Abstract
In intact adult mammalian brains, there are two neurogenic regions: the subependymal zone and the subgranular layer of the hippocampus. Even outside these regions, small numbers of proliferating precursors do exist. Many studies suggest that the majority of these are oligodendrocyte precursors that express NG2, a chondroitin sulfate proteoglycan, and most of the residual proliferating cells seem to be endothelial cells. However, it is still unclear whether NG2-immunonegative proliferating precursors are present, because previous studies have neglected their possible existence. In this study, we systematically analyzed the phenotypes of the proliferating cells in the intact adult rat cortical gray matter. We improved our techniques and carefully characterized the proliferating cells, because there were several problems with identifying and quantifying the proliferating cells: the detection of NG2-expressing cells was dependent on the fixation condition; there were residual proliferating leukocytes in the blood vessels; and two anti-NG2 antibodies gave rise to different staining patterns. Moreover, we used two methods, BrdU and Ki67 immunostaining, to quantify the proliferating cells. Our results strongly suggest that in the intact adult cerebral cortical gray matter, there were only two types of proliferating cells: the majority were NG2-expressing cells, including pericytes, and the rest were endothelial cells.
Keywords: NG2, adult brain, precursor, quantification
I. Introduction
There are two neurogenic sites in the adult mammalian brain, the subependymal zone (SEZ) around the lateral ventricle and the subgranular layer of the hippocampal dentate gyrus (SGL) [26]. Although normally there is no neurogenesis in the adult cerebral cortical gray matter, recently it has been suggested that neurogenesis can be induced under special conditions, i.e. animal models with specific lesions [25], animal models of ischemia [3, 27], and even in normal animals [9, 11, 15]. However, the presence of neurogenesis under normal conditions is still controversial [18, 19]. In animal models of stroke, at least some newborn neurons might be recruited from the SEZ [37], but it is possible that there are other sources of new neurons in the brain parenchyma [3].
There are proliferating cells in the parenchyma in the normal adult mammalian brain. The majority of them are oligodendrocyte precursors that express NG2, one of the chondroitin sulfate proteoglycans [8, 14]. Normally, NG2 immunopositive (+)-cycling cells differentiate into mature oligodendrocytes over a long period of time [34, 35]. Almost all adult cortical NG2(+) cells express Olig2 [23], a basic helix-loop-helix transcription factor and a critical gene for oligodendrocyte development [24, 33, 39].
In normal adult brains, endothelial cells also proliferate, but they comprise a much smaller population than NG2(+) cells [36]. It is still not clear what proportion of proliferating cells are NG2(+) or endothelial cells. Dawson et al. [8] reported that about 77% of proliferating cells in the adult cerebral cortex were NG2(+), and the majority of the residual proliferating cells were endothelial cells. Other studies suggest that more than 90% of all proliferating cells are NG2(+) [1, 9]. Tamura et al. [35] found that more than 95% of proliferating cells were NG2(+), with the exception of vascular cells. These findings are consistent with the idea that the majority of proliferating cells are NG2(+) cells, but there is the possibility that NG2-immunonegative (−) proliferating cells are also present. Indeed, Buffo et al. [6] reported that about 25% of proliferating cells are NG2(−) and unidentified cells. Also, in the adult basal ganglia and spinal cord, about 50-90% of cells were 5-bromo-2’-deoxyuridine (BrdU)-incorporated NG2(+) cells and endothelial cells [12, 13, 36]. It has also been suggested that unidentified cycling cells exist in dissociated adult brain cultures [10]. Stem cells always comprise a small proportion of the cells in various types of adult tissue [21, 22], but a perfect marker for stem cells is not yet available. Do unidentified proliferating cells exist in the normal brain? If there are small numbers of unidentified proliferating precursors in the brain parenchyma, it is possible that NG2(+) cells may make up a subpopulation of oligodendrocyte precursors: unidentified proliferating cells can be derived from NG2(+) cells, and vice versa; or, alternatively, the cells could make up an independent population, such as neural precursors. Indeed, neural precursors have been isolated from adult brain parenchyma [29].
The purpose of this study was to systematically characterize the proliferating cells in the intact brain parenchyma by optimizing tissue fixation and immunostaining conditions, testing antibody specificity, and establishing criteria for proliferating cells in the brain parenchyma.
II. Materials and Methods
Subjects
Eight-week-old male Wistar rats and 6-week-old male C57BL/6 mice were used. All experimental protocols were approved by the animal ethics committee of Kansai Medical University and were performed in accordance with the Principles of Laboratory Animal Care (NIH publication no. 85-23, revised 1985).
BrdU administration
BrdU (Sigma, St. Louis, MO, USA) was used to detect proliferating cells. BrdU is a thymidine analogue which is incorporated into the genomic DNA of proliferating cells during the S phase. To label the maximum number of proliferating cells and to achieve maximal labeling intensity in the short-term pulse labeling paradigm, 50 mg/Kg of BrdU was injected intraperitoneally (IP) every hour for 5 hours (total 250 mg/Kg) and animals were sacrificed 1 hour after the last injection. We adopted this time frame because the G2 and M phases of the glial precursors in the adult mouse brain were reported to be about 5 hours long [20, 31]. Under this BrdU administration protocol, almost all of the BrdU(+) cells in the cortical gray matter were single cells, and 2.70±0.85% (mean±S.E.M., n=3) of all the BrdU(+) cells were twin cells. Thus, virtually all the BrdU(+) cells highlighted using this administration protocol were in the cell cycle. Twin BrdU(+) cells were counted as single cells, because it is reasonable that they were just exiting the cell cycle [15]. In the long-term pulse labeling paradigm, 50 mg/Kg of BrdU was injected by IP 3 times a day (9:00 A.M., 15:00 P.M., and 21:00 P.M.) for either 3 days or 7 days, and the animals were sacrificed 5 hours following the last injection.
Histological procedure
For histological analysis, rats were deeply anesthetized with pentobarbital (50 mg/Kg), and then they were perfused transcardially with ice-cold phosphate buffered saline (PBS) followed by formaldehyde in PBS. To optimize fixation condition, 1, 2, 3, or 4% of formaldehyde in PBS were used (Fig. 1). The brains were removed and further fixed with the same fixative overnight, then cryo-protected with 20% sucrose in PBS. Brains were embedded in O.C.T. compound (Sakura Finetek, Tokyo, Japan), snap frozen in dry ice, sectioned at 30 µm with a cryostat, and processed for immunohistochemistry.
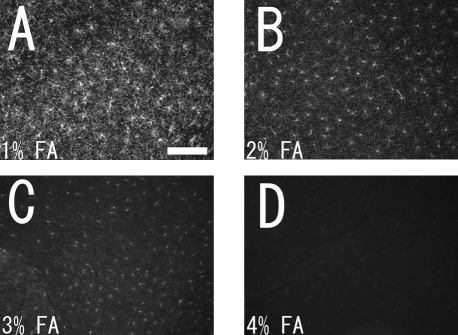
Fig. 1.
Photomicrographs showing NG2 immunopositive cells in the intact adult mouse cortex. Intact adult mice were fixed with 1% (A), 2% (B), 3% (C), and 4% (D) formaldehyde (FA), and labeled with rabbit polyclonal anti-NG2 antibody. Images were taken with a epifluorescence microscope in the cortical gray matter. Bar=0.2 mm.
Free floating sections were labeled using the following primary antibodies: rat anti-BrdU (1:200, Abcam, Cambridge, UK); rabbit anti-Ki67 (1:1,000, Novocastra, Newcastle, UK); rabbit anti-NG2 (1:500, Chemicon, Temecula, CA, USA); mouse anti-NG2 (1:200, Chemicon); mouse anti-RECA-1 (1:2,000, Serotec, Oxford, UK); rabbit anti-Iba-1 (1:1,000, Wako, Osaka, Japan); rabbit anti-glutamine synthetase (1:20,000, Sigma); mouse anti-S100β (1:1,000, Sigma); mouse anti-CD45 (1:3,000, Biolegend, San Diego, CA, USA); goat anti-Olig2 (1:400, Santa Cruz Biotechnology, Santa Cruz, CA, USA); and mouse anti-NeuN antibody (1:6,000, Chemicon). Primary antibodies were detected by species-specific donkey secondary antibodies conjugated to Cy2, Cy3, or Cy5 (1:200, Jackson ImmunoResearch, West Grove, PA, USA), or Alexa488 (1:400, Invitrogen, Carlsbad, CA, USA). After staining, sections were mounted on glass slides with a medium containing 100 mM DTT, 50% glycerol, and 5 µg/ml Hoechst 33258 to visualize nuclei. 0.3% PBST (PBS containing 0.3% Triton-X 100) was used for antibody dilution and to wash sections. For BrdU immunohistochemistry, sections were pre-treated with 2 N HCl for 30 min at room temperature, followed by neutralization with 0.1 M boric acid. We compared two methods for double-staining combining the anti-BrdU antibody with another antibody. First, after pre-treatment, sections were incubated with anti-BrdU antibody and another primary antibody simultaneously, followed by incubation of a secondary antibody cocktail (one-step staining). Second, sections were incubated with another primary antibody followed by a secondary antibody, fixed with 4% formaldehyde in PBS, pre-treated, and incubated with anti-BrdU antibody followed by an anti-rat secondary antibody (two-step staining) [16]. Fluorescence images were acquired using a confocal microscope (LSM510-Meta, Carl Zeiss, Oberkochen, Germany).
Quantification
Cell counting analysis was performed using an epifluorescence microscope with a ×40 objective (Eclipse-E1000M, Nikon, Japan). Coronal brain sections were selected between +0.2 mm to −2.8 mm from the bregma, and quantification was performed from layers 1 to 6 in the neocortical gray matter of the motor cortex, somatosensory cortex, and insular cortex, but excluding the cingulate cortex and piriform cortex.
III. Results
Improvement and optimization of histochemical analysis
Before quantifying the proliferating cells, in order to acquire precise data we examined and improved on the following four technical points described below. First, we found that NG2 immunostaining was very sensitive to the concentration of formaldehyde used during perfusion. When the concentration of formaldehyde was reduced, NG2 immunostaining was dramatically improved, especially in the adult mouse brains (Fig. 1). Even in the adult rat brains, this staining problem sometimes happened. Because of this, in this study rats were also fixed with a mild fixative (2% formaldehyde).
Second, we established quantification criteria for proliferating cells. We found some residual proliferating leukocytes inside the blood vessels even after rats were perfused (Fig. 2). In the intact adult brains, proliferating NG2(+) cells were few. Although the absolute number of proliferating leukocytes was small, these were excluded from quantification to acquire precise data. We used anti-BrdU antibody raised in rats to detect proliferating cells in the rat brain. This was an advantage, because a subset of the rat leukocytes was non-specifically labeled by anti-rat secondary antibody, and these were easily recognized by the staining patterns. Non-specific stained cells appeared larger than their nuclei, and this staining pattern was also detected on the negative control sections incubated with only anti-rat secondary antibody. Most of these cells were clearly located inside the blood vessels. Sometimes they appeared to be enclosed by the nuclei of endothelial cells with tubular morphology (Fig. 2), and their nuclei had a very complex morphology, with several segments and/or foldings. We characterized these as leukocytes using anti-CD45 antibody, a marker for pan-leukocytes [32] (Fig. 2).
Fig. 2.
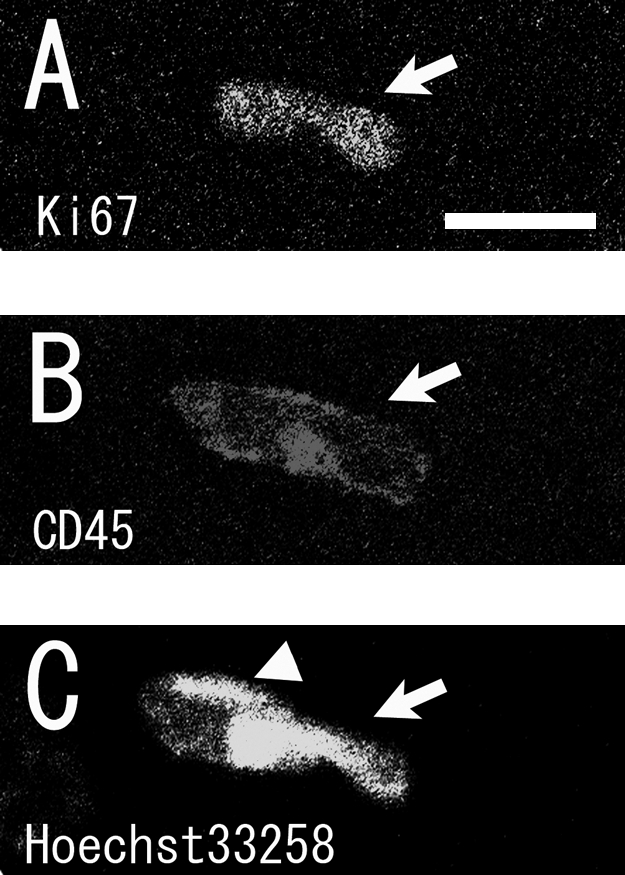
Single optical confocal microscopy images showing examples of proliferating leukocytes in the blood vessels. A Ki67(+) proliferating cell (A) expressing CD45 (B) (arrows in A–C) was clearly visible inside the blood vessel. (C) indicates the nuclei stained by Hoechst 33258. The arrowhead in (C) shows the nucleus of an endothelial cell with tubular morphology. Bar=10 µm.
Third, we examined the double immunostaining procedure, which combines anti-BrdU antibody with another antibody. Several research groups claim that a two-step staining method is required to minimize the reduced signal intensity of another antigen by pre-treating sections with HCl [16, 30], but other studies have used a one-step staining method [8, 35]. When we compared these two staining methods during double staining with anti-BrdU antibody and anti-Ki67 antibody (a marker for proliferating cells), Ki67 immunoreactivity was clearly lost during the one-step staining method (Table 1). Because all the BrdU(+) cells were cycling cells under our short-term pulse labeling protocol, all of these should be Ki67(+). However, when we characterized the phenotype of BrdU(+) cells using cell type-specific makers, we did not find a significant difference in the number of NG2(+) cells that were labeled by rabbit polyclonal anti-NG2 (rb-NG2) antibody, but found a slight improvement in the numbers of RECA-1(+) endothelial cells, Iba-1(+) microglia, and glutamine synthetase/S100β(+) astrocytes (Table 1). To detect astrocytes, a cocktail of anti-glutamine synthetase antibody and anti-S100β antibody was used, because astrocytes are highly heterogeneous, and a single marker cannot label all astrocytes [17].
Table 1.
Phenotypes of BrdU(+) proliferating cells in the intact cortical gray matter of the adult rat brain
| % of BrdU(+) | Ki67(+) | rb-NG2(+) | ms-NG2(+) | RECA-1(+) | Iba-1(+) | GS/S100β(+) | Olig2(+) |
|---|---|---|---|---|---|---|---|
| One-step staining | 92.39±1.15 | 89.56±1.43 | N.A. | 5.42±0.08 | 0 | 0 | 95.39±1.91 |
| Two-step staining | 100 | 89.02±0.68 | 91.78±1.78 | 8.28±1.82 | 0.51±0.51 | 0.93±0.93 | 93.83±0.82 |
All data were collected from 3 rats (n=3). Data are reported as mean±S.E.M. N.A.=not analyzed; GS=glutamine synthetase.
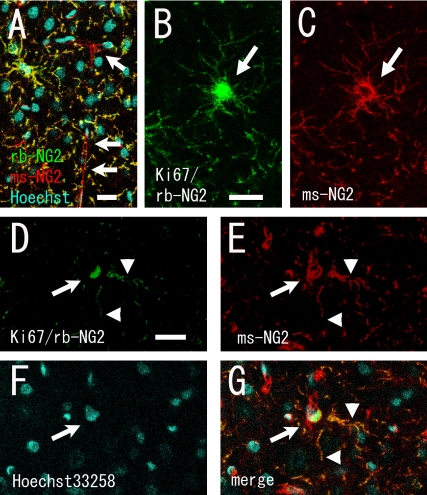
Fourth, we tested the specificity of two anti-NG2 antibodies. We found clear differences in staining intensity in the pericytes when we compared rb-NG2 antibody and mouse anti-NG2 (ms-NG2) antibody. Pericytes have fewer processes and extend their processes along the blood vessels, and NG2 is one of the markers for pericytes [28]. Pericytes were poorly stained by rb-NG2 antibody, but both antibodies equally stained the majority of NG2(+) cells with stellate morphology, which are known as synantocytes [7] (Fig. 3A–C). When we compared the quantification results for the BrdU(+) cells between the rb-NG2 and ms-NG2 antibodies in the two-step staining protocol, the ratio of BrdU(+)/ms-NG2(+) cells was slightly higher than that of BrdU(+)/rb-NG2(+) cells (Table 1). This result was supported by the results of the quantification of the number of ms-NG2(+) or RECA-1(+) cells among the Ki67(+) cells (Table 2). These results suggest that a few proliferating pericytes were present. Indeed, we were able to detect Ki67(+)/rb-NG2(−)/ms-NG2(+) proliferating pericytes (Fig. 3D–G). This finding of the presence of a small number of proliferating pericytes is consistent with another previous report [36].
Fig. 3.
Single optical confocal microscopy images showing differential signal intensities in pericytes between polyclonal and monoclonal anti-NG2 antibodies. (A) Pericytes (arrows) were only poorly stained by rabbit polyclonal anti-NG2 antibody (rb-NG2, green), but were stained intensely by mouse monoclonal anti-NG2 antibody (ms-NG2, red). A merged image is shown. (B–G) To detect Ki67(+)/rb-NG2(−)/ms-NG2(+) pericytes, a cocktail of anti-Ki67/rb-NG2/ms-NG2 antibodies was used. Ki67 and rb-NG2 were visualized in green and ms-NG2 in red. (B, C) Rb-NG2 and ms-NG2 antibodies equally labeled Ki67(+) proliferating cells with stellate morphology. (B) and (C) show a Ki67(+)/rb-NG2(+)/ms-NG2(+) cell (arrows). Note that the Ki67 protein localizes to the nucleus (B), but the NG2 signal is localized to the surface of the cell, and is not present in the nucleus (C). (D–G) An example of a small number of rb-NG2(−)(green)/ms-NG2(+) (red) pericytes in the cell cycle (Ki67(+), green; arrows). The arrowheads indicate the processes of another NG2(+) cell with stellate morphology. Bars=20 µm.
Table 2.
Phenotypes of Ki67(+) cells in the intact cortical gray matter of the adult rat brain
| % of Ki67(+) | ms-NG2(+) | RECA-1(+) |
|---|---|---|
| 92.80±0.93 (n=6) | 8.38±0.43 (n=3) |
± represents S.E.M.
Quantification and characterization of proliferating cells
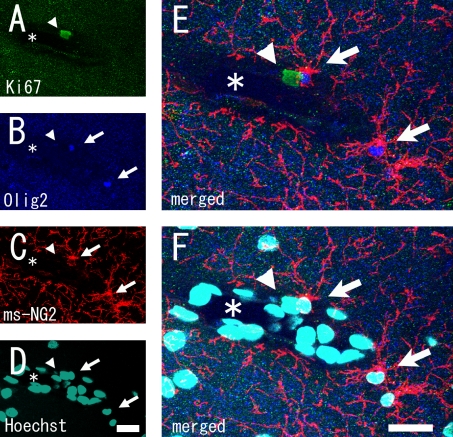
After we optimized and improved these conditions, we were not able to find NG2(−) proliferating cells except proliferating endothelial cells in the intact adult cortical gray matter (Tables 1 and 2). The sum of proliferating NG2(+) cells, including pericytes and proliferating RECA-1(+) cells, corresponded to the overall number of proliferating cells. To confirm this, we examined Olig2 expression in the proliferating cells. The ratio of Olig2(+) proliferating cells was almost the same as the ratio of NG2(+) proliferating cells (Table 1). The morphology of any residual Olig2(−) proliferating cells resembled either pericytes or endothelial cells (Fig. 4).
Fig. 4.
Stacked confocal microscopy images showing proliferating endothelial cell. Stacked images of (A) Ki67, (B) Olig2, (C) NG2, and (D) Hoechst 33258 staining. (E) and (F) are merged images of (A–C) and (A–D) respectively. Note that a Ki67(+) proliferating endothelial cell was Olig2(−) (arrowheads). Arrows indicate ms-NG2(+)/Olig2(+) cells. Asterisks indicate a large blood vessel. Bars=20 µm.
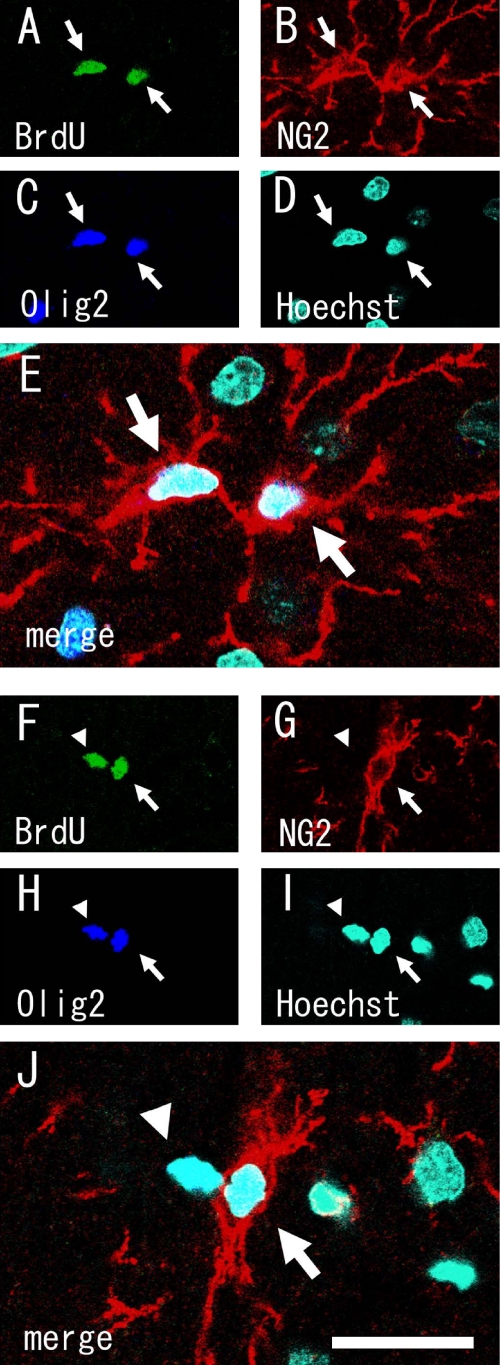
Finally, we analyzed the phenotypes of the precursors and their descendants in long-term BrdU pulse-labeled brains. After 3 days, in BrdU-labeled rat brains most of the BrdU(+) cells were twins, suggesting that one precursor had divided into two cells. The number of the BrdU(+) cells that were NG2(+) was not markedly different from that found in the short-pulse labeling experiment (92.2 ± 0.01%, mean ± S.E.M., n=2, with ms-NG2 antibody). Again, we were not able to find a large population of BrdU(+)/NG2(−) cells, as had been described previously [6]. Even in a 7-day BrdU labeling experiment, we did not find a large number of BrdU(+)/NG2(−) cells, although there was a trend toward an increased number of BrdU(+)/NG2(−) cells (preliminary data not shown). Because some divided NG2(+) cells differentiate into oligodendrocytes very slowly [34, 35] with down-regulating NG2 expression level, and some are eliminated by cell death [8, 20], it is possible that almost all of the BrdU(+)/NG2(−) cells differentiate into oligodendrocytes. In fact, most BrdU(+) twin cells were BrdU(+)/NG2(+)/Olig2(+), while in some of the BrdU(+) twin cells, one was NG2(+)/Olig2(+) but the other was NG2(−)/Olig2(+) (Fig. 5).
Fig. 5.
Single optical confocal microscopy images showing two types of twin BrdU(+) cells in 7-day labeled brain. Triple immunostaining was done for BrdU (A, F), rb-NG2 (B, G), Olig2 (C, H), and Hoechst 33258 (D, I). (E) and (J) are merged images. Most BrdU(+) twin cells were BrdU(+)/NG2(+)/Olig2(+) (arrows, A–E). While in small number of BrdU(+) twin cells, one was NG2(−) (arrowheads, F–J), but both cells expressed oligodendrocyte lineage marker, Olig2 (F–J). These data suggest that BrdU(+)/NG2(−) cells are differentiating into mature oligodendrocytes. Bar=20 µm.
IV. Discussion
In this study, we systematically quantified and characterized proliferating cells using immunohistochemical techniques. We did not neglect the small number of proliferating pericytes and leukocytes, which had been largely ignored in previous studies. To our knowledge, this is the first time that proliferating cells in adult cortical gray matter have been precisely quantified.
In most immunohistochemical studies, 4% formaldehyde is used to fix tissues. We found that the fixation condition was critical for detecting NG2(+) cells. A mild fixative was necessary for NG2 staining, but did not make an obvious difference to the other antigens used in the present study. Although mild fixatives tend to spoil the fine structure of sections, the conditions have to be adjusted depending on the antigens being used.
Characterizing proliferating cells using anti-BrdU or anti-Ki67 antibodies was difficult because these antibodies only stained the nuclei. It is impossible to remove all of the leukocytes from the blood vessels by perfusion, and we found a substantial number of CD45(+) proliferating leukocytes remained after perfusion. The nuclei of leukocytes have a complex morphology with numerous segments and/or foldings, and it was easy to identify leukocytes by staining the nuclei with Hoechst 33258. However, it was sometimes difficult to completely discriminate proliferating residual leukocytes from other cells in the brain parenchyma, because, depending on the slice angle, some leukocytes inside the blood vessels appeared to have round nuclei. Such cells might result in the false-positive identification of proliferating cells. In this study, we used anti-BrdU antibody raised in rats to exclude proliferating leukocytes from quantification in the rat sections. This method was the easiest and most effective way to precisely quantify the number of BrdU(+) cells with immunohistochemical analysis.
BrdU is a reliable tracer for the identification of cell proliferation by immunohistochemistry [4]. However, detecting BrdU by this method always requires the pre-treatment of sections with HCl. In double staining, which combines anti-BrdU antibody with another antibody, this process might reduce the immunoreactivity of the other antigen in the one-step staining method, or may dissociate the antigen-antibody complex in the two-step staining method, resulting in signal reduction. Our results indicate that the two-step staining method was better than the one-step method for double immunostaining. The anti-Ki67 antibody used in this study does not require antigen retrieval treatment, unlike other commercially available anti-Ki67 antibodies. Although using this anti-Ki67 antibody might be the mildest and best way to detect proliferating cells in the double immunostaining method, it is sometimes difficult to discriminate proliferating leukocytes, as discussed above.
We found differential staining intensities in pericytes when stained with rb-NG2 and ms-NG2 antibodies. This may derive from the different immunogens used in antibody production. This led to the incompatibility between the quantification results of the two anti-NG2 antibodies. Even though the number of proliferating pericytes was small, for the sake of precision they should not be neglected.
The present study strongly suggests that there were no NG2(−) proliferating precursors in the intact adult rat cortical gray matter. We cannot completely exclude the possibility that NG2(−) proliferating precursors exist in the brain parenchyma, but the likelihood that they are present is very low. Olig2 was expressed in more than 90% of proliferating cells in the intact brain. This value fits well with the ratio of cycling NG2(+) cells. Olig2 is known to be exclusively expressed in NG2(+) cells with stellate morphology [23], but not in endothelial cells or pericytes (unpublished observation). Thus, virtually all the proliferating cells except pericytes and endothelial cells were NG2(+)/Olig2(+) cells.
Except in the SEZ and GCL, astrocytes do not exhibit the properties of neural stem cells in the intact adult brain. However, it was reported that a subset of quiescent astrocytes respond to injury, begin to proliferate, and contribute to glial scar formation [5] in concert with NG2(+) cells [2]. Notably, the astrocytes that respond to injury exhibit neural stem cell properties in in vitro analysis, but this is not the case with NG2(+) cells [5]. In addition, a subset of microglia can acquire multipotency in specific culture conditions [38]. Based on our quantification analyses, there was a small proportion of BrdU-labeled astrocytes and microglia, but no reports have suggested that astrocytes or microglia can transform or differentiate into NG2(+) cells in intact adult brains in vivo. Throughout our analyses, there were no BrdU(+)/NeuN(+) neurons, even during the long BrdU pulse-labeling paradigm. Thus, NG2(+) cells were the only proliferating precursor population in the intact adult cortical gray matter. Recently it was suggested that NG2(+) cells might differentiate into neurons in the intact adult cortex [9]. Neurogenesis in the adult cortex is still controversial [18, 19], and further studies are needed to clarify this possibility.
In conclusion, in the adult rat cortical gray matter, there were just two populations of proliferating cells: the majority were NG2(+) cells, including a small number of pericytes, and the rest were endothelial cells.
V. Acknowledgements
This study was supported in part by Grants-in-Aid for Scientific Research (C) from the Ministry of Education, Culture, Sports, Science and Technology of Japan to T. M. (19500281) and H. Y. (19890199).
Footnotes
The authors declare that they have no competing financial interests.
VI. References
- 1.Alonso G. Prolonged corticosterone treatment of adult rats inhibits the proliferation of oligodendrocyte progenitors present throughout white and gray matter regions of the brain. Glia. 2000;31:219–231. doi: 10.1002/1098-1136(200009)31:3<219::aid-glia30>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 2.Alonso G. NG2 proteoglycan-expressing cells of the adult rat brain: possible involvement in the formation of glial scar astrocytes following stab wound. Glia. 2005;49:318–338. doi: 10.1002/glia.20121. [DOI] [PubMed] [Google Scholar]
- 3.Arvidsson A., Collin T., Kirik D., Kokaia Z., Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 4.Bauer S., Patterson P. H. The cell cycle-apoptosis connection revisited in the adult brain. J. Cell Biol. 2005;171:641–650. doi: 10.1083/jcb.200505072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Buffo A., Rite I., Tripathi P., Lepier A., Colak D., Horn A. P., Mori T., Gotz M. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc. Natl. Acad. Sci. U S A. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buffo A., Vosko M. R., Erturk D., Hamann G. F., Jucker M., Rowitch D., Gotz M. Expression pattern of the transcription factor Olig2 in response to brain injuries: implications for neuronal repair. Proc. Natl. Acad. Sci. U S A. 2005;102:18183–18188. doi: 10.1073/pnas.0506535102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Butt A. M., Hamilton N., Hubbard P., Pugh M., Ibrahim M. Synantocytes: the fifth element. J. Anat. 2005;207:695–706. doi: 10.1111/j.1469-7580.2005.00458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dawson M. R., Polito A., Levine J. M., Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol. Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 9.Dayer A. G., Cleaver K. M., Abouantoun T., Cameron H. A. New GABAergic interneurons in the adult neocortex and striatum are generated from different precursors. J. Cell Biol. 2005;168:415–427. doi: 10.1083/jcb.200407053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gensert J. M., Goldman J. E. Heterogeneity of cycling glial progenitors in the adult mammalian cortex and white matter. J. Neurobiol. 2001;48:75–86. [PubMed] [Google Scholar]
- 11.Gould E., Reeves A. J., Graziano M. S., Gross C. G. Neurogenesis in the neocortex of adult primates. Science. 1999;286:548–552. doi: 10.1126/science.286.5439.548. [DOI] [PubMed] [Google Scholar]
- 12.Horky L. L., Galimi F., Gage F. H., Horner P. J. Fate of endogenous stem/progenitor cells following spinal cord injury. J. Comp. Neurol. 2006;498:525–538. doi: 10.1002/cne.21065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horner P. J., Power A. E., Kempermann G., Kuhn H. G., Palmer T. D., Winkler J., Thal L. J., Gage F. H. Proliferation and differentiation of progenitor cells throughout the intact adult rat spinal cord. J. Neurosci. 2000;20:2218–2228. doi: 10.1523/JNEUROSCI.20-06-02218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kataoka Y., Tamura Y., Cui Y., Yamada H. Neural Activity-dependent cellular proliferation in the rat cerebral cortex. Acta Histochem. Cytochem. 2005;38:93–98. [Google Scholar]
- 15.Kataoka Y., Tamura Y., Takamori Y., Cui Y., Yamada H. Perineuronal germinal cells in the rat cerebral cortex. Med. Mol. Morphol. 2006;39:28–32. doi: 10.1007/s00795-006-0307-x. [DOI] [PubMed] [Google Scholar]
- 16.Kee N., Sivalingam S., Boonstra R., Wojtowicz J. M. The utility of Ki-67 and BrdU as proliferative markers of adult neurogenesis. J. Neurosci. Methods. 2002;115:97–105. doi: 10.1016/s0165-0270(02)00007-9. [DOI] [PubMed] [Google Scholar]
- 17.Kimelberg H. K. The problem of astrocyte identity. Neurochem. Int. 2004;45:191–202. doi: 10.1016/j.neuint.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 18.Koketsu D., Mikami A., Miyamoto Y., Hisatsune T. Nonrenewal of neurons in the cerebral neocortex of adult macaque monkeys. J. Neurosci. 2003;23:937–942. doi: 10.1523/JNEUROSCI.23-03-00937.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kornack D. R., Rakic P. Cell proliferation without neurogenesis in adult primate neocortex. Science. 2001;294:2127–2130. doi: 10.1126/science.1065467. [DOI] [PubMed] [Google Scholar]
- 20.Korr H., Schultze B., Maurer W. Autoradiographic investigations of glial proliferation in the brain of adult mice. II. Cycle time and mode of proliferation of neuroglia and endothelial cells. J. Comp. Neurol. 1975;160:477–490. doi: 10.1002/cne.901600405. [DOI] [PubMed] [Google Scholar]
- 21.Kuang S., Kuroda K., Le Grand F., Rudnicki M. A. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell. 2007;129:999–1010. doi: 10.1016/j.cell.2007.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kucia M., Reca R., Campbell F. R., Zuba-Surma E., Majka M., Ratajczak J., Ratajczak M. Z. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- 23.Ligon K. L., Kesari S., Kitada M., Sun T., Arnett H. A., Alberta J. A., Anderson D. J., Stiles C. D., Rowitch D. H. Development of NG2 neural progenitor cells requires Olig gene function. Proc. Natl. Acad. Sci. U S A. 2006;103:7853–7858. doi: 10.1073/pnas.0511001103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lu Q. R., Sun T., Zhu Z., Ma N., Garcia M., Stiles C. D., Rowitch D. H. Common developmental requirement for Olig function indicates a motor neuron/oligodendrocyte connection. Cell. 2002;109:75–86. doi: 10.1016/s0092-8674(02)00678-5. [DOI] [PubMed] [Google Scholar]
- 25.Magavi S. S., Leavitt B. R., Macklis J. D. Induction of neurogenesis in the neocortex of adult mice. Nature. 2000;405:951–955. doi: 10.1038/35016083. [DOI] [PubMed] [Google Scholar]
- 26.Mori T., Buffo A., Gotz M. The novel roles of glial cells revisited: the contribution of radial glia and astrocytes to neurogenesis. Curr. Top Dev. Biol. 2005;69:67–99. doi: 10.1016/S0070-2153(05)69004-7. [DOI] [PubMed] [Google Scholar]
- 27.Nakatomi H., Kuriu T., Okabe S., Yamamoto S., Hatano O., Kawahara N., Tamura A., Kirino T., Nakafuku M. Regeneration of hippocampal pyramidal neurons after ischemic brain injury by recruitment of endogenous neural progenitors. Cell. 2002;110:429–441. doi: 10.1016/s0092-8674(02)00862-0. [DOI] [PubMed] [Google Scholar]
- 28.Ozerdem U., Grako K. A., Dahlin-Huppe K., Monosov E., Stallcup W. B. NG2 proteoglycan is expressed exclusively by mural cells during vascular morphogenesis. Dev. Dyn. 2001;222:218–227. doi: 10.1002/dvdy.1200. [DOI] [PubMed] [Google Scholar]
- 29.Palmer T. D., Markakis E. A., Willhoite A. R., Safar F., Gage F. H. Fibroblast growth factor-2 activates a latent neurogenic program in neural stem cells from diverse regions of the adult CNS. J. Neurosci. 1999;19:8487–8497. doi: 10.1523/JNEUROSCI.19-19-08487.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Palmer T. D., Willhoite A. R., Gage F. H. Vascular niche for adult hippocampal neurogenesis. J. Comp. Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 31.Schultze B., Korr H. Cell kinetic studies of different cell types in the developing and adult brain of the rat and the mouse: a review. Cell Tissue Kinet. 1981;14:309–325. doi: 10.1111/j.1365-2184.1981.tb00535.x. [DOI] [PubMed] [Google Scholar]
- 32.Sunderland C. A., McMaster W. R., Williams A. F. Purification with monoclonal antibody of a predominant leukocyte-common antigen and glycoprotein from rat thymocytes. Eur. J. Immunol. 1979;9:155–159. doi: 10.1002/eji.1830090212. [DOI] [PubMed] [Google Scholar]
- 33.Takebayashi H., Nabeshima Y., Yoshida S., Chisaka O., Ikenaka K. The basic helix-loop-helix factor olig2 is essential for the development of motoneuron and oligodendrocyte lineages. Curr. Biol. 2002;12:1157–1163. doi: 10.1016/s0960-9822(02)00926-0. [DOI] [PubMed] [Google Scholar]
- 34.Tamura Y., Kataoka Y., Cui Y., Takamori Y., Watanabe Y., Yamada H. Intracellular translocation of glutathione S-transferase pi during oligodendrocyte differentiation in adult rat cerebral cortex in vivo. Neuroscience. 2007;148:535–540. doi: 10.1016/j.neuroscience.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 35.Tamura Y., Kataoka Y., Cui Y., Takamori Y., Watanabe Y., Yamada H. Multi-directional differentiation of doublecortin- and NG2-immunopositive progenitor cells in the adult rat neocortex in vivo. Eur. J. Neurosci. 2007;25:3489–3498. doi: 10.1111/j.1460-9568.2007.05617.x. [DOI] [PubMed] [Google Scholar]
- 36.Westin J. E., Lindgren H. S., Gardi J., Nyengaard J. R., Brundin P., Mohapel P., Cenci M. A. Endothelial proliferation and increased blood-brain barrier permeability in the basal ganglia in a rat model of 3,4-dihydroxyphenyl-L-alanine-induced dyskinesia. J. Neurosci. 2006;26:9448–9461. doi: 10.1523/JNEUROSCI.0944-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamashita T., Ninomiya M., Hernandez Acosta P., Garcia-Verdugo J. M., Sunabori T., Sakaguchi M., Adachi K., Kojima T., Hirota Y., Kawase T., Araki N., Abe K., Okano H., Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J. Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yokoyama A., Sakamoto A., Kameda K., Imai Y., Tanaka J. NG2 proteoglycan-expressing microglia as multipotent neural progenitors in normal and pathologic brains. Glia. 2006;53:754–768. doi: 10.1002/glia.20332. [DOI] [PubMed] [Google Scholar]
- 39.Zhou Q., Choi G., Anderson D. J. The bHLH transcription factor Olig2 promotes oligodendrocyte differentiation in collaboration with Nkx2.2. Neuron. 2001;31:791–807. doi: 10.1016/s0896-6273(01)00414-7. [DOI] [PubMed] [Google Scholar]