Abstract
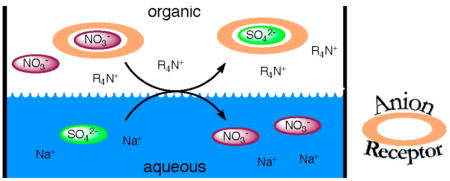
In this Communication, a new approach to enhancing the efficacy of liquid-liquid anion exchange is demonstrated. It involves the concurrent use of appropriately chosen hydrogen-bond-donating (HBD) anion receptors in combination with a traditional quaternary ammonium extractant. The fluorinated calixpyrroles 1 and 2 and the tetraamide macrocycle 4 were found to be particularly effective receptors. Specifically, their use allowed the extraction of sulfate by tricaprylmethylammonium nitrate to be effected in the presence of excess nitrate. As such, the present work provides a rare demonstration of overcoming the Hofmeister bias in a competitive environment and the first to the authors’ knowledge wherein this difficult-to-achieve objective is attained using a neutral HBD-based anion binding agent under conditions of solvent extraction.
Synergism in liquid-liquid extraction, typified by the combination of a neutral extractant with a cation-exchanger to enhance selectively cation extraction strength, has been used and understood for over five decades.1 Surprisingly, analogous enhancements in anion extraction have yet to be reported. In this Communication we present a simple way to achieve non-Hofmeister selectivity in liquid-liquid anion exchange by combining a synthetic hydrogen-bond-donating (HBD) anion receptor with a standard quaternary ammonium type extractant. Specifically, we show that fluorinated calixpyrroles 1 and 22 and the tetraamide macrocycles 3–5,3 may be used to enhance the solvent extraction of sulfate from nitrate by Aliquat 336-nitrate (CH3N[(CH2)7CH3]3NO3; A336N).

Liquid-liquid anion exchange is an established industrial technology for anion separations,4 which are recently enjoying rapid progress5 driven in part by advances in understanding anion binding.6,7 In traditional practice, extraction-based anion exchange relies on the use of lipophilic cationic sites, such as ammonium, phosphonium, imidazolium, or pyridinium centers (protonated or quaternary), whose associated anions exchange in favor of more charge-diffuse anions. Despite its many uses, this solvation-based selectivity,6b a manifestation of the ubiquitous Hofmeister bias,8 effectively inhibits the anion exchange of hydrophilic anions. This inherent limitation has deterred broader application of this technology, which might otherwise provide alternative solutions to such problems as nuclear-waste and environmental remediation.5b
In view of promising earlier results,9 we have recently suggested that the use of appropriately chosen anion receptors might allow the Hofmeister bias to be overcome.3b Whereas a number of examples are known for receptors containing non-hydrogen bond donor (HBD) Lewis acid sites,6a,7,10 in fact non-Hofmeister selectivity has been rarely seen in extractive systems based on receptors possessing only HBD sites. This is particularly true in the case of sulfate,11 a highly hydrated anion whose selective removal from nitrate-rich matrices is particularly difficult (hydration energies ΔGh = −1103 vs −314 kJ mol−1 for sulfate vs nitrate, respectively12), but which would be potentially beneficial in the context of current nuclear waste remediation.3b,5b Recently, we demonstrated that the extraction of sulfate from nitrate-rich mixtures could be achieved through the use of a charged oligopyrrolic receptor.11 However, a potentially simpler approach to this and a multitude of other anion-separation problems would involve combining a neutral HBD receptor, whose anion-recognition properties could be separately optimized, with a traditional quaternary ammonium extractant.
The choice of receptors 1–5 for the present studies was based on their neutral charge, their ability to bind preferentially various anions through HBD interactions, and the fact that they represent two separate classes of anion-binding agents.7 Since their “rediscovery” in 1996, calix[4]pyrroles have been shown to be good receptors for anions and certain neutral substrates.13 Moreover, the use of β-pyrrolic fluorine substitution and expansion of the core has led to the generation of systems, such as 1 and 2, with enhanced affinities and modulated selectivities.2 The tetraamide macrocycles 3–5 are noteworthy for their ability to bind nitrate and sulfate in organic solution and in the solid state.3 We thus considered that systems 1–5 would allow the concept of receptor-mediated enhancement to be tested as a general phenomenon in anion exchange.
The ability to enhance sulfate anion extraction was tested using 35S-labeled sulfate to monitor the exchange of sulfate from an aqueous matrix, consisting of sodium sulfate and excess sodium nitrate, into an organic solvent containing Aliquat 336-nitrate and varying concentrations of the receptors 1–5. Toluene and chloroform were chosen as the water-immiscible organic-phase diluents, as they lack strong HBD strength and were thus expected to minimize competitive anion-solvation effects. Standard beta liquid-scintillation counting techniques were used to determine the distribution of sulfate between the aqueous and organic phases.
Figure 1 illustrates the distribution ratio (Dsulfate = [SO4]org/[SO4]aq) recorded for various concentrations of the anion receptors 1 and 2 with A336-nitrate in the organic (toluene) phase. The ion exchange between nitrate and the more hydrophilic sulfate was notably increased in the presence of both the receptors (1 or 2) and the quaternary ammonium salt. For the stronger receptor 2, we showed through use of higher reagent concentrations that Dsulfate could be pushed to technologically useful values above 1 (i.e., where stage-wise extraction is viable). We ascribe this apparent enhancement to the specific binding interactions between the calixpyrroles and sulfate anion. However, the matter of stoichiometry, expected to involve competitive binding of both nitrate and sulfate in the organic phase and aggregation phenomena, is not easily inferred from the data without detailed equilibrium modeling, which is the object of ongoing investigation.
Figure 1.
Extraction of sulfate from water using a toluene solution with 10 mM or 100 mM Aliquat 336-nitrate and varying concentrations of anion receptors 1 and 2. Aqueous phase: 10 mM NaNO3, 0.1 mM Na2SO4 (spiked with 35SO4), and 0.1 mM HNO3. Equal volumes of organic and aqueous phases were placed on a rotating wheel for 1 h at 25 °C. Experimental uncertainty in the sulfate distribution ratio, Dsulfate, is ±5–10%. The tics on the Y-axis indicate the position at the limit of zero receptor concentration.
The enhancement was extended to chloroform as a diluent (Fig. 2), both for demonstrating the generality of the effect to other solvent environments and for the practical necessity of overcoming solubility limitations of 3–5. The aqueous phase was made slightly alkaline with 1 mM NaOH to ensure that 3–5 were in their neutral form. While both 3 and 4 bind sulfate strongly in chloroform,3 the less-lipophilic system 3 did not show any significant sulfate extraction behavior (below detection limit), but 4 was quite effective. The butyl-substituted analog of 3, 5, showed intermediate behavior.
Figure 2.
Extraction of sulfate from water using a chloroform solution with 10 mM Aliquat 336-nitrate and varying concentrations of receptors 1–5. Aqueous phase: 10 mM NaNO3, 0.1 mM Na2SO4 (spiked with 35SO4), and 1.0 mM NaOH. Other conditions are as described in Figure 1. Values of Dsulfate <2.5 × 10−5 (dotted line) are considered below the detection limit (1.3× background count rate).
In the case of 1 and 2, a comparison of the extraction behavior in chloroform and toluene was possible. Such a comparison (cf. Figs. 1 and 2) serves to highlight a significant solvent dependence. In particular, in toluene the behavior of these two fluorinated calixpyrrole receptors was essentially identical, whereas in chloroform, the larger system was ca. 10x more effective. While further work will be required to rationalize these findings, they could reflect differences in aggregation, conformational effects, or specific ion-pairing interactions, for which a strong solvent dependence has been suggested in the case of non-fluorinated calix[4]pyrrole.14
In summary, we have shown that the use of specifically chosen neutral anion receptors can be used to overcome the Hofmeister bias in liquid-liquid extraction. This finding is noteworthy given the weak nature of the recognition forces involved and the expectation that little direct anion-receptor interaction would be observed in purely aqueous environments. The approach reported here is attractive given its inherent simplicity and the fact that various anion receptors could be made and tested as extractants without having to develop fundamental new approaches to anion separation.
Supplementary Material
Procedures for extraction studies.
Acknowledgments
This work was sponsored by the Environmental Management Science Program of the Offices of Science and Environmental Management, U.S. Department of Energy, under contract number DE-AC05-00OR22725 with Oak Ridge National Laboratory, managed and operated by UT-Battelle, LLC, and under grants no. DE-FG-96ER62307 and DE-FG-02-04ER63741 to The Univ. of Kansas and The Univ. of Texas, respectively. Support was also provided by the National Institutes of Health (grant GM 58907 to J.L.S.) and the INEST Group (PMUSA).
References
- 1.Baes CF., Jr Nucl Sci Eng. 1963;16:405–412. [Google Scholar]
- 2.(a) Anzenbacher P, Jr, Try AC, Miyaji H, Jurisíková K, Lynch VM, Marquez M, Sessler JL. J Am Chem Soc. 2000;122:10268–10272. [Google Scholar]; (b) Sessler JL, Anzenbacher P, Jr, Shriver JA, Jurisíková K, Miyaji H, Lynch VM, Marquez M. J Am Chem Soc. 2000;122:12061–12062. [Google Scholar]; (c) Sessler JL, Cho WS, Gross DE, Shriver JA, Lynch VM, Marquez M. J Org Chem. 2005;70:5982–5986. doi: 10.1021/jo050662c. [DOI] [PubMed] [Google Scholar]
- 3.(a) Hossain MA, Llinares JM, Powell D, Bowman-James K. Inorg Chem. 2001;40:2936–2937. doi: 10.1021/ic015508x. [DOI] [PubMed] [Google Scholar]; (b) Moyer BA, Delmau LH, Fowler CJ, Ruas A, Bostick DA, Sessler JL, Katayev E, Pantos GD, Llinares JM, Hossain MA, Kang SO, Bowman-James K. Supramolecular Chemistry of Environmentally Relevant Anions. In: van Eldik R, Bowman-James K, editors. Advances in Inorganic Chemistry. Vol. 59. Academic Press; 2006. pp. 175–204. [Google Scholar]
- 4.Ritcey GM. Solvent Extraction, Principles and Applications to Process Metallurgy. 1 and 2 G. M. Ritcey & Associates; Ottawa, Canada: 2006. [Google Scholar]
- 5.Moyer BA, Singh RP, editors. Fundamentals and Applications of Anion Separations. Kluwer Academic/Plenum; New York: 2004. [Google Scholar]; (b) Lumetta GJ. In: Fundamentals and Applications of Anion Separations. Moyer BA, Singh RP, editors. Kluwer Academic/Plenum; New York: 2004. pp. 107–114. [Google Scholar]
- 6.(a) Bianchi A, Bowman-James K, García-España E, editors. Supramolecular Chemistry of Anions. Wiley-VCH, Inc.; 1997. [Google Scholar]; (b) Moyer BA, Bonnesen PV. In: Supramolecular Chemistry of Anions. Bianchi A, Bowman-James K, García-España E, editors. Wiley-VCH, Inc.; 1997. pp. 1–44. [Google Scholar]
- 7.For recent reviews of anion receptors, see Sessler JL, Gale PA, Cho WS. Synthetic Anion Receptor Chemistry. Royal Society of Chemistry; 2006. p. 413.Gale PA, García-Garrido SE, Garric J. Chem Soc Rev. 2008;37:151–190. doi: 10.1039/b715825d.
- 8.Custelcean R, Moyer BA. Eur J Inorg Chem. 2007:1321–1340. [Google Scholar]
- 9.(a) Levitskaia TG, Marquez M, Sessler JL, Shriver JA, Vercouter T, Moyer BA. Chem Comm. 2003:2248–2249. doi: 10.1039/b306385m. [DOI] [PubMed] [Google Scholar]; (b) Sisson AL, Clare JP, Taylor LH, Charmant JPH, Davis AP. Chem Comm. 2003:2246–2247. doi: 10.1039/b305261c. [DOI] [PubMed] [Google Scholar]
- 10.Reetz MT. In: Molecular Recognition: Receptors for Molecular Guests. Vögtle F, editor. Pergamon Press; Oxford: 1996. pp. 553–562. [Google Scholar]
- 11.Eller LR, Stępień M, Fowler CJ, Lee JT, Sessler JL, Moyer BA. J Am Chem Soc. 2007;129:11020–11021. 14523. doi: 10.1021/ja074568k. [DOI] [PubMed] [Google Scholar]
- 12.Marcus Y. Ion Properties. Marcel Dekker; New York: 1997. [Google Scholar]
- 13.(a) Gale PA, Sessler JL, Král V, Lynch V. J Am Chem Soc. 1996;118:5140–5141. [Google Scholar]; (b) Allen WE, Gale PA, Brown CT, Lynch VM, Sessler JL. J Am Chem Soc. 1996;118:12471–12472. [Google Scholar]
- 14.(a) Sessler JL, Gross DE, Cho WS, Lynch VM, Schmidtchen FP, Bates GW, Light ME, Gale PA. J Am Chem Soc. 2006;128:12281–12288. doi: 10.1021/ja064012h. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Custelcean R, Moyer BA, Sessler JL, Cho WS, Gross D, Bates GW, Brooks SJ, Light ME, Gale PA. Angew Chem Int Ed. 2005;44:2537–2542. doi: 10.1002/anie.200462945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Procedures for extraction studies.




