Abstract
Prevotella bivia is associated with pelvic inflammatory disease. A 77-year-old man developed a rapidly growing chest wall abscess due to P. bivia within days. He underwent surgical resection of the infected area; his postoperative course was uneventful. This is the first case of chest wall abscess due to P. bivia infection. Its correct diagnosis cannot be underestimated because fulminant infections can occur in aged or immunocompromised patients if treated incorrectly. Prompt, appropriate surgical management, and antibiotic therapy affect treatment outcome.
Keywords: Prevotella bivia, Chest wall abscess, Resection, Reconstruction
CASE REPORT
A 77-year-old male Taiwanese retired farmer experienced a left, anterior chest wall soreness followed by a rapidly growing mass lesion in the infraclavicular area within two weeks. He had no history of major trauma in the previous year. He developed and was nourished moderately. He experienced no anorexia or body weight loss recently. He had no major medical disease such as diabetes mellitus, cardiovascular disease, or renal disease. He smoked 1.5 packs of cigarettes per day, but did not drink alcohol or chew betal nuts. He did not take any medicine or herbal drugs regularly in the last few years.
At the onset of pain, he took medicines for pain relieve only. However, his chest pain worsened and a rapidly growing mass of greater than 10 cm in diameter was noted locally within 2 weeks. The overlying skin looked erythematous and tense, and felt warm to the touch. On examination in the clinic, the patient had a painful, protruding mass of 10 cm in diameter in the left upper parasternal area, with overlying skin discoloration and local heat on palpation (Fig.1). He also had a low-grade fever of 38.0 °C. He had no cervical lymphadenopathy or venous engorgement. His breathing sounds were vesicular in both lung fields. Laboratory data revealed leukocytosis (white blood cell count was 18 330 mm−3) with shift to the left (neutrophils ratio in white blood cell count was 85.5%). Chest roentgenography revealed an opaque mass shadow in the left upper medial lung field, just under the left clavicle. Chest computed tomography (CT) showed a soft tissue mass measuring 10 cm in diameter, with enhanced wall and central low density just anterior to the left border of the sternum (Fig.2). Infectious lesion was impressed because local heat and redness were noted. This mass grew rapidly even though continuous parenteral antibiotics with the first to the second generation cephalosporins were treated. Focal cortical breakthrough of the sternum suggested a contiguous spread. A 2.3-cm fibrocalcified lesion in the left upper lung was also incidentally found, which was probably an old tuberculosis lesion.
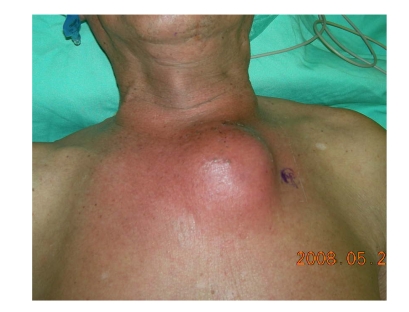
Fig. 1.
A mass up to 10 cm in diameter protruded at the left upper parasternal chest wall, with overlying skin discoloration and local heat on palpation
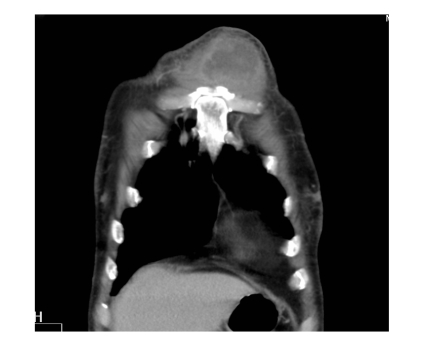
Fig. 2.
Chest computed tomography (CT) showing a soft tissue mass measuring up to 10 cm in diameter with enhancing wall and central low density just anterior to the left border of sternum
The patient underwent surgery to remove this lesion since it had been refractory to be under control. We resected all of the infected tissue en bloc with some of the overlying skin, and curetted the necrotic bone and soft tissue of the underlying thoracic cage. Tissues and fluids were collected during the surgery for further cultures for aerobes, anaerobes, fungi, and mycobacteria.
The pathology examination revealed that the specimen consisted of one mass with overlying skin, measuring 10 cm×8 cm×6 cm (Fig.3). The sections of the specimen showed skin tissue with acute and chronic inflammatory cell infiltration, granulation tissue, fibrosis, necrotic cellular debris, and aggregation of degenerative leukocytes. Gram and periodic acid-Schiff (PAS) stains failed to demonstrate microorganism in the specimens examined. The tissue and pus culture revealed abundant growth of anaerobic gram-negative rods, which were classified as Prevotella bivia. P. bivia was isolated on supplemented blood agar plates containing Brucella agar, L-cysteine, hemin, and vitamin K1; it was an obligate anaerobic. The isolate was classified as P. bivia using the Crystal Anaerobe ID kit (Becton Dickinson Diagnostic Systems, Baltimore, Maryland, USA). It did not grow in the presence of bile nor did it hydrolyze esculin. The bacterial organism was susceptible to ampicillin/sulbactam, clindamycin, cefmetazole, cefoxitin, and metronidazole as examined by agar dilution antimicrobial susceptibility testing for minimum inhibitory concentrations of antibiotics based on criteria defined by the Clinical and Laboratory Standards Institute (NCCLS, 2004).
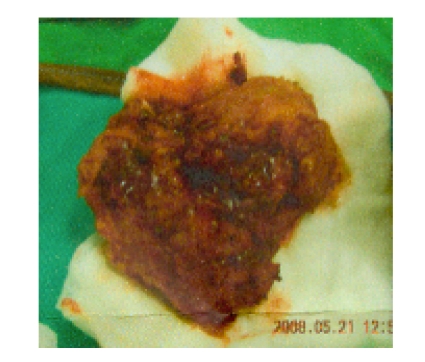
Fig. 3.
The gross picture of the resected specimen
The infection was effectively controlled postoperatively by parenteral treatment and then oral amoxicillin/clavulanate treatment. The patient was discharged uneventfully 11 d later. Small areas of necrosis on the skin edges were noted but healed into granulation tissue shortly thereafter, as seen during later follow-up visits to the clinic.
DISCUSSION
Prevotella belongs to one of the major genera of anaerobic, gram-negative rods (Finegold, 1995). P. bivia, a member of the non-pigmented group, is predominantly associated with infections in the female urogenital tract, presenting as endometritis, pelvic inflammatory disease, or peri-rectal or anal abscess (Jousimies-Somer, 1997). This infection also increases the risk of preterm delivery in pregnant women (Strömbeck et al., 2007). Some rare locations of P. bivia infection, such as bones/joints, nail bed, male external genitalia, oral cavity, and endocardium, are reported (Alegre-Sancho et al., 2000; Riesbeck, 2003; Nalmas et al., 2007; Schindl and Schön, 1999; Kentos et al., 1994). Infection manifesting as a large chest wall abscess was not previously reported in the literature. Other species of Prevotella (P. oralis and P. ruminicola) can result in lung abscesses in human immunodeficiency virus (HIV)-infected patients (Marcos Sánchez et al., 2003).
P. bivia is isolated from infectious tissues or fluids as the only pathogen or in association with other pathogenic microorganisms (Nalmas et al., 2007; Falagas and Siakavellas, 2000). Other anaerobes, such as Bacteroides or Porphyromonas species, Gardnerelia vaginalis, and Streptococcus constellatus, were reported to be associated with P. bivia in mixed anaerobic infections (Nalmas et al., 2007; Falagas and Siakavellas, 2000). On the contrary, some of the vaginal normal flora, such as Lactobacillus species, inhibited the growth of P. bivia (Atassi et al., 2006).
P. bivia usually results in low-virulence infections in the female urogenital tract. An experimental study showed that it invades epithelial cells, but induces weak pro-inflammatory responses (Strömbeck et al., 2007). In cases of preterm labor induced by P. bivia infection, the mechanism could be related to the increased prostaglandin formation (Puapermpoonsiri et al., 1997). In our patient, the infection in the chest wall was rapid and fulminant. Although severe and systemic infections can be caused in immunocompromised hosts (Nagy et al., 1995), our patient was well and immunocompetent. We postulated that the behavior of P. bivia differs by site of infection. An animal study in a mouse subcutaneous infection model revealed that P. bivia needed to be mixed with other bacteria, such as E. coli or Peptostreptococcus spp., to start an effective initial infection; then, the P. bivia became the predominant organism in the chronic abscess (Citron et al., 2005). We believe that our patient was an example of this model of the P. bivia infection, because P. bivia was the only isolated organism in the infected tissue and fluid of our patient. Most P. bivia is β-lactamase positive (Kuzucu et al., 2004), although the positive rate is lower than that for other anaerobes such as Bacteroides (Herrak et al., 2007). P. bivia are susceptible to clindamycin, amoxicillin/clavulanate, metronidazole, and imi-penam (Riesbeck, 2003; Stein et al., 2007; Citron et al., 2005). In our patient, P. bivia isolated from the chest wall was sensitive to amoxicillin/clavulanate, which was administered postoperatively and achieved satisfactory results.
The common infectious agents on the chest wall are Mycobacterium tuberculosis, Actinomyces sp., fungi, and other aerobes and anaerobes (Kuzucu et al., 2004; Herrak et al., 2007; Lin et al., 2007; Luh et al., 2007). Chest wall abscesses caused by P. bivia have not been reported previously. Our patient’s infection could have arisen from the bony chest wall instead of from the lung parenchyma or skin. Nonetheless, his lung lesion was old and seemed unrelated to the chest wall abscess. The overlying skin of the abscess, in spite of erythematous change, was intact without defects or draining sinuses. The precise mechanism of this infection remains unclear.
In summary, we report the first case of chest wall abscess due to P. bivia infection. Its correct diagnosis cannot be underestimated because fulminant infections can occur in aged or immunocompromised patients if treated incorrectly. Prompt, appropriate surgical management, and antibiotic therapy affect treatment outcome.
References
- 1.Alegre-Sancho JJ, Juanola X, Narvaez FJ, Roig-Escofet D. Septic arthritis due to Prevotella bivia in a patient with rheumatoid arthritis. Joint Bone Spine. 2000;67:228–229. [PubMed] [Google Scholar]
- 2.Atassi F, Brassart D, Grob P, Graf F, Servin AL. Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol Med Microbiol. 2006;48(3):424–432. doi: 10.1111/j.1574-695X.2006.00162.x. [DOI] [PubMed] [Google Scholar]
- 3.Citron DM, Tyrrell KL, Warren YA, Fernandez H, Merriam CV, Goldstein EJ. In vitro activities of tinidazole and metronidazole against Clostridium difficile, Prevotella bivia and Bacteroides fragilis . Anaerobe. 2005;11(6):315–317. doi: 10.1016/j.anaerobe.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 4.Falagas ME, Siakavellas E. Bacteroides, Prevotella, and Porphyromonas species: a review of antibiotic resistance and therapeutic options. Int J Antimicrob Agents. 2000;15(1):1–9. doi: 10.1016/S0924-8579(99)00164-8. [DOI] [PubMed] [Google Scholar]
- 5.Finegold SM. Overview of clinically important anaerobes. Clin Infect Dis. 1995;20(Suppl. 2):205–207. doi: 10.1093/clinids/20.supplement_2.s205. [DOI] [PubMed] [Google Scholar]
- 6.Herrak L, Msouger Y, Ouadnouni Y, Bouchikh M, Benosmane A. Pulmonary actinomycosis with chest wall fistula formation. Rev Mal Respir. 2007;24(3 Pt 1):349–352. doi: 10.1016/s0761-8425(07)91068-2. [DOI] [PubMed] [Google Scholar]
- 7.Jousimies-Somer H. Recently described clinically important anaerobic bacteria: taxonomic aspects and update. Clin Infect Dis. 1997;25(Suppl. 2):S78–S87. doi: 10.1086/516227. [DOI] [PubMed] [Google Scholar]
- 8.Kentos A, Motte S, Nonhoff C, Jacobs F, de Smet JM, Serruys E, Thys JP. Prevotella bivia as an unusual cause of endocarditis. Eur J Clin Microbiol Infect Dis. 1994;13(2):142–145. doi: 10.1007/BF01982187. [DOI] [PubMed] [Google Scholar]
- 9.Kuzucu A, Soysal O, Günen H. The role of surgery in chest wall tuberculosis. Interact Cardiovasc Thorac Surg. 2004;3(1):99–103. doi: 10.1016/S1569-9293(03)00192-0. [DOI] [PubMed] [Google Scholar]
- 10.Lin MS, Lin WL, Luh SP, Tsao TC, Wu TC. Pulmonary actinomycosis: a case undergoing resection through video-assisted thoracic surgery (VATS) J Zhejiang Univ Sci B. 2007;8(10):721–724. doi: 10.1631/jzus.2007.B0721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luh SP, Hsu JD, Lai YS, Chen SW. Primary tuberculous infection of breast: experiences of surgical resection for aged patients and review of literature. J Zhejiang Univ Sci B. 2007;8(8):580–583. doi: 10.1631/jzus.2007.B0580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marcos Sánchez F, Albo Castaño I, Arbol Linde F, Celdrán Gil J. Lung abscess caused by Prevotella oralis and Prevotella ruminicola in an HIV-positive patient. Ann Intern Med. 2003;20:164–165. [PubMed] [Google Scholar]
- 13.Nagy E, Szóke I, Gacs M, Csiszár K. Resistance to beta-lactam antibiotics and beta-lactamase production of Bacteroides, Porphyromonas and Prevotella strains. Acta Microbiol Immunol Hung. 1995;42:287–299. [PubMed] [Google Scholar]
- 14.Nalmas S, Bishburg E, Chan T. Streptococcus constellatus and Prevotella bivia penile abscess. Scientific World Journal. 2007;7(10):1631–1633. doi: 10.1100/tsw.2007.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NCCLS (National Committee for Clinical Laboratory Standards) Methods for Antimicrobial Susceptibility Testing of Anaerobic Bacteria. 6th Ed. Wayne, PA: National Committee for Clinical Laboratory Standards; 2004. [Google Scholar]
- 16.Puapermpoonsiri S, Watanabe K, Kato N, Ueno K. In vitro activities of 10 antimicrobial agents against bacterial vaginosis-associated anaerobic isolates from pregnant Japanese and Thai women. Antimicrob Agents Chemother. 1997;41:2297–2299. doi: 10.1128/aac.41.10.2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Riesbeck K. Paronychia due to Prevotella bivia that resulted in amputation: fast and correct bacteriological diagnosis is crucial. J Clin Microbiol. 2003;41(10):4901–4903. doi: 10.1128/JCM.41.10.4901-4903.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schindl AH, Schön H. Foot infection with Prevotella bivia, P. oralis and P. loescheii after wound licking. J Med Microbiol. 1999;48:109. [PubMed] [Google Scholar]
- 19.Stein GE, Schooley S, Tyrrell KL, Citron DM, Goldstein EJ. Human serum activity of telithromycin, azithromycin and amoxicillin/clavulanate against common aerobic and anaerobic respiratory pathogens. Int J Antimicrob Agents. 2007;29:39–43. doi: 10.1016/j.ijantimicag.2006.08.041. [DOI] [PubMed] [Google Scholar]
- 20.Strömbeck L, Sandros J, Holst E, Madianos P, Nannmark U, Papapanou P, Mattsby-Baltzer I. Prevotella bivia can invade human cervix epithelial (HeLa) cells. APMIS. 2007;115(3):241–251. doi: 10.1111/j.1600-0463.2007.apm_512.x. [DOI] [PubMed] [Google Scholar]