Abstract
Purpose
To evaluate the effect of adding exercise to a hypocaloric diet on changes in appendicular lean mass and strength in frail obese older adults undergoing voluntary weight loss.
Methods
Thirty frail older (age, 70 ± 5 yr) obese (body mass index, 37 ± 5 kg·m−2) adults were randomly assigned to 6 months of diet/behavioral therapy (diet group, n = 15) or diet or behavioral therapy plus exercise that incorporated progressive resistance training (PRT; diet + exercise group; n = 15). Body composition was assessed using dual-energy x-ray absorptiometry, and muscle strength was assessed using one-repetition maximum. The volume of upper extremity (UE) and lower extremity (LE) exercise training was determined by multiplying the average number of repetitions performed by the average weight lifted during the first three exercise sessions and during the last three exercise sessions of the study.
Results
The diet and the diet + exercise groups had similar (P > 0.05) decreases in weight (10.7 ± 4.5 vs 9.7 ± 4.0 kg) and fat mass (6.8 ± 3.7 vs 7.7 ± 2.9 kg). However, the diet + exercise group lost less fat-free mass (FFM; 1.8 ± 1.5 vs 3.5 ± 2.1 kg), LE lean mass (0.9 ± 0.8 vs 2.0 ± 0.9 kg), and UE lean mass (0.1 ± 0.2 vs 0.2 ± 0.2 kg) than the diet group (P < 0.05). The diet + exercise group had greater increases in percent of weight as FFM (FFM/weight × 100) than the diet group (7.9 ± 3.3 vs 5.4 ± 3.7%; P < 0.05). Despite lean mass losses, the diet + exercise group increased UE and LE strength in response to exercise (17–43%), whereas the diet group maintained strength. The volume of UE and LE exercises correlated with the amount of UE and LE lean mass (r = 0.64–0.84; P < 0.05).
Conclusion
Exercise added to diet reduces muscle mass loss during voluntary weight loss and increases muscle strength in frail obese older adults. Regular exercise that incorporates PRT should be used to attenuate muscle mass loss in frail obese older adults on weight-loss therapy.
Keywords: EXERCISE TRAINING, OBESITY, DIET, MUSCLE STRENGTH
Obesity in the older population is a major public health issue in the United States (32) because both the number of older adults and the prevalence of obesity among older adults are rapidly increasing (3,23,24). In older persons, obesity not only causes serious medical complications but also exacerbates the age-related decline in physical function leading to frailty (6,34,37), impaired quality of life (34), and increased nursing home admissions (19,38). Therefore, weight-loss therapy is recommended for older persons who are obese and who have medical complications or functional impairments that can benefit from weight loss (6,33). However, aging is accompanied by loss of muscle mass, termed sarcopenia, which contributes to loss of strength and risk of disability (31). In addition, both voluntary and involuntary weight loss particularly in older adults are accompanied by loss of muscle mass (26). Therefore, there is concern that weight-loss therapy will accelerate sarcopenia by causing further loss of muscle superimposed on age-related muscle loss.
Resistance training has been investigated as an approach to counteract sarcopenia in older adults by stimulating muscle protein synthesis and causing muscle hypertrophy (29). The increase in muscle mass with resistance training is not well understood but may involve recruitment of satellite cells resulting in the hypertrophy of mature myofibers (15). However, little is known regarding the capacity of resistance training to counter the loss of muscle that accompanies voluntary weight loss in older adults (>65 yr old) who are at risk for worsening sarcopenia. Most previous studies that combined weight loss with exercise training were conducted in nonsarcopenic young and middle-aged adults (4,10). Therefore, the purpose of this study was to evaluate the effect of adding exercise training to diet-induced weight loss on changes in fat-free mass (FFM) and lean mass in frail obese older adults. We directly compared two groups of older obese adults randomized to 6 months of diet alone or diet + exercise. In addition, because the volume of exercise associated with the attenuation of muscle loss during weight loss is not known, we also characterized exercise volume in relation to muscle mass in these participants.
METHODS
Subjects
The study was approved by the Human Research Protection Office and the General Clinical Research Center Advisory Committee at Washington University School of Medicine in St. Louis, MO. Study participants provided their written, informed consent to participate in the study.
Participants were 30 community-living obese [body mass index (BMI), ≥30 kg·m−2] older (age, ≥ 65 yr old) adults. They were randomly assigned to 6 months of diet or behavioral intervention (diet group, n = 15) or 6 months of diet or behavioral intervention plus exercise intervention (diet + exercise group, n = 15). To be included in the study, obese older participants had to be sedentary (did not participate in exercise more than twice per week) and had stable medications and stable weight (± 2 kg over the past year). In addition, they had to meet at least two of three of the following criteria for mild–moderate physical frailty (5,7,34): 1) modified physical performance test (PPT) score between 18 and 32 (maximum score = 36); 2) peak aerobic power (V̇O2peak) between 10 and 18 mL·kg−1·min−1; and 3) self-reported difficulty and/or assistance with up to two instrumental activities of daily living and/or one basic activity of daily living. Exclusion criteria were 1) severe cardiopulmonary disease, 2) diabetes mellitus, 3) musculoskeletal or neuromuscular impairments, 4) sensory or cognitive deficits, 5) cancer diagnosis within last 5 yr, and 6) use of corticosteroids, androgens, or estrogen-containing compounds within the last year. Participants who dropped out early (n = 3) due to difficulty with diet compliance were also excluded from the study.
Experimental Protocol
Diet group
Participants in the diet group were prescribed a balanced diet providing an energy deficit of approximately 750 kcal·d−1. The prescribed diet consisted of 20% protein, 30% fat, and 50% carbohydrate. The weight-loss goal for each participant was 10% body weight with no more than 1.5% body weight loss per week. Participants met weekly as a group with a study dietician for caloric intake adjustments and standard behavioral strategies (problem-solving skills, identification of high-risk situations, and relapse prevention training) (17) aimed at modifying eating habits. They were prohibited from initiating an exercise program during the study.
Diet + exercise group
Participants in the diet + exercise group also received weekly group diet and behavioral intervention as described above, which were conducted separately from the diet alone group. In addition, they participated in an exercise program, which was closely supervised by a physical therapist. The exercise-training sessions were conducted as a group at our indoor exercise facility and consisted of three 90-min sessions per week. Participants performed make up sessions if they missed a class, so each participant performed a minimum of 72 sessions. Each session consisted of 15 min of flexibility exercises, 30 min of low-impact aerobic exercise, 30 min of high-intensity progressive resistance training (PRT), and 15 min of balance activities. The PRT program consisted of nine exercises (squats, leg press, knee extension, knee flexion, seated row, upright row, seated chest press, biceps curl, and triceps extension) performed using a squat rack and Hoist machine (Hoist Fitness Systems, San Diego, CA). One-repetition maximums (1-RM), the maximal amount of weight that can be lifted once, were measured to provide the information needed to adjust exercise intensity, that is, the amount of weight lifted during the training sessions as the participants became stronger. Initially, the weightlifting sessions consisted of two sets of each exercise using a weight that allowed completion of six to eight repetitions of each exercise at ~65% of 1-RM. After ~4 wk, they progressed to three sets of 8–12 repetitions performed at 85% of initial 1-RM (5,18). Participants tracked the amount of PRT by using a training log to document the weight and the number of repetitions performed. Measurements of 1-RM were repeated monthly so that workloads could be progressed for each participant.
Assessments
All assessments were performed by individuals blinded to group assignment at baseline and after 6 months of diet plus exercise therapy.
Muscle Strength Testing
1-RM testing
1-RM testing was performed using the same Hoist machines used for training. The participants lifted increasingly heavy weights, and the maximal amount of weight that they could lift was recorded as the 1-RM for each exercise (5). Participants were initially given instructions and shown how to perform the exercises and then practiced during a trial session before the baseline measurements. All exercise testing sessions were medically supervised.
Body Composition Analyses
Total body mass, fat mass, and FFM were measured by using whole-body DXA (dual energy-x-ray absorptiometry, enhanced whole-body 11.2 software version, Hologic Delphi 4500/w; Hologic Inc., Waltham, MA). Body regions were defined according to the manufacturer’s recommendations as follows. Appendages were isolated from the trunk and the head by using DXA regional computer-generated default lines, with manual adjustment, on the anterior view planogram. The legs and the arms were defined using specific landmarks: the soft tissue extending from a line drawn through and perpendicular to the axis of the femoral neck and angled with the pelvic brim to the phalangeal tips and the soft tissue extending from the center of the arm socket to the phalangeal tips, respectively. The bone-mineral-free portion of the appendicular, upper extremity (UE), and lower extremity (LE) lean mass represents primarily skeletal muscle in the extremities (13). Phantom calibration was performed before each scan. The coefficients of variation for these measurements in our laboratory are less than 2%.
Statistical Analysis
Baseline characteristics between groups were compared by using the t-test for unpaired samples for continuous variables and Fisher exact test for categorical variables. Two-way (group-by-time) repeated-measures ANOVA was used to compare treatment effects over time, with a treatment factor (group) and a trial factor (time). Sex and baseline values were entered as covariates in the ANOVA. When a significant group-by-time interaction was observed, the t-test for paired samples was performed to determine whether there were significant within-group changes in outcomes.
Training volumes were expressed as the average number of repetitions performed multiplied by the average weight lifted for the diet + exercise group during the first three exercise sessions and during the three exercise sessions at 6 months of PRT for each exercise (14). Of the 15 participants in the diet + exercise group, 3 did not complete the leg press, 4 did not complete the leg extension, 4 did not complete the leg flexion, 7 did not complete the bench press, and 7 did not complete the seated row exercises because they were either discharged from the exercise, did not like the exercise, or it was deemed unsafe or harmful (as determined by the research team, which included a physician). Therefore, training volumes for these exercises for these individuals were omitted from the analysis. Pearson correlations were performed to assess associations between changes in exercise volume and lean mass for each exercise. We used SPSS software (version 15.0; SPSS Inc, Chicago, IL) for all statistical analyses. A P value of <0.05 was considered statistically significant. Results are reported as mean ± SD, except in the figures, in which data are given as mean ± SE.
RESULTS
Baseline characteristics including age, sex, weight, BMI, and body composition in the diet alone group and the diet + exercise group were similar Table 1). In addition, indicators of physical frailty were not different between groups as demonstrated by PPT (28.4 ± 2.0 vs 29.3 ± 1.9) and V̇O2peak (17.7 ± 2.1 vs 17.1 ± 3.0) in the diet group and the diet + exercise group, respectively (both P > 0.05). Because all participants in the diet + exercise group were required to complete the 72 exercise sessions, compliance with the exercise program was 100%. Consistent with PRT, mean volumes of exercise training for both UE and LE significantly increased (by ~60 to 100%; all P < 0.05 from baseline) (Table 2).
TABLE 1.
Baseline characteristics of the study participants*.
| Characteristics | Diet Group (n = 15) |
Diet + Exercise Group (n = 15) |
P |
|---|---|---|---|
| Age (yr) | 70.3 ± 4.8 | 68.7 ± 4.3 | 0.34 |
| Women/men | 9/6 | 9/6 | 1.00 |
| Weight (kg) | 102.9 ± 14.6 | 97.3 ± 13.5 | 0.29 |
| Body mass index (kg·m−2) | 36.9 ± 4.9 | 36.7 ± 5.1 | 0.91 |
| Fat mass (kg) | 42.2 ± 5.7 | 39.5 ± 9.3 | 0.34 |
| FFM (kg) | 59.8 ± 13.2 | 57.8 ± 11.3 | 0.66 |
| Relative FFM (%) | 57.8 ± 6.1 | 57.5 ± 7.9 | 0.52 |
| Appendicular FFM (kg) | 25.8 ± 6.6 | 23.7 ± 5.1 | 0.33 |
| UE FFM (kg) | 5.9 ± 1.9 | 5.8 ± 1.6 | 0.91 |
| LE FFM (kg) | 20.0 ± 4.7 | 17.9 ± 3.6 | 0.17 |
Values are presented as mean ± SD.
TABLE 2.
Exercise-training volumesa for the diet plus exercise group during the first three exercise sessions (baseline) and during the last three exercise sessions (final) of the 6 months of progressive resistance exercise training.
| Exercise | Baseline (kg) | Final (kg) | Change (%) |
|---|---|---|---|
| Biceps curl | 167 ± 108 | 286 ± 133* | 100 ± 20 |
| Seated row | 467 ± 218 | 698 ± 403* | 59 ± 45 |
| Bench press | 432 ± 208 | 635 ± 256* | 61 ± 16 |
| Leg press | 525 ± 275 | 915 ± 647* | 71 ± 12 |
| Knee flexion | 550 ± 270 | 792 ± 380* | 54 ± 12 |
| Knee extension | 549 ± 254 | 958 ± 510* | 77 ± 48 |
Exercise-training volumes were expressed as the average number of repetitions performed multiplied by the average weight lifted. Values are presented as mean ± SD.
P < 0.05 compared with baseline.
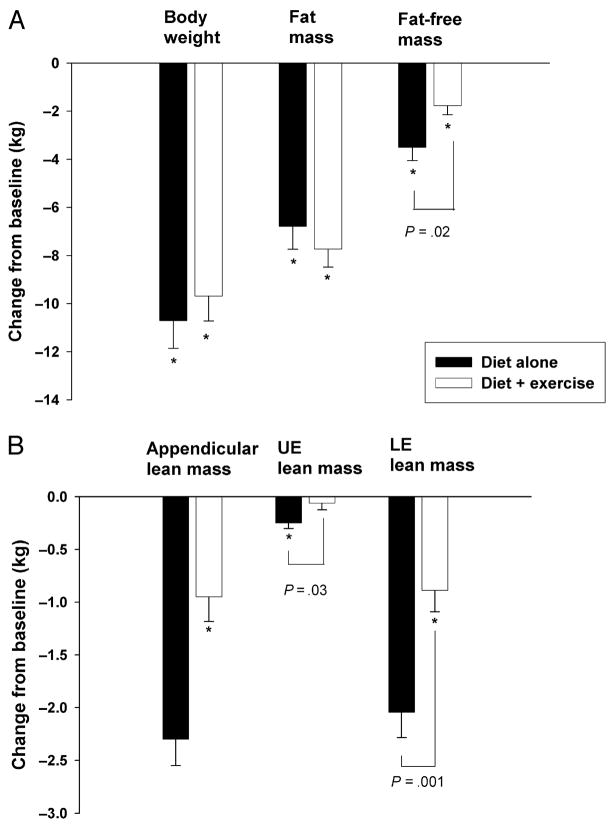
At 6 months, the diet group and the diet + exercise group lost similar amounts of body weight [10.7 ± 4.5 kg (10.6 ± 4.6%) vs 9.7 ± 4.0 kg (10.0 ± 3.9%); P = 0.52] and fat mass (6.8 ± 3.7 vs 7.7 ± 2.9 kg; P = 0.45) (Fig. 1A). However, despite similar quantity of weight and fat losses, the diet + exercise group lost significantly less FFM than the diet group (1.8 ± 1.5 vs 3.5 ± 2.1 kg; P = 0.02) (Fig. 1A). As a result, the percentage of weight loss as FFM was significantly less in the diet + exercise group than that in the diet alone group (15.6 ± 14.3% vs 33.2 ± 20.7%; P = 0.02). In addition, the diet + exercise group had a greater increase in their relative FFM than the diet group (7.9 ± 3.3% vs 5.4 ± 3.7%; P = 0.04).
FIGURE 1.
A. Changes in body weight, fat mass, and FFM in older obese adults randomized to 6 months of diet alone or 6 months of diet + exercise. *Significant change from baseline, P < 0.05. Values are mean ± SE. B. Change in appendicular lean mass, UE lean mass, and LE lean mass in older obese adults randomized to 6 months of diet alone or 6 months of diet + exercise. *Significant change from baseline, P < 0.05. Values are mean ± SE.
Similarly, at 6 months, the diet + exercise group lost significantly less lean mass in the UE (0.1 ± 0.2 vs 0.2 ± 0.2 kg; P = 0.03) and the LE (0.9 ± 0.8 vs 2.0 ± 0.9 kg; P = 0.001) than the diet group (Fig. 1B). In fact, weight loss alone did not result in a significant loss of lean mass at the UE in the diet + exercise group (P = 0.35 compared with baseline) (Fig. 1B).
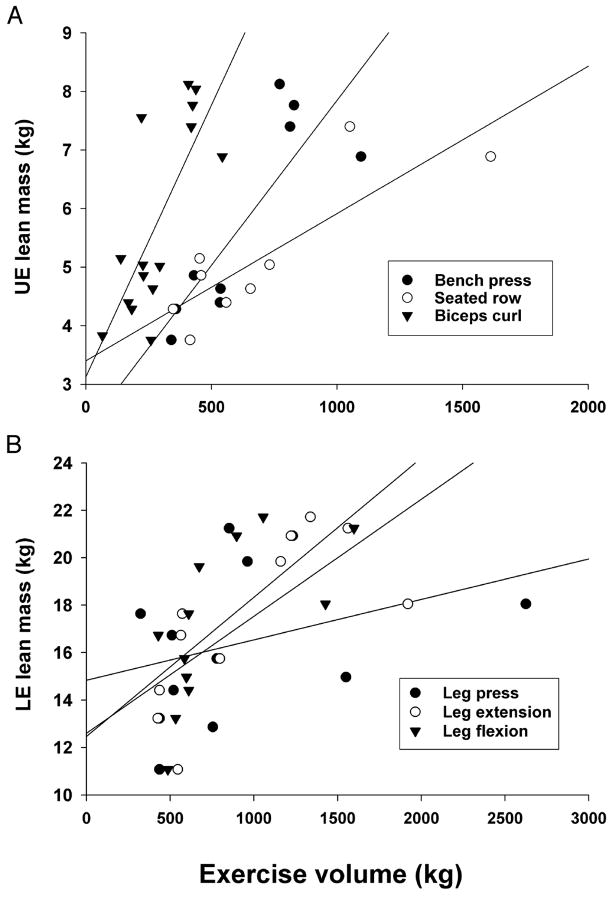
At 6 months, the volumes of weight lifted for both UE and LE directly correlated with the corresponding amounts of lean mass in the UE [bench press (r = 0.84; P < 0.01), seated row (r = 0.84; P < 0.01), biceps curl (r = 0.76; P = 0.001)] (Fig. 2A) and the LE [leg extension (r = 0.72; P = 0.01), leg flexion (r = 0.67; P = 0.02), leg press (r = 0.34, P = 0.28)] (Fig. 2B). The volumes of weight lifted did not correlate strongly with the changes in lean mass for the diet + exercise group (data not shown).
FIGURE 2.
A. Relationships between UE lean skeletal mass and exercise-training volumes at 6 months: bench press (r = 0.84; P < 0.01), seated row (r = 0.84; P < 0.01), biceps curl (r = 0.76; P = 0.001), and UE lean mass (kg) after 6 months of intervention for the diet plus exercise group. B. Relationships between LE lean mass and exercise-training volumes at 6 months: leg extension (r = 0.72; P = 0.01), leg flexion (r = 0.67; P = 0.02), and leg press (r = 0.34, P = 0.28).
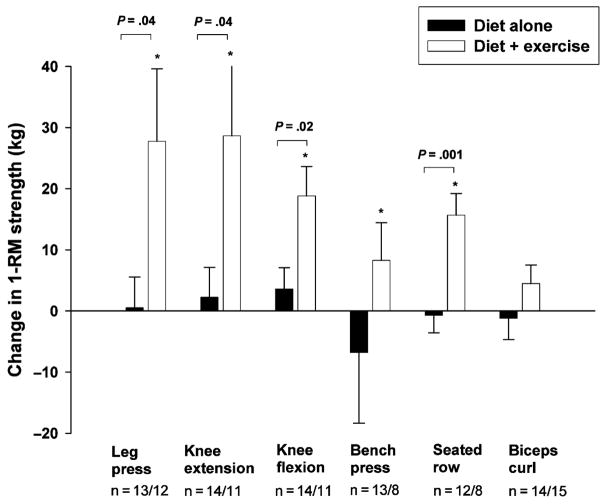
At 6 months, the diet + exercise group made significant increases in 1-RM strength for all LE exercises (leg press, 43 ± 45%; knee extension, 33 ± 41%; knee flexion, 19 ± 21%) and two out of three UE exercises (bench press, 17 ± 21%; seated row, 19 ± 20%), whereas the diet group demonstrated no changes in 1-RM muscle strength over 6 months (P < 0.05 for between-group differences) (Fig. 3).
FIGURE 3.
Changes in 1-RM strength in older obese adults randomized to 6 months of diet alone or 6 months of diet + exercise. *Significant change from baseline, P < 0.05. Values are mean ± SE.
DISCUSSION
Although weight-loss therapy is recommended to improve obesity-related medical complications and functional impairments (2,33), a prevailing concern in the therapy of obese older adults is that weight loss alone will accelerate sarcopenia. However, the precise amount of muscle loss that result from voluntary weight loss in obese older adults and the efficacy of exercise in protecting against this weight-loss-induced reduction in muscle mass in this population are unclear. Therefore, we used a randomized trial to directly compare the effects of diet alone versus diet + exercise on changes in total FFM, appendicular UE and LE lean mass, and UE and LE muscle strength in obese older adults. Our data show that diet only reduces FFM by ~3.5 kg, which corresponds to a loss of appendicular lean mass of ~2.5 kg. These losses of lean tissue are effectively reduced (by one-half) through an exercise program that incorporates PRT. In fact, the loss of UE lean mass is completely prevented by regular exercise. Although the loss of LE lean mass is not completely prevented, exercise still increases LE muscle strength (despite lower muscle mass after weight loss). The volume of exercise training correlates with the amount of UE and LE lean mass, suggesting a dose response to resistance exercise training. These findings demonstrate that weight-loss-induced loss of FFM can be attenuated or prevented by regular exercise, which additionally leads to significant gains in UE and LE muscle function.
The 3.5 ± 2.1 kg (5.9%) loss of FFM that we observed in obese older adults consisted of 32% of the total absolute ~11 kg weight loss over the 6-month study interval. Data from previous weight-loss studies that were conducted in young and middle-aged adults found 2.5–2.8 kg loss of FFM with a weight loss of ~10 kg (4,10). Importantly, we also found that the addition of exercise to diet reduced the FFM loss from 3.5 to 1.8 kg in older adults. Previous weight-loss studies conducted in younger adults reported that exercise combined with diet reduced the expected FFM loss to 1.7 kg (10). Therefore, the present data further demonstrate that regular exercise added to diet is just as effective in attenuating FFM loss in older adults despite the presence of underlying age-related sarcopenia.
Older adults are particularly susceptible to the adverse effects of excessive body weight on physical function due to 1) the decreased muscle mass and strength that occur with aging and 2) a need to carry greater weight due to obesity (30). Indeed, obese older adults have been shown to have sarcopenia (low relative muscle mass, low muscle strength per muscle mass) despite more than adequate body weight which is opposite the stereotypical frail older adult (30,34). In the present study, we found that relative FFM (the percent of body weight as FFM) improved in the diet group and the diet + exercise group, but the improvement was greater in the diet + exercise group due to the additional exercise-induced preservation of FFM. In addition, we found that muscle quality (muscle strength per muscle mass) (21) improved in both groups, but the improvement was also greater in the diet + exercise group due to the additional exercise-induced increase in muscle strength. Because muscle strength increased whereas muscle mass decreased, the improvement in muscle quality in the diet + exercise group could best be explained by exercise-induced neuromuscular activation (22,25). Additional mechanisms for the improvement in muscle quality observed in the diet + exercise group are possible and may include improvement in muscle architecture (16) and fiber type area (20) and increase in high-energy phosphate availability (15). The reason for the improvement in muscle quality in the diet group is unknown. However, both obesity and aging are accompanied by increased muscle lipid content, which correlates with decreased muscle strength (11), and weight loss decreases muscle fat infiltration (12). Therefore, muscle quality may improve due to a decrease in muscle fat infiltration as well as a reduction in inflammation (27) as a result of weight loss. Indeed, there is an association between inflammation and muscle mass and strength (8,35), and thus it is possible that a loss of muscle fat may benefit through a decrease in inflammation (28). Our findings are consistent with a recent report by Wang et al. (36) that knee strength was maintained and muscle quality was improved despite 3 kg FFM loss during weight loss in obese older adults. However, although our data demonstrate that 6 months of diet alone does not decrease muscle strength despite muscle loss, they also demonstrate that the addition of an exercise program is essential for inducing significant increases in muscle strength, thus potentially improving overall function in frail obese older adults (9).
A primary goal of our study was to determine the efficacy of an exercise program to minimize the loss of lean tissue during voluntary weight loss. Therefore, because the adaptations to exercise are muscle specific and overload dependent, we incorporated different resistance exercises that stimulated major muscle groups of the UE and the LE and progressively increased the exercise-training volumes (1,12). In response to exercise added to diet, we found no loss of lean mass in the UE, whereas the loss of lean mass in the LE was reduced by ~50%. To our knowledge, this is the first study to report a differential protective effect of exercise in the UE and the LE extremities in older adults during voluntary weight loss. As the quantity and the quality of UE and LE exercises were similar, a possible explanation for this finding is that the UE may be more responsive to acute high-intensity PRT because it is more novel, whereas the weight-bearing LE may be used more often during daily activities (e.g., walking and stair climbing) in community-living obese adults. Regardless of the amount of muscle mass lost, we found that the addition of exercise to dietary restriction significantly improved muscle strength in both UE and LE.
Our study is the first direct comparison between a diet alone group and a diet + exercise group within a randomized clinical trial specifically conducted in frail obese older adults. We used PRT following ACSM guidelines (18) supervised by a physical therapist at our exercise facility to ensure safe and effective implementation of the exercise regimen. Although a few participants were unable to complete some exercises due to site-specific orthopedic or arthritic impairments expected in this frail population, overall, the compliance was high and all participants completed the 72 sessions of exercise intervention. A limitation of our study is that we did not have a control group that did not participate in weight loss or a group that participated in exercise only. These groups are essential for future research to understand how weight loss may affect FFM in this population and how resistance exercise may assist in preserving FFM. Another limitation of our study is that we were not able to examine sex differences in response to diet and exercise due to the small sample size. However, we controlled for the effect of sex by including it as a covariate in the analyses of variance. In addition, our study was limited to 6-month duration, so longer studies are needed to evaluate long-term compliance with diet and exercise in this older population.
In conclusion, our study provides evidence that the addition of exercise training to diet reduces the amount of muscle mass loss during voluntary weight loss in frail obese older adults and significantly increases muscle strength. Therefore, diet should be combined with regular exercise to reduce the loss of FFM in older obese adults undergoing weight-loss therapy to attenuate obesity-related medical and functional complications.
Acknowledgments
The authors wish to thank Nicole Wright, Stacie Metzger, Kathy Obert, Laura Weber, and Ellen Frye for subject recruitment, training, and technical assistance and the study subjects for their participation.
The study was supported by US National Institute of Health grants AG 025501 and RR 00036 (General Clinical Research Center) and DK56341 (Clinical Nutrition Research Unit).
Tiffany Frimel, PhD, PT, was supported by a fellowship from the Foundation for Physical Therapy, Inc.
References
- 1.American College of Sports Medicine Position Stand. The recommended quantity and quality of exercise for developing and maintaining cardiorespiratory and muscular fitness, and flexibility in healthy adults. Med Sci Sports Exerc. 1998;30(6):975–91. doi: 10.1097/00005768-199806000-00032. [DOI] [PubMed] [Google Scholar]
- 2.National Institutes of Health. Clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—the evidence report. Obes Res. 1998;6(Suppl 2):51S–209S. [PubMed] [Google Scholar]
- 3.Arterburn DE, Crane PK, Sullivan SD. The coming epidemic of obesity in elderly Americans. J Am Geriatr Soc. 2004;52:1907–12. doi: 10.1111/j.1532-5415.2004.52517.x. [DOI] [PubMed] [Google Scholar]
- 4.Ballor DL, Poehlman ET. Exercise-training enhances fat-free mass preservation during diet-induced weight loss: a meta-analytical finding. Int J Obes Relat Metab Disord. 1994;18:35–40. [PubMed] [Google Scholar]
- 5.Binder EF, Schechtman KB, Ehsani AA, et al. Effects of exercise training on frailty in community-dwelling older adults: results of a randomized, controlled trial. J Am Geriatr Soc. 2002;50:1921–8. doi: 10.1046/j.1532-5415.2002.50601.x. [DOI] [PubMed] [Google Scholar]
- 6.Blaum CS, Xue QL, Michelon E, Semba RD, Fried LP. The association between obesity and the frailty syndrome in older women: the women’s health and aging studies. J Am Geriatr Soc. 2005;53:927–34. doi: 10.1111/j.1532-5415.2005.53300.x. [DOI] [PubMed] [Google Scholar]
- 7.Brown M, Sinacore DR, Binder EF, Kohrt WM. Physical and performance measures for the identification of mild to moderate frailty. J Gerontol A Biol Sci Med Sci. 2000;55:M350–5. doi: 10.1093/gerona/55.6.m350. [DOI] [PubMed] [Google Scholar]
- 8.Cesari M, Penninx BW, Pahor M, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci. 2004;59:242–8. doi: 10.1093/gerona/59.3.m242. [DOI] [PubMed] [Google Scholar]
- 9.Chandler JM, Hadley EC. Exercise to improve physiologic and functional performance in old age. Clin Geriatr Med. 1996;12:761–84. [PubMed] [Google Scholar]
- 10.Garrow JS, Summerbell CD. Meta-analysis: effect of exercise, with or without dieting, on the body composition of overweight subjects. Eur J Clin Nutr. 1995;49:1–10. [PubMed] [Google Scholar]
- 11.Goodpaster BH, Carlson CL, Visser M, et al. Attenuation of skeletal muscle and strength in the elderly: the Health ABC Study. J Appl Physiol. 2001;90:2157–65. doi: 10.1152/jappl.2001.90.6.2157. [DOI] [PubMed] [Google Scholar]
- 12.Goodpaster BH, Theriault R, Watkins SC, Kelley DE. Intramuscular lipid content is increased in obesity and decreased by weight loss. Metabolism. 2000;49:467–72. doi: 10.1016/s0026-0495(00)80010-4. [DOI] [PubMed] [Google Scholar]
- 13.Heymsfield SB, Gallagher D, Visser M, Nunez C, Wang ZM. Measurement of skeletal muscle: laboratory and epidemiological methods. J Gerontol A Biol Sci Med Sci. 1995;50:23–9. doi: 10.1093/gerona/50a.special_issue.23. [DOI] [PubMed] [Google Scholar]
- 14.Host HH, Sinacore DR, Bohnert KL, Steger-May K, Brown M, Binder EF. Training-induced strength and functional adaptations after hip fracture. Phys Ther. 2007;87:292–303. doi: 10.2522/ptj.20050396. [DOI] [PubMed] [Google Scholar]
- 15.Hunter GR, McCarthy JP, Bamman MM. Effects of resistance training on older adults. Sports Med. 2004;34:329–48. doi: 10.2165/00007256-200434050-00005. [DOI] [PubMed] [Google Scholar]
- 16.Kawakami Y, Abe T, Fukunaga T. Muscle-fiber pennation angles are greater in hypertrophied than in normal muscles. J Appl Physiol. 1993;74:2740–4. doi: 10.1152/jappl.1993.74.6.2740. [DOI] [PubMed] [Google Scholar]
- 17.Klein S, Wadden T, Sugerman HJ. AGA technical review on obesity. Gastroenterology. 2002;123:882–932. doi: 10.1053/gast.2002.35514. [DOI] [PubMed] [Google Scholar]
- 18.Kraemer WJ, Adams K, Cafarelli E, et al. American College of Sports Medicine position stand. Progression models in resistance training for healthy adults. Med Sci Sports Exerc. 2002;34(2):364–80. doi: 10.1097/00005768-200202000-00027. [DOI] [PubMed] [Google Scholar]
- 19.Lapane KL, Resnik L. Obesity in nursing homes: an escalating problem. J Am Geriatr Soc. 2005;53:1386–91. doi: 10.1111/j.1532-5415.2005.53420.x. [DOI] [PubMed] [Google Scholar]
- 20.Lexell J. Human aging, muscle mass, and fiber type composition. J Gerontol A Biol Sci Med Sci. 1995;50:11–16. doi: 10.1093/gerona/50a.special_issue.11. [DOI] [PubMed] [Google Scholar]
- 21.Lynch NA, Metter EJ, Lindle RS, et al. Muscle quality. I. Age-associated differences between arm and leg muscle groups. J Appl Physiol. 1999;86:188–94. doi: 10.1152/jappl.1999.86.1.188. [DOI] [PubMed] [Google Scholar]
- 22.McCarthy JP, Pozniak MA, Agre JC. Neuromuscular adaptations to concurrent strength and endurance training. Med Sci Sports Exerc. 2002;34(3):511–9. doi: 10.1097/00005768-200203000-00019. [DOI] [PubMed] [Google Scholar]
- 23.Mokdad AH, Bowman BA, Ford ES, Vinicor F, Marks JS, Koplan JP. The continuing epidemics of obesity and diabetes in the United States. JAMA. 2001;286:1195–200. doi: 10.1001/jama.286.10.1195. [DOI] [PubMed] [Google Scholar]
- 24.Mokdad AH, Ford ES, Bowman BA, et al. Prevalence of obesity, diabetes, and obesity-related health risk factors, 2001. JAMA. 2003;289:76–9. doi: 10.1001/jama.289.1.76. [DOI] [PubMed] [Google Scholar]
- 25.Moritani T, deVries HA. Neural factors versus hypertrophy in the time course of muscle strength gain. Am J Phys Med. 1979;58:115–30. [PubMed] [Google Scholar]
- 26.Newman AB, Lee JS, Visser M, et al. Weight change and the conservation of lean mass in old age: the Health, Aging and Body Composition Study. Am J Clin Nutr. 2005;82:872–8. doi: 10.1093/ajcn/82.4.872. [DOI] [PubMed] [Google Scholar]
- 27.Nicklas BJ, Ambrosius W, Messier SP, et al. Diet-induced weight loss, exercise, and chronic inflammation in older, obese adults: a randomized controlled clinical trial. Am J Clin Nutr. 2004;79:544–51. doi: 10.1093/ajcn/79.4.544. [DOI] [PubMed] [Google Scholar]
- 28.Nicklas BJ, You T, Pahor M. Behavioural treatments for chronic systemic inflammation: effects of dietary weight loss and exercise training. CMAJ. 2005;172:1199–209. doi: 10.1503/cmaj.1040769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porter MM. The effects of strength training on sarcopenia. Can J Appl Physiol. 2001;26:123–41. doi: 10.1139/h01-009. [DOI] [PubMed] [Google Scholar]
- 30.Roubenoff R. Sarcopenic obesity: the confluence of two epidemics. Obes Res. 2004;12:887–8. doi: 10.1038/oby.2004.107. [DOI] [PubMed] [Google Scholar]
- 31.Roubenoff R, Hughes VA. Sarcopenia: current concepts. J Gerontol A Biol Sci Med Sci. 2000;55:M716–24. doi: 10.1093/gerona/55.12.m716. [DOI] [PubMed] [Google Scholar]
- 32.van Baak MA, Visscher TL. Public health success in recent decades may be in danger if lifestyles of the elderly are neglected. Am J Clin Nutr. 2006;84:1257–8. doi: 10.1093/ajcn/84.6.1257. [DOI] [PubMed] [Google Scholar]
- 33.Villareal DT, Apovian CM, Kushner RF, Klein S. Obesity in older adults: technical review and position statement of the American Society for Nutrition and NAASO, The Obesity Society. Am J Clin Nutr. 2005;82:923–34. doi: 10.1093/ajcn/82.5.923. [also published in: Obes Res. 2005: 13:1849–1863] [DOI] [PubMed] [Google Scholar]
- 34.Villareal DT, Banks M, Siener C, Sinacore DR, Klein S. Physical Frailty and Body Composition in Obese Elderly Men and Women. Obes Res. 2004;12:913–20. doi: 10.1038/oby.2004.111. [DOI] [PubMed] [Google Scholar]
- 35.Visser M, Pahor M, Taaffe DR, et al. Relationship of interleukin-6 and tumor necrosis factor-alpha with muscle mass and muscle strength in elderly men and women: the Health ABC Study. J Gerontol A Biol Sci Med Sci. 2002;57:M326–32. doi: 10.1093/gerona/57.5.m326. [DOI] [PubMed] [Google Scholar]
- 36.Wang X, Miller GD, Messier SP, Nicklas BJ. Knee strength maintained despite loss of lean body mass during weight loss in older obese adults with knee osteoarthritis. J Gerontol A Biol Sci Med Sci. 2007;62:866–71. doi: 10.1093/gerona/62.8.866. [DOI] [PubMed] [Google Scholar]
- 37.Woods NF, LaCroix AZ, Gray SL, et al. Frailty: emergence and consequences in women aged 65 and older in the Women’s Health Initiative Observational Study. J Am Geriatr Soc. 2005;53:1321–30. doi: 10.1111/j.1532-5415.2005.53405.x. [DOI] [PubMed] [Google Scholar]
- 38.Zizza CA, Herring A, Stevens J, Popkin BM. Obesity affects nursing-care facility admission among whites but not blacks. Obes Res. 2002;10:816–23. doi: 10.1038/oby.2002.110. [DOI] [PubMed] [Google Scholar]