Abstract
Many early metal-on-polyethylene hip resurfacing arthroplasty designs were abandoned after reports of high short-term and midterm failure rates. To investigate factors associated with failure, we retrospectively reviewed our experience with early-design hip resurfacing implants in 75 patients during a 25-year period (median followup, 7.9 years; range, 0.1–25.2 years). Implant failure was defined as revision for any reason. One of 75 patients was lost to followup. The estimated rate of implant survival was 73% at 5 years, 34% at 10 years, 27% at 15 years, 12% at 20 years, and 8% at 25 years. Of the many clinical and radiographic factors considered, only age, implant type, and gender were associated with implant survival independent of other variables considered. Hip resurfacing arthroplasty showed poor overall long-term survival in this series. Particular attention should be paid to the identified risk factors as long-term followup data become available for modern designs.
Level of Evidence: Level IV, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The introduction of THA by Sir John Charnley [21] was a remarkable advance in the treatment of end-stage arthritic degeneration of the hip. Although early results were successful in older patients, outcomes were less promising in younger patients. To address this issue, more physiologic surface replacement systems were developed in the 1970s. Capello et al. [18] introduced the Indiana system in 1973, and in 1975, Amstutz et al. [6] introduced a hip resurfacing system called THARIES (total hip articular replacement using internal eccentric shells). Anticipated advantages of these resurfacing systems were increased durability in younger patients and preservation of femoral bone stock to decrease the technical difficulty of and increase the clinical function after subsequent revisions. Although early results were promising, interest waned after additional followup showed disappointingly high failure rates of 21% to 49.4% at 22 months to 8 years [18, 19, 24, 26, 44].
With the introduction of metal-on-metal bearing surfaces, interest in hip resurfacing arthroplasty has been renewed. Early results with modern implants are again promising [2, 3, 22, 36, 41, 43], but longer-term followup is pending. Intermediate followup of early-design implants is limited to two studies showing an estimated implant survival of 34% at 9.7 years [39] and 40% at 8 years [30] with major causes of failure in both studies being aseptic loosening of either or both components. Only one study analyzed clinical factors such as age, gender, and diagnosis in relation to failure and found no association with the outcomes [30].
The primary aims of our study were to (1) ascertain implant survival in our cohort of patients; and (2) determine whether specific clinical (age, gender, diagnosis, BMI), operative (surgeon, implant type, geometry of reconstruction), or radiographic factors (presence of cysts) were associated with failure of early-design total hip resurfacing arthroplasty. Specifically, we hypothesized that age and gender would be associated with implant survival.
Materials and Methods
We used the institution’s joint registry to identify all total hip resurfacing arthroplasties performed at our institution between 1977 and 1982. Ninety primary hip resurfacing procedures were performed in 75 patients and represent the first resurfacing arthroplasty to the last performed with these designs. Fifteen patients underwent resurfacing of their contralateral hip after the index resurfacing arthroplasty. Since the time of the original operation, the joint registry has been following each patient every 5 years until death or revision with either direct evaluation, including patient history, physical examination, and radiographs, or a questionnaire completed by mail or telephone. The median age of the 75 patients at the time of index surgery was 42 years (range, 17–69 years); 42 patients were male and 33 female. The median height was 172 cm (range, 155–193 cm), the median weight was 74.5 kg (range, 45.9–110.9 kg), and median BMI was 24.7 kg/m2 (range, 18.1–38.8 kg/m2). The diagnosis leading to hip arthroplasty was osteoarthritis in 39 patients (52%), posttraumatic degenerative joint disease in 10 patients (13%), congenital hip dysplasia in 11 patients (15%), avascular necrosis in seven patients (9%), ankylosing spondylitis in five patients (7%), rheumatoid arthritis in two patients (3%), and synovial chondromatosis in one patient (1%). The left hip was replaced in 31 patients, the right hip in 29 patients, and both hips in 15 patients. Fifty-nine arthroplasties (66%) were performed by one of the authors (MEC) and 31 (34%) by Mark B. Coventry, MD. The THARIES system was used in 62 hips (69%) and the Indiana system in 28 hips (31%) (Table 1). A transtrochanteric approach was used in all cases. All patients were followed until death, revision of the resurfacing arthroplasty, or for 25 years after surgery. One patient in this group of 75 was lost to followup; this patient had a Harris hip score of 93 at 15 years postoperatively according to his response to a questionnaire sent to him in Ecuador before he moved and could not be located again. The minimum followup was 0.1 year (median, 7.9 years; range, 0.1–25.2 years). We conducted this study with approval from the Mayo Clinic Institutional Review Board.
Table 1.
Hip and operative information
| Characteristic | Value* |
|---|---|
| Side of surgery | |
| Left | 46 (51) |
| Right | 44 (49) |
| Surgeon | |
| MEC | 59 (66) |
| MBC | 31 (34) |
| Implant type | |
| Indiana | 28 (31) |
| THARIES | 62 (69) |
| Femoral component diameter (mm) | 47 (39–54) |
| Neck shaft angle (degrees) | 132 (123–156) |
| Head diameter (mm) | 55 (45–75) |
| Neck diameter (mm) | 39 (30–51) |
| Head-to-neck ratio | 1.37 (1.18–1.97) |
| Preoperative offset | 42 (15–55) |
| Femoral cyst greater than 1 cm | 20 (26) |
| Component shaft angle (degrees) | 142 (96–172) |
| Postoperative offset | 33 (13–60) |
| Heterotopic ossification class | |
| 0–1 | 49 (62) |
| 2–4 | 30 (38) |
| Offset (preoperative minus postoperative; mm) | 7 (–27–31) |
| Shaft angle (neck minus component; mm) | −3 (−26–20) |
* Values are reported as sample median and range or number (percentage); there were, at most, 13 values missing for any one variable; THARIES = total hip articular replacement using internal eccentric shells.
We reviewed medical records to record pertinent clinical information. Demographic information included age, gender, height, weight, BMI, and hip disorder diagnosis. We used operative notes to identify the surgeon, implant type, femoral implant diameter, operative side, and intraoperative complications. Postoperative records were reviewed for complications, including dislocation, nerve palsy, deep vein thrombus, pulmonary embolism, and infection. We gathered hip scores from a system used at our institution from preoperative, 1-year postoperative, and prerevision assessments; these scores were converted to Harris hip scores [25, 34]. Revision operative notes were used to determine the reason for revision, and specifically, failure of specific components (femoral, acetabular, or both). We recorded for each primary hip resurfacing implant survival or time to revision of the primary implant within the context of patient survival and availability of followup data.
Intraoperative complications at index surgery included two intraoperative acetabular fractures of the medial wall, which were treated with bone grafting; acetabular implants were placed successfully in these patients. Additional complications included femoral neck fracture (three), dislocation (one), hematoma (one), and pulmonary embolism (one).
Of the 75 patients having index arthroplasties, 63 (84%) had preoperative radiographs available, 73 (97%) had postoperative radiographs, and 69 (92%) had prerevision radiographs. One author (EJY) reviewed all radiographic records to record information using methods similar to those described by Silva et al. [42]. Preoperative neck-shaft angle, head diameter, neck diameter, head-neck ratio, and femoral offset were measured, and the presence of any femoral cysts larger than 1 cm was recorded. Postoperative component shaft angle and femoral offset were measured and the presence of any exposed area of reamed femoral neck was noted. We also calculated the difference between preoperative and postoperative offset and neck-shaft angle to estimate the accuracy of the reconstruction from a biomechanical viewpoint. Prerevision radiographs were examined for evidence of gross femoral or acetabular component migration, the presence of lucent lines greater than 1 mm wide around the acetabular component in the zones described by De Lee and Charnley [23], and the Brooker grade of heterotopic ossification [17]. We did not use prerevision radiographic findings as an indication of implant failure; rather, we used only the occurrence of revision surgery as noted by the joint registry.
Operative and radiographic variables (Table 1) were summarized with the sample median and range (numeric variables) or number and percentage (categorical variables). The Kaplan-Meier method was used to estimate implant survival after surgery, censoring at the date of death or date of last followup for patients who did not experience implant failure [33]. Associations between clinical, operative, and radiographic variables and implant survival were investigated using Cox proportional hazards models; univariate models and multivariate models using stepwise variable selection were considered. Relative risks (RRs) and corresponding 95% confidence intervals (CIs) were estimated. For patients who had bilateral arthroplasties, we considered only the first (index) procedure in Kaplan-Meier and Cox proportional hazards analysis to satisfy the statistical assumption of independence. We did not make any adjustments for multiple comparisons in these exploratory analyses. For purposes of display in tables, the sample median was used to find an appropriate cutoff point for numeric variables.
Results
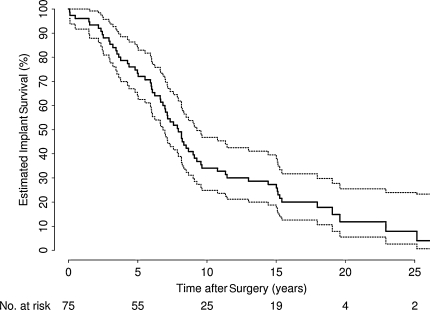
Sixty-four of 75 patients underwent revision of their resurfacing implants. Estimated implant survival was 73% (95% CI, 64%–84%) at 5 years, 34% (95% CI, 25%–47%) at 10 years, and 8% (95% CI, 3%–24%) at 25 years after surgery (Table 2; Fig. 1).
Table 2.
Estimated implant survival after surgery
| Time after surgery (years) | Estimated implant survival (95% CI; %)* |
|---|---|
| 1 | 96 (92–100) |
| 2 | 93 (88–99) |
| 3 | 85 (78–94) |
| 4 | 79 (70–89) |
| 5 | 73 (64–84) |
| 10 | 34 (25–47) |
| 15 | 27 (19–40) |
| 20 | 12 (6–26) |
| 25 | 8 (3–24) |
* Kaplan-Meier method was used to estimate implant survival after surgery (censoring at date of death or last followup); CI = confidence interval.
Fig. 1.
Overall estimated implant survival is summarized in this graph. With longer followup, implant failure increased. The dashed lines represent 95% confidence intervals.
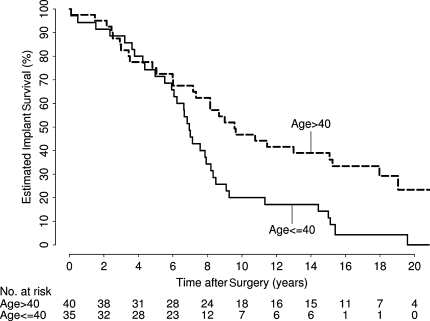
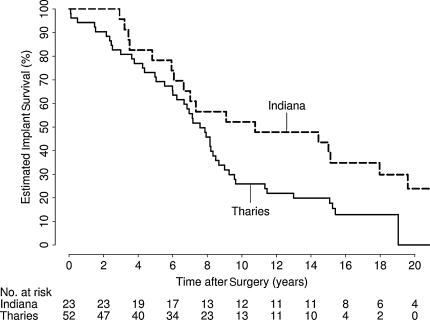
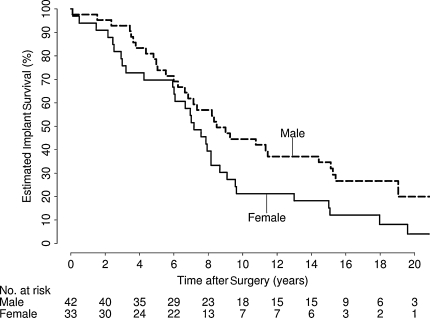
In univariate analysis, eight variables were identified as showing evidence of an association with implant survival: age (p < 0.001), gender (p = 0.043), implant type (p = 0.039), diagnosis (p = 0.056), femoral implant diameter (p = 0.011), neck diameter (p = 0.016), postoperative offset (p = 0.010), and heterotopic ossification class (p < 0.001) (Table 3). In the multivariate analysis, only age (RR, 1.42 [10-year decrease]; 95% CI, 1.19–1.71; p < 0.001) (Fig. 2) and implant type (RR, 2.17; 95% CI, 1.19–3.95; p = 0.011) (Fig. 3) independently predicted survival; gender was weakly associated with implant survival (RR, 1.61; 95% CI, 0.98–2.66; p = 0.062) (Table 4; Fig. 4). After adjusting for age, implant type, and gender, there was no association (p > 0.17) between implant survival and any other variable considered. We identified no difference in mode of failure between the two implant designs.
Table 3.
Estimated 5- and 10-year implant survival and univariate associations
| Variable | Estimated implant survival (95% CI; %) | p Value* | |
|---|---|---|---|
| 5-year rate | 10-year rate | ||
| Age (years) | < 0.001 | ||
| 40 or younger | 71 (58–88) | 20 (10–39) | |
| Older than 40 | 75 (63–90) | 47 (33–65) | |
| Gender | 0.043 | ||
| Male | 76 (64–90) | 45 (32–62) | |
| Female | 60 (56–87) | 21 (11–41) | |
| Implant type | 0.039 | ||
| Indiana | 78 (63–97) | 52 (35–77) | |
| THARIES | 71 (60–85) | 26 (16–41) | |
| Diagnosis | 0.056 | ||
| Degenerative joint disease | 77 (65–91) | 45 (32–64) | |
| Congenital dislocation of the hip | 55 (32–94) | 9 (1–59) | |
| Other | 76 (61–95) | 28 (15–53) | |
| Femoral component diameter (mm) | 0.011 | ||
| 47 or less | 66 (52–83) | 26 (13–42) | |
| Greater than 47 | 81 (69–95) | 45 (31–65) | |
| Neck diameter (mm) | 0.016 | ||
| 39 or less | 72 (58–89) | 38 (24–59) | |
| Greater than 39 | 84 (72–98) | 41 (26–63) | |
| Postoperative offset (mm) | 0.010 | ||
| 33 or less | 73 (60–88) | 30 (19–48) | |
| Greater than 33 | 76 (62–92) | 41 (27–63) | |
| Heterotopic ossification class | < 0.001 | ||
| 0–1 | 80 (69–93) | 25 (15–43) | |
| 2–4 | 76 (61–95) | 59 (43–82) | |
* p values result from univariate Cox proportional hazards models; age, gender, femoral implant diameter, neck diameter, postoperative offset, and heterotopic ossification class were considered as continuous variables in these models; in univariate analysis, there was no notable evidence of an association between implant survival and any other variable considered (p > 0.15); CI = confidence interval; THARIES = total hip articular replacement using internal eccentric shells.
Fig. 2.
There was strong evidence of increased risk of implant failure in younger patients (relative risk, 1.42 [10-year decrease]; 95% confidence interval, 1.19–1.71; p < 0.001).
Fig. 3.
Evidence of increased risk of implant failure in patients with a THARIES implant (relative risk, 2.17; 95% confidence interval, 1.19–3.95; p = 0.011) as compared with an Indiana implant is shown. THARIES = total hip articular replacement using internal eccentric shells.
Table 4.
Implant survival associated with age, gender, and implant type in multivariate analysis
| Variable | Estimated RR (95% CI)* | p Value* |
|---|---|---|
| Age (10-year decrease) | 1.42 (1.19–1.71) | < 0.001 |
| Gender (female) | 1.61 (0.98–2.66) | 0.062 |
| Implant type (THARIES) | 2.17 (1.19–3.95) | 0.011 |
* p values and estimated RRs result from Cox proportional hazards models adjusted for age, gender, and implant type; estimated RRs correspond to the group of patients (categorical variables) or change given in parentheses (numeric variables); after adjusting for age, implant type, and gender, there was no notable evidence of an association between implant survival and any other variable considered (p > 0.17); RR = relative risk; CI = confidence interval; THARIES, total hip articular replacement using internal eccentric shells.
Fig. 4.
There was weak evidence of increased risk of implant failure in women (relative risk, 1.61; 95% confidence interval, 0.98–2.66; p = 0.062).
Although initial clinical results of hip resurfacing arthroplasty were favorable, with hip function, as measured by Harris hip scores, improving from a median of 58.7 preoperatively up to a median of 96.7 1 year postoperatively, hip scores then declined to a median of 61 before revision. Sixty (94%) of the 64 patients underwent revision for aseptic loosening. In 45 of these patients, operative notes or prerevision radiographs were available to ascertain which components were loose; the other 19 revisions were noted by the patient or the treating surgeon in the medical records, and more detailed information was unavailable. Of these 45 patients, aseptic loosening of the acetabular and femoral components occurred in 13 cases, loosening of the acetabular component only in 13 cases, and loosening of the femoral component only in 19 cases (Fig. 5).
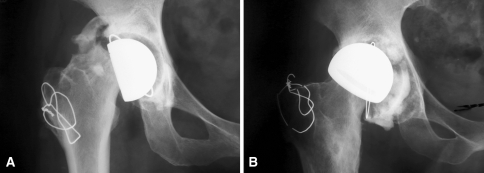
Fig. 5A–B.
(A) The radiograph shows aseptic loosening of the femoral component with fracture of the bone at the base of the implant in a 43-year-old woman with a history of developmental hip dysplasia 1.5 years after implantation. (B) This radiograph of a 50-year-old man who underwent arthroplasty for severe osteoarthritis shows aseptic loosening of an acetabular component 8 years after implantation.
Four revisions were performed for causes other than aseptic loosening. Three revisions were performed for femoral neck fracture: one for a fracture 4 weeks postoperatively in a 37-year-old man with ankylosing spondylitis, one 6 weeks postoperatively in a 61-year-old woman with degenerative joint disease, and one 6 months postoperatively in a 24-year-old woman with congenital dislocation of the hip. One revision was performed for infection in a 29-year-old man with posttraumatic degenerative joint disease who had a leg ulcer develop 2 years postoperatively followed by hip pain. He was treated with Girdlestone resection followed by revision THA 1 year later.
Discussion
We performed this retrospective review of early-design total hip resurfacing arthroplasty to determine clinical and radiographic factors that could influence implant survival. We specifically hypothesized age and gender would be associated with implant survival. We found estimated implant survival to be 73% at 5 years, 34% at 10 years, and 8% at 25 years. Of the many clinical and radiographic factors considered, only age and implant type were associated with implant survival independent of the other variables considered, although weak evidence of an association between gender and implant survival also was identified.
Limitations of our study included use of a retrospective design with loss of some clinical and radiographic data. Missing clinical information included loss of BMI data in 27 of 75 patients and some loss of Harris hip scores in the 90 patients (2 preoperative, 17 one year and 37 at latest followup or prior to revision). The nearest prerevision radiographs that were available were taken a median of 130 days before revision, potentially introducing a bias toward underreporting the amount of heterotopic ossification and periacetabular radiolucent lines. However, we had nearly complete capture of the primary implant survival data with only one patient lost to followup after 15 years.
Young age was the most important factor predicting implant survival with these early designs. The association of young age, increased activity, and bearing surface wear to increased failure rates is generally well established, but the degree to which these factors dominated the others was revealing. A recent report by Amstutz et al. [2] suggested no clinical difference with modern metal-on-metal resurfacing arthroplasty in 295 patients younger than 50 years compared with 281 patients 50 years or older at 2 to 9 years of followup.
Our data suggest better survival with the Indiana design as compared with the THARIES implant. The literature does not suggest any clear difference in outcomes of early-design implants although no studies include both designs (Table 5). The Indiana and THARIES designs have one major difference between them; the Indiana system uses a hemispheric reamer, whereas the THARIES system uses a cylindrical reamer. Nevertheless, their overall design is similar, and the degree of difference in outcome was unexpected.
Table 5.
Summary of literature for implant survival
| Source | Implant type | Followup | Revision |
|---|---|---|---|
| Capello et al. [18] | Indiana | 22 months | 17/65 (26%) |
| Capello et al. [20] | Indiana | 2–7 years | 17/116 (15%) |
| Bell et al. [15] | Wagner | 1–4 years | 22/219 (10%) |
| Head [26] | Wagner | 2.4 years | 14/41 (34%) |
| Head [27] | Total articular resurfacing arthroplasty | 3.3 years | 8/67 (12%) |
| Freeman and Bradley [24] | ICLH | 3.2 years | 43/204 (21%) |
| Trentani and Vaccarino [44] | Paltrineiri-Trentani | 8 years | 49.4% of 114 |
| Jolley et al. [32] | ICLH and THARIES | 3 years | 7/55 (13%) |
| Amstutz et al. [8] | THARIES | 4–32 months | 3/100 (3%) |
| Amstutz et al. [9] | THARIES | 2–5 years | 11/200 (6%) |
| Amstutz et al. [7] | THARIES | 1–10 years | 72/584 (12%) |
| Howie et al. [30] | Wagner | 8–10 years | 55/100 (55%) |
| Mai et al. [35] | THARIES | 10 years | 80/170 (47%) |
| Ritter et al. [39] | Indiana | 9.7 years | 41/62 (66%) |
| Current authors | THARIES and Indiana | 0.1–25 years | 92% at 25 years |
ICLH = Imperial College of London Hospital; THARIES = total hip articular replacement using internal eccentric shells.
We hypothesized female gender would be a negative factor in implant survival. Although weak, we did find some evidence of an increased risk of implant failure in women of 1.61 (95% CI, 0.98–2.66). A comparison with other publications, including those pertaining to THA, reveals mixed results with some showing improved implant survival for women [3, 28] and others showing contrary findings [16, 20, 38]. These mixed results may be related to the high degree of association between gender and other factors such as height, weight, diagnosis, and implant size, which may have differed between this study and others.
With modern metal-on-metal resurfacing arthroplasty, being female may be associated more frequently with implant failure, including femoral neck fracture [29, 41]. In a prospective multicenter US Food and Drug Administration investigational device exemption clinical trial of a modern-design implant, poor bone quality (loss of femoral head bone and dual-energy xray absorptiometry showing severe osteopenia) was identified as a risk factor for femoral neck fracture in 1016 hips (906) patients, 27.6% male and 72.4% female. The authors of that study subsequently altered the selection criteria to exclude such patients [37]. Such severe osteopenia typically is more common in women than men. Moreover, smaller components would be placed more frequently in women, another potential factor in implant survival [10, 28].
We chose to track several predictive factors identified in other studies, including those looking at modern designs, that are associated with implant failure. These included clinical factors such as weight [12, 13, 15], height [3], BMI, and diagnosis such as rheumatoid arthritis [4, 20]. We also considered radiographic factors that have been a part of biomechanical analysis in other publications such as implant shaft angle [5, 11–14], implant size [3], head-neck ratio, neck-shaft angle [40], femoral cysts [3, 12, 13], and femoral offset [40, 42]. Larger implant size has been believed to have a protective effect on implant survival; more time was required for disruption of the larger fixation area of larger femoral resurfacing components than smaller ones [31, 35]. However, in contrast, in our series, none of these factors showed associations with implant survival independent of age, implant type, and gender.
We found the choice of surgeon who performed the procedure was not associated with implant survival after adjusting for patient age, implant type, and gender. Moreover, the year the procedure was performed was not a factor in relation to implant survival, indicating there was no evidence of a learning curve phenomenon. Finally, in contrast to another report [9], the rate of implant failure on the acetabular side was similar to that on the femoral side, and these failures did not occur at considerably different times.
Our estimated cumulative rate of revision for any reason in this series was 27% at 5 years. In the largest series of THARIES implants, Amstutz et al. [7] reported an estimated 23% revision rate at 5 years after surgery, which suggests our followup to 25 years was not biased by an unexpectedly high early failure rate. The failure rates for various followup periods (Table 5) indicate the early designs of hip resurfacing arthroplasty had a high failure rate at early followup and up to 10 years; we have not identified any followup to 25 years. The history and evolution of this design are well documented, and the reader is referred to several excellent reviews by Amstutz et al. [1, 7, 10].
Our series shows the long-term rate of failure for the early hip resurfacing implants was unacceptably high. The thin polyethylene bearing surface of these early designs contributed to this failure rate. However, isolated aseptic loosening of the femoral component was not uncommon in our series. Newer metal-on-metal designs have been introduced that promise to overcome previous problems related to polyethylene debris-induced osteolytic wear and aseptic loosening. However, current implants are of similar femoral component designs. Factors we identified as associated with implant failure, specifically patient age, implant design, and gender, need long-term followup study for the modern implant designs.
Acknowledgments
We acknowledge the contributions of the late Mark B. Coventry, MD, who performed many of the procedures included in this study.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
References
- 1.Amstutz HC. Innovations in design and technology: the story of hip arthroplasty. Clin Orthop Relat Res. 2000;378:23–30. [DOI] [PubMed]
- 2.Amstutz HC, Ball ST, Le Duff MJ, Dorey FJ. Resurfacing THA for patients younger than 50 years: results of 2- to 9-year followup. Clin Orthop Relat Res. 2007;460:159–164. [DOI] [PubMed]
- 3.Amstutz HC, Beaule PE, Dorey FJ, Le Duff MJ, Campbell PA, Gruen TA. Metal-on-metal hybrid surface arthroplasty: two to six-year follow-up study. J Bone Joint Surg Am. 2004;86:28–39. [PubMed]
- 4.Amstutz HC, Campbell P, Nasser S, Kossovsky N. Modes of failure of surface replacements. In: Amstutz HC, ed. Hip Arthroplasty. New York, NY: Churchill Livingstone; 1991:507–534.
- 5.Amstutz HC, Campbell PA, Le Duff MJ. Fracture of the neck of the femur after surface arthroplasty of the hip. J Bone Joint Surg Am. 2004;86:1874–1877. [DOI] [PubMed]
- 6.Amstutz HC, Clarke IC, Christie J, Graff-Radford A. Total hip articular replacement by internal eccentric shells: the ‘THARIES’ approach to total surface replacement arthroplasty. Clin Orthop Relat Res. 1977;128:261–284. [PubMed]
- 7.Amstutz HC, Dorey F, O’Carroll PF. THARIES resurfacing arthroplasty: evolution and long-term results. Clin Orthop Relat Res. 1986;213:92–114. [PubMed]
- 8.Amstutz HC, Graff-Radford A, Gruen TA, Clarke IC. THARIES surface replacements: a review of the first 100 cases. Clin Orthop Relat Res. 1978;134:87–101. [PubMed]
- 9.Amstutz HC, Graff-Radford A, Mai LL, Thomas BJ. Surface replacement of the hip with the THARIES system: two to five-year results. J Bone Joint Surg Am. 1981;63:1069–1077. [PubMed]
- 10.Amstutz HC, Grigoris P, Dorey FJ. Evolution and future of surface replacement of the hip. J Orthop Sci. 1998;3:169–186. [DOI] [PubMed]
- 11.Amstutz HC, Le Duff MJ, Campbell PA, Dorey FJ. The effects of technique changes on aseptic loosening of the femoral component in hip resurfacing: results of 600 Conserve Plus with a 3 to 9 year follow-up. J Arthroplasty. 2007;22:481–489. [DOI] [PubMed]
- 12.Beaule PE, Dorey FJ, Le Duff M, Gruen T, Amstutz HC. Risk factors affecting outcome of metal-on-metal surface arthroplasty of the hip. Clin Orthop Relat Res. 2004;418:87–93. [DOI] [PubMed]
- 13.Beaule PE, Le Duff M, Campbell P, Dorey FJ, Park SH, Amstutz HC. Metal-on-metal surface arthroplasty with a cemented femoral component: a 7–10 year follow-up study. J Arthroplasty. 2004;19(suppl 3):17–22. [DOI] [PubMed]
- 14.Beaule PE, Lee JL, Le Duff MJ, Amstutz HC, Ebramzadeh E. Orientation of the femoral component in surface arthroplasty of the hip: a biomechanical and clinical analysis. J Bone Joint Surg Am. 2004;86:2015–2021. [DOI] [PubMed]
- 15.Bell RS, Schatzker J, Fornasier VL, Goodman SB. A study of implant failure in the Wagner resurfacing arthroplasty. J Bone Joint Surg Am. 1985;67:1165–1175. [PubMed]
- 16.Berry DJ, Harmsen WS, Cabanela ME, Morrey BF. Twenty-five-year survivorship of two thousand consecutive primary Charnley total hip replacements: factors affecting survivorship of acetabular and femoral components. J Bone Joint Surg Am. 2002;84:171–177. [DOI] [PubMed]
- 17.Brooker AF, Bowerman JW, Robinson RA, Riley LH Jr. Ectopic ossification following total hip replacement: incidence and a method of classification. J Bone Joint Surg Am. 1973;55:1629–1632. [PubMed]
- 18.Capello WN, Ireland PH, Trammell TR, Eicher P. Conservative total hip arthroplasty: a procedure to conserve bone stock. Part I: analysis of sixty-six patients. Part II: analysis of failures. Clin Orthop Relat Res. 1978;134:59–74. [PubMed]
- 19.Capello WN, Misamore GW, Trancik TM. Conservative total hip arthroplasty. Orthop Clin North Am. 1982;13:833–842. [PubMed]
- 20.Capello WN, Misamore GW, Trancik TM. The Indiana conservative (surface-replacement) hip arthroplasty. J Bone Joint Surg Am. 1984;66:518–528. [PubMed]
- 21.Charnley J. Arthroplasty of the hip: a new operation. Lancet. 1961;1:1129–1132. [DOI] [PubMed]
- 22.Daniel J, Pynsent PB, McMinn DJ. Metal-on-metal resurfacing of the hip in patients under the age of 55 years with osteoarthritis. J Bone Joint Surg Br. 2004;86:177–184. [DOI] [PubMed]
- 23.De Lee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res. 1976;121:20–32. [PubMed]
- 24.Freeman MA, Bradley GW. ICLH double cup arthroplasty. Orthop Clin North Am. 1982;13:799–811. [PubMed]
- 25.Harris WH. Traumatic arthritis of the hip after dislocation and acetabular fractures: treatment by mold arthroplasty: an end-result study using a new method of result evaluation. J Bone Joint Surg Am. 1969;51:737–755. [PubMed]
- 26.Head WC. Wagner surface replacement arthroplasty of the hip: analysis of fourteen failures in forty-one hips. J Bone Joint Surg Am. 1981;63:420–427. [PubMed]
- 27.Head WC. Total articular resurfacing arthroplasty: analysis of component failure in sixty-seven hips. J Bone Joint Surg Am. 1984;66:28–34. [PubMed]
- 28.Herberts P, Malchau H. How outcome studies have changed total hip arthroplasty practices in Sweden. Clin Orthop Relat Res. 1997;344:44–60. [DOI] [PubMed]
- 29.Hing C, Back D, Shimmin A. Hip resurfacing: indications, results, and conclusions. Instr Course Lect. 2007;56:171–178. [PubMed]
- 30.Howie DW, Campbell D, McGee M, Cornish BL. Wagner resurfacing hip arthroplasty: the results of one hundred consecutive arthroplasties after eight to ten years. J Bone Joint Surg Am. 1990;72:708–714. [PubMed]
- 31.Howie DW, Cornish BL, Vernon-Roberts B. Resurfacing hip arthroplasty: classification of loosening and the role of prosthesis wear particles. Clin Orthop Relat Res. 1990;255:144–159. [PubMed]
- 32.Jolley MN, Salvati EA, Brown GC. Early results and complications of surface replacement of the hip. J Bone Joint Surg Am. 1982;64:366–377. [PubMed]
- 33.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [DOI]
- 34.Kavanagh BF, Fitzgerald RH Jr. Clinical and roentgenographic assessment of total hip arthroplasty: a new hip score. Clin Orthop Relat Res. 1985;193:133–140. [PubMed]
- 35.Mai MT, Schmalzried TP, Dorey FJ, Campbell PA, Amstutz HC. The contribution of frictional torque to loosening at the cement-bone interface in THARIES hip replacements. J Bone Joint Surg Am. 1996;78:505–511. [DOI] [PubMed]
- 36.Mont MA, Ragland PS, Etienne G, Seyler TM, Schmalzried TP. Hip resurfacing arthroplasty. J Am Acad Orthop Surg. 2006;14:454–463. [DOI] [PubMed]
- 37.Mont MA, Seyler TM, Ulrich SD, Beaule PE, Boyd HS, Grecula MJ, Goldberg VM, Kennedy WR, Marker DR, Schmalzried TP, Sparling EA, Vail TP, Amstutz HC. Effect of changing indications and techniques on total hip resurfacing. Clin Orthop Relat Res. 2007;465:63–70. [DOI] [PubMed]
- 38.Older J. Charnley low-friction arthroplasty: a worldwide retrospective review at 15 to 20 years. J Arthroplasty. 2002;17:675–680. [DOI] [PubMed]
- 39.Ritter MA, Lutgring JD, Berend ME, Pierson JL. Failure mechanisms of total hip resurfacing: implications for the present. Clin Orthop Relat Res. 2006;453:110–114. [DOI] [PubMed]
- 40.Schmalzried TP, Silva M, de la Rosa MA, Choi ES, Fowble VA. Optimizing patient selection and outcomes with total hip resurfacing. Clin Orthop Relat Res. 2005;441:200–204. [DOI] [PubMed]
- 41.Shimmin AJ, Back D. Femoral neck fractures following Birmingham hip resurfacing: a national review of 50 cases. J Bone Joint Surg Br. 2005;87:463–464. [DOI] [PubMed]
- 42.Silva M, Lee KH, Heisel C, Dela Rosa MA, Schmalzried TP. The biomechanical results of total hip resurfacing arthroplasty. J Bone Joint Surg Am. 2004;86:40–46. [DOI] [PubMed]
- 43.Treacy RB, McBryde CW, Pynsent PB. Birmingham hip resurfacing arthroplasty: a minimum follow-up of five years. J Bone Joint Surg Br. 2005;87:167–170. [DOI] [PubMed]
- 44.Trentani C, Vaccarino FP. The Paltrinieri-Trentani hip joint resurface arthroplasty. Orthop Clin North Am. 1982;13:857–867. [PubMed]