted earlier, protein levels could not be evaluated). Notably, steroid withdrawal reduced and R5020 addition restored TNF-α mRNA levels. In contrast, LH and/or progestin reduced expression of several other components at the mRNA and/or protein level. For example, R5020 addition reduced TNF-R1 mRNA levels and eliminated the intense immunostaining for TNF-R1 observed in discrete groups of luteal cells after steroid withdrawal (trilostane treatment). Although mRNA levels were not altered, steroid withdrawal versus R5020 administration appeared to have similar effects of enhancing versus reducing TNF-R2 immunostaining, as LH withdrawal and replacement. But the most remarkable effects were observed on the FAS-FASL system, where steroid withdrawal markedly increased their immunostaining in CL, but R5020 addition prevented this effect and reduced FASL (but not FAS) mRNA levels.
There are limited reports in the literature of progestin regulation of the TNF-R family. For example, Amory et al (Amory et al., 2005) demonstrated that progesterone increases TNF-α production after lipopolysaccharide stimulation of whole blood from nonpregnant women (Amory et al., 2004). Previous reports suggest that progesterone suppresses apoptosis and luteal regression in species with long luteal phases (Duffy and Stouffer, 1997;Okuda et al., 2004;Rueda et al., 2000;Svensson et al., 2001;Young and Stouffer, 2004). If, as we hypothesized earlier, the increased expression of components of the TNF-R system during the late luteal phase plays a role in death receptor-mediated apoptosis during luteolysis in primates, then the anti-apoptotic actions of progesterone may be mediated at least in part by suppressing the TNF-R, FAS-FASL pathways in the CL. However, further studies are needed to address the regulation and roles of TNF-R components and pathways in the macaque CL. Regulation appears complex, with some actions of LH/progesterone at the transcriptional and post-transcriptional levels.
In conclusion, our findings demonstrate the expression of multiple members of the TNF-receptor system (including FAS and its ligand) in the primate CL during luteinization and luteolysis in the natural menstrual cycle. Both the TNF-R and FAS systems are more highly expressed in theca luteal cells compared to granulosa luteal cells, suggesting a differential regulation of theca-derived cells during luteolysis. The dynamic and increased expression of members of the TNF-receptor family in the CL during the late luteal phase may signify a role for death receptor-mediated apoptosis during luteolysis in the natural menstrual cycle in the primate CL. Several components of the TNF-R and FAS-FASL systems in the developed CL appear regulated by LH, most likely indirectly via progestin action. As such, further studies are warranted to determine if the reported anti-apoptotic actions of progesterone in the CL are mediated in part by suppressing the TNF-R, FAS-FASL pathways.
Materials and Methods
Animals
The general care and housing of the monkeys (Macaca mulatta) at the Oregon National Primate Research Center (ONPRC) was previously described (Wolf et al., 1990). Animal protocols and experiments were approved by the ONPRC Animal Care and Use Committee. The studies were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals. Menstrual cycles of adult female rhesus monkeys were monitored daily as described previously (Duffy et al., 1999a).
Treatments and tissues
CL (n=3–4 per stage) were obtained from anaesthetized monkeys (Duffy et al., 2000) at specific stages during the luteal phase [early (ECL, day 3–5 post LH surge), mid (MCL, day 7–8), mid-late (MLCL, day 10–12), late (LCL, day 14–16), and very-late (VLCL, day 17–19) luteal phase] of the natural menstrual cycle, according to the protocol reported previously by our laboratory (Young and Stouffer, 2004). These stages represent CL formation (early luteal phase, ECL), peak CL function (mid luteal phase, MCL), CL on the verge of regression (mid-late luteal phase, MLCL), and the regressing CL (late and very-late luteal phase, LCL and VLCL). A portion of the CL was frozen in liquid nitrogen and stored at −80°C for later isolation of total RNA using Trizol (Invitrogen, CA, USA). The other portion was fixed in formalin and used for immunohistochemical analyses.
Hormone ablation/replacement treatment was performed in adult rhesus monkeys on day 9 through day 11 of the luteal phase. These days were selected because the developed, functional CL begins to regress near the end of this time period; in addition, this is the window of CL rescue in the primate by chorionic gonadotropin in fertile cycles (Stouffer, 2003). To assess the potential regulatory role of LH and P, females were assigned randomly to one of five treatment groups (n = 4/group): control (no treatment), antide (GnRH antagonist, 3 mg/kg/day, s.c. injection; previously demonstrated to suppress circulating bioactive LH levels and luteal function (Duffy et al., 1999b), antide + recombinant human LH (LH; 40 IU three times a day, i.m. injection; Serono Reproductive Biology Institute, Rockland, MA; previously shown to restore CL function (Duffy et al., 1999b)), antide + LH + trilostane (TRL; a 3β-hydroxysteroid dehydrogenase inhibitor previously demonstrated to ablate luteal P production (Duffy et al., 2000); 600 mg/day administered in an oral dose [8 ml of sucrose and Tang vehicle]; Sanofi Res. Div., Northumberland, UK), and antide + LH + TRL + R5020 (a nonmetabolizable progestin, 2.5 mg/day administered as a 2.5-ml [total volume] s.c. injection in a vehicle of sesame oil; Dupont, Boston, MA; previously shown to restore progestin actions (Duffy et al., 2000)). Serum estradiol and P levels were analyzed daily starting on Day 6 postmenstruation of the follicular phase through the end of treatment on Day 12 of the luteal phase. On Day 12, the CL was removed from anesthetized monkeys during an aseptic ventral midline surgery (Duffy et al., 2000) and weighed, and portions were divided and either immediately frozen for subsequent RNA isolation or processed for immunohistochemistry (Young and Stouffer, 2004).
sq RT-PCR Analysis of TNF-α and β, TNF-RI and TNF-RII, Fas and Fas-L
Total RNA extracted from individual CL was treated with 1 µg DNase (Invitrogen, CA, USA), and reverse transcription (RT) performed using Molony Murine Leukemia Virus reverse transcriptase (Invitrogen) for 2 h at 37 °C in a 20-µl reaction volume as previously described (Chaffin and Stouffer, 1999). PCR was carried out in a 25 µl volume containing an empirically determined amount of the RT reaction, 1 µl of the 10 mM specific primer set based on human sequences (Table 1), 2.5 µl 10x Taq buffer (Invitrogen), 0.5 µl 50 mM MgCl2, 0.5 µl 10 mM deoxy-NTPs Mix, 0.25 µl Taq (Invitrogen). Primer sequences were designed using Vector NTI Software (Invitrogen). The reaction was initiated at 94 °C for 1.5 min, followed by 94°C for 30 sec, 55 to 60 °C (depending on the gene; Table 1) for 30 sec, and 72 °C for 2 min for 31–40 cycles (Table 1), and a final extension at 72 °C for 5 min. The number of cycles used for each gene was determined previously to be in the linear part of the amplification curve. Aliquots of PCR products were electrophoresed through a 1.4% agarose gel containing 0.1 µg/ml ethidium bromide. Cyclophilin was used as an internal control as no changes in CL content were observed during the luteal phase in previous studies (Duffy and Stouffer, 1995). Gels were visualized on a UV transilluminator as well as photographed, and gel bands were quantified using Quantity One software (BioRad, CA, USA). Values for cDNA products of the various TNF-R components were standardized to their tissues’ cyclophilin level. PCR products were also purified for later sequencing by the ONPRC Molecular Biology Core facility, using an ABI 3100 automated sequencer. The resultant sequences were aligned using BLAST analyses to verify amplification of the expected cDNA product and to establish the homology between individual macaque and human genes.
Table I.
Sets of forward (FOR) and reverse (REV) primers designed for RT-PCR amplification of luteal TNF-R family mRNAs.
| Gene | Sequence Type | Sequence | Ta (°C) | # Cycles |
|---|---|---|---|---|
| TNF-α | FOR Primer | CCCGAGTGACAAGCCTG | 58.4 | 40 |
| NM_000594 | REV Primer | CCAAAGTAGACCTGCCCA | ||
| TNF-β | FOR Primer | ACTGTCTTCTTTGGAGCCTTC | 60 | 33 |
| NM_000595 | REV Primer | TGCTCTTCCTCTGTGTGTGG | ||
| TNF-RI | FOR Primer | CCCAGTTCCACCTTCACCTCCAGC | 60 | 35 |
| NM_001065 | REV Primer | GGCATAGCGTCCCTCATCCTCG | ||
| TNF-RII | FOR Primer | ACCGTGTGTGACTCCTGTG | 55 | 34 |
| NM_001066 | REV Primer | GGATGAAGTCGTGTTGGAGA | ||
| Fas | FOR Primer | CATCTGGACCCTCCTACCTCTGGT | 56 | 31 |
| NM_152876 | REV Primer | CCTTCATCACACAATCTACATCTTCTG | ||
| Fas-L | FOR Primer | CCATGCAGCAGCCCTTCAATT | 58.4 | 40 |
| NM_000639 | REV Primer | CCAAGGCAACCAGAACCATGAA | ||
Ta, PCR annealing temperature; #Cycles, number of PCR cycless
Immunohistochemistry for TNF-β, TNF-RI and TNF-RII, Fas and Fas-L
Portions of the CL were fixed in 10% neutral buffered formalin (Richard-Allen Scientific, Kalamazoo, MI, USA) for 1 week. Tissue was then dehydrated in a series of ethanol solutions (50, 70 and 100%) and paraffin-embedded. Then, 6 µm sections were deparaffinized and hydrated through xylene and a graded series of ethanol, as reported previously (Hazzard et al., 2000). Sections were incubated in phosphate-buffered saline (PBS) prior to pressure cooker-antigen retrieval in citrate buffer (Citra; BioGenex Laboratories, Inc., San Ramon, CA, USA). Endogenous peroxidases were then quenched with a 10 min incubation in 3% H2O2. Sections were placed in a blocking buffer [1.5% normal horse serum (NHS) in PBS], then incubated with primary antibody in NHS PBS buffer for 1 h at room temperature and overnight at 4°C. Concentrations of primary antibodies to human TNF-RI, TNF-RII, TNF-β, Fas and Fas-L (sc-1067, sc-1074, sc-8302, sc-715, sc-834; Santa Cruz Biotechnology, Inc., CA, USA), were 1:100, 1:100, 1:200, 1:100, 1:50 respectively. Primary antibody was detected using a biotinylated anti-rabbit or -goat IgG secondary antibody (1:1000; Vector Laboratories, Burlingame, CA, USA) and the Vector ABC-Elite Kit, visualized with Nickel-enhanced Sigma Fast diaminobenzidine substrate (Sigma, St Louis, MO, USA) and counter-stained with hematoxylin. As negative controls, adjacent tissue sections were exposed to primary antibody preabsorbed with its specific antigen. Unlike the above antibodies, human TNF-α antibodies (sc-7317; Santa Cruz Biotechnology, MAB610 and AF210NA, R&D systems Inc., Mineapolis, MN) failed to detect monkey antigen in paraffin sections.
Statistical analysis
Differences among experimental groups were analyzed using the Sigma Stat software package (SPSS, Chicago, IL). Data sets were typically not transformed, except for FASL mRNA levels (to log + 1, due to heterogeneity of variance), prior to one-factor analysis of variance (ANOVA) followed by Student-Newman-Keuls or Bonferroni’s Multiple Comparison tests. Differences were considered significant at p< 0.05.
Acknowledgement
We are grateful for the expert contributions of the animal care staff and surgical unit of the Division of Animal Resources, the outstanding technical support of the Endocrine Services, Imaging & Morphology, and Molecular & Cell Biology Core Laboratories at ONPRC. Recombinant human LH (Serono Reproductive Biology Institute) and TRL (Sanofi Pharmaceutical Inc., Grand Valley, PA) were generously donated for this project. A special thanks to Dr. Jon Hennebold’s laboratory associates (Wilma Perez and Melinda Murphy) for their scientific advice and assistance, and Natalia Lobato for helping in the preparation of photographs.
This research was supported by NIH NICHD HD20869 (RLS) through a cooperative agreement, U54 HD18185, as part of the Specialized Cooperative Centers Program in Infertility and Reproduction Research (SCCPIR). Support was also provided by NICHD/Fogarty D43 TW00668 (MCP), NRSA HD042896 (KAY) and NCCR RR00163 (RLS, JDH).
References
- Abdo M, Hisheh S, Dharmarajan A. Role of tumor necrosis factor-alpha and the modulating effect of the caspases in rat corpus luteum aptosis. Biol Reprod. 2003;68:1241–1248. doi: 10.1095/biolreprod.102.010819. [DOI] [PubMed] [Google Scholar]
- Amory J, Lawler R, Shields L. Hydroxyprogesterone caproate and progesterone increase tumor necrosis factor-alpha production in lipopolysaccharide stimulated whole blood from non-pregnant women. J Perinat Med. 2005;33:506–509. doi: 10.1515/JPM.2005.089. [DOI] [PubMed] [Google Scholar]
- Amory JH, Lawler R, Hitti J. Increased tumor necrosis factor-alpha in whole blood during the luteal phase of ovulatory cycles. J Reprod Med. 2004;49:678–682. [PubMed] [Google Scholar]
- Beg AA, Baltimore D. An essential role for NF-kappaB in preventing TNF-alpha-induced cell death. Science. 1996;274:782–784. doi: 10.1126/science.274.5288.782. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Friden B. Immune regulation of corpus luteum function. Semin Reprod Endocrinol. 1997;15:363–370. doi: 10.1055/s-2008-1068374. [DOI] [PubMed] [Google Scholar]
- Brannstrom M, Giesecke L, van den Heuvel CJ, Moore IC, Robertson SA. Leukocyte subpopulations in the rat corpus luteum during pregnancy and pseudopregnancy. Biol Reprod. 1994;50:1161–1167. doi: 10.1095/biolreprod50.5.1161. [DOI] [PubMed] [Google Scholar]
- Chaffin CL, Stouffer RL. Expression of matrix metalloproteinases and their tissue inhibitor messenger ribonucleic acids in macaque periovulatory granulosa cells: time course and steroid regulation. Biol Reprod. 1999;61:14–21. doi: 10.1095/biolreprod61.1.14. [DOI] [PubMed] [Google Scholar]
- Chen HL, Marcinkiewicz JL, Sancho-Tello M, Hunt JS, Terranova PF. Tumor necrosis factor-alpha gene expression in mouse oocytes and follicular cells. Biol Reprod. 1993;48:707–714. doi: 10.1095/biolreprod48.4.707. [DOI] [PubMed] [Google Scholar]
- Davis JS, Rueda BR. The corpus luteum: an ovarian structure with maternal instincts and suicidal tendencies. Front Biosci. 2002;7:1949–1978. doi: 10.2741/davis1. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Abdelgadir SE, Stott KR, Resko JA, Stouffer RL, Zelinski-Wooten MB. Androgen receptor messenger RNA expression in the rhesus monkey ovary. Endocrine. 1999a;11:23–30. doi: 10.1385/ENDO:11:1:23. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Chaffin CL, Stouffer RL. Expression of estrogen receptor α and β in the rhesus monkey corpus luteum during the menstrual cycle: regulation by luteinizing hormone and progesterone. Endocrinology. 2000;141:1711–1717. doi: 10.1210/endo.141.5.7477. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stewart DR, Stouffer RL. Titrating LH replacement to sustain the structure and function of the corpus luteum after GnRH antagonist treatment in rhesus monkeys. J Clin Endocrinol Metab. 1999b;84:342–349. doi: 10.1210/jcem.84.1.5362. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. Gonadotropin versus steroid regulation of the corpus luteum of the rhesus monkey during simulated early pregnancy. Biol Reprod. 1997;57:1451–1460. doi: 10.1095/biolreprod57.6.1451. [DOI] [PubMed] [Google Scholar]
- Duffy DM, Stouffer RL. Progesterone receptor messenger ribonucleic acid in the primate corpus luteum during the menstrual cycle: Possible regulation by progesterone. Endocrinology. 1995;136:1869–1876. doi: 10.1210/endo.136.5.7720632. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Lunn SF, Cowen GM, Illingworth PJ. Induced luteal regression in the primate: evidence for apoptosis and changes in c-myc protein. J Endocrinol. 1995;147:131–137. doi: 10.1677/joe.0.1470131. [DOI] [PubMed] [Google Scholar]
- Fraser HM, Lunn SF, Harrison DJ, Kerr JB. Luteal regression in the primate: different forms of cell death during natural and gonadotropin-releasing hormone antagonist or prostaglandin analogue-induced luteolysis. Biol Reprod. 1999;61:1468–1479. doi: 10.1095/biolreprod61.6.1468. [DOI] [PubMed] [Google Scholar]
- Friedman A, Weiss S, Levy N, Meidan R. Role of tumor necrosis factor alpha and its type 1 receptor in luteal regression: induction of programmed cell death in bovine corpus luteumderived endothelial cells. Biol Reprod. 2000;63:1905–1912. doi: 10.1095/biolreprod63.6.1905. [DOI] [PubMed] [Google Scholar]
- Gaytan F, Morales C, Garcia-Pardo L, Reymundo C, Bellido C, Sanchez-Criado JE. Macrophages, cell proliferation, and cell death in the human menstrual corpus luteum. Biol Reprod. 1998;59:417–425. doi: 10.1095/biolreprod59.2.417. [DOI] [PubMed] [Google Scholar]
- Hazzard TM, Christenson LK, Stouffer RL. Changes in expression of vascular endothelial growth factor and angiopoietin -1 and -2 in the macaque corpus luteum during the menstrual cycle. Mol Hum Reprod. 2000;6:993–998. doi: 10.1093/molehr/6.11.993. [DOI] [PubMed] [Google Scholar]
- Hueber AO, Zornig M, Lyon D, Suda T, Nagata S, Evan GI. Requirement for the CD95 receptor-ligand pathway in c-Myc-induced apoptosis. Science. 1997;278:1305–1309. doi: 10.1126/science.278.5341.1305. [DOI] [PubMed] [Google Scholar]
- Kondo H, Maruo T, Peng X, Mochizuki M. Immunological evidence for the expression of the Fas antigen in the infant and adult human ovary during follicular regression and atresia. J Clin Endocrinol Metab. 1996;81:2702–2710. doi: 10.1210/jcem.81.7.8675599. [DOI] [PubMed] [Google Scholar]
- Morales C, Garcia-Pardo L, Reymundo C, Bellido C, Sanchez-Criado JE, Gaytan F. Different patterns of structural luteolysis in the human corpus luteum of menstruation. Hum Reprod. 2000;15:2119–2128. doi: 10.1093/humrep/15.10.2119. [DOI] [PubMed] [Google Scholar]
- Morrison LJ, Marcinkiewicz JL. Tumor necrosis factor-alpha enhances oocyte/follicle apoptosis in the neonatal rat ovary. Biol Reprod. 2002;66:450–457. doi: 10.1095/biolreprod66.2.450. [DOI] [PubMed] [Google Scholar]
- Okuda K, Korzekwa A, Shibaya M. Progesterone is a suppressor of apoptosis in bovine luteal cells. Biol Reprod. 2004;71:2065–2071. doi: 10.1095/biolreprod.104.028076. [DOI] [PubMed] [Google Scholar]
- Peluffo MC, Young KA, Stouffer RL. Dynamic expression of caspase-2, -3, -8, and -9 proteins and enzyme activity, but not mRNA, in the primate corpus luteum during the menstrual cycle. J Clin Endocrinol Metab. 2005;90:2327–2335. doi: 10.1210/jc.2004-2214. [DOI] [PubMed] [Google Scholar]
- Petroff MG, Petroff BK, Pate JL. Expression of cytokine messenger ribonucleic acids in the bovine corpus luteum. Endocrinology. 1999;140:1018–1021. doi: 10.1210/endo.140.2.6676. [DOI] [PubMed] [Google Scholar]
- Pru JK, Lynch MP, Davis JS, Rueda BR. Signaling mechanisms in tumor necrosis factor alpha-induced death of microvascular endothelial cells of the corpus luteum. Reprod Biol Endocrinol. 2003;1:17. doi: 10.1186/1477-7827-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quatacker JR. Formation of autophagic vacuoles during human corpus luteum involution. Z Zellforsch Mikrosk Anat. 1971;122:479–487. doi: 10.1007/BF00936082. [DOI] [PubMed] [Google Scholar]
- Quirk SM, Cowan RG, Joshi SG, Henrikson KP. Fas antigen-mediated apoptosis in human granulosa/luteal cells. Biol Reprod. 1995;52:279–287. doi: 10.1095/biolreprod52.2.279. [DOI] [PubMed] [Google Scholar]
- Quirk SM, Harman RM, Huber SC, Cowan RG. Responsiveness of mouse corpora luteal cells to Fas antigen (CD95)-mediated apoptosis. Biol Reprod. 2000;63:49–56. doi: 10.1095/biolreprod63.1.49. [DOI] [PubMed] [Google Scholar]
- Robker RL, Russell DL, Espey LL, Lydon JP, O'Malley BW, Richards JS. Progesterone-regulated genes in the ovulation process: ADAMTS-1 and cathepsin L proteases. Proc Natl Acad Sci. 2000;97:4689–4694. doi: 10.1073/pnas.080073497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roughton SA, Lareu RR, Bittles AH, Dharmarajan AM. Fas and Fas ligand messenger ribonucleic acid and protein expression in the rat corpus luteum during apoptosis-mediated luteolysis. Biol Reprod. 1999;60:797–804. doi: 10.1095/biolreprod60.4.797. [DOI] [PubMed] [Google Scholar]
- Rueda BR, Hendry IR, Hendry WJ, III, Stormshak F, Slayden OD, Davis JS. Decreased progesterone levels and progesterone receptor antagonists promote apoptotic cell death in bovine luteal cells. Biol Reprod. 2000;62:269–276. doi: 10.1095/biolreprod62.2.269. [DOI] [PubMed] [Google Scholar]
- Sakamaki K, Yoshida H, Nishimura Y, Nishikawa S, Manabe N, Yonehara S. Involvement of Fas antigen in ovarian follicular atresia and luteolysis. Mol Reprod Dev. 1997;47:11–18. doi: 10.1002/(SICI)1098-2795(199705)47:1<11::AID-MRD2>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- Sakumoto R, Berisha B, Kawate N, Schams D, Okuda K. Tumor necrosis factor-alpha and its receptor in bovine corpus luteum throughout the estrous cycle. Biol Reprod. 2000;62:192–199. doi: 10.1095/biolreprod62.1.192. [DOI] [PubMed] [Google Scholar]
- Shikone T, Yamoto M, Kokawa K, Yamashita K, Nishimori K, Nakano R. Apoptosis of human corpora lutea during cyclic luteal regression and early pregnancy. J Clin Endocrinol Metab. 1996;81:2376–2380. doi: 10.1210/jcem.81.6.8964880. [DOI] [PubMed] [Google Scholar]
- Stouffer RL. Progesterone as a mediator of gonadotropin action in the primate corpus luteum: beyond steroidogenesis. Hum Reprod Update. 2003;9:99–117. doi: 10.1093/humupd/dmg016. [DOI] [PubMed] [Google Scholar]
- Sugino N, Suzuki T, Kashida S, Karube A, Takiguchi S, Kato H. Expression of Bcl-2 and Bax in the human corpus luteum during the menstrual cycle and in early pregnancy: Regulation by human chorionic gonadotropin. J Clin Endocrinol Metab. 2000;85:4379–4386. doi: 10.1210/jcem.85.11.6944. [DOI] [PubMed] [Google Scholar]
- Svensson EC, Markstrom E, Shao R, Andersson M, Billig H. Progesterone receptor antagonists Org 31710 and RU 486 increase apoptosis in human periovulatory granulosa cells. Fertil Steril. 2001;76:1225–1231. doi: 10.1016/s0015-0282(01)02891-6. [DOI] [PubMed] [Google Scholar]
- Taniguchi H, Yokomizo Y, Okuda K. Fas-Fas ligand system mediates luteal cell death in bovine corpus luteum. Biol Reprod. 2002;66:754–759. doi: 10.1095/biolreprod66.3.754. [DOI] [PubMed] [Google Scholar]
- Terranova PF, Rice VM. Review: Cytokine involvement in ovarian processes. Am J Reprod Immunol. 1997;37:50–63. doi: 10.1111/j.1600-0897.1997.tb00192.x. [DOI] [PubMed] [Google Scholar]
- Vaskivuo TE, Ottander U, Oduwole O. Role of apoptosis, apoptosis-related factors and 17beta-hydroxysteroid dehydrogenases in human corpus luteum regression. Mol Cell Endocrinol. 2002a;194:191–200. doi: 10.1016/s0303-7207(02)00087-4. [DOI] [PubMed] [Google Scholar]
- Vaskivuo TE, Stenback F, Tapanainen JS. Apoptosis and apoptosis-related factors Bcl-2, Bax, tumor necrosis factor-alpha, and NF-kappaB in human endometrial hyperplasia and carcinoma. Cancer. 2002b;95:1463–1471. doi: 10.1002/cncr.10876. [DOI] [PubMed] [Google Scholar]
- Wang CY, Mayo MW, Baldwin AS., Jr TNF- and cancer therapy-induced apoptosis: protentiation by inhibition of NF-kappaB. Science. 1996;274:784–787. doi: 10.1126/science.274.5288.784. [DOI] [PubMed] [Google Scholar]
- Wang LJ, Brännström M, Robertson SA, Norman RJ. Tumor necrosis factor α in the human ovary: presence in follicular fluid and effects on cell proliferation and prostaglandin production. Fertil Steril. 1992;58:934–940. doi: 10.1016/s0015-0282(16)55438-7. [DOI] [PubMed] [Google Scholar]
- Wolf DP, Thomson JA, Zelinski-Wooten MB, Stouffer RL. In vitro fertilization-embryo transfer in nonhuman primates: The technique and its applications. Mol Reprod Dev. 1990;27:261–280. doi: 10.1002/mrd.1080270313. [DOI] [PubMed] [Google Scholar]
- Wu M, Lee H, Bellas RE, Schauer SL, Arsura M, Katz D, FitzGerald MJ, Rothstein TL, Sherr DH, Sonenshein GE. Inhibition of NF-kappaB/Rel induces apoptosis of murine B cells. EMBO J. 1966;15:4682–4690. [PMC free article] [PubMed] [Google Scholar]
- Young KA, Stouffer RL. Gonadotropin and steroid regulation of matrix metalloproteinases and their endogenous tissue inhibitors in the developed corpus luteum of the rhesus monkey during the menstrual cycle. Biol Reprod. 2004;70:244–252. doi: 10.1095/biolreprod.103.022053. [DOI] [PubMed] [Google Scholar]
- Yuan W, Giudice LC. Programmed cell death in human ovary is a function of follicle and corpus luteum status. J Clin Endocrinol Metab. 1997;82:3148–3155. doi: 10.1210/jcem.82.9.4191. [DOI] [PubMed] [Google Scholar]
- Zapata JM, Pawlowski K, Haas E, Ware CF, Godzik A, Reed JC. A diverse family of proteins containing tumor necrosis factor receptor-associated factor domains. J Biol Chem. 2001;276:24242–24252. doi: 10.1074/jbc.M100354200. [DOI] [PubMed] [Google Scholar]
- Zhaoc Y, Burbach JA, Roby KF, Terranova PF, Brannian JD. Macrophages are the major source of tumor necrosis factor α in the porcine corpus luteum. Biol Reprod. 1998;59:1385–1391. doi: 10.1095/biolreprod59.6.1385. [DOI] [PubMed] [Google Scholar]