Abstract
Reperfusion injury induced by cardiac arrest and resuscitation leads to secondary challenges to the brainstem. A 12-minute cardiac arrest results in about 50% survival rate in the resuscitated rats over a 4-day recovery period. We investigated hypoxic ventilatory response (HVR) to mild hypoxia by measuring the minute volume before and during a brief exposure to 10% oxygen before and following cardiac arrest and resuscitation. Our results indicate that after cardiac arrest and resuscitation the baseline spontaneous ventilation was elevated significantly in all rats due to both increased frequency and tidal volume; HVR in the non-survivor group was essentially absent while the brainstem responsiveness to hypoxia is fully maintained in the survivor group. Thus, the HVR was shown in this study to be a reliable indicator of survival vs. non-survival during early days of recovery following cardiac arrest and resuscitation.
Keywords: Brainstem, transient global ischemia, post-resuscitation survival, reperfusion injury
1. Introduction
Cardiac arrest and resuscitation induces transient global ischemia and results in ischemia and reperfusion injury in the brain leading to post-resuscitation mortality and morbidity. The estimated deaths associated with sudden cardiac arrest in the United States can reach as much as half-million each year (Goldberger et al. 1497-518), many of which are initially successfully resuscitated. However, the survival rate is less than 15% in the first 5 years following resuscitation. The statistics concerning cardiac arrest and resuscitation have been previously reviewed by (Safar et al. 110-21). Roughly 250,000 annual candidates for cardiopulmonary resuscitation, about 100,000 resuscitation attempts are made. About half of these cardiac arrests each year are initially resuscitated. Of the half that survive, half will never leave the hospital. Of the half that leave the hospital about half again have residual neurologic deficit. Thus, there is a significant number of cases each year that die or suffer chronically from the central nervous system effects of cardiac arrest.
Brain ischemia, focal or global, has been extensively studied in animal models, especially in those vulnerable regions such as hippocampus where delayed neuronal death occurs (Kirino 57-69;Lipton 1431-568;Durukan and Tatlisumak 179-97). The role of the brainstem following transient brain ischemia has been mostly ignored. The brainstem contains respiratory and cardiovascular centers that play important roles in regulating cardiac and respiratory function necessary for survival. Reperfusion injury induced by cardiac arrest and resuscitation can lead to secondary challenges to the brainstem (LaManna et al. 213-23). For example, Bax and activated caspase 3 were detected in brainstem for a few days after resuscitation, which may indicate the reperfusion injury. Signs of neuronal apoptosis were also found in brainstem regions, e.g. TUNEL-positive cell were detected 2-4 days after resuscitation in the sensitive regions such as rostral ventral lateral medulla (RVLM), pyramidal tract, raphe magnus nucleus, raphe pallidus nucleus, raphe obscurus nucleus, predorsal bundle, prepositus hypoglossal nucleus, medial vestibular nucleus, facial nucleus, perifacial zone, trapezoid body, C1 adrenaline cells, spinal trigeminal tract and medial lemniscus. The rat cardiac arrest and resuscitation model produces transient global ischemia in brain that allows regional studies of the brain including the brainstem (Crumrine and LaManna 272-82;Xu et al. 208-17). In this model, the duration of arrest can be manipulated to result in about 50% 4-day survival rates (Hoxworth et al. 467-79;Xu et al. 208-17), producing both survivor and non-survivor groups that can be examined for physiological variables that differentiate the 2 groups. The non-surviving rats tend to die from cardio-respiratory collapse which suggested that brainstem function might become compromised during the first few days after resuscitation. We have previously reported brainstem hypoperfusion and edema in the first few days following resuscitation (Xu et al. 208-17). The present study determined if these alterations in brainstem were represented by physiological consequences, such as the ability to adapt to hypoxia. We used a relatively non-invasive test of the brainstem response to mild hypoxic challenge by measuring the minute volume before and during a brief exposure to 10% oxygen from which the hypoxic ventilatory response (HVR) was calculated.
HVR is a physiological response to decreased environmental oxygen levels. Exposure to hypoxia triggers immediate hyperventilation in rats (LaManna, Vendel, and Farrell 2238-43) through the chemoreflex, a reflex arc which is initiated at the carotid bodies, modulated in brainstem (e.g. nucleus tractus solitatius in medulla oblongata), to the respiratory muscle effectors such as diaphragm (Finley and Katz 108-16;Koshiya and Guyenet R1273-R1278;Prabhakar 2287-95;Bianchi, Denavit-Saubie, and Champagnat 1-45). Therefore, HVR reflects brainstem function with respect to regulation of respiration. If the post-resuscitation mortality is associated with impairment of brainstem function then the HVR can serve as a differentiating variable between survivors and non-survivors. To test this hypothesis, using the rat cardiac arrest and resuscitation model, we investigated the HVR before and after transient global ischemia over the first four days of recovery. Physiological variables, such as mean arterial blood pressure, arterial blood gases, plasma glucose and lactate concentrations, were also measured.
2. Results
2.1 Physiological variables
There were no differences in the pre-arrest physiological variables of the rats that survived for at least 4 days (survivor group) compared to the rats that did not survive (non-survivor group) for 4 days after cardiac arrest and resuscitation (Table 1). There were also no differences in any variable between these 2 groups when measured 1 hour after resuscitation. At the 1 hour recovery the mean arterial blood pressure was significantly decreased in both groups compared to the pre-arrest values. In addition, the plasma lactate levels were significantly elevated. Body weights decreased (about 5 -10%) during the early days of recovery in both survivor and non-survivor groups.
Table 1.
Physiological variables
| Variable | Survivor (n = 10) |
Non-survivor (n = 9) |
|---|---|---|
| Body weight (g) | ||
| Pre-arrest | 357 ± 28 | 364 ± 33 |
| 1 d post | 340 ± 27 | 339 ± 32 (n = 7) |
| 2 d post | 328 ± 26* | 325 ± 33 (n = 5) |
| 3 d post | 325 ± 25* | 330 ± 30 (n = 2) |
| 4 d post | 334 ± 24 | NA |
| HVR pre-arrest | 2.9 ± 0.3 | 2.9 ± 0.3 |
| MABP (mmHg) | ||
| pre-arrest | 113 ± 6 | 113 ± 7 |
| 1 hr post | 97 ± 8* | 98 ± 10* |
| Arterial pH (unit) | ||
| pre-arrest | 7.43 ± 0.03 | 7.41 ± 0.04 |
| 1 hr post | 7.38 ± 0.08 | 7.35 ± 0.08 |
| PaO2 (mmHg) | ||
| pre-arrest | 96 ± 17 | 92 ± 7 |
| 1 hr post | 99 ± 14 | 95 ± 8 |
| PaCO2 (mmHg) | ||
| Pre-arrest | 38 ± 3 | 40 ± 2 |
| 1 hr | 36 ± 4 | 38 ± 3 |
| Hematocrit (%) | ||
| pre-arrest | 48 ± 2 | 48 ± 2 |
| 1 hr post | 48 ± 2 | 48 ± 2 |
| Glucoseplasma (mM) | ||
| pre-arrest | 7.9 ± 1.1 | 8.2 ± 0.3 |
| 1 hr post | 9.6 ± 1.2 | 9.3 ± 0.8 |
| Lactateplasma (mM) | ||
| pre-arrest | 1.5 ± 0.3 | 1.7 ± 0.4 |
| 1 hr post | 2.9 ± 0.8* | 3.3 ± 0.6* |
Values are mean + SD; 1 hr post, 1 d post, 2 d post 3 d post and 4 d post: 1 hour, 1, 2, 3, and 4 days post-resuscitation values, respectively; MABP: mean arterial blood pressure;
indicated the post-resuscitation value was significantly different (t-test, p < 0.05) from the pre-arrest value in the same group.
2.2. Hypoxic ventilatory response (HVR)
HVR, as measured by the ratio of hypoxic minute volume to normoxic baseline minute volume, was determined in each rat the day before cardiac arrest. When exposed to hypoxic gas, mammals respond with an increase in respiration frequency and tidal volume, resulting in an increase in the minute volume. Fig. 1 is an example of ventilation patterns during normoxic and hypoxic exposure in a rat. On average, the mean increase in minute volume (tidal volume × frequency) in response to hypoxic exposure to a gas mixture (10% O2 balanced with N2) was 3-fold higher (HVR = 2.9 ± 0.3) compared to normoxic baseline. There were no differences in pre-arrest HVR between survivors and non-survivors (Table 1). Fig.1A displays the individual data points and the means (± SD) for HVR over the four day survival period. The first post-resuscitation HVR was determined at 1 day recovery; all rats were conscious and able to walk around. In the survivor group, the daily HVR was significantly depressed to about the same extent (30% from pre-arrest control values) as determined over the 4-day survival period (average daily HVR was 1.9 ± 0.2). In the non-survivor group, during 1-3 days of recovery, HVR ranged from 0.8 to1.3, indicating a 60 - 70% decrease compared to the pre-arrested values. In the non-survivor rats where HVR was not distinguishable from 1.0, the hypoxia-induced increased ventilation response was completely lost.
Fig. 1.
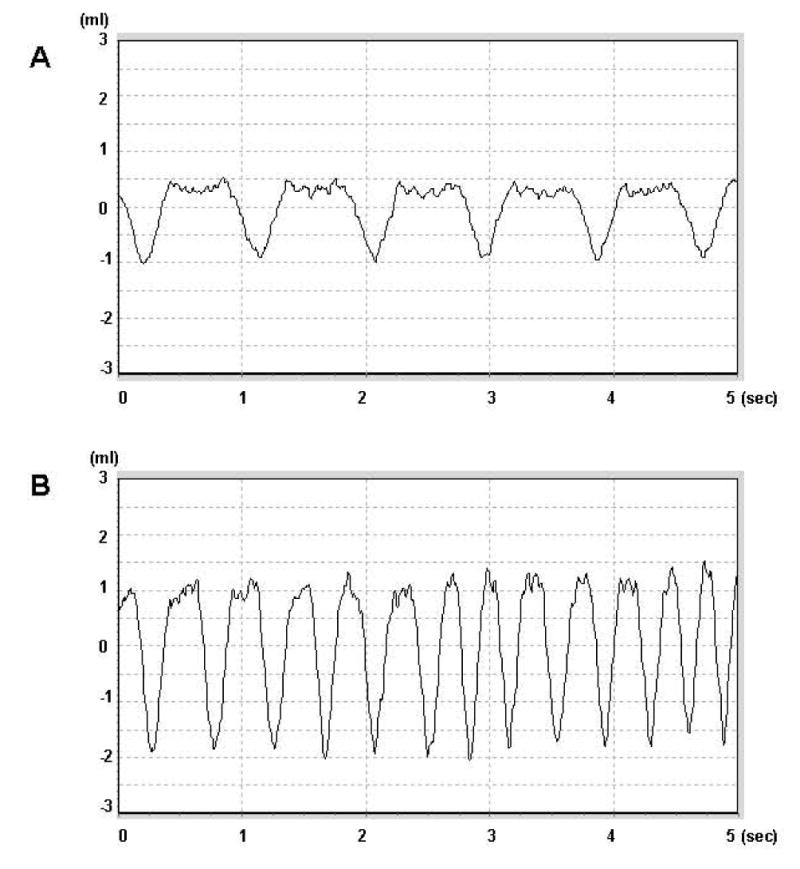
An example of respiration traces during normoxic (air, A) and hypoxic exposure (10% O2, B) in a rat pre-arrest. X-axis shows the total recording time (5 seconds); Y- axis indicates the volume during inspiration time (negative) and expiration time (positive), respectively. Note the significant changes in pattern when challenged with hypoxia.
In the recovery period, no rat with an HVR below 1.5 survived for 4 days. Conversely, every rat with HVR higher than 1.5 survived for at least 4 days. At each day of recovery, there was no overlap in the daily ranges of HVR in survivor and non-survivors (Fig. 2A). In the non-survivor group, HVR progressively worsened in each case. This was not the case in the survivor group where no pattern was discernable (Fig. 2B).
Fig. 2.

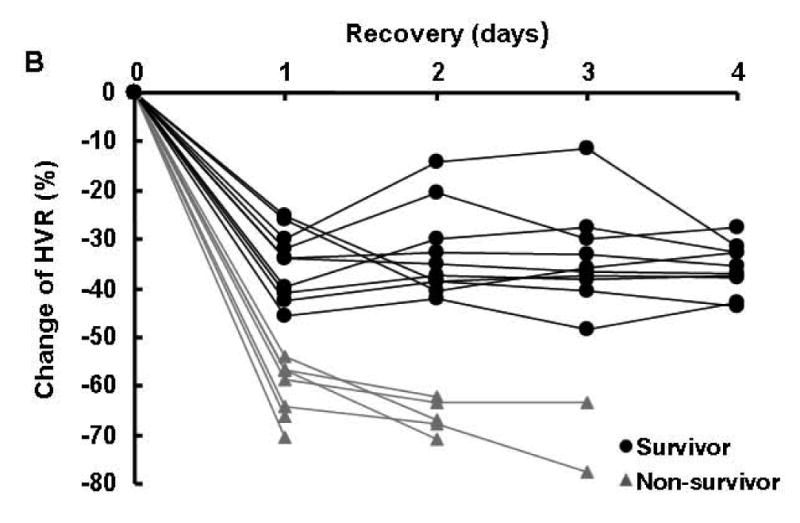
A: HVR (individual data points and mean ± SD) before and after cardiac arrest and resuscitation in both survivor and non-survivor groups. Survivor: black solid circle; n =10 for each day of recovery; non-survivor: gray solid triangle; B: Percent change (individual data point) of HVR (vs. pre-arrest values) following cardiac arrest and resuscitation in both survivor (black solid circle and line) and non-survivor groups (Gray solid triangle and line).
2.3. Minute volume, frequency and tidal volume
The HVR is calculated as the ratio of the respiratory minute volume during and before exposure to hypoxia. The minute volume comprised of 2 components, respiratory frequency and pulmonary tidal volume. Fig. 3 shows the minute volume, frequency and tidal volume of both survivor and non-survivor groups under normoxic and hypoxic exposure, before and after cardiac arrest and resuscitation. As expected, pre-arrest values for frequency or tidal volume between the 2 groups before cardiac arrest during normoxia or hypoxia showed no differences (Figs. 3A and B). Before cardiac arrest hypoxic minute volume was 3-fold the normoxic minute volume, in both survivor and non-survivor groups (Fig. 2C). The increase was attributed to the increased frequency with a smaller contribution from a 35% increase in tidal volume (Figs. 3A and B).
Fig. 3.


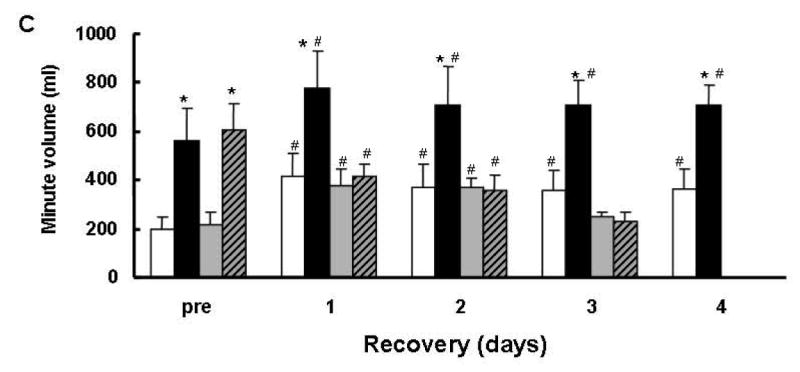
Frequency (A) and tidal volumes (B) and minute volume (C) before and after cardiac arrest and resuscitation in both survivor and non-survivor groups. Survivor-N: normoxic baseline for survivors, Survivor-H: hypoxic values for survivors, n = 10 for each day; Non-survivor-N: normoxic baseline for non-survivors, Non-survivor-H: hypoxic values for non-survivors, n = 9, 7, 5, and 2 for 1, 2, and 3 days of recovery, respectively. Values are mean ± SD;* indicated the hypoxic value was significantly different (t-test, p < 0.05) from normoxic baseline; # indicated the post-resuscitation value was significantly different (t-test, p < 0.05) from the pre-arrest value in the same group.
During the recovery period the normoxic minute volume increased significantly in both survivors and non-survivors compared to their respective pre-arrest controls (Fig. 3C). This increase in normoxic baseline minute volume during recovery was explained by equal contributions from frequency and tidal volume (Figs. 3A and B) which persisted throughout the 4 recovery days. Whereas the hypoxic minute volume significantly increased in the survivor group and decreased in the non-survivor group.
Since HVR is calculated by the ratio of hypoxic minute volume (numerator) to normoxic minute volume (denominator); the attenuated HVR during recovery in the survivor group was, therefore, due to the elevated normoxic baseline rather than to an attenuated hypoxic response. The hypoxia-induced tidal volume increase during recovery remained about the same in the survivor group, but the frequency increase was less, without significant differences in the absolute rate pre- or post-arrest. Perhaps indicating the maximum rate in frequency was reached (Fig. 3A). On the other hand in the non-survivors, the hypoxic drive was completely absent which displayed neither a frequency nor a tidal volume response to hypoxia (Figs. 3A and B).
3. Discussion
Long-term survival rates in humans after cardiac arrest and resuscitation are still disappointing and there are very few indicators that can be used for early prognosis. The rat model of cardiac arrest and resuscitation can be used to study potential variables that might allow early prediction of survival after cardiac arrest and resuscitation. Previous studies have demonstrated that in this model 12 minutes of cardiac arrest will result in an approximately 50% 4-day survival, and delayed hippocampal neuronal death in survivors (Hoxworth et al. 467-79;Xu et al. 208-17).
Previous studies had suggested that brainstem function might be the limiting factor in determining long term survival after cardiac arrest and resuscitation (Xu et al. 208-17) and this current study confirms that at least one aspect of brainstem responsiveness, i.e. the HVR, correlates closely with survival during the first few days of recovery. The first observation to note was that the baseline spontaneous ventilation was elevated significantly in all rats after cardiac arrest and resuscitation. The increase in minute ventilation was due to the similar increase in both tidal volume and frequency, at least at the 1 day measurement interval, in both survivor and non-survivor groups. Perhaps this represents a common stress due to continued hypoxia (Chavez and LaManna 8922-31)or metabolic imbalance (Carden and Granger 255-66;Eltzschig and Collard 71-86;White et al. 1-33) as suggested by the continued hypoperfusion throughout the brain that persists for at least the first 24 hours following resuscitation (Xu et al. 208-17).
The second observation is that the HVR in the non-survivor groups is essentially absent after cardiac arrest and resuscitation. The survivors still exhibit brisk increases in minute volume in response to hypoxia after cardiac arrest and resuscitation. In fact, the hypoxic induced minute volume after cardiac arrest and resuscitation in the survivor group is significantly greater than the hypoxic induced minute volume pre arrest. This suggests that the brainstem responsiveness to hypoxia is fully maintained in the survivor group and completely lost in the non-survivors. The attenuated HVR in the survivor group is attributed to the increased minute volume at baseline and not the hypoxic response.
In conclusion, the HVR was shown in this study to be a reliable indicator of survival vs. non-survival during the first several days of recovery following cardiac arrest and resuscitation. In the first few days after resuscitation, if the HVR was below 1.5, then the rat invariably died. But, if the HVR remained above 1.5 then the rat invariably survived.
4. Experimental procedures
4.1. Animal preparation
The experimental protocol employed by this study was approved by the Institutional Animal Care and Use Committee (IACUC) at Case Western Reserve University. Male adult Wistar rats (aged 2-3 months, 250 – 300g) were purchased and allowed to acclimate in the animal facility at Case Western Reserve University for one week before experiment. On the day of experiment, surgical procedures were performed as previously described (Hoxworth et al. 467-79;Xu et al. 208-17). Briefly, anesthesia was induced by 2.5% isoflurane and maintained on 1.5% through a nasal cone. Ventral tail artery was cannulated for the purpose of monitoring of arterial blood pressure and collecting blood samples; external jugular vein was cannulated into the right atrium for administration of drug. Rats were allowed to recover for at least 1 hour after surgery while restrained in plastic cages. The body temperature was maintained at 37°C by an infrared heat lamp (250W, 45 cm above the body) regulated by feedback from a rectal probe during the surgery and the following experiment.
4.2. Induction of transient global brain ischemia in rat
Transient global brain ischemia was achieved using a rat model of cardiac arrest and resuscitation (Hoxworth et al. 467-79;Xu et al. 208-17). Cardiac arrest was induced in the conscious rat by rapid sequential intra-atrial injection of d-tubocurare (0.3 mg) and ice-cold KCl solution (0.5 M; 0.12 ml/100g of body weight). Resuscitation was initiated 7 min after arrest. The animal was intubated orotreacheally with a 14-gauge catheter attached to a rodent ventilator (100% O2, tidal volume: 10cc/kg, respiratory rate: 80 breaths min). Simultaneous chest compressions and the infusion of normal saline (0.5 ml/min) were given until a spontaneous heartbeat returned. Epinephrine (4-10 μg) was given to establish a mean blood pressure greater than 80% of the pre-arrest value, at which point the animal was considered to be resuscitated. The total ischemic time was about 12 minutes. Ventilation was then adjusted to achieve blood gases in the normal range, until spontaneous respiration was regained. Nineteen rats were subjected to the cardiac arrest protocol; all were initially successfully resuscitated. Rats were subsequently grouped as “survivor”, i.e., those that survived for at least 4 days after resuscitation (n = 10) and “non-survivor”, i.e., those that died within 4 days (n = 9). In the non-survivor group, 2 rats died in the first 24 hours, 2 more died between 24 and 48 hours, 3 rats died between 48 and 72 hours and 2 rats died between 72 and 96 hours.
4.3. Measurement of Hypoxic Ventilatory Response (HVR)
A plethysmograph system (Buxco Electronics, Troy, NY) was used to measure HVR of rats before cardiac arrest and following resuscitation. Conscious, unrestrained rats were placed individually in a pre-calibrated 3 L barometric chamber and continuously ventilated with humidified room air or gas mixtures passing through the chamber at a rate of 2 L/min. Pressure changes in the chamber due to animal inspiration and expiration were measured by a high gain differential pressure transducer. Analog signals was amplified, continuously digitized and analyzed by Buxco software. A rejection algorithm was induced in the breath-by-breath analysis routine which allows accurate rejection of motion-induced artifact. The room air and 10% O2 in nitrogen were ventilated for 10 min each to determine the normoxic baseline and hypoxic ventilation, respectively. Tidal volume, breath frequency, and minute volume were computed and stored for subsequent analysis. HVR was represented as ratio of hypoxic minute volume vs. normoxic minute volume baseline; minute volume = tidal volume × frequency.
4.4. Statistical Methods
All values are presented as mean ± SD. Statistical analyses were performed using SPSS v13.0 for Windows. Group comparisons were made by paired or unpaired Student's t-test. Significance was considered at the level of p < 0.05.
Acknowledgments
We would like to thank Dr. Michelle A. Puchowicz for her help in preparing the manuscript. This work was supported by NIH grant NS 46074.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75(1):1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Carden DL, Granger DN. Pathophysiology of ischaemia-reperfusion injury. J Pathol. 2000;190(3):255–66. doi: 10.1002/(SICI)1096-9896(200002)190:3<255::AID-PATH526>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Chavez JC, LaManna JC. Activation of hypoxia-inducible factor-1 in the rat cerebral cortex after transient global ischemia: potential role of insulin-like growth factor-1. J Neurosci. 2002;22(20):8922–31. doi: 10.1523/JNEUROSCI.22-20-08922.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumrine RC, LaManna JC. Regional cerebral metabolites, blood flow, plasma volume, and mean transit time in total cerebral ischemia in the rat. J Cereb Blood Flow Metab. 1991;11(2):272–82. doi: 10.1038/jcbfm.1991.59. [DOI] [PubMed] [Google Scholar]
- Durukan A, Tatlisumak T. Acute ischemic stroke: overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav. 2007;87(1):179–97. doi: 10.1016/j.pbb.2007.04.015. [DOI] [PubMed] [Google Scholar]
- Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70:71–86. doi: 10.1093/bmb/ldh025. [DOI] [PubMed] [Google Scholar]
- Finley JC, Katz DM. The central organization of carotid body afferent projections to the brainstem of the rat. Brain Res. 1992;572(12):108–16. doi: 10.1016/0006-8993(92)90458-l. [DOI] [PubMed] [Google Scholar]
- Goldberger JJ, et al. American Heart Association/American College of Cardiology Foundation/Heart Rhythm Society scientific statement on noninvasive risk stratification techniques for identifying patients at risk for sudden cardiac death: a scientific statement from the American Heart Association Council on Clinical Cardiology Committee on Electrocardiography and Arrhythmias and Council on Epidemiology and Prevention. Circulation. 2008;118(14):1497–518. [PubMed] [Google Scholar]
- Hoxworth JM, et al. Cerebral metabolic profile, selective neuron loss, and survival of acute and chronic hyperglycemic rats following cardiac arrest and resuscitation. Brain Res. 1999;821(2):467–79. doi: 10.1016/s0006-8993(98)01332-8. [DOI] [PubMed] [Google Scholar]
- Kirino T. Delayed neuronal death in the gerbil hippocampus following ischemia. Brain Res. 1982;239(1):57–69. doi: 10.1016/0006-8993(82)90833-2. [DOI] [PubMed] [Google Scholar]
- Koshiya N, Guyenet PG. NTS neurons with carotid chemoreceptor inputs arborize in the rostral ventrolateral medulla. Am J Physiol. 1996;270(6 Pt 2):R1273–R1278. doi: 10.1152/ajpregu.1996.270.6.R1273. [DOI] [PubMed] [Google Scholar]
- LaManna JC, et al. Brain Stem Sensitivity to Hypoxia and Ischemia. In: Haddad GG, Yu SP, editors. Brain hypoxia and ischemia. Humana Press, a part of Springer Science + Bussiness Media, LLC; 2009. pp. 213–23. 2009. [Google Scholar]
- LaManna JC, Vendel LM, Farrell RM. Brain adaptation to chronic hypobaric hypoxia in rats. J Appl Physiol. 1992;72(6):2238–43. doi: 10.1152/jappl.1992.72.6.2238. [DOI] [PubMed] [Google Scholar]
- Lipton P. Ischemic cell death in brain neurons. Physiol Rev. 1999;79(4):1431–568. doi: 10.1152/physrev.1999.79.4.1431. [DOI] [PubMed] [Google Scholar]
- Prabhakar NR. Oxygen sensing by the carotid body chemoreceptors. J Appl Physiol. 2000;88(6):2287–95. doi: 10.1152/jappl.2000.88.6.2287. [DOI] [PubMed] [Google Scholar]
- Safar P, et al. Systematic development of cerebral resuscitation after cardiac arrest. Three promising treatments: cardiopulmonary bypass, hypertensive hemodilution, and mild hypothermia. Acta Neurochir Suppl (Wien) 1993;57:110–21. doi: 10.1007/978-3-7091-9266-5_16. [DOI] [PubMed] [Google Scholar]
- White BC, et al. Brain ischemia and reperfusion: molecular mechanisms of neuronal injury. J Neurol Sci. 2000;179(S 12):1–33. doi: 10.1016/s0022-510x(00)00386-5. [DOI] [PubMed] [Google Scholar]
- Xu K, et al. Adenosine treatment delays postischemic hippocampal CA1 loss after cardiac arrest and resuscitation in rats. Brain Res. 2006;1071(1):208–17. doi: 10.1016/j.brainres.2005.11.060. [DOI] [PubMed] [Google Scholar]


