Abstract
Understanding of the mechanism of colorectal carcinogenesis has been gaining momentum for some years on account of its high incidence and impact on the lives of individuals affected. Different genetic abnormalities have been found in colorectal cancers from different sites. For example, proximal colon cancer is usually related to the nucleotide instability pathway, as microsatellite instability (MSI). However, distal colon cancer is usually associated with specific chromosomal instability (CIN). The development of cancer at the rectum, though similar to that at the colon, displays its own unique features. These differences might be partially attributed to different embryological development and physiological circumstances. Environmental factors such as diet and alcohol intake also differ in their role in the development of tumors in the three segments, proximal colon, distal colon, and rectum. “Proximal shift” of colon cancer has been known for some time, and survival rates of colorectal cancer are higher when rectal cancers are excluded, both of which emphasize the three different segments of colorectal cancer and their different properties. Meanwhile, colonic and rectal cancers are distinctive therapeutic entities. The concept of three entities of colorectal cancer may be important in designing clinical trails or therapeutic strategies. However, the dispute about the inconsistency of data concerning the site-specific mechanism of colorectal carcinoma does exist, and more evidence about molecular events of carcinogenesis and targeted therapy needs to be collected to definitely confirm the conception.
Keywords: Colorectal cancer, Proximal colon, Distal colon, Rectum, Carcinogenesis, Epidemiology and prognosis
INTRODUCTION
Colorectal cancer (CRC) is one of the commonest cancers and the third leading cause of cancer death. CRC incidence has decreased as a result of effective intervention and life-style changes in the West. However, in developing counties like China, economic development and the adoption of earlier Western life-styles have led to an increased CRC incidence similar to that of developed countries more than two decades ago. Interaction of genetics and environmental effects has an important role in colorectal carcinogenesis. The prognosis of CRC, especially advanced cancer, has not substantially improved although the molecular mechanism of CRC has become better understood compared with other solid tumors in the past two decades. According to the Surveillance, Epidemiology and End Results (SEER) Program database analysis, 5-year survival rates have risen from 56.5% for patients diagnosed in the early 1980s to as much as 63.2% for those diagnosed in the early 1990s and most recently to 64.9%, a trend due mostly to earlier diagnosis and treatment (Ries et al., 2008). One reason for the improving trend is that the prognosis for patients with CRC is highly dependent on stage: 5-year survival rates are over 90% for Dukes A, but only 5% for Dukes D. Unfortunately, only 10% of CRCs are diagnosed early, most patients presenting themselves with advanced disease (AHCPR, 1998). Another reason is probably that CRCs are heterogeneous. More than a decade ago, it was proposed that two distinct categories of CRC existed based on tumor location (proximal or distal to the splenic flexure of the colon) (Bufill, 1990). Cancers of these two anatomical segments show a different predominance in terms of the molecular genetic pathway. Numerous reports thereafter supported the “two types of CRCs” hypothesis (Beart et al., 1983). Currently, rectal cancer, which is usually discussed together with colon cancer, in the term of “colorectal cancer”, was addressed specifically as a unique type of colorectal cancer. Rectal cancers, once accounting for more than 50% of CRCs in the West, have now decreased to less than colon cancers; the prognosis of rectal cancers is worse than that of colon cancers (Enblad et al., 1988); clinical treatment of rectal cancers is different from that of colon cancers, so related analysis of prognosis and treatment of CRC as a whole is not logical. A more appropriate classification will avoid neglecting useful information and be beneficial for treatment application and study of the molecular mechanism.
This review presents evidence that supports the concept that CRC should be divided into proximal colon cancer, distal colon cancer, and rectal cancer.
ANATOMY, EMBRYOLOGY, AND PHYSIOLOGY
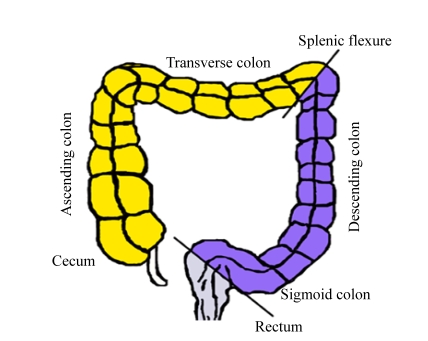
In order to understand the mechanism of CRC in the different segments of the large bowel, we study the differences between these segments in anatomy, embryology, and physiology (Fig.1 and Table 1). From the anatomical and embryological standpoints, the proximal and distal colons are located within the peritoneal cavity, while the rectum lies within the pelvis, the location of which is relatively inaccessible. Proximal colon embryologically originates from the midgut, while segments from the splenic flexure to the upper anal canal containing the distal colon and rectum arise from the hindgut.
Fig. 1.
Three segments of colorectum: right-sided tumours are classified as originating proximal to the splenic flexure (cecum, ascending colon, and transverse colon); left-sided tumors arise distally to this site (descending colon, sigmoid colon) and rectum
Table 1.
Differences in the three subgroups of CRC
| Feature | Proximal colon | Distal colon | Rectum |
| Physiology | |||
| Embryological origin | Midgut | Hindgut | Hindgut |
| Artery supply | Branches of superior mesenteric artery | Tributaries from the inferior mesenteric artery | Tributaries from the inferior mesenteric artery |
| Innervation | Vague nerve | S2, S3, S4 | S2, S3, S4 |
| Histology | Dense mucous apical vesicles | Highest proportion of goblet cells | Richer in endocrine cells |
| Major physical function | Nutrient and water absorption | Nutrient and water absorption | Fecal storage |
| Other physical features | Short-chain fatty acids and ethanol fermentation | Protein fermentation; neutral mucopolysaccharide predominance | Acidic mucin predominance |
| Carcinogenesis | |||
| Molecular mechanism | MSI | CIN | MSS; CIN; more contribution of TP53, APC/β-catenin pathway; more COX2 overexpression; less K-ras mutation |
| Hereditary disease predominant | HNPCC | FAP | |
| Risk factors | |||
| Dietary fiber | Greatest protective effect | Protective effect | Less or null protective effect |
| Meat consumption | Roasted meat | Processed meat | Red meat |
| Calcium and vitamin D | Less protective effect | More protective effect | More protective effect |
| Physical activity | Protective effect | Protective effect | No protective effect |
CIN: chromosomal instability; MSI: microsatellite instability; MSS: microsatellite stability; HNPCC: hereditary nonpolyposis colorectal cancer; FAP: familial adenomatous polyposis; TP53: tumor protein P53; APC: adenomatous polyposis coli; COX2: cyclooxygenase-2
Anatomically, the proximal colon is nourished by branches of the superior mesenteric artery; distal colon and rectum are supplied by tributaries from the inferior mesenteric artery. The innervation to the proximal colon derives from the vagus nerve, while fibers from S2~S4 innervate distal colon and rectum.
Nutrient and water absorption and fecal storing are major functions of colon and rectum. However, these functions take place differentially in different segments of the large bowel. Sodium and water absorption rates are greatest in the cecum and decrease progressively towards the rectum. The functional difference may be explained by the structural and physiological differences of various segments of the large bowel: evident difference indicates functional heterogeneity of various segments of the large bowel. The thickness of the total wall of colon and rectum visualized by endoscopic ultrasonography is not identical, with a thinner wall in the colon compared with the rectum. The capillary networks of the ascending colon are multi-layered, while those of the distal colon are almost single-layered (Araki et al., 1996; Skinner and O′Brien, 1996). It has been noted that there is a gradual reduction toward the more distal colon, in both the mucosal capillary density and the average width of the mucosal capillary bed (Browning and Gannon, 1986). These findings correspond well with the fact that the proximal colon is more related to water absorption and electrolyte transport activities compared with the distal colon.
Histologically, the proportion of goblet cells, functioning in mucin secretion on the sigmoid colon and rectum is higher than that on the right-sided colon, which is believed to be a defense of the mucosal surface against increased physical and/or chemical stimuli in the distal colon and rectum (Arai and Kino, 1989). The rectum shows an unusually high concentration of endocrine cells compared with other segments, which is suggestive of a possible relationship with the high incidence of carcinoma in the rectum (Shamsuddin et al., 1982).
Short-chain fatty acids and ethanol concentrations are highest in the proximal colon; however, in the distal colon, products of protein account for the major part of fermentation content. In the descending colon, neutral mucopolysaccharides are predominant, whereas the rectum is characterized by acid mucins (Shamsuddin et al., 1982). Thus, different regions of the human gut harbor diverse bacterial fermentation products, which have been found to influence many aspects of colon function, such as epithelial cell metabolism, absorptive capacity and, possibly, motor activity.
GENETIC PATHWAY
Much progress has been made in understanding the molecular mechanism of CRC since 1990, when a genetic model for CRC tumorigenesis was proposed (Fearon and Vogelstein, 1990). A progression from normal mucosa to adenoma to carcinoma was supported by the demonstration of accumulating mutations in genes of K-ras, adenomatous polyposis coli (APC), tumor protein P53 (TP53), and deleted in colorectal carcinoma (DCC), all of which are thought to be of significance, but are not able successfully to account for all CRCs. There is heterogeneity in the pathogenetic pathway leading to CRCs, and there are two major tumorigenic pathways. The first is driven by chromosomal instability (CIN), namely the model mentioned above, the progress of which involves both oncogenes and tumor-suppressor genes including chromosomes 5q, 17p, and 18q (Delattre et al., 1989; Fearon and Vogelstein, 1990; Gervaz et al., 2001). Chromosome 5q genes are responsible for APC, 17p for TP53, and 18q for DCC or Mothers against decapentaplegic homolog 4 (SMAD4), respectively. K-ras is the most common oncogene following this pattern. As far as tumor-suppressor genes are concerned, genes of APC, TP53, DCC/SMAD4 play important roles in this sequential adenoma to carcinoma pattern.
An alternative genetic pathway related to genetic instability may well be depicted as a consequence of the alteration in mismatch repair (MMR) genes (Gervaz et al., 2001; Miyakura et al., 2001; Thibodeau et al., 1993). When the alteration happens in germinal cells, the hereditary cancer known as hereditary nonpolyposis colorectal cancer (HNPCC) occurs. When somatic cells are affected, microsatellite instability (MSI) would be unavoidable, which is responsible for a subset of sporadic colorectal tumors.
What is of interest is that the former is predominant in the distal colon, typically when familial adenomatous polyposis (FAP) is concerned. It was reported that the frequency of allelic loss on the three chromosomes was more than double in distal tumors compared with proximal tumors (Delattre et al., 1989), and that close to 100% of FAP individuals will develop CRC in the left colon (Iacopetta, 2002). However, as for the proximal colon, the second pathway is predominant, and this is reflected in the high incidence of MSI phenotype in the proximal colon (Miyakura et al., 2001; Thibodeau et al., 1993), which is up to as much as ten times higher than that in distal tumors in sporadic CRCs.
The preferential location of the two notable familial colorectal cancers helps to make our view more persuasive—that carcinogenesis arises from a different genetic pathway according to proximal or distal location to the splenic colon.
FAP and HNPCC, the two major familial forms of CRC, exhibit a distal location preference and a proximal location preference, respectively. The former has an involvement in the CIN pathway, while the latter, in contrast, in the MSI pathway (Miyakura et al., 2001; Patchett et al., 1997; Thibodeau et al., 1993). It has been reported that 60%~70% of HNPCC carcinomas are located proximal to the splenic flexure, compared with 30% among the sporadic cases.
Recently, epigenetics has been considered as an important mechanism of colorectal carcinogenesis, though it had long been relegated to an almost non-existent role in tumorigenesis (Jubb et al., 2001). HNPCC is mostly due to mutations of MMR genes, which show MSI phenotype. However, MSI is also present in 10%~15% of sporadic colorectal cancer cases, the majority of which do not show mutations in MMR genes. Therefore, the supposition is made that there are other mechanisms responsible for the defect in MMR genes. It has been demonstrated that epigenetic, rather than genetic means silence the transcription of MMR genes in sporadic colorectal cancers with MSI (Anacleto et al., 2005). Methylation is believed to be a crucial epigenetic regulation in colorectal carcinoma. It has been verified that 89% of MSI-positive sporadic tumors could be accounted for by full methylation of hMLH1 that is an important DNA mismatch repair gene (Miyakura et al., 2001). To sum up, tumors with MSI, though sharing relatively identical features such as proximal location preference, poor differentiation, Crohn-like reaction, increased signet cells, and a lymphocytic infiltrate, experience a different carcinogenesis pathway of the MMR mutation phenotype or an MMR hypermethylation phenotype.
There are studies favoring the assumption that colon cancer and rectal cancer are different entities, though relatively few studies have addressed molecular and/or biological differences between the colonic and rectal cancers, compared with subsites within the colon. Based on the data of the Nurses’ Health Study and the Health Professionals Follow-Up Study, Wei et al.(2004) reported that a family history of CRC appears to affect relative risk (RR) of colon cancer more strongly than RR of rectal cancer, the RR being 1.94 (95% CI=1.80~2.10) and 1.27 (95% CI=1.08~1.50), respectively.
It has been observed that MSI is rare in rectal carcinoma (Nilbert et al., 1999), whereas the incidence of CIN is high in rectal cancer (Aaltonen et al., 1998; Fernebro et al., 2002; Frattini et al., 2004). Nevertheless, compared with colon cancer, the number of mutations detected is significantly higher in rectal cancer (Frattini et al., 2004). Kapiteijn et al.(2001) demonstrated that rectal cancers showed significantly more nuclear β-catenin than colon cancers, which is a critical mediator of the WNT signaling pathway negatively regulated by APC. Additionally, compared with colon cancer, rectal cancer is less K-ras-dependent and the APC gene-restricting pattern is more common (Frattini et al., 2004). Therefore, the APC/catenin pathway in rectal cancer is suggested as the predominant one. A high level of TP53 expression in rectal cancer was also revealed. The cyclooxygenase-2 (COX2) is overexpressed in 90% of rectal tumors but in only 20% of colonic tumors (Dimberg et al., 1999). In these respects, therefore, rectal cancer might be viewed as a different entity from colon cancer to some extent.
EPIDEMIOLOGY
CRC continues to be one of the commonest malignancies worldwide. According to statistics of the American Cancer Society, the incidence of CRC was estimated to rank third in both genders for all malignant diseases in 2008 (Jemal et al., 2008). Over recent decades, the site-specific incidence in the large bowel has been investigated. The data of the SEER program of the National Cancer Institute showed that proximal colon cancer, distal colon cancer, and rectal cancer contribute approximately 41.5%, 28.7%, and 29.8% respectively to all the colorectal cancer in 12 SEER areas during 1988~2001 (Ries et al., 2007). Numerous reports revealed an increased incidence of colon cancer and a decreased incidence of rectal cancer; and a proximal shift in the incidence of CRCs was noticed. Despite the controversy (Crerand et al., 1991; Erkek et al., 2007; Ponz de Leon et al., 2004; Vobecky et al., 1984), the shift is evidenced by various population-based studies relating to both ethnicity (White (Troisi et al., 1999), African American (Nelson et al., 1997), and Asian (Ji et al., 1998)) and geography (Rochester (Beart et al., 1983), Canada (Obrand and Gordon, 1998), and New Zealand (Jass, 1991)). Association of the subsite distribution of colorectal cancer with race, gender, age, and other epidemiological factors has been detected through population-based data (Tables 2 and 3).
Table 2.
Summary of epidemiology evidence of subsite-specific CRC
| Studies | Details of studies | Results |
| Wei et al., 2004 | Data from 2 prospective cohort studies, including 134 365 women | Family history of CRC affects risk for colon cancer more than risk for rectal cancer |
| Ponz de Leon et al., 2004 | A specialized CRC registry including 265 227 residents and 2 462 cases | An increase in tumor incidence in all colonic segments more than a shift to the right colon is indicated |
| Troisi et al., 1999 | CRC registry of SEER program including 220 000 cases | Proximal colon carcinoma rates were higher than distal colon or rectal carcinoma rates throughout the study period in the US |
| Nelson et al., 1997 | A descriptive population-based study including 38 931 cases | African Americans were more likely than Whites to develop proximal CRC (odds ratio (OR)=1.19), whereas Whites were more likely to have distal CRC than African Americans (OR=1.2) |
| Gervaz et al., 2001; Skinner and O′Brien, 1996; Wu et al., 2004 | Twenty-three population-based central cancer registries including 336 798 cases | Age-adjusted CRC incidence rates were significantly lower in Asians and Pacific Islanders (API) than in Whites and African Americans particularly for proximal colon cancer; Among API, the rate of rectal cancer (19.2 per 100 000) was significantly higher than the rates of proximal (15.2 per 100 000) and distal (17.7 per 100 000) colon cancers in males |
| Gervaz et al., 2004; Iacopetta, 2002; Ji et al., 1998 | A population-based cancer registry including 30 693 CRCs | The increase in rates was much more rapid for colon cancer, with rates approximately doubling, than that for rectal cancer; proximal colon cancer was more common than distal colon cancer over the whole study period |
| Irby et al., 2006 | CRC registry of SEER program including 323 888 cases | Proximal CRCs were more common and rectal cancers were less common among Blacks than among Whites |
| Rothberg et al., 1985; Stang et al., 2007 | National Cancer Registry of the German Democratic Republic (GDR) including 147 790 cases | With the incidence increase of CRCs in the GDR, the ratio of colon to rectal cancer incidence became larger and surpassed the reference value |
| Svensson et al., 2002 | An age-period-cohort analysis including 32 981 and 32 812 cases for men and women | The age distribution differs for subsites and between men and women. Men have more distal colon and rectal cancers, while women have more proximal colon cancers |
| Wu et al., 2001 | Twenty-eight population-based central cancer registries including 134 724 CRCs | The male-to-female ratios progressively increased from the proximal colon to the distal colon; the ratios of proximal-to-distal CRC gradually increased with advancing age |
| Saltzstein and Behling, 2007 | California cancer registry including 213 383 cases | The left-to-right shift increases significantly with increasing age and year of diagnosis, is greater in women than in men, and is greater in Whites than in other racial/ethnic groups |
Table 3.
Summary of evidence of different risk factors in subsite-specific CRC
| Studies | Details of studies | Results |
| Terry et al., 2001b | A population-based cohort of 61 463 Swedish women | Total consumption of fruit and vegetables combined was inversely associated with colon and rectal cancers, but the association was stronger for fruit, especially in relation to rectal cancer. High body mass was associated with risk for distal colon and rectal cancers, but not risk for proximal colon cancer |
| Voorrips et al., 2000 | The Netherlands cohort study of 62 573 women and 58 279 men | Inverse associations were stronger for distal colonic tumors than for proximal colonic tumors; no statistically significant associations were found for vegetable or fruit consumption for rectal cancer |
| Bingham et al., 2003 | An observational study of 519 978 individuals | Adjusted RR of dietary fiber intake for the highest versus lowest quintile is 0.72 (95% CI=0.43~0.99) for the colon and 0.80 (95% CI=0.53~1.22) for rectal cancer. The association was stronger for colon cancer, especially left-sided colon cancer [RR=0.65 (95% CI=0.43~0.99)], than for rectal cancer |
| Delattre et al., 1989; Sandhu et al., 2001 | A meta-analysis | A daily increase of 100 g of all meat or red meat is associated with a significant 12%~17% increased risk of CRC; a significant 49% increased risk was found for a daily increase of 25 g of processed meat |
| Larsson and Wolk, 2006 | A meta-analysis | High consumption of processed meat was associated with an increased risk of distal colon cancer but not of proximal colon cancer; association with red meat appeared to be stronger for rectal cancer than for colon cancer (p-heterogeneity between cancer sites was 0.06) |
| Welfare et al., 1997 | A case-control study of 174 cases and 174 matches | Consumption of roast meat [OR=3.0 (95% CI=1.14~9.23)] at least once a week [OR=2.83 (95% CI=1.07~8.78)] was a significant risk factor for proximal colon cancer |
| Cho et al., 2004; Obrand and Gordon, 1998 | A pooled analysis of 10 cohort studies | The inverse association for milk was limited to cancers of the distal colon (P<0.001) and rectum (P=0.02) |
| Friedenreich et al., 2006 | A prospective cohort study of 366 521 women and 153 457 men | Physical activity reduced colon cancer risk, specifically for right-sided tumors, but not rectal cancer |
| Gatta et al., 1998; Larsson et al., 2006 | A cohort study of 100 303 Swedish men | The inverse association between leisure-time physical activity and colon cancer risk was somewhat stronger for distal colon cancer than for proximal colon cancer |
| Li et al., 2007; Steindorf et al., 2005 | Hospital-based case control study of 239 cases and 239 controls | An inverse association of colon cancer risk and physical activity was found [OR=0.37 (95% CI=0.17~0.83)]. For rectal cancer, no consistent association with physical activity was found |
| Thune and Lund, 1996 | A population-based cohort of 53 242 males and 28 274 females | Physical activity reduced risk of colon cancer, particularly the proximal colon [RR=0.51 (95% CI=0.28~0.93)]. No association between physical activity and rectal cancer was observed in males or females |
Black individuals are more likely to develop proximal colon cancer than distal colon cancer (Irby et al., 2006; Nelson et al., 1997; Troisi et al., 1999; Wu et al., 2004), while Asians and Pacific islanders are more susceptible to local distal colon and rectal cancers than Americans (Ji et al., 1998; Wu et al., 2004). A slightly higher incidence of rectal cancer has been observed in White compared with Black individuals. The sex distribution of colon cancer is equal, but rectal cancer is more common in males (Wu et al., 2004). The incidence of colon cancer among women is higher than that of rectal cancer, usually in the ratio of 2:1; however, Stang et al.(2007) reported that an inversion of the ratio (less than 1) was observed among men. Within the colon, women have more proximal colon cancers (Svensson et al., 2002), whereas in men there is a predominance of distal colon cancer, in particular in White males; moreover, the male-to-female ratios progressively increase from the proximal colon to the distal colorectum (Wu et al., 2001). The left-to-right shift of incidence is greater among women than among men (Saltzstein and Behling, 2007). Furthermore, a more increased incidence of proximal colon cancer in women was reported early in 1983 by Beart et al.(1983), and the continuing trends could be found afterwards (Obrand and Gordon, 1998). The ratio of proximal-to-distal colorectal cancer gradually increases with age according to several population-based studies (Nelson et al., 1998; Wu et al., 2004), which is suggestive of a greater effect of age on proximal colon carcinoma risk than on distal.
Many etiologic factors have been established as contributing to the risk of CRC. However, when the statistical models that evaluate the endpoint of CRC assume a constant relative risk for cancer of the whole large bowel, or ignore the possibility of heterogeneity between subsites, the conclusions have to be considered tentative, because the risk factors for colorectal cancer as a whole may or may not be risk factors for proximal colonic, distal colonic, or rectal cancer, considered separately.
To further our understanding of etiologic similarities and differences among cancers of different anatomic locations within the colorectum, it is necessary to compare the risk estimates on the different regions of large bowel cancers across a range of lifestyle factors. On the question of diet, a study from Sweden reported that consumption of fruit is inversely related to rectal cancer to a more significant degree than to colon cancer (Terry et al., 2001b). However, Voorrips et al.(2000) found that the incidence of distal colon cancer seemed to be more influenced by vegetable consumption than that of proximal colon cancer, while a less inverse association or no association with vegetable consumption was found for rectal cancer. In a large study of the European Prospective Investigation into Cancer and Nutrition (EPIC) recruiting individual cases from 10 European countries, the adjusted RR of dietary fiber for the highest versus lowest quintile of intake is 0.72 (95% CI=0.43~0.99) for the colon and 0.80 (95% CI=0.53~1.22) for rectal cancer. The association was stronger for colon cancer, especially left-sided colon cancer [RR=0.65 (95% CI=0.43~0.99)], than for rectal cancer (Bingham et al., 2003). In a second report from EPIC with more adjusted models, the association with left-side colon cancer was strengthened and with rectal cancer weakened (Bingham et al., 2005). Thus, the protective effect is likely greatest for the left side of the colon, and least or null for the rectum.
High consumption of red meat and processed meat has been associated with an increased risk of CRC in two meta-analyses (Norat et al., 2002; Sandhu et al., 2001). In an update meta-analysis of prospective studies published through March 2006, the association with red meat appeared to be stronger for rectal cancer; a high consumption of processed meat was associated with an increased risk of distal colon cancer but not of proximal colon cancer (Larsson et al., 2006). In addition, consumption of roasted meat as well as the regular use of animal fat in cooking was a significant factor for the proximal colon (Welfare et al., 1997).
The association between the intake of calcium and vitamin D and the risk of CRC is inconsistent; nevertheless, a pooled analysis of 10 cohort studies showed that the intake of calcium and vitamin D conferred a potential protective effect on CRC and was more frequently in inverse association with distal and rectal cancers than with proximal colonic cancer (Cho et al., 2004).
Sufficient evidence for a cancer-preventive effect of physical activity on colon cancer (although inadequate for rectal cancer) has been found in the EPIC (Friedenreich et al., 2006), Swedish nationwide censuses (Larsson and Wolk, 2006), and others (Steindorf et al., 2005; Thune and Lund, 1996; Wei et al., 2004). Within the colon, proximal colon cancer may be more sensitive to a low physical activity level [RR=0.51 (95% CI=0.28~0.93)] (Thune and Lund, 1996). High body mass is consistently suggestive of risk for colon cancer among men and is less consistent for women: when it is positive in women, the association tends to be weak (Potter, 1999). However, it was reported that body mass is associated with risk for distal colon and rectal cancers (rectal cancer especially), but not proximal colon cancer among females at a relatively young age (Terry et al., 2001a).
TNM STAGING AND PROGNOSIS
Anatomical and biological factors present colonic and rectal cancers as two different entities for treatment and prognosis. In contrast to colonic tumors, which extend directly to the serosal surface, advanced rectal cancers cannot gain direct access to the peritoneal cavity. TNM staging is an important prognostic factor in colon cancer, whereas lateral margin involvement, in addition to TNM, is of important prognostic value to rectal cancer. Accurate determination of the circumferential resection margin (CRM) in rectal cancer is important for the determination of local recurrence risk either after conservative surgery or after total mesorectal excision (TME) (Nagtegaal et al., 2002). In 1986, Quirke et al.(1986) first described the importance of lateral spread and the role of involvement of the radical margin in predicting rectal cancer recurrence. Positive radical margins predict not only local recurrence but also survival (Adam et al., 1994). Adjuvant preoperative radiotherapy is highly effective for rectal cancer by reducing disease recurrence and increasing overall survival (Guyot et al., 2005), while patients with colon cancer benefit from adjuvant chemotherapy (Casillas et al., 1997). The different pattern of survival of colon and rectal cancer has been observed (Berrino et al., 1995; Roncucci et al., 1996). Colonic carcinomas are reported to have a significantly better survival than rectal cancer (Gatta et al., 1998). Our own data (Xu et al., 2006) showed that the survival rate of CRC is partly determined by the constitutive proportion of rectal cancer, which contributes to a worse survival rate. From the comparative point of view, the significant prognostic factor for colon cancer is the age of the patient at diagnosis, while for rectal cancer, it is the pattern of tumor growth (Roncucci et al., 1996). A DNA ploidy pattern has been proved to be an important prognostic factor. Diploid tumors conferred a better survival compared with aneuploid tumors (Bazan et al., 2002; Lanza et al., 1998). Tumors of the proximal colon are more frequently diploid than those localized distally to the splenic flexure. The overall survival rate is reported to be lower in the cases of mucinous carcinomas (Papadopoulos et al., 2004; Taal et al., 2001), a high proportion of which are proximally located. TP53 overexpression is significantly more common in rectal than in colon cancers, which is associated with worse disease-free survival in rectal but not in colon tumors (Kapiteijn et al., 2001). A significant survival benefit with 5-fluorouracil was shown in proximal colon tumors, whereas patients with distal colon cancer showed only a slight benefit from adjuvant treatment compared with proximal colon tumors (Elsaleh et al., 2000; Taal et al., 2001).
CONCLUDING REMARKS
Colorectal cancers from different anatomic sites should not be assumed to be constant in their biological behavior or relative risk factors. It is more rational to divide the colorectum into proximal colon, distal colon, and rectum than the traditional division of colon and rectum or colorectal cancer as a whole, between which heterogeneity exists. Differences in physiology and anatomy, environmental carcinogens, genetic mechanisms, and prognosis between the three segments support the ‘three entities’ concept. The dispute about the inconsistency of data on the site-specific mechanism of colorectal carcinoma does exist; more evidence about molecular events of carcinogenesis and targeted therapy needs to be further collected to definitely confirm the conception. This classification directs scientists to pay more attention to the comparative differences between the three subgroups, which in turn may help to refine prognosis and develop appropriate treatments.
Footnotes
Project supported by the Program of Science and Technology in the National “Eleventh 5-year Plan” of China (No. 2006BAI0214) and the Foundation of Science and Technology of Hangzhou City, China (No. 20061123B02)
References
- 1.Aaltonen LA, Salovaara R, Kristo P, Canzian F, Hemminki A, Peltomaki P, Chadwick RB, Kaariainen H, Eskelinen M, Jarvinen H, et al. Incidence of hereditary nonpolyposis colorectal cancer and the feasibility of molecular screening for the disease. N Engl J Med. 1998;338(21):1481–1487. doi: 10.1056/NEJM199805213382101. [DOI] [PubMed] [Google Scholar]
- 2.Adam IJ, Mohamdee MO, Martin IG, Scott N, Finan PJ, Johnston D, Dixon MF, Quirke P. Role of circumferential margin involvement in the local recurrence of rectal cancer. Lancet. 1994;344(8924):707–711. doi: 10.1016/S0140-6736(94)92206-3. [DOI] [PubMed] [Google Scholar]
- 3.AHCPR (Agency for Health Care Policy and Research) Colorectal Cancer Screening. Technical Review 1. Rockville, MD: Agency for Health Care Policy and Research; 1998. AHCPR Publication No. 98-0033. [PubMed] [Google Scholar]
- 4.Anacleto C, Leopoldino AM, Rossi B, Soares FA, Lopes A, Rocha JC, Caballero O, Camargo AA, Simpson AJ, Pena SD. Colorectal cancer “methylator phenotype”: fact or artifact? Neoplasia. 2005;7(4):331–335. doi: 10.1593/neo.04502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arai T, Kino I. Morphometrical and cell kinetic studies of normal human colorectal mucosa. Comparison between the proximal and the distal large intestine. Acta Pathol Jpn. 1989;39(11):725–730. doi: 10.1111/j.1440-1827.1989.tb02421.x. [DOI] [PubMed] [Google Scholar]
- 6.Araki K, Furuya Y, Kobayashi M, Matsuura K, Ogata T, Isozaki H. Comparison of mucosal microvasculature between the proximal and distal human colon. J Electron Microsc (Tokyo) 1996;45(3):202–206. doi: 10.1093/oxfordjournals.jmicro.a023433. [DOI] [PubMed] [Google Scholar]
- 7.Bazan V, Migliavacca M, Zanna I, Tubiolo C, Corsale S, Calo V, Amato A, Cammareri P, Latteri F, Grassi N, et al. DNA ploidy and S-phase fraction, but not p53 or NM23-H1 expression, predict outcome in colorectal cancer patients. Result of a 5-year prospective study. J Cancer Res Clin Oncol. 2002;128(12):650–658. doi: 10.1007/s00432-002-0394-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beart RW, Melton LJ3rd, Maruta M, Dockerty MB, Frydenberg HB, O′Fallon WM. Trends in right and left-sided colon cancer. Dis Colon Rectum. 1983;26(6):393–398. doi: 10.1007/BF02553382. [DOI] [PubMed] [Google Scholar]
- 9.Berrino F, Sant M, Verdecchia A, et al. Cancer Survival in Europe. Lyon: International Agency for Research on Cancer; 1995. IARC Scientific Publication No. 132. [Google Scholar]
- 10.Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H, et al. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361(9368):1496–1501. doi: 10.1016/S0140-6736(03)13174-1. [DOI] [PubMed] [Google Scholar]
- 11.Bingham SA, Norat T, Moskal A, Ferrari P, Slimani N, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H, Tjonneland A, et al. Is the association with fiber from foods in colorectal cancer confounded by folate intake? Cancer Epidemiol Biomarkers Prev. 2005;14(6):1552–1556. doi: 10.1158/1055-9965.EPI-04-0891. [DOI] [PubMed] [Google Scholar]
- 12.Browning J, Gannon B. Mucosal microvascular organization of the rat colon. Cells Tissues Organs. 1986;126(2):73–77. doi: 10.1159/000146191. [DOI] [PubMed] [Google Scholar]
- 13.Bufill JA. Colorectal cancer: evidence for distinct genetic categories based on proximal or distal tumor location. Ann Intern Med. 1990;113(10):779–788. doi: 10.7326/0003-4819-113-10-779. [DOI] [PubMed] [Google Scholar]
- 14.Casillas S, Pelley RJ, Milsom JW. Adjuvant therapy for colorectal cancer: present and future perspectives. Dis Colon Rectum. 1997;40(8):977–992. doi: 10.1007/BF02051209. [DOI] [PubMed] [Google Scholar]
- 15.Cho E, Smith-Warner SA, Spiegelman D, Beeson WL, van den Brandt PA, Colditz GA, Folsom AR, Fraser GE, Freudenheim JL, Giovannucci E, et al. Dairy foods, calcium, and colorectal cancer: a pooled analysis of 10 cohort studies. J Natl Cancer Inst. 2004;96(13):1015–1022. doi: 10.1093/jnci/djh185. [DOI] [PubMed] [Google Scholar]
- 16.Crerand S, Feeley TM, Waldron RP, Corrigan T, Hederman W, O′Connell FX, Heffernan SJ. Colorectal carcinoma over 30 years at one hospital: no evidence for a shift to the right. Int J Colorectal Dis. 1991;6(4):184–187. doi: 10.1007/BF00341386. [DOI] [PubMed] [Google Scholar]
- 17.Delattre O, Olschwang S, Law DJ, Melot T, Remvikos Y, Salmon RJ, Sastre X, Validire P, Feinberg AP, Thomas G. Multiple genetic alterations in distal and proximal colorectal cancer. Lancet. 1989;334(8659):353–356. doi: 10.1016/S0140-6736(89)90537-0. [DOI] [PubMed] [Google Scholar]
- 18.Dimberg J, Samuelsson A, Hugander A, Soderkvist P. Differential expression of cyclooxygenase 2 in human colorectal cancer. Gut. 1999;45(5):730–732. doi: 10.1136/gut.45.5.730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elsaleh H, Joseph D, Grieu F, Zeps N, Spry N, Iacopetta B. Association of tumour site and sex with survival benefit from adjuvant chemotherapy in colorectal cancer. Lancet. 2000;355(9217):1745–1750. doi: 10.1016/S0140-6736(00)02261-3. [DOI] [PubMed] [Google Scholar]
- 20.Enblad P, Adami HO, Bergstrom R, Glimelius B, Krusemo U, Pahlman L. Improved survival of patients with cancers of the colon and rectum? J Natl Cancer Inst. 1988;80(8):586–591. doi: 10.1093/jnci/80.8.586. [DOI] [PubMed] [Google Scholar]
- 21.Erkek B, Ozkan N, Bayar S, Genc V, Ekrem U, Kuzu A, Aribal D. Subsite distribution of colorectal carcinoma and implications for screening: a retrospective audit of 1771 cases. Hepatogastroenterology. 2007;54(73):77–80. [PubMed] [Google Scholar]
- 22.Fearon ER, Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61(5):759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 23.Fernebro E, Halvarsson B, Baldetorp B, Nilbert M. Predominance of CIN versus MSI in the development of rectal cancer at young age. BMC Cancer. 2002;2(1):25. doi: 10.1186/1471-2407-2-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frattini M, Balestra D, Suardi S, Oggionni M, Alberici P, Radice P, Costa A, Daidone MG, Leo E, Pilotti S, et al. Different genetic features associated with colon and rectal carcinogenesis. Clin Cancer Res. 2004;10(12 Pt 1):4015–4021. doi: 10.1158/1078-0432.CCR-04-0031. [DOI] [PubMed] [Google Scholar]
- 25.Friedenreich C, Norat T, Steindorf K, Boutron-Ruault MC, Pischon T, Mazuir M, Clavel-Chapelon F, Linseisen J, Boeing H, Bergman M, et al. Physical activity and risk of colon and rectal cancers: the European prospective investigation into cancer and nutrition. Cancer Epidemiol Biomarkers Prev. 2006;15(12):2398–2407. doi: 10.1158/1055-9965.EPI-06-0595. [DOI] [PubMed] [Google Scholar]
- 26.Gatta G, Faivre J, Capocaccia R, Ponz de Leon M. Survival of colorectal cancer patients in Europe during the period 1978-1989. Eur J Cancer. 1998;34(14):2176–2183. doi: 10.1016/S0959-8049(98)00327-X. [DOI] [PubMed] [Google Scholar]
- 27.Gervaz P, Bouzourene H, Cerottini JP, Chaubert P, Benhattar J, Secic M, Wexner S, Givel JC, Belin B. Dukes B colorectal cancer: distinct genetic categories and clinical outcome based on proximal or distal tumor location. Dis Colon Rectum. 2001;44(3):364–372. doi: 10.1007/BF02234734. [DOI] [PubMed] [Google Scholar]
- 28.Gervaz P, Bucher P, Morel P. Two colons-two cancers: paradigm shift and clinical implications. J Surg Oncol. 2004;88(4):261–266. doi: 10.1002/jso.20156. [DOI] [PubMed] [Google Scholar]
- 29.Guyot F, Faivre J, Manfredi S, Meny B, Bonithon-Kopp C, Bouvier AM. Time trends in the treatment and survival of recurrences from colorectal cancer. Ann Oncol. 2005;16(5):756–761. doi: 10.1093/annonc/mdi151. [DOI] [PubMed] [Google Scholar]
- 30.Iacopetta B. Are there two sides to colorectal cancer? Int J Cancer. 2002;101(5):403–408. doi: 10.1002/ijc.10635. [DOI] [PubMed] [Google Scholar]
- 31.Irby K, Anderson WF, Henson DE, Devesa SS. Emerging and widening colorectal carcinoma disparities between Blacks and Whites in the United States (1975-2002) Cancer Epidemiol Biomarkers Prev. 2006;15(4):792–797. doi: 10.1158/1055-9965.EPI-05-0879. [DOI] [PubMed] [Google Scholar]
- 32.Jass JR. Subsite distribution and incidence of colorectal cancer in New Zealand, 1974-1983. Dis Colon Rectum. 1991;34(1):56–59. doi: 10.1007/BF02050208. [DOI] [PubMed] [Google Scholar]
- 33.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, Thun MJ. Cancer statistics, 2008. CA Cancer J Clin. 2008;58(2):71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- 34.Ji BT, Devesa SS, Chow WH, Jin F, Gao YT. Colorectal cancer incidence trends by subsite in urban Shanghai, 1972-1994. Cancer Epidemiol Biomarkers Prev. 1998;7(8):661–666. [PubMed] [Google Scholar]
- 35.Jubb AM, Bell SM, Quirke P. Methylation and colorectal cancer. J Pathol. 2001;195(1):111–134. doi: 10.1002/path.923. [DOI] [PubMed] [Google Scholar]
- 36.Kapiteijn E, Liefers GJ, Los LC, Kranenbarg EK, Hermans J, Tollenaar RA, Moriya Y, van de Velde CJ, van Krieken JH. Mechanisms of oncogenesis in colon versus rectal cancer. J Pathol. 2001;195(2):171–178. doi: 10.1002/path.918. [DOI] [PubMed] [Google Scholar]
- 37.Lanza G, Gafa R, Santini A, Maestri I, Dubini A, Gilli G, Cavazzini L. Prognostic significance of DNA ploidy in patients with stage II and stage III colon carcinoma: a prospective flow cytometric study. Cancer. 1998;82(1):49–59. doi: 10.1002/(SICI)1097-0142(19980101)82:1<49::AID-CNCR6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 38.Larsson SC, Wolk A. Meat consumption and risk of colorectal cancer: a meta-analysis of prospective studies. Int J Cancer. 2006;119(11):2657–2664. doi: 10.1002/ijc.22170. [DOI] [PubMed] [Google Scholar]
- 39.Larsson SC, Rutegard J, Bergkvist L, Wolk A. Physical activity, obesity, and risk of colon and rectal cancer in a cohort of Swedish men. Eur J Cancer. 2006;42(15):2590–2597. doi: 10.1016/j.ejca.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 40.Li M, Li JY, Zhao AL, Gu J. Colorectal cancer or colon and rectal cancer? Clinicopathological comparison between colonic and rectal carcinomas. Oncology. 2007;73(1-2):52–57. doi: 10.1159/000120628. [DOI] [PubMed] [Google Scholar]
- 41.Miyakura Y, Sugano K, Konishi F, Ichikawa A, Maekawa M, Shitoh K, Igarashi S, Kotake K, Koyama Y, Nagai H. Extensive methylation of hMLH1 promoter region predominates in proximal colon cancer with microsatellite instability. Gastroenterology. 2001;121(6):1300–1309. doi: 10.1053/gast.2001.29616. [DOI] [PubMed] [Google Scholar]
- 42.Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH. Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit. Am J Surg Pathol. 2002;26(3):350–357. doi: 10.1097/00000478-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 43.Nelson RL, Dollear T, Freels S, Persky V. The relation of age, race, and gender to the subsite location of colorectal carcinoma. Cancer. 1997;80(2):193–197. doi: 10.1002/(SICI)1097-0142(19970715)80:2<193::AID-CNCR4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 44.Nelson RL, Persky V, Turyk M. Time trends in distal colorectal cancer subsite location related to age and how it affects choice of screening modality. J Surg Oncol. 1998;69(4):235–238. doi: 10.1002/(SICI)1096-9098(199812)69:4<235::AID-JSO8>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 45.Nilbert M, Planck M, Fernebro E, Borg A, Johnson A. Microsatellite instability is rare in rectal carcinomas and signifies hereditary cancer. Eur J Cancer. 1999;35(6):942–945. doi: 10.1016/S0959-8049(99)00045-3. [DOI] [PubMed] [Google Scholar]
- 46.Norat T, Lukanova A, Ferrari P, Riboli E. Meat consumption and colorectal cancer risk: dose-response meta-analysis of epidemiological studies. Int J Cancer. 2002;98(2):241–256. doi: 10.1002/ijc.10126. [DOI] [PubMed] [Google Scholar]
- 47.Obrand DI, Gordon PH. Continued change in the distribution of colorectal carcinoma. Br J Surg. 1998;85(2):246–248. doi: 10.1046/j.1365-2168.1998.00507.x. [DOI] [PubMed] [Google Scholar]
- 48.Papadopoulos VN, Michalopoulos A, Netta S, Basdanis G, Paramythiotis D, Zatagias A, Berovalis P, Harlaftis N. Prognostic significance of mucinous component in colorectal carcinoma. Tech Coloproctol. 2004;8(Suppl. 1):s123–s125. doi: 10.1007/s10151-004-0131-z. [DOI] [PubMed] [Google Scholar]
- 49.Patchett SE, Alstead EM, Saunders BP, Hodgson SV, Farthing MJ. Regional proliferative patterns in the colon of patients at risk for hereditary nonpolyposis colorectal cancer. Dis Colon Rectum. 1997;40(2):168–171. doi: 10.1007/BF02054982. [DOI] [PubMed] [Google Scholar]
- 50.Ponz de Leon M, Marino M, Benatti P, Rossi G, Menigatti M, Pedroni M, Di Gregorio C, Losi L, Borghi F, Scarselli A, et al. Trend of incidence, subsite distribution and staging of colorectal neoplasms in the 15-year experience of a specialised cancer registry. Ann Oncol. 2004;15(6):940–946. doi: 10.1093/annonc/mdh224. [DOI] [PubMed] [Google Scholar]
- 51.Potter JD. Colorectal cancer: molecules and populations. J Natl Cancer Inst. 1999;91(11):916–932. doi: 10.1093/jnci/91.11.916. [DOI] [PubMed] [Google Scholar]
- 52.Quirke P, Durdey P, Dixon MF, Williams NS. Local recurrence of rectal adenocarcinoma due to inadequate surgical resection. Histopathological study of lateral tumour spread and surgical excision. Lancet. 1986;328(8514):996–999. doi: 10.1016/S0140-6736(86)92612-7. [DOI] [PubMed] [Google Scholar]
- 53.Ries LAG, Young JL, Keel GE, et al., editors. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001, Patient and Tumor Characteristics. National Cancer Institute; 2007. SEER Program. [Google Scholar]
- 54.Ries LAG, Melbert D, Krapcho M, et al., editors. SEER Cancer Statistics Review, 1975-2005. National Cancer Institute; 2008. (Available from: http://seer.cancer.gov/csr/1975_2005/results_merged/topic_survival.pdf) [Accessed 06/08/2008] [Google Scholar]
- 55.Roncucci L, Fante R, Losi L, Di Gregorio C, Micheli A, Benatti P, Madenis N, Ganazzi D, Cassinadri MT, Lauriola P, et al. Survival for colon and rectal cancer in a population-based cancer registry. Eur J Cancer. 1996;32A(2):295–302. doi: 10.1016/0959-8049(95)00532-3. [DOI] [PubMed] [Google Scholar]
- 56.Rothberg PG, Spandorfer JM, Erisman MD, Staroscik RN, Sears HF, Petersen RO, Astrin SM. Evidence that c-myc expression defines two genetically distinct forms of colorectal adenocarcinoma. Br J Cancer. 1985;52(4):629–632. doi: 10.1038/bjc.1985.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saltzstein SL, Behling CA. Age and time as factors in the left-to-right shift of the subsite of colorectal adenocarcinoma: a study of 213 383 cases from the California Cancer Registry. J Clin Gastroenterol. 2007;41(2):173–177. doi: 10.1097/01.mcg.0000225550.26751.6a. [DOI] [PubMed] [Google Scholar]
- 58.Sandhu MS, White IR, McPherson K. Systematic review of the prospective cohort studies on meat consumption and colorectal cancer risk: a meta-analytical approach. Cancer Epidemiol Biomarkers Prev. 2001;10(5):439–446. [PubMed] [Google Scholar]
- 59.Shamsuddin AM, Phelps PC, Trump BF. Human large intestinal epithelium: light microscopy, histochemistry, and ultrastructure. Hum Pathol. 1982;13(9):790–803. doi: 10.1016/S0046-8177(82)80075-0. [DOI] [PubMed] [Google Scholar]
- 60.Skinner SA, O′Brien PE. The microvascular structure of the normal colon in rats and humans. J Surg Res. 1996;61(2):482–490. doi: 10.1006/jsre.1996.0151. [DOI] [PubMed] [Google Scholar]
- 61.Stang A, Stabenow R, Stegmaier C, Eisinger B, Bischof-Hammes E, Jockel KH. Unexplained inversion of the incidence ratio of colon and rectal cancer among men in East Germany. A time trend analysis including 147,790 cases. Eur J Epidemiol. 2007;22(4):245–255. doi: 10.1007/s10654-007-9114-5. [DOI] [PubMed] [Google Scholar]
- 62.Steindorf K, Jedrychowski W, Schmidt M, Popiela T, Penar A, Galas A, Wahrendorf J. Case-control study of lifetime occupational and recreational physical activity and risks of colon and rectal cancer. Eur J Cancer Prev. 2005;14(4):363–371. doi: 10.1097/00008469-200508000-00009. [DOI] [PubMed] [Google Scholar]
- 63.Svensson E, Grotmol T, Hoff G, Langmark F, Norstein J, Tretli S. Trends in colorectal cancer incidence in Norway by gender and anatomic site: an age-period-cohort analysis. Eur J Cancer Prev. 2002;11(5):489–495. doi: 10.1097/00008469-200210000-00012. [DOI] [PubMed] [Google Scholar]
- 64.Taal BG, van Tinteren H, Zoetmulder FA. Adjuvant 5FU plus levamisole in colonic or rectal cancer: improved survival in stages II and III. Br J Cancer. 2001;85(10):1437–1443. doi: 10.1054/bjoc.2001.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Terry P, Giovannucci E, Bergkvist L, Holmberg L, Wolk A. Body weight and colorectal cancer risk in a cohort of Swedish women: relation varies by age and cancer site. Br J Cancer. 2001;85(3):346–349. doi: 10.1054/bjoc.2001.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Terry P, Giovannucci E, Michels KB, Bergkvist L, Hansen H, Holmberg L, Wolk A. Fruit, vegetables, dietary fiber, and risk of colorectal cancer. J Natl Cancer Inst. 2001;93(7):525–533. doi: 10.1093/jnci/93.7.525. [DOI] [PubMed] [Google Scholar]
- 67.Thibodeau SN, Bren G, Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260(5109):816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 68.Thune I, Lund E. Physical activity and risk of colorectal cancer in men and women. Br J Cancer. 1996;73(9):1134–1140. doi: 10.1038/bjc.1996.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Troisi RJ, Freedman AN, Devesa SS. Incidence of colorectal carcinoma in the U.S.: an update of trends by gender, race, age, subsite, and stage, 1975-1994. Cancer. 1999;85(8):1670–1676. doi: 10.1002/(SICI)1097-0142(19990415)85:8<1670::AID-CNCR5>3.3.CO;2-D. [DOI] [PubMed] [Google Scholar]
- 70.Vobecky J, Leduc C, Devroede G. Sex differences in the changing anatomic distribution of colorectal carcinoma. Cancer. 1984;54(12):3065–3069. doi: 10.1002/1097-0142(19841215)54:12<3065::AID-CNCR2820541242>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 71.Voorrips LE, Goldbohm RA, van Poppel G, Sturmans F, Hermus RJ, van den Brandt PA. Vegetable and fruit consumption and risks of colon and rectal cancer in a prospective cohort study: the Netherlands cohort study on diet and cancer. Am J Epidemiol. 2000;152(11):1081–1092. doi: 10.1093/aje/152.11.1081. [DOI] [PubMed] [Google Scholar]
- 72.Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, Colditz GA. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108(3):433–442. doi: 10.1002/ijc.11540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Welfare MR, Cooper J, Bassendine MF, Daly AK. Relationship between acetylator status, smoking, and diet and colorectal cancer risk in the north-east of England. Carcinogenesis. 1997;18(7):1351–1354. doi: 10.1093/carcin/18.7.1351. [DOI] [PubMed] [Google Scholar]
- 74.Wu XC, Chen VW, Steele B, Ruiz B, Fulton J, Liu L, Carozza SE, Greenlee R. Subsite-specific incidence rate and stage of disease in colorectal cancer by race, gender, and age group in the United States, 1992-1997. Cancer. 2001;92(10):2547–2554. doi: 10.1002/1097-0142(20011115)92:10<2547::AID-CNCR1606>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 75.Wu X, Chen VW, Martin J, Roffers S, Groves FD, Correa CN, Hamilton-Byrd E, Jemal A. Subsite-specific colorectal cancer incidence rates and stage distributions among Asians and Pacific Islanders in the United States, 1995 to 1999. Cancer Epidemiol Biomarkers Prev. 2004;13(7):1215–1222. [PubMed] [Google Scholar]
- 76.Xu FY, Zhai MJ, Dong JK, Wang FJ, Jin YS, Zhu YM, Lai MD. Clinical pathological factors function differently in colonic and rectal cancer prognosis. Journal of Zhejiang University (Medical Science) 2006;3(35):303–310. doi: 10.3785/j.issn.1008-9292.2006.03.013. (in Chinese) [DOI] [PubMed] [Google Scholar]