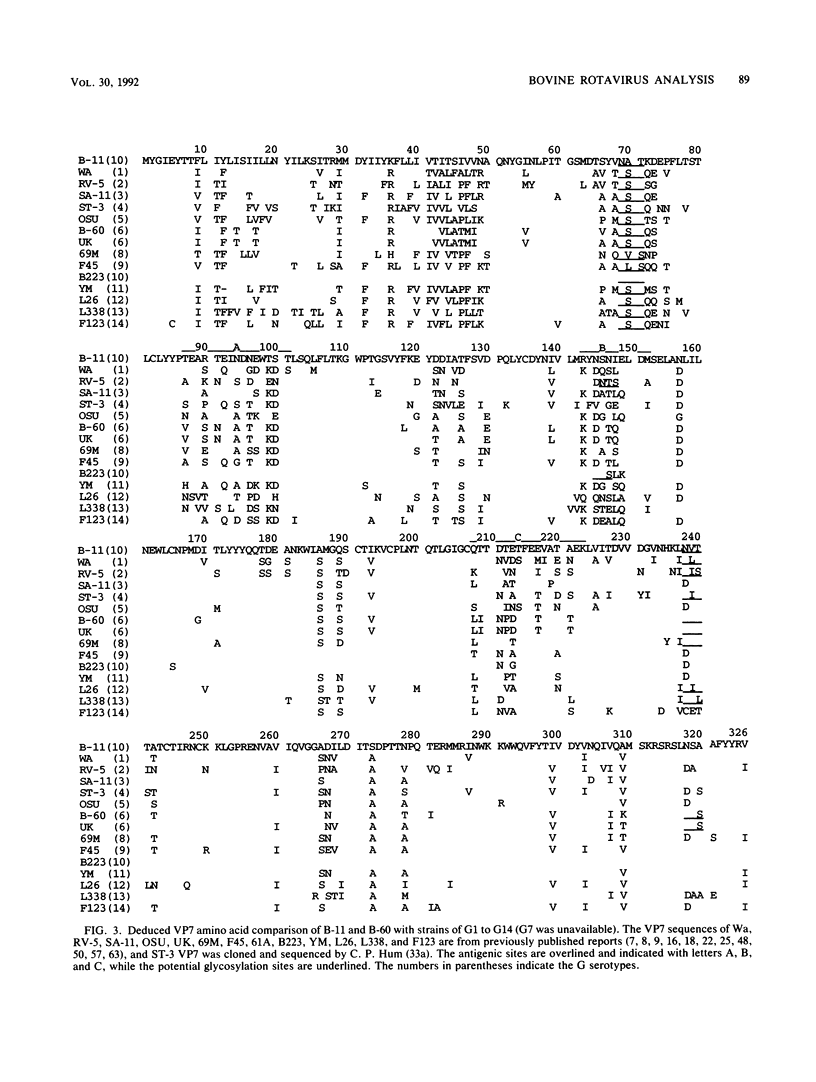
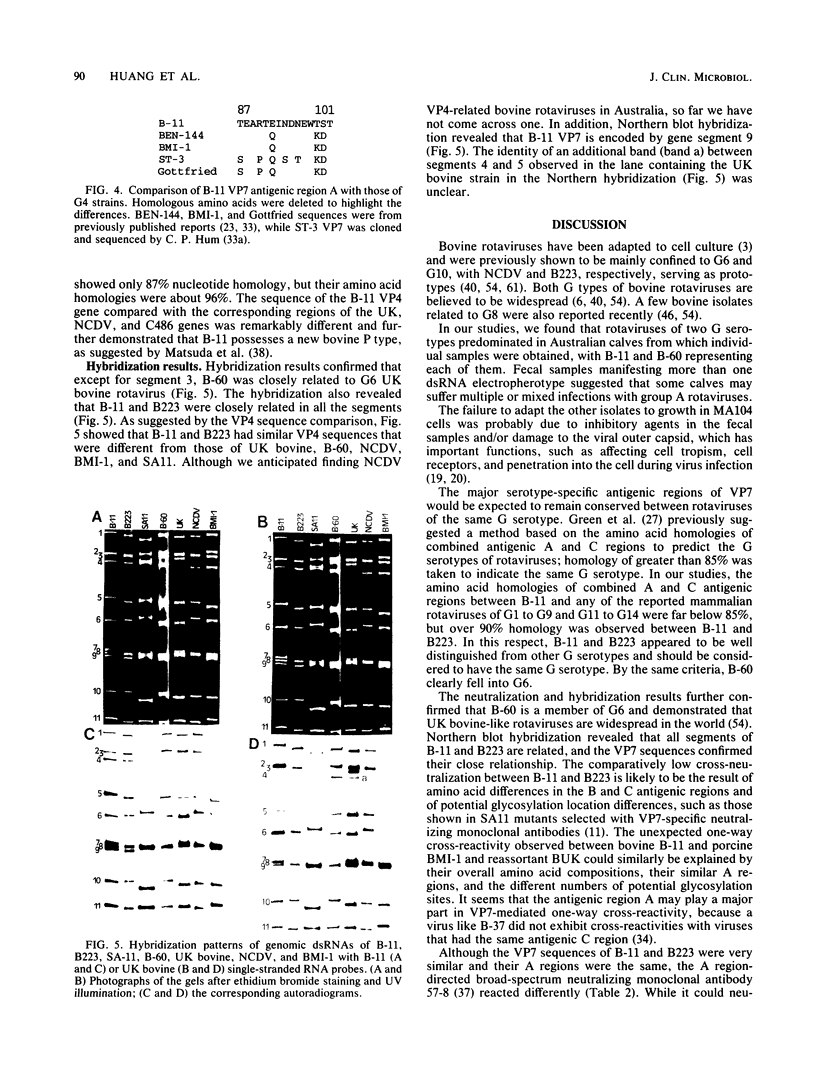
Abstract
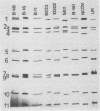
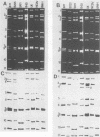
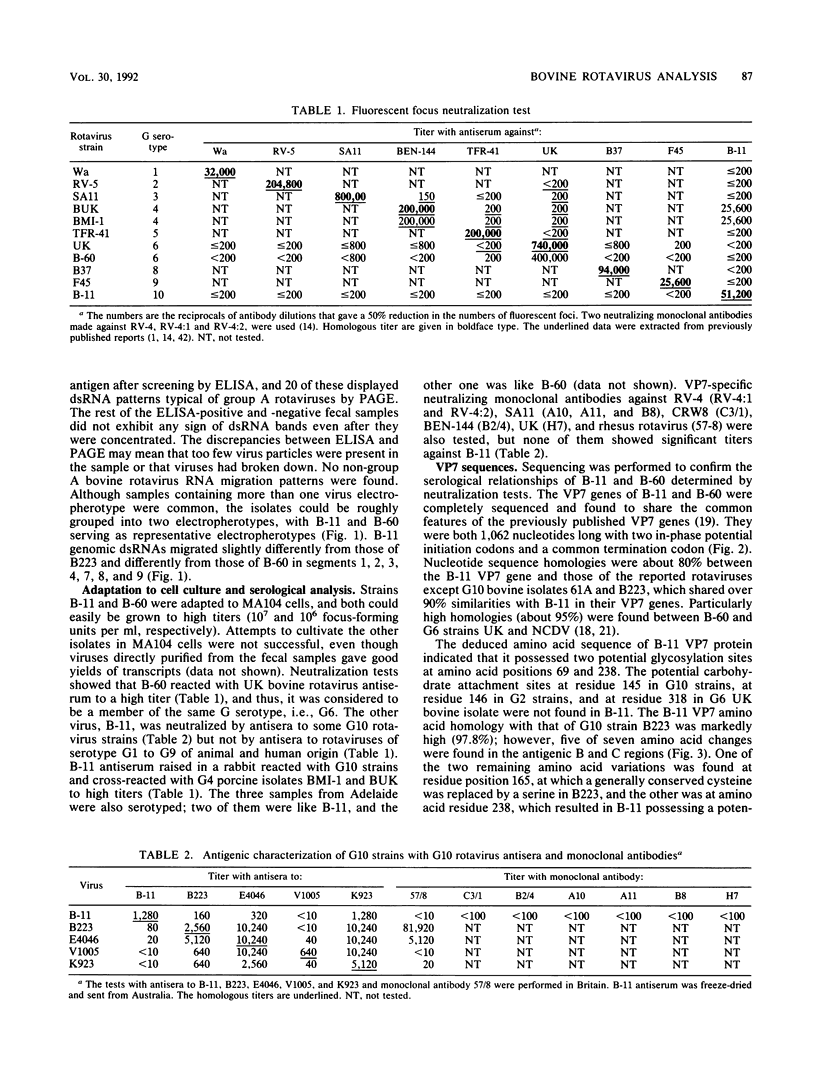
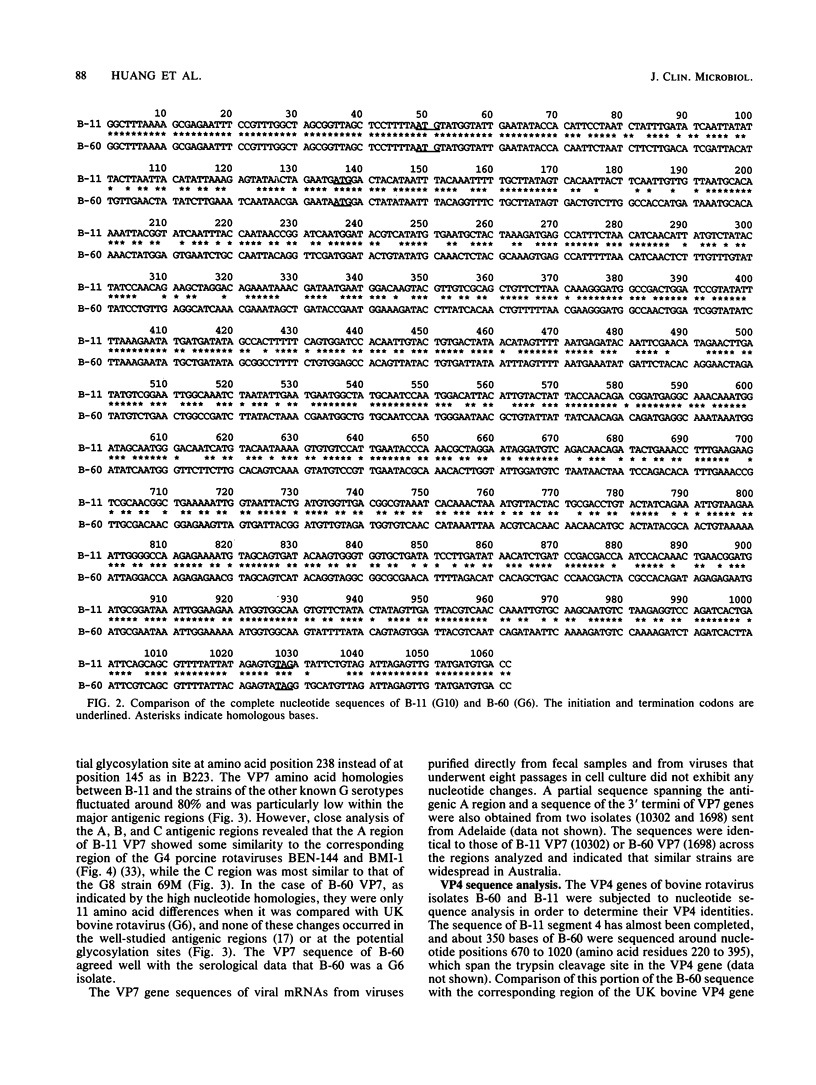
Fecal specimens from 78 calves involved in outbreaks of calf diarrhea which occurred in three farms in Victoria, Australia, in 1988 were analyzed for rotaviruses. Thirty-eight samples were positive for group A virus antigen by enzyme-linked immunosorbent assay, and 20 of these contained viral double-stranded RNAs that could be detected by polyacrylamide gel electrophoresis. Two major electropherotypes could be observed, and a representative isolate of each electropherotype (isolates B-11 and B-60) was successfully adapted to grow in MA104 cells. Sequencing of the VP7 genes directly from RNA transcripts of fecal and cell culture-adapted viruses demonstrated that no base changes occurred in this gene upon adaptation to growth in MA104 cells. Sequencing also revealed that the VP7 protein of B-60 was closely related to G serotype 6 (G6) strains, whereas the B-11 sequence was significantly different from all previously published sequences except the recently reported VP7 sequences of bovine isolates 61A and B223, particularly across the antigenic regions A, B, and C. The other strains most closely related to B-11 by VP7 amino acid sequence analysis were G4 porcine strains BMI-1 and BEN-144 and G8 human strain 69M. Serotyping of B-11 and B-60 gave results that were in good agreement with the sequencing data. Hyperimmune typing sera clearly identified B-60 as a member of G6, whereas the B-11 strain reacted to moderate titers only with antisera to some G10 strains. Antiserum raised against B-11 neutralized some strains of G10 cross-reacted with porcine G4 type isolates BMI-1 and BEN-144 but not with other G4 strains or with rotaviruses of other mammalian G serotypes. Northern blot hybridization showed that B-11 was closely related to the recently reported bovine G10 strain B223, and they both possessed a similar segment 4 that was different from that of either UK bovine or NCDV rotavirus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albert M. J., Unicomb L. E., Bishop R. F. Cultivation and characterization of human rotaviruses with "super short" RNA patterns. J Clin Microbiol. 1987 Jan;25(1):183–185. doi: 10.1128/jcm.25.1.183-185.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert M. J., Unicomb L. E., Tzipori S. R., Bishop R. F. Isolation and serotyping of animal rotaviruses and antigenic comparison with human rotaviruses. Brief report. Arch Virol. 1987;93(1-2):123–130. doi: 10.1007/BF01313898. [DOI] [PubMed] [Google Scholar]
- Bachmann P. A., Hess R. G. Routine isolation and cultivation of bovine rotaviruses in cell culture. Am J Vet Res. 1981 Dec;42(12):2149–2150. [PubMed] [Google Scholar]
- Bastardo J. W., McKimm-Breschkin J. L., Sonza S., Mercer L. D., Holmes I. H. Preparation and characterization of antisera to electrophoretically purified SA11 virus polypeptides. Infect Immun. 1981 Dec;34(3):641–647. doi: 10.1128/iai.34.3.641-647.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beards G. M., Pilfold J. N., Thouless M. E., Flewett T. H. Rotavirus serotypes by serum neutralisation. J Med Virol. 1980;5(3):231–237. doi: 10.1002/jmv.1890050307. [DOI] [PubMed] [Google Scholar]
- Bellinzoni R. C., Blackhall J. O., Mattion N. M., Estes M. K., Snodgrass D. R., LaTorre J. L., Scodeller E. A. Serological characterization of bovine rotaviruses isolated from dairy and beef herds in Argentina. J Clin Microbiol. 1989 Nov;27(11):2619–2623. doi: 10.1128/jcm.27.11.2619-2623.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., Mattick J. S., Bellamy A. R. Serotype-specific glycoprotein of simian 11 rotavirus: coding assignment and gene sequence. Proc Natl Acad Sci U S A. 1983 May;80(10):3091–3095. doi: 10.1073/pnas.80.10.3091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browning G. F., Chalmers R. M., Fitzgerald T. A., Snodgrass D. R. Serological and genomic characterization of L338, a novel equine group A rotavirus G serotype. J Gen Virol. 1991 May;72(Pt 5):1059–1064. doi: 10.1099/0022-1317-72-5-1059. [DOI] [PubMed] [Google Scholar]
- Browning G. F., Fitzgerald T. A., Chalmers R. M., Snodgrass D. R. A novel group A rotavirus G serotype: serological and genomic characterization of equine isolate FI23. J Clin Microbiol. 1991 Sep;29(9):2043–2046. doi: 10.1128/jcm.29.9.2043-2046.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüssow H., Snodgrass D., Fitzgerald T., Eichhorn W., Gerhards R., Bruttin A. Antigenic and biochemical characterization of bovine rotavirus V1005, a new member of rotavirus serotype 10. J Gen Virol. 1990 Nov;71(Pt 11):2625–2630. doi: 10.1099/0022-1317-71-11-2625. [DOI] [PubMed] [Google Scholar]
- Caust J., Dyall-Smith M. L., Lazdins I., Holmes I. H. Glycosylation, an important modifier of rotavirus antigenicity. Arch Virol. 1987;96(3-4):123–134. doi: 10.1007/BF01320955. [DOI] [PubMed] [Google Scholar]
- Cohen J., Laporte J., Charpilienne A., Scherrer R. Activation of rotavirus RNA polymerase by calcium chelation. Arch Virol. 1979;60(3-4):177–186. doi: 10.1007/BF01317489. [DOI] [PubMed] [Google Scholar]
- Coulson B. S., Fowler K. J., Bishop R. F., Cotton R. G. Neutralizing monoclonal antibodies to human rotavirus and indications of antigenic drift among strains from neonates. J Virol. 1985 Apr;54(1):14–20. doi: 10.1128/jvi.54.1.14-20.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson B. S., Tursi J. M., McAdam W. J., Bishop R. F. Derivation of neutralizing monoclonal antibodies to human rotaviruses and evidence that an immunodominant neutralization site is shared between serotypes 1 and 3. Virology. 1986 Oct 30;154(2):302–312. doi: 10.1016/0042-6822(86)90456-3. [DOI] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Holmes I. H. Sequence homology between human and animal rotavirus serotype-specific glycoproteins. Nucleic Acids Res. 1984 May 11;12(9):3973–3982. doi: 10.1093/nar/12.9.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyall-Smith M. L., Lazdins I., Tregear G. W., Holmes I. H. Location of the major antigenic sites involved in rotavirus serotype-specific neutralization. Proc Natl Acad Sci U S A. 1986 May;83(10):3465–3468. doi: 10.1073/pnas.83.10.3465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elleman T. C., Hoyne P. A., Dyall-Smith M. L., Holmes I. H., Azad A. A. Nucleotide sequence of the gene encoding the serotype-specific glycoprotein of UK bovine rotavirus. Nucleic Acids Res. 1983 Jul 25;11(14):4689–4701. doi: 10.1093/nar/11.14.4689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes M. K., Cohen J. Rotavirus gene structure and function. Microbiol Rev. 1989 Dec;53(4):410–449. doi: 10.1128/mr.53.4.410-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuhara N., Yoshie O., Kitaoka S., Konno T. Role of VP3 in human rotavirus internalization after target cell attachment via VP7. J Virol. 1988 Jul;62(7):2209–2218. doi: 10.1128/jvi.62.7.2209-2218.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass R. I., Keith J., Nakagomi O., Nakagomi T., Askaa J., Kapikian A. Z., Chanock R. M., Flores J. Nucleotide sequence of the structural glycoprotein VP7 gene of Nebraska calf diarrhea virus rotavirus: comparison with homologous genes from four strains of human and animal rotaviruses. Virology. 1985 Mar;141(2):292–298. doi: 10.1016/0042-6822(85)90260-0. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Aguirre Y., Hoshino Y., Esparza J., Blumentals I., Askaa J., Thompson M., Glass R. I., Kapikian A. Z., Chanock R. M. VP7 serotype-specific glycoprotein of OSU porcine rotavirus: coding assignment and gene sequence. J Gen Virol. 1986 Nov;67(Pt 11):2445–2454. doi: 10.1099/0022-1317-67-11-2445. [DOI] [PubMed] [Google Scholar]
- Gorziglia M., Nishikawa K., Green K., Taniguchi K. Gene sequence of the VP7 serotype specific glycoprotein of Gottfried porcine rotavirus. Nucleic Acids Res. 1988 Jan 25;16(2):775–775. doi: 10.1093/nar/16.2.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V., Glass R. I., Woods P., Taniguchi K., Clark H. F., Forrester B., Fang Z. Y. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990 Feb;28(2):276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green K. Y., Hoshino Y., Ikegami N. Sequence analysis of the gene encoding the serotype-specific glycoprotein (VP7) of two new human rotavirus serotypes. Virology. 1989 Feb;168(2):429–433. doi: 10.1016/0042-6822(89)90289-4. [DOI] [PubMed] [Google Scholar]
- Green K. Y., Sears J. F., Taniguchi K., Midthun K., Hoshino Y., Gorziglia M., Nishikawa K., Urasawa S., Kapikian A. Z., Chanock R. M. Prediction of human rotavirus serotype by nucleotide sequence analysis of the VP7 protein gene. J Virol. 1988 May;62(5):1819–1823. doi: 10.1128/jvi.62.5.1819-1823.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg H., McAuliffe V., Valdesuso J., Wyatt R., Flores J., Kalica A., Hoshino Y., Singh N. Serological analysis of the subgroup protein of rotavirus, using monoclonal antibodies. Infect Immun. 1983 Jan;39(1):91–99. doi: 10.1128/iai.39.1.91-99.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herring A. J., Inglis N. F., Ojeh C. K., Snodgrass D. R., Menzies J. D. Rapid diagnosis of rotavirus infection by direct detection of viral nucleic acid in silver-stained polyacrylamide gels. J Clin Microbiol. 1982 Sep;16(3):473–477. doi: 10.1128/jcm.16.3.473-477.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Sereno M. M., Midthun K., Flores J., Kapikian A. Z., Chanock R. M. Independent segregation of two antigenic specificities (VP3 and VP7) involved in neutralization of rotavirus infectivity. Proc Natl Acad Sci U S A. 1985 Dec;82(24):8701–8704. doi: 10.1073/pnas.82.24.8701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino Y., Wyatt R. G., Greenberg H. B., Flores J., Kapikian A. Z. Serotypic similarity and diversity of rotaviruses of mammalian and avian origin as studied by plaque-reduction neutralization. J Infect Dis. 1984 May;149(5):694–702. doi: 10.1093/infdis/149.5.694. [DOI] [PubMed] [Google Scholar]
- Huang J., Nagesha H. S., Dyall-Smith M. L., Holmes I. H. Comparative sequence analysis of VP7 genes from five Australian porcine rotaviruses. Arch Virol. 1989;109(3-4):173–183. doi: 10.1007/BF01311079. [DOI] [PubMed] [Google Scholar]
- Hum C. P., Dyall-Smith M. L., Holmes I. H. The VP7 gene of a new G serotype of human rotavirus (B37) is similar to G3 proteins in the antigenic c region. Virology. 1989 May;170(1):55–61. doi: 10.1016/0042-6822(89)90351-6. [DOI] [PubMed] [Google Scholar]
- Kantharidis P., Dyall-Smith M. L., Holmes I. H. Marked sequence variation between segment 4 genes of human RV-5 and simian SA 11 rotaviruses. Arch Virol. 1987;93(1-2):111–121. doi: 10.1007/BF01313897. [DOI] [PubMed] [Google Scholar]
- Mackow E. R., Shaw R. D., Matsui S. M., Vo P. T., Benfield D. A., Greenberg H. B. Characterization of homotypic and heterotypic VP7 neutralization sites of rhesus rotavirus. Virology. 1988 Aug;165(2):511–517. doi: 10.1016/0042-6822(88)90595-8. [DOI] [PubMed] [Google Scholar]
- Matsuda Y., Nakagomi O., Offit P. A. Presence of three P types (VP4 serotypes) and two G types (VP7 serotypes) among bovine rotavirus strains. Arch Virol. 1990;115(3-4):199–207. doi: 10.1007/BF01310530. [DOI] [PubMed] [Google Scholar]
- Murakami Y., Nishioka N., Hashiguchi Y., Kuniyasu C. Neutralizing patterns of anti-bovine rotavirus (Lincoln) serum against cytopathic bovine rotaviruses isolated from calves in Japan. Microbiol Immunol. 1981;25(10):1097–1100. doi: 10.1111/j.1348-0421.1981.tb00117.x. [DOI] [PubMed] [Google Scholar]
- Nagesha H. S., Brown L. E., Holmes I. H. Neutralizing monoclonal antibodies against three serotypes of porcine rotavirus. J Virol. 1989 Aug;63(8):3545–3549. doi: 10.1128/jvi.63.8.3545-3549.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagesha H. S., Holmes I. H. New porcine rotavirus serotype antigenically related to human rotavirus serotype 3. J Clin Microbiol. 1988 Feb;26(2):171–174. doi: 10.1128/jcm.26.2.171-174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagomi O., Oyamada H., Nakagomi T. Use of alkaline northern blot hybridization for the identification of genetic relatedness of the fourth gene of rotaviruses. Mol Cell Probes. 1989 Sep;3(3):263–271. doi: 10.1016/0890-8508(89)90007-8. [DOI] [PubMed] [Google Scholar]
- Nishikawa K., Taniguchi K., Torres A., Hoshino Y., Green K., Kapikian A. Z., Chanock R. M., Gorziglia M. Comparative analysis of the VP3 gene of divergent strains of the rotaviruses simian SA11 and bovine Nebraska calf diarrhea virus. J Virol. 1988 Nov;62(11):4022–4026. doi: 10.1128/jvi.62.11.4022-4026.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offit P. A., Blavat G., Greenberg H. B., Clark H. F. Molecular basis of rotavirus virulence: role of gene segment 4. J Virol. 1986 Jan;57(1):46–49. doi: 10.1128/jvi.57.1.46-49.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojeh C. K., Snodgrass D. R., Herring A. J. Evidence for serotypic variation among bovine rotaviruses. Arch Virol. 1984;79(3-4):161–171. doi: 10.1007/BF01310809. [DOI] [PubMed] [Google Scholar]
- Reynolds D. J., Morgan J. H., Chanter N., Jones P. W., Bridger J. C., Debney T. G., Bunch K. J. Microbiology of calf diarrhoea in southern Britain. Vet Rec. 1986 Jul 12;119(2):34–39. doi: 10.1136/vr.119.2.34. [DOI] [PubMed] [Google Scholar]
- Richardson M. A., Iwamoto A., Ikegami N., Nomoto A., Furuichi Y. Nucleotide sequence of the gene encoding the serotype-specific antigen of human (Wa) rotavirus: comparison with the homologous genes from simian SA11 and UK bovine rotaviruses. J Virol. 1984 Sep;51(3):860–862. doi: 10.1128/jvi.51.3.860-862.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodger S. M., Holmes I. H. Comparison of the genomes of simian, bovine, and human rotaviruses by gel electrophoresis and detection of genomic variation among bovine isolates. J Virol. 1979 Jun;30(3):839–846. doi: 10.1128/jvi.30.3.839-846.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A. M., López I. V., López S., Espejo R. T., Arias C. F. Molecular and antigenic characterization of porcine rotavirus YM, a possible new rotavirus serotype. J Virol. 1988 Nov;62(11):4331–4336. doi: 10.1128/jvi.62.11.4331-4336.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethabutr O., Unicomb L. E., Holmes I. H., Taylor D. N., Bishop R. F., Echeverria P. Serotyping of human group A rotavirus with oligonucleotide probes. J Infect Dis. 1990 Aug;162(2):368–372. doi: 10.1093/infdis/162.2.368. [DOI] [PubMed] [Google Scholar]
- Shaw R. D., Vo P. T., Offit P. A., Coulson B. S., Greenberg H. B. Antigenic mapping of the surface proteins of rhesus rotavirus. Virology. 1986 Dec;155(2):434–451. doi: 10.1016/0042-6822(86)90205-9. [DOI] [PubMed] [Google Scholar]
- Snodgrass D. R., Fitzgerald T., Campbell I., Scott F. M., Browning G. F., Miller D. L., Herring A. J., Greenberg H. B. Rotavirus serotypes 6 and 10 predominate in cattle. J Clin Microbiol. 1990 Mar;28(3):504–507. doi: 10.1128/jcm.28.3.504-507.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snodgrass D. R., Ojeh C. K., Campbell I., Herring A. J. Bovine rotavirus serotypes and their significance for immunization. J Clin Microbiol. 1984 Sep;20(3):342–346. doi: 10.1128/jcm.20.3.342-346.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Pongsuwanna Y., Choonthanom M., Urasawa S. Nucleotide sequence of the VP7 gene of a bovine rotavirus (strain 61A) with different serotype specificity from serotype 6. Nucleic Acids Res. 1990 Aug 11;18(15):4613–4613. doi: 10.1093/nar/18.15.4613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Kobayashi N., Gorziglia M., Urasawa S. Nucleotide sequence of VP4 and VP7 genes of human rotaviruses with subgroup I specificity and long RNA pattern: implication for new G serotype specificity. J Virol. 1990 Nov;64(11):5640–5644. doi: 10.1128/jvi.64.11.5640-5644.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Morita Y., Greenberg H. B., Urasawa S. Direct serotyping of human rotavirus in stools by an enzyme-linked immunosorbent assay using serotype 1-, 2-, 3-, and 4-specific monoclonal antibodies to VP7. J Infect Dis. 1987 Jun;155(6):1159–1166. doi: 10.1093/infdis/155.6.1159. [DOI] [PubMed] [Google Scholar]
- Taniguchi K., Urasawa T., Urasawa S. Reactivity patterns to human rotavirus strains of a monoclonal antibody against VP2, a component of the inner capsid of rotavirus. Brief report. Arch Virol. 1986;87(1-2):135–141. doi: 10.1007/BF01310550. [DOI] [PubMed] [Google Scholar]
- Tzipori S., Chandler D., Smith M., Makin T., Hennessy D. Factors contributing to postweaning diarrhoea in a large intensive piggery. Aust Vet J. 1980 Jun;56(6):274–278. doi: 10.1111/j.1751-0813.1980.tb05723.x. [DOI] [PubMed] [Google Scholar]
- Woode G. N., Kelso N. E., Simpson T. F., Gaul S. K., Evans L. E., Babiuk L. Antigenic relationships among some bovine rotaviruses: serum neutralization and cross-protection in gnotobiotic calves. J Clin Microbiol. 1983 Aug;18(2):358–364. doi: 10.1128/jcm.18.2.358-364.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyatt R. G., James H. D., Jr, Pittman A. L., Hoshino Y., Greenberg H. B., Kalica A. R., Flores J., Kapikian A. Z. Direct isolation in cell culture of human rotaviruses and their characterization into four serotypes. J Clin Microbiol. 1983 Aug;18(2):310–317. doi: 10.1128/jcm.18.2.310-317.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Harbour D., McCrae M. A. Sequence of the gene encoding the major neutralization antigen (VP7) of serotype 10 rotavirus. J Gen Virol. 1991 Jan;72(Pt 1):177–180. doi: 10.1099/0022-1317-72-1-177. [DOI] [PubMed] [Google Scholar]
- Zheng B. J., Lam W. P., Yan Y. K., Lo S. K., Lung M. L., Ng M. H. Direct identification of serotypes of natural human rotavirus isolates by hybridization using cDNA probes derived from segment 9 of the rotavirus genome. J Clin Microbiol. 1989 Mar;27(3):552–557. doi: 10.1128/jcm.27.3.552-557.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Leeuw P. W., Ellens D. J., Talmon F. P., Zimmer G. N., Kommerij R. Rotavirus infections in calves: efficacy of oral vaccination in endemically infected herds. Res Vet Sci. 1980 Sep;29(2):142–147. doi: 10.1016/S0034-5288(18)32654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]