Abstract
Background
Research suggests that glucose levels in cancer patients may be an important prognostic indicator. In ovarian tumors, increased expression of GLUT1, a transmembrane protein responsible for glucose uptake, is related to shorter survival time in ovarian cancer patients. This study tested the hypothesis that higher pre-surgical glucose levels predict shorter disease-specific survival time and time to recurrence in ovarian cancer patients.
Methods
Non-fasting plasma glucose levels were determined for 74 patients with ovarian cancer at the time of their pre-surgical consultation and for 125 ovarian cancer patients in an independent validation set. Survival time and time to recurrence (disease free interval--DFI) were ascertained from medical records. Cox proportional hazards regression models were used to estimate the hazard ratio (HR) for survival time and DFI in relation to glucose level, adjusting for body mass index, stage, grade, and cytoreduction as appropriate.
Results
Higher glucose levels were associated with shorter survival times in univariate analyses (HR, 1.88; P = 0.05). Multivariate analysis adjusting for stage showed that higher glucose levels were associated with shorter survival times (HR, 2.01; P = 0.04) and DFI (HR, 2.32; P = 0.05). In the validation set, higher glucose levels were associated with shorter survival times (HR, 2.01; P = 0.02) and DFI (HR, 2.48; P = 0.001) in univariate analysis, although glucose was not independent of the effect of cytoreduction when predicting survival time in this latter set.
Conclusion
These findings contribute to mounting evidence that glucose levels have prognostic value in ovarian carcinoma.
Keywords: glucose, cancer, ovarian cancer, prognosis, recurrence, survival rate
INTRODUCTION
Ovarian carcinoma is the deadliest of gynecological cancers and the fifth leading cause of cancer mortality in women, with a five-year relative survival rate of 45% in the United States.1 The generally poor prognosis for ovarian cancer is largely due to late presentation by patients whose symptoms remain clinically silent until after the tumor has disseminated locally or metastasized.2 Also, recurrent disease commonly develops, even in patients whose initial response to therapy was positive. Prognosis at time of diagnosis has been shown to vary according to standard clinical variables such as the disease stage, tumor grade, and success of surgical debulking of the tumor.3 However, given the poor prognosis for ovarian cancer in general, efforts to identify other factors that influence recurrence and patient survival are needed for this disease.
Recent clinical research suggests that plasma glucose levels in cancer patients may be an important prognostic indicator. Elevated blood glucose levels, in both diabetic and non-diabetic persons, before or around the time of diagnosis have been shown to predict shorter survival times in head and neck cancer, stomach cancer, lung cancer, and acute lymphocytic leukemia.4,5,6 Although such findings have not been reported for ovarian cancer, one study of gynecological cancers found that patients in remission had significantly lower glucose levels than new patients presenting with active disease.7 The biological rationale for these clinical associations may be explained by data demonstrating increased expression in ovarian tumors of glucose transporter protein 1 (GLUT 1), a transmembrane protein responsible for glucose uptake.8 Increased expression of GLUT1 has been related to shorter survival time in ovarian cancer patients.9
Given the biological plausibility of glucose influence on cancer outcome, we examined the prognostic value of plasma glucose levels at the time of pre-surgical consultation in ovarian cancer patients. We hypothesized that higher glucose levels in ovarian cancer patients before surgery would be associated with shorter disease-specific survival times. We also hypothesized that higher glucose levels would predict shorter time to recurrence (disease free interval [DFI]) in patients who achieved remission.
MATERIALS AND METHODS
Patients
We retrospectively evaluated 74 patients, aged 33 to 87 (median = 62), with primary epithelial ovarian, papillary peritoneal, or fallopian tube cancer who were treated at the University of Iowa Hospitals and Clinics (UIHC). Patients underwent surgery between January 2001 and December 2005 for cytoreduction and were followed until death or the end of June 2007. Patients were surgically staged according to the International Federation of Gynecologists and Obstetricians (FIGO) guidelines (stages I, II, III, or IV). Tumor grade was assessed by a gynecological pathologist (grades 1, 2, or 3). Cytoreduction below 1 cm was considered optimal. At 2–3 weeks post-surgery, 88% of the patients began adjuvant treatment with platinum and taxane combination chemotherapy. The majority of these patients received six or more cycles of therapy (Table 1). These patients were part of a larger prospective study to evaluate the influence of biobehavioral factors on cancer progression. Individuals with a primary cancer of non-ovarian origin, a non-epithelial ovarian tumor, an ovarian tumor of low malignant potential, a history of systemic corticosteroid medication use in the last 4 months, or comorbidities known to alter the immune response such as an immunomodulatory or inflammatory disease were excluded from this study.
TABLE 1.
Patient Characteristics (N = 74)
| Age (years) | |
| median, range | 62, 33–87 |
| Glucose (mg/dL) | |
| mean, SD | 108, 38.11 |
| range | 60–308 |
| % of Patients | |
| Stage | |
| I | 8.2 |
| II | 5.5 |
| III | 65.8 |
| IV | 20.5 |
| Grade | |
| 1 | 8.7 |
| 2 | 21.7 |
| 3 | 69.5 |
| Cytoreduction | |
| suboptimal | 36.1 |
| optimal | 63.9 |
| Chemotherapy Cycles | |
| none | 12.2 |
| 1–5 | 10.9 |
| 6 | 51.4 |
| > 6 | 24.4 |
For validation of our findings with preoperative glucose levels from the test set, we obtained preoperative glucose measurements from an additional 125 patients with epithelial ovarian carcinoma who were treated at the University of Texas M. D. Anderson Cancer Center (MDACC). This cohort served as an independent validation set to allow further evaluation of the significance of our results. Eligibility, glucose levels, and the other clinicopathological data were determined for these patients in the same manner as described above for the UIHC test set. The Institutional Review Boards at both institutions approved this research, and all patients provided informed consent before participating.
Plasma Glucose
Peripheral blood was collected at the pre-surgical visit and, thus, before postoperative adjuvant chemotherapy (no neoadjuvant chemotherapy before surgery). Plasma glucose (mg/dL) was determined by the UIHC Department of Pathology Laboratory as part of a panel of pre-surgical laboratory tests or by the MDACC Core Laboratory for the validation set. For two patients, two readings were taken and the means were used for all analyses. Patients were not fasting, and blood was collected at various recorded times of the day.
Because previous studies suggest diabetes status may be predictive of reduced survival time,4 preliminary analyses using diabetes diagnosis as a predictor variable were conducted. Although non-fasting, pre-surgical glucose level was moderately correlated with diabetic status (r = 0.29; P = 0.01), diabetes diagnosis as a predictor variable yielded non-significant results. Thus, glucose levels were examined as a continuous variable in the present analysis. The American Diabetes Association (ADA) has determined that normal postprandial glucose levels range from 70 mg/dL to 139 mg/dL10 and marks 140 mg/dL as the start point for impaired glucose tolerance in the oral glucose tolerance test.11 Thus, after testing for an association between higher glucose levels and survival times and DFIs, the significant final models were used to compute the difference in hazard (i.e., the risk of death from disease, risk of disease recurrence) for a patient at 70 mg/dL versus a patient at 140 mg/dL, as representative data points for normoglycemia and hyperglycemia, respectively. As such, the hazard ratio (HR) for glucose in all analyses is based on an increase of 70 mg/dL in glucose level, in order to make these clinically relevant comparisons.
Survival Time and DFI
For the analysis of survival time, date of death and cause were ascertained from patient medical records. Information for seven patients was ascertained through state death records. Survival time was calculated as the number of days between date of tumor resection and date of death. Analysis of DFI was conducted on a subset of patients who achieved remission (n = 61). Date of recurrence was ascertained from medical records, and DFI was calculated as the number of days between date of tumor resection and date of recurrence.
Statistical Methods
Median survival time and median DFI for the whole test set were estimated using the Kaplan-Meier product limit method.12 Univariate associations between survival time, DFI, and glucose were examined using Cox proportional hazards regression models.13 These analyses examined glucose as a continuous variable, using an increment of 70 mg/dL to derive hazard ratios, and adjusted for time of blood draw to control for circadian effects on glucose levels.14
Univariate associations between survival time, DFI, and other covariates were examined; weight, standardized as body mass index (BMI); stage (advanced-stage: III, IV vs. early-stage: I, II), grade (high: 3 vs. low: 1, 2), and cytoreduction (suboptimal vs. optimal). Wald Chi-square P values were used to calculate univariate statistical significance, and 95% confidence intervals were estimated.
Survival time and DFI were then examined in a multivariate setting using a fitted Cox proportional hazards regression model for each outcome variable. The entry criterion for candidate covariates in this multivariate model was a univariate significance level of ≤ 0.25, and the staying requirement was ≤ 0.10. Standard diagnostics were utilized to evaluate model adequacy.15 These same procedures were used to analyze the independent validation set. SPSS 15.0 software was used, and P values ≤ 0.05 were considered significant in all analyses.
RESULTS
Of the 74 patients observed, the mean follow-up time was 2.01 years (range, 0.02–6.08). Eighty-six percent of patients were diagnosed with advanced-stage disease, and 69.5% had high grade tumor. Mean preoperative glucose level was 108 mg/dL (SD = 38.11 mg/dL) (Table 1). Twenty-nine patients were still alive at the end of the observation period and were censored for all survival time analyses. One patient died of causes unrelated to ovarian cancer and was also censored. For the remaining 44, cause of death was persistent or recurrent ovarian cancer, or complications associated with cancer disease and treatment (e.g., bowel obstruction, sepsis). Median survival time was 2.54 years; 95% CI, 1.60 to 3.49. A subset of patients (n = 61) achieved remission during the observation period. Of these, 38 experienced recurrence of ovarian cancer. The remaining 23 were censored for all DFI analyses. Median DFI was 1.19 years; 95% CI, 0.58 to 1.80.
As expected, advanced-stage disease was significantly associated with shorter survival time (HR, 10.79; 95% CI, 1.48 to 78.56; P = 0.02) and shorter DFI (HR, 7.63; 95% CI, 1.82 to 31.98; P = 0.005) in univariate analyses. Univariate analyses of BMI, grade, and cytoreduction revealed no significant associations with survival time (all P values > 0.17), which may be due to the relatively short follow-up time. There were no significant associations with DFI (all P values > 0.22) (Table 2).
TABLE 2.
Univariate Hazard Ratios for Survival Time and Disease Free Interval (DFI)
| Survival Time (N = 74) | DFI (N = 61) | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Glucose (per 70 mg/dL)† | 1.88 (1.00–3.73) | 0.05 | 1.63 (0.76–3.73) | 0.21 |
| Advanced Stage | 10.79 (1.48–78.56) | 0.02 | 7.63 (1.82–31.98) | 0.005 |
| High Grade | 1.42 (0.71–2.89) | 0.32 | 0.82 (0.41–1.63) | 0.58 |
| Suboptimal Cytoreduction | 1.54 (0.82–2.87) | 0.18 | 0.95 (0.47–1.91) | 0.89 |
| Body Mass Index | 1.01 (0.97–1.04) | 0.66 | 1.02 (0.98–1.06) | 0.29 |
Abbreviations: DFI, Disease Free Interval; HR, Hazard Ratio; CI, Confidence Interval.
Time of blood draw was statistically controlled when calculating glucose HR to account for circadian effects.
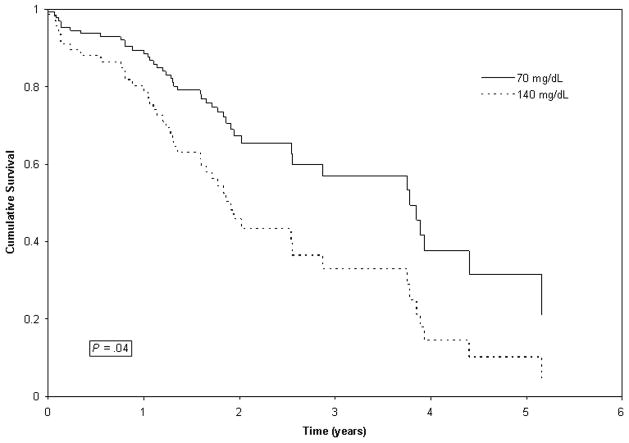
For survival time, univariate analysis of preoperative glucose levels revealed a significant association between higher glucose levels and shorter survival time (HR, 1.88; 95% CI, 1.00 to 3.73; P = 0.05), controlling for circadian effect on glucose (Table 2). In the multivariate analysis, this relationship was also observed (HR, 2.01; 95% CI, 1.00 to 3.73; P = 0.04), adjusting for stage of disease (Table 3). Thus, for every increase of 70 mg/dL of glucose, a patient was two times more likely to die of disease. Median survival time for a hyperglycemic patient at 140 mg/dL was 1.86 years versus 3.78 years for a normoglycemic patient at 70 mg/dL (Figure 1).
TABLE 3.
Multivariate-Adjusted Hazard Ratios of Glucose for Survival Time and Disease Free Interval (DFI)
| Survival Time (N = 74) | DFI (N = 61) | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Glucose (per 70 mg/dL) | 2.01 (1.00–3.73) | 0.04 | 2.32 (1.00–5.26) | 0.05 |
| Advanced Stage | 10.49 (1.44–76.62) | 0.02 | 9.04 (2.06–39.64) | 0.004 |
Abbreviations: DFI, Disease Free Interval; HR, Hazard Ratio; CI, Confidence Interval.
Final multivariate models for survival time and DFI included time of blood draw to control for circadian effect on glucose.
Figure 1.
Survival time for a hyperglycemic patient with a postprandial glucose level of 140 mg/dL versus a patient at the low end of the normal postprandial range with a glucose level of 70 mg/dL, adjusting for stage and time of blood draw. Cox regression indicates that patients with higher glucose levels before surgery had shorter survival times (P = 0.04).
For DFI, in the patients who achieved remission, univariate analysis of preoperative glucose levels showed no significant relationship with DFI (P = 0.21), adjusting for time of blood draw (Table 2). However, after adjusting for stage, higher glucose levels were significantly associated with shorter DFI (HR, 2.32; 95% CI, 1.00 to 5.26; P = 0.05) (Table 3). Thus, for every increase of 70 mg/dL of glucose, a patient in remission was 2.32 times more likely to experience a recurrence. Median DFI for a hyperglycemic patient at 140 mg/dL was 1.09 years versus 2.18 years for a normoglycemic patient at 70 mg/dL (Figure 2).
Figure 2.
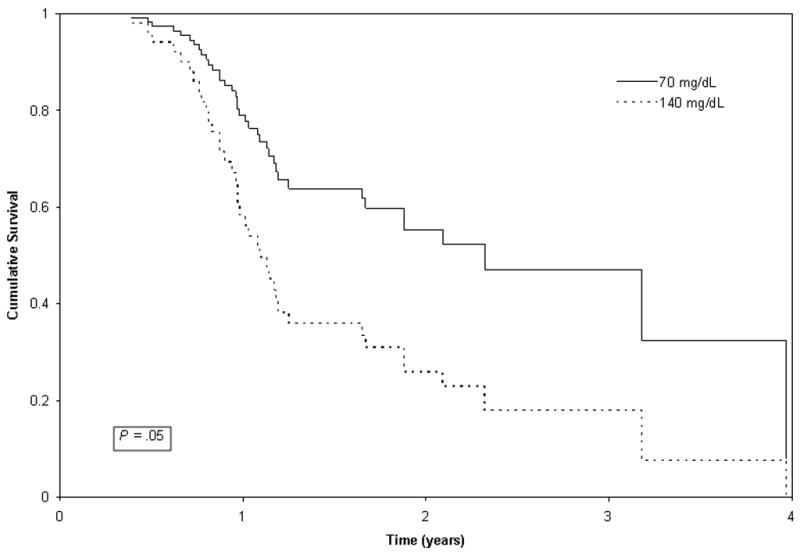
DFI for a hyperglycemic patient with a postprandial glucose of 140 mg/dL versus a patient at the low end of the normal postprandial range with a glucose level of 70 mg/dL, adjusting for stage and time of blood draw. Cox regression indicates that patients with higher glucose levels before surgery had shorter DFI (P = 0.05).
We next asked whether these associations would hold true in a separate and independent validation set. To answer this question, a validation set of 125 ovarian cancer patients, aged 31 to 87 (median = 61), from a different institution were examined. Among these patients, 91.2% had advanced-stage disease, and 90.4% had high grade tumor. The associations between pre-surgical glucose levels and survival time and DFI were confirmed in this validation set. Univariate analyses showed that higher glucose levels were significantly associated with shorter survival time (HR, 2.01; 95% CI, 1.07 to 3.49; P = 0.02) and shorter DFI (HR, 2.48; 95% CI, 1.42 to 4.28; P = 0.001) in the validation set. Thus, similar to the initial test set, for every increase of 70 mg/dL of glucose, patients in the validation set were two times as likely to die of disease, and, for those in remission, 2.48 times as likely to recur. Advanced-stage disease (HR, 9.40; 95% CI, 1.28 to 68.912; P = 0.03) and suboptimal cytoreduction (HR, 2.35; 95% CI, 1.32 to 4.18; P = 0.004) were also significantly associated with shorter survival time in univariate analyses of the validation set. Grade of disease and BMI were not associated with survival time in the validation set (all P values > 0.59). For univariate analyses of DFI in the validation set, all other clinical variables were non-significant (all P values > 0.14) (Table 4).
TABLE 4.
Validation Set Univariate Hazard Ratios for Survival Time and Disease Free Interval (DFI)
| Survival Time (N = 125) | DFI (N = 103) | |||
|---|---|---|---|---|
| HR (95% CI) | P | HR (95% CI) | P | |
| Glucose (per 70 mg/dL)† | 2.01 (1.07–3.49) | 0.02 | 2.48 (1.42–4.28) | 0.001 |
| Advanced Stage | 9.40 (1.28–68.91) | 0.03 | 1.64 (0.70–3.85) | 0.25 |
| High Grade | 1.27 (0.50–3.25) | 0.62 | 0.62 (0.28–1.35) | 0.22 |
| Suboptimal Cytoreduction | 2.35 (1.32–4.18) | 0.004 | 1.44 (0.88–2.34) | 0.14 |
| Body Mass Index | 1.02 (0.96–1.08) | 0.59 | 1.02 (0.97–1.06) | 0.50 |
Abbreviations: DFI, Disease Free Interval; HR, Hazard Ratio; CI, Confidence Interval.
Time of blood draw was statistically controlled when calculating glucose HR to account for circadian effects.
In the multivariate analysis of the validation set, after adjusting for other clinical variables, the relationship between higher glucose levels and DFI persisted (HR, 2.47; 95% CI, 1.42 to 4.28; P = 0.001). For survival time, the relationship was independent of BMI, stage, and grade but moved out of the range of significance (HR, 1.63; 95% CI, 0.99 to 2.84; P = 0.13) when cytoreduction was included in the model.
DISCUSSION
This clinical study is, to the best of our knowledge, the first to observe a relationship between higher pre-surgical glucose levels in ovarian cancer patients and decreased, disease-specific survival time and DFI. In the initial test set, after controlling for other clinical variables and circadian effects on glucose metabolism, the model showed that a hyperglycemic patient with a glucose level of 140 mg/dL at the time of pre-surgical consultation was twice as likely to die of disease during the observation period in comparison to a patient at the bottom of the normal range (70 mg/dL). For a patient who achieved remission, a glucose level of 140 mg/dL before surgery meant being more than two times as likely to recur in comparison to a patient at 70 mg/dL. These findings were further strengthened by separate analyses using an independent patient sample in our validation set, which confirmed the associations between glucose, survival time, and DFI.
These findings are consistent with other studies that have shown an inverse relationship between glucose levels and length of survival time in head and neck, stomach, and lung cancers, and acute lymphocytic leukemia.4,5,6 These findings are also consistent with the larger corpus of research that shows a positive relationship between glucose levels and incidence and mortality of many cancer types, including cancers of the liver, pancreas, colon, endometrium, and breast.16
Previous findings include both diabetic and non-diabetic patients. While our results are also based on both diabetic and non-diabetic patients, it is pertinent to note that preliminary analyses using diabetes diagnosis as a prognostic factor yielded non-significant results in our study. Although higher pre-surgical glucose levels were moderately correlated with diabetes status, the lack of association between that status and survival time and DFI may reflect good glucose control by those diabetic patients in the weeks and months following surgery. Thus, we also conducted a post hoc analysis to see if patients falling into the postprandial hyperglycemic range before surgery were driving the results. In this instance, patients with a postprandial glucose level of 140 mg/dL or higher were removed from the sample but similar significant results were still obtained.
The mounting evidence about glucose and cancer outcomes is not surprising given the phenomenon, first noted by Otto Warburg in 1930, that fully transformed cancer cells have an increased rate of glycolysis.17 These classical observations have stood the test of time, and it is commonly recognized today that increased glucose uptake and metabolism is a near-universal phenomenon in cancer cells, although the underlying mechanisms governing these metabolic abnormalities remain controversial.18,19,20 In addition to the previously mentioned findings on GLUT1 and ovarian carcinoma,8,9 recent research suggests that many tumors of epithelial origin rely on a second type of glucose transporter, sodium/glucose cotransporter 1 (SGLT1), to actively increase intracellular levels of glucose for glycolysis and cell survival. Surprisingly, this has been shown to be dependent on epidermal growth factor receptor (EGFR) but independent of its tyrosine kinase activity.21
In addition to increased glycolysis, it is recognized that a second major pathway for glucose metabolism, the pentose phosphate cycle, is also increased in neoplastic cells.19,20 Recent findings strongly suggest that cancer cells may increase glucose utilization to derive pyruvate and nicotinamide adenine dinucleotide phosphate in reduced form (NADPH) via glycolysis and pentose phosphate pathways, respectively, in order to detoxify excess hydroperoxides produced by aberrant oxidative metabolism.22,23,24,25,26
Thus, although the present prognostic findings do not demonstrate causality, the documented survival strategy of increased glucose metabolism by cancer cells, in order to compensate for an oxidative metabolic defect, suggests one mechanism by which plasma glucose levels may affect prognosis in cancer patients. Researchers have referred to this mechanism as a potential “Achilles heel” of cancer because it may represent a vulnerability in an otherwise impenetrable disease.27,28 For ovarian carcinoma and other specific malignancies that appear to show this vulnerability, further investigation into the role of diet and glycemic control medications is warranted and could potentially yield new adjunctive therapies for these diseases.
There are certain limitations to the current study that should be considered. The glucose levels were measured without regard to fasting, which introduces more variability. The degree to which this is a limitation is arguable based on studies that suggest postload plasma glucose level may be the more salient risk factor for cancer mortality than fasting glucose level29 and have confirmed it as a risk factor.30 Although the patients in this study most likely varied in the amount of glucose loading prior to testing, it is noteworthy that a prognostic relationship was detected in spite of this variability. Future prospective work is needed to examine non-fasting glucose levels more continuously in the months and years following surgery so that a patient’s mean plasma glucose concentration can be examined in relation to survival time and/or DFI. In this regard, use of the glycated hemoglobin assay, which provides an accurate assessment of average blood glucose over the previous two to three months, may provide information that is both simple and economical to obtain.7
In conclusion, this investigation provides evidence regarding the prognostic value of glucose levels in ovarian cancer patients and may have implications for the importance of glycemic control in patients with this disease.
Acknowledgments
Sources of Support: Supported in part by NIH grant nos. R21CA88293 and R01-CA104825 to Susan K. Lutgendorf, R01-CA100045 and P30-CA086862 to Douglas R. Spitz, LAF CF2002-0000832, K07-CA 093512 to Eileen H. Shinn, and CA110793, CA199298, and the University of Texas M. D. Anderson Ovarian Cancer Spore P50 CA083639 to Anil K. Sood.
We gratefully acknowledge the assistance of Heena Maiseri, Kelsey Flaten, and Christiana Taylor in data collection.
References
- 1.Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2008. CA Cancer J Clin. 2007;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 2.Chen T, Pengetnze Y, Taylor CC. Src inhibition enhances paclitaxel cytotoxicity in ovarian cancer cells by caspase-9-independent activation of caspase-3. Mol Cancer Ther. 2005;4:217–224. [PubMed] [Google Scholar]
- 3.Markmann S, Gerber B, Briese V. Prognostic value of Ca 125 levels during primary therapy. Anticancer Res. 2007;27:1837–1840. [PubMed] [Google Scholar]
- 4.Park SM, Lim MK, Shin SA, et al. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National health insurance corporation study. J Clin Oncol. 2006;24:5017–5024. doi: 10.1200/JCO.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- 5.Ali NA, O’Brien JM, Blum W, et al. Hyperglycemia in patients with acute myeloid leukemia is associated with increased hospital mortality. Cancer. 2007;110:96–102. doi: 10.1002/cncr.22777. [DOI] [PubMed] [Google Scholar]
- 6.Weiser MA, Cabanillas ME, Konopleva M, et al. Relation between the duration of remission and hyperglycemia during induction chemotherapy for acute lymphocytic leukemia with a hyperfractionated cyclophosphamide, vincristine, doxorubicin, and dexamethasone/methotrexate-cytarabine regimen. Cancer. 2004;100:1179–1185. doi: 10.1002/cncr.20071. [DOI] [PubMed] [Google Scholar]
- 7.Krone CA, Ely JTA. Controlling hyperglycemia as an adjunct to cancer therapy. Integr Cancer Ther. 2005;4:25–31. doi: 10.1177/1534735404274167. [DOI] [PubMed] [Google Scholar]
- 8.Rudlowski C, Moser M, Becker AJ, et al. GLUT1 mRNA and protein expression in ovarian borderline tumors and cancer. Oncology. 2004;66:404–410. doi: 10.1159/000079489. [DOI] [PubMed] [Google Scholar]
- 9.Cantuaria G, Fagotti A, Ferrandina G, et al. GLUT-1 expression in ovarian carcinoma. Cancer. 2001;92:1144–1150. doi: 10.1002/1097-0142(20010901)92:5<1144::aid-cncr1432>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Postprandial blood glucose. Diabetes Care. 2001;24:775–778. doi: 10.2337/diacare.24.4.775. [DOI] [PubMed] [Google Scholar]
- 11.American Diabetes Association. Standards of medical care in diabetes-2007. Diabetes Care. 2007;30(Supp1):S4–S41. doi: 10.2337/dc07-S004. [DOI] [PubMed] [Google Scholar]
- 12.Kaplan EL, Meier P. Nonparametric estimation from incomplete data. J Amer Stat Assc. 1958;53:457–481. [Google Scholar]
- 13.Cox DR. Regression models and life-tables. J R Stat Soc. 1972;34:187–202. [Google Scholar]
- 14.Van Cauter E, Polonsky KS, Scheen AJ. Roles of circadian rhythmicity and sleep in human glucose regulation. Endocr Rev. 1997;18:716–738. doi: 10.1210/edrv.18.5.0317. [DOI] [PubMed] [Google Scholar]
- 15.Singer JD, Willett JB. Applied Longitudinal Data Analysis - Modeling Change and Event Occurrence. New York: Oxford University Press; 2003. [Google Scholar]
- 16.Richardson LC, Pollack LA. Therapy insight: Influence of type 2 diabetes on the development, treatment, and outcomes of cancer. Nat Clin Pract Oncol. 2005;2:48–53. doi: 10.1038/ncponc0062. [DOI] [PubMed] [Google Scholar]
- 17.Warburg O. The Metabolism of Tumors. London: Constable Press; 1930. [Google Scholar]
- 18.Gatenby RA, Gawlinski ET. The glycolytic phenotype in carcinogenesis and tumor invasion: Insights through mathematical models. Cancer Res. 2003;63:3847–3854. [PubMed] [Google Scholar]
- 19.Weber G. The enzymology of cancer cells (second of two parts) N Eng J Med. 1977;296:541–551. doi: 10.1056/NEJM197703102961005. [DOI] [PubMed] [Google Scholar]
- 20.Maity A, Tuttle S. 2-Deoxyglucose and Radiosensitization: Teaching an Old DOG New Tricks? Cancer Biol Ther. 2006;5:824–826. doi: 10.4161/cbt.5.7.3024. [DOI] [PubMed] [Google Scholar]
- 21.Weihua Z, Tsan R, Huang W, et al. Survival of cancer cells is maintained by EGFR independent of its kinase activity. Cancer Cell. 2008;13:385–393. doi: 10.1016/j.ccr.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Spitz DR, Sim JE, Ridnour LA, et al. Glucose deprivation-induced oxidative stress in human tumor cells: a fundamental defect in metabolism? Ann NY Acad Sci. 2000;899:349–362. doi: 10.1111/j.1749-6632.2000.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 23.Ahmad IM, Aykin-Burns N, Sim JE, et al. Mitochondrial O2•− and H2O2 mediate glucose deprivation-induced cytotoxicity and oxidative stress in human cancer cells. J Biol Chem. 2005;280:4254–4263. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 24.Andringa KK, Coleman MC, Aykin-Burns N, et al. Inhibition of glutamate cysteine ligase (GCL) activity sensitizes human breast cancer cells to the toxicity of 2-deoxy-D-glucose. Cancer Res. 2006;66:1605–1610. doi: 10.1158/0008-5472.CAN-05-3462. [DOI] [PubMed] [Google Scholar]
- 25.Slane BG, Aykin-Burns N, Smith BJ, et al. Mutation of succinate dehydrogenase subunit C (SDHC) results in increased O2•−, oxidative stress, and genomic instability. Cancer Res. 2006;66:7615–7620. doi: 10.1158/0008-5472.CAN-06-0833. [DOI] [PubMed] [Google Scholar]
- 26.Simons AL, Ahmad IM, Mattson DM, et al. 2-Deoxy-D-glucose (2DG) combined with cisplatin enhances cytotoxicity via metabolic oxidative stress in human head and neck cancer cells. Cancer Res. 2007;67:3364–3370. doi: 10.1158/0008-5472.CAN-06-3717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fruehauf JP, Meyskens FL. Reactive oxygen species: A breath of life or death? Clin Cancer Res. 2007;13:789–794. doi: 10.1158/1078-0432.CCR-06-2082. [DOI] [PubMed] [Google Scholar]
- 28.Simons AL, Fath MA, Mattson DM, et al. Enhanced response of human head and neck cancer xenograft tumors to Cisplatin combined with 2-deoxy-D-glucose correlates with increased 18F-FDG uptake as determined by PET imaging. Int J Radiat Oncol Biol Phys. 2007;69:1220–1230. doi: 10.1016/j.ijrobp.2007.07.2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gapstur SM, Gann PH, Lowe W, et al. Abnormal glucose metabolism and pancreatic cancer mortality. JAMA. 2000;283:2552–2558. doi: 10.1001/jama.283.19.2552. [DOI] [PubMed] [Google Scholar]
- 30.Shaw JE, Hodge AM, de Courten M, et al. Isolated post-challenge hyperglycemia confirmed as a risk factor for mortality. Diabetologia. 1999;42:1050–1054. doi: 10.1007/s001250051269. [DOI] [PubMed] [Google Scholar]