Abstract
Facioscapulohumeral muscular dystrophy (FSHD) seems to be caused by a complex epigenetic disease mechanism as a result of contraction of the polymorphic macrosatellite repeat D4Z4 on chromosome 4qter. Currently, the exact mechanism causing the FSHD phenotype is still not elucidated. In this review, we discuss the genetic and epigenetic changes observed in patients with FSHD and the possible disease mechanisms that may be associated with FSHD pathogenesis.
Keywords: FSHD, muscular dystrophy, D4Z4 repeat, epigenetics, chromatin, nuclear organization
1. Introduction
Facioscapulohumeral muscular dystrophy (FSHD [OMIM 158900]), an inherited myopathy that is predominantly characterized by progressive, often asymmetric, weakness and wasting of the facial, shoulder and upper arm muscles [1], does not seem to be caused by structural mutations within a specific disease gene. Instead, increasing evidence suggests a significant role for a complex epigenetic mechanism, resulting in the perturbation of transcriptional control over multiple disease genes. This review aims to discuss the epigenetic changes observed in the FSHD locus and the possible epigenetic disease mechanisms that may be associated with and contribute to FSHD pathogenesis.
2. Genetic changes associated with FSHD
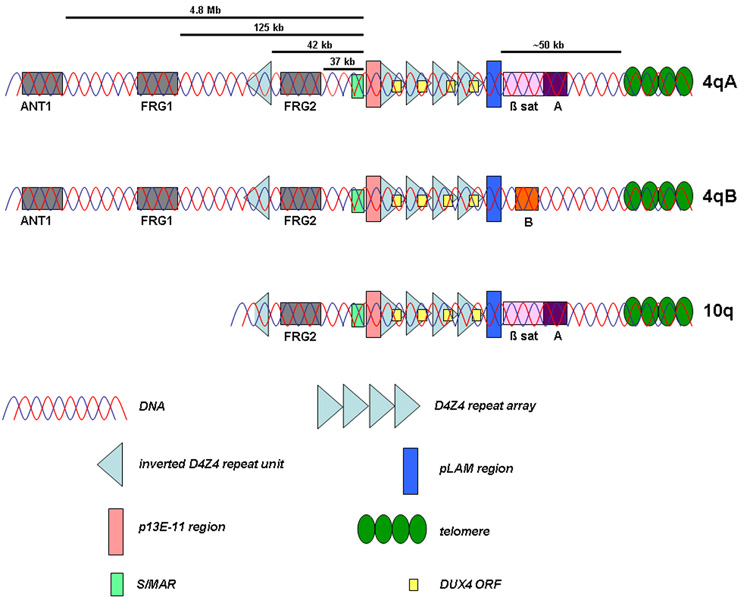
FSHD is inherited in an autosomal dominant fashion. The majority of FSHD cases shows linkage to the subtelomere of chromosome 4q which harbors the macrosatellite repeat D4Z4 [2]. In the general population, this polymorphic repeat array varies between 11 and 100 units of 3.3 kb each. In patients with FSHD, the D4Z4 repeat array is contracted to 1–10 units on one allele [3;4]. The smallest residual repeat sizes are correlated with the more severe phenotypes, although a clear linear inverse relationship between residual repeat size and clinical severity has not been observed [5–7]. As monosomy of 4qter is not associated with FSHD, a critical role for the D4Z4 repeat array and flanking sequences in FSHD pathogenesis is to be expected [8]. Interestingly, in ~1% of patients presenting with a classic FSHD phenotype, a partial D4Z4 deletion extending in the proximal direction has been identified [9–11]. In these cases, an inverted D4Z4 repeat unit that is present 42 kb upstream of the D4Z4 repeat array and the candidate gene FRG2 (FSHD region gene 2) can be deleted [11]. Thus far, the role of this inverted repeat in FSHD is unknown. The role of FRG2 in FSHD pathogenesis will be discussed below.
D4Z4-like repeat arrays are not restricted to chromosome 4qter. Sequences homologous to D4Z4 have been identified on many chromosomes, especially on the acrocentric chromosomes [12]. In addition, as a result of an ancient duplication, the subtelomere of chromosome 10q contains a repeat array that is highly homologous to D4Z4 [13;14] (figure 1). In ~10% of the population, subtelomeric exchanges between the D4Z4 repeats on 4qter and 10qter have been observed [15]. These rearrangements can result in the formation of hybrid alleles containing a mixture of 4-type and 10-type repeat units [9]. Translocated repeat arrays on chromosome 10 are more homogeneous than translocated repeat arrays on chromosome 4, the latter being almost always comprised of both 4- and 10-derived repeat units [16]. Importantly, FSHD is uniquely linked to chromosome 4. Although ~10% of chromosomes 10 have been identified with a repeat array <11 repeat units, no contractions on 10qter have been reported to result in FSHD [17;18]. Contraction of a translocated 4-type allele on chromosome 10 does not result in disease either [9;15;16].
Figure 1. Schematic map of 4qA, 4qB and 10q.
The subtelomere of chromosome 10q contains a repeat array that is highly homologous to D4Z4 on 4qter. The homology extends both in proximal (~40 kb upstream) and distal direction. In addition, two allelic variants of the 4q subtelomere have been identified. The presence of beta satellite DNA distal to D4Z4 on 4qA-type alleles is the most prominent difference between these allelic variants.
Some years ago, two allelic variants of the 4q subtelomere, termed “4qA” and “4qB”, were identified [19] (figure 1). Although both variants are equally common in the population, FSHD is exclusively associated with a shortened D4Z4 repeat on a 4qA-type allele [20]. A FSHD-sized repeat array on a 4qB-type allele does not cause FSHD [21]. The most prominent difference between these two allelic variants is the presence of 6.2 kb beta satellite DNA distal to D4Z4 on 4qA-type alleles [19]. An additional rare 4qter subtype was identified in two FSHD cases [22]. Recently, by the identification of 9 different haplotypes of chromosome 4q on basis of sequence variations in the FSHD locus, the picture became even more complex. Thus far, only contraction in one of these haplotypes, termed “4qA161”, was found to cause FSHD, while contractions in other common 4q haplotypes such as “4qA166” and “4qB163” are nonpathogenic [23]. Currently, it is unclear what determines the difference in pathogeneticity between the different haplotypes. Haplotype-specific single nucleotide polymorphisms (SNPs) can be identified in the FSHD locus and are speculated to have an effect on the transcriptional activity of FSHD candidate genes or on the binding of proteins to the D4Z4 repeat array.
Finally, a small percentage of FSHD cases (<5%), referred to as patients with phenotypic FSHD, shows no contraction of D4Z4 on one of their chromosomes 4 [24]. Currently, no disease locus has been identified for this heterogeneous patient group. Genes encoding the components of the D4Z4 repressor complex (see below), MYOD1 and several genes encoding proteins that play a role in chromatin structure, like DNA methyltransferase 3B (DNMT3B), have been excluded as disease genes for this group of patients [25;26].
3. Epigenetic changes associated with FSHD
Over the years, because of the lack of evidence for transcription emanating from D4Z4 (see below), FSHD studies shifted towards understanding the chromatin structure of D4Z4. Each D4Z4 repeat unit harbors two classes of GC-rich sequences, namely the low-copy-repeats hhspm3 and LSau. This type of repetitive DNA is predominantly found in heterochromatic regions of the genome [27]. Moreover, D4Z4 is overall very GC rich and has characteristics of a CpG island. Therefore, it has been hypothesized that repeat contraction-induced changes in chromatin conformation leading to inappropriate regulation of FSHD candidate genes, thus an epigenetic mechanism, may underlie FSHD pathogenesis. Major epigenetic mechanisms accounting for and contributing to human disease are changes in DNA methylation and histone modifications. An overview of studies on changes in DNA methylation and histone modifications at the D4Z4 repeat array in FSHD is given below and is summarized in figure 2.
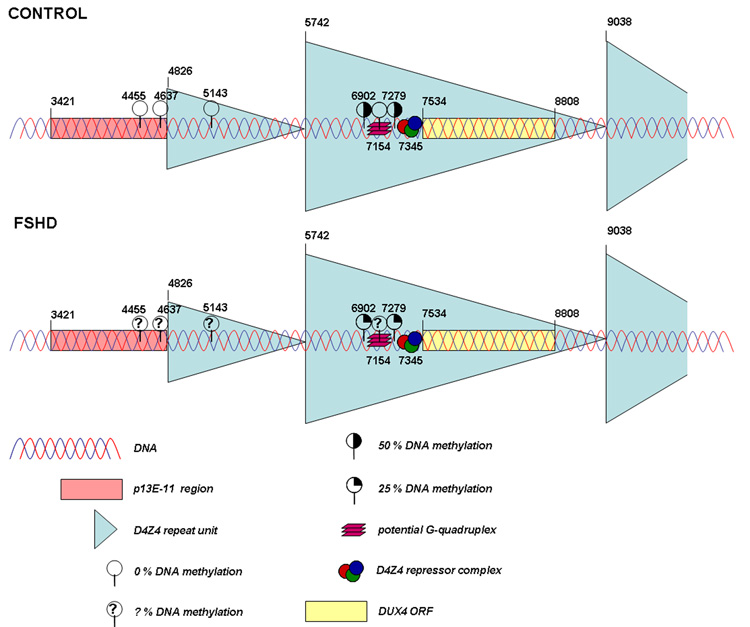
Figure 2. Epigenetic modifications at the D4Z4 repeat array in controls and patients with FSHD.
In control individuals methylation levels of 50 % are identified at two CpG dinucleotides while in patients with FSHD significant hypomethylation is found at these sites (25 % methylation). Interestingly, part of the proximal repeat unit seems to be resistant to DNA methylation (0 % methylation) and is more accessible to DNaseI in cancer tissues, suggesting the presence of a boundary element at the junction of D4Z4 and p13E-11. Finally, a subregion within each D4Z4 repeat unit that may form a G-quadruplex also shows resistance to cancer-linked hypermethylation (0 % methylation). In somatic control DNA samples, similar results were obtained, although at a lower intensity. FSHD DNA samples have not yet been tested (? % DNA methylation). The presented nucleotide positions are based on AF117653 (GenBank).
3.1 DNA methylation in FSHD
In mammalian DNA, the cytosine of CpG dinucleotides can be methylated by DNA methyltransferases like DNMT1, DNMT3A and DNMT3B. Generally, the presence of methyl groups on DNA is associated with increased chromatin condensation and gene silencing. When a promoter region is methylated, transcription factors with CpG dinucleotides in their DNA recognition sequence cannot bind. Reports on the methylation-sensitive binding of proteins, including E2F, CTCF (CCCTC-binding factor) and YY1 (Ying Yang 1), are numerous [28–30]. On the other hand, the methyl binding domain (MBD) proteins bind specifically to methylated DNA. Subsequently, these proteins can recruit histone deacetylases and histone methyltransferases, resulting in increased chromatin condensation and recruitment of the chromatin silencer heterochromatin protein 1 (HP1), respectively [31;32].
An initial study on DNA methylation in the D4Z4 repeat array did not show a change in this epigenetic marker in FSHD since high methylation levels, consistent with heterochromatin, were observed at several CpG dinucleotides in both normal and FSHD cell lines and somatic tissues. However, the methylation level of both chromosome 4 and 10 repeat arrays was analyzed simultaneously [33]. A few years later, studying two different CpG dinucleotides and discriminating between chromosomes 4 and 10, significant hypomethylation of the contracted allele was observed in patients with FSHD compared to controls and individuals with non-FSHD muscular dystrophies. Although this study was predominantly performed on lymphoblast DNA, a similar level of hypomethylation was identified in a small group of DNA samples isolated from FSHD muscle. Importantly, low D4Z4 methylation levels were observed at both chromosome 4 alleles in phenotypic FSHD patients who are clinically indistinguishable from 4q-linked FSHD patients but who show no D4Z4 contraction [34].
Interestingly, part of the proximal D4Z4 repeat unit seems to be resistant to DNA methylation, as was observed in cancer tissues presenting with high DNA methylation throughout the D4Z4 repeat array. In addition, this 2 kb region showed differential DNaseI accessibility compared to the remainder of the repeat array. These results may suggest the presence of a boundary element at the junction of D4Z4 and the proximal AT-rich p13E-11 region [35]. Such a boundary element can be essential in physically separating active and inactive genomic regions [36]. Further, a subregion within each D4Z4 repeat unit, 1.4 kb from the single KpnI site within D4Z4, also showed resistance to cancer-linked hypermethylation. This subregion contains stretches of G residues that are hypothesized to form stable G-quadruplexes that can play an important role in D4Z4 chromatin organization [35]. Intriguingly, homodimers of the myogenic regulatory factor MyoD may specifically recognize these G-quadruplexes [37]. These results on cancer-linked hypermethylation were only confirmed at a lower intensity in somatic control DNA samples and not in FSHD DNA samples [35].
Currently, the precise role of D4Z4 hypomethylation in FSHD pathogenesis remains to be established. Altogether, FSHD alleles are hypomethylated compared to controls, but methylation levels can vary substantially between individuals. Generally, patients with residual repeat sizes between 10 and 20 kb are severely affected and show very low DNA methylation levels, while patients with repeat sizes between 20 and 31 kb show large interindividual variation in both clinical severity and D4Z4 hypomethylation [38]. In addition, asymptomatic gene carriers show the same reduction in D4Z4 methylation as 4q-linked FSHD patients and strong D4Z4 hypomethylation is also reported in patients with immunodeficiency, centromeric instability and facial anomalies syndrome (ICF syndrome [OMIM 242860]) [34;39]. Patients with ICF syndrome present with severe immunodeficiency, resulting in recurrent respiratory and gastrointestinal infections, and non-myopathic facial anomalies. In ~60% of patients with ICF, mutations in the DNA methyltransferase gene DNMT3B have been identified [40]. As these mutations reduce the methyltransferase activity of DNMT3B, hypomethylation of several repeat arrays, including satellite 2 (Sat2), satellite 3 (Sat3), the NBL2 repeat and the D4Z4 repeat, is observed in patients with ICF [39–41]. However, the commonalities between FSHD and ICF seem to be restricted to D4Z4 hypomethylation. Therefore, other (epigenetic) factors that differ between FSHD and ICF may contribute to the development of FSHD [26].
3.2 Histone modifications in FSHD
Chromatin is the assembly of DNA, histone proteins and other chromosomal proteins. A major function of chromatin is to accommodate the packaging of the DNA in the nucleus. The smallest structural unit of packaging is the nucleosome that consists of ~146 bp of DNA wrapped around eight core histone proteins. Histone proteins may undergo several posttranslational modifications, such as acetylation, methylation, phosphorylation and ubiquitination [42]. Currently, two models explaining the function of these histone modifications prevail. Histone modifications may directly affect chromatin structure by preventing transcription factor binding, altering the interactions between nucleosomes or changing the interactions of the histone tails with the DNA in the nucleosome [43]. On the other hand, histone modifications may serve as a site for recruitment of chromatin-associating proteins that recognize a specific histone code. As a consequence, downstream events generating a particular chromatin state may occur [44]. Specific histone modifications seem to be associated with either transcriptional activation or transcriptional repression. Methylation at lysine residues 4, 36 and 79 of histone H3 has been correlated with transcriptional activation. Acetylation of arginine residues of histone H3 and H4 is also characteristic for euchromatin and gene activation [45]. In contrast, methylation at lysine residues 9 and 27 of histone H3 and at lysine residue 20 of histone H4 has been linked to heterochromatin and gene repression [46;47].
Using chromatin immunoprecipitation (ChIP) assays, the hypothesized heterochromatic nature of the D4Z4 repeat array was studied. The level of histone H4 acetylation of D4Z4 in chromosome 4-containing somatic cell hybrids was higher than expected for a heterochromatic structure. Further, histone H4 acetylation levels at the p13E-11 region immediately proximal to D4Z4 were similar to those observed in the 5’ regions of the FSHD candidate genes FRG1 (FSHD region gene 1) and ANT1 (adenine nucleotide translator 1) and did not differ significantly between control and FSHD lymphoid cells. In conclusion, these results suggested that the nature of D4Z4 chromatin is that of unexpressed euchromatin rather than that of constitutive heterochromatin [48]. In a second study, other heterochromatin marks were studied using immuno-fluorescence in situ hybridization (immuno-FISH) methods. The FSHD locus at 4qter did neither colocalize in control and FSHD myoblasts with DAPI-intense loci, nor with heterochromatic foci in interphase nuclei and nor with chromatin regions enriched in HP1α or histone H3 methylated at lysine 9. In addition, no late replication in S-phase, characteristic for constitutive heterochromatin, was observed. On the other hand, histone H3 methylation at lysine 4 and histone H4 acetylation at lysine 8, both characteristics for highly expressed gene regions, was observed in FSHD and control myoblasts [49]. Again these results indicated a more euchromatic or facultative heterochromatic structure at the D4Z4 repeat.
4. Epigenetic disease mechanism of FSHD
The exact pathogenetic mechanism causing FSHD is still unknown. Over the years, several disease mechanisms for FSHD have been postulated, implying either a direct (protein coding) or an indirect (non-protein coding) role for D4Z4 in the development of FSHD. A number of observations need to be considered when proposing a disease mechanism for FSHD. First, a critical number of D4Z4 repeat units is associated with FSHD pathogenesis. In general, patients with FSHD carry a D4Z4 repeat array that is contracted to 1–10 repeat units [3;4] while monosomy of 4qter does not cause FSHD [8]. Second, despite the high homology between D4Z4 repeat arrays derived from chromosomes 4qter and 10qter, only contraction in one of the 4qter haplotypes, termed 4qA161, results in FSHD [20;23]. FSHD-sized repeat arrays on chromosome 10 or on 4qA166 and 4qB163 alleles do not cause FSHD [21;23]. Third, a specific change in chromatin structure is observed, namely D4Z4 hypomethylation [34]. At present, it is unknown whether this change in chromatin structure is causative for FSHD or arises as a consequence of the primary genetic defect. Therefore, it is also unclear what the contribution of this chromatin change is to the FSHD phenotype. However, a small group of patients presents with a FSHD phenotype but does not show a D4Z4 contraction. Importantly, these patients show D4Z4 hypomethylation [34]. Thus, it will be imperative to study the functional consequences of this chromatin change.
4.1 Role of D4Z4 transcription in FSHD pathogenesis
Initially, a putative promoter and the putative double homeodomain gene DUX4 were identified within each D4Z4 repeat unit. As D4Z4 was considered to be of heterochromatic nature, it was hypothesized that partial deletion of the D4Z4 repeat array resulted in destabilization of the D4Z4 heterochromatin and in the inappropriate upregulation of DUX4 [27;50]. DUX4 overexpression may induce cell death by apoptosis, induce caspase 3/7 activation and alter emerin distribution at the nuclear envelope [51]. In addition, DUX4 overexpression may activate PITX1 (paired-like homeodomain transcription factor 1), as was determined for both a reporter gene fused to the Pitx1 promoter and the endogenous Pitx1 gene. Interestingly, upregulation of the PITX1 protein was also observed in muscle biopsies of patients with FSHD [51;52].
Nevertheless, for a long time, the functionality of the DUX4 gene was questioned, because of lack of introns and polyadenylation signals and absence of evidence for in vivo transcription [27;50;53–55]. Recently however, D4Z4 homologues have been identified in several mammalian species and it was established that the DUX4 open reading frame (ORF) shows evolutionary conservation, challenging the non-functionality of DUX4 and suggesting a coding role, possibly during development. Interestingly, not only the ORF of DUX4, but also their organization in an array is evolutionary conserved. Importantly, this study provided evidence for bidirectional transcription of the mouse Dux array [56]. Next, expression of two different DUX4 transcripts in cells transfected with D4Z4 elements and in FSHD myoblasts was reported. The first transcript lacks introns and is transcribed from internal D4Z4 repeat units, while the second transcript has two introns and is transcribed from the most distal D4Z4 repeat unit. Interestingly, the pLAM sequence distal to the second transcript may provide a polyadenylation signal. Thus far, DUX4 expression seems to be restricted to FSHD myoblasts [51;52]. As most homeodomain proteins have a function as transcriptional regulators in developmental processes, DUX4 expression may normally be restricted to embryogenesis [57]. In fact, the DUX4 homeodomain shares high homology with the homeodomain of the proteins Pax3 and Pax7, which are involved in the development of skeletal muscle [58].
As FSHD is specifically linked to the 4qA161 haplotype [23], sequence variations residing within or close to the D4Z4 repeat array may play a role in the regulation of DUX4 transcription. Therefore, it is very interesting that differences between 4qA and 4qB alleles are observed in the pLAM region, possibly affecting the polyadenylation signal [20;52]. However, these data need to be extended. At the same time, lower DNA methylation levels at D4Z4 may also influence the regulatory process of DUX4, explaining the occurrence of FSHD in phenotypic patients without a D4Z4 contraction.
4.2 Role of gene deregulation in cis in FSHD pathogenesis
Other models have predicted an indirect role for the D4Z4 contraction in FSHD pathogenesis. Chromatin structure alterations at D4Z4, like D4Z4 hypomethylation, may cause loss of transcriptional control over the expression of candidate genes in cis. The identification of a DNA-binding complex, consisting of YY1, HMGB2 (high-mobility group box 2) and nucleolin and acting as a transcriptional repressor, supported the cis-model of gene deregulation. In controls, the presence of a threshold number of D4Z4 repeats may repress 4q35 genes, while in FSHD patients, because of a strong reduction in the number of bound YY1-HMGB2-nucleolin complexes, the transcriptional repression is abrogated, resulting in inappropriate overexpression [59]. A second line of evidence for deregulation in cis was recently provided by the identification of a nuclear matrix attachment site (S/MAR) associating with the nuclear matrix immediately upstream of D4Z4 [60]. S/MAR sequences are important for the organization of DNA into loop domains as part of a higher-order chromatin structure [61]. In normal cells, the S/MAR is located between the upstream FSHD candidate genes FRG1 and FRG2 and the D4Z4 repeat array, thus separating them into two distinct DNA loop domains. In myoblasts from patients with FSHD, dissociation of the S/MAR from the nuclear matrix seems to occur, what may result in the presence of the FRG1 and FRG2 genes in the same loop as the D4Z4 repeat array [60]. Since the 5’ end of the D4Z4 repeat array was shown to contain a strong transcriptional enhancer, as a consequence FRG1 and FRG2 expression may be upregulated in patients with FSHD [62].
Although initial testing showed that FRG1, FRG2 and ANT1 were indeed transcriptionally upregulated in FSHD muscle [59], several follow-up studies could not reproduce these findings [48;53;54;63]. The use of different techniques and different sources of RNA may partly explain this lack of reproducibility. The highly conserved nuclear protein FRG1 is a component of the human spliceosome and may have a role in pre-messenger RNA splicing [64–66]. Importantly, mice that overexpress FRG1 25- or 40-fold in skeletal muscle develop a muscular dystrophy phenotype. In addition, missplicing of muscle-specific mRNAs was observed in skeletal muscle of these transgenic mice, in FRG1-expressing C2C12 cells and in FSHD myoblasts [67]. Although an independent follow-up study could not confirm a splicing defect in FSHD muscle [54], a potential role for FRG1 in FSHD pathogenesis has to be considered. FRG2, mapping 37 kb proximal to D4Z4 and specifically upregulated in differentiating myoblasts of patients with FSHD, is a less attractive FSHD candidate gene, as it is absent in some FSHD patients with a proximally extended deletion [9–11;68]. Also, mice that overexpress FRG2 do not present with muscular dystrophy. The same holds for mice overexpressing ANT1; these mice do not seem to develop a muscular dystrophy phenotype [67]. Interestingly, ANT1 protein levels were shown to be increased in both unaffected and affected FSHD muscles compared to muscles from controls and patients with Duchenne muscular dystrophy (DMD). An increased expression of ANT1 may sensitize muscle cells to oxidative stress and apoptosis [69]. Thus, ANT1 remains an attractive candidate gene and further studies addressing the role of ANT1 in FSHD pathogenesis are warranted.
4.3 Role of gene deregulation in trans in FSHD pathogenesis
Several studies support an important trans-sensing effect in FSHD. An initial study on global gene expression profiles of FSHD muscle suggested a FSHD-specific defect in myogenic differentiation [53]. Since then, both gene and protein expression follow-up studies have been performed, presenting new interesting affected pathways, such as an impairment of slow to fast fiber differentiation, increased sensitivity to oxidative stress and a possible link with retinal vasculopathy [54;63]. As the somatic pairing frequency between the 4q subtelomere and the 10q subtelomere was observed to be slightly but significantly increased in patients with FSHD, a trans-sensing effect of the D4Z4 contraction on gene regulation on 10qter is expected [70]. Evidence supporting this hypothesis is the observation of a distinct level of FRG2 expression on chromosome 10 in differentiating myoblasts of patients with FSHD [68] and a significant trans effect on myotube formation when D4Z4 repeats were transfected in C2C12 myoblasts [71].
As discussed above, each D4Z4 unit contains a 27 bp D4Z4 binding element (DBE) which binds a multi-protein complex consisting of YY1, HMGB2 and nucleolin [59]. Loss of this repressor complex at the disease allele in patients with FSHD may not only have an effect on transcriptional regulation of 4q35 genes. Genome-wide effects can be expected as well as a result of a local imbalance of D4Z4 binding of these proteins and subsequent interaction with different proteins at the disease allele. HMGB2 is a chromatin-associated DNA binding protein and member of the high mobility group (HMG) proteins [72]. Binding of HMGB2 to DNA may have a profound effect on the maintenance of heterochromatic regions, as HMGB2 interacts with SP100B which in turn binds to HP1, which has a function in the establishment and maintenance of higher-order chromatin structures [73;74]. Nucleolin, a nucleolar RNA-binding protein involved in several steps of ribosome biogenesis [75], may have the opposite effect on heterochromatin maintenance. Nucleolin has been shown to interact with histone H1 which may result in chromatin decondensation by displacement of histone H1 from linker DNA [76]. Finally, YY1 may also effect the chromatin structure at D4Z4, since it is the homologue of the Drosophila PcG protein pleiohomeotic (PHO). PcG multi-protein complexes control chromatin accessibility and maintain transcriptional repression during embryogenesis [77]. Depending on its relative concentration, the presence of coactivators or corepressors and the promoter context, YY1 can act as a transcriptional activator or repressor. A local imbalance in YY1 binding at D4Z4 may have multiple consequences. First, YY1 binding to regulatory regions of transcriptionally inactive muscle-specific genes seems to be required for recruitment of the histone lysine methyltransferase Ezh2 in proliferating mouse myoblasts. During myoblast differentiation, the YY1-Ezh2 complex disassociates from the DNA and consequently the transcription factor MyoD, having a key role in the differentiation of all skeletal muscle lineages, is recruited [78]. Thus, an imbalance in YY1 binding at D4Z4 in patients with FSHD may affect muscle differentiation, especially during embryonic development when Ezh2 is expressed [79]. Second, an unbalance in YY1 binding may influence chromatin structure at or around its target site by recruiting the histone H4-specific methyltransferase PRMT1, resulting in methylation of arginine residue 3 of histone H4 and gene activation [80]. Third, an imbalance in YY1 binding may influence the interaction with the protein CTCF, a chromatin insulator that seems to be essential for homologous X-chromosome pairing [81]. Possibly, YY1-CTCF may have a similar function in pairing between the 4q subtelomere and the 10q subtelomere.
4.4 Role of nuclear organization in FSHD pathogenesis
Appropriate nuclear organization is essential for normal gene expression. Chromosomes are compartmentalized into discrete nuclear territories. The location of a gene within such a nuclear territory determines the availability of regulatory proteins and the accessibility of the DNA to the transcriptional apparatus [82]. The nuclear envelope (NE), consisting of an inner (INM) and outer nuclear membrane (ONM), forms the boundary of the nucleus [83]. The INM is covered with a protein meshwork, the nuclear lamina, which maintains the shape of the nucleus and provides mechanical strength to the nucleus. Besides, it has a role in many nuclear activities, including DNA replication, RNA transcription, nuclear and chromatin organization, cell cycle regulation, cell development and differentiation, nuclear migration and apoptosis [84]. A large group of inherited human diseases, collectively termed the “laminopathies”, is caused by mutations in components of the nuclear lamina. Most commonly, adipose tissue, bone and connective tissue, heart and importantly skeletal muscle are affected by these mutations [85].
The 4q subtelomere is preferentially localized in the outer nuclear rim, both in controls and in patients with FSHD. Other subtelomeric regions, including 10qter, localize more to the interior of the nucleus [86;87]. This peripheral localization of 4qter seems to be caused by an intrinsic property of 4qter as the X-chromosome showed a more peripheral localization in a cell line with a X;4 translocation containing the distal 4 Mb of 4qter [87]. A region proximal to D4Z4 seems to be primarily responsible for the perinuclear localization [86;87]. These results may explain the different nuclear localization of 10q subtelomeres, since the homology between 4qter and 10qter is restricted to the 40 kb proximal to D4Z4. A major role for a correct integrity of the nuclear lamina in the peripheral organization of 4qter is to be expected as the peripheral localization of 4qter is lost in fibroblasts lacking lamin A/C, a protein of the nuclear lamina [86;87].
Although no change in the localization of disease chromosomes compared to healthy chromosomes was observed, the interaction between 4qter and the nuclear envelope may be disturbed in FSHD because of alterations in chromatin structure at D4Z4 and the consequent loss of binding of specific proteins that may interact with the nuclear lamina. Interestingly, other neuromuscular disorders, like X-linked and autosomal dominant Emery-Dreifuss muscular dystrophies (EDMD), are caused by mutations in emerin and lamin A/C, respectively [85]. Moreover, six nuclear envelope transmembrane proteins (NETs) were identified that are predicted to have an important function in myoblast differentiation and/or muscle maintenance [88]. Finally, transcriptome studies showed that FSHD and EDMD are highly related [89] and DUX4 overexpression may redistribute emerin at the nuclear envelope [51]. In conclusion, it is hypothesized that FSHD may arise from improper chromatin interactions at the nuclear envelope.
5. An integrative model for FSHD pathogenesis
Although the D4Z4 repeat contraction in patients with FSHD was discovered more than 15 years ago, the exact molecular mechanism causing the FSHD phenotype is still not elucidated. It seems unlikely that a single candidate gene is responsible for the development of FSHD. Probably, a complex epigenetic disease mechanism involving the deregulation of multiple genes, both in cis and in trans, underlies its pathogenesis. Therefore, all disease mechanisms described above may be correct in essence. One of the major challenges for future FSHD research will be to integrate these disease mechanisms into a single model and to obtain consistent evidence supporting this model.
We predict that the D4Z4 repeat array and its chromatin structure will be central in such a unifying model, because every patient with FSHD, either 4q-linked, with a proximally extended deletion or phenotypic, shows genetic and/or epigenetic changes at this repeat array. We propose that in control individuals the D4Z4 repeat array is tightly packaged, probably as facultative heterochromatin. In patients with FSHD, this chromatin structure becomes more open. As a consequence, proteins may bind to D4Z4, influencing the regulation of candidate genes and the interaction with the nuclear envelope (figure 3). Sequence variations residing within or close to the D4Z4 repeat array may be important for binding of such proteins and thus determine why FSHD is specifically linked to the 4qA161 haplotype [23].
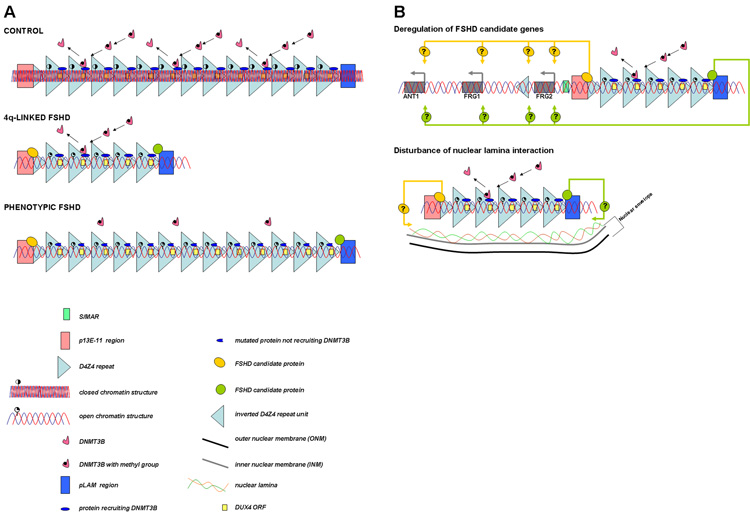
Figure 3. An integrative model for FSHD pathogenesis.
A. In controls the D4Z4 repeat array is packed as facultative heterochromatin. In patients with 4q-linked FSHD only below 11 D4Z4 repeat units, a more open chromatin structure is induced. Below this threshold an insufficient level of a protein (complex) responsible for D4Z4 methylation or a presently unidentified chromatin modifier (complex) may be present at D4Z4. In patients with phenotypic FSHD, changes in chromatin structure may occur because of a yet unidentified gene defect responsible for the epigenetic modifications at D4Z4. Therefore, an open chromatin structure is present in these patients even above the critical threshold of ten D4Z4 repeat units. Because of the open chromatin structure in patients with FSHD, binding of protein(s) to D4Z4 that normally do not bind may occur. This will most likely occur either proximal or distal to D4Z4 or to a critical D4Z4 element, since the amount of repeat units differs significantly between 4q-linked and phenotypic FSHD, while their phenotypes are highly similar. In addition, specific SNPs discriminating between the different haplotypes may be important for the binding of these proteins.
B. When the chromatin structure is in a more open conformation, candidate genes may be deregulated in cis (upper panel) and the interaction with the nuclear envelope may be disturbed (lower panel).
In 4q-linked FSHD, the open chromatin structure is only reached at a certain threshold. If more than 10 D4Z4 repeat units are still present, the chromatin structure can be kept in a closed state, for example because of a sufficient level of binding of proteins that attract DNMT3B, the DNA methyltransferase responsible for DNA methylation at D4Z4, or the binding of a yet unidentified chromatin modifier. When less than 11 D4Z4 repeat units are left, the critical threshold is reached, resulting in loss of DNA methylation at D4Z4. In phenotypic FSHD, a presently unidentified gene defect may disturb the recruitment of DNMT3B or another chromatin modifier to D4Z4 with consequent loss of D4Z4 methylation, thus also resulting in an open chromatin structure at D4Z4. We predict the presence of at least one 4qA161 allele in phenotypic FSHD patients to explain the occurrence of FSHD in these individuals.
Since the number of D4Z4 repeat units with an open chromatin structure differs significantly between 4q-linked and phenotypic FSHD patients (1–10 versus >10 repeats), it seems unlikely that a protein that binds to each D4Z4 repeat unit, e.g. the D4Z4 repressor complex, plays an important role in FSHD development. If this was the case, differences in phenotype between 4q-linked and phenotypic FSHD patients were to be expected. More likely, binding of a protein just upstream or downstream of D4Z4 or binding to a specific D4Z4 element (e.g. the proximal or distal unit) is critical. Interestingly, the chromatin structure of the p13E-11 region just upstream of D4Z4 is different compared to the remainder of the D4Z4 repeat array [35;48] and this open chromatin configuration extends into the proximal D4Z4 repeat unit just distal to p13E-11 in both controls and patients with FSHD [35]. Also, this region harbors haplotype-specific SNPs that may be critical to disease development. Apparently, there is an unusual small transition zone from a very open to a compact chromatin structure. Changes in this transition zone may uncover presently unidentified D4Z4 elements essential for the pathogenesis of FSHD. It is therefore imperative to investigate whether such a transition zone also exists at the distal end of the repeat.
Currently, we cannot explain the large clinical intra- and interfamilial variability observed in FSHD, varying from gene carriers without symptoms and patients that eventually become wheelchair-bound [90]. This intrafamilial variability also applies for phenotypic FSHD families, where a non-affected family member with significant D4Z4 hypomethylation was identified [26]. Therefore, further analysis of chromatin changes in gene carriers will be essential. Second, because of the lack of the FSHD phenotype when D4Z4 is contracted on 10q alleles, 4qB alleles and 4qA166 alleles, it will be important to study chromatin changes in controls carrying such a short repeat array [17;18;21;23]. In addition, the differences between all recently identified haplotypes need to be studied in detail as SNPs in the D4Z4 repeat array may affect binding of proteins and thus may explain the lack of FSHD development on non-4qA161 alleles [23]. We speculate that the field of FSHD research will move forward significantly by studying individuals that carry 4qter changes not resulting in FSHD and by studying FSHD cases that carry non-standard alleles, as recently described [22].
Acknowledgements
We apologize to the many investigators whose work we could not cite because of space limitations. We thank Stephen Tapscott and Kyoko Yokomori for critical reading of the manuscript and we thank all patients and family members for their participation in our studies. Our FSHD research is supported by grants from the Netherlands Organization for Scientific Research, the Muscular Dystrophy Association USA, the FSH Society, the National Institutes of Health and the Fields Center for FSHD & Neuromuscular Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest statement
The authors declare that there are no conflicts of interest.
References
- 1.Padberg GW. Facioscapulohumeral disease, PhD thesis. Leiden: Leiden University; 1982. [Google Scholar]
- 2.Wijmenga C, Frants RR, Brouwer OF, Moerer P, Weber JL, Padberg GW. Location of facioscapulohumeral muscular dystrophy gene on chromosome 4. Lancet. 1990;336:651–653. doi: 10.1016/0140-6736(90)92148-b. [DOI] [PubMed] [Google Scholar]
- 3.van Deutekom JC, Wijmenga C, van Tienhoven EA, Gruter AM, Hewitt JE, Padberg GW, van Ommen GJ, Hofker MH, Frants RR. FSHD associated DNA rearrangements are due to deletions of integral copies of a 3.2 kb tandemly repeated unit. Hum. Mol. Genet. 1993;2:2037–2042. doi: 10.1093/hmg/2.12.2037. [DOI] [PubMed] [Google Scholar]
- 4.Wijmenga C, Hewitt JE, Sandkuijl LA, Clark LN, Wright TJ, Dauwerse HG, Gruter AM, Hofker MH, Moerer P, Williamson R. Chromosome 4q DNA rearrangements associated with facioscapulohumeral muscular dystrophy. Nat.Genet. 1992;2:26–30. doi: 10.1038/ng0992-26. [DOI] [PubMed] [Google Scholar]
- 5.Lunt PW, Jardine PE, Koch MC, Maynard J, Osborn M, Williams M, Harper PS, Upadhyaya M. Correlation between fragment size at D4F104S1 and age at onset or at wheelchair use, with a possible generational effect, accounts for much phenotypic variation in 4q35-facioscapulohumeral muscular dystrophy (FSHD) Hum.Mol.Genet. 1995;4:951–958. doi: 10.1093/hmg/4.5.951. [DOI] [PubMed] [Google Scholar]
- 6.Tawil R, Forrester J, Griggs RC, Mendell J, Kissel J, McDermott M, King W, Weiffenbach B, Figlewicz D. Evidence for anticipation and association of deletion size with severity in facioscapulohumeral muscular dystrophy, The FSH-DY Group. Ann.Neurol. 1996;39:744–748. doi: 10.1002/ana.410390610. [DOI] [PubMed] [Google Scholar]
- 7.Ricci E, Galluzzi G, Deidda G, Cacurri S, Colantoni L, Merico B, Piazzo N, Servidei S, Vigneti E, Pasceri V, Silvestri G, Mirabella M, Mangiola F, Tonali P, Felicetti L. Progress in the molecular diagnosis of facioscapulohumeral muscular dystrophy and correlation between the number of KpnI repeats at the 4q35 locus and clinical phenotype. Ann.Neurol. 1999;45:751–757. doi: 10.1002/1531-8249(199906)45:6<751::aid-ana9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 8.Tupler R, Berardinelli A, Barbierato L, Frants R, Hewitt JE, Lanzi G, Maraschio P, Tiepolo L. Monosomy of distal 4q does not cause facioscapulohumeral muscular dystrophy. J.Med.Genet. 1996;33:366–370. doi: 10.1136/jmg.33.5.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lemmers RJ, van der Maarel SM, van Deutekom JC, van der Wielen MJ, Deidda G, Dauwerse HG, Hewitt J, Hofker M, Bakker E, Padberg GW, Frants RR. Inter- and intrachromosomal sub-telomeric rearrangements on 4q35: implications for facioscapulohumeral muscular dystrophy (FSHD) aetiology and diagnosis. Hum.Mol.Genet. 1998;7:1207–1214. doi: 10.1093/hmg/7.8.1207. [DOI] [PubMed] [Google Scholar]
- 10.Lemmers RJ, Osborn M, Haaf T, Rogers M, Frants RR, Padberg GW, Cooper DN, van der Maarel SM, Upadhyaya M. D4F104S1 deletion in facioscapulohumeral muscular dystrophy: phenotype, size, and detection. Neurology. 2003;61:178–183. doi: 10.1212/01.wnl.0000078889.51444.81. [DOI] [PubMed] [Google Scholar]
- 11.Deak KL, Lemmers RJ, Stajich JM, Klooster R, Tawil R, Frants RR, Speer MC, van der Maarel SM, Gilbert JR. Genotype-phenotype study in an FSHD family with a proximal deletion encompassing p13E-11 and D4Z4. Neurology. 2007;68:578–582. doi: 10.1212/01.wnl.0000254991.21818.f3. [DOI] [PubMed] [Google Scholar]
- 12.Lyle R, Wright TJ, Clark LN, Hewitt JE. The FSHD-associated repeat, D4Z4, is a member of a dispersed family of homeobox-containing repeats, subsets of which are clustered on the short arms of the acrocentric chromosomes. Genomics. 1995;28:389–397. doi: 10.1006/geno.1995.1166. [DOI] [PubMed] [Google Scholar]
- 13.Bakker E, Wijmenga C, Vossen RH, Padberg GW, Hewitt J, van der Wielen M, Rasmussen K, Frants RR. The FSHD-linked locus D4F104S1 (p13E-11) on 4q35 has a homologue on 10qter. Muscle Nerve. 1995;2:S39–S44. [PubMed] [Google Scholar]
- 14.Deidda G, Cacurri S, Grisanti P, Vigneti E, Piazzo N, Felicetti L. Physical mapping evidence for a duplicated region on chromosome 10qter showing high homology with the facioscapulohumeral muscular dystrophy locus on chromosome 4qter. Eur.J.Hum.Genet. 1995;3:155–167. doi: 10.1159/000472291. [DOI] [PubMed] [Google Scholar]
- 15.van Deutekom JC, Bakker E, Lemmers RJ, van der Wielen MJ, Bik E, Hofker MH, Padberg GW, Frants RR. Evidence for subtelomeric exchange of 3.3 kb tandemly repeated units between chromosomes 4q35 and 10q26: implications for genetic counselling and etiology of FSHD1. Hum.Mol.Genet. 1996;5:1997–2003. doi: 10.1093/hmg/5.12.1997. [DOI] [PubMed] [Google Scholar]
- 16.Van Overveld PG, Lemmers RJ, Deidda G, Sandkuijl L, Padberg GW, Frants RR, van der Maarel SM. Interchromosomal repeat array interactions between chromosomes 4 and 10: a model for subtelomeric plasticity. Hum.Mol.Genet. 2000;9:2879–2884. doi: 10.1093/hmg/9.19.2879. [DOI] [PubMed] [Google Scholar]
- 17.Lemmers RJL, de Kievit P, van Geel M, van der Wielen MJ, Bakker E, Padberg GW, Frants RR, van der Maarel SM. Complete allele information in the diagnosis of facioscapulohumeral muscular dystrophy by triple DNA analysis. Ann.Neurol. 2001;50:816–819. doi: 10.1002/ana.10057. [DOI] [PubMed] [Google Scholar]
- 18.Zhang Y, Forner J, Fournet S, Jeanpierre M. Improved characterization of FSHD mutations. Ann.Genet. 2001;44:105–110. doi: 10.1016/s0003-3995(01)01075-9. [DOI] [PubMed] [Google Scholar]
- 19.van Geel M, Dickson MC, Beck AF, Bolland DJ, Frants RR, van der Maarel SM, de Jong PJ, Hewitt JE. Genomic analysis of human chromosome 10q and 4q telomeres suggests a common origin. Genomics. 2002;79:210–217. doi: 10.1006/geno.2002.6690. [DOI] [PubMed] [Google Scholar]
- 20.Lemmers RJ, de Kievit P, Sandkuijl L, Padberg GW, van Ommen GJ, Frants RR, van der Maarel SM. Facioscapulohumeral muscular dystrophy is uniquely associated with one of the two variants of the 4q subtelomere. Nat.Genet. 2002;32:235–236. doi: 10.1038/ng999. [DOI] [PubMed] [Google Scholar]
- 21.Lemmers RJ, Wohlgemuth M, Frants RR, Padberg GW, Morava E, van der Maarel SM. Contractions of D4Z4 on 4qB subtelomeres do not cause facioscapulohumeral muscular dystrophy. Am.J.Hum.Genet. 2004;75:1124–1130. doi: 10.1086/426035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas NS, Wiseman K, Spurlock G, MacDonald M, Ustek D, Upadhyaya M. A large patient study confirming that facioscapulohumeral muscular dystrophy (FSHD) disease expression is almost exclusively associated with an FSHD locus located on a 4qA-defined 4qter subtelomere. J.Med.Genet. 2007;44:215–218. doi: 10.1136/jmg.2006.042804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lemmers RJ, Wohlgemuth M, van der Gaag KJ, van der Vliet P, van Teijlingen CM, de Knijff P, Padberg GW, Frants RR, van der Maarel SM. Specific sequence variations within the 4q35 region are associated with facioscapulohumeral muscular dystrophy. Am.J.Hum.Genet. 2007;81:884–894. doi: 10.1086/521986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gilbert JR, Stajich JM, Wall S, Carter SC, Qiu H, Vance JM, Stewart CS, Speer MC, Pufky J, Yamaoka LH, Rozear M, Samson F, Fardeau M, Roses AD, Pericak-Vance A. Evidence for heterogeneity in facioscapulohumeral muscular dystrophy (FSHD) Am.J.Hum.Genet. 1993;53:401–408. [PMC free article] [PubMed] [Google Scholar]
- 25.Bastress KL, Stajich JM, Speer MC, Gilbert JR. The genes encoding for D4Z4 binding proteins HMGB2, YY1, NCL, and MYOD1 are excluded as candidate genes for FSHD1B. Neuromuscul.Disord. 2005;15:316–320. doi: 10.1016/j.nmd.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 26.de Greef JC, Wohlgemuth M, Chan OA, Hansson KB, Smeets D, Frants RR, Weemaes CM, Padberg GW, van der Maarel SM. Hypomethylation is restricted to the D4Z4 repeat array in phenotypic FSHD. Neurology. 2007;69:1018–1026. doi: 10.1212/01.wnl.0000271391.44352.fe. [DOI] [PubMed] [Google Scholar]
- 27.Hewitt JE, Lyle R, Clark LN, Valleley EM, Wright TJ, Wijmenga C, van Deutekom JC, Francis F, Sharpe PT, Hofker M, Frants RR, Williamson R. Analysis of the tandem repeat locus D4Z4 associated with facioscapulohumeral muscular dystrophy. Hum.Mol.Genet. 1994;3:1287–1295. doi: 10.1093/hmg/3.8.1287. [DOI] [PubMed] [Google Scholar]
- 28.Campanero MR, Armstrong MI, Flemington EK. CpG methylation as a mechanism for the regulation of E2F activity. Proc.Natl.Acad.Sci.U.S.A. 2000;97:6481–6486. doi: 10.1073/pnas.100340697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hark AT, Schoenherr CJ, Katz DJ, Ingram RS, Levorse JM, Tilghman SM. CTCF mediates methylation-sensitive enhancer-blocking activity at the H19/Igf2 locus. Nature. 2000;405:486–489. doi: 10.1038/35013106. [DOI] [PubMed] [Google Scholar]
- 30.Kim J, Kollhoff A, Bergmann A, Stubbs L. Methylation-sensitive binding of transcription factor YY1 to an insulator sequence within the paternally expressed imprinted gene, Peg3. Hum.Mol.Genet. 2003;12:233–245. doi: 10.1093/hmg/ddg028. [DOI] [PubMed] [Google Scholar]
- 31.Ballestar E, Wolffe AP. Methyl-CpG-binding proteins, Targeting specific gene repression. Eur.J.Biochem. 2001;268:1–6. doi: 10.1046/j.1432-1327.2001.01869.x. [DOI] [PubMed] [Google Scholar]
- 32.Fujita N, Watanabe S, Ichimura T, Tsuruzoe S, Shinkai Y, Tachibana M, Chiba T, Nakao M. Methyl-CpG binding domain 1 (MBD1) interacts with the Suv39h1-HP1 heterochromatic complex for DNA methylation-based transcriptional repression. J.Biol.Chem. 2003;278:24132–24138. doi: 10.1074/jbc.M302283200. [DOI] [PubMed] [Google Scholar]
- 33.Tsien F, Sun B, Hopkins NE, Vedanarayanan V, Figlewicz D, Winokur S, Ehrlich M. Methylation of the FSHD syndrome-linked subtelomeric repeat in normal and FSHD cell cultures and tissues. Mol.Genet.Metab. 2001;74:322–331. doi: 10.1006/mgme.2001.3219. [DOI] [PubMed] [Google Scholar]
- 34.Van Overveld PG, Lemmers RJ, Sandkuijl LA, Enthoven L, Winokur ST, Bakels F, Padberg GW, van Ommen GJ, Frants RR, van der Maarel SM. Hypomethylation of D4Z4 in 4q-linked and non-4q-linked facioscapulohumeral muscular dystrophy. Nat.Genet. 2003;35:315–317. doi: 10.1038/ng1262. [DOI] [PubMed] [Google Scholar]
- 35.Tsumagari K, Qi L, Jackson K, Shao C, Lacey M, Sowden J, Tawil R, Vedanarayanan V, Ehrlich M. Epigenetics of a tandem DNA repeat: chromatin DNaseI sensitivity and opposite methylation changes in cancers. Nucleic Acids Res. 2008 doi: 10.1093/nar/gkn055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou J, Berger SL. Good fences make good neighbors: barrier elements and genomic regulation. Mol.Cell. 2004;16:500–502. doi: 10.1016/j.molcel.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 37.Etzioni S, Yafe A, Khateb S, Weisman-Shomer P, Bengal E, Fry M. Homodimeric MyoD preferentially binds tetraplex structures of regulatory sequences of muscle-specific genes. J.Biol.Chem. 2005;280:26805–26812. doi: 10.1074/jbc.M500820200. [DOI] [PubMed] [Google Scholar]
- 38.Van Overveld PG, Enthoven L, Ricci E, Rossi M, Felicetti L, Jeanpierre M, Winokur ST, Frants RR, Padberg GW, van der Maarel SM. Variable hypomethylation of D4Z4 in facioscapulohumeral muscular dystrophy. Ann.Neurol. 2005;58:569–576. doi: 10.1002/ana.20625. [DOI] [PubMed] [Google Scholar]
- 39.Kondo T, Bobek MP, Kuick R, Lamb B, Zhu X, Narayan A, Bourc'his D, Viegas-Pequignot E, Ehrlich M, Hanash SM. Whole-genome methylation scan in ICF syndrome: hypomethylation of non-satellite DNA repeats D4Z4 and NBL2. Hum.Mol.Genet. 2000;9:597–604. doi: 10.1093/hmg/9.4.597. [DOI] [PubMed] [Google Scholar]
- 40.Xu GL, Bestor TH, Bourc'his D, Hsieh CL, Tommerup N, Bugge M, Hulten M, Qu X, Russo JJ, Viegas-Pequignot E. Chromosome instability and immunodeficiency syndrome caused by mutations in a DNA methyltransferase gene. Nature. 1999;402:187–191. doi: 10.1038/46052. [DOI] [PubMed] [Google Scholar]
- 41.Jeanpierre M, Turleau C, Aurias A, Prieur M, Ledeist F, Fischer A, Viegas-Pequignot E. An embryonic-like methylation pattern of classical satellite DNA is observed in ICF syndrome. Hum.Mol.Genet. 1993;2:731–735. doi: 10.1093/hmg/2.6.731. [DOI] [PubMed] [Google Scholar]
- 42.Bhaumik SR, Smith E, Shilatifard A. Covalent modifications of histones during development and disease pathogenesis. Nat.Struct.Mol.Biol. 2007;14:1008–1016. doi: 10.1038/nsmb1337. [DOI] [PubMed] [Google Scholar]
- 43.Peterson CL, Laniel MA. Histones and histone modifications. Curr.Biol. 2004;14:R546–R551. doi: 10.1016/j.cub.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 44.Strahl BD, Allis CD. The language of covalent histone modifications. Nature. 2000;403:41–45. doi: 10.1038/47412. [DOI] [PubMed] [Google Scholar]
- 45.Eberharter A, Becker PB. Histone acetylation: a switch between repressive and permissive chromatin. Second in review series on chromatin dynamics. EMBO Rep. 2002;3:224–229. doi: 10.1093/embo-reports/kvf053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fischle W, Wang Y, Allis CD. Histone and chromatin cross-talk. Curr.Opin.Cell Biol. 2003;15:172–183. doi: 10.1016/s0955-0674(03)00013-9. [DOI] [PubMed] [Google Scholar]
- 47.Lachner M, O'Sullivan RJ, Jenuwein T. An epigenetic road map for histone lysine methylation. J.Cell Sci. 2003;116:2117–2124. doi: 10.1242/jcs.00493. [DOI] [PubMed] [Google Scholar]
- 48.Jiang G, Yang F, Van Overveld PG, Vedanarayanan V, van der Maarel S, Ehrlich M. Testing the position-effect variegation hypothesis for facioscapulohumeral muscular dystrophy by analysis of histone modification and gene expression in subtelomeric 4q. Hum.Mol.Genet. 2003;12:2909–2921. doi: 10.1093/hmg/ddg323. [DOI] [PubMed] [Google Scholar]
- 49.Yang F, Shao C, Vedanarayanan V, Ehrlich M. Cytogenetic and immuno-FISH analysis of the 4q subtelomeric region, which is associated with facioscapulohumeral muscular dystrophy. Chromosoma. 2004;112:350–359. doi: 10.1007/s00412-004-0280-x. [DOI] [PubMed] [Google Scholar]
- 50.Gabriels J, Beckers MC, Ding H, De Vriese A, Plaisance S, van der Maarel SM, Padberg GW, Frants RR, Hewitt JE, Collen D, Belayew A. Nucleotide sequence of the partially deleted D4Z4 locus in a patient with FSHD identifies a putative gene within each 3.3 kb element. Gene. 1999;236:25–32. doi: 10.1016/s0378-1119(99)00267-x. [DOI] [PubMed] [Google Scholar]
- 51.Kowaljow V, Marcowycz A, Ansseau E, Conde CB, Sauvage S, Matteotti C, Arias C, Corona ED, Nunez NG, Leo O, Wattiez R, Figlewicz D, Laoudj-Chenivesse D, Belayew A, Coppee F, Rosa AL. The DUX4 gene at the FSHD1A locus encodes a pro-apoptotic protein. Neuromuscul.Disord. 2007;17:611–623. doi: 10.1016/j.nmd.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 52.Dixit M, Ansseau E, Tassin A, Winokur S, Shi R, Qian H, Sauvage S, Matteotti C, van Acker AM, Leo O, Figlewicz D, Barro M, Laoudj-Chenivesse D, Belayew A, Coppee F, Chen YW. DUX4, a candidate gene of facioscapulohumeral muscular dystrophy, encodes a transcriptional activator of PITX1. Proc.Natl.Acad.Sci.U.S.A. 2007;104:18157–18162. doi: 10.1073/pnas.0708659104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Winokur ST, Chen YW, Masny PS, Martin JH, Ehmsen JT, Tapscott SJ, van der Maarel SM, Hayashi Y, Flanigan KM. Expression profiling of FSHD muscle supports a defect in specific stages of myogenic differentiation. Hum.Mol.Genet. 2003;12:2895–2907. doi: 10.1093/hmg/ddg327. [DOI] [PubMed] [Google Scholar]
- 54.Osborne RJ, Welle S, Venance SL, Thornton CA, Tawil R. Expression profile of FSHD supports a link between retinal vasculopathy and muscular dystrophy. Neurology. 2007;68:569–577. doi: 10.1212/01.wnl.0000251269.31442.d9. [DOI] [PubMed] [Google Scholar]
- 55.Alexiadis V, Ballestas ME, Sanchez C, Winokur S, Vedanarayanan V, Warren M, Ehrlich M. RNAPol-ChIP analysis of transcription from FSHD-linked tandem repeats and satellite DNA. Biochim.Biophys.Acta. 2007;1769:29–40. doi: 10.1016/j.bbaexp.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Clapp J, Mitchell LM, Bolland DJ, Fantes J, Corcoran AE, Scotting PJ, Armour JA, Hewitt JE. Evolutionary conservation of a coding function for D4Z4, the tandem DNA repeat mutated in facioscapulohumeral muscular dystrophy. Am.J.Hum.Genet. 2007;81:264–279. doi: 10.1086/519311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deschamps J, Meijlink F. Mammalian homeobox genes in normal development and neoplasia. Crit Rev.Oncog. 1992;3:117–173. [PubMed] [Google Scholar]
- 58.Buckingham M, Relaix F. The role of Pax genes in the development of tissues and organs: Pax3 and Pax7 regulate muscle progenitor cell functions. Annu.Rev.Cell Dev.Biol. 2007;23:645–673. doi: 10.1146/annurev.cellbio.23.090506.123438. [DOI] [PubMed] [Google Scholar]
- 59.Gabellini D, Green MR, Tupler R. Inappropriate gene activation in FSHD: a repressor complex binds a chromosomal repeat deleted in dystrophic muscle. Cell. 2002;110:339–348. doi: 10.1016/s0092-8674(02)00826-7. [DOI] [PubMed] [Google Scholar]
- 60.Petrov A, Pirozhkova I, Carnac G, Laoudj D, Lipinski M, Vassetzky YS. Chromatin loop domain organization within the 4q35 locus in facioscapulohumeral dystrophy patients versus normal human myoblasts. Proc.Natl.Acad.Sci.U.S.A. 2006;103:6982–6987. doi: 10.1073/pnas.0511235103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Razin SV, Iarovaia OV, Sjakste N, Sjakste T, Bagdoniene L, Rynditch AV, Eivazova ER, Lipinski M, Vassetzky YS. Chromatin domains and regulation of transcription. J.Mol.Biol. 2007;369:597–607. doi: 10.1016/j.jmb.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 62.Petrov A, Allinne J, Pirozhkova I, Laoudj D, Lipinski M, Vassetzky YS. A nuclear matrix attachment site in the 4q35 locus has an enhancer-blocking activity in vivo: implications for the facio-scapulo-humeral dystrophy. Genome Res. 2008;18:39–45. doi: 10.1101/gr.6620908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Celegato B, Capitanio D, Pescatori M, Romualdi C, Pacchioni B, Cagnin S, Vigano A, Colantoni L, Begum S, Ricci E, Wait R, Lanfranchi G, Gelfi C. Parallel protein and transcript profiles of FSHD patient muscles correlate to the D4Z4 arrangement and reveal a common impairment of slow to fast fibre differentiation and a general deregulation of MyoD-dependent genes. Proteomics. 2006;6:5303–5321. doi: 10.1002/pmic.200600056. [DOI] [PubMed] [Google Scholar]
- 64.van Koningsbruggen S, Straasheijm KR, Sterrenburg E, de Graaf N, Dauwerse HG, Frants RR, van der Maarel SM. FRG1P-mediated aggregation of proteins involved in pre-mRNA processing. Chromosoma. 2007;116:53–64. doi: 10.1007/s00412-006-0083-3. [DOI] [PubMed] [Google Scholar]
- 65.Grewal PK, Todd LC, van der Maarel S, Frants RR, Hewitt JE. FRG1, a gene in the FSH muscular dystrophy region on human chromosome 4q35, is highly conserved in vertebrates and invertebrates. Gene. 1998;216:13–19. doi: 10.1016/s0378-1119(98)00334-5. [DOI] [PubMed] [Google Scholar]
- 66.van Deutekom JC, Lemmers RJ, Grewal PK, van Geel M, Romberg S, Dauwerse HG, Wright TJ, Padberg GW, Hofker MH, Hewitt JE, Frants RR. Identification of the first gene (FRG1) from the FSHD region on human chromosome 4q35. Hum.Mol.Genet. 1996;5:581–590. doi: 10.1093/hmg/5.5.581. [DOI] [PubMed] [Google Scholar]
- 67.Gabellini D, D'Antona G, Moggio M, Prelle A, Zecca C, Adami R, Angeletti B, Ciscato P, Pellegrino MA, Bottinelli R, Green MR, Tupler R. Facioscapulohumeral muscular dystrophy in mice overexpressing FRG1. Nature. 2006;439:973–977. doi: 10.1038/nature04422. [DOI] [PubMed] [Google Scholar]
- 68.Rijkers T, Deidda G, van Koningsbruggen S, van Geel M, Lemmers RJ, van Deutekom JC, Figlewicz D, Hewitt JE, Padberg GW, Frants RR, van der Maarel SM. FRG2, an FSHD candidate gene, is transcriptionally upregulated in differentiating primary myoblast cultures of FSHD patients. J.Med.Genet. 2004;41:826–836. doi: 10.1136/jmg.2004.019364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Laoudj-Chenivesse D, Carnac G, Bisbal C, Hugon G, Bouillot S, Desnuelle C, Vassetzky Y, Fernandez A. Increased levels of adenine nucleotide translocator 1 protein and response to oxidative stress are early events in facioscapulohumeral muscular dystrophy muscle. J.Mol.Med. 2005;83:216–224. doi: 10.1007/s00109-004-0583-7. [DOI] [PubMed] [Google Scholar]
- 70.Stout K, van der Maarel S, Frants RR, Padberg GW, Ropers HH, Haaf T. Somatic pairing between subtelomeric chromosome regions: implications for human genetic disease? Chromosome.Res. 1999;7:323–329. doi: 10.1023/a:1009287111661. [DOI] [PubMed] [Google Scholar]
- 71.Yip DJ, Picketts DJ. Increasing D4Z4 repeat copy number compromises C2C12 myoblast differentiation. FEBS Lett. 2003;537:133–138. doi: 10.1016/s0014-5793(03)00110-8. [DOI] [PubMed] [Google Scholar]
- 72.Thomas JO. HMG1 and 2: architectural DNA-binding proteins. Biochem.Soc.Trans. 2001;29:395–401. doi: 10.1042/bst0290395. [DOI] [PubMed] [Google Scholar]
- 73.Lehming N, Le Saux A, Schuller J, Ptashne M. Chromatin components as part of a putative transcriptional repressing complex. Proc.Natl.Acad.Sci.U.S.A. 1998;95:7322–7326. doi: 10.1073/pnas.95.13.7322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lomberk G, Wallrath L, Urrutia R. The Heterochromatin Protein 1 family. Genome Biol. 2006;7:228. doi: 10.1186/gb-2006-7-7-228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ginisty H, Sicard H, Roger B, Bouvet P. Structure and functions of nucleolin. J.Cell Sci. 1999;112:761–772. doi: 10.1242/jcs.112.6.761. [DOI] [PubMed] [Google Scholar]
- 76.Erard MS, Belenguer P, Caizergues-Ferrer M, Pantaloni A, Amalric F. A major nucleolar protein, nucleolin, induces chromatin decondensation by binding to histone H1. Eur.J.Biochem. 1988;175:525–530. doi: 10.1111/j.1432-1033.1988.tb14224.x. [DOI] [PubMed] [Google Scholar]
- 77.Orlando V. Polycomb, epigenomes, and control of cell identity. Cell. 2003;112:599–606. doi: 10.1016/s0092-8674(03)00157-0. [DOI] [PubMed] [Google Scholar]
- 78.Caretti G, Di Padova M, Micales B, Lyons GE, Sartorelli V. The Polycomb Ezh2 methyltransferase regulates muscle gene expression and skeletal muscle differentiation. Genes Dev. 2004;18:2627–2638. doi: 10.1101/gad.1241904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Laible G, Wolf A, Dorn R, Reuter G, Nislow C, Lebersorger A, Popkin D, Pillus L, Jenuwein T. Mammalian homologues of the Polycomb-group gene Enhancer of zeste mediate gene silencing in Drosophila heterochromatin and at S. cerevisiae telomeres. EMBO J. 1997;16:3219–3232. doi: 10.1093/emboj/16.11.3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rezai-Zadeh N, Zhang X, Namour F, Fejer G, Wen YD, Yao YL, Gyory I, Wright K, Seto E. Targeted recruitment of a histone H4-specific methyltransferase by the transcription factor YY1. Genes Dev. 2003;17:1019–1029. doi: 10.1101/gad.1068003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Xu N, Donohoe ME, Silva SS, Lee JT. Evidence that homologous X-chromosome pairing requires transcription and Ctcf protein. Nat.Genet. 2007;39:1390–1396. doi: 10.1038/ng.2007.5. [DOI] [PubMed] [Google Scholar]
- 82.Lanctot C, Cheutin T, Cremer M, Cavalli G, Cremer T. Dynamic genome architecture in the nuclear space: regulation of gene expression in three dimensions. Nat.Rev.Genet. 2007;8:104–115. doi: 10.1038/nrg2041. [DOI] [PubMed] [Google Scholar]
- 83.Gerace L, Burke B. Functional organization of the nuclear envelope. Annu.Rev.Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- 84.Gruenbaum Y, Goldman RD, Meyuhas R, Mills E, Margalit A, Fridkin A, Dayani Y, Prokocimer M, Enosh A. The nuclear lamina and its functions in the nucleus. Int.Rev.Cytol. 2003;226:1–62. doi: 10.1016/s0074-7696(03)01001-5. [DOI] [PubMed] [Google Scholar]
- 85.Worman HJ, Bonne G. "Laminopathies": a wide spectrum of human diseases. Exp.Cell Res. 2007;313:2121–2133. doi: 10.1016/j.yexcr.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Masny PS, Bengtsson U, Chung SA, Martin JH, van Engelen B, van der Maarel SM, Winokur ST. Localization of 4q35.2 to the nuclear periphery: is FSHD a nuclear envelope disease? Hum.Mol.Genet. 2004;13:1857–1871. doi: 10.1093/hmg/ddh205. [DOI] [PubMed] [Google Scholar]
- 87.Tam R, Smith KP, Lawrence JB. The 4q subtelomere harboring the FSHD locus is specifically anchored with peripheral heterochromatin unlike most human telomeres. J.Cell Biol. 2004;167:269–279. doi: 10.1083/jcb.200403128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen IH, Huber M, Guan T, Bubeck A, Gerace L. Nuclear envelope transmembrane proteins (NETs) that are up-regulated during myogenesis. BMC.Cell Biol. 2006;7:38. doi: 10.1186/1471-2121-7-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bakay M, Wang Z, Melcon G, Schiltz L, Xuan J, Zhao P, Sartorelli V, Seo J, Pegoraro E, Angelini C, Shneiderman B, Escolar D, Chen YW, Winokur ST, Pachman LM, Fan C, Mandler R, Nevo Y, Gordon E, Zhu Y, Dong Y, Wang Y, Hoffman EP. Nuclear envelope dystrophies show a transcriptional fingerprint suggesting disruption of Rb-MyoD pathways in muscle regeneration. Brain. 2006;129:996–1013. doi: 10.1093/brain/awl023. [DOI] [PubMed] [Google Scholar]
- 90.Padberg GW, Lunt PW, Koch M, Fardeau M. Diagnostic criteria for facioscapulohumeral muscular dystrophy. Neuromuscul.Disord. 1991;1:231–234. doi: 10.1016/0960-8966(91)90094-9. [DOI] [PubMed] [Google Scholar]