Abstract
Objective: To study the effects of the generation 4 polyamidoamine/vascular endothelial growth factor antisense oligodeoxynucleotide (G4PAMAM/VEGFASODN) compound on the expressions of vascular endothelial growth factor (VEGF) and its mRNA of breast cancer cells and on the inhibition of vascular endothelial cells. Methods: We examined the morphology of G4PAMAM/VEGFASODN compound and its pH stability, in vitro transfection efficiency and toxicity, and the expressions of VEGF and its mRNA. Methyl thiazolyl tetrazolium assay was used to detect the inhibitory function of the compound on vascular endothelial cells. Results: The compound was about 10 nm in diameter and was homogeneously netlike. From pH 5 to 10, it showed quite a buffered ability. The 48-h transfection rate in the charge ratio of 1:40 was 98.76%, significantly higher than that of the liposome group (P<0.05). None of the transfection products showed obvious toxicity on the cells. The expressions of both VEGF protein and its mRNA after G4PAMAM/VEGFASODN transfection decreased markedly. Conclusion: With a low toxicity, high safety, and high transfection rate, G4PAMAM/VEGFASODN could be a promising gene vector. Specifically, it inhibits VEGF gene expression efficiently, laying a basis for further in vivo animal studies.
Keywords: Breast cancer, Vascular endothelial growth factor (VEGF), VEGF mRNA, Generation 4 polyamidoamine (G4PAMAM), Generation 4 polyamidoamine/vascular endothelial growth factor antisense oligodeoxynucleotide (G4PAMAM/VEGFASODN), Vascular endothelial cell
INTRODUCTION
Currently, gene transfer systems mainly include two types: viral and non-viral vectors. Among the non-viral vectors, while cationic liposome is the most commonly used one, the nanoparticle, which has been developed in recent years, is the most prospective gene transfer system. Researches in vivo and in vitro have shown that the cationic liposome and the nanoparticle can efficiently transfer target genes, such as the endostatin gene, into various human tumor cells and then kill the tumor cells (Hattori et al., 2007; Wang et al., 2000). In this experiment, we used generation 4 polyamidoamine (G4PAMAM) as the gene vector and vascular endothelial growth factor antisense oligodeoxynucleotide (VEGFASODN) as the target gene to synthesize the generation 4 polyamidoamine/vascular endothelial growth factor antisense oligodeoxynucleotide (G4PAMAM/VEGFASODN) compound, and used it to transfect a breast cancer cell line, MDA-MB-231 cells. We examined the morphology of G4PAMAM/VEGFASODN using transmission electron microscope (TEM), and also observed its pH stability. The transfection efficiency was detected by flow cytometry (FCM) and the positive transfection cells were detected by laser scanning confocal microscope. The survival rate of post-transfection cells was detected by methyl thiazolyl tetrazolium (MTT) assay. The expression of vascular endothelial growth factor (VEGF) protein was determined by immunohistochemical method and the expression of VEGF mRNA by reverse transcription-polymerase chain reaction (RT-PCR). The inhibitory function of the compound on vascular endothelial cells was detected by MTT assay.
MATERIALS AND METHODS
Materials
MDA-MB-231 cell line was purchased from Shanghai Institute of Chinese Academy of Sciences; fetal bovine serum was purchased from Shanghai Fumeng Company, China; MTT and dimethyl sulfoxide (DMSO) were from Sigma Company, USA; mouse monoclonal antibody against human VEGF, immunohistochemical SP staining kit, diaminobenzidine (DAB) color kit and Trizol RNA extraction kit were from Invitrogen, USA; reverse transcription kit was from MBI Co., USA; vascular endothelial growth factor scrambled oligodeoxynucleotide (VEGFSODN), VEGFASODN, vascular endothelial growth factor missense oligodeoxynucleotide (VEGFMSODN), VEGF and beta-actin primers were synthesized by Beijing Aoke Co., China.
Preparation of the transfection compound
The G4PAMAM colloid was dissolved in the triple-distilled water, and then oscillated to blend with the water, forming 1 g/L working fluid. 66 μg VEGFASODN was dissolved and blended in the triple-distilled water to prepare 0.1 mg/L working solution. After incubation at room temperature for 10 min, the VEGFASODN and G4PAMAM were mixed at charge ratios of 1:10, 1:20, 1:30 and 1:40. Every 1 μg DNA contained 1.96×1012 base groups, and every 1 μg G4PAMAM contained 2.751×1021 amino groups. All the solutions were oscillated and blended, incubated at room temperature for 20 min, and stored at 4 °C until use. About 2 μl G4PAMAM/VEGFASODN solution was dropped onto the copper mesh with Formvr+C membrane. After 30 min, the copper mesh was taken out to dry naturally and then examined by a TEM. The G4PAMAM/VEGFASODN compounds with different charge ratios were analyzed by 160 g/L degenerated acrylamide gel electrophoresis at pH 10, 7, and 5.
Detection of the transfection efficiency in vitro
MDA-MB-231 cells (1×105 ml−1) were seeded in a 24-well plate and grown in the growth medium for 48 h at 37 °C. When cells went into the flush stage of growth, they were starved for 4 h in serum-free medium. Then the cells were divided into pure cell group, liposome group, and G4PAMAM/VEGFASODN groups of different charge ratios, and were grown back in the growth medium with serum for 24, 48, and 72 h. Then the cells were tripsinized by 2.5 g/L trypsin and collected into numbered tubes as cell suspension. Cells were centrifuged and resuspended in 1 ml phosphate buffer solution (PBS). The cell suspension was examined and quantified by FCM. The cells were firstly observed under a fluorescence microscope and then pictured under a laser scanning confocal microscope before digesting and centrifugalization.
Detection of MTT
About 0.5 ml MDA-MB-231 cell suspension (1×105 ml−1) was dropped into each well of a 96-well plate. The cells were divided, washed, starved, and grown back in the growth medium with serum as the procedure above. Then the cells were collected for MTT detection. The cells without transfection were set as blank control. To each well, 20 μl MTT (5 g/L) was added. The plate was incubated at room temperature for another 4 h. Cells were washed twice by PBS, 150 μl DMSO was added to each well, and the plate was oscillated horizontally for 10 min for the crystal to be dissolved and colored. At last, the absorbance (A) at 570 nm was read by the multi-function minipoles and the relative growth rate (RGR) was calculated: RGR (%)=A E/A C×100, where A E is the absorbance in the experiment group and A C is the absorbance in the blank control group.
Immunohistochemical staining
The cells were prepared as above. Forty-eight hours after the transfection, cells were washed twice by PBS and fixed in 40 g/L paraformaldehyde for 30 min. Then the cells were incubated in deionized water containing 30 ml/L H2O2 for 10 min so as to eliminate the activity of endogenous peroxidase. After rinse in distilled water and immersion in PBS for 5 min, the working solution of normal goat serum was added into the cell suspension and incubated for 15 min at room temperature. Then the goat serum was discarded, and the primary antibody (mouse monoclonal antibody against human VEGF with the dilution of 1:100, which was the best concentration chosen by the Chessboard method) was added without pre-wash, and incubated overnight at 4 °C. The cells were washed with PBS 3 times, 3 min each. The biotin conjugated goat IgG against mouse VEGF was added, incubated for 15 min at room temperature, and then washed with PBS 3 times, 3 min each. Next, the horseradish peroxidase (HRP) conjugated streptavidin working solution was added, incubated for 15 min at room temperature, and washed 3 times with PBS, 3 min each. The peroxidase activity was demonstrated by using the DAB substrate, which was controlled under microscope and followed by fully washing in tap water. Then the cells were mildly stained by hematoxylin, gradually dehydrated by ethanol, transparented by dimethyl benzene, and mounted by intermediate polar resin. The samples were scored according to (1) the intensity of staining: 0 for achromatism, 1 for straw yellow, and 2 for brown; and (2) the positive staining cell rate: 0 for below 20%, 1 for between 20% and 50%, and 2 for above 50%. During cell counting, for each section, 10 representative fields of vision under high-power microscope were chosen, and 100 cells were counted in each field. Taking (1) and (2) together, the results were graded into 4 levels: 0 is considered as negative, 1 weak positive, 2 and 3 positive, and 4 strong positive.
VEGF mRNA expression
The cells were cultivated and processed as mentioned above and divided into control group, G4PAMAM/VEGFSODN group, G4PAMAM/VEGFASODN group, and G4PAMAM/VEGFMSODN group. Total RNA was extracted according to the instruction of Trizol extraction kit 48 h after the transfection. Cells (5×106) were spun down, blended with 1 ml Trizol, and incubated for 5 min at room temperature. 0.2 ml chloroform was added to the solution, vortexed for 15 s, and incubated for 5 min at room temperature. Samples were centrifuged at 12 000×g at 4 °C for 15 min, and the upper achromatic aqueous phase was removed. After adding 0.5 ml isopropanol, samples were incubated at room temperature for 10 min and then centrifuged at 12 000×g at 4 °C for 10 min. Next, the supernatant was removed and 1 ml 75% ethanol was added. The reverse transcription was carried out according to the instruction of the first cDNA chain synthesis reaction kit. Total RNA (1 μl) and random primer (1 μl) were blended with 10 μl treated water. After 5 min incubation at 70 °C, the solution was cooled by ice. After a transient centrifugalization, samples were blended with 4 μl 5× reaction buffer, 1 μl RNase inhibitor and 2 μl 10 mmol/L dNTP mix. Then the samples were placed into a 25 °C water bath for 5 min, incubated at 42 °C for 60 min and later at 70 °C for 10 min. The primer sequences of VEGF were CCCACTGAGGAGTCCAACAT (sense chain) and CATTTACACGTCAGCGGATC (antisense chain). The primer sequences of β-actin were CCTTCCTGGGCATGGAGTCCTG (sense chain) and GGAGCAATGATCTTGATCTTC (antisense chain).
The PCR started with the solution containing 1 μl of each primer and 12.5 μl Master Mix diluted to 25 μl by ddH2O. The conditions were 3 min predenaturation at 94 °C, 1 min denaturation at 94 °C, and 50 s annealing at 55 °C, 40 s extension at 72 °C, and one more extension for 5 min after 32 cycles. The fruit of RT-PCR was put in the 15 g/L agarose gel electrophoresis for 30 min and then scanned by the gel imaging system to assay the grey valve. Taking β-actin as the internal standard, the relative valve of VEGF expression to β-actin was calculated.
Vascular endothelial cell growth inhibition
The conventionally cultivated MDA-MB-231 cells were inoculated on a 96-well plate, 1×104 cells for each well, and divided into control group, G4PAMAM group, G4PAMAM/VEGFSODN group, G4PAMAM/VEGFASODN group, and G4PAMAM/VEGFMSODN group, five wells per group. When the cells completely adhered to the wall, they were washed in PBS buffer 3 times. Then the cell fluid was changed, and various compounds prepared by RPMI 1640 culture medium without calf serum were added. After 72 h incubation, the culture medium of each group was collected into aseptic cryopreservation tubes and centrifuged at 800 r/min for 8 min. Then the supernatant was collected and reserved in the cryogenic refrigerator.
To examine vascular endothelial cell growth inhibition, calf aorta endothelial cells in passages 3 to 5 with the growth medium were seeded into a 96-well plate for 5 groups, 5 wells each, 1×104 cells per 200 μl per well. We also set up a group for negative control in which there were no calf aortic endthelial cells. When the cells grew attached to the wall, they were changed into the 200 μl PRMI 1640 culture medium with 100 ml/L calf serum and incubated for 24 h. Then the culture medium was sucked out, and 150 μl RPMI 1640 complete culture medium containing 100 ml/L calf serum and 50 μl corresponding breast cancer cell supernatant were added to each group. In the control group, 200 μl RPMI 1640 culture medium with 100 ml/L calf serum was added. The cells were collected after 24, 48, and 72 h incubation in the 50 ml/L CO2 incubator at 37 °C. In the end, the MTT method was performed to detect the growth of the vascular endothelial cells of each group, the absorbance (A) at 570 nm was read by the multi-function minipoles, and the RGR was calculated.
Statistical analysis
All the results were expressed as mean±SD, and all the data were analyzed by SPSS 12.0 software. P<0.05 was considered statistically significant.
RESULTS
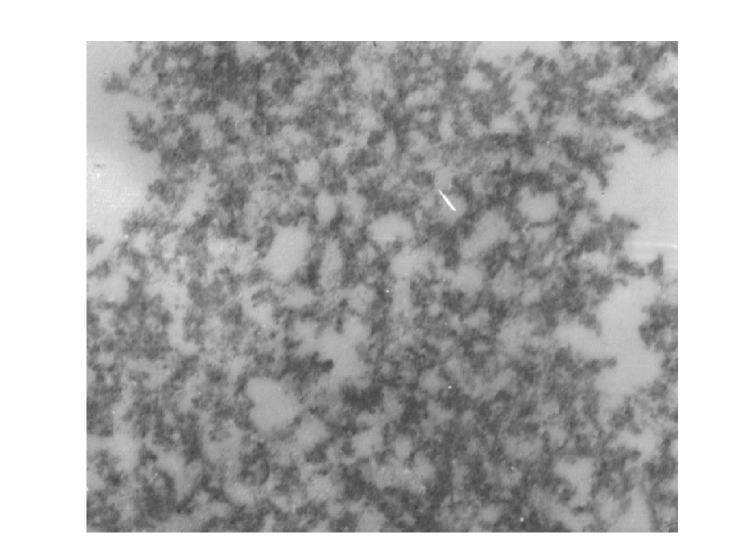
Electron microscopy of the transfection compound
Under a TEM, we observed that the G4PAMAM/VEGFASODN compound was about 10 nm in diameter and was homogeneously netlike (Fig.1).
Fig. 1.
The ultrastructure of G4PAMAM/VEGFASODN under TEM (magnification ×100 000)
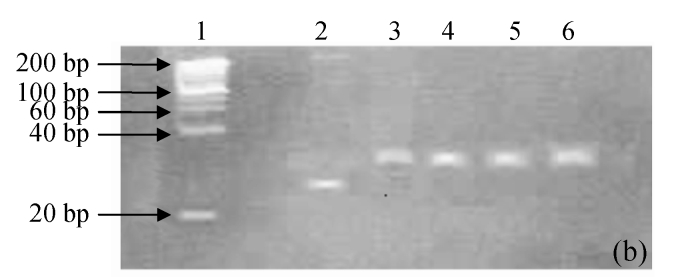
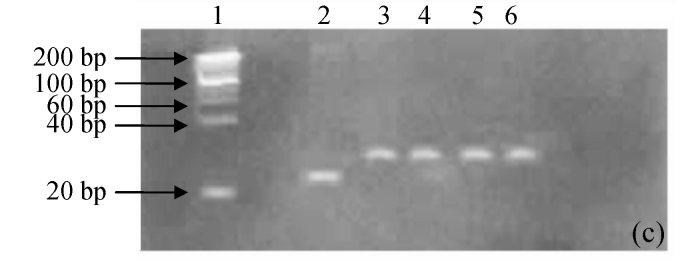
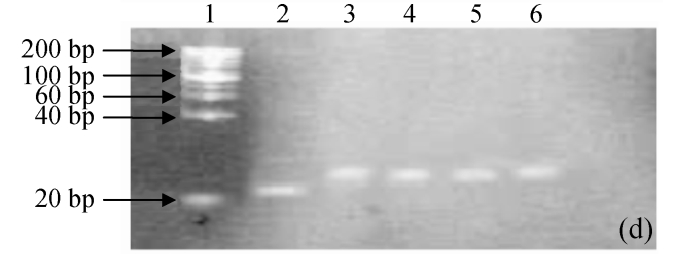
pH stability of G4PAMAM/VEGFASODN compound
The degenerated acrylamide gel electrophoresis showed that the G4PAMAM/VEGFASODN had quite a buffered ability from pH 5 to 10. No dissociation was seen in all the G4PAMAM/VEGFASODN compounds with different charge ratios (Fig.2), indicating that these compounds were fairly stable and ready to transfect the cells.
Fig. 2.
The pH stability electrophoresis experiment of G4PAMAM/VEGFASODN of different charge ratios. (a) 1:10; (b) 1:20; (c) 1:30; (d) 1:40
Lane 1: maker; Lane 2: ASODN; Lane 3: G4PAMAM/VEGFASODN; Lane 4: G4PAMAM/VEGFASODN at pH 10; Lane 5: G4PAMAM/VEGFASODN at pH 7; Lane 6: G4PAMAM/VEGFASODN at pH 5
Transfection efficiency
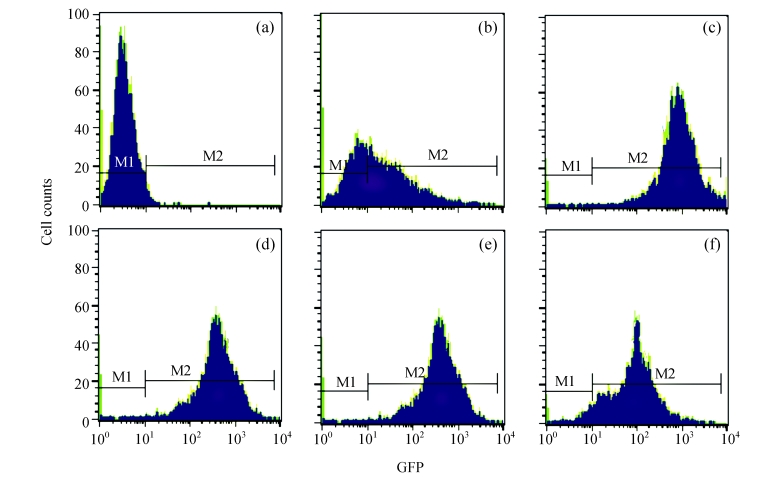
Under a laser scanning confocal microscope, we observed that the green fluorescence was seen in cells after 24 h of transfection and its intensity gradually increased thereafter, indicating that G4PAMAM had successfully transfected VEGFASODN into MDA-MB-231 cells (Fig.3). At 48 h the highest transfection efficiency was achieved and the expression maintained at a high level until 72 h before declining. When VEGFASODN mixed with G4PAMAM in a charge ratio of 1:40, the green fluorescent protein in the cells was obviously higher than that of other charge ratios. The transfection rates were 1.47% in the blank control group, 56.44% in the liposome group, and 98.13%, 98.36%, 98.41% and 98.76% in the G4PAMAM/VEGFASODN groups of different charge ratios (1:10, 1:20, 1:30, and 1:40, respectively), measured by FCM (Fig.4).
Fig. 3.
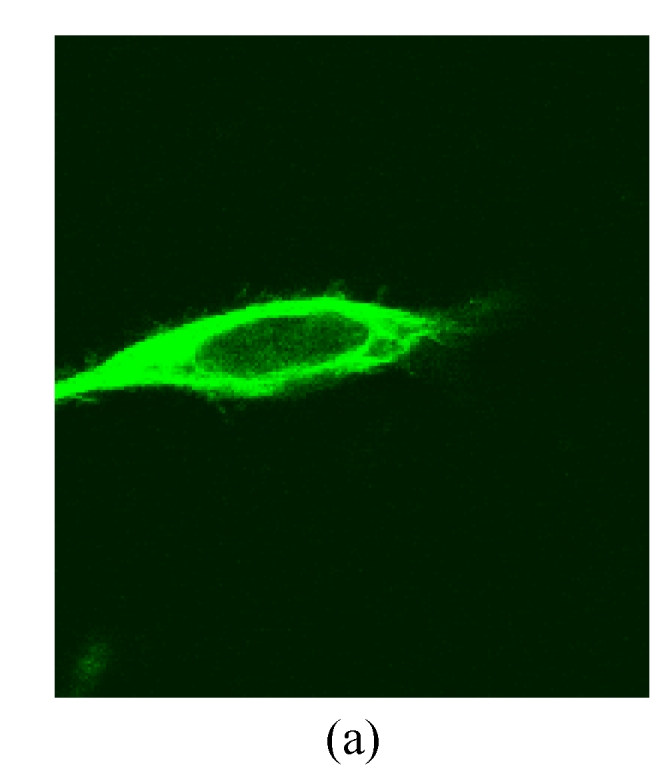

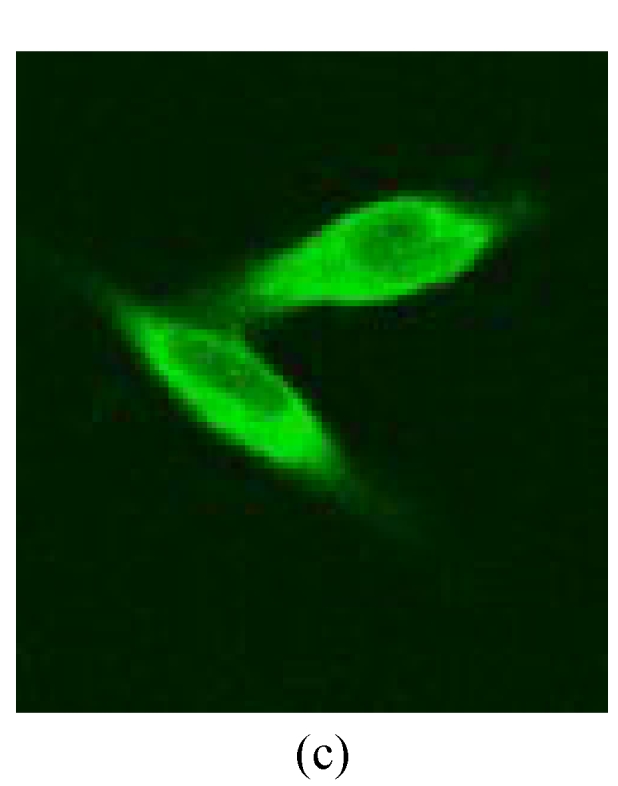
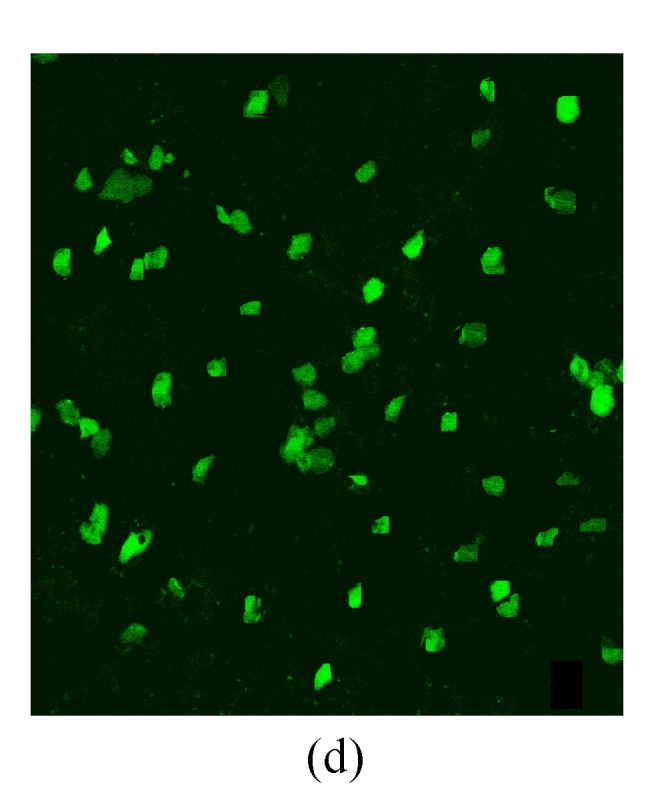
The green fluorescent protein expression of MDA-MB-231 cells at 48 h
(a), (b) Transfection in liposome at different magnifications (×400 and ×40, respectively); (c), (d) Transfection in 1:40 G4PAMAM/VEGFASODN at different magnifications (×400 and ×40, respectively)
Fig. 4.
The transfection efficiency of MDA-MB-231 cells. The difference of transfection rates demonstrated statistic difference (P<0.05)
(a) Without disposition of the compound; (b) Liposome; (c) 1:10 G4PAMAM/VEGFASODN; (d) 1:20 G4PAMAM/VEGFASODN; (e) 1:30 G4PAMAM/VEGFASODN; (f) 1:40 G4PAMAM/VEGFASODN. GFP: green fluorescent protein
Detection of RGR by MTT assay
MTT assay showed that all the transfection products of each group did not have toxicity to cells. The activity of cells decreased as the electric charge ratio of G4PAMAM/VEGFASODN increased and gradually recovered as time passed (Table 1). The cell activity was significantly higher in the control group than in the transfection groups (P<0.05). When compared in pairs, there were no significant differences between liposome, 1:10 and 1:20 G4PAMAM/VEGFASODN groups (P>0.05), nor between 1:30 and 1:40 G4PAMAM/VEGFASODN groups (P>0.05); however, significant differences were found among liposome, 1:10, 1:20, 1:30 and 1:40 G4PAMAM/VEGFASODN groups (P<0.05). These results indicated that the cell activity might be affected by the presence and increasing concentration of G4PAMAM.
Table 1.
The cell activity in each group detected by MTT
| Groups |
A |
||
| 24 h | 48 h | 72 h | |
| Control | 1.722±0.101 | 2.539±0.127 | 2.506±0.092 |
| Liposome | 1.571±0.059 | 2.382±0.089 | 2.361±0.395 |
| G4PAMAM/VEGFASODN | |||
| 1:10 | 1.477±0.041 | 2.375±0.059 | 2.416±0.453 |
| 1:20 | 1.421±0.104 | 2.349±0.076 | 2.403±0.472 |
| 1:30 | 1.416±0.115 | 2.291±0.085 | 2.333±0.446 |
| 1:40 | 1.410±0.027 | 2.298±1.177 | 2.298±0.056 |
A: absorbance at 570 nm; All data are expressed as mean±SD
Expression of VEGF protein
VEGF positivity was displayed as the brownish yellow cytoplasm in MDA-MB-231 tumor cells according to different degrees of staining. The expression rate of VEGF protein before transfection was 48.9%, and the positive staining spot was mainly located in the cytoplasm and few located in the nucleus (Table 2, Fig.5). However, the VEGF protein after G4PAMAM/VEGFASODN transfection was observed as straw yellow, mainly located in the cytoplasm and few in the nucleus, and the positive expression rate decreased to 29.3%. The positive expression rates of G4PAMAM/VEGFSODN group and G4PAMAM/VEGFMSODN group were 41.3% and 39.2% respectively, and the proteins were also mainly in the cytoplasm and few in the nucleus. These results indicated that G4PAMAM/VEGFASODN markedly inhibited the expression of VEGF protein in MDA-MB-231 cells.
Table 2.
The positive expression of VEGF protein in MDA-MB-231 cells
| Groups | Positive expression |
|
| BT | AT | |
| G4PAMAM/VEGFSODN | +++ | ++ |
| G4PAMAM/VEGFASODN | +++ | + |
| G4PAMAM/VEGFMSODN | +++ | ++ |
BT: before transfection; AT: after transfection
Fig. 5.
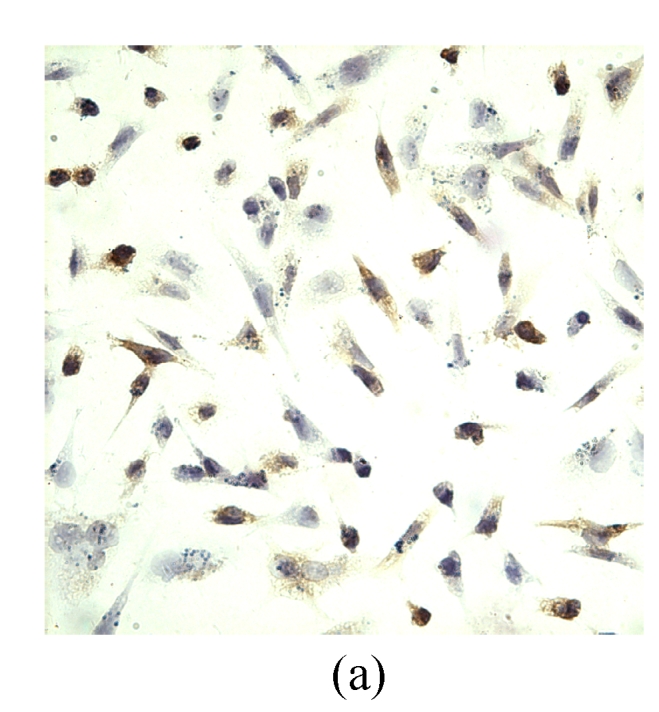
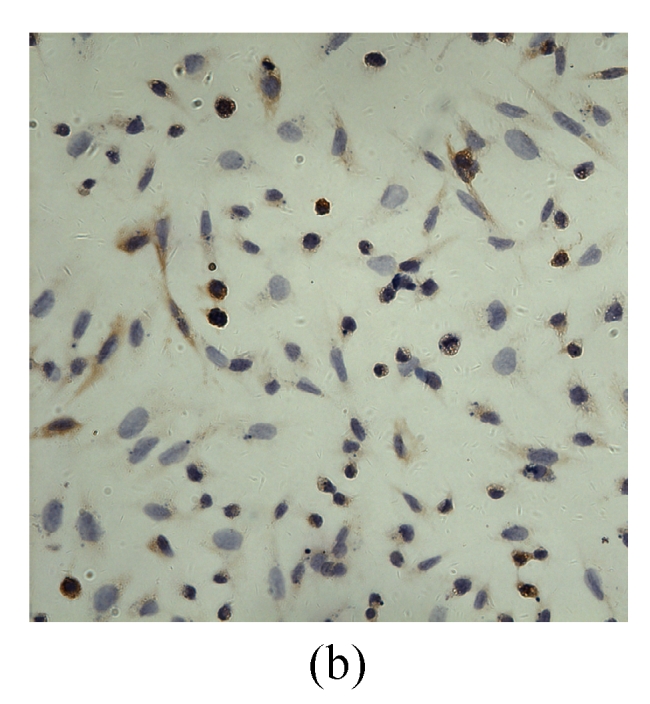
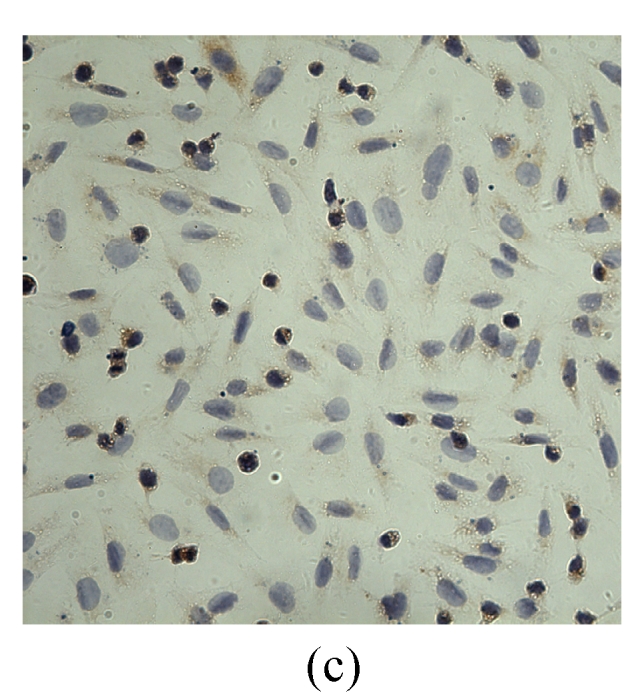
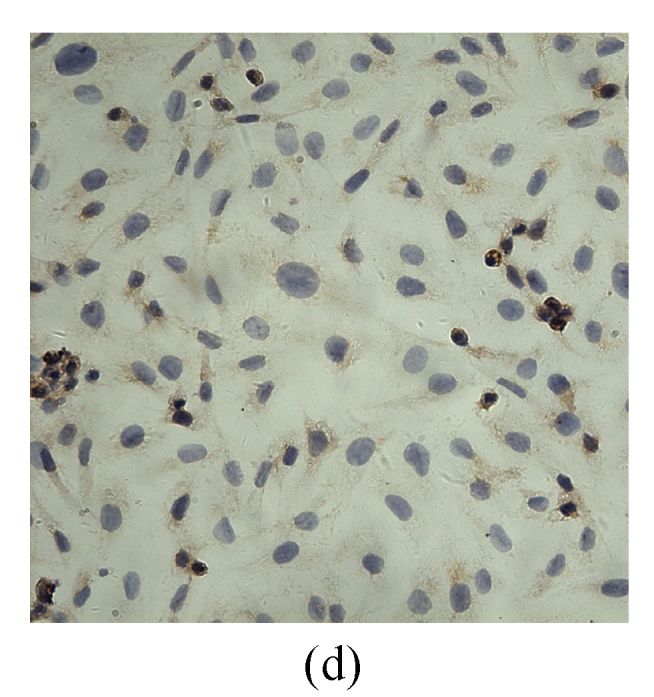
The positive expression of VEGF protein in MDA-MB-231 cells at 48 h (magnification ×400)
(a) Without disposition of the compound; (b) G4PAMAM/VEGFSODN; (c) G4PAMAM/VEGFASODN; (d) G4PAMAM/VEGFMSODN
Expression of VEGF mRNA
RT-PCR results showed a 200-bp band for VEGF mRNA and a 150-bp band for β-actin. The relative expression amount of VEGF mRNA was 0.85±0.02 in pre-transfection cells, and 0.43±0.05 in the post-transfection G4PAMAM/VEGFASODN group, showing a significant difference (P<0.05). In G4PAMAM/VEGFSODN group and G4PAMAM/VEGFMSODN group, the relative expression amounts were 0.79±0.03 and 0.71±0.023 respectively, similar to that in the control group (P>0.05). These results demonstrated that antisense VEGF gene greatly inhibited VEGF mRNA in MDA-MB-231 cells (Fig.6).
Fig. 6.
The relative expression level of VEGF mRNA of MDA-MB-231 cell line
Lane 1: maker; Lane 2: control group; Lane 3: G4PAMAM/VEGFASODN; Lane 4: G4PAMAM/VEGFSODN; Lane 5: G4PAMAM/VEGFMSODN
Growth of vascular endothelial cell
After 24 h of transfection, the G4PAMAM/VEGFASODN group was observed to have obvious cytotoxicity, whereas the G4PAMAM/VEGFSODN group and G4PAMAM/VEGFMSODN group had only mild to modest cytotoxicity. After 48 h, all the groups were observed to have mild cytotoxicity. The G4PAMAM group was shown to have only mild cytotoxicity and the endothelial cells gradually recovered to have activity after 72 h. Differences could be seen among different groups and time (P<0.01). For instance, the 24-h endothelial cell growth was 0.396±0.029 in G4PAMAM group, 0.387±0.046 in G4PAMAM/VEGFSODN group, 0.297±0.015 in G4PAMAM/VEGFASODN group, and 0.376±0.019 in G4PAMAM/VEGFMSODN group, significantly lower than that in control group (0.517±0.051, P<0.05, Table 3).
Table 3.
The growth of vascular endothelial cells in different groups
| Groups |
A |
||
| 24 h | 48 h | 72 h | |
| Control | 0.517±0.051 | 1.189±0.019 | 1.518±0.037 |
| G4PAMAM | 0.396±0.029 | 1.081±0.084 | 1.445±0.056 |
| G4PAMAM/VEGFSODN | 0.387±0.046 | 1.067±0.076 | 1.426±0.059 |
| G4PAMAM/VEGFASODN | 0.297±0.015 | 0.896±0.049* | 1.349±0.045 |
| G4PAMAM/VEGFMSODN | 0.376±0.019 | 0.980±0.088 | 1.383±0.069 |
A: absorbance at 570 nm; All data are expressed as mean±SD;
P<0.05 among G4PAMAM/VEGFASODN group, G4PAMAM/VEGFMSODN group, and control group separately
DISCUSSION
The occurrence of breast cancer is a multi-step process based on the activation of several oncogenes at different time and space through different pathways and the inactivation of several tumor suppressor genes. Therefore, the gene therapy, showing a great hope, gradually becomes one of the most prospective methods in biologic treatment of tumor (Edelstein et al., 2007). It is the fifth treatment modality after surgery, radiotherapy, chemotherapy, and immunotherapy (Flotte, 2007). It transfects the external functional gene or other germ plasm into the patient’s body by certain ways so that it could decrease and/or increase expressions of certain genes to compensate the clinical symptom caused by the defected gene. Because of the rapid development of tumor molecular pathology as well as recombinant DNA technology, the clinical application of gene therapy in tumors is practicable (Alton, 2007). At present time, the gene therapy of breast cancer mainly focuses on immuno-gene therapy, tumor suppressor gene therapy, apoptosis gene therapy, chemical gene therapy, gene knockout therapy, anti-angiogenesis gene therapy, and combination gene therapy (Vattemi and Claudio, 2007).
In recent years, the non-viral vectors, like polyethyleneimine, chitosan, polylysine, and PAMAM in this experiment, are being deeply researched (Miller, 2003). PAMAM is a new artificial synthetic nano-high molecular polymer and is the most prospective non-viral vector of late years (Esfand and Tomalia, 2001). In the common physiologic condition, because of the N-terminal, the PAMAM carries positive charge and can band the phosphate group of DNA carrying the negative charge, forming the nano-polyelectrolyte compound to act as gene vector (Mo et al., 2007). As a nano-particle, it can easily pass the gap of the tumor vascular endothelial cells that are hypoplasia and then be phagocytized by the tumor cells. The phagocytic function of tumor cell is 8- to 100-fold stronger than that of the normal cell. In this way, the passive targeting function is achieved (Guo et al., 2004). This compound has a good stability and solubility. It can exist stably in aqueous solution for weeks and in different buffers or pHs, without dissociation (Devarakonda et al., 2007). Tightly bound by means of static electricity effort, the compound can protect DNA from being degraded by enzymes either in or out of cells. Similar to the mechanism of other non-viral gene vectors, like liposome and polyamine, the compound formed by PAMAM and DNA can enter the cells by endocytosis, and consequently transfer the gene into cells (Yoo and Juliano, 2000).
While the gene therapy of tumor is being taken into great account in recent years, the research of antisense oligonucleotide inhibiting the growth of tumor is still in cellular level, and the research of VEGFASODN is just at its very beginning. However, VEGFASODN has already been qualified to be used in clinical treatment of breast cancer for its clear mechanism, stability, small toxicity and side effect, and low cost. VEGF has a strong effect of stimulating the bovine aortic vascular endothelial cells and can raise the vasopermeability of the capillary vessel. The normal tissues do not express or little express VEGF, whereas the tumor tissues express VEGF highly and specifically the effect on the vascular endothelial cell by means of exocrine. Moreover, there are already some researches (Hayes et al., 2007; Zelnak and O′Regan, 2007) of using monoclonal antibody against VEGF and cDNA to inhibit the growth of the blood vessel of tumor. After entering the nucleus, ASODN specifically binds with target mRNA by sequence. On one hand, it blocks the translation; on the other hand, it inhibits the maturity and transfer of mRNA in the cytoplasm. That is to say, it can directly, efficiently block and inhibit the expression of target gene on the transcription and translation levels (Schiavone et al., 2004).
In the current study, we found that the VEGFASODN with phosphorothioate modification was successfully transfected into MDA-MB-231 cells by the mediating of G4PAMAM. As time passed, the transfection rate increased, with the highest transfection rate occurring at 48 h. The immunohistochemical test and RT-PCR results suggest that the morphology of MDA-MB-231 cells after transfection was basically normal, showing no obvious necrosis. The expressions of VEGF mRNA and protein in MDA-MB-231 cells with VEGFASODN transfection were lower than those of VEGFSODN, VEGFMSODN, and control groups, indicating that intracellular VEGFASODN can block and inhibit the expressions of VEGF mRNA and protein. Our observation shows that antisense VEGF gene inhibited the expression of VEGF mRNA, and thus broke the synthesis and secretion of VEGF. In this experiment, the VEGF protein was not quantified and therefore needs further investigation. After the decrease of the VEGF secretion of tumor cells, the growth of tumor blood vessels decreases, because the stimulations through autocrine and paracrine to the growth of endothelial cells and tumor cells decrease. And so does the density of the tumor capillary vessels. The signal translation of VEGF is broken, so the growth and infiltration of tumor are inhibited (Bertolini et al., 2007). Thus, compared with other angiogenic growth factors, the transfection of VEGF has double functions in anti-generation and vasopermeability of the tumor blood vessels.
In the experiment, G4PAMAM/VEGFASODN did not completely stop the MDA-MB-231 cells from expressing VEGF, but decreased its expression level. Because the G4PAMAM/VEGFASODN can only inhibit tumor capillary vessels, other approaches must be also combined to achieve a better inhibition effect. We, therefore, conclude that G4PAMAM successfully transfected VEGFASODN into MDA-MB-231 cells and inhibited the expressions of the VEGF mRNA and protein. With a low toxicity and high safety and transfection rate, G4PAMAM could be a promising gene vector for further in vivo animal experiment.
Acknowledgments
The work is carried out in the First Affiliated Hospital, College of Medicine, Xi’an Jiaotong University. We sincerely thank Prof. Tian-bao SONG and Feng-ling PENG, Medical School of Xi’an Jiaotong University, for their selfless sacrifice and helpful advice on modification of this paper.
Footnotes
Project (No. 30572140) supported by the National Natural Science Foundation of China
References
- 1.Alton E. Progress and prospects: gene therapy clinical trials (part 1) Gene Ther. 2007;14(20):1439–1447. doi: 10.1038/sj.gt.3303001. [DOI] [PubMed] [Google Scholar]
- 2.Bertolini F, Mancuso P, Shaked Y, Kerbel RS. Molecular and cellular biomarkers for angiogenesis in clinical oncology. Drug Discov Today. 2007;12(19-20):806–812. doi: 10.1016/j.drudis.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 3.Devarakonda B, Otto DP, Judefeind A, Hill RA, de Villiers MM. Effect of pH on the solubility and release of furosemide from polyamidoamine (PAMAM) dendrimer complexes. Int J Pharm. 2007;345(1-2):142–153. doi: 10.1016/j.ijpharm.2007.05.039. [DOI] [PubMed] [Google Scholar]
- 4.Edelstein ML, Abedi MR, Wixon J. Gene therapy clinical trials worldwide to 2007—an update. J Gene Med. 2007;9(10):833–842. doi: 10.1002/jgm.1100. [DOI] [PubMed] [Google Scholar]
- 5.Esfand R, Tomalia DA. Poly(amidoamine) (PAMAM) dendrimers: from biomimicry to drug delivery and biomedical applications. Drug Discov Today. 2001;6(8):427–436. doi: 10.1016/S1359-6446(01)01757-3. [DOI] [PubMed] [Google Scholar]
- 6.Flotte TR. Gene therapy: the first two decades and the current state-of-the-art. J Cell Physiol. 2007;213(2):301–305. doi: 10.1002/jcp.21173. [DOI] [PubMed] [Google Scholar]
- 7.Guo C, Wang H, Lin Y, Cai Q. Application of Starburst (TM) PAMAM dendrimers as DNA carriers in vitro. Prog Biochem Biophys. 2004;31(9):804–811. [Google Scholar]
- 8.Hattori Y, Ding W, Maitani Y. Highly efficient cationic hydroxyethylated cholesterol-based nanoparticle-mediated gene transfer in vivo and in vitro in prostate carcinoma PC-3 cells. Journal of Controlled Release. 2007;120(1-2):122–130. doi: 10.1016/j.jconrel.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 9.Hayes DF, Miller K, Sledge G. Angiogenesis as targeted breast cancer therapy. Breast. 2007;16(2):17–19. doi: 10.1016/j.breast.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 10.Miller AD. The problem with cationic liposome/micelle-based non-viral vector systems for gene therapy. Curr Med Chem. 2003;10(14):1195–1211. doi: 10.2174/0929867033457485. [DOI] [PubMed] [Google Scholar]
- 11.Mo Z, Liu Y, Chen H, Li H, Sun Y. Preparation and molecular dynamics simulation of Sm/PAMAM nano-composite. Rare Metal Mat Eng. 2007;36(1):32–36. (in Chinese) [Google Scholar]
- 12.Schiavone N, Donnini M, Nicolin A, Capaccioli S. Antisense oligonucleotide drug design. Curr Pharm Design. 2004;10(7):769–784. doi: 10.2174/1381612043452956. [DOI] [PubMed] [Google Scholar]
- 13.Vattemi E, Claudio PP. Advances and perspectives in gene-based therapy for breast cancer. Drug Future. 2007;32(6):507–516. doi: 10.1358/dof.2007.032.06.1096368. [DOI] [Google Scholar]
- 14.Wang Y, Boros P, Liu J, Qin L, Bai Y, Bielinska AU, Kukowska-Latallo JF, Baker JR, Bromberg JS. DNA/dendrimer complexes mediate gene transfer into murine cardiac transplants ex vivo. Mol Ther. 2000;2(6):602–608. doi: 10.1006/mthe.2000.0201. [DOI] [PubMed] [Google Scholar]
- 15.Yoo H, Juliano RL. Enhanced delivery of antisense oligonucleotides with fluorophore-conjugated PAMAM dendrimers. Nucleic Acids Res. 2000;28(21):4225–4231. doi: 10.1093/nar/28.21.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zelnak AB, O′Regan RM. Targeting angiogenesis in advanced breast cancer. BioDrugs. 2007;21(4):209–214. doi: 10.2165/00063030-200721040-00001. [DOI] [PubMed] [Google Scholar]