Abstract
The fibrocartilage transition zone in the direct bone-tendon junction reduces stress concentration and protects the junction from failure. Unfortunately, bone-tendon junctions often heal without fibrocartilage transition zone regeneration. We hypothesized articular cartilage grafts could increase fibrocartilage transition zone regeneration. Using a goat partial patellectomy repair model, autologous articular cartilage was harvested from the excised distal third patella and interposed between the residual proximal two-thirds bone fragment and tendon during repair in 36 knees. We evaluated fibrocartilage transition zone regeneration, bone formation, and mechanical strength after repair at 6, 12, and 24 weeks and compared them with direct repair. Autologous articular cartilage interposition resulted in more fibrocartilage transition zone regeneration (69.10% ± 14.11% [mean ± standard deviation] versus 8.67% ± 7.01% at 24 weeks) than direct repair at all times. There was no difference in the amount of bone formation and mechanical strength achieved. Autologous articular cartilage interposition increases fibrocartilage transition zone regeneration in bone-tendon junction healing, but additional research is required to ascertain the mechanism of stimulation and to establish the clinical applicability.
Introduction
A direct bone-tendon junction typically consists of four zones of tissue: tendon, uncalcified fibrocartilage, calcified fibrocartilage, and bone. The uncalcified fibrocartilage and calcified fibrocartilage together form the transition zone [2, 8]. The transition zone fibrocartilage is characterized by chains of enlarged, round chondrocytes residing in extracellular matrix-filled lacunae, embedded in parallel bundles of collagen fibers [8]. The arrangement of the transition zone between tendon and bone ensures a gradual transition in stiffness and other material properties between tendon, a soft tissue, and bone, a hard tissue.
The normal bone-tendon junction is a strong structure protected by the transition zone. Traumatic failures near the bone-tendon junction usually occur as bone avulsion or tendon/ligament failure, but not at the junction [9, 22, 34]. The transition zone in the direct bone-tendon junction is thought to reduce stress concentration, tearing, or shearing at the interface [33]. The organization of uncalcified fibrocartilage and calcified fibrocartilage between tendon and bone ensures a gradual transition in stiffness and other material properties between tendon and bone. The presence of mechanical property gradients at the interface, instead of a sudden change in material property between tendon and bone, decreases stress concentration, improves the strength of tissue bonding, and decreases the chance of failure [29]. It also has been proposed [2] that fibrocartilage in the transition zone allows movement of tendon at the interface but prevents bending at the hard tissue. This notion is supported by the finding of increased fibrocartilage thickness at insertion sites that have greater freedom of movements [3, 10, 11]. The absence of transition zone regeneration presumably would result in a junction more prone to injury. One might presume, therefore, restoration of the transition zone in bone-tendon junction healing would be important after injury to maintain this unique protective mechanism.
Unfortunately, the bone-tendon junction often heals without fibrocartilage transition zone regeneration: MRI and biopsy studies in humans show there is no fibrocartilage zone regeneration or basophilic line formation years after bone-patellar tendon-bone graft harvest [18]. The absence of fibrocartilage transition zone regeneration also has been observed in supraspinatus reattachment [12], infraspinatus reattachment [1, 23], and partial patellectomy repair in animal models [17, 24, 32]. The unique protective mechanism is not restored.
Distal-third partial patellectomy with attachment of the tendon to the residual proximal two thirds provides a model to study the process of direct bone-tendon junction healing [17, 24, 32] (Fig. 1). Earlier tissue incorporation and better junction healing between articular cartilage and tendon than between bone and tendon have been observed after partial patellectomy in rabbits [24]. Wong et al. [32] reported increased fibrocartilage formation in the healing bone-tendon junction adjacent to the articular cartilage cut surface in a goat model. The formation of fibrocartilage resulted in a transition zone-like structure.
Fig. 1A–C.
(A) A transverse osteotomy was performed between the proximal two thirds and distal third of the patella. The patellar tendon was transected at the tip of the patella with a surgical knife. The distal-third patella and its attached tendon segment were removed. (B) A Bunnell tendon suture was applied to the patellar tendon. The two ends of the suture then were passed through the two sagittal drill holes in the patella, pulling the patellar tendon against the proximal patella, and tied at the superior pole of the patella. (C) A figure-of-eight tension band wiring was applied to protect the patella-to-patellar tendon repair.
We hypothesized autologous articular cartilage interposition grafting would increase fibrocartilage transition zone regeneration in bone-tendon junction healing, bone formation, and mechanical strength.
Materials and Methods
We obtained 36 mature 24- to 28-month-old male Chinese goats with body weight between 25 and 30 kg from a licensed dealer and housed them in the university animal laboratory center. We randomly allocated either knee to the autologous articular cartilage interposition group (n = 36) or the direct repair group (n = 36) for histologic studies (fibrocartilage transition zone regeneration and bone formation) and mechanical studies (ultimate failure load and ultimate stress). The knee samples were harvested at 6 (n = 24), 12 (n = 24), and 24 (n = 24) weeks. At each time, 12 samples (six from each group) were used for histologic studies and 12 samples (six from each group) were used for mechanical studies. A power analysis was performed with estimated fibrocartilage regeneration data (unpublished data) from a previous goat partial patellectomy study [32]. With a normalized mean fibrocartilage length of 10.0 (%), assuming a common standard deviation of 5.0, the sample size of six in each group would have 80% power to detect a doubling (ie, one fold increase) in fibrocartilage regeneration using a two-group comparison with a 0.05 two-sided significance level. Animal research ethics approval was obtained from our institute. The institute’s guidelines for the care and use of laboratory animals were followed throughout the study.
The procedure for partial patellectomy repair was as follows [32]. Gas induction was given by halothane inhalation via a conical breathing mask. After intubation, we administered general anesthesia with a mixture of halothane, nitrous oxide, and oxygen. One of the knees was shaved and disinfected with 0.05% chlorhexidene gluconate solution (Zeneca, Cheshire, UK). The patella and patellar tendon were exposed though a midline skin incision. We performed a transverse osteotomy at a level between the proximal two thirds and distal third of the patella using an oscillating saw (Mini Compressed Air; Synthes, Bettlach, Switzerland). The patellar tendon was transected at the tip of the patella with a surgical knife. Two sagittal drill holes were made from the osteotomy surface of the patella toward the superior pole of the patella. We applied a Bunnell tendon suture [16] to the patellar tendon using Ethibond® suture (Ethicon Ltd, San Angelo, TX). The two ends of the suture then were passed through the two patellar drill holes, pulling the patellar tendon against the proximal patella, and tied at the proximal end of the patella. The patellar tendon was attached to the center of the patella osteotomy surface. A figure-of-eight tension band wiring was applied using 0.9-mm-diameter steel wire to protect the patella-to-patellar tendon repair as in usual partial patellectomy repair [15] (Fig. 1). The figure-of-eight tension band wiring protected the repair, while allowing as much as 90° knee flexion. We closed the patellar retinaculum, paratenon, and skin with 3/0 chromic catgut and Mersilk® sutures (Ethicon Ltd). Antibiotic spray (Nebacetin®; Byk Gulden, Konstanz, Germany) was applied.
In knees allocated to the autologous articular cartilage interposition group (n = 36), a piece of articular cartilage was inserted between the proximal patella and patellar tendon during the repair. Autologous articular cartilage was harvested from the distal-third patella, which was excised during the partial patellectomy. The articular surface first was abraded by scraping with a surgical blade to remove the most superficial layer to expose the chondrocytes. Full-thickness articular cartilage then was harvested by tangential excision with a surgical blade, taking care to avoid lacerations in the graft. The average size of articular cartilage harvested measured 8.5 mm in width and 7.0 mm in length. The thickness of the cartilage graft averaged 0.80 mm (standard deviation, 0.073 mm). The harvested articular cartilage then was trimmed to the size of the partial patellectomy cross section. The articular cartilage was interposed between the proximal patellar segment and the patellar tendon, with the cut surface facing the patella. The articular cartilage was secured by passing the two ends of the Bunnell suture through the articular cartilage before they were passed into the patellar drill holes (Fig. 2). There was no dislodgement of cartilage observed on completion of the surgical repair.
Fig. 2A–B.
(A) The articular cartilage was interposed between the proximal patellar segment and the patellar tendon. The articular cartilage was secured by passing the two ends of the Bunnell suture through the articular cartilage before they were passed into the patellar drill holes. (B) The articular cartilage autograft was kept between the proximal patella segment and patellar tendon. A figure-of-eight tension band wiring protected the patella-to-patellar tendon repair.
A long-leg cast with the knee in resting position was applied for 6 weeks (Scotchcast™; 3 M Health Care, Borken, Germany). Intramuscular buprenorphine injection (5 μg/kg, Temgesic®; Schering-Plough, Welwyn Garden City, UK) was given as postoperative analgesia. A standard laboratory animal diet and water were provided ad libitum. The goats were housed individually in 4- × 5-m animal rings after the surgery and allowed free activity. All goats were able to resume full-weightbearing ambulation in a couple of days. Anteroposterior and lateral radiographs of the operated knees were taken immediately after surgery to ensure proper placement of the figure-of-eight tension band wiring.
The goats were euthanized at 6, 12, and 24 weeks after surgery by an overdose of intravenous 25% sodium pentobarbital (100 mg/kg intravenously). The figure-of-eight tension band wires were carefully removed and the patella-patellar tendon complexes were harvested. Samples for histologic studies were fixed in 4% paraformaldehyde solution and cut in the midsagittal section into medial and lateral halves with a hand saw. One half was sent for decalcified tissue processing and the other half for undecalcified tissue processing. Patella-patellar tendon complexes for mechanical tests were harvested together with a block of tibial tuberosity to facilitate fixation in a mechanical testing jig. Fresh samples were wrapped in normal saline gauze, sealed in a freezer bag, and stored in a −20°C freezer until mechanical testing.
Samples for undecalcified tissue processing were fixed and dehydrated and then embedded in methyl methacrylate. Serial 200-μm-thick sections were cut using a saw microtome (SP1600; Leica Instruments, Nussloch, Germany), mounted onto polyethylene slides, and polished down to 10 μm thick using a grinding machine (Rotopol-21; Struers, Rodovre, Denmark). The methyl methacrylate sections were stained with toluidine blue.
Samples for decalcified tissue processing were decalcified in 9% formic acid-formalin solution for 8 weeks. The formic acid-formalin solution was changed every week. The completeness of the decalcification process was determined by calcium oxalate test. The decalcified samples then were fixed and dehydrated in a Shandon Pathcentre® Tissue Processor (Thermo Electron Corp, Pittsburgh, PA) before paraffin embedding in a Tissue Embedding Center (Thermolyne Sybron, Dubuque, IA). Serial 7-μm-thick sagittal sections were cut with a microtome (RM2165; Leica). The mounted sections were stained in an automatic slide stainer (Shandon Varistain®; Thermo Electron Corp) with hematoxylin and eosin for general histologic evaluation [31].
Immunohistochemical localization of Type I and Type II collagen was performed with monoclonal antibodies (Lab Vision Co, Fremont, CA). Paraffin sections were deparaffinized and rehydrated. The rehydrated sections were immersed in 0.1% phenylhydrazine solution for 10 minutes to quench all endogenous hydrogen peroxidase activity. After rinsing with phosphate-buffered saline, trypsin digestion was performed, followed by hyaluronidase digestion. Nonimmune bovine serum was applied for 20 minutes at room temperature to block nonspecific antibody binding. The sections then were incubated with the primary antibody at 4°C overnight (dilutions at 1:100 for Type I collagen and 1:200 for Type II collagen). A StreptABComplex/HRP Duet, Mouse/Rabbit Kit (DAKO, Glostrup, Denmark) was used for color development. Biotinylated secondary antibody to rabbit IgG then was added. Streptavidin-biotin-horseradish peroxidase complex solution was applied to amplify the signals. Diaminobenzidine tetrahydrochloride was used as chromogen and applied until the sections were light brown. Once the signal appeared, the sections were counterstained with Mayer’s hematoxylin and then dehydrated in graded ethanol and xylene, followed by mounting. Negative controls were run in each case by omitting the primary antibody.
One researcher (MWNW) trained in histologic assessment evaluated the histologic samples and performed the histomorphometric analysis for all samples. All histologic sections were evaluated under light microscopy and polarizing microscopy using a Leica Q500MC microscope image analysis system (Leica Microsystems, Wetzlar, Germany). Four midsagittal sections were used for analysis. Sections were examined under low magnifications (×16 and ×40) for general histologic analysis, integration of cartilage graft to bone and tendon, and new bone formation. Cellular organization and the presence of fibrocartilage and basophilic line at the healing interface were examined under high magnifications (×100 and ×200). The images were captured with a digital video camera and digitized at a resolution of 3264 × 2448 pixels. The digitalized images were analyzed using an image analysis system (MetaMorph® 4.5; Universal Imaging Corp, Downingtown, PA) to quantify the lengths of fibrocartilage transition zone regeneration and basophilic line regeneration along the healing bone-tendon junction and the amount of bone formation after the repair. With segmental transition zone and basophilic line regeneration, the segmental lengths were summed together to obtain the total length. Percentage regeneration was calculated by dividing the lengths measured by the length of the individual healing junction, to adjust for size differences between samples.
Bone formation was measured by (1) the area, (2) the maximum length, and (3) the average length of bone formation. The original partial patellectomy level could be identified by the articular cut surface, which remained distinct. All bone distal to the original partial patellectomy level was defined as bone formation after partial patellectomy. The new junction between bone and patellar tendon was outlined as the healing bone-tendon junction. The area of bone formation was measured by the area between the partial patellectomy line and the healing junction. The maximum length of bone formation was measured by the longest perpendicular distance between the partial patellectomy line and the healing junction. The average length of bone formation was calculated by dividing the area of bone formation by the length of the partial patellectomy line.
For mechanical testing, the patella-patellar tendon complexes were thawed at 4°C for 24 hours. The anteroposterior and mediolateral widths of the healing bone-tendon junction interface were measured with a precision caliper (Davis Calibration, Baltimore, MD). Assuming a near-rectangular shape, the cross-sectional area of the healing interface was approximated by multiplying the anteroposterior width with the mediolateral width. The two ends of the patella-patellar tendon complexes were fixed onto a mechanical test machine (H25KM; Hounsfield Test Equipment, Redhill, UK) with two specially designed clamps at fixed distances from the healing bone-tendon junction. A 1-N tensile preload was applied. The load to failure tensile test was performed at a load speed of 50 mm per minute. The loading speed of 50 mm per minute corresponded to a strain rate of 3% per second on the patellar tendon. This strain rate was chosen as it is within physiologic range, and the mechanical properties of the bone-ligament-bone complex differed little between strain rates of 1% per second to 100% per second [34]. The resultant force displacement curves were recorded. Ultimate stress was calculated by dividing the failure load over cross-sectional areas of the specimens.
Differences in the amount of fibrocartilage transition zone regeneration and bone formation among the three times in each group were determined by the Kruskal-Wallis test, followed by the post hoc Bonferroni test. Differences in the amount of fibrocartilage transition zone regeneration and bone formation achieved at the same time between the two groups were determined using the Mann-Whitney U test. Quantitative data were analyzed using SPSS® Version 14.0 (SPSS Inc, Chicago, IL).
Results
There was no clinical failure observed in either group. Remnants of the autologous articular cartilage could be seen in some of the 6-week histologic sections, with good integration to adjacent tissues (Fig. 3). The interposed articular cartilage could not be identified in the 12-week and 24-week samples. Fibrocartilage transition zone regeneration can be well identified on hematoxylin and eosin-stained sections along the healing bone-tendon junction by the presence of chains of chondrocytes embedded in collagen fibers. Areas with fibrocartilage transition zone regeneration had increased collagen II stain and showed well-aligned collagen fibers under polarized microscopy. Collagen I stain was less than surrounding tendon tissue. No collagen II stain was detected in areas where tendon healed directly to bone by fibrous tissue. Fibrocartilage transition zone regeneration was absent in two 6-week and one 24-week direct repair samples. Even by 24 weeks, the percentage lengths of fibrocartilage transition zone formed remained less than 10% of the healing bone-tendon junction lengths (Fig. 4). Autologous articular cartilage interposition resulted in more fibrocartilage transition zone regeneration than direct repair at all times (Table 1) (Fig. 5). The mean (± standard deviation) length of fibrocartilage transition zone regenerated at 24 weeks measured 7760 ± 1665 μm with articular cartilage interposition, which was higher (p = 0.002) than the 787 ± 726 μm after direct repair. When the data were normalized with individual sample’s new bone-tendon junction length, articular cartilage interposition resulted in 69.10% ± 14.11% length with fibrocartilage, again higher (p = 0.002) than the 8.67% ± 7.01% after direct repair. Basophilic line formation was variable in both groups. The amount of basophilic line formation varied from none to 57% of the total new bone-tendon junction length. Two or three of the six samples from each time showed no basophilic line formation. This was true for the articular cartilage interposition group and the direct repair group.
Fig. 3.
A photomicrograph shows a 6-week sample with autologous articular cartilage interposition. A remnant of the interposed autologous articular cartilage (AC) can be seen in the bone (B)-tendon (T) interface (Stain, hematoxylin and eosin; original magnification, ×50). Bar = 1 mm.
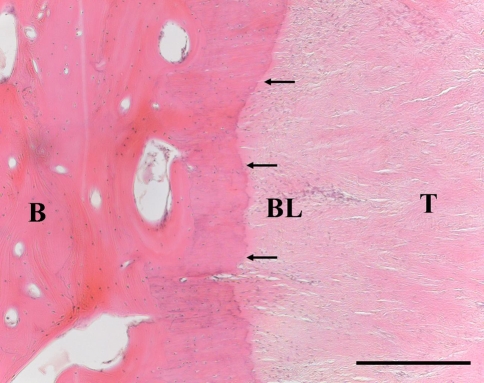
Fig. 4.
A photomicrograph shows a 24-week sample with direct repair. The bone (B)-tendon (T) junction healed with fibrous tissue, without fibrocartilage transition zone formation. A basophilic line (BL) can be seen (arrows) (Stain, hematoxylin and eosin; original magnification, ×100). Bar = 500 μm.
Table 1.
Fibrocartilage transition zone regeneration
| Measurement | Direct repair (n = 6) | Autologous articular cartilage interposition (n = 6) | p Value* |
|---|---|---|---|
| Length of fibrocartilage regenerated (μm) | |||
| 6 weeks | 910 ± 818 | 4938 ± 1126 | 0.010 |
| 12 weeks | 1747 ± 670 | 6833 ± 887 | 0.004 |
| 24 weeks | 787 ± 726 | 7760 ± 1665 | 0.002 |
| % length with fibrocartilage† | |||
| 6 weeks | 10.49 ± 9.64 | 62.24 ± 11.97 | 0.010 |
| 12 weeks | 19.20 ± 7.01 | 59.73 ± 14.43 | 0.004 |
| 24 weeks | 8.67 ± 7.01 | 69.10 ± 14.11 | 0.002 |
| Length of basophilic line formed (μm) | |||
| 6 weeks | 586 ± 981 | 809 ± 935 | 0.631 |
| 12 weeks | 326 ± 322 | 442 ± 502 | 0.740 |
| 24 weeks | 1384 ± 2358 | 1445 ± 1356 | 0.464 |
| % length with basophilic line† | |||
| 6 weeks | 7.19 ± 11.81 | 10.33 ± 12.22 | 0.631 |
| 12 weeks | 3.48 ± 3.28 | 3.39 ± 3.97 | 0.740 |
| 24 weeks | 13.95 ± 22.51 | 12.07 ± 11.33 | 0.642 |
Values are expressed as mean ± standard deviation; *Mann-Whitney test; †percentage values adjusted for size difference between samples.
Fig. 5.
A photomicrograph shows a 24-week sample with autologous articular cartilage interposition. Fibrocartilage (FC) formation with transition zone regeneration can be seen at the bone (B)-tendon (T) junction (Stain, hematoxylin and eosin; original magnification, ×100). Bar = 500 μm.
The area and the maximum length of bone formation increased with time in the autologous articular cartilage interposition group and the direct repair group (Table 2). The area of bone formation increased (p = 0.015) from 7.55 mm2 at 6 weeks to 20.15 mm2 at 24 weeks with articular cartilage interposition and increased (p = 0.005) from 11.15 mm2 at 6 weeks to 25.49 mm2 at 24 weeks in the direct repair group. The maximum bone length also increased with time after articular cartilage interposition (p = 0.011) and direct repair (p = 0.021). The area of bone formation, maximum bone length, and average bone length achieved at the same time did not differ between the groups.
Table 2.
Bone formation at the healing bone-tendon junction
| Measurement | Direct repair (n = 6) | Autologous articular cartilage interposition (n = 6) | p Value* |
|---|---|---|---|
| Area of bone formed (mm2) | |||
| 6 weeks | 11.15 ± 4.99 | 7.55 ± 3.94 | 0.201 |
| 12 weeks | 14.85 ± 5.61 | 23.72 ± 7.38 | 0.025 |
| 24 weeks | 25.49 ± 6.82 | 20.15 ± 7.14 | 0.180 |
| p value for difference with time | 0.005 | 0.015 | |
| Maximum new bone length (μm) | |||
| 6 weeks | 2182 ± 886 | 1470 ± 651 | 0.201 |
| 12 weeks | 2929 ± 974 | 3147 ± 790 | 0.522 |
| 24 weeks | 4175 ± 1370 | 3502 ± 949 | 0.406 |
| p value for difference with time | 0.021 | 0.011 | |
| Average new bone length (μm) | |||
| 6 weeks | 1635 ± 652 | 941 ± 500 | 0.055 |
| 12 weeks | 1876 ± 762 | 2413 ± 702 | 0.337 |
| 24 weeks | 2856 ± 1120 | 2351 ± 752 | 0.406 |
| p value for difference with time | 0.104 | 0.024 | |
Values are expressed as mean ± standard deviation; *Mann-Whitney test.
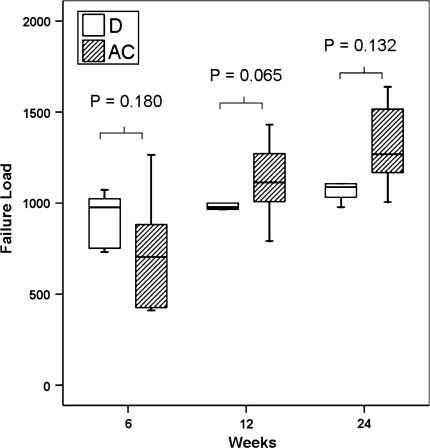
The ultimate failure load increased (p = 0.020) from 732 N at 6 weeks to 1310 N at 24 weeks after articular cartilage interposition and increased (p = 0.040) from 922 N at 6 weeks to 1113 N at 24 weeks after direct repair (Fig. 6). The ultimate stress did not show substantial change with time. There was no difference in ultimate failure load and ultimate stress achieved after articular cartilage interposition and after direct repair.
Fig. 6.
Box plots show the changes in ultimate failure load against time. The mechanical strength increased with time in both groups. There was no difference between the two groups. D = direct repair; AC = autologous articular cartilage interposition. Horizontal line = median; box = first and third quartiles; bars = 1.5× interquartile range.
Discussion
The fibrocartilage transition zone in direct bone-tendon junction reduces stress concentration, tearing, or shearing at the interface. The regeneration of fibrocartilage transition zone in bone-tendon junction healing is important to restore this unique protective mechanism. Unfortunately, normal bone-tendon junction healing often occurs without fibrocartilage transition zone regeneration. Increased fibrocartilage formation in the healing bone-tendon junction adjacent to the articular cartilage cut surface, with formation of a transition zone-like structure, has been reported [32]. The observation led to the hypothesis that articular cartilage could increase fibrocartilage transition zone regeneration in bone-tendon junction healing: the amounts of fibrocartilage transition zone regeneration, bone formation, and mechanical strength achieved with autologous articular cartilage interposition were studied and compared with direct repair.
This study has some limitations. Outcome assessment for histology in this study was performed on midsagittal sections. The healing bone-tendon junction is a three-dimensional structure, with a cross-sectional area and a depth. Accurate assessment of the amounts of bone formation and transition zone regeneration ideally would be performed in a three-dimensional manner. This is technically very difficult. There is no way to detect fibrocartilage formation inside an intact specimen, which is the subject of interest. The use of micro-CT theoretically can show bone microarchitecture and bone volume. However, as the junction heals, it is impossible to tell where old bone ends and new bone starts on micro-CT. However, delineation of this boundary could be ascertained accurately on histology sections, as the articular cut surface remained very well demarcated despite adjacent bone growth. It therefore was resolved to assess the outcome on histologic sections, knowing they were two-dimensional representations of the healing junction. One researcher assessed all the specimens and performed the histomorphometric analysis. Variability in assessment is minimized with one blinded observer but one observer can introduce systematic bias. The small sample size of the study was another possible source of error. Parameters with large variability may escape detection of differences. The reader needs to be aware of the chance of a Type 2 error for parameters that failed to show a difference.
The transition zone in a direct bone-tendon junction is marked by the presence of fibrocartilage. The direct repair group showed little fibrocartilage formation, indicating suboptimal regeneration of the transition zone. This finding was similar to that observed in tendon healing in a bone trough, in which there was reestablishment of continuity between bone and tendon but poor regeneration of the fibrocartilage zone [32]. The lack of fibrocartilage zone regeneration after partial patellectomy also was observed by Leung et al. and Qin et al. [17, 24] in rabbits. Previous anatomic studies in rat Achilles tendons and medial collateral ligaments have suggested fibrocartilage at the enthesis differentiate from tendon cells [13, 25]. It was proposed tenocytes lose contact with adjacent tenocytes, round up and enlarge, differentiating into chondrocytes. It is not known how these changes take place or how they are regulated. Tendon subjected to compressive force also develops fibrocartilage at the site of compression [30]. This metaplasia from tendon cells might explain how fibrocartilage forms after bone-tendon junction repair. However, our data suggest fibrocartilage regeneration after direct bone-tendon junction repair is limited in extent. Natural differentiation from tendon cells, if present, is not enough to restore a normal fibrocartilage transition zone.
Autologous articular cartilage interposition during bone-tendon junction repair substantially increased fibrocartilage transition zone regeneration. Articular cartilage is responsible for resisting compression, whereas fibrocartilage functions mainly to resist tension. The rounded chondrocytes residing in lacunae found in fibrocartilage resembles the chondrocytes in hyaline cartilage. However, the microstructural arrangement, extracellular matrix components, and mechanical functions of fibrocartilage differ from those of hyaline cartilage. The matrix of fibrocartilage contains coarse Type I collagen fibers arranged in thick bundles. Type II collagen is concentrated around the fibrocartilage cells, and its amount varies among anatomic sites [4]. The matrix in hyaline cartilage appears homogeneous, with collagen fibers arranged in a loose meshwork. Most of the collagen in articular cartilage is Type II. Mechanical loading plays an important role in the development, function, and repair of all tissues in the musculoskeletal system, including bone, tendon, ligament, and muscle. Connective tissue cells change their extracellular matrix according to the mechanical stress received [6, 28]. The exact mechanism and signaling pathways by which these adaptive changes occur are unknown. Altered mechanical stimulation causes chondrocytes to proliferate and to change matrix macromolecules and collagen-type synthesis [5, 20]. Articular cartilage defects often heal with fibrocartilage instead of hyaline cartilage [20]. Fibrocartilage formation is unwanted in articular cartilage resurfacing but actually desired in bone-tendon junction healing. The piece of autologous articular cartilage was removed from its normal environment of compression and placed in the healing junction, which was subjected to tensile force. It is uncertain whether the altered mechanical environment caused the piece of interposed articular cartilage to develop into fibrocartilage. It is also possible the regenerated fibrocartilage might have originated from bone, or from tendon, as proposed by Gao et al. and Ralphs et al. [13, 25] in enthesis formation. The interposed articular cartilage might have acted as a catalyst or a provider of mediators, instead of as a substance in the production of the fibrocartilage transition zone. The exact mechanism of action whereby the interposed articular cartilage resulted in increased fibrocartilage transition zone formation needs additional investigation and is beyond the scope of this study.
Bone formation increased with time in the direct repair group and autologous articular cartilage interposition group. Bone formation is reportedly an essential step in the process of bone-tendon junction healing [26]. The application of bone morphogenetic proteins (BMP) increases bone formation and mechanical strength of tendon healing inside the bone tunnel [19, 21, 27]. BMP-7, which is expressed in adult human, bovine, rabbit, and goat articular cartilage [7], promotes tendon-graft integration in anterior cruciate ligament reconstruction in sheep [21]. Insertion of autologous articular cartilage in direct bone-tendon junction repair in goats did not increase the amount of bone formed in our study. The amount of BMP-7 in normal articular cartilage is probably too low to affect the bone formation process.
Improved mechanical strength has been reported in association with increased bone formation in bone-tendon junction healing [14, 19, 21, 27]. In our study, bone formation did not differ between the two groups. The structural improvement as evidenced by increased fibrocartilage transition zone regeneration after autologous articular cartilage interposition did not result in a corresponding increase in mechanical strength. The failure stress in either group only reached 10% of previously published failure stress of the normal goat patella-patellar tendon complex [32]. Mechanical properties other than ultimate failure load and failure stress have not been tested. It is not known whether soft tissue mechanical properties, eg, stress relaxation and viscoelasticity, are improved by the presence of increased fibrocartilage transition zone regeneration.
Partial patellectomy is an ideal model for autologous articular cartilage interposition as the articular cartilage graft can be harvested from the discarded distal-third patella segment with no additional donor site morbidity. There are few other situations as ideal as partial patellectomy in providing a readily available articular cartilage autograft nearby with no donor site morbidity. In most situations of bone-tendon repair, a separate cartilage donor site would be required, with subsequent risk of donor site morbidity. Additional research is required to investigate the mechanism of increased fibrocartilage regeneration in bone-tendon junction healing, and to establish the applicability of autologous articular cartilage interposition in clinical settings.
Acknowledgments
We thank J. Tai and W. Chong for assistance in animal surgery and sample processing.
Footnotes
One or more of the authors (MWNW, LQ, KSL) have received funding from Research Grants Council Earmarked Grant (Ref. CUHK 427597M).
Each author certifies that his or her institution has approved the animal protocol for this investigation and that all investigations were conducted in conformity with ethical principles of research.
References
- 1.Aoki M, Oguma H, Fukushima S, Ishii S, Ohtani S, Murakami G. Fibrous connection to bone after immediate repair of the canine infraspinatus: the most effective bony surface for tendon attachment. J Shoulder Elbow Surg. 2001;10:123–128. [DOI] [PubMed]
- 2.Benjamin M, Evans EJ. Fibrocartilage: a review. J Anat. 1990;171:1–15. [PMC free article] [PubMed]
- 3.Benjamin M, Newell RL, Evans EJ, Ralphs JR, Pemberton DJ. The structure of the insertions of the tendons of biceps brachii, triceps and brachialis in elderly dissecting room cadavers. J Anat. 1992;180:327–332. [PMC free article] [PubMed]
- 4.Benjamin M, Ralphs JR. Fibrocartilage in tendons and ligaments: an adaptation to compressive load. J Anat. 1998;193:481–494. [DOI] [PMC free article] [PubMed]
- 5.Buckwalter J, Mow VC, Ratcliffe A. Restoration of injured or degenerated articular cartilage. J Am Acad Orthop Surg. 1994;2:192–201. [DOI] [PubMed]
- 6.Chiquet M. Regulation of extracellular matrix gene expression by mechanical stress. Matrix Biol. 1999;18:417–426. [DOI] [PubMed]
- 7.Chubinskaya S, Kuettner KE. Regulation of osteogenic proteins by chondrocytes. Int J Biochem Cell Biol. 2003;35:1323–1340. [DOI] [PubMed]
- 8.Cooper RR, Misol S. Tendon and ligament insertion: a light and electron microscopic study. J Bone Joint Surg Am. 1970;52:1–20. [PubMed]
- 9.Crowninshield RD, Pope MH. The strength and failure characteristics of rat medial collateral ligaments. J Trauma. 1976;16:99–105. [DOI] [PubMed]
- 10.Evans EJ, Benjamin M, Pemberton DJ. Fibrocartilage in the attachment zones of the quadriceps tendon and patellar ligament of man. J Anat. 1990;171:155–162. [PMC free article] [PubMed]
- 11.Frowen P, Benjamin M. Variations in the quantity of uncalcified fibrocartilage at the insertion of the extrinsic calf muscles in the foot. J Anat. 1995;186:417–421. [PMC free article] [PubMed]
- 12.Galatz LM, Sandell LJ, Rothermich SY, Das R, Mastny A, Havlioglu N, Silva MJ, Thomopoulos S. Characteristics of the rat supraspinatus tendon during tendon-to-bone healing after acute injury. J Orthop Res. 2006;24:541–550. [DOI] [PubMed]
- 13.Gao J, Messner K, Ralphs JR, Benjamin M. An immunohistochemical study of enthesis development in the medial collateral ligament of the rat knee joint. Anat Embryol. 1996;194:399–406. [DOI] [PubMed]
- 14.Huangfu X, Zhao J. Tendon-bone healing enhancement using injectable tricalcium phosphate in a dog anterior cruciate ligament reconstruction model. Arthroscopy. 2007;23:455–462. [DOI] [PubMed]
- 15.Hung LK, Lee SY, Leung KS, Chan KM, Nicholl LA. Partial patellectomy for patellar fracture: tension band wiring and early mobilization. J Orthop Trauma. 1993;7:252–260. [DOI] [PubMed]
- 16.Leddy JP. Flexor tendons: acute injuries. In: Green DP, ed. Green’s Operative Hand Surgery. 5th ed. Philadelphia, PA: Elsevier/Churchill Livingstone; 2005:219–276.
- 17.Leung KS, Qin L, Fu LK, Chan CW. A comparative study of bone to bone repair and bone to tendon healing in patella-patellar tendon complex in rabbits. Clin Biomech. 2002;17:594–602. [DOI] [PubMed]
- 18.Liu SH, Hang DW, Gentili A, Finerman GA. MRI and morphology of the insertion of the patellar tendon after graft harvesting. J Bone Joint Surg Br. 1996;78:823–826. [PubMed]
- 19.Ma CB, Kawamura S, Deng XH, Ying L, Schneidkraut J, Hays P, Rodeo SA. Bone morphogenetic proteins-signaling plays a role in tendon-to-bone healing: a study of rhBMP-2 and noggin. Am J Sports Med. 2007;35:597–604. [DOI] [PubMed]
- 20.Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–466. [PubMed]
- 21.Mihelic R, Pecina M, Jelic M, Zoricic S, Kusec V, Simic P, Bobinac D, Lah B, Legovic D, Vukicevic S. Bone morphogenetic protein-7 (osteogenic protein-1) promotes tendon graft integration in anterior cruciate ligament reconstruction in sheep. Am J Sports Med. 2004;32:1619–1625. [DOI] [PubMed]
- 22.Noyes FR, DeLucas JL, Torvik PJ. Biomechanics of anterior cruciate ligament failure: an analysis of strain-rate sensitivity and mechanisms of failure in primates. J Bone Joint Surg Am. 1974;56:236–253. [PubMed]
- 23.Oguma H, Murakami G, Takahashi-Iwanaga H, Aoki M, Ishii S. Early anchoring collagen fibers at the bone tendon interface are conducted by woven bone formation: light microscope and scanning electron microscope observation using a canine model. J Orthop Res. 2001;19:873–880. [DOI] [PubMed]
- 24.Qin L, Leung KS, Chan CW, Fu LK, Rosier R. Enlargement of remaining patella after partial patellectomy in rabbits. Med Sci Sports Exerc. 1999;31:502–506. [DOI] [PubMed]
- 25.Ralphs JR, Benjamin M, Wagget AD, Russell DC, Messner K, Gao J. Regional differences in cell shape and gap junction expression in rat Achilles tendon: relation to fibrocartilage differentiation. J Anat. 1998;193:215–222. [DOI] [PMC free article] [PubMed]
- 26.Rodeo SA, Arnoczky SP, Torzilli PA, Hidaka C, Warren RF. Tendon-healing in a bone tunnel: a biomechanical and histological study in the dog. J Bone Joint Surg Am. 1993;75:1795–1803. [DOI] [PubMed]
- 27.Rodeo SA, Suzuki K, Deng XH, Wozney J, Warren RF. Use of recombinant human bone morphogenetic protein–2 to enhance tendon healing in a bone tunnel. Am J Sports Med. 1999;27:476–488. [DOI] [PubMed]
- 28.Sarasa-Renedo A, Chiquet M. Mechanical signals regulating extracellular matrix gene expression in fibroblasts. Scand J Med Sci Sports. 2005;15:223–230. [DOI] [PubMed]
- 29.Suresh S. Graded materials for resistance to contact deformation and damage. Science. 2001;292:2447–2451. [DOI] [PubMed]
- 30.Vogel KG, Koob TJ. Structural specialization in tendons under compression. Int Rev Cytol. 1989;115:267–293. [DOI] [PubMed]
- 31.Wilson I, Gamble M. The hematoxylins and eosin. In: Bancroft JD, Gamble M, eds. Theory and Practice of Histological Techniques. 6th ed. London, UK: Churchill Livingstone; 2007:135–136.
- 32.Wong MW, Qin L, Lee KM, Tai KO, Chong WS, Leung KS, Chan KM. Healing of bone-tendon junction in a bone trough: a goat partial patellectomy model. Clin Orthop Relat Res. 2003;413:291–302. [DOI] [PubMed]
- 33.Woo SL, Buckwalter JA. Injury and repair of the musculoskeletal soft tissues. J Orthop Res. 1988;6:907–931. [DOI] [PubMed]
- 34.Woo SL, Peterson RH, Ohland KJ, Sites TJ, Danto MI. The effects of strain rate on the properties of the medial collateral ligament in skeletally immature and mature rabbits: a biomechanical and histological study. J Orthop Res. 1990;8:712–721. [DOI] [PubMed]