Abstract
Biodegradable poly(2-hydroxyethyl methacrylate) hydrogels for engineered tissue constructs were developed using atom transfer radical polymerization (ATRP), a degradable crosslinker and a macroinitiator. Hydrogels are appropriate materials for tissue engineering scaffolds due to their tissue-like mechanical compliance and mass transfer properties. However, many hydrogels that have seen wide application in medicine are not biodegradable or cannot be easily cleared from the body. Poly(2-hydroxyethyl methacrylate) (pHEMA) was selected for the scaffold material due to its reasonable mechanical strength, elasticity, long history of successful use in medicine and because it can be easily fabricated into numerous configurations. pHEMA was studied at various molecular weights between 2 kDa and 50 kDa. The molecular weight range suitable for renal clearance was an important factor in the experimental design. The fabricated hydrogels contain oligomeric blocks of polycaprolactone (PCL), a hydrolytically and enzymatically degradable polymer, as a crosslinking agent. In addition a degradable macroinitiator also containing oligomeric PCL was used to initiate the ATRP. The chain length, crosslink density, and polymerization solvent were found to greatly affect the mechanical properties of the pHEMA hydrogels. Degradation of the pHEMA hydrogels was characterized using 0.007 M NaOH, lipase solutions and phosphate buffered saline. Mass loss, swelling ratio and tensile modulus were evaluated. Degradation products from the sodium hydroxide were measured using gel permeation chromatography (GPC) to verify the polymer lengths and polydispersity. Erosion was only observed in the sodium hydroxide and lipase solutions. However, swelling ratio and tensile modulus indicate bulk degradation in all PCL containing samples. Degradable hydrogels in enzymatic solutions showed 30% mass loss in 16 weeks. Initial cell toxicity studies indicate no adverse cellular response to the hydrogels or their degradation products. These hydrogels have appropriate mechanical properties, a tunable degradation rate, and are composed of materials currently in FDA approved devices. Thus the degradable pHEMA developed in this study has considerable potential as a scaffold for tissue engineering in cardiac and other applications.
Keywords: PolyHEMA, Polycaprolactone, ATRP, Cardiac Tissue Engineering, Degradation
Introduction
This study addresses scaffold polymers that will be used in heart muscle tissue engineering. Heart failure and related cardiovascular disease continues to be the leading cause of death in the United States[1]. Myocardial infarctions result from coronary artery blockages and often leads to heart failure. Because cardiomyocytes have limited regenerative capability, tissue damaged by an acute myocardial infarction is replaced with nonfunctional scar tissue. Despite advances in pharmacological, interventional and surgical therapies, the prognosis for patients with heart failure is unfavorable[2]. Consequently, research has focused on regenerating functional myocardium often using tissue engineered scaffolds. It has been proposed that scaffolds seeded with functional cardiomyocytes can be placed in the infracted region to restore viable myocardium[3-6]. The role of the scaffold in this process is to support and direct three dimensional cellular growth leading to restored tissue. Compared to direct injection of cells, a seeded scaffold allows for a higher cell density and, more importantly, localizes injected cells[7]. As cells develop and lay down extracellular matrix, the scaffold should begin to degrade. The rate of degradation can affect macroscopic shape and the timely development of new tissue[8, 9]. Therefore, it is important to have a scaffold material with a tunable degradation rate. A suitable scaffold for cardiac tissue engineering should also be biocompatible, integrate with the host tissue, and exhibit tissue-like mechanical properties[10]. Additionally, to assure that scaffold degradation products can easily egress by renal clearance, the molecular weight should be less than 50 kDa and products should be soluble in the bloodstream[11].
The majority of the scaffolds used in tissue engineering studies published to date have been based on poly(glycolic acid)(PGA), poly(lactic acid)(PLA) and their copolymers. These polymers, though having a long history in medicine and wide acceptance, exhibit a number of disadvantages for application as tissue engineering scaffolds, especially for heart muscle tissue engineering. These disadvantages include (1) they are not elastomeric and thus do not match the modulus of soft tissue, (2) they break down into acidic products incompatible with cell growth, (3) the acidic breakdown products auto-catalyze further polymer breakdown, often leading to catastrophic disintegration of larger masses of polymer, (4) they are hydrophobic, and (5) they are difficult to chemically derivatize making surface immobilization problematical. These issues are addressed in the new polymer developed in this work.
Methacrylate and acrylate polymers are widely used in medicine and biology because they are well tolerated in vivo. Several methacrylates and acrylates with hydrophilic substituent groups such as poly(2-hydroxyethyl methacrylate) (pHEMA) can form hydrogels that, due to the water content and favorable mechanical properties, have found use in applications such as contact lenses, drug delivery vehicles and tissue engineered scaffolds[12]. Hydrogels of pHEMA meet several of the scaffold requirements. They elicit an in vivo response considered biocompatible, can be fabricated in various architectures, and have mechanical properties similar to those of natural tissue[13-16]. However, pHEMA has not been previously used as a bioresorbable scaffold because of its excellent biostablility. Polycaprolactone (PCL) is a semi-crystalline polyester that contains aliphatic ester linkages that are susceptible to both hydrolytic and enzymatic degradation [17-19]. Extensive research has been performed with PCL that has led to FDA approval of several PCL containing medical and drug delivery devices[20, 21]. The degradation kinetics of PCL are considerably slower than other aliphatic polyesters due to its hydrophobicity and crystallinity. This slow degradation can be desirable for scaffolding material as it may reflect the rate of tissue development.
Atom transfer radical polymerization (ATRP) can be used to prepare controlled molecular weight pHEMA with low polydispersity and the water solubility of that pHEMA has been evaluated [23]. ATRP is a versatile technique that can be used to control the polymerization of several monomer classes including methacrylates[22, 23]. ATRP involves polymer radicals as opposed to ionic species and thus is well suited for the polymerization of functional monomers such as pHEMA [24]. This technique is used here to ensure that the degradation products are an appropriately small for excretion from the body.
In this study we have combined pHEMA and PCL using the ATRP technique and advanced macromolecular design ideas to develop a scaffold that can meet all the aforementioned requirements. The specific rationale for the polymers described in this paper is: (1) for pHEMA to be cleared after biodegradation, fragments must be smaller than 10kDa (5kDa is preferable); (2) to provide hydrolytically labile sites for the breakdown of the stable pHEMA, dimethacrylated oligo-PCL segments were incorporated; (3) When pHEMA segments were 5kDa, poor mechanical properties were observed in the resulting polymer. Thus, a difunctional PCL oligomer terminated with ATRP initiator was used to create “two pHEMA” units with molecular weights of 10 kDa + the molecular weight of the backbone PCL unit. Here, we describe synthesis, characterization and degradation of low molecular weight pHEMA initiated and crosslinked with PCL moieties.
Materials and Methods
Materials
2-Hydroxyethyl methacrylate monomer (ophthalmologic grade; 99% minimum purity, acid content maximum % = 0.05; crosslinker content maximum % = 0.15) (HEMA) and tetraethylene glycol dimethacrylate (TEGDMA) were purchased from Polysciences Inc.(Warrington, PA) and used without further purification. Polycaprolactone diols (Mw = 530, 1250, 2000), methacryloyl chloride (97%), copper (I) chloride, 2,2’-bipyridyl, ethyl 2-bromoisobutyrate, and α-bromoisobutyryl bromide were purchased from Sigma-Aldrich (Milwaukee, WI) and used with no further purification. Amano Lipase PS and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) were also purchased from Sigma along with all other reagent grade solvents.
Instrumentation
1H NMR spectra were recorded on a Bruker AVance series instrument (300 MHz) using a Bruker BBI probe. A Tosoh Corp gel permeation chromatograph was used with TSRGel α-3000 and α-4000 columns eluted at 1 mL/min. Dimethylformamide containing 1 wt % lithium bromide was used as the solvent and poly(methyl methacrylate) standards were used for calibrating the molecular weights. Mechanical properties of the hydrogels such as tensile modulus, ultimate tensile strength, and ultimate tensile strain were evaluated using an Instron universal tester 3340 series single column system equipped with a 10 N load cell.
Polycaprolactone Crosslinker Synthesis
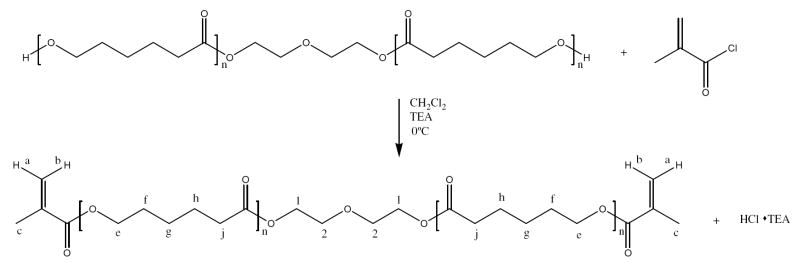
Synthesis of the polycaprolactone crosslinker was performed as previously reported by Sawhney et al [25]. The polycaprolactone crosslinker (PCLX) was made by reacting an oligomeric caprolactone diol (Mw =530) with methacryloyl chloride to produce a degradable crosslinker, PCLX. Figure 1 shows the reaction scheme for the synthesis of PCLX. Briefly, polycaprolactone diol (25g, 0.047 mol), MW =530, was dissolved in dichloromethane (100 mL) in a round bottom flask. Triethylamine (19.7 mL, 0.14 mol) was added directly to the flask and the solution was cooled to 0° C. Methacryloyl chloride (13.8 mL, 0.14 mol) and dichloromethane (50 mL) were place in an addition funnel over the round bottom flask. The air was removed and replaced with nitrogen. The methacryloyl chloride solution was added dropwise to the stirred solution over a two hour period. The solution was allowed to react for 12 hours at 0° C and then 24 hours at room temperature. The triethylamine hydrogen chloride salts were removed by filtration and then the solvent was removed under vacuum. The remaining oil was precipitated into cold hexanes to yield a slightly yellow wax. The purchased PCL diol (MW=530) has an average n of 2.3. The product was evaluated by NMR (Figure 1) and the chemical shifts were obtained relative to tetramethylsilane. 1H NMR (300 MHz, CDCl3, ppm): δ 6.13 (b), 5.58 (a), 4.28 (1), 4.08 (e), 3.68 (2), 2.35 (j), 1.95 (c), 1.68 (h), 1.39 (g).
Figure 1.
PCLX reaction scheme. PCL diols of various molecular weights (530, 1250 and 2000 g/mol) were reacted with methacryloyl chloride to produce a dimethacrylated PCL moiety used as a crosslinker (PCLX).
Polycaprolactone Initiator Synthesis
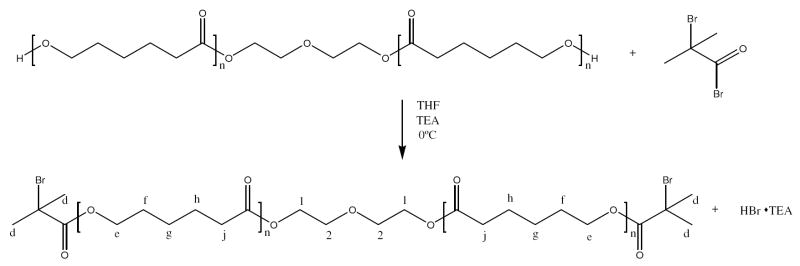
An ATRP macroinitiator was also synthesized in order to improve mechanical properties of the hydrogel while maintaining low molecular weight degradation products. The polycaprolactone macroinitiator (PCLI) was synthesized again by reacting an oligomeric caprolactone diol with α-bromoisobutyryl bromide resulting in a difunctional initiator [26]. Polycaprolactone diol (Mw = 530) (10 g, 0.02 mol) was dissolved in anhydrous tetrahydrofuran (300 mL). Triethylamine (13.1 mL, 0.1 mol) was added and to the stirred solution followed by the addition of α-bromoisobutyryl bromide (5.8 mL, 0.05 mol). The reaction was allowed to proceed overnight at ambient temperature. The resulting hydrogen chloride salt was removed by filtration and the THF was removed under vacuum. The residual yellow oil was redissolved in dichloromethane (300 mL) and then washed with a saturated solution of bicarbonate three times. The organic layer was separated and dried over magnesium sulphate, filtered and the dichloromethane was removed under vacuum. The final product, a yellow oil, was characterized with NMR [26]. Figure 2 shows the reaction scheme for the synthesis of PCLI. 1H NMR (300 MHz, CDCl3, ppm): δ 4.31 (1), 4.11 (e), 3.72 (2), 2.35 (j), 1.95 (d), 1.65 (h), 1.53 (f), 1.25 (g).
Figure 2.
PCLI reaction scheme. A PCL diol (Mw = 530) was reacted with α bromoisobutyrl bromide to produce a difunctional macroinitiator (PCLI).
Preparation and Characterization of Scaffold and Linear Polymer
The synthesis of linear pHEMA for solubility and macroinitiator studies was done according to Weaver et al. [23]. In a typical experiment HEMA monomer (7.16 mmol, 0.9 mL), the initiator ethyl 2-bromoisobutyrate (EBiB) (0.094 mmol, 14 μL), and methanol (1 mL) were degassed with nitrogen for 30 minutes. A catalyst solution containing CuCl (0.096 mmol, 9.5 mg), 2,2’-bipyridyl (0.24 mmol, 37.5 mg), and methanol (100 μL) was also degassed and then added under nitrogen to the previous monomer solution. The methanolic solution was stirred for 24 hours and then upon exposure to air the solution changed from dark brown to blue indicating oxidation of the copper. Additional methanol (5 mL) was added to the polymer solution before passing through a silica column to remove the catalyst. Excess methanol was removed by vacuum and then the reduced volume solution was precipitated into cold diethyl ether. The white precipitate was then collected and dried under vacuum for 12 hours. pHEMA hydrogels with various molecular weight block segments and crosslink densities were prepared using atom transfer radical polymerization (ATRP) techniques. The initiator (PCLI) and crosslinker (PCLX) used for the degradable gels were synthesized as described above. The following experiments were all conducted on hydrogels prepared using PCLX synthesized from PCL diol Mw =530 g/mol. EBiB and tetraethylene glycol dimethacrylate (TEGDMA) were used in the non-degradable controls as initiator and crosslinker, respectively. The ATRP transition metal and ligand used for both polymerizations were copper(I) chloride and 2,2’-bipyridyl.
For a typical polymerization to form the degradable pHEMA (Mw = 10 kDa), PCL-I (0.46 mmol, 0.4 g) and PCL-X (1.16 mmol, 1.0 g) were dissolved in dimethylformamide (1.5 mL). HEMA (35.8 mmol, 4.5 mL) and ethylene glycol (3.0 mL) were then added to the solution and the solution was purged with nitrogen for 30 minutes. The catalyst solution was prepared separately by dissolving CuCl (0.93 mmol, 92.1 mg) and 2,2’-bipyridyl (2.33 mmol, 363 mg) in dimethylformamide (0.3 mL) and ethylene glycol (0.7 mL). The catalyst solution was also purged with nitrogen for 30 minutes. In a nitrogen environment the two solutions were mixed, cast into molds, and allowed to polymerize for 24 hours. The hydrogels were then removed from the molds and rinsed several times with 90 % acetone 10% water to remove copper and unreacted monomer. It should be noted that the term crosslinking density will be used in this manuscript to refer to the mol percent of crosslinking agent in the initial polymerization solution.
The uncrosslinked pHEMA and hydrogel degradation products were characterized using gel permeation chromatography (GPC). Mechanical properties were evaluated from dog-bone shaped samples (4.5mm × 0.65mm × 20 mm) punched from a film. The crosshead speed was set to stretch at 10 % strain/min until failure. At least three samples were used for each measurement.
Scaffold Degradation and Cytotoxicity
In vitro degradation profiles and rates were evaluated by measuring percent mass loss and volumetric swelling ratio. The percent mass loss and swelling ratio were determined using the following equations,
where mo is the original dry mass of the sample, mD is the residual dry mass of the sample after a degradation period, and mw is the mass of the hydrated sample after a degradation period [6]. Degradation was studied in several environments including 0.007 M NaOH, 1.0 mg/ml lipase, 0.5 mg/ml lipase, and phosphate buffered saline (PBS). Before degradation studies all hydrogels were lyophilized and weighed to determine the original dry weight. In the accelerated degradation study samples were place in 0.007 M NaOH at room temperature and placed on an orbital shaker rotating at approximately 60 rpm. Samples were taken twice a day for 5 days and the hydrated weight was measured before the samples were frozen and lyophilized. The dry weight was again recorded for use in calculations. The degradation studies in lipase and PBS solutions were conducted in a similar fashion only the temperature was controlled at 37 °C and samples were drawn over a 20 week time period. Degradation media was changed every 4 days to prevent contamination and to ensure enzyme activity. The Young’s modulus of the hydrogels was measured over the degradation time. The modulus was determined by examining the slope of the linear region of the stress vs. strain curve (< 10% strain).
Solubility of the degradation products was evaluated in a 0.15 M NaCl solution. In each case, 20 mg of polymer was dissolved in 2 mL solution and stirred at 0-5 °C for 24 hours. The solution was then filtered and remaining insoluble material was vacuum dried and weighed [23].
Cytotoxicity of the hydrogels and their degradation products was measured using a MTT assay. Hydrogels were soaked in media for 24 hours before the eluent was removed and placed on 3T3 mouse fibroblasts plated 24 hours earlier at a concentration below confluency. The cells were then evaluated at 12, 24, and 48 hour time points. The degradation products were dissolved into media at concentrations ranging from 5-15 mg/mL and evaluated in a similar procedure as the hydrogel eluent. Tissue culture polystyrene (TCPS) and latex were used as the negative and positive controls for cytotoxicity, respectively.
Results and Discussion
Figures 1 and 2 show the reaction scheme for both PCLX and PCLI production. The extent of methacrylation of the PCL diol was calculated by comparing the integral area under the NMR peaks at δ 6.13 (methacrylate functional group) to 3.68 (PCL diol backbone). The functionality of the initiator was determined by comparing the peak areas of δ 1.95 (bromine functional group) to 3.72(PCL diol backbone). The functionality of the PCLX and PCLI were determined to range between 80-95% and 70-85% respectively. These percentages correspond closely to those reported by Sawhney et al. and Rice et al for similar reaction schemes[18, 25, 27]. Additionally, the comparison of HNMR shifts between the initial PCL diol to either PCLX or PCLI shows the disappearance of the peak at 3.53 which corresponds to OH terminals. This again confirms the end group functionality of the PCL has been converted from a diol to either a dimethacrylate or diorganohalide.
To verify the living nature of ATRP/HEMA system and the ability to synthesize pHEMA with low molecular weights, various monomer to initiator ratios were examined utilizing ethyl 2-bromoisobutyate as the initiator. Table 1 lists the monomer to initiator ratios, theoretical molecular weights and measured molecular weights (GPC) for several polymerizations of linear pHEMA. As Table 1 indicates the molecular weight as measured by GPC is significantly higher than theoretical; however, discrepancies in the low molecular weight range have been reported as being systematic errors in the GPC analysis [23]. Increased polydispersities and standard deviations were observed at higher molecular weights. This is thought to be due in part to impurities in the HEMA monomer. Additionally, measurement errors for low molecular weights (less than 5 kDa) may be due in part to the fact that this approaches the lower limits of the GPC column. Polydispersities were measured as low as 1.17 indicating low variance in the molecular weight of the pHEMA chains.
Table 1.
A summary of monomer to initiator ratios used and the correlating theoretical and measured molecular weight for linear pHEMA initiated with ethyl 2-bromoisobutyrate.
| Mo:I Ratio | Mn (theory) | Mn (GPC) | PDI |
|---|---|---|---|
| 15:1 | 2000 | 6000 ± 120 | 1.17 ± 0.009 |
| 30:1 | 4000 | 8200 ± 270 | 1.22 ± 0.005 |
| 60:1 | 8000 | 11600 ± 500 | 1.26 ± 0.008 |
| 120:1 | 16000 | 17600 ± 730 | 1.35 ± 0.05 |
| 240:1 | 32000 | 33500 ± 3800 | 1.71 ± 0.05 |
| 360:1 | 48000 | 46500 ± 4800 | 2.17 ± 0.2 |
The molecular weight of the polymer is an important factor for ultimate excretion. There are literature reports that molecular weights suitable for renal clearance are in the range of 10-50 kDa [11, 28]. This corresponds to monomer to initiator ratios of 75:1 to 375:1. The linear correlation between measured and theoretical molecular weight, as shown in Figure 3, indicates that the polymerization is controlled.
Figure 3.
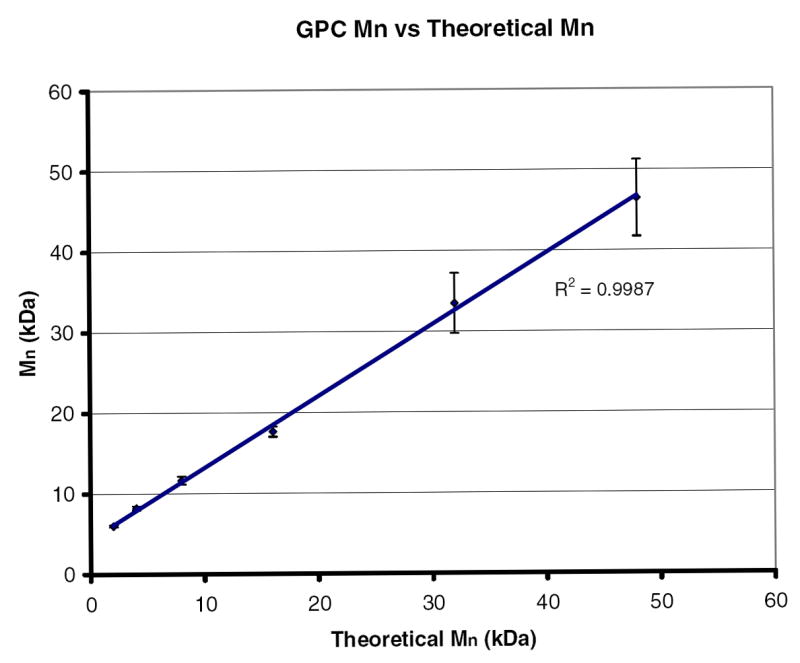
The molecular weight of linear pHEMA initiated with ethyl 2-bromoisobutyrate as measured by GPC verses the theoretical molecular weight calculated from the monomer to initiator ratio. The linear correlation indicates a controlled polymerization.
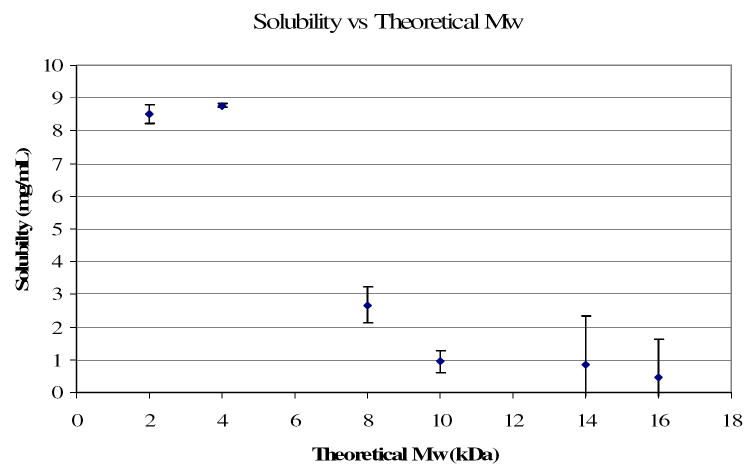
Water solubility is another significant factor for renal clearance. As shown by Figure 4, there is a significant decline in water solubility as molecular weight increases. A molecular weight above 5 kDa (monomer to initiator ratio 38:1) results in limited water solubility. Taking into consideration the GPC systematic errors, this solubility limit is in agreement with that reported by Weaver et al [23]. Unfortunately, the low molecular weights required for renal clearance and water solubility produce hydrogels that do not have mechanical properties that are in the natural tissue range. Therefore a macroinitiator was synthesized to allow the backbone chain length to be double the length of the degradation products.
Figure 4.
Single pass solubility of linear pHEMA initiated with ethyl 2-bromoisobutyrate in 0.15 M NaCl verses the theoretical molecular weight calculated from monomer to initiator ratios.
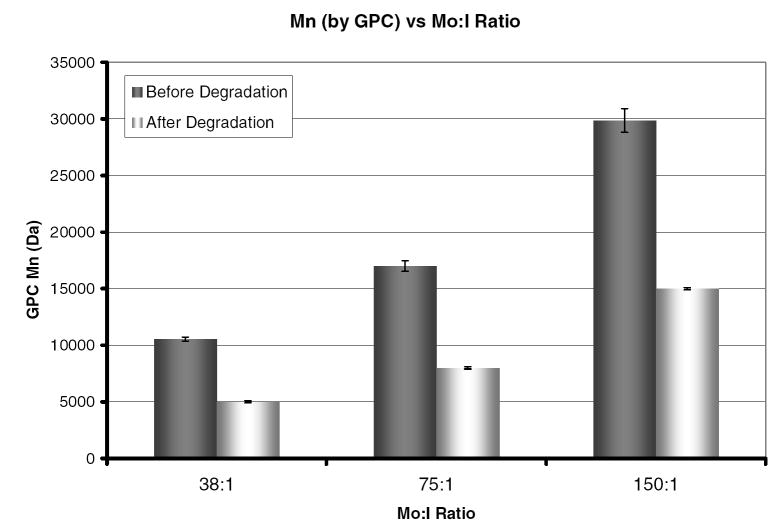
Polymerizations of various molecular weights were performed to evaluate the effectiveness of the macroinitiator PCLI. GPC was used to evaluate the chain length before and after the polymer was placed in 1 M NaOH to accelerate degradation (incubation time 24 hours). As shown by Figure 5 the molecular weight of the degraded polymer chain is half the original length. This indicates that the macroinitiator is indeed difunctional and does not affect the ability to control the polymerization. The contribution of the PCL in the polymer chain is small and thus falls within the standard error of the measurements. Therefore the hydrogels used for degradation experiments were polymerized with a ratio of 75:1 which has a predegradation theoretical backbone length of 9.76 kDa and a degraded backbone length of 4.88 kDa.
Figure 5.
Molecular weight as measured by GPC for linear pHEMA initiated with PCLI at various monomer to initiator ratios both before and after exposure to 1 M NaOH.
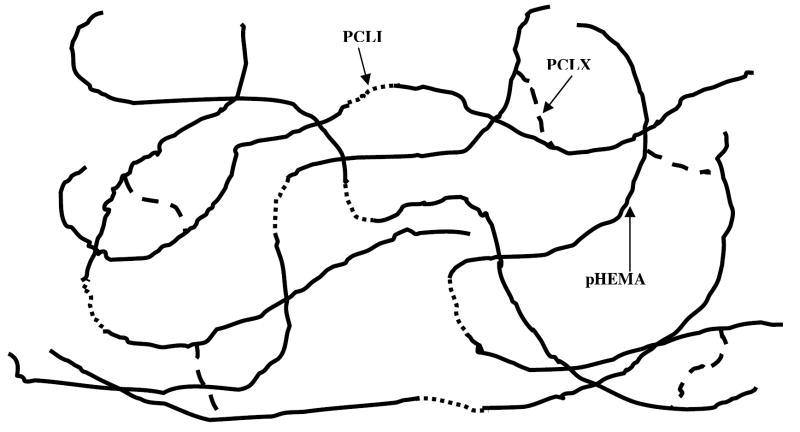
Hydrogels were polymerized with crosslinker concentrations ranging from 0.8 to13.5 mol% and backbone lengths ranging from 10 to 50 kDa. Mechanical properties were found to be greatly influenced by polymerization solvent, crosslinking density and monomer to initiator ratio. The minimum crosslinking density is dependant on the monomer to initiator ratio used. A minimum of 2 crosslinks per chain was used to ensure the formation of an elastic hydrogel. Thus, for a backbone chain length of 10 kDa the minimum crosslinking density was calculated to be approximately 4 mol%. Figures 6 shows a schematic of the hydrogel polymerized with PCLI and PCLX.
Figure 6.
Schematic of pHEMA hydrogel crosslinked and initiated with PCLX ( ) and PCLI (
) and PCLI ( ). pHEMA backbone chain length can be varied from 5 to 20 and a minimum of 2 crosslinks per chain is necessary for hydrogel formation.
). pHEMA backbone chain length can be varied from 5 to 20 and a minimum of 2 crosslinks per chain is necessary for hydrogel formation.
The Young’s modulus of the hydrogel was measured for several different polymerizations. The polymerization details and the resulting Young’s moduli are shown in Table 2. As expected, significant increases in the Young’s modulus result from increasing the crosslinking density, crosslinking with PCLX instead of TEGDMA, and by decreasing the molecular weight (Table 2). The modulus of all the samples measured was comparable to the modulus of rat heart tissue and pHEMA polymerized with a TEGDMA crosslinker. The incorporation of the PCLX nearly doubles the tensile modulus of the material increasing it from 0.29 to 0.43 MPa (experiments 1 and 2). This effect could be due to the fact that PCL can crystallize and this increase in crystallinity may be contributing to the increased tensile modulus. Further studies will be performed using differential scanning calorimetry to elucidate this effect. Doubling the crosslinking density also doubles the tensile modulus (experiments 2 and 3). It should be noted that the modulus is also significantly impacted by the type and amount of solvent used in the polymerization. Increasing the amount of ethylene glycol in the polymerization by 50% resulted in a decrease of modulus from 0.76 ± 0.05 to 0.43 ± 0.02 MPa (experiments 2 and 5). All hydrogels were subjected to a pre-test cycle that extended the sample to 10% strain follow by relaxation three times. Hysteresis was only observed on the first cycle indicating that after an initial conditioning, the hydrogels exhibit elastic behavior.
Table 2.
A summary of experimental factors affecting the tensile modulus of the hydrogels. Crosslinker types tetraethylene glycol dimethacrylate (TEGDMA) and polycaprolactone dimethacyrlate (PCLX) were used.
| Experiment Number | Mo:I Ratio | Theoretical Mn (Da) | Crosslinker | Crosslink Density (mol %) | Ethylene Glycol (mL) | Tensile Modulus (MPa) |
|---|---|---|---|---|---|---|
| 1 | 75:1 | 10,000 | TEGDMA | 4.5 | 3 | 0.29 ± 0.03 |
| 2 | 75:1 | 10,000 | PCLX | 4.5 | 3 | 0.43 ± 0.02 |
| 3 | 75:1 | 10,000 | PCLX | 9 | 3 | 0.83 ± 0.07 |
| 4 | 75:1 | 10,000 | TEGDMA | 4.5 | 2 | 0.48 ± 0.03 |
| 5 | 75:1 | 10,000 | PCLX | 4.5 | 2 | 0.76 ± 0.05 |
| 6 | 150:1 | 20,000 | TEGDMA | 2.5 | 2 | 0.31 ± 0.02 |
| 7 | 150:1 | 20,000 | PCLX | 2.5 | 2 | 0.49 ± 0.03 |
| Conventional pHEMA* | - | TEGDMA | 1.8 | - | 0.63 ± 0.04 | |
| Heart Tissue (rat)** | - | - | - | - | 0.59 ± 0.22 | |
| Scar Tissue** | - | - | - | - | 4.7 ± 1.4 |
Conventional pHEMA is polymerized with ammonium persulfate and sodium bisulfate.
Heart and scar tissue provided by Steve Korte, Alicia Gonzalez, and Mike Reigner.
Conventional pHEMA was polymerized by a free radical mechanism using TEGDMA crosslinker and ammonium persulfate and sodium bisulfate as the initiator system. Unfortunately, reliable measurements could not be obtained from hydrogels with crosslinking densities of 13.5 mol% (approximate mass equivalent to the PCLX) due to cracking and therefore were not included in the subsequent table.
Degradation of the hydrogels was investigated in sodium hydroxide solutions of varying concentrations, enzymatic solutions and phosphate buffered saline (PBS) by following the changes in the weight loss, swelling ratio, and tensile modulus. The hydrogels undergo bulk degradation uniformly throughout the gels. As PCL crosslinks or chain segments are cleaved, the effective crosslink density will be reduced which increases the polymer mesh size, lowers the tensile modulus, and increases the swelling ratio or water content of the hydrogel. When enough PCL bonds have been broken to free a pHEMA backbone segment, the chain will go into solution and the mass of the hydrogel will decrease.
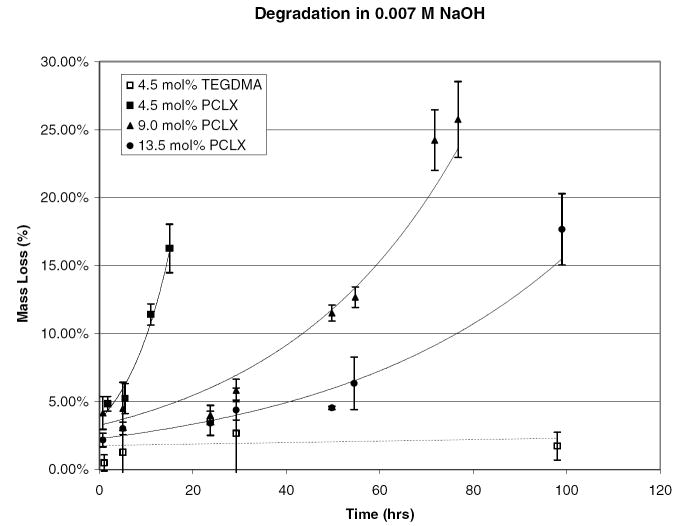
Accelerated degradation studies were performed primarily to ensure that the hydrogel’s hydrolytically labile bonds are accessible and that the degradation products will be soluble. This was confirmed by degradation in 0.007 M NaOH at room temperature. The monomer to initiator ratio for these hydrogels was 75:1 (Mw = 10 kDa), the crosslink density varied from 4.5 to 13 mol percent, and the solvent to monomer ratio was kept constant at a 1:3 ratio. The degradation profile is shown in Figures 7 and 8 as assessed by the swelling ratio and percent mass loss. Degradation profiles followed the expected trend. The lowest crosslink density hydrogel completely degraded in under 24 hours while the gel with the highest crosslink density did not completely degrade within the experimental time frame. This verifies that crosslink density can be used to tailor the degradation rate of the hydrogel. The nondegradable control, initiated with ethyl 2-bromoisobutyrate and crosslinked with TEGDMA, showed no statistically significant mass loss or change in swelling ratio.
Figure 7.
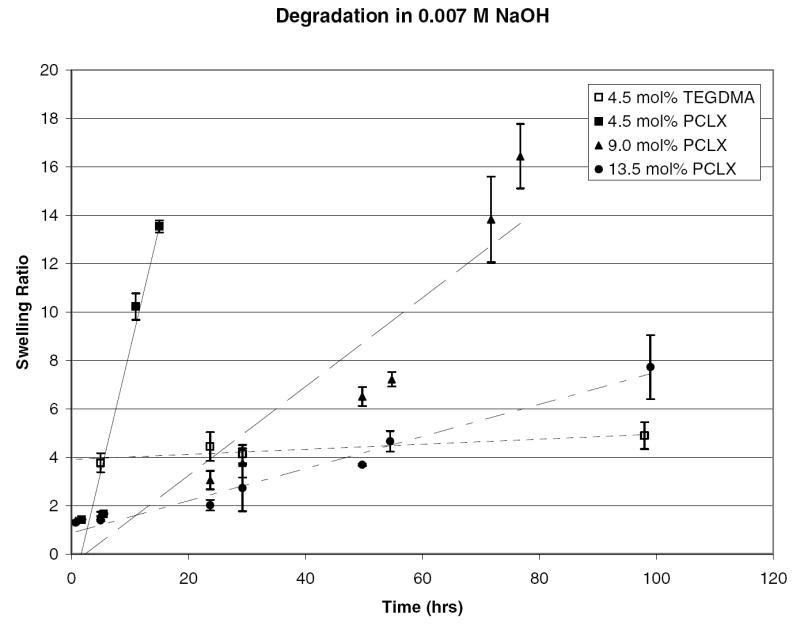
Swelling ratio as a function of time for pHEMA hydrogels crosslinked with 4.5, 9.0, and 13.5 mol % PCLX and 4.5 mol% TEGDMA in a 7 mmol solution of NaOH.
Figure 8.
Percent mass loss as a function of time for pHEMA hydrogels crosslinked with 4.5, 9.0, and 13.5 mol % PCLX and 4.5 mol% TEGDMA in a 7 mmol solution of NaOH.
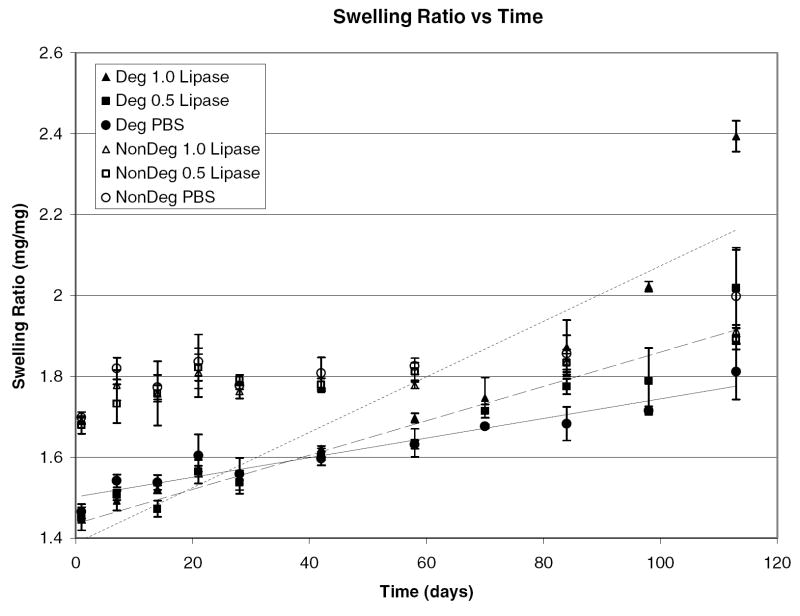
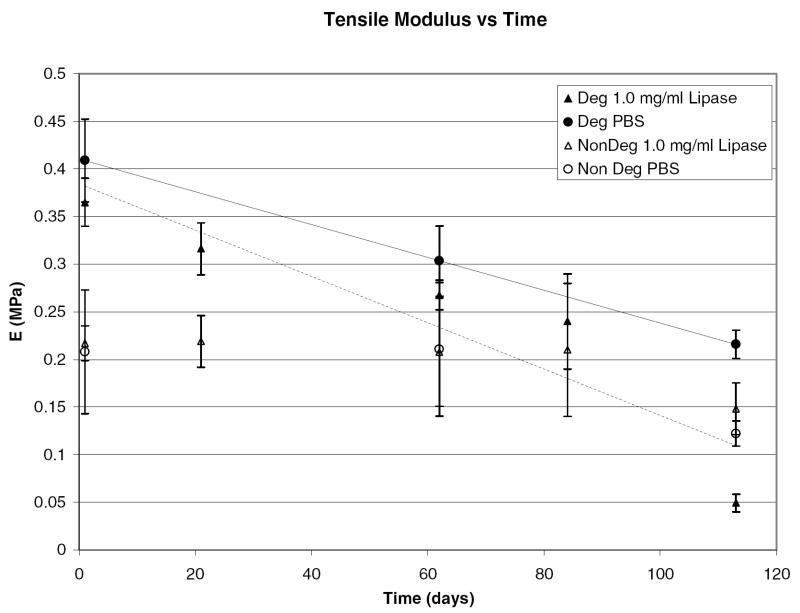
Experiments in NaOH confirm degradation, but, to more closely match the in vivo environment, degradation was examined in lipase solutions of 1.0 and 0.5 mg/mL and in PBS. The monomer to initiator ratio for these hydrogels was 75:1 (Mw = 10 kDa), the crosslink density varied from 4.5 to 13 mol percent, and the solvent to monomer ratio was kept constant at a 1:3 ratio. Figures 9 and 10 illustrate how the swelling ratio and tensile modulus change over degradation time.
Figure 9.
Swelling ratio as a function of time for pHEMA hydrogels crosslinked and initiated with PCLX /PCLI or TEGDMA/EBiB in solutions of 1.0 mg/mL lipase, 0.5 mg/mL lipase, and PBS.
Figure 10.
Tensile modulus of pHEMA hydrogels crosslinked and initiated with either PCLX/PCLI or TEGDMA/EBiB in 1.0 mg/mL lipase and PBS.
Degradation as measured by swelling and weight loss is observed in all media for the PCLX hydrogels while there is no statistical difference for control samples (crosslinked with TEGDMA). Both swelling ratio and tensile modulus for the degradable hydrogels vary linearly with degradation time with correlation values above 0.95. For PCLX/PCLI hydrogels, the swelling ratio increases from 1.4 to 2.0 over 16 weeks while nondegradable hydrogel start at 1.8 and remain constant over that time period. The nondegradable hydrogel swelling ratio is initially higher because the PCLX and PCLI contribute hydrophobic moieties that lower the water uptake by the hydrogel. As expected, the degradation observed by monitoring swelling ratio is more pronounced in the enzymatic solutions and the rate correlates with enzyme concentration. The tensile modulus for degradable hydrogels decreases from 0.4 to 0.05 MPa over the 16 weeks. Again, no statistical difference is observed in the nondegradable TEGDMA/EBiB hydrogels. From these data it is clear the hydrogel is subjected to bulk degradation as there are measurable initial changes in properties for all degradable samples. Figures 11 and 12 show the mass loss for control and degradable hydrogels respectively.
Figure 11.
Percent mass loss verses time for pHEMA hydrogels crosslinked and initiated with TEGDMA/EBiB in 1.0 mg/mL lipase, 0.5 mg/mL lipase, and PBS.
Figure 12.
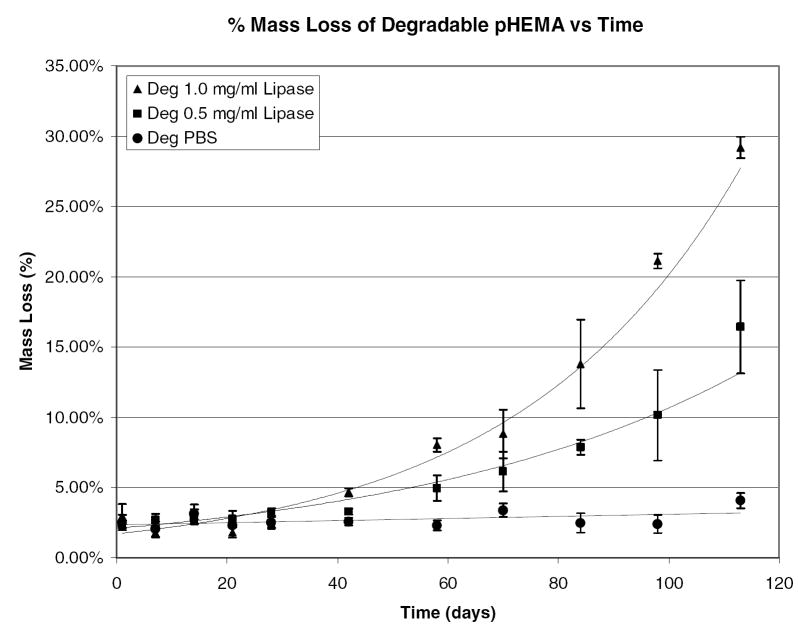
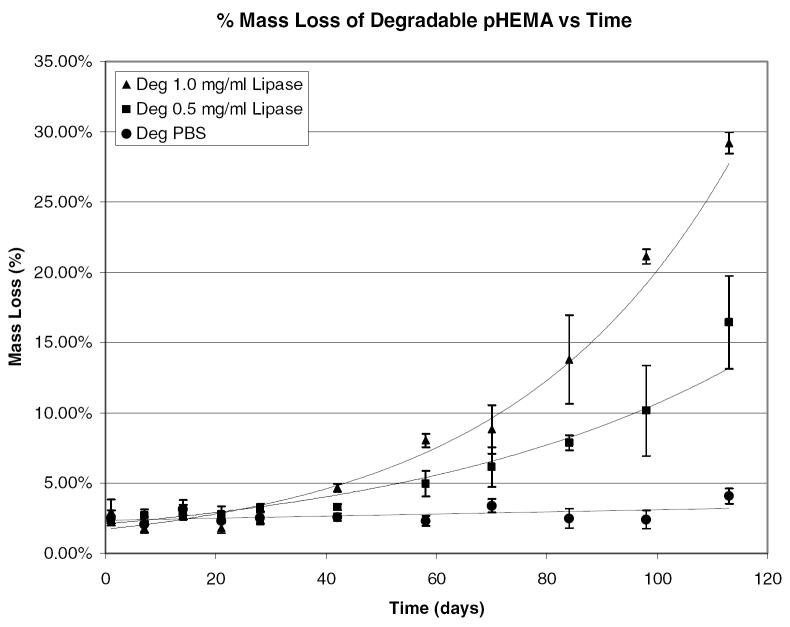
Percent mass loss verses time for degradable pHEMA hydrogels crosslinked and initiated with PCLX/PCLI in 1.0 mg/mL lipase, 0.5 mg/mL lipase, and PBS.
There is a significant lag time in mass loss where the hydrogel properties are changing but no erosion has occurred. Day 42 is the first time point where a statistically significant mass loss can be measured. After that time, mass loss proceeds quickly until the gels completely dissolve into the degradation media. Again, there is no statistically measurable mass loss for the control samples. Mass loss is only observed for the degradable samples in the enzymatic solutions. At 16 weeks the PCLX/PCLI hydrogels degraded in 1.0 mg/mL lipase have lost 30% of their original weight. As shown by the accelerated degradation profiles, the hydrogel rapidly erodes after this point and reliable measurements are difficult. While it is clear from swelling studies and tensile measurements that the hydrogel is subjected to bulk degradation, the samples exposed to enzymatic solutions may also be undergoing surface degradation leading to measurable mass loss. The enzymatically accelerated degradation is likely surface erosion as the diffusion of lipase into the bulk of the hydrogel in limited by the size of the enzyme. The mass loss varies exponentially with time and correlation coefficients for the two enzymatic experiments were found to be 0.85 and 0.95 for the 1.0 and 0.5 mg/mL respectively. This slow rate of mass loss is not unexpected. Kwoen et al. reported weight loss of 10% at 40 days for PCL and PCL networks in PBS [17]. However, Rice et al. reported complete degradation of PEG-PCL hydrogels in 1.0 mg/mL lipase solutions in less than 9 days[18]. The swelling ratio of the PEG-PCL hydrogels in that study were ten fold higher than these pHEMA gels and the PCL segments were presumably more accessible to hydrolysis and hydrolytic enzyme. The enzyme concentrations used were not selected based on known esterase concentrations in vivo, and thus may have a different activity than what will be encountered intramuscularly. Additionally, to address the slow rate of degradation current studies are investigating the use of poly(lactic acid) (PLA) and poly(glycolic acid) (PGA) as a replacement for the PCL segments in the degradable crosslinker. Preliminary results for PLA crosslinked hydrogels indicate a much faster degradation compared to PCL crosslinked hydrogels. Further investigations into tuning the degradation rate are in progress.
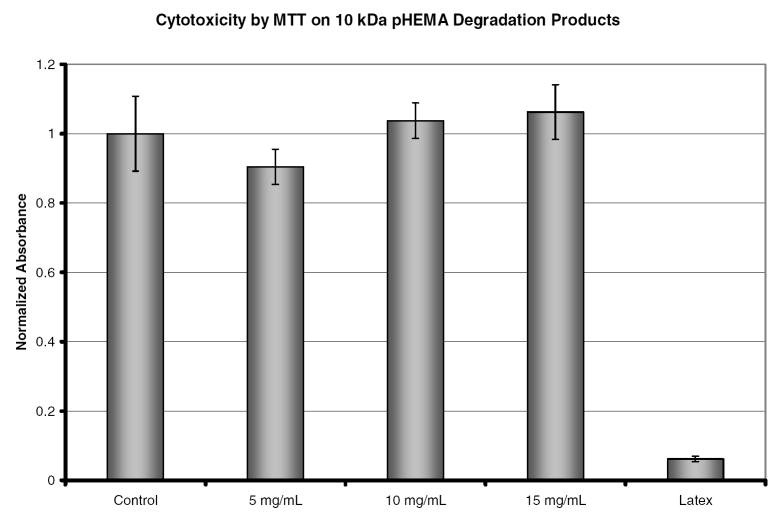
Prior to in vivo experiments the cytoxicity of the hydrogels and their degradation products must be evaluated. The MTT assay is a colorimetric method used to measure cell proliferation and cytotoxicity. Mitochondria of live cells reduce the yellow tetrazole to purple formazan which can be solubilized by acidic isopropanol. The absorbance of this colored solution can then be used to quantify the number of live cells. For this study the absorbance values of all samples were normalized to the negative control (TCPS). The hydrogels tested were not cytotoxic to fibroblasts and the degradation products showed no cytotoxicity at any of the concentrations examined. Cytotoxicity was evaluated at time points of 12, 24, and 48 hours. No statistical difference was observed in this time period. Figure 13 shows the normalized absorbance for the three concentrations tested as well as the positive (latex) and negative controls at 24 hours. These results justify examining these polymers in in vivo models for degradation and this will be the subject of a future study.
Figure 13.
Cytotoxicity as measured by MTT of degradation products at 5 mg/mL, 10 mg/mL, and 15 mg/mL. TCPS and latex were used as the negative and positive, respectively, controls for cytotoxicity. Absorbance has been normalized to the value of TCPS.
Conclusions
A novel degradable hydrogel has been synthesized and characterized. The addition of degradable PCL segments both in the initiator and crosslinker as well as control over the pHEMA molecular weight resulted in a bioresorbable pHEMA hydrogel. The degradation rate of this material can be controlled by the crosslinking density, the PCL chain length, and the pHEMA backbone chain length. The hydrogel exhibited mechanical properties that are suitable for cardiac tissue engineering scaffolds. Degradation products are soluble in solutions with ionic strength similar to blood and we expect that renal clearance take place. Bulk degradation was observed over a period of 16 weeks and seen only in enzymatic solutions. Because of the long history of successful application of methacrylate polymers in medical devices, we envision this new material to be readily accepted and to have applications in tissue engineering and drug delivery systems.
Acknowledgments
This work was supported by the National Institutes of Health (Grant No. HL64387). Thanks to Steve Korte, Alicia Gonzalez, and Mike Reigner for heart and scar tissue mechanical data.
Literature Cited
- 1.Heart Disease and Stoke Statistics-2006 Update, in American Heart Association. Dallas: American Heart Association; 2006. [Google Scholar]
- 2.Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Circ Res. 2007;100:263–72. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- 3.Leor J, Gerecht S, Cohen S, Miller L, Holbova R, Ziskind A, Shachar M, Feinberg MS, Guetta E, Itskovitz-Eldor J. Heart. 2007;93:1278–84. doi: 10.1136/hrt.2006.093161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leor J, Aboulafia-Etzion S, Dar A, Shapiro L, Barbash IM, Battler A, Granot Y, Choen S. Circulation. 2000;102:III56–61. doi: 10.1161/01.cir.102.suppl_3.iii-56. [DOI] [PubMed] [Google Scholar]
- 5.Hidalgo-Bastida LA, Barry JJ, Everitt NM, Rose FR, Buttery LD, Hall IP, Claycomb WC, Shaksheff KM. Acta Biomater. 2007;3:457–62. doi: 10.1016/j.actbio.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Eschenhagen T, Zimmermann WH. Circ Res. 2005;97:1220–31. doi: 10.1161/01.RES.0000196562.73231.7d. [DOI] [PubMed] [Google Scholar]
- 7.Park H, Cannizzarro C, Vunjak-Novakovic G, Langer R, Vacanti CA, Farokhzad OC. Tissue Eng. 2007;13:1867–77. doi: 10.1089/ten.2006.0198. [DOI] [PubMed] [Google Scholar]
- 8.Bryant SJ, Anseth KS. J Biomed Mater Res. 2002;59:63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 9.Rice MA, Anseth KS. J Biomed Mater Res A. 2004;70:560–8. doi: 10.1002/jbm.a.30106. [DOI] [PubMed] [Google Scholar]
- 10.Nair LS, Laurencin CT. Progress in Polymer Science. 2007;32:762–798. [Google Scholar]
- 11.Healy JM, Lewis SD, Kurz M, Boomer RM, Thompson KM, Wilson C, McCauley TG. Pharm Res. 2004;21:2234–46. doi: 10.1007/s11095-004-7676-4. [DOI] [PubMed] [Google Scholar]
- 12.Metters AT, Anseth KS, Bowman CN. J Phys Chem B. 2001;105:8069–76. [Google Scholar]
- 13.Homsy CA. J Biomed Mater Res. 1970;4:341–56. doi: 10.1002/jbm.820040306. [DOI] [PubMed] [Google Scholar]
- 14.Peppas NA, Moynihan HJ, Lucht LM. Journal of Biomedical Materials Research. 1985;19:397–411. doi: 10.1002/jbm.820190405. [DOI] [PubMed] [Google Scholar]
- 15.Ratner BD, Miller IF. Journal of Biomedical Materials Research. 1973;7:353–367. doi: 10.1002/jbm.820070407. [DOI] [PubMed] [Google Scholar]
- 16.Bakshi A, Fisher O, Dagci T, Himes BT, Fischer I, Lowman A. J Neurosurg Spine. 2004;1:322–9. doi: 10.3171/spi.2004.1.3.0322. [DOI] [PubMed] [Google Scholar]
- 17.Kweon H, Yoo MK, Park IK, Kim TH, Lee HC, Lee HS, Oh JS, Akaike T, Cho CS. Biomaterials. 2003;24:801–8. doi: 10.1016/s0142-9612(02)00370-8. [DOI] [PubMed] [Google Scholar]
- 18.Rice MA, Sanchez-Adams J, Anseth KS. Biomacromolecules. 2006;7:1968–75. doi: 10.1021/bm060086+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tay BY, Zhang SX, Myint MH, Ng FL, Chandrasekaran M, Tan LKA. Journal of Materials Processing Technology. 2007;182:117–121. [Google Scholar]
- 20.Woodward SC, Brewer PS, Moatamed F, Schindler A, Pitt CG. J Biomed Mater Res. 1985;19:437–44. doi: 10.1002/jbm.820190408. [DOI] [PubMed] [Google Scholar]
- 21.Darney PD, Monroe SE, Klaisle CM, Alvarado A. Am J Obstet Gynecol. 1989;160:1292–5. doi: 10.1016/s0002-9378(89)80015-8. [DOI] [PubMed] [Google Scholar]
- 22.Beers KL, Boo S, Gaynor SG, Matyjaszewski K. Macromolecules. 1999;32:5772–5776. [Google Scholar]
- 23.Weaver JVM, Bannister I, Robinson KL, Bories-Azeau X, Armes SP. Macromolecules. 2004;37:2395–2403. [Google Scholar]
- 24.Matyjaszewski K, Xia J. Chem Rev. 2001;101:2921–90. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- 25.Sawhney AS, Pathak CP, Hubbell JA. Macromolecules. 1993;26:581–587. [Google Scholar]
- 26.Huan K, Bes L, Haddleton DM, Khoshdel E. Journal of Polymer Science. 2001;39:1833–1842. [Google Scholar]
- 27.Baez JE, Marcos-Fernandez A, Lebron-Aguilar R, Martinez-Richa A. Polymer. 2006;47:8420–8429. [Google Scholar]
- 28.Jiang X, Lok MC, Hennink WE. Bioconjug Chem. 2007;18:2077–84. doi: 10.1021/bc0701186. [DOI] [PubMed] [Google Scholar]