Abstract
C-reactive protein (CRP), the prototypic marker of inflammation, is a cardiovascular risk marker and recent in vitro studies suggest that it may promote atherogenesis. CRP promotes oxidative stress in vitro and induces tissue factor (TF) release. However, there is a paucity of data examining the effects of CRP on oxidative stress and tissue factor procoagulant activity (PCA) in vivo. Thus, we tested the effects of CRP administration on superoxide anion release and tissue factor activity and examined mechanistic pathways using a rat sterile air pouch model. Intraperitoneal administration of CRP (20 mg/kg body weight) compared to human serum albumin (HuSA) increased superoxide anion release and tissue factor activity from peritoneal macrophages in vivo (p < 0.01). This was confirmed using intrapouch administration of CRP (25 μg/mL) compared to HuSA. Pretreatment with reactive oxygen species (ROS) scavengers or protein kinase C (PKC) inhibitor significantly abrogated CRP-induced superoxide anion release and tissue factor activity. Pretreatment with extracellular signal-regulated kinase (ERK) and Jun N-terminal kinase (JNK) inhibitors, but not p38 mitogen-activated protein kinase (p38MAPK) significantly decreased CRP-induced superoxide anion release from macrophages in vivo. CRP-induced tissue factor activity in vivo was abrogated by pretreatment with inhibitors to p38MAPK, JNK and NFκb (nuclear factor-κb), but not ERK. Antibodies to Fc gamma receptors, CD32 and CD64 resulted in significant reduction in CRP-induced superoxide and tissue factor activity in vivo. Thus, CRP appears to induce oxidative stress in vivo by stimulating NADPH oxidase via PKC, ERK and JNK phosphorylation, and induces tissue factor PCA in vivo via upregulation of PKC, p38MAPK, JNK, ROS and NFκb. CRP-induced ROS appears to precede tissue factor release. These effects are abrogated by blocking Fc gamma receptors, CD32 and CD64. This in vivo demonstration provides further evidence for a role for CRP in atherothrombosis.
Keywords: Macrophages, Oxidative stress, Rat, Mechanistic insights, Tissue factor, Procoagulant
1. Introduction
Increasing evidence supports the involvement of inflammation and oxidative stress in the pathogenesis of atherosclerosis [1,2]. C-reactive protein (CRP), the prototypic marker of inflammation, has been shown in numerous studies to predict cardiovascular events. In addition to being a risk marker, CRP by enhancing inflammation, oxidative stress and procoagulant activity (PCA), appears to mediate atherothrombosis [3].
CRP induces oxidative stress in vitro, i.e., superoxide production in various cells including endothelial cells, smooth muscle cells and monocyte–macrophages, which are involved in the process of atherosclerosis [4–8]. Furthermore, in situ hybridization revealed the presence of CRP mRNA in coronary vasculature and CRP was frequently colocalized with p22phox, an essential component of NADPH oxidase, which is an important source of reactive oxygen species (ROS) in vasculature [9]. Kobayashi et al. [9] showed that the incubation of cultured coronary artery smooth muscle cells with CRP resulted in enhanced p22phox protein expression and in the generation of intracellular ROS. We have previously shown, in vitro in human aortic endothelial cells (HAEC), that CRP stimulates superoxide anion release and results in increased nitration of prostaglandin synthase, resulting in decreased prostacyclin release [4]. We have recently demonstrated in vivo that CRP promotes oxidized LDL (Ox-LDL) uptake and matrix metalloproteinase-9 (MMP-9) release from rat macrophages [10]. Thus, in this study, we wished to examine the effect of CRP in vivo on superoxide anion release and examine plausible mechanisms.
Several studies indicate that CRP appears to promote a procoagulant diathesis as reviewed previously [3]. CRP upregulates tissue factor (TF) activity in monocytes and cultured vascular smooth muscle cells, CRP upregulates PAI-1 and decreases tPA, eNOS and prostacyclin in HAEC [3,5,11,12]. Furthermore, mice carrying the human CRP (HCRP) transgene exhibit accelerated thrombosis after vascular injury [13]. However, mice do not appear to be an appropriate model to test CRP’s effects in vivo and the data appear to be equivocal [3].
As reviewed previously [3], the rat appears to be the preferred model to test CRP’s effects due to the following reasons: in mice the main acute-phase reactant is serum amyloid P (SAP) component and serum amyloid A, not CRP. Human CRP is anti-inflammatory in mice and rat CRP shares 79% identity with human CRP. Griselli et al. [14] showed that parenteral injection of human CRP in experimental acute myocardial infarction produced by coronary artery ligation reproducibly enhanced infarct size by ~40%. Gill et al. [15] also showed that adult rats subjected to middle cerebral artery occlusion and then treated with human CRP similarly developed significantly larger cerebral infarcts compared with control animals receiving human serum albumin (HuSA). Human CRP activates complement in rats. Human CRP has been shown to be pro-inflammatory in the rat and increases inducible nitric oxide synthase expression in cytokine-stimulated rat cardiac myocytes, as well as inducible nitric oxide synthase in rat macrophages, and stimulates nuclear factor-κb (NFκb) in rat vascular smooth muscle cells [3,12,16,17]. Also, the Pepys group [18] has shown that a small molecule inhibitor (bis-phosphatidylcholine) to CRP prevents the increase in infarct size following coronary ligation. We recently showed that CRP induced Ox-LDL uptake and MMP-9 from rat macrophages in vivo using the sterile pouch model [10]. Thus, we chose this model to test CRP’s effects on tissue factor procoagulant activity in vivo.
In this report, we demonstrate for the first time that CRP induces superoxide anion release and tissue factor activity in rat macrophages in vivo.
2. Materials and methods
HCRP was purified from human ascitic/pleural fluids as described by Du Clos et al. [19] and had endotoxin levels <12.5 pg/mL as described previously [5,10]. Recently, we have shown that our in-house purified, dialyzed CRP mediates its inflammatory effects in TLR4 knocked down cells providing further cogent data that CRP-mediated effects are not due to endotoxin contamination [20].
Ficoll, SB203580 (a p38 mitogen-activated protein kinase (MAPK) inhibitor), PD 098059 (an extracellular signal-regulated kinase (ERK) 1/2 ERK inhibitor), caffeic acid phenethyl ester (CAPE) (an NFκb inhibitor), SP600125 (a Jun N-terminal kinase (JNK) inhibitor), apocynin, N-acetyl cysteine (NAC), diphenylene iodonium (DPI) and HuSA were from Sigma Chemical Company.
2.1. Animal treatment
Male hooded Wistar rats (weighing 125–150 g) were obtained from Charles River Lab. Animals were housed in a controlled environment at an ambient temperature of 21 ± 2 °C on a 12 h light:dark cycle. Food and water were provided ad libitum. The rats were acclimatized to animal housing conditions for 1 week. The protocol was approved by the Animal Committee of University of California at Davis. Two different routes of administration for HCRP or HuSA were used in vivo—(i) intraperitoneal model: CRP administered intraperitoneally and (ii) sterile pouch model: CRP administered in sterile air pouch. The pouch model was used mainly for mechanistic studies since the purification of CRP is time-consuming, laborious and very expensive and the sterile pouch model requires smaller amounts of protein [10].
2.2. Intraperitoneal model
HCRP/HuSA (20 mg/kg body weight for 3 days, n = 5 in each group) was injected in the peritoneal cavity of the rats. The rats were sacrificed on the 4th day by overdose of pentobarbital and peritoneal macrophages were isolated as described previously [10] and used for further analysis.
2.3. Sterile air pouch model
The sterile air pouches were formed on the dorsal surface of the rats as described previously [10,21]. The sterile air pouch model has been very recently published and validated by our group to examine pro-inflammatory effects of CRP in rat in vivo [10]. Briefly, under mild isofluorane sedation, the backs of Wistar rats were shaved and cleaned with alcohol swabs. At approximately 1.5 in. from mid neck 20 mL of sterile air attached to 23-G needle syringe was subcutaneously injected. HCRP/HuSA (25 μg/mL intrapouch) was directly injected into the pouch cavities of lightly restrained (hand-held) conscious animals on the 3rd day after the formation of pouch. For the mechanistic studies, the inhibitors/antibodies were injected into the pouch 2–4 h before injection of HCRP. The rats were euthanized on the following day by overdose of pentobarbital. The washouts of the pouch were made by deflating the pouch, irrigating with normal saline and its aspiration. The cells were pelleted by centrifugation and supernatants were used as pouch exudates.
2.4. Superoxide anion release
Superoxide anion release from peritoneal and pouch macrophages was assessed by acetylated ferricytochrome C reduction as described previously [4]. Also, superoxide release was assessed by dihydroethidium (DHE) fluorescence [4,5]. The results were standardized to milligram protein content.
2.5. Tissue factor activity
Peritoneal/pouch macrophages were stored in octylpyranoside to measure tissue factor activity by the method of Pendurthi and Rao [22]. Lysates in RIPA buffer were placed in a 96-well plate and 10 nM of FVIIa was added, after 5 min, factor X (175 nM) was added and incubated for 15 min at 37 °C. To measure Xa generation, 25 μL of S-2765 was added to each well and absorbance measured for 2 min at 405 nm. TF activity was calculated using relipidated TF as standard in the assay and standardized to milligram protein content.
2.6. Mechanistic studies for CRP-induced superoxide anion release and tissue factor activity
These experiments were performed using the pouch model. The rats were divided into different groups (n = 4 rats per group): (1) HuSA; (2) HCRP; (3) SB203580 (1 mg/kg bw) + HCRP; (4) PD 098059 (0.5 mg/kg bw) + HCRP; (5) SP600125 (1 mg/kg bw) + HCRP; (6) CAPE (0.2 mg/kg bw) + HCRP; (7) apocynin (100 μg/mL) + HCRP; (8) calphostin C (10 μM) + HCRP; (9) diphenylene iodonium (DPI, 10 μM) + HCRP and (10) NAC (10 μg/mL) + HCRP. On the 3rd day after the formation of the pouches, various inhibitors were injected in the pouches at specific concentrations as described in literature for in vivo use [10]. HuSA or HCRP was injected 2 h after the injection of inhibitors followed by euthanization of rats after 12–16 h. The pouch exudates and cells were obtained for measurement of super-oxide release and tissue factor activity.
2.7. Involvement of the receptor type in CRP-mediated superoxide anion release and tissue factor activity
The pouch model was used to explore the specific receptor type involved. Blocking antibodies (10 μg/mL intrapouch) to CD32, CD64, CD16 or irrelevant IgG either alone or their combinations (CD32 + CD64) were injected in the rats (3 per group) pouches 2 h prior to HCRP injection. Rats were euthanized 12–16 h later as described [10] and pouch macrophages and exudates were collected for measurement of superoxide release and tissue factor activity.
2.8. Statistical analysis
All experiments were performed at least three times in duplicate. The comparisons between group means were analyzed using ANOVA. The experimental results are presented as the mean ± S.D. Paired t-tests were used to compute differences in the variables, and the level of significance was set at p < 0.05.
3. Results
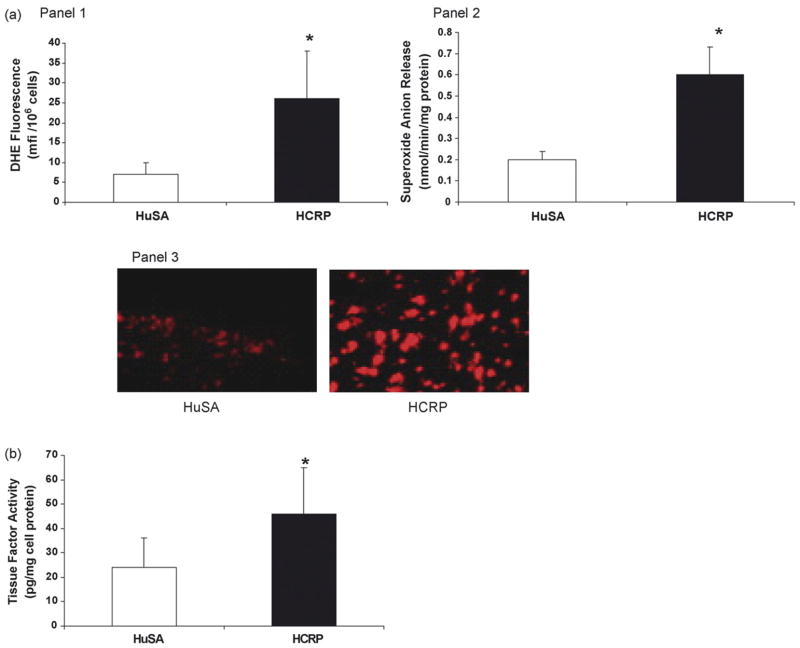
Intraperitoneal administration of human CRP (20 mg/kg) compared to HuSA stimulated superoxide anion release (Fig. 1a) and tissue factor activity from peritoneal macrophages in vivo (p < 0.01) (Fig. 1b). This was also confirmed using intrapouch administration of CRP (25 μg/mL) compared to HuSA (Fig. 2a and b).
Fig. 1.
(a) Effect of CRP on superoxide anion release in rat macrophages: HCRP/HuSA (20 mg/kg bw for 3 days, n = 5 in each group) was injected in the peritoneal cavity of the rats. The rats were sacrificed on the 4th day and peritoneal macrophages were isolated. Superoxide anion release was assessed by DHE fluorescence (Panel 1) and ferricytochrome C reduction (Panel 2) as depicted in Section 2. Panel 3 is a representative picture of macrophages loaded with DHE. The results are mean ± S.D. of five different experiments and are expressed as per mg cell protein. *p < 0.01 compared to HuSA. (b) Effect of CRP on tissue factor activity in rat macrophages: HCRP/HuSA (20 mg/kg bw for 3 days, n = 5 in each group) was injected in the peritoneal cavity of the rats. The rats were sacrificed on the 4th day and peritoneal macrophages were isolated. Tissue factor activity was assessed as described in Section 2. *p < 0.05 compared to HuSA.
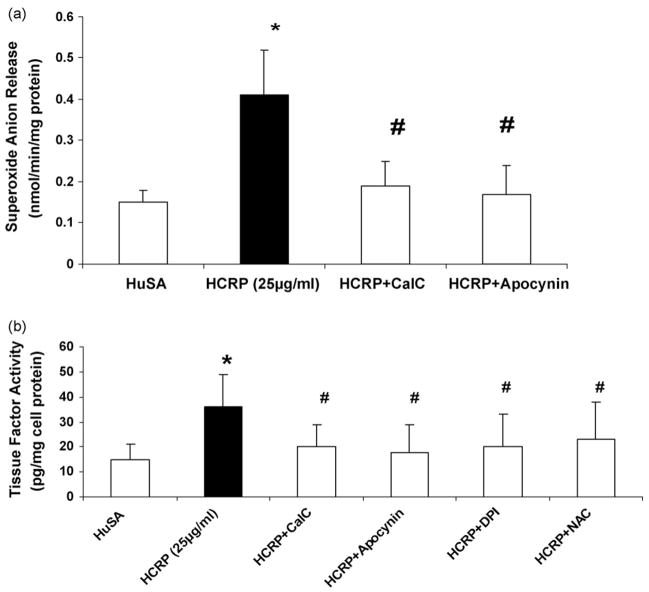
Fig. 2.
(a) Effect of inhibitors to PKC (10 μM calphostin C), NADPH oxidase (100 μg/mL apocynin), on superoxide anion release and (b) effect of inhibitors to PKC (10 μM calphostin C), ROS scavengers (NAC, 10 μg/mL; DPI, 10 μM; apocynin, 100 μg/mL), on tissue factor activity in pouch macrophages. Inhibitors were injected into sterile air pouches of Wistar rats (n = 3 in each group) at least 2 h prior to administration of HCRP (25 μg/mL) or HuSA and superoxide and tissue factor were assessed as described in Section 2. The results are mean ± S.D. *p < 0.05 compared to HuSA and #p < 0.05 compared to HCRP.
3.1. Mechanistic insights for CRP-mediated superoxide release and tissue factor in vivo
Since protein kinase C (PKC) drives superoxide release from monocytes via activation of NADPH oxidase [23], we examined the effect of PKC and NADPH oxidase inhibitors on CRP-induced superoxide anion release as well as tissue factor activity in vivo.
Pretreatment with the PKC inhibitor, calphostin C, and the NADPH oxidase inhibitor, apocynin, significantly abrogated CRP-induced superoxide anion release (Fig. 2a). To examine if ROS and PKC upregulation affect CRP-induced tissue factor activity in vivo, we examined three ROS scavengers, NAC, DPI, apocynin (NADPH oxidase inhibitor) and calphostin C (PKC inhibitor). Inhibitors of PKC and ROS significantly abrogated CRP-induced tissue factor activity in vivo (Fig. 2b).
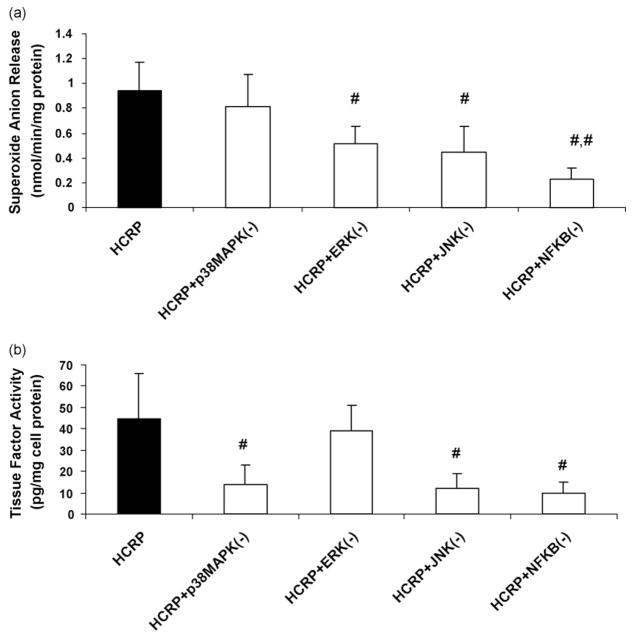
Furthermore, we have shown previously that CRP-induced MMP-9 in rat macrophages in vivo is abrogated by MAPK inhibitors [10]. In this study, pretreatment with ERK and JNK inhibitors and NFκb inhibitor, but not p38MAPK significantly decreased CRP-induced superoxide anion release from pouch macrophages in vivo (Fig. 3a). With regards to CRP-induced tissue factor activity in vivo, this was abrogated by pretreatment with inhibitors to p38MAPK, JNK and NFκb, but not by the ERK inhibitor (Fig. 3b).
Fig. 3.
Effect of inhibitors to MAPK and NFκb on (a) superoxide anion release and (b) tissue factor activity in pouch macrophages. Inhibitors to p38MAPK, ERK, JNK or NFκb were injected into sterile air pouches of Wistar rats (n = 3 in each group) at least 2 h prior to administration of HCRP (25 μg/mL) or HuSA and superoxide and tissue factor were assessed as described in Section 2. The results are mean ± S.D. #p < 0.05 compared to CRP.
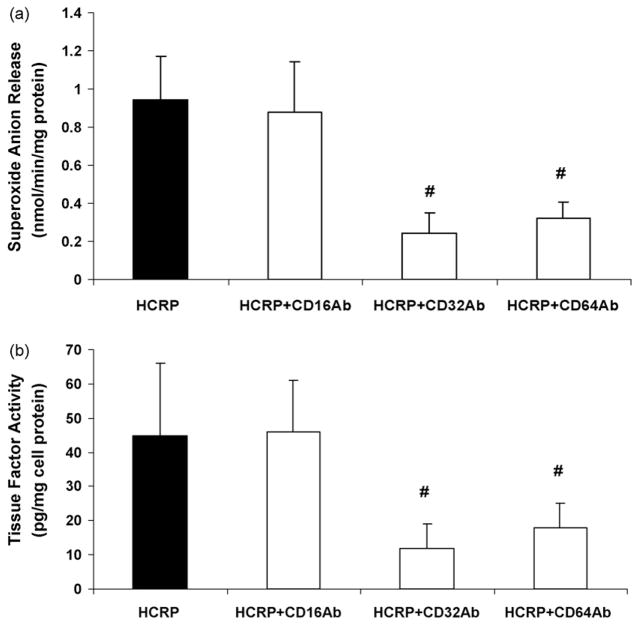
Previously, CRP has been shown to mediate its biological effects in endothelial cells and monocyte–macrophages via CD32 and CD64 [3–5,10–12,24–28]. Thus, we examined the effects of antibodies to CD16, CD32 and CD64 on CRP-induced superoxide anion release and tissue factor activity. We demonstrate that antibodies to Fc gamma receptors, CD32 and CD64, but not CD16 resulted in significant reduction in CRP-induced superoxide and tissue factor activity in vivo (Fig. 4a and b).
Fig. 4.
Effect of pretreatment with antibodies (10 μg/mL each separately) to Fc gamma receptors; CD32, CD64, CD16 on (a) superoxide anion release and (b) tissue factor activity in pouch macrophages as described in Section 2. Antibodies to CD16, CD32 and CD64 were injected into sterile air pouches of Wistar rats (n = 3 in each group) at least 2 h prior to administration of HCRP (25 μg/mL) or HuSA and superoxide and tissue factor were assessed as described in Section 2. The results are mean ± S.D. #p < 0.05 compared to CRP.
4. Discussion
In this paper, we demonstrate that CRP appears to induce oxidative stress in vivo via upregulation of PKC, NADPH oxidase, ERK and JNK phosphorylation, while CRP also induces tissue factor procoagulant activity in vivo via upregulation of PKC, NADPH oxidase, ROS, p38MAPK, JNK and NFκb. These effects are abrogated by blocking Fc gamma receptors, CD32 and CD64.
In the present study, CRP induced the release of super-oxide anion and tissue factor from pouch macrophages at a concentration of 25 μg/mL. Importantly, Ridker et al. [29] have shown that CRP levels >20 μg/mL predict future cardiovascular events. Also, several investigators have reported that CRP concentrations in the setting of acute coronary syndromes range up to 50 μg/mL [30,31]. Thus, the levels of CRP shown to induce superoxide/tissue factor release in vivo in this study can clearly be attained in patients.
With regards to superoxide anion release, several investigators have demonstrated that CRP induces superoxide anion release from cells in vitro. Prasad [32] have shown that in neutrophils, CRP significantly and dose-dependently induces superoxide anion release in vitro, however, no mechanistic studies were undertaken. Exposure of rat mesangial cells to CRP for 60 min led to a dose-dependent increase in superoxide anion release that was nearly threefold greater than that in the control conditions (p < 0.0001) [33]. They also showed that co-incubation with DPI (10 μM) prevented the increase in superoxide production by rat mesangial cells induced by CRP (100 μg/mL). While they used high concentrations of CRP in their in vitro studies, we show that at lower concentrations of CRP (25 μg/mL) in vivo, CRP induces superoxide anion release and that this is attenuated by inhibition of PKC and apocynin, a specific inhibitor of NADPH oxidase. Previously, we showed that CRP promotes superoxide anion release from HAEC and induces eNOS uncoupling and this is inhibited by siRNA to p22phox and p47phox [5]. Also, Kobayashi et al. [9] have shown that CRP in lesions colocalizes with p22phox, another component of NADPH oxidase, and this is in support of our findings. Furthermore, Qamirani et al. [7] showed that in porcine coronary arterioles treated with CRP (7 μg/mL; 1 h), using DHE staining that CRP produced SB203850- and TEMPOL-sensitive superoxide production in the arteriolar endothelium. CRP treatment of coronary arterioles significantly increased NAD(P)H oxidase activity and this was mediated via p38MAPK activation. In this report, in rat pouch macrophages, we demonstrate that CRP’s in vivo effects on superoxide anion release were inhibited via inhibition of ERK, but not p38MAPK.
With regards to tissue factor release, one of the first groups that demonstrated that human CRP-induced tissue factor release in vitro was Cermak et al. [34]. Cermak showed that CRP (10–100 μg/mL) induced a 75-fold increase in TF PCA of human peripheral blood mononuclear cells, with a parallel increase in TF antigen levels. Later studies have shown that peripheral blood mononuclear cells also respond in a similar fashion to CRP [35]. Previously, CRP has been shown to induce procoagulant activity as observed by increased endothelial PAI-1 and decreased tPA [3]. Cirillo et al. [36] demonstrated that incubation of endothelial cells and smooth muscle cells with CRP resulted in a dose-dependent activation of cell proliferation, which was mediated by activation of the ERK 1/2 pathway. In addition, CRP also induced TF expression in both cell types in a dose-dependent fashion, exerting its effect at the transcriptional level, as demonstrated by semiquantitative and by real-time PCR. Activation of the transcription factor, NFκb, by CRP was demonstrated by EMSA and they also demonstrated suppression of TF expression by the NFκb inhibitor, pyrrolidine-dithio-carbamate ammonium [12,36]. In rat pouch macrophages, in vivo, we show for the first time that CRP-induced TF is inhibited by inhibitors to p38MAPK and JNK, but not ERK inhibitor. Furthermore, we have previously shown in vivo that CRP augments NFκb activity in rat pouch macrophages. In this paper, in support of the work of Cirillo et al. [36], we show that inhibition of NFκb activity with CAPE, resulted in significant inhibition of CRP-induced TF activity in vivo. Furthermore, using three scavengers of ROS, we demonstrate a link between CRP-induced superoxide release and TF activity, since all three inhibitors significantly abrogate CRP-induced TF activity. Mechanisms by which increased oxidative stress results in increased CRP-induced TF activity in vivo will be explored in future studies. Also, we have previously shown that CRP upregulates pro-inflammatory cytokines in vivo [10]. In future studies, we will examine the autocrine/paracrine contribution of these cytokines to CRP-induced superoxide and tissue factor activity in vivo.
Immunocytochemical detection of Fc gamma (Fcγ) receptors in human atherosclerotic lesions has been demonstrated [37]. The activation of FcγRs generates oxygen radicals by phagocytes and immune cells. Ryu et al. [6] have demonstrated that FcγRIIa exclusively mediates CRP-induced intracellular ROS generation by human vascular smooth muscle cells [6]. Also, they showed that CRP-induced FcγRIIa activation modulates activation of AP-1 and NFκb and p22phox and inhibition of p22phox abrogates ROS in vascular smooth muscle cells in vitro. We have previously shown that blocking of CD32/CD64 in endothelial cells abrogate effects of CRP [5,10,11,24,28]. Also, recently we showed that CRP induces superoxide anion release in endothelial cells and promotes eNOS uncoupling, this is reversed by inhibition of CD32 and CD64 [5]. In addition, in human monocytes and U937 histiocytes, CRP has been shown to induce CCR2 expression via CD64 [26] and matrix met-alloproteinase (MMP)-1 expression via CD32, respectively. In neutrophils, CRP-mediated effects have been reported to be via CD16. Furthermore, the Fay group recently reported CRP-mediated TF release appears to be via CD16 in vascular smooth muscle cells in vitro [12]. In this paper, we report that CRP-mediated superoxide anion release and tissue factor activity are abrogated by inhibition of CD32 and CD64, with no effect of CD16 inhibition in rat pouch macrophages in vivo.
To conclude, in the present study we make the novel observation that CRP induces superoxide anion release and tissue factor activity in vivo using the rat sterile air pouch model and that superoxide release appears to precede tissue factor release. These data provide further evidence for the role of CRP in promoting atherothrombosis.
Acknowledgments
This work was supported by National Institutes of Health Grant RO1 HL074360 as well as NIHK24 AT00596 to I. Jialal.
References
- 1.Libby P. Inflammatory mechanisms: the molecular basis of inflammation and disease. Nutr Rev. 2007;65:S140–6. doi: 10.1111/j.1753-4887.2007.tb00352.x. [DOI] [PubMed] [Google Scholar]
- 2.Stocker R, Keaney JF., Jr New insights on oxidative stress in the artery wall. J Thromb Haemost. 2005;3:1825–34. doi: 10.1111/j.1538-7836.2005.01370.x. [DOI] [PubMed] [Google Scholar]
- 3.Verma S, Devaraj S, Jialal I. Is C-reactive protein an innocent bystander or proatherogenic culprit? C-reactive protein promotes atherothrombosis. Circulation. 2006;113:2135–50. [PubMed] [Google Scholar]
- 4.Venugopal SK, Devaraj S, Jialal I. C-reactive protein decreases prostacyclin release from human aortic endothelial cells. Circulation. 2003;108:1676–8. doi: 10.1161/01.CIR.0000094736.10595.A1. [DOI] [PubMed] [Google Scholar]
- 5.Singh U, Devaraj S, Vasquez-Vivar J, Jialal I. C-reactive protein decreases endothelial nitric oxide synthase activity via uncoupling. J Mol Cell Cardiol. 2007;43:780–91. doi: 10.1016/j.yjmcc.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryu J, Lee CW, Shin JA, et al. FcgammaRIIa mediates C-reactive protein-induced inflammatory responses of human vascular smooth muscle cells by activating NADPH oxidase 4. Cardiovasc Res. 2007;75:555–65. doi: 10.1016/j.cardiores.2007.04.027. [DOI] [PubMed] [Google Scholar]
- 7.Qamirani E, Ren Y, Kuo L, Hein TW. CRP inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2005;25:995–1001. doi: 10.1161/01.ATV.0000159890.10526.1e. [DOI] [PubMed] [Google Scholar]
- 8.Zeller JM, Sullivan BL. C-reactive protein selectively enhances the intracellular generation of reactive oxygen products by IgG-stimulated monocytes and neutrophils. J Leukoc Biol. 1992;52:449–55. doi: 10.1002/jlb.52.4.449. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi S, Inoue N, Ohashi Y, et al. Interaction of oxidative stress and inflammatory response in coronary plaque instability: important role of C-reactive protein. Arterioscler Thromb Vasc Biol. 2003;23:1398–404. doi: 10.1161/01.ATV.0000081637.36475.BC. [DOI] [PubMed] [Google Scholar]
- 10.Singh U, Dasu MR, Yancey PG, et al. Human C-reactive protein promotes oxidized low-density lipoprotein uptake and matrix metalloproteinase-9 release in Wistar rats. J Lipid Res. 2008;49:1015–23. doi: 10.1194/jlr.M700535-JLR200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Devaraj S, Xu DY, Jialal I. C-reactive protein increases plasminogen activator inhibitor-1 expression and activity in human aortic endothelial cells: implications for the metabolic syndrome and atherothrombosis. Circulation. 2003;107:398–404. doi: 10.1161/01.cir.0000052617.91920.fd. [DOI] [PubMed] [Google Scholar]
- 12.Wu J, Stevenson MJ, Brown JM, et al. C-reactive protein enhances tissue factor expression by vascular smooth muscle cells: mechanisms and in vivo significance. Arterioscler Thromb Vasc Biol. 2008;28:698–704. doi: 10.1161/ATVBAHA.107.160903. [DOI] [PubMed] [Google Scholar]
- 13.Danenberg HD, Szalai AJ, Swaminathan RV, et al. Increased thrombosis after arterial injury in human C-reactive protein-transgenic mice. Circulation. 2003;108:512–5. doi: 10.1161/01.CIR.0000085568.13915.1E. [DOI] [PubMed] [Google Scholar]
- 14.Griselli M, Herbert J, Hutchinson WL, et al. C-reactive protein and complement are important mediators of tissue damage in acute myocardial infarction. J Exp Med. 1999;190:1733–40. doi: 10.1084/jem.190.12.1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gill R, Kemp JA, Sabin C, Pepys MB. Human C-reactive protein increases cerebral infarct size after middle cerebral artery occlusion in adult rats. J Cereb Blood Flow Metab. 2004;24:1214–8. doi: 10.1097/01.WCB.0000136517.61642.99. [DOI] [PubMed] [Google Scholar]
- 16.Ikeda U, Maeda Y, Yamamoto K, Shimada K. C-reactive protein augments inducible nitric oxide synthase expression in cytokine-stimulated cardiac myocytes. Cardiovasc Res. 2002;56:86–92. doi: 10.1016/s0008-6363(02)00496-0. [DOI] [PubMed] [Google Scholar]
- 17.Hattori Y, Matsumura M, Kasai K. Vascular smooth muscle cell activation by C-reactive protein. Cardiovasc Res. 2003;58:186–95. doi: 10.1016/s0008-6363(02)00855-6. [DOI] [PubMed] [Google Scholar]
- 18.Pepys MB, Hirschfield GM, Tennent GA, et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature. 2006;440:1217–21. doi: 10.1038/nature04672. [DOI] [PubMed] [Google Scholar]
- 19.Du Clos TW, Zlock LT, Marnell L. Definition of a C-reactive protein binding determinant on histones. J Biol Chem. 1991;266:2167–71. [PubMed] [Google Scholar]
- 20.Dasu MR, Devaraj S, Du Clos TW, Jialal I. The biological effects of CRP are not attributable to endotoxin contamination: evidence from TLR4 knockdown human aortic endothelial cells. J Lipid Res. 2007;48:509–12. doi: 10.1194/jlr.C600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Davies DE, Stevens AJ, Houston JB. Use of the rat air pouch model of inflammation to evaluate regional drug delivery. Agents Actions. 1992 Spec No.: C109–11. [PubMed] [Google Scholar]
- 22.Pendurthi UR, Rao LV. Effect of wine phenolics and stilbene analogues on tissue factor expression in endothelial cells. Thromb Res. 2002;106:205–11. doi: 10.1016/s0049-3848(02)00143-3. [DOI] [PubMed] [Google Scholar]
- 23.Venugopal SK, Devaraj S, Yang T, Jialal I. Alpha-tocopherol decreases superoxide anion release in human monocytes under hyperglycemic conditions via inhibition of protein kinase C-alpha. Diabetes. 2002;51:3049–54. doi: 10.2337/diabetes.51.10.3049. [DOI] [PubMed] [Google Scholar]
- 24.Devaraj S, Davis B, Simon SI, Jialal I. CRP promotes monocyte–endothelial cell adhesion via Fcgamma receptors in human aortic endothelial cells under static and shear flow conditions. Am J Physiol Heart Circ Physiol. 2006;291:H1170–6. doi: 10.1152/ajpheart.00150.2006. [DOI] [PubMed] [Google Scholar]
- 25.Williams TN, Zhang CX, Game BA, He L, Huang Y. C-reactive protein stimulates MMP-1 expression in U937 histiocytes through Fc[gamma]RII and extracellular signal-regulated kinase pathway: an implication of CRP involvement in plaque destabilization. Arterioscler Thromb Vasc Biol. 2004;24:61–6. doi: 10.1161/01.ATV.0000104014.24367.16. [DOI] [PubMed] [Google Scholar]
- 26.Han KH, Hong KH, Park JH, et al. CRP promotes MCP-1 mediated chemotaxis through upregulating CCR2 expression in human monocytes. Circulation. 2004;109:2566–71. doi: 10.1161/01.CIR.0000131160.94926.6E. [DOI] [PubMed] [Google Scholar]
- 27.Singh U, Devaraj S, Dasu MR, et al. C-reactive protein decreases interleukin-10 secretion in activated human monocyte-derived macrophages via inhibition of cyclic AMP production. Arterioscler Thromb Vasc Biol. 2006;26:2469–75. doi: 10.1161/01.ATV.0000241572.05292.fb. [DOI] [PubMed] [Google Scholar]
- 28.Devaraj S, Du Clos TW, Jialal I. Binding and internalization of C-reactive protein by Fcgamma receptors on human aortic endothelial cells mediates biological effects. Arterioscler Thromb Vasc Biol. 2005;25:1359–63. doi: 10.1161/01.ATV.0000168573.10844.ae. [DOI] [PubMed] [Google Scholar]
- 29.Ridker PM, Cannon CP, Morrow D, et al. Pravastatin or atorvastatin evaluation and infection therapy—thrombolysis in myocardial infarction 22(PROVE IT-TIMI 22) investigators. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–8. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 30.Niccoli G, Biasucci LM, Biscione C, et al. Independent prognostic value of C-reactive protein and coronary artery disease extent in patients affected by unstable angina. Atherosclerosis. 2008;196:779–85. doi: 10.1016/j.atherosclerosis.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 31.Brunetti ND, Troccoli R, Correale M, Pellegrino PL, Di Biase M. C-reactive protein in patients with acute coronary syndrome: correlation with diagnosis, myocardial damage, ejection fraction and angiographic findings. Int J Cardiol. 2006;109:248–56. doi: 10.1016/j.ijcard.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Prasad K. C-reactive protein increases oxygen radical generation by neutrophils. J Cardiovasc Pharmacol Ther. 2004;9:203–9. doi: 10.1177/107424840400900308. [DOI] [PubMed] [Google Scholar]
- 33.Trachtman H, Futterweit S, Arzberger C, et al. Nitric oxide and super-oxide in rat mesangial cells: modulation by C-reactive protein. Pediatr Nephrol. 2006;21:619–26. doi: 10.1007/s00467-006-0066-x. [DOI] [PubMed] [Google Scholar]
- 34.Cermak J, Key NS, Bach RR, et al. C-reactive protein induces human peripheral blood monocytes to synthesize tissue factor. Blood. 1993;82:513–20. [PubMed] [Google Scholar]
- 35.Paffen E, Vos HL, Bertina RM. C-reactive protein does not directly induce tissue factor in human monocytes. Arterioscler Thromb Vasc Biol. 2004;24:975–81. doi: 10.1161/01.ATV.0000126681.16619.69. [DOI] [PubMed] [Google Scholar]
- 36.Cirillo P, Golino P, Calabrò P, et al. C-reactive protein induces tissue factor expression and promotes smooth muscle and endothelial cell proliferation. Cardiovasc Res. 2005;68:47–55. doi: 10.1016/j.cardiores.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 37.Ratcliffe NR, Kennedy SM, Morganelli PM. Immunocytochemical detection of Fc γR in human atherosclerotic lesions. Immunol Lett. 2001;77:169–74. doi: 10.1016/s0165-2478(01)00217-6. [DOI] [PubMed] [Google Scholar]