Abstract
The alarm anti‐protease secretory leukocyte protease inhibitor (SLPI) is frequently overexpressed in ovarian cancer cells and has been proposed for inclusion in biomarker panels but function remains unclear. We hypothesized that SLPI overexpression promotes ovarian cancer growth and survival. Low SLPI‐expressing Hey‐A8 ovarian cancer cells were engineered to produce functional (WT) or protease inhibitor‐null (PI‐) mutant SLPI; lack of PI activity was confirmed by enzymatic assay. WT/SLPI and PI‐ mutants stimulated significant proliferation and survival of Hey‐A8 ovarian cancer cells under basal culture conditions (P ≤ 0.02), in soft agar colony number and size (P ≤ 0.05), and in anoikis resistance (P ≤ 0.005). SLPI protected the ovarian cancer survival factor, progranulin (PRGN), and HEY‐A8 cells from degradation and apoptosis due to neutrophil elastase. PI‐/SLPI cells had greater protective activity than WT/SLPI cells. HEY‐A8 murine xenografts revealed enhanced solid tumor formation, dissemination, and invasion in WT/SLPI and PI‐/SLPI mutants. Increased proliferation was demonstrated by Ki‐67 staining (P ≤ 0.02). Increased secreted PRGN was seen in culture and was also observed by immunohistochemistry in the SLPI transfectant xenografts. This study describes a PI‐independent function for SLPI in ovarian cancer growth and dissemination. (Cancer Sci 2009; 100: 434–440).
Abbreviations:
- SLPI
secretory leukocyte protease inhibitor
- pAb
polyclonal antibody
- CM
conditioned medium
- I.P.
immunoprecipitation
- hNE
human neutrophil elastase
- PI‐
protease‐inhibitor null
- PRGN
progranulin
- WT
wild type
Ovarian cancer remains the most lethal gynecologic cancer, with approximately 15 000 ovarian cancer‐related deaths estimated in the United States in 2008.( 1 ) Modest improvements in survival of ovarian cancer patients have been achieved over the last decade, driving a need for a better understanding of the mechanisms underlying ovarian cancer formation and progression. Secretory leukocyte protease inhibitor (SLPI) has been reported as one of the most commonly amplified genes in epithelial ovarian cancer,( 2 , 3 , 4 , 5 ) an observation that is explained in part by genomic amplification of the SLPI locus, 20q12–13.( 6 ) Significantly elevated serum concentrations of SLPI have been found in ovarian cancer patients compared to healthy women or women with benign ovarian cysts, leading to the inclusion of SLPI in some experimental ovarian cancer biomarker panels.( 7 )
Secretory leukocyte protease inhibitor is a secreted pleiotropic protein with tissue‐protective, regenerative, and anti‐inflammatory properties.( 8 ) It protects normal mucosal tissues against detrimental proteolytic actions of inflammatory serine proteases, such as neutrophil elastase, through direct inhibition of these enzymes.( 9 , 10 ) Evidence also points toward direct roles for SLPI in tumor development and progression.( 2 , 11 , 12 ) Some of these studies are conflicting depending upon the model used, suggesting context dependence for SLPI function. SLPI was reported to both stimulate and counteract tumor formation, invasion and/or metastasis of cancer cells.( 10 , 12 , 13 , 14 , 15 ) The role of the anti‐protease properties of SLPI in cancer remains under investigation.
Progranulin (PRGN) is a potent growth and survival factor for both normal and cancer cells.( 16 , 17 , 18 , 19 , 20 , 21 ) Mutations in PRGN are associated with frontotemporal dementia and other neuromuscular degenerative syndromes,( 22 , 23 ) consistent with our reported prosurvival role. We have shown that anti‐sense silencing of PRGN in Hey‐A8 ovarian cancer cells reduced in vitro proliferation rates and soft agar colonization.( 16 ) Treatment of ovarian cancer cell lines with neutralizing anti‐PRGN antibodies induced apoptosis shown by an increased percentage of cells in the sub‐GoG1 phase of the cell cycle, condensation of 4′,6‐diamidino‐2‐phenylindole (DAPI)‐stained nuclei, and intracellular cleavage of poly (ADP‐ribose) polymerase (PARP) and caspase 3.( 19 )
Zhu et al. demonstrated in a wound‐healing model that PRGN activity is protected by SLPI.( 24 ) They identified a protective role of SLPI in blocking elastase degradation of PRGN resulting in prevention of wound closure. This occurred through both direct inhibition of proteolytic activity and binding to and stabilization of PRGN through steric hindrance preventing access to elastase.( 24 ) We confirmed in vitro protection of PRGN by recombinant human SLPI resulting in ovarian cancer cell survival.( 10 ) We and Zhu et al. have shown direct binding of SLPI and PRGN. We now hypothesize SLPI will promote ovarian cancer malignant potential and aggressiveness. Low‐expressing HEY‐A8 cells were engineered to express wild‐type SLPI (WT/SLPI) or protease inhibition‐null mutant (PI‐/SLPI). These were used to demonstrate an in vitro and in vivo proliferative drive of SLPI, arguing a role for SLPI as an autocrine proliferative and malignancy factor in ovarian cancer.
Materials and Methods
Western blot, immunoprecipitation and antibodies. Anti‐HA antibody was from Sigma (St Louis, MO, US), anti‐PARP from Santa Cruz Biotechnology (Santa Cruz, CA, US), anti‐cleaved caspase 3 from Cell Signaling Technology (Danvers, MA, US), and anti‐Ki67 from Vector Laboratories (Burlingame, CA, US). Anti‐SLPI and anti‐PRGN pAbs have been reported.( 10 , 16 ) Cell lysis, immunoprecipitation, and immunoblot were performed using Ultralink Immobilized protein A/G beads from Pierce (Rockford, IL, US). All reagents were of molecular or cell culture grade.
Generation and stable transfection of HA‐tagged SLPI forms. We have used plasmids expressing wild‐type SLPI (WT/SLPI), and PI‐/SLPI mutants Leu72Phe (F‐SLPI) and Leu72Arg (R‐SLPI) as described,( 12 ) with addition of a genetically fused C‐terminal HA‐tag. The Leu72 mutation has been shown to cause attenuation of inhibitory activity toward elastase‐like serine proteases.( 9 ) Hey‐A8 cells, a generous gift of Dr G. Mills (M.D. Anderson Cancer Center, Houston, TX, US), were cultured as described.( 10 ) Cells were transfected in bulk using Fugene reagent (Roche Diagnostics, Indianapolis, IN, US). Transfectants were selected and maintained in complete medium containing 1 mg/mL G418 (Invitrogen, Carlsbad, CA, US).
Plasmin activity/activation assay. The protease inhibitory activity of SLPI forms was tested in a plasminogen cleavage assay. WT/SLPI and PI‐/SLPI cells were adhered overnight in complete medium, washed with phosphate‐buffered saline (PBS), and medium replaced with serum‐ and phenol red‐free culture medium (RPMI‐1640) containing 0.5 mg/mL plasminogen for 4 h at 37°C; plasminogen‐free conditioned medium (CM) served as the control. Plasmin activity in CM was measured using a modified Chromozym PL assay (Roche Applied, Manneheim, Germany). Data presented, mean ± SD, are representative of replicate experiments, read out at the 60‐min time point.
In vitro proliferation assay. Cells were plated in a monolayer for 72 h after which the WST‐1 reagent was added and the assay proceeded according to manufacturer's instructions (Roche Diagnostics, Indianapolis, IN, US). Experiments were done in triplicate and reported as mean ± SEM.
Analysis of anchorage‐independent growth. Cells were plated in triplicate in 24‐well plates at 5000 cells per well in 0.3% top agar containing RPMI, antibiotics, G418 and 10% serum, as described previously.( 16 ) After 2–3 weeks of culture, digital pictures of representative z‐planes were made using the Ultraphot Light microscope (Zeiss, Thornwood, NY, US) at 9.5 × magnification and the Dicomed software/hardware package from BetterLight (San Carlos, CA, US). Colony formation was evaluated by quantification of number and size of colonies using the ImageJ software (NIH, Bethesda, MD, US).
Anoikis resistance. Petri plastic was treated with poly‐hydroxyethylmethacrylate (poly‐HEMA) in ethanol at 5 mg/cm2 surface area and left to evaporate overnight. Serum‐starved cells were grown for 5 days on treated plastic in serum‐free medium after which( 1 ) proliferation was measured using the XTT assay (Roche Diagnostics, Indianapolis, IN, US), or( 2 ) cells were harvested, dissociated with Accumax (Innovative Cell Technologies, San Diego, CA, US) and analyzed by flow cytometry for cell cycle progression, as described.( 10 , 19 )
Cytostatic and cytotoxic activities of neutrophil elastase (hNE). Serum‐starved cells were treated for 24 or 48 h with recombinant hNE (Calbiochem, San Diego, CA, US) in serum‐free medium, as reported.( 10 ) Cells were analyzed in flow cytometry as reported( 10 ) and CM and lysates were analyzed by immunoblot.
Mouse xenografts. 106 transfectants were injected i.p. into 6–8‐week‐old female athymic Nu/Nu mice (Harlan, Indianapolis, IN, US) in a protocol approved by the NCI Animal Care and Use Committee. Animals were sacrificed humanely and necropsied 5 weeks post‐injection. Tumors and tissue samples from five randomly selected mice were fixed in neutral‐buffered formalin or snap frozen in Tissue‐Tek O.C.T. Compound (Sakura, Tokyo, Japan). Hematoxylin and eosin staining was performed on paraffin‐embedded sections to evaluate general morphology.
Immunohistochemistry. Formalin‐fixed paraffin embedded mouse xenograft tumor slices (5 µm) were processed according to standard immunohistochemistry (IHC) protocols with microwave antigen retrieval using Target Retrieval Solution citrate pH 6.0 (Dako North America, Carpentaria, CA, US) for 20 min followed by cooling to room temperature. Slides were incubated with either the anti‐Ki‐67 antibody and detected with biotinyland secondary with the Vectastain Elite ABC kit and diamino benzidine (DAB) substrate (Vector Laboratories, Burlingame, CA, US)( 16 ) or anti‐PRGN (Invitrogen; Carlsbad, CA, US) at 1:100 dilution for 1 h and stained using the Dako Envision + system (Dako North America, Carpentaria, CA, US). Color was developed with DAB solution and slides were counterstained with hematoxylin prior to mounting.
Statistical analysis. Statistical analyses were performed by the two‐tailed unpaired t‐test or Kruskal‐Wallace one‐way analysis of variance (ANOVA). All P‐values shown are compared to the mock control, unless otherwise indicated.
Results
PI‐/SLPI mutants cannot inhibit serine protease activity. Endogenous SLPI expression in HEY‐A8 cells is low, making it an ideal setting in which to evaluate the pro‐malignant behavior of SLPI and its PI‐ mutants. Hey‐A8 cells were stably transfected with WT/SLPI and both PI‐ mutant SLPI forms, F‐ and R‐SLPI. Secreted HA‐tagged SLPI is detected in both serum‐free CM and in stable bulk transfectant lysates (Fig. 1a). Successful abrogation of protease inhibitory activity was tested using the chromozym assay. Plasminogen is provided as the substrate from which to measure plasmin activation in the presence or absence of CM expressed from the control, WT/SLPI, or PI‐/SLPI expressing HEY‐A8 cells. F‐SLPI and control CM yielded the same active cleavage of plasminogen (Fig. 1b). WT/SLPI CM reduced plasmin activity 35% (P ≤ 0.0001), confirming the PI‐/SLPI transfectants had reduction of protease inhibitory activity. In order to demonstrate focal activity of this mutation, lack of inhibition of partner protein binding to PRGN was demonstrated. Co‐immunoprecipitation of PRGN by SLPI in CM of the bulk transfectants is shown in Fig. 1c. There is an increasing quantity of PRGN with overexpression of SLPI and a net reduction in immunocomplex when corrected for the increased PRGN.
Figure 1.
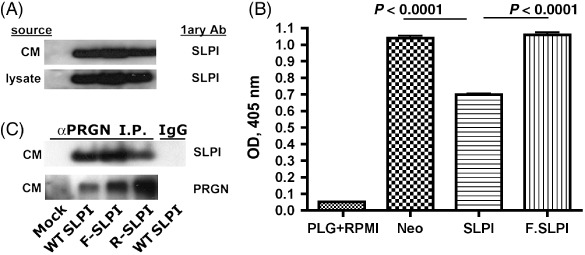
Secretory leukocyte protease inhibitor (SLPI) but not protease‐inhibitor null (PI‐) transfectants reduces plasmin activity. (A) HA‐tagged SLPI forms are found in Hey‐A8 transfectant lysates and conditioned medium (CM). (B) Wild type (WT)/SLPI but not mock or PI‐ cell CM reduce plasminogen cleavage (P ≤ 0.0001). (C) No difference between WT/SLPI and PI‐/SLPI cells in progranulin (PRGN) binding. Co‐immunoprecipitation of PRGN with SLPI is shown. An isotype‐matched polyclonal antibody (pAb) (IgG) confirmed specificity of immunoprecipitation (PI). F‐SLPI, PI‐/SLPI mutant Leu72Phe; R‐SLPI, PI‐/SLPI mutant Leu72Arg; PLG, Plasminogen.
Growth and colony formation is induced in SLPI transfectants independently of PI activity. Tumor proliferation is a net function of cell growth and death. Our previous findings indicated an anti‐apoptotic activity of SLPI. We now examined growth and transforming potential of PI‐ and WT/SLPI transfectants. A statistically significant increase in proliferation was observed in all transfectants (Fig. 2a; P ≤ 0.02), indicating that this property of SLPI is independent of its protease‐inhibitory activity. A marked difference was seen in soft agar (Fig. 2b) with all SLPI mutants forming colonies more rapidly than controls. Colonization was approximately 50% greater by colony size (Fig. 2c; P ≤ 0.05) with a growth advantage (Fig. 2d; P ≤ 0.001) for both WT and PI‐/SLPI mutants. Thus, SLPI promotes growth and the transforming potential of the ovarian cancer cell lines.
Figure 2.

Growth and colony‐formation is induced in secretory leukocyte protease inhibitor (SLPI) transfectants independently of protease inhibitor (PI) activity. (A, B) No difference in relative proliferation rates (A) or soft agar colonization (B) of stable transfectants between wild type (WT)/SLPI and the protease‐inhibitor null (PI‐) mutants (n = 5). (C, D) Similar growth advantage in soft agar was observed for WT/SLPI and PI‐ mutants. Number of colonies (C) and colony size (D) were quantitated using digital pictures taken at a 9.5 × magnification. Representative data of three experiments are shown. All P‐values are compared to the mock transfection control. F‐SLPI, PI‐/SLPI mutant Leu72Phe; R‐SLPI, PI‐/SLPI mutant Leu72Arg.
WT/SLPI and PI‐/SLPI overexpression protects from anoikis. Anoikis is defined as apoptosis induced by inadequate or inappropriate cell‐matrix interactions.( 25 ) Cancer cells spread and grow in suspension in the peritoneal cavity in the advanced stages of ovarian cancer progression. Therefore, cells need mechanisms to protect themselves against cell death triggered by the absence of adhesion signals. Parental and transfected Hey‐A8 cells were unable to attach to poly‐HEMA‐treated Petri plastic dishes. Serum‐free conditions were used to minimize exposure to lysophosphatidic acid, a strong survival and growth factor for ovarian cancer cells, and one that overcomes anoikis.( 26 ) Under these culture conditions, the majority of the cells appeared to die. However, after several days of growth, spheroids containing viable cells formed and expanded. Proliferation was equally and statistically significantly increased in WT/SLPI, and PI‐/SLPI mutants (P ≤ 0.005, Fig. 3a). The viability of the spheroid cells was reflected in their increased S‐phase fraction (Fig. 3b) and reduced percentage of apoptotic cells in the sub‐GoG1 region of the cell cycle (Fig. 3c). SLPI provided both survival and proliferation signals in absence of trophic extracellular matrix survival signals.
Figure 3.

Protection from anoikis for WT/secretory leukocyte protease inhibitor (SLPI) and protease‐inhibitor null (PI‐) mutants. (A) SLPI transfectants grew more successfully when cultured in serum‐free medium on poly‐hydroxyethylmethacrylate (poly‐HEMA)‐treated plastic. XTT assay was used to quantitate spheroid proliferation (n = 2). (B, C) Increased S phase and decreased sub GoG1 fraction in SLPI transfectants. The sub‐G0G1‐phase is used as a marker of apoptotic cells. Representative data of three experiments are shown. All P‐values are compared to the mock transfection control. F‐SLPI, PI‐/SLPI mutant Leu72Phe; R‐SLPI, PI‐/SLPI mutant Leu72Arg.
Growth and survival‐promoting activities of SLPI may be mediated in part through PRGN. One mechanism through which SLPI may promote net proliferation in ovarian cancer is protection of, or binding to the growth factor, PRGN. We showed previously that recombinant human SLPI protected PRGN from neutrophil elastase (hNE)‐induced degradation and that silencing of SLPI was associated with a loss of PRGN.( 10 ) This is supported by the protection of PRGN from hNE‐mediated degradation by overexpression of WT/SLPI and PI‐/SLPI (Fig. 4a). Elastase caused a G1 arrest followed by DNA fragmentation shown by increase in the sub G0G1 phase in mock transfected cells. This was progressively reduced with the WT/SLPI and PI‐/SLPI transfectants (Fig. 4b). PARP cleavage is nearly absent in SLPI transfectants (Fig. 4c) as is activation of caspase 3 with WT/SLPI and PI‐/SLPI cells (Fig. 4d). These protective effects of SLPI were independent of protease inhibition activity. These data suggest that SLPI may also function as a survival chaperone for PRGN, in addition to its enzymatic inhibition of PRGN degradation.
Figure 4.
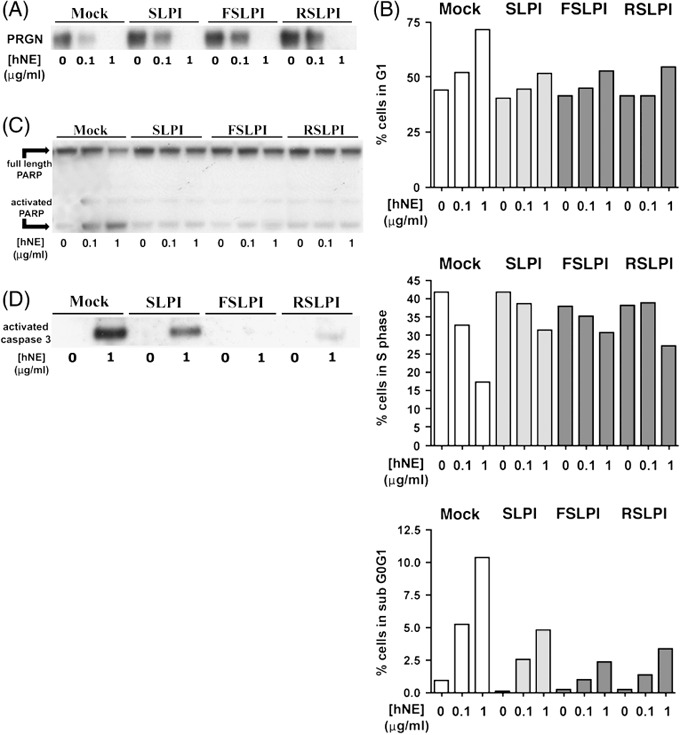
Growth and survival‐promoting secretory leukocyte protease inhibitor (SLPI) activity may be mediated in part through progranulin (PRGN). (A) Protection of PRGN from elastase‐mediated loss. (B) Elastase causes apoptosis that is abrogated by wild type (WT)/SLPI and protease‐inhibitor null (PI‐) mutants. Cell cycle measures of G1‐phase (top) and S phase (middle) were done in transfectants treated for 24 h with increasing concentrations of human neutrophil elastase (hNE) in serum‐free conditions. Those at 48 h measure percent of cells in subG0G1, (bottom) and demonstrate progressive loss of apoptosis in WT/SLPI and the PI‐/SLPI mutants (representative of n = 2). (C, D) Reduction of poly‐(ADP‐ribose) polymerase (PARP) (C) and caspase‐3 (D) cleavage in response to neutrophil elastase in WT/SLPI and PI‐ mutants (n = 2). Floating and adherent cells were collected for all analyses. F‐SLPI, PI‐/SLPI mutant Leu72Phe; R‐SLPI, PI‐/SLPI mutant Leu72Arg.
SLPI overexpression increases aggressiveness of Hey‐A8 tumor formation and dissemination. We have reported that SLPI expression promoted growth of mouse lung cancer cells in vivo that was dependent upon SLPI protease‐inhibitory activity.( 12 , 13 ) Ovarian cancer disseminates by shedding into the peritoneal cavity yielding peritoneal carcinomatosis and serosal spread, and ascites. The Hey‐A8 transfectant series were injected intraperitoneally into athymic nude mice in an orthotopic model of ovarian cancer. Tumor take was complete in all mice, but mice bearing any of the SLPI transfectants gained weight more rapidly than their mock transfectant counterparts, a surrogate measure for greater tumor burden (P ≤ 0.007 one‐way ANOVA at each time point; Fig. 5a). The macroscopic pattern of tumor formation in mice challenged with mock and SLPI transfectants differed dramatically. All mice had patches of small, soft and highly vascularized tumor nodules attached to the peritoneum (Fig. 5b left panel). Mice injected with WT/SLPI and PI‐/SLPI transfectants had greater omental and mesenteric disease (Fig. 5b middle) and hepatic serosal implants (Fig. 5b right). Pathologic review confirmed invasion into neighboring organs, such as duodenum, liver, spleen, pancreas, skeletal muscle, and lung, in WT/SLPI and PI‐/SLPI mice (Fig. 5c). A statistically significantly higher Ki‐67 proliferative index was demonstrated in all SLPI transfectants (Figs 5d,e, P ≤ 0.02). The pattern of Ki‐67 staining in the mock and WT/SLPI transfectants was peripheral, suggesting proliferation only at the leading edges, whereas, proliferating cells were found diffusely in the PI‐/SLPI mutants. Increased PRGN staining was observed in the WT/SLPI transfectants and was increased in the PI‐xenografts, notably in the invasive cells (Fig. 5f, note lymph node invasion in R‐SLPI). No difference in circulating vascular endothelial growth factor concentration was found between transfectant xenograft mouse groups. These findings indicate that the growth, survival, and anoikis effects of SLPI in vitro translated to more aggressive tumors in vivo with maintenance or augmented activity in the PI‐/SLPI cells.
Figure 5.

Secretory leukocyte protease inhibitor (SLPI) expression increases aggressiveness of Hey‐A8 orthotopic xenografts. (A) Wild type (WT)/SLPI and protease‐inhibitor null (PI‐)/SLPI transfectants induce greater tumor burden than mock transfected Hey‐A8 cells. Animal weight was used as a surrogate for tumor burden; animals were weighed at indicated times after inoculation and weight compared statistically across groups at each time point. Data presented are 10 animals/group from the second of 2 experiments. (B) Representative pictures of intraperitoneal disease. Peritoneal nodules were found in all inoculated mice (a). Solid omental and mesenteric disease (b) and macroscopic serosal liver nodules (c) were seen in the WT/SLPI and PI‐mutants. (C) Local invasion was seen with the PI‐/SLPI mutants (50 × magnification). (D, E) Higher proliferative index in WT/SLPI and PI‐/SLPI mutants. Ki‐67 staining of available sections from mock (n= 4) WT/SL Pl (n= 5), PI‐/SLPI mutants F‐SLPI (n= 5) and R‐SLPI (n= 5) tumors are shown, (representative stains), (D) and Ki‐67‐positive nuclei counted (E). At least 200 cells in three random fields were counted per tumor sections (P ≤ 0.02). (F) Increased progranulin (PRGN) is found in SLPI overexpressors. PRGN immunostain was done on available xenograft fixed sections and representative examples are shown. Note staining in invasive cells (arrows). F‐SLPI, PI‐/SLPI mutant Leu72Phe; R‐SLPI, PI‐/SLPI mutant Leu72Arg. H&E, hematoxylin and eosin.
Discussion
The biological consequence of SLPI amplification and overexpression in ovarian cancer is poorly understood. We examined this question through overexpression of SLPI and mutants lacking full protease inhibitory activity in HEY‐A8 human ovarian cancer cells with low endogenous SLPI expression. SLPI and its protease inhibitor null mutants stimulated growth and survival under both adherent and density‐independent conditions. Further, SLPI and its PI‐mutants protected the cells from anoikis. These markers of malignancy translated to enhanced aggressiveness in orthotopic xenografts, resulting in more serosal, omental, and mesenteric disease. Invasion of the serosal implants into organ parenchyma was absent in the mock transfectants at the time of experimental closure, yet progressively greater from WT/SLPI to the PI‐mutants. Where the PI‐mutants could not inhibit serine protease activity, they remained able to bind the SLPI partner protein and growth factor, PRGN.( 16 , 17 , 19 ) SLPI prevented degradation of PRGN and increased its net quantity in vitro and in xenografts, a potential mechanism through which SLPI functions. These cellular and PRGN‐protective effects of SLPI on ovarian cancer cells are independent of the ability of SLPI to inhibit elastase. We conclude that SLPI is not be simply a biomarker for presence of ovarian cancer, but its amplification and expression may be necessary, though not fully sufficient, for ovarian cancer growth and dissemination.
Secretory leukocyte protease inhibitor has been proposed as a potential biomarker for ovarian cancer diagnosis, because of its amplification and overexpression.( 2 , 3 , 4 , 5 , 7 ) Devoogdt and colleagues identified SLPI associated with increased tumorigenic and metastatic potential in a murine Lewis lung cancer model.( 2 , 12 ) SLPI overexpression in a poorly metastatic mouse mammary cancer cell line resulted in augmented growth and spontaneous metastases to lung in vivo.( 15 ) In contrast, Wang and coworkers found that SLPI overexpression in the same cell background attenuated host proinflammatory response and resulted in significantly fewer hepatic metastases.( 14 ) It is possible that different functions of SLPI may be dominant in different cancer microenvironments. Anti‐inflammatory activities of SLPI include reduction in monocyte production of proteases in response to LPS or concanavalin A stimulation,( 27 ) antimicrobial properties in mucosal fluids, and regulation of tumor growth factor (TGF)‐ß in wound healing.( 28 ) SLPI−/– mice showed impaired wound healing associated with increased inflammation and neutrophil elastase activity, and enhanced production of TGF‐ß. Our in vivo studies were done in nude mice where the immune system is attenuated and may allow the non‐inflammation inhibitory activity of SLPI to be more prominent.
Our findings of increased aggressiveness with increased SLPI are similar to those seen by Devoogdt in the syngeneic lung cancer model. There, SLPI was associated with increased tumorigenicity and pulmonary metastases. However, these effects were attenuated by mutation of Leu72 which abrogates the protease inhibitory activity. In contrast, our model showed equivalent proliferation in vitro and in vivo of WT and the PI‐/SLPI transfectants. There was increased aggressiveness in the form of the quality and quantity of metastases with the WT and PI‐/SLPI xenografts. Invasion of PI‐/SLPI tumors was more single‐cell and finger‐like; these cells also stained more strongly and more consistently for PRGN. Further, we found in vitro that the WT and PI‐/SLPI cells were more resistant to anoikis in vitro. Anoikis has been considered a key component of ovarian cancer dissemination. Shed ovarian cancer cells float in the peritoneum and move through the gutters until finding a point of secondary implantation and invasion. Cells must have an intrinsic anoikis survival capacity for successful shedding. Our findings suggest that SLPI may have a differential effect depending upon the cellular background, through the protease inhibition role, and as a putative chaperone and/or growth factor.
Secretory leukocyte protease inhibitor may function either through its protease inhibitory activity as previously reported, or as our current mutation studies show independently of that function, and therefore independently of elastase. The serine protease inhibitory activity has been reported as broader in outreach than regulation of elastase; our demonstration that SLPI is an inhibitor of plasmin serine protease function and overcome elastase apoptosis argues that SLPI may modify multiple serine protease events in ovarian cancer biology. SLPI may interact in a protease‐inhibitor independent fashion in ovarian cancer cells with growth‐stimulating signals, such as PRGN. Our neutralizing and silencing studies suggested SLPI may function with PRGN as a partner or chaperone.( 10 ) Partnering of SLPI and PRGN was reported by Zhu et al. who found this relationship stimulated wound‐healing.( 24 ) SLPI‐binding to PRGN prevented PRGN degradation by inflammatory elastase‐like proteases and restored the PRGN anti‐inflammatory and growth‐promoting properties. They suggested this occurs by direct inhibition of elastase enzymatic activity and also through stabilization of PRGN to prevent elastase access to PRGN cleavage sites. Our study confirms the indirect blocking role, showing the PI‐ mutants can partner with PRGN and also that they provide equal or greater protection of PRGN from elastase.
Progranulin itself is an important regulator of cell growth, survival and migration.( 16 , 17 , 18 , 24 ) We previously reported high PRGN expression in various ovarian cancer tumor specimens and cell lines,( 16 , 29 ) and we and others showed that antisense and antibody‐mediated blockade of PRGN in ovarian cancer cell lines reduced proliferation, agar colony formation, invasion, and triggered apoptosis.( 16 , 19 ) PRGN has been shown to contribute to transformation of ovarian surface epithelium (OSE) or uterine smooth muscle cells through activation of the Ras pathway.( 20 , 21 ) These studies underscore the pro‐survival function of PRGN, and through PRGN of its partner SLPI.
We thus advance our initial report of a functional role for amplified SLPI in ovarian cancer. The current findings coupled with activity of exogenously provided SLPI and apoptosis in response to neutralizing anti‐SLPI antibodies suggest that SLPI might be successfully abrogated using blocking approaches. SLPI should be targeted for development of molecular therapeutics for ovarian cancer.
Disclosure
None of the authors declare a conflict of interest.
Acknowledgments
This research was supported by the Intramural Research Program of the National Cancer Institute, Center for Cancer Research. N.D. was supported by Fonds voor Wetenschappelijk Onderzoek Vlaanderen.
References
- 1. Jemal A, Siegel R, Ward E et al . Cancer statistics, 2008. CA: a cancer journal for clinicians 2008; 58 (2): 71–96. [DOI] [PubMed] [Google Scholar]
- 2. Devoogdt N, Revets H, Ghassabeh GH, De Baetselier P. Secretory leukocyte protease inhibitor in cancer development. Ann N Y Acad Sci 2004; 1028: 380–9. [DOI] [PubMed] [Google Scholar]
- 3. Hough CD, Cho KR, Zonderman AB, Schwartz DR, Morin PJ. Coordinately up‐regulated genes in ovarian cancer. Cancer Res 2001; 61 (10): 3869–76. [PubMed] [Google Scholar]
- 4. Shigemasa K, Tanimoto H, Underwood LJ et al . Expression of the protease inhibitor antileukoprotease and the serine protease stratum corneum chymotryptic enzyme (SCCE) is coordinated in ovarian tumors. Int J Gynecol Cancer 2001; 11 (6): 454–61. [DOI] [PubMed] [Google Scholar]
- 5. Israeli O, Goldring‐Aviram A, Rienstein S et al . In silico chromosomal clustering of genes displaying altered expression patterns in ovarian cancer. Cancer Genet Cytogenet 2005; 160 (1): 35–42. [DOI] [PubMed] [Google Scholar]
- 6. Tanner MM, Grenman S, Koul A et al . Frequent amplification of chromosomal region 20q12–q13 in ovarian cancer. Clin Cancer Res 2000; 6 (5): 1833–9. [PubMed] [Google Scholar]
- 7. Tsukishiro S, Suzumori N, Nishikawa H, Arakawa A, Suzumori K. Use of serum secretory leukocyte protease inhibitor levels in patients to improve specificity of ovarian cancer diagnosis. Gynecol Oncol 2005; 96 (2): 516–9. [DOI] [PubMed] [Google Scholar]
- 8. Doumas S, Kolokotronis A, Stefanopoulos P. Anti‐inflammatory and antimicrobial roles of secretory leukocyte protease inhibitor. Infect Immun 2005; 73 (3): 1271–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Eisenberg SP, Hale KK, Heimdal P, Thompson RC. Location of the protease‐inhibitory region of secretory leukocyte protease inhibitor. J Biol Chem 1990; 265 (14): 7976–81. [PubMed] [Google Scholar]
- 10. Simpkins FA, Devoogdt NM, Rasool N et al . The alarm anti‐protease, secretory leukocyte protease inhibitor, is a proliferation and survival factor for ovarian cancer cells. Carcinogenesis 2008; 29 (3): 466–72. [DOI] [PubMed] [Google Scholar]
- 11. Bouchard D, Morisset D, Bourbonnais Y, Tremblay GM. Proteins with whey‐acidic‐protein motifs and cancer. Lancet Oncol 2006; 7 (2): 167–74. [DOI] [PubMed] [Google Scholar]
- 12. Devoogdt N, Hassanzadeh Ghassabeh G, Zhang J, Brys L, De Baetselier P, Revets H. Secretory leukocyte protease inhibitor promotes the tumorigenic and metastatic potential of cancer cells. Proc Natl Acad Sci USA 2003; 100 (10): 5778–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Devoogdt N, Revets H, Kindt A, Liu YQ, De Baetselier P, Ghassabeh GH. The tumor‐promoting effect of TNF‐{alpha} involves the induction of secretory leukocyte protease inhibitor. J Immunol 2006; 177 (11): 8046–52. [DOI] [PubMed] [Google Scholar]
- 14. Wang N, Thuraisingam T, Fallavollita L, Ding A, Radzioch D, Brodt P. The secretory leukocyte protease inhibitor is a type 1 insulin‐like growth factor receptor‐regulated protein that protects against liver metastasis by attenuating the host proinflammatory response. Cancer Res 2006; 66 (6): 3062–70. [DOI] [PubMed] [Google Scholar]
- 15. Sugino T, Yamaguchi T, Ogura G et al . The secretory leukocyte protease inhibitor (SLPI) suppresses cancer cell invasion but promotes blood‐borne metastasis via an invasion‐independent pathway. J Pathol 2007; 212 (2): 152–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Jones MB, Michener CM, Blanchette JO et al . The granulin‐epithelin precursor/PC‐cell‐derived growth factor is a growth factor for epithelial ovarian cancer. Clin Cancer Res 2003; 9 (1): 44–51. [PubMed] [Google Scholar]
- 17. Jones MB, Spooner M, Kohn EC. The granulin‐epithelin precursor: a putative new growth factor for ovarian cancer. Gynecol Oncol 2003; 88 (1 Part 2): S136–9. [DOI] [PubMed] [Google Scholar]
- 18. He Z, Ismail A, Kriazhev L, Sadvakassova G, Bateman A. Progranulin (PC‐cell‐derived growth factor/acrogranin) regulates invasion and cell survival. Cancer Res 2002 October 1; 62 (19): 5590–6. [PubMed] [Google Scholar]
- 19. Kamrava M, Simpkins F, Alejandro E, Michener C, Meltzer E, Kohn EC. Lysophosphatidic acid and endothelin‐induced proliferation of ovarian cancer cell lines is mitigated by neutralization of granulin‐epithelin precursor (GEP), a prosurvival factor for ovarian cancer. Oncogene 2005; 24 (47): 7084–93. [DOI] [PubMed] [Google Scholar]
- 20. Miyanishi M, Mandai M, Matsumura N et al . Immortalized Ovarian Surface Epithelial Cells Acquire Tumorigenicity Acrogranin Gene Overexpression Oncol Reports 2007; 17 (2): 329–33. [PubMed] [Google Scholar]
- 21. Matsumura N, Mandai M, Miyanishi M et al . Oncogenic property of acrogranin in human uterine leiomyosarcoma: direct evidence of genetic contribution in in vivo tumorigenesis. Clin Cancer Res 2006; 12 (5): 1402–11. [DOI] [PubMed] [Google Scholar]
- 22. Baker M, Mackenzie IR, Pickering‐Brown SM et al . Mutations in progranulin cause tau‐negative frontotemporal dementia linked to chromosome 17. Nature 2006; 442 (7105): 916–9. [DOI] [PubMed] [Google Scholar]
- 23. Van Damme P, Van Hoecke A, Lambrechts D et al . Progranulin functions as a neurotrophic factor to regulate neurite outgrowth and enhance neuronal survival. J Cell Bio 2008; 181 (1): 37–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhu J, Nathan C, Jin W, Sim D et al . Conversion of proepithelin to epithelins: roles of SLPI and elastase in host defense and wound repair. Cell 2002; 111 (6): 867–78. [DOI] [PubMed] [Google Scholar]
- 25. Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol 2001; 13 (5): 555–62. [DOI] [PubMed] [Google Scholar]
- 26. Eder AM, Sasagawa T, Mao M, Aoki J, Mills GB. Constitutive and lysophosphatidic acid (LPA) ‐induced LPA production: role of phospholipase D and phospholipase A2. Clin Cancer Res 2000; 6 (6): 2482–91. [PubMed] [Google Scholar]
- 27. Zhang Y, DeWitt DL, McNeely TB, Wahl SM, Wahl LM. Secretory leukocyte protease inhibitor suppresses the production of monocyte prostaglandin H synthase‐2, prostaglandin E2, and matrix metalloproteinases. J Clin Invest 1997; 99 (5): 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ashcroft GS, Lei K, Jin W et al . Secretory Leukocyte Protease Inhibitor Med Non-Redundant Functions Necessary for Normal Wound Healing Nat Med 2000; 6 (10): 1147–53. [DOI] [PubMed] [Google Scholar]
- 29. Davidson B, Alejandro E, Florenes VA et al . Granulin‐epithelin precursor is a novel prognostic marker in epithelial ovarian carcinoma. Cancer 2004; 100 (10): 2139–47. [DOI] [PubMed] [Google Scholar]


