Abstract
Several studies show cytokine concentrations in the peripheral blood are associated with inflammatory activity and surgical trauma. Cytokine concentrations have more rapid increase and quicker return to normal values than either C-reactive protein or erythrocyte sedimentation rate – a matter of hours rather than weeks; some studies suggest they are better predictors of postoperative infection than C-reactive protein and erythrocyte sedimentation rate. Threshold levels of interleukin-6 after joint arthroplasty have been determined, but levels of other potentially useful cytokines (tumor necrosis factor-α, interleukin-8, interleukin-10, etc) are not known. We measured the serum levels of 25 different cytokines before and after hip and knee arthroplasties and identified those associated with surgical trauma. Peripheral venous blood samples (one preoperative and three postoperative) from 49 patients undergoing hip or knee arthroplasty were analyzed by laser chromatography. Three of the 25 cytokines had a relationship with postsurgical trauma, which included one deep infection. Serum levels of these three cytokines might be useful to identify periprosthetic infections during the early postoperative period when C-reactive protein and erythrocyte sedimentation rate remain elevated.
Level of Evidence: Level II, prognostic study. See the Guidelines for Authors for a complete description of levels of evidence.
Introduction
Major surgery causes various changes in the neuroendocrine and inflammatory cytokine systems. Many of these physiologic responses have been investigated extensively, but in particular C-reactive protein (CRP) has been studied extensively [19, 25, 29]. CRP serum levels are elevated to their peak value 2 to 3 days after arthroplasty and return to normal in approximately 3 weeks [5, 26].
Elevation beyond this 3-week period is associated with acute infection at the arthroplasty site [33]. However, it is during this 3-week period that initiation of bacterial infection occurs [24]. Prompt diagnosis and treatment at this stage potentially could eradicate infection, whereas a delay in diagnosis allows the microorganisms to form a biofilm and become resistant to any subsequent treatment other than revision.
Unfortunately, measuring CRP during the first 3 weeks is inconclusive because it remains elevated in the absence of infection. Various cytokine concentrations (tumor necrosis factor [TNF]-α, interleukin [IL]-6, IL-8, IL-10) in the peripheral blood have been reported to undergo a more rapid increase (hours) and quicker return (days) to normal values after surgery than CRP [18, 30]. Potentially these cytokine measurements could diagnose infection before it becomes clinically apparent during the early weeks after joint arthroplasty [13, 18, 21, 29, 33].
The aims of our study were to measure the levels of 25 different cytokines before and after joint arthroplasty and to identify any that were changed with postsurgical trauma.
Materials and Methods
We performed a prospective case-control study. We invited all patients admitted to our institution between January 2006 and May 2006 for hip and knee arthroplasties (including resurfacing arthroplasty of the hip and unicompartmental arthroplasty of the knee) for osteoarthritis to participate in the study. Patients with known chronic inflammatory disease (rheumatoid arthritis, systemic lupus erythematosus, Crohn’s disease, Hashimoto’s thyroiditis, psoriasis), recent antibiotic treatment or intercurrent infections before surgery, Paget’s disease, revision arthroplasty, vascular disorders (lymphoproliferative disorders, autoimmune hemolytic anemia), or cancer were excluded because the cytokine response can be abnormal in these conditions [6, 8, 12]. Eighty potential patients with osteoarthritis were available during the time of the study of which 49 fulfilled all the eligibility criteria and were included in the study. The mean age of the patients in the study was 63 years, with the majority having hip arthroplasty under a general anesthetic (Table 1). Most of the early postoperative complications were urinary tract infections (UTIs) and upper respiratory tract infections (URTIs) (Table 2). There was one proven deep infection leaving 48 patients for study. We performed 25 cytokine measurements (IL-1β, IL-1ra, IL-2, IL-2R, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p40, IL-13, IL-15, IL-17, TNF-α, interferon-α, interferon-γ, granulocyte-macrophage colony-stimulating factor, macrophage inflammatory protein-1α, macrophage inflammatory protein-1β, interferon-γ-inducible protein-10, monokine induced by interferon-γ, eotaxin, RANTES, monocyte chemoattractant protein [MCP]-1). Ethical approval was obtained from The Central Research and Ethics Committee.
Table 1.
Patient characteristics
| Patient demographics | Value |
|---|---|
| Age (years)* | 63 (36–79) |
| Gender | |
| Male | 15 |
| Female | 34 |
| Procedure | |
| Resurfacing | 14 |
| THA | 17 |
| TKA | 16 |
| UKA | 2 |
| Anesthetic | |
| General | 34 |
| Spinal | 15 |
| Body mass index* | 31.8 (22–42) |
| Charlson age-related comorbidity index* | 2.4 (55–300) |
| Surgical time (minutes)* | 85 (55–300) |
* Values expressed as means, with ranges in parentheses; UKA = unicompartmental knee arthroplasty.
Table 2.
Early postoperative complications
| Complications | Number of patients |
|---|---|
| Urinary tract infection | 6 |
| Upper respiratory tract infection | 3 |
| Chest infection | 4 |
| Deep vein thrombosis | 3 |
We recorded the index procedure, comorbidity (diabetes mellitus, liver disease, renal disease, congestive heart failure, chronic pulmonary disease, peripheral vascular disease), body mass index, surgical duration, and any postoperative complications such as UTIs or URTIs and deep venous thrombosis (DVT). Each patient had four blood samples taken. We obtained the first sample (P1) in the preoperative assessment clinic 2 weeks before surgery. The second sample (P2) was obtained 6 hours after surgery, the third sample (P3) was 48 hours after surgery, and the fourth sample (P4) was 6 weeks after surgery.
Peripheral venous blood samples were centrifuged and stored in pyrogen- and endotoxin-free polypropylene tubes at −70°C until analysis. All samples were clarified by centrifugation (14,000 rpm for 10 minutes) before analysis to prevent clogging of filter plates. We determined the cytokine concentrations using multiplex assay reagents (Human Cytokine 25-plex; BioSource Europe SA, Nivelles, Belgium) and the xMAP® technology (Luminex Corp, Austin, TX) according to the manufacturer’s protocol. Although the kit measures levels of cytokines not involved in immune response, we used it because it was inexpensive and easily available. We measured Luminex color code tiny microspheres with the Luminex compact analyzer. A standard curve was developed on the basis of the concentrations of known standards provided. We diluted samples with concentrations that exceeded the standard curve in assay diluents and reanalyzed them; this dilution factor was used to correct for the concentration of factors from the original sample. We obtained the mean value, at each time, for each of the 25 cytokines based on the 49 patients.
Deep infection was diagnosed based on the Centers for Disease Control and Prevention guidelines [25]. We recalculated the mean values after excluding the infected cases. A parametric ANOVA model was used to measure each set of cytokine data across the four times. When appropriate, we used Tukey’s post hoc test to ascertain where any differences occurred. A 5% level of significance was set for all hypothesis testing. For cytokines with a significant difference across the times, we used the mean value of the normal subject data as a reference against that of the one patient with infection.
Results
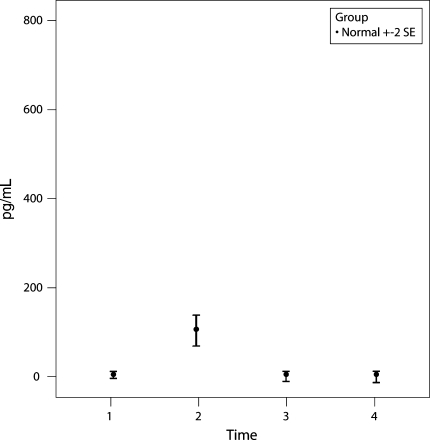
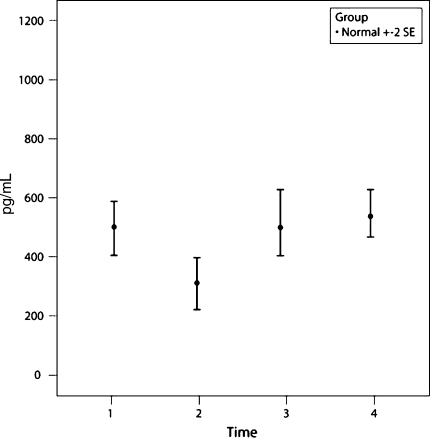
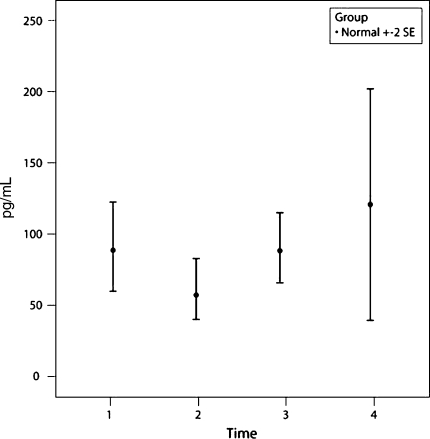
Three of the 25 cytokines measured in 48 control subjects showed a distinct change with time from P1 to P2 to P3; IL-6, MCP-1, and IL-2R. IL-6 and MCP-1 showed an increase (p = 0.0006 and p = 0.002, respectively) at time P2 compared with times P1 and P3 (Figs. 1, 2). IL-6 and MCP-1 recovered to within the baseline (preoperative) value at P3 (48 hours postoperatively). There were no abnormal results seen at P4. IL-2R values dropped slightly at P2 (p = 0.12) but returned to baseline at P3 (Fig. 3).
Fig. 1.
A graph shows the mean ± two standard error (SE) values of IL-6 for the group of 48 control patients. IL-6 showed an increase (p = 0.0006) at P2 (6 hours postoperatively) compared with P1 and P3 but recovered to within the baseline (preoperative) value at P3 (48 hours postoperatively).
Fig. 2.
A graph shows the mean ± two standard error (SE) values of MCP-1 for the group of 48 control patients. MCP-1 showed an increase (p = 0.002) at P2 (6 hours postoperatively) compared with P1 and P3 but recovered to within the baseline (preoperative) value at P3 (48 hours postoperatively).
Fig. 3.
A graph shows the mean ± two standard error (SE) values of IL-2R for the group of 48 control patients. IL-2R values dropped slightly at P2 (p = 0.12) but returned to baseline at P3.
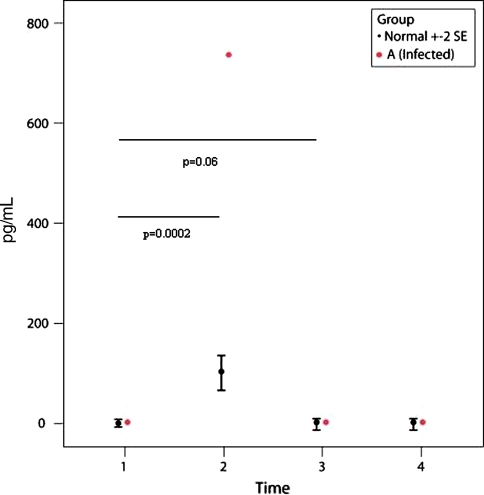
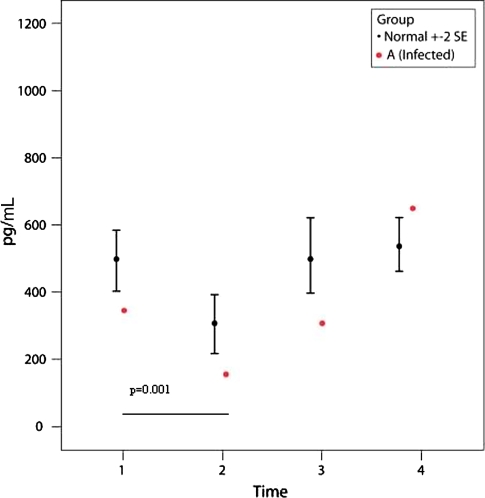
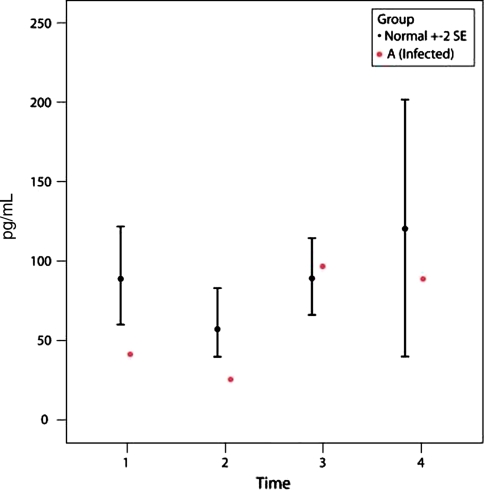
Patient A had a deep infection after TKA and had a subsequent washout and exchange of the polyethylene insert. Intraoperative cultures were positive for methicillin-resistant Staphylococcus aureus and the patient underwent two-stage revision of the TKA several months later. The IL-6 level of this patient was high at P2 (Fig. 4) and the MCP-1 level was low at P2 (Fig. 5) compared with the control group (p = 0.0002 and p = 0.001, respectively). However, by P3, the IL-6 level had decreased to baseline level and the MCP-1 level had increased but still was lower than controls (Figs. 4, 5). IL-2R values dropped slightly at P2 (p = 0.14) compared to controls (Fig. 6).
Fig. 4.
A graph shows the IL-6 values for the 48 control subjects (mean ± two standard error [SE]) and one patient with a deep infection (Patient A). Patient A had a higher IL-6 value (p = 0.0002) compared with control subjects at P2 (6 hours after surgery). The differences between P2 compared with P1 and P3 for Patient A are significant (p = 0.06).
Fig. 5.
A graph shows the MCP-1 values in the 48 control subjects (mean ± two standard error [SE]) and one patient with a deep infection (Patient A). Patient A shows a lower MCP-1 value (p < 0.001) compared with the control subjects at P2 (6 hours after surgery). The differences between P2 compared with P1 and P3 for Patient A are significant (p = 0.08).
Fig. 6.
A graph shows the IL-2R values in the 48 control subjects (mean ± two standard error [SE]) and one patient with a deep infection (Patient A). Patient A had a lower IL-2R value compared with the control subjects at P2 (6 hours after surgery).
Discussion
Prosthetic joint infection is one of the most catastrophic complications of arthroplasty and is associated with considerable morbidity and healthcare costs. The pathogenesis of prosthetic joint infections is influenced by microorganisms growing in biofilms, making these infections difficult to diagnose and eradicate. As much as 1/3 of infections after joint arthroplasty occur during the first 3 months after surgery [15]. If detected early and treated aggressively, there is a better chance of eradicating the infection [31]. Measurement of CRP and erythrocyte sedimentation rate (ESR) has been used to assess infection after joint arthroplasty [22, 29, 33]. However, their value is limited during the first few weeks after surgery because their levels are normally elevated during this period. In the later postoperative period when CRP and ESR are expected to have returned to normal levels, their use is limited to excluding infection reliably by a combination of normal ESR and CRP levels [32] or suggesting infection with increasing levels of CRP [33]. To rely on increasing levels of CRP for diagnosis of infection has the disadvantage of delaying treatment and permitting microorganisms time to establish biofilms, making these infections difficult to eradicate. Therefore, there is a need to identify an inflammatory marker with a rapid increase and a quicker return to normal compared with CRP. We therefore measured the serum levels of 25 different cytokines before and after hip and knee arthroplasties and determined whether any were associated with normal postsurgical trauma.
The major limitation of our study was that there was no way to distinguish between changes from postsurgical trauma and other sources of inflammation, including infection. We also could not account for other variables (eg, gender of the patient, type of anesthetic used, etc) that may influence the levels of circulating cytokines. Although it was not the aim of the study, we calculated and found no statistical difference in cytokine levels between male and female patients, whether the anesthetic was general or regional, or according to the type of arthroplasty. There was also no correlation with body mass index, comorbidity, surgical time, or complications of UTI, URTI, lower respiratory tract infection, or DVT. Autologous blood obtained from cell salvage systems also is associated with higher levels of IL-6 [1, 20]. None of our patients had retransfusion of autologous blood. Units that use this reperfusion will be reinfusing blood with elevated levels of IL-6 [2]. The consequence of this on morbidity and infection rates needs to be established. We used a mixture of regional and general anesthesia. However, others [4, 15, 17] suggest regional versus general anesthetic do not differentially influence IL-6 levels and this was confirmed by our study.
Three of the 25 cytokines we measured revealed a distinct postoperative pattern after arthroplasty: IL-6, MCP-1, and IL-2R. IL-6 measurement has been used to predict rejection of lung transplants, development of major complications after elective surgery, and neonatal sepsis [3, 11, 35]. Sustained levels of IL-6 after polytrauma also have been associated with multiorgan failure [27]. For arthroplasties, it has been studied in chronic infection [10], but its role in the immediate postoperative period has been more debatable. IL-6 peaks in the first 6 to 12 hours after surgery and decreases to its baseline range by 48 to 72 hours postoperatively after THA or TKA [16, 19, 34]. We observed similar results between hip and knee primary procedures, with a major difference in the levels of IL-6 among the measured times (P2, P1, P3).
Other studies have looked at TNF-α, IL-1β, and IL-10. In general, TNF-α and IL-1β are elevated only with severe trauma and associated hypoxia and not arthroplasty [9, 30]. Plasma IL-10 reportedly has been elevated after major trauma but not necessarily postsurgery [7, 14, 16, 28]. The observation that IL-10 plasma levels reportedly undergo only modest changes after arthroplasty [9] is logical because it is an antiinflammatory cytokine [23] and its consumption will depend on the degree of tissue trauma, which would be expected to be higher in major trauma compared with surgery. The unique finding in our study is MCP-1 showed a distinct postoperative pattern and a difference in levels among the measured times (P2, P1, P3). Although MCP-1 is not known to be purely antiinflammatory, its levels decreased considerably at P2 (6 hours postoperatively) and recovered to baseline levels at P3 and P4 in all 48 normal subjects, implying it potentially has antiinflammatory properties. Additional study is needed to determine if this could be used as a preoperative risk predictor of infection.
There was a major difference among the three times (P2, P1, P3) for IL-6 and MCP-1 in Patient A with a deep infection (Figs. 4, 5). In addition, IL-6 levels were too high at P2 and MCP-1 levels were too low at P2 compared with the control group. These changes in IL-6 and MCP-1 seem to reflect the increased inflammation because of the infection in this patient. Although levels of IL-2R in Patient A did not show a marked decrease with deep infection, its value was lower than the overall average (Fig. 6). It is possible, as a result of the washout, the infection remained under control and IL-6 levels did not increase after the first abnormal value in Patient A. It would be interesting to repeat these cytokine measurements in other known infected cases. IL-6 returned to baseline control levels by 48 hours in Patient A despite the ultimate persistence of infection for which a two-stage revision was performed. However, MCP-1 was still below normal values at 48 hours. Unfortunately, we did not obtain a blood sample before revision to measure IL-6 and MCP-1. Results of the study of Di Cesare et al. [10] suggest IL-6 should be elevated. Nevertheless, our data suggest a combination of increased IL-6 at 6 hours and reduced levels of MCP-1 at 48 hours is associated with infection.
We identified two cytokines that were associated with surgical trauma after joint arthroplasty. IL-6 and MCP-1 have an exaggerated change in plasma levels at an early stage in the presence of deep infection before the onset of clinical signs. However, there was only one case of deep infection in our study; therefore, we cannot comment on the predictive value of these cytokines for infection. A multicenter study would be useful to investigate this further.
Acknowledgments
We thank Alastair Gracie and Helen Murray for invaluable contributions.
Footnotes
Each author certifies that he or she has no commercial associations (eg, consultancies, stock ownership, equity interest, patent/licensing arrangements, etc) that might pose a conflict of interest in connection with the submitted article.
Each author certifies that his or her institution has approved the human protocol for this investigation, that all investigations were conducted in conformity with ethical principles of research, and that informed consent for participation in the study was obtained.
References
- 1.Andersson I, Tylman M, Bengtson JP, Bengtsson A. Complement split products and pro-inflammatory cytokines in salvaged blood after hip and knee arthroplasty. Can J Anaesth. 2001;48:251–255. [DOI] [PubMed]
- 2.Arnestad JP, Bengtsson A, Bengtson JP, Tylman M, Redl H, Schlag G. Formation of cytokines by retransfusion of shed whole blood. Br J Anaesth. 1994;72:422–425. [DOI] [PubMed]
- 3.Baigrie RJ, Lamont PM, Kwiatkowski D, Dallman MJ, Morris PJ. Systemic cytokine response after major surgery. Br J Surg. 1992;79:757–760. [DOI] [PubMed]
- 4.Bew SA, Bryant AE, Desborough JP, Hall GM. Epidural analgesia and arterial reconstructive surgery to the leg: effects on fibrinolysis and platelet degranulation. Br J Anaesth. 2001;86:230–235. [DOI] [PubMed]
- 5.Bilgen O, Atici T, Durat K, Karaeminogullari O, Bilgen MS. C-reactive protein values and erythrocyte sedimentation rates after total hip and total knee arthroplasty. J Int Med Res. 2001;29:7–12. [DOI] [PubMed]
- 6.Bottner F, Wegner A, Winkelmann W, Becker K, Erren M, Gotze C. Interleukin-6, procalcitonin and TNF-alpha: markers of peri-prosthetic infection following total joint replacement. J Bone Joint Surg Br. 2007;89:94–99. [DOI] [PubMed]
- 7.Brune IB, Wilke W, Hensler T, Holzmann B, Siewert JR. Downregulation of T helper type 1 immune response and altered pro-inflammatory and anti-inflammatory T cell cytokine balance following conventional but not laparoscopic surgery. Am J Surg. 1999;177:55–60. [DOI] [PubMed]
- 8.Chikanza IC, Roux-Lombard P, Dayer JM, Panavi GS. Dysregulation of the in vivo production of interleukin-1 receptor antagonist in patients with rheumatoid arthritis: pathogenetic implications. Arthritis Rheum. 1995;38:642–648. [DOI] [PubMed]
- 9.Clementsen T, Krohn CD, Reikeras O. Systemic and local cytokine patterns during total hip surgery. Scand J Clin Lab Invest.. 2006;66:535–542. [DOI] [PubMed]
- 10.Di Cesare P, Chang E, Preston C, Liu CJ. Serum interleukin-6 as a marker of periprosthetic infection following total hip and knee arthroplasty. J Bone Joint Surg Am. 2005;87:1921–1927. [DOI] [PubMed]
- 11.Dollner H, Vatten L, Austgulen R. Early diagnostic markers for neonatal sepsis: comparing C-reactive protein, interleukin-6, soluble tumour necrosis factor receptors and soluble adhesion molecules. J Clin Epidemiol. 2001;54:1251–1257. [DOI] [PubMed]
- 12.Ehrlich LA, Roodman GD. The role of immune cells and inflammatory cytokines in Paget’s disease and multiple myeloma. Immunol Rev. 2005;208:252–266. [DOI] [PubMed]
- 13.Franke A, Lante W, Fackeldey V, Becker HP, Kurig E, Zöller LG, Weinhold C, Markewitz A. Pro-inflammatory cytokines after different kinds of cardio-thoracic surgical procedures: is what we see what we know? Eur J Cardiothorac Surg. 2005;28:569–575. [DOI] [PubMed]
- 14.Giannoudis PV, Smith RM, Perry SL, Windsor AJ, Dickson RA, Bellamy MC. Immediate IL-10 expression following major orthopaedic trauma: relationship to anti-inflammatory response and subsequent development of sepsis. Intensive Care Med. 2000;26:1076–1081. [DOI] [PubMed]
- 15.Goto Y, Ho SL, McAdoo J, Fanning NF, Wang J, Redmond HP, Shorten GD. General versus regional anaesthesia for cataract surgery: effects on neutrophil apoptosis and the postoperative pro-inflammatory state. Eur J Anaesthesiol. 2000;17:474–480. [DOI] [PubMed]
- 16.Hensler T, Hecker H, Heeg K, Heidecke CD, Bartels H, Barthlen W, Wagner H, Siewert JR, Holzmann B. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect Immun. 1997;65:2283–2291. [DOI] [PMC free article] [PubMed]
- 17.Hogevold HE, Lyberg T, Kahler H, Haug E, Reikeras O. Changes in plasma IL-1beta, TNF-alpha and IL-6 after total hip replacement surgery in general or regional anaesthesia. Cytokine. 2000;12:1156–1159. [DOI] [PubMed]
- 18.Kimura F, Shimizu H, Yoshidome H, Ohtsuka M, Kato A, Yoshitomi H, Nozawa S, Furukawa K, Mitsuhashi N, Sawada S, Takeuchi D, Ambiru S, Miyazaki M. Increased plasma levels of IL-6 and IL-8 are associated with surgical site infection after pancreaticoduodenectomy. Pancreas. 2006;32:178–185. [DOI] [PubMed]
- 19.Kragsbjerg P, Holmberg H, Vikerfors T. Serum concentrations of interleukin-6, tumour necrosis factor-alpha, and C-reactive protein in patients undergoing major operations. Eur J Surg. 1995;161:17–22. [PubMed]
- 20.Krohn CD, Reikeras O, Mollnes TE, Aasen AO. Complement activation and release of interleukin-6 and tumour necrosis factor-alpha in drained and systemic blood after major orthopaedic surgery. Eur J Surg. 1998;164:103–108. [DOI] [PubMed]
- 21.Küster H, Weiss M, Willeitner AE, Detlefsen S, Jeremias I, Zbojan J, Geiger R, Lipowsky G, Sumbruner G. Interleukin-1 receptor antagonist and interleukin-6 for early diagnosis of neonatal sepsis 2 days before clinical manifestation. Lancet. 1998;352:1271–1277. [DOI] [PubMed]
- 22.Larsson S, Thelander U, Friberg S. C-reactive protein (CRP) levels after elective orthopaedic surgery. Clin Orthop Relat Res. 1992;275:237–242. [PubMed]
- 23.Lyons A, Kelly JL, Rodrick ML, Mannick JA, Lederer JA. Major injury induces increased production of interleukin-10 by cells of the immune system with a negative impact on resistance to infection. Ann Surg. 1997;226:450–458. [DOI] [PMC free article] [PubMed]
- 24.Mackie PH, Crockson RA, Stuart J. C-reactive protein for rapid diagnosis of infections of leukaemia. J Clin Pathol. 1979;32:1253–1256. [DOI] [PMC free article] [PubMed]
- 25.Mangram AJ, Horan TC, Pearson ML, Silver LC, Jarvis WR. Guideline for Prevention of Surgical Site Infection, 1999. Centers for Disease Control and Prevention (CDC) Hospital Infection Control Practices Advisory Committee. Am J Infect Control. 1999,27:97–132; quiz 133–134; discussion 96. [DOI] [PubMed]
- 26.Moreschini O, Greggi G, Giordano MC, Nocente M, Margheritini F. Postoperative physiopathological analysis of inflammatory parameters in patients undergoing hip or knee arthroplasty. Int J Tissue React. 2001;23:151–154. [PubMed]
- 27.Nast-Kolb D, Waydhas C, Gippner-Steppert C, Schneider I, Trupka A, Ruchholtz S, Zettl R, Schweiberer L, Jochum M. Indicators of the posttraumatic inflammatory response correlate with organ failure in patients with multiple injuries. J Trauma. 1997;42:446–454. [DOI] [PubMed]
- 28.Neidhardt R, Keel M, Steckholzer U, Safret A, Ungethuem U, Trentz O, Ertel W. Relationship of interleukin-10 plasma levels to severity of injury and clinical outcome in injured patients. J Trauma. 1997;42:863–870. [DOI] [PubMed]
- 29.Niskanen RO, Korkola O, Pammo H. Serum C-reactive protein levels after total hip and knee arthroplasty. J Bone Joint Surg Br. 1996;78:431–433. [PubMed]
- 30.Pape HC, Schmidt RE, Rice J, van Griensven M, Das Gupta R, Krettek C, Tscherne H. Biochemical changes after trauma and skeletal surgery of the lower extremity: quantification of the operative burden. Crit Care Med. 2000;28:3441–3448. [DOI] [PubMed]
- 31.Phillips JE, Crane TP, Noy M, Elliot TS, Grimer RJ. The incidence of deep prosthetic infections in a specialist orthopaedic hospital: a 15-year prospective survey. J Bone Joint Surg Br. 2006;88:943–948. [DOI] [PubMed]
- 32.Spengehl MJ, Masri BA, O’Connell JX, Duncan CP. Prospective analysis of perioperative and intraoperative investigations for the diagnosis of infection at the sites of two hundred and two revision total hip arthroplasties. J Bone Joint Surg Am. 1999;81:672–683. [DOI] [PubMed]
- 33.White J, Kelly M, Dunsmir R. C-reactive protein level after total hip and total knee replacement. J Bone Joint Surg Br. 1998;80:909–911. [DOI] [PubMed]
- 34.Wirtz DC, Heller KD, Miltner O, Zilkens KW, Wolff JM. Interleukin-6: a potential inflammatory marker after total joint replacement. Int Orthop. 2000;24:194–196. [DOI] [PMC free article] [PubMed]
- 35.Yoshida Y, Iwaki Y, Pham S, Dauber JH, Yousem SA, Zeevi A, Morita S, Griffith BP. Benefits of posttransplantation monitoring of interleukin 6 in lung transplantation. Ann Thorac Surg. 1993;55:89–93. [DOI] [PubMed]