Abstract
The optimal method for reconstructing the proximal humerus in patients with tumors is controversial. To determine functional outcomes and complication rates after different types of reconstructions, we reviewed a consecutive series of 49 patients who underwent proximal humerus resection and osteoarticular allograft (17 patients), allograft-prosthetic composite (16), or endoprosthetic (16) reconstruction. Operative indications included primary malignancies (24 patients), metastatic disease (19), and benign aggressive disease (six). Implant revision was more common after osteoarticular reconstruction (five of 17) than after allograft-prosthetic composite (one of 16) or endoprosthetic (zero of 16) reconstructions. At a minimum followup of 24 months (median, 98 months; range, 24–214 months) in surviving patients, Musculoskeletal Tumor Society functional scores averaged 79% for the allograft-prosthetic composite, 71% for the osteoarticular allograft, and 69% for the endoprosthetic reconstruction cohorts. Shoulder instability was associated with abductor mechanism compromise and was more common after endoprosthetic reconstruction. Allograft fractures occurred in 53% of patients receiving osteoarticular allografts. We recommend allograft-prosthetic composite reconstruction for younger patients with primary tumors of bone and endoprosthetic reconstruction for older patients with metastatic disease. Because of the unacceptable complication rate, we do not recommend osteoarticular allograft reconstruction for routine use in the proximal humerus.
Level of Evidence: Level III, therapeutic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
The proximal humerus is a relatively common location for primary and metastatic tumors of bone [2, 8, 9, 20, 26, 28, 31]. The increasing and often unpredictable longevity of patients with metastatic disease [2, 19] coupled with higher-than-expected failure rates after internal fixation with or without intralesional treatment and radiotherapy [11, 29, 30] has led to renewed interest in more aggressive local control efforts through proximal humeral resection and reconstruction. Compared with other anatomic regions, the proximal humerus has had favorable functional results and implant longevity profiles after endoprosthetic reconstruction [18, 20, 28, 31].
The most commonly used reconstructive techniques after proximal humeral resection include osteoarticular allografts [6, 7, 12, 13, 24, 25], allograft- or autograft-prosthesis composites [3, 5, 15], and endoprosthetic reconstruction [1, 4, 6, 8, 17, 18, 20, 24–26, 28, 31]. Alternative techniques include allograft arthrodesis, Tikhoff-Linberg resection and modifications, claviculo pro humero, vascularized fibula transfers, and concomitant scapular replacement [21, 22, 25]. Because of decreased technical complexity and anecdotally more favorable results, we prefer the former techniques for cases in which glenoid resection is not required, particularly if the axillary nerve and/or rotator cuff can safely be preserved.
To determine which type of reconstruction might be most appropriate for specific patients, we compared the functional results, postoperative complications, and implant survival of osteoarticular allograft (OA), allograft-prosthesis composite (APC), and endoprosthetic (EP) reconstructions after proximal humeral resection for treatment of primary and metastatic bone tumors. We presumed biologic reconstructions (OA and APC) would have decreased implant longevity because of an increased rate of major complications (eg, infection, fracture, nonunion) but would provide superior functional results and joint stability.
Materials and Methods
We retrospectively reviewed patients who underwent proximal humeral resection and reconstruction between 1989 and 2005. Forty-nine patients who underwent glenoid-preserving segmental OA, APC, or EP reconstruction of the proximal humerus for treatment of primary bone tumors or metastatic disease with actual or impending pathologic fracture were included in the study. We excluded patients treated with internal or intramedullary fixation with or without curetting of the lesion, arthrodesis, amputation, or alternative techniques of reconstruction. The indication for surgery was primary malignancy of bone in 24 patients (12 chondrosarcomas, eight osteosarcomas, two primary lymphomas of bone, one Ewing’s sarcoma, one leiomyosarcoma), metastatic disease in 19 patients (eight renal carcinoma, three breast, three lung, three multiple myeloma, one colon, one melanoma), and benign aggressive disease in seven patients (six giant cell tumors of bone, one renal osteodystrophy). Seventeen patients underwent OA, 16 patients APC, and 16 patients EP reconstruction. Twenty-nine patients were male and 20 were female, with a mean age of 48.5 years (range, 10–80 years). All patients were followed for a minimum of 24 months (median, 98 months; mean, 113 months; range, 24–214 months) postoperatively unless death supervened. Institutional Review Board approval was obtained before study initiation.
Medical records were reviewed and abstracted data included patient demographics, diagnoses, indications for surgery, operative technique, humeral resection length as measured from the tip of the greater tuberosity, postoperative complications, reoperations, local recurrence, and functional outcomes. Functional outcomes were assessed using the Musculoskeletal Tumor Society (MSTS) functional scoring instrument [10] (score of 0–30 and presented here as a percentage; raw score/30), with subgroup analyses performed on the basis of the initial reconstruction technique (OA, APC, or EP), humeral resection length, tumor diagnosis (metastatic disease versus primary tumor), abductor compromise, and the presence of preoperative pathologic fracture or prior surgery. The shoulder abductor mechanism was considered compromised when the rotator cuff or axillary nerve was sacrificed and/or greater than 50% of the deltoid was resected.
Power analysis revealed a sample size of 17 in the OA control group with 16 patients in each of the comparative treatment groups would provide only a 45% power to detect a difference in implant survival outcomes based on the log rank test, assuming a constant hazard ratio of 2.0 during followup and two-sided alpha of 0.05. This means the study had a 45% chance of detecting a twofold increase in the risk of implant revision for any reason between study cohorts. Likewise (assuming 16 patients in each treatment group and three groups), the study had a 74% power to detect a difference in MSTS functional scores between groups in one-way ANOVA, assuming the observed standard deviation of 10.7% for MSTS functional scores and a two-sided alpha of 0.05. Power analysis calculations were determined using PASS 2008 software package (NCSS, Kaysville, UT).
Patients in the OA group were younger (p = 0.02) than patients in the other groups, with mean ages of 36.5, 56.3, and 53.6 years for the OA, APC, and EP cohorts, respectively (Table 1). Fourteen patients (29%) sustained pathologic fractures preoperatively and there was no major difference between groups regarding pathologic fracture rate. Overall, six patients (12%) had prior internal or intramedullary fixation of metastatic lesions and these patients comprised a greater (p = 0.02) percentage of patients undergoing EP reconstruction (31%) versus OA (6%) or APC (0%). The mean humeral resection length was 13.8 cm (range, 6–24 cm). The mean followup for surviving patients was longer (p = 0.01) after OA than after APC or EP reconstruction (154 months versus 82 and 34 months, respectively). At the conclusion of the study period, 17 of the 49 patients remained alive without evidence of disease and two were alive with disease. Twenty-five patients died of disease at a mean of 30.7 months postoperatively (range, 2–117 months), and five patients died of other causes at a mean of 68.4 months (range, 19–132 months).
Table 1.
Summary data for all patients
| Treatment factors and outcomes | All | OA | APC | EP | p Value |
|---|---|---|---|---|---|
| Number of patients | 49 | 17 | 16 | 16 | |
| Mean age (years) | 48.5 | 36.5 | 56.3 | 53.6 | 0.01 |
| Diagnosis | |||||
| Primary | 24 | 10 | 8 | 6 | 0.45 |
| Metastatic | 19 | 4 | 6 | 9 | |
| Benign | 6 | 3 | 2 | 1 | |
| Pathologic fracture | 14 (29%) | 4 (24%) | 4 (25%) | 6 (35%) | 0.67 |
| Prior surgery | 6 (12%) | 1 (6%) | 0 (0%) | 5 (31%) | 0.02 |
| Mean resection length (cm) | 13.8 | 15.1 | 12.5 | 13.6 | 0.14 |
| Abductor compromised | 9 (18%) | 2 (12%) | 1 (6%) | 6 (38%) | 0.09 |
| Patients with complications | 25 (51%) | 11 (65 %) | 7 (44%) | 7 (44%) | 0.38 |
| Mean MSTS score | 73% | 71% | 79% | 69% | 0.008 |
| Final patient status | |||||
| DOD | 25 | 7 | 6 | 12 | |
| DOC | 5 | 0 | 4 | 1 | |
| AWOD | 17 | 9 | 6 | 2 | |
| AWD | 2 | 1 | 1 | ||
OA = osteoarticular allograft; APC = allograft-prosthesis composite; EP = endoprosthesis; MSTS = Musculoskeletal Tumor Society; DOD = died of disease; DOC = died of other causes; AWOD = alive without disease; AWD = alive with disease.
All surgical procedures were performed by a fellowship-trained musculoskeletal oncologist. Except in rare instances in which biopsy sites or soft tissue extension of tumor was prohibitive, an anterior transdeltoid approach (paralleling the deltopectoral approach through the anterior 1/5 of the deltoid) was used and biopsy tracks, when present, were elliptically excised en bloc with the specimen. The humeral diaphysis was isolated and cut distally using an oscillating saw at a point at least 2 cm from the distal extent of the lesion. The lesion was measured on a coronal MR image from the tip of the greater tuberosity, which was localized intraoperatively through direct palpation or observation. If possible, based on the length of resection, the distal deltoid insertion was preserved. Cancellous bone was curetted from the medullary canal distal to the diaphyseal osteotomy and sent for intraoperative frozen section to confirm a negative margin. The humerus then was dissected circumferentially with a cuff of normal muscle tissue. Except in rare instances in which prior surgery or tumor proximity required sacrifice, the rotator cuff was preserved and released from near its insertions on the native humerus for later reattachment to the reconstruction. Likewise, the axillary nerve was preserved when practical, particularly in patients with metastatic disease undergoing palliative surgery. No other major nerves were sacrificed in any patient. We measured the resected specimen on the back table to estimate the required replacement length, after which it was sent for pathologic analysis.
Bulk allografts (OA and APC) were templated and ordered preoperatively from a well-established tissue bank (University of Miami Tissue Bank, Miami, FL). After aseptic recovery and processing, all allografts for OA reconstructions were cryopreserved by slow-freezing with dimethyl sulfoxide and maintained at –150°C in liquid nitrogen vapor to optimize articular cartilage integrity, and all APC allografts were fresh-frozen. No grafts were secondarily sterilized with chemicals or radiation. All EP reconstructions were performed with Global Modular Replacement System™ endoprostheses (Stryker Orthopaedics, Mahwah, NJ) and APC reconstructions used either the Neer® II (Smith and Nephew, Inc, Memphis, TN) or Solar® (Stryker) prostheses. All EP reconstructions were cemented using second- or third-generation techniques, and APC reconstructions were sequentially cemented first into the allograft and subsequently into the remaining host distal humerus without supplemental plate fixation. Compression plate and screw fixation was used for all OA reconstructions. Plate stabilization was performed along the entire length of the graft while seeking to minimize the total number of screws in the graft without intramedullary cementation. The capsule and rotator cuff tendons were repaired directly to their allograft counterparts (OA and APC) in a “pants over vest” (native tendon over allograft) fashion or to the prosthesis using Number 5 braided nylon suture (Ethibond™; Ethicon, Inc, Somerville, NJ) with the glenohumeral joint in abduction. When practicable, the rotator cuff and capsule were closed in separate layers. When the capsule and/or rotator cuff were deficient, this repair was augmented with 5-mm woven polyester tape (Mersilene™; Ethicon) and/or fresh-frozen fascia lata allograft. The long head of the biceps tendon was routinely resected and tenodesed distally to the adjacent soft tissue. If the distal deltoid tendon had been released, this was reconstructed to its allograft counterpart or the prosthesis with Mersilene™ tape and Number 5 Ethibond™ suture. Implant fixation and soft tissue reconstruction techniques generally were consistent throughout the study period as described previously. The specific reconstruction technique for each patient was not standardized and was selected by the attending surgeon based on individual patient characteristics and the putative need for postoperative radiotherapy and after counseling patients regarding the risks, benefits, and alternatives to each method.
We used postoperative radiotherapy only in patients with metastatic disease based on the margin of resection; patients with marginal or intralesional excisions generally received postoperative radiotherapy whereas those with negative final margins (wide excisions) did not. Two patients in the OA, two patients in the APC, and five patients in the EP groups received postoperative external beam radiotherapy. One patient in the APC group and four patients in the EP group had received radiation therapy before surgery.
Descriptive statistics were performed for all groups. Implant survivorship was assessed using the Kaplan-Meier method [16], which assumes censored patients (those dying of disease or other causes or lost to further followup) continued to fail at the same rate as those remaining in the analysis with differences in survival assessed by the log rank (Mantel-Cox [23]) test. Frequency analysis of complications and confounding variables was determined by chi square analysis (eg, total complications and complications requiring reoperation) or Fisher’s exact test (eg, diagnoses, preoperative pathologic fractures, prior surgery, abductor compromise, prosthesis revision, postoperative infection, fracture, instability, nonunion, or hematoma, and frequency of patients with multiple complications). Differences between cohorts in potentially confounding continuous variables (humeral resection length, patient age, duration of followup) and in MSTS functional scores were analyzed using one-way ANOVA. All p values reported are two-tailed. Analysis was performed using SPSS® Version 15.0 (SPSS Inc, Chicago, IL).
Results
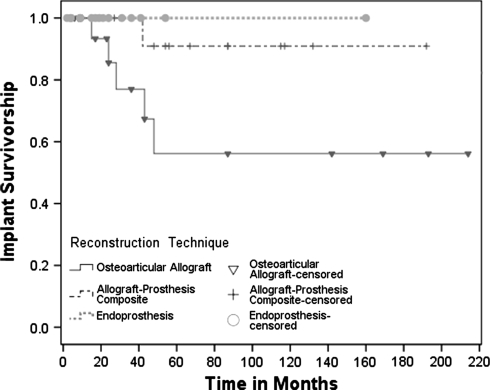
The Kaplan-Meier 5-year implant survival estimates, with revision for any reason as the end point (p = 0.07), were 56%, 91%, and 100% for the OA, APC, and EP cohorts, respectively (Fig. 1). Implant revision was more common (p = 0.03) in the OA cohort, with five patients in the OA (four patients had revision to an APC for fracture, one patient had revision to an EP for infection) and one patient in the APC cohorts (revision to an EP for infection) requiring revision of their implants. The diagnoses of patients requiring implant revision were chondrosarcoma (three patients, including the APC cohort revision), osteosarcoma (one), giant cell tumor (one), and metastatic lung cancer (one). There were no cases of aseptic loosening in either the APC or EP groups, and no patient in the EP cohort required implant revision for any reason.
Fig. 1.
The Kaplan-Meier implant survivorship estimate curves with revision for any reason as the end point show an apparent difference (p = 0.07) in revision rates at 5 years for OA (56%; 95% confidence interval, 26%–86%), compared with APC (91%; 95% confidence interval, 74%–100%) and EP (100%; 95% confidence interval, not applicable) reconstructions. Inverted triangles (OA), plus signs (APC), and solid circles (EP) along the curves represent patients censored owing to patient death or loss to followup.
Excluding local disease recurrence, postoperative complications occurred in 25 patients (51%), with 16 patients (33%) experiencing complications requiring operative intervention (Table 2). Including late revisions, complications requiring reoperation occurred in eight patients (47%) of the OA group, compared with only four patients each (25%) in the APC and EP groups. Five patients (29%) experienced multiple complications after OA reconstruction. Specifically, allograft fracture occurred in nine patients (53%) in the OA cohort, including six episodes of humeral head fragmentation and four fractures of the graft body with one patient sustaining both types of allograft fracture. One patient in the APC cohort had an allograft fracture, and no patient in the EP cohort experienced prosthesis or periprosthetic fracture. One patient in the OA cohort underwent radial nerve exploration and neurolysis for a transient postoperative palsy (attributed to an offending screw, which was removed) and another underwent irrigation and débridement of a superficial infection, which subsequently resolved. Nonunion developed in one patient in the OA cohort (who also sustained a fracture of the allograft body and had not received radiotherapy) and one patient in the APC cohort (who had received preoperative radiotherapy). The mean time to union was 10.3 months (range, 8–14 months) in the OA group and 9.1 months (range, 6–12 months) in the APC group. Subluxation occurred in three patients in the OA (18%) and APC (19%) cohorts versus two patients in the EP cohort (13%), with three additional patients (19%) in the EP cohort requiring reoperation for overt dislocation. Only one patient in the OA cohort required reoperation for retensioning and augmenting of the shoulder capsule and rotator cuff. No patient in the APC cohort required reoperation for instability-related complications. Three patients (13%) with primary tumors of bone, two in the OA cohort and one in the APC cohort, experienced soft tissue local recurrence of disease requiring reexcision. No patient underwent amputation for either local recurrence or postoperative complications, and no reconstructions were revised because of local recurrence.
Table 2.
Summary of complications and reoperations
| Complications | OA | APC | EP | p Value |
|---|---|---|---|---|
| Number of patients | 17 | 16 | 16 | |
| Total complications | 11 (65%) | 7 (44%) | 7 (44%) | 0.38 |
| Patients with complications requiring reoperation | 8 (47%) | 4 (25%) | 4 (25%) | 0.29 |
| Prosthesis revision | 5 (29%) | 1 (6%) | 0 (0%) | 0.03 |
| Infection | 2 (12%) | 2 (13%) | 0 (0%) | 0.45 |
| Superficial | 1 (6%) | 0 (0%) | ||
| Deep | 1 (6%) | 2 (13%) | ||
| Fracture | 9 (53%) | 1 (6%) | NA | 0.0002 |
| Humeral head fracture | 6 (35%) | NA | ||
| Graft body fracture | 4 (24%) | 1 (6%) | ||
| Instability | 3 (18%) | 3 (19%) | 5 (31%) | 0.63 |
| Subluxation | 3 (18%) | 3 (19%) | 2 (13%) | |
| Dislocation | 0 (0%) | 0 (0%) | 3 (19%) | 0.03 |
| Nonunion | 1 (6%) | 1 (6%) | NA | 0.74 |
| Bleeding/hematoma | 0 (0%) | 0 (0%) | 2 (13%) | 0.10 |
| Patients with multiple complications | 5 (29%) | 0 (0%) | 0 (0%) | 0.008 |
OA = osteoarticular allograft; APC = allograft-prosthesis composite; EP = endoprosthesis; NA = not applicable.
The mean MSTS functional score at last followup for all groups was 73%. The MSTS functional scores for the APC cohort (79%) were greater (p = 0.008) than those of the OA (71%) and EP (69%) cohorts. Abductor function was better (p = 0.02) after APC reconstruction, with abduction to 90° or greater achieved after seven OA (41%), 12 APC (75%), and four EP (25%) reconstructions. There was no difference in mean humeral resection length between cohorts (p = 0.14). We found no relationship between history of a pathologic fracture (p = 0.49) or prior surgery (p = 0.12) and subsequent MSTS scores. The abductor mechanism was compromised in nine patients, with a greater (p = 0.04) percentage of these patients comprising the EP (38%) than the APC cohort (6%). The mean MSTS scores were similar (p = 0.07) for patients with an intact versus compromised abductor mechanism (74% versus 68%) and for patients with primary tumors of bone (p = 0.09) versus those with metastatic disease (75% versus 70%). Abductor mechanism compromise consisted of rotator cuff and axillary nerve sacrifice in one patient each from the OA cohort, one rotator cuff resection in the APC cohort, and five rotator cuff resections and one total abductor resection (rotator cuff and greater than 50% of deltoid with axillary nerve sacrifice) in the EP group. Abductor mechanism competence was associated with greater (p = 0.02) shoulder stability, with subluxation or dislocation occurring in five of nine patients (56%) with compromised abductors, compared with only six of 40 patients (15%) with intact abductor mechanisms.
Discussion
The optimal reconstructive technique after proximal humerus resection is controversial. We sought to evaluate the outcomes and complications of three of the more common reconstructive techniques by means of a retrospective cohort study to further elucidate the best technique(s) for individual patients. We hypothesized allograft (OA and APC) proximal humerus reconstructions would have decreased implant longevity as compared with EP reconstructions because of an increased rate of major complications requiring revision, but the biologic tendon and capsular reconstructions afforded by allograft soft tissue attachments would result in superior patient function and joint stability.
Although our hypotheses generally were confirmed by our study, they must be interpreted in light of our study limitations. This study was retrospective and the study period included a relatively broad time frame. As such, patient selection between reconstruction cohorts was not standardized. OA reconstructions generally were performed earlier during the study period as we became increasingly aware of the high complication rate associated with this technique. Despite the longer followup in the OA cohort, all revision procedures were performed within 5 years of the index reconstruction. The younger patient age and slightly greater number of primary tumors in the OA cohort might be expected to improve functional outcomes in this group. However, the generally even distribution of diagnoses among treatment cohorts might limit potential bias regarding the self-fulfilling prophecy of favoring outcomes with a specific technique for a specific diagnostic group. Although the numbers of patients in each cohort and the study as a whole are relatively small, these numbers are comparable or greater than in previous reports of OA and APC reconstructions and all three treatment cohorts contained comparable numbers of patients. Although we believe our key study findings generally are applicable to similar diverse groups of patients, the limited number of patients precluded meaningful multivariable analyses of functional outcomes and complications, which might have shed further light on potential biases resulting from differences in confounding variables between cohorts (eg, diagnosis, abductor mechanism competence, humeral resection length, pathologic fracture, prior surgery, preoperative or postoperative radiotherapy).
We found 5-year Kaplan-Meier implant survival estimates, with revision for any reason as the end point, of 56%, 91%, and 100% for the OA, APC, and EP groups, respectively. This did not reach the arbitrary statistical significance cutoff of p = 0.05 in log rank analysis (p = 0.07), but our study was underpowered to detect a twofold difference in implant survival (1 − β = 45%). However, revision surgery was more common (p = 0.03) in the OA group. In previous reports of OA graft survivorship, Getty and Peabody [13] found 68% Kaplan-Meier graft survival at 5 years, and Gebhardt et al. [12] reported 80% actuarial graft survival at a mean followup of 63 months. Actuarial survivorship from small series of APC reconstructions has ranged from 83% of six patients at 55 months [3] to 100% of four reconstructions at 26 months [15]. Our implant survivorship rate of 100% for EP reconstructions is slightly greater than the reported range of 70% at 5 years [26] to 86.5% at 20 years [18]. Although prior comparative studies are lacking, the implant survivorship findings in our study are thus consistent with those previously reported.
Major complications requiring reoperation (including deep infection, symptomatic instability, fracture, and aseptic loosening) occurred in 47% of the OA group, compared with only 25% each in the APC and EP groups. Author enthusiasm for OA reconstructions has varied, with major complication rates commonly exceeding 45% [6, 12, 13, 24]. Fracture of the graft body and, in particular, fracture or fragmentation of the humeral head frequently has been problematic, occurring in 25% to 67% of cases [6, 12, 13, 24]. Although not always symptomatic, humeral head collapse causing declining OA reconstruction function with time also has been noted [13, 24, 25]. DeGroot et al. [7] proposed filling grafts with cement for fracture prevention, but fractures still occurred in 18% of patients in their series, and this technique may complicate subsequent revision surgery. Although dislocation rates as much as 50% have been reported for OA reconstructions [13], our series had generally favorable joint stability after OA and APC reconstruction. However, because of the considerable complication rate related to fracture and fragmentation in our series, we concur with previous authors’ recommendations [13, 25] and caution against routine use of OA proximal humerus reconstructions.
Although OA reconstruction resulted in high complication rates in our series, APC and EP reconstructions provided some clear advantages. In comparison to OA reconstructions, APC reconstructions may afford lower rates of host-graft nonunion and graft body fracture while avoiding humeral head fragmentation [14, 22, 27]. We noted a lower rate of reoperation and revision after APC reconstruction. Likewise, the high cumulative risks of infection, nonunion, and fracture associated with OA and APC reconstructions are largely avoided with EP reconstructions [14, 22, 27]. However, instability has been reported in as many as 55% of EP reconstructions [18, 24] with frank dislocation observed in 10% to 14% [1, 25, 26]. The rates in our series for instability and frank dislocation of 31% and 19%, respectively, are consistent with these reported rates.
Functional outcomes as assessed by MSTS functional scores were greater (p = 0.008) in the APC cohort than in the OA and EP groups (79% versus 71% and 69%, respectively) in our study. Two previous reports of functional outcomes after OA reconstruction found mean MSTS functional scores of 70% [13] and 71% [24], respectively, which are equivalent to our results. Previous reports of APC proximal humerus reconstructions have been limited, with only two case series reporting functional outcomes. Black et al. [3] reported generally favorable results in six patients, with a mean MSTS score of 74%, and Jensen and Johnston [15] reported on 11 patients with autoclaved APCs and four patients with APCs, with the ability to abduct to greater than 80° in the majority of patients. Our results are consistent with this, with abduction to 90° or greater in 75% of patients receiving APC. EP proximal humerus reconstructions have had reported MSTS scores ranging from 61% to 87%, which are consistent with our mean score of 69% [20, 24]. As noted, meaningful subgroup analysis of our instability rates and functional outcomes based on confounding variables was not possible because of the small number of patients in each cohort. However, we did not find a major effect of abductor mechanism competence or metastatic disease diagnosis on MSTS scores. We found good functional results and an acceptable complication rate after APC reconstruction and reasonable function and excellent implant longevity with a similar complication rate after EP reconstruction. However, OA resulted in an unacceptably high rate of complications and reoperations, with no functional advantage over EP and worse functional results than APC. In general, we recommend APC reconstruction for younger patients with primary tumors of bone. For patients with metastatic disease, EP reconstruction is technically less challenging and provides acceptable and reproducible results, with implant longevity likely to exceed that of the patient. We cannot recommend OA for routine use in the proximal humerus.
Footnotes
This study was supported by a restricted educational grant from Stryker and one of the authors (HTT) is a paid consultant of Stryker.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Asavamongkolkul A, Eckardt JJ, Eilber FR, Dorey FJ, Ward WG, Kelly CM, Wirganowicz PZ, Kabo JM. Endoprosthetic reconstruction for malignant upper extremity tumors. Clin Orthop Relat Res. 1999;360:207–220. [DOI] [PubMed]
- 2.Bell RS. Treatment of axial skeleton bone metastases. Clin Orthop Relat Res. 2003;415(suppl):S198–S200. [DOI] [PubMed]
- 3.Black AW, Szabo RM, Titelman RM. Treatment of malignant tumors of the proximal humerus with allograft-prosthesis composite reconstruction. J Shoulder Elbow Surg. 2007;16:525–533. [DOI] [PubMed]
- 4.Bos G, Sim F, Pritchard D, Shives T, Rock M, Askew L, Chao E. Prosthetic replacement of the proximal humerus. Clin Orthop Relat Res. 1987;224:178–191. [PubMed]
- 5.Chen WM, Chen TH, Huang CK, Chiang CC, Lo WH. Treatment of malignant bone tumours by extracorporeally irradiated autograft-prosthetic composite arthroplasty. J Bone Joint Surg Br. 2002;84:1156–1161. [DOI] [PubMed]
- 6.Damron TA, Rock MG, O’Connor MI, Johnson M, An KN, Pritchard DJ, Sim FH. Functional laboratory assessment after oncologic shoulder joint resections. Clin Orthop Relat Res. 1998;348:124–134. [DOI] [PubMed]
- 7.DeGroot H, Donati D, Di Liddo M, Gozzi E, Mercuri M. The use of cement in osteoarticular allografts for proximal humeral bone tumors. Clin Orthop Relat Res. 2004;427:190–197. [DOI] [PubMed]
- 8.Eckardt JJ, Kabo M, Kelly CM, Ward WG, Cannon CP. Endoprosthetic reconstructions for bone metastases. Clin Orthop Relat Res. 2003;415(suppl):S254–S262. [DOI] [PubMed]
- 9.Enneking WF, Dunham W, Gebhardt M, Malawer M, Pritchard DJ. A system for the classification of skeletal resections. Chir Organi Mov. 1990;75(suppl 1):217–240. [PubMed]
- 10.Enneking WF, Dunham W, Gebhardt MC, Malawer M, Pritchard DJ. A system for the functional evaluation of reconstructive procedures after surgical treatment of tumors of the musculoskeletal system. Clin Orthop Relat Res. 1993;286:241–246. [PubMed]
- 11.Gainor BJ, Buchert P. Fracture healing in metastatic bone disease. Clin Orthop Relat Res. 1983;178:297–302. [PubMed]
- 12.Gebhardt MC, Roth YF, Mankin HJ. Osteoarticular allografts for reconstruction in the proximal part of the humerus after excision of a musculoskeletal tumor. J Bone Joint Surg Am. 1990;72:334–345. [PubMed]
- 13.Getty PJ, Peabody TD. Complications and functional outcomes of reconstruction with an osteoarticular allograft after intra-articular resection of the proximal aspect of the humerus. J Bone Joint Surg Am. 1999;81:1138–1146. [DOI] [PubMed]
- 14.Hornicek FJ, Gebhardt MC, Tomford WW, Sorger JI, Zavatta M, Menzner JP, Mankin HJ. Factors affecting nonunion of the allograft-host junction. Clin Orthop Relat Res. 2001;382:87–98. [DOI] [PubMed]
- 15.Jensen KL, Johnston JO. Proximal humeral reconstruction after excision of a primary sarcoma. Clin Orthop Relat Res. 1995;311:164–175. [PubMed]
- 16.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [DOI]
- 17.Kiss J, Sztrinkai G, Antal I, Kiss J, Szendroi M. Functional results and quality of life after shoulder girdle resections in musculoskeletal tumors. J Shoulder Elbow Surg. 2007;16:273–279. [DOI] [PubMed]
- 18.Kumar D, Grimer RJ, Abudu A, Carter SR, Tillman RM. Endoprosthetic replacement of the proximal humerus: long-term results. J Bone Joint Surg Br. 2003;85:717–722. [PubMed]
- 19.Lin PP, Mirza AN, Lewis VO, Cannon CP, Tu SM, Tannir NM, Yasko AW. Patient survival after surgery for osseous metastases from renal cell carcinoma. J Bone Joint Surg Am. 2007;89:1794–1801. [DOI] [PubMed]
- 20.Malawer MM, Chou LB. Prosthetic survival and clinical results with use of large-segment replacements in the treatment of high-grade bone sarcomas. J Bone Joint Surg Am. 1995;77:1154–1165. [DOI] [PubMed]
- 21.Malawer MM, Meller I, Dunham WK. A new surgical classification system of shoulder-girdle resections: analysis of 38 patients. Clin Orthop Relat Res. 1991;267:33–44. [PubMed]
- 22.Mankin HJ, Gebhardt MC, Jennings LC, Springfield DS, Tomford WW. Long-term results of allograft replacement in the management of bone tumors. Clin Orthop Relat Res. 1996;324:86–97. [DOI] [PubMed]
- 23.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed]
- 24.O’Connor MI, Sim FH, Chao EYS. Limb salvage for neoplasms of the shoulder girdle. J Bone Joint Surg Am. 1996;78:1872–1888. [DOI] [PubMed]
- 25.Rodl RW, Gosheger G, Gebert C, Lindner N, Ozaki T, Winkelman W. Reconstruction of the proximal humerus after wide resection of tumors. J Bone Joint Surg Br. 2002;84:1004–1008. [DOI] [PubMed]
- 26.Sharma S, Turcotte RE, Isler MH, Wong C. Experience with cemented large segment endoprostheses for tumors. Clin Orthop Relat Res. 2007;459:54–59. [DOI] [PubMed]
- 27.Sorger JI, Hornicek FJ, Zavatta M, Menzner JP, Gebhardt MC, Tomford WW, Mankin HJ. Allograft fractures revisited. Clin Orthop Relat Res. 2001;382:66–74. [DOI] [PubMed]
- 28.Torbert JT, Fox EJ, Hosalkar HS, Ogilvie CM, Lackman RD. Endoprosthetic reconstructions: long-term followup of 139 patients. Clin Orthop Relat Res. 2005;438:51–59. [DOI] [PubMed]
- 29.Wedin R, Bauer HCF, Wersall P. Failures after operation for skeletal metastatic lesions of long bones. Clin Orthop Relat Res. 1999;358:128–139. [DOI] [PubMed]
- 30.Yazawa Y, Frassica FJ, Chao EY, Pritchard DJ, Sim FH, Shives TC. Metastatic bone disease: a study of the surgical treatment of 166 pathologic humeral and femoral fractures. Clin Orthop Relat Res. 1990;251:213–219. [PubMed]
- 31.Zeegen EN, Aponte-Tinao LA, Hornicek FJ, Gebhardt MC, Mankin HJ. Survivorship analysis of 141 modular metallic endoprostheses at early follow-up. Clin Orthop Relat Res. 2004;420:239–250. [DOI] [PubMed]