Abstract
This is the second in a series of canonical reviews on invertebrate muscle. We cover here thin and thick filament structure, the molecular basis of force generation and its regulation, and two special properties of some invertebrate muscle, catch and asynchronous muscle. Invertebrate thin filaments resemble vertebrate thin filaments, although helix structure and tropomyosin arrangement show small differences. Invertebrate thick filaments, alternatively, are very different from vertebrate striated thick filaments and show great variation within invertebrates. Part of this diversity stems from variation in paramyosin content, which is greatly increased in very large diameter invertebrate thick filaments. Other of it arises from relatively small changes in filament backbone structure, which results in filaments with grossly similar myosin head placements (rotating crowns of heads every 14.5 nm) but large changes in detail (distances between heads in azimuthal registration varying from three to thousands of crowns). The lever arm basis of force generation is common to both vetebrates and invertebrates, and in some invertebrates this process is understood on the near atomic level. Invertebrate actomyosin is both thin (tropomyosin:troponin) and thick (primarily via direct Ca++ binding to myosin) filament regulated, and most invertebrate muscles are dually regulated. These mechanisms are well understood on the molecular level, but the behavioral utility of dual regulation is less so. The phosphorylation state of the thick filament associated giant protein, twitchin, has been recently shown to be the molecular basis of catch. The molecular basis of the stretch activation underlying asynchronous muscle activity, however, remains unresolved.
Keywords: actin, myosin, insect, mollusc, nematode, C. elegans, scallop, crustacea, Limulus
1. Introduction
This is the second of a projected total of six reviews covering invertebrate muscle. The first review covered invertebrate muscle genes and proteins (Hooper and Thuma, 2005). This review covers thin and thick filament structure and the molecular basis of force production and its regulation in invertetebrate muscle, and two properties of invertebrate muscle that arise on the level of the actomyosin, catch and asynchronous activity. The later reviews will cover (third) muscle anatomy, (fourth) ionotropic channels and excitation/contraction coupling, (fifth) metabotropic channels and modulation, and (sixth) integrative and whole muscle properties. In several places in the present review the presentation is made phylogenetically. This organization follows the tree of Animalia presented in Hooper and Thuma (2005) as substantial revisions of this scheme have not occurred in the interim.
1.1. Why study invertebrate muscles?
A primary justification given in the first review for studying invertebrate muscle was the opportunity the great diversity of invertebrate muscle genes and proteins provided for studying gene regulation and protein interaction. This theme of great diversity is continued in the work presented here, which shows that thick filament structure and regulatory mechanisms are more variable in invertebrate than in vertebrate striated muscle, and that invertebrate muscle has two properties, catch and asynchronous activity, that are not or are only slightly present in vertebrates. This diversity provides a rich arena in which to study protein assembly into macromolecules and protein interaction. Thin filament structure and the molecular basis of force production, alternatively, are similar in vertebrates and invertebrates. This similarity raises two additional, not fully resolved, questions. First, given the apparent latitude for variation in other aspects of muscle, why are these two characteristics so well conserved across Animalia? Second, given the need of all muscle constituents to function as a unified whole, how is function preserved when only parts of the system vary?
1.2. Scope of review and literature database
A goal of these reviews is to cover, for the first time to our knowledge, every journal article ever published on invertebrate muscle (abstracts are not included due to their general lack of presented data and books are not included due to their limited availability), excepting papers on metabolism and development. This review covers articles published before 2007 on invertebrate thin and thick filament structure, force production and actomyosin regulation, and catch and asynchronous muscle (some 1,300 of the 7,100 articles presently in the database). However, we cannot cover articles in languages we cannot read; articles completely in languages other than English, French, German, Italian, and Spanish are therefore not included (not included but on-topic articles in Japanese, Chinese, or Korean: Hozawa, 1911; Sheng et al., 1956; Yazawa and Yoshida, 1979; Fan and Chen, 1986; Nishita, 1998; Katoh, 1999; Ojima, 2003; Funabara, 2004; in Russian: Samosudova and Frank, 1962; Razumova et al., 1966, 1968, 1972, 1973a, 1973b, 1975). We have also not included articles in which the species used is not identified or cannot now be identified (requiring a book long out of print) (Aubert, 1944; Kuschinski and Turba, 1950; Szent-Györgyi, 1953; Philpott and Szent-Györgyi, 1954; Szent-Györgyi and Borbiro, 1956; Cohen and Szent-Györgyi, 1957; Szent-Györgyi and Cohen, 1957; Szent-Györgyi et al., 1960; Maruyama and Ishikawa, 1963; Shechter and Blout, 1964; Baier and Zobel, 1966; Kominz and Maruyama, 1967; Botts et al., 1972; Moos, 1972; Ogawa, 1985; Mehta et al., 1997).
2. Review of vertebrate muscle
Vertebrate muscles have been extensively studied, and are often exclusively presented in textbook explanations of muscle. Although invertebrate muscles have many differences from vertebrate muscles, the muscles of both groups nonetheless share several fundamentally similarities. To remind the reader of these issues, and to provide a background for comparison, we briefly review relevant aspects of vertebrate muscle structure and function. In light of the number of references in this review, this section is only sparsely referenced. Aidley (1988) is an excellent but dated review of vertebrate muscle and Brading (1999) of smooth muscle. Three excellent recent compendiums of primarily vertebrate articles covering all aspects of muscle are volumes 538, Molecular and Cellular Aspects of Muscle Contraction (2003) and 565, Sliding Filament Mechanism in Contraction Fifty Years of Research (2005) in the Advances in Experimental Medicine and Biology series. Reviews covering filament structure and actomyosin force generation and regulation include {Amos, 1997 37667 /id;Cooke, 1995 36069 /id;Cooke, 2004 37674 /id;Geeves, 2005 37044 /id;Grabarek, 1992 37268 /id;Harrington, 1984 14 /id;Holmes, 1984 37657 /id;Holmes, 1995 37267 /id;Holmes, 1997 37709 /id;Holmes, 1998 943 /id;Holmes, 2000 38469 /id;Houdusse, 2001 93 /id;Huxley, 2000 24067 /id;Huxley, 2000 4000 /id;Huxley, 2004 1170 /id;Jontes, 1995 38754 /id;Murphy, 2000 37675 /id;Offer, 2006 37684 /id;Rayment, 1993 380 /id;Root, 2002 36624 /id;Rüegg, 2002 RUEGG2002 /id;Ruppel, 1996 RUPPEL1996 /id;Sellers, 2004 37677 /id;Sheterline, 1995 37743 /id;Spudich, 1995 192 /id;Spudich, 2001 37702 /id;Squire, 1975 23411 /id;Vale, 2000 51 /id;Volkmann, 2000 23101 /id;Warshaw, 2004 37681 /id}.
2.1. Vertebrate thin and thick filament structure
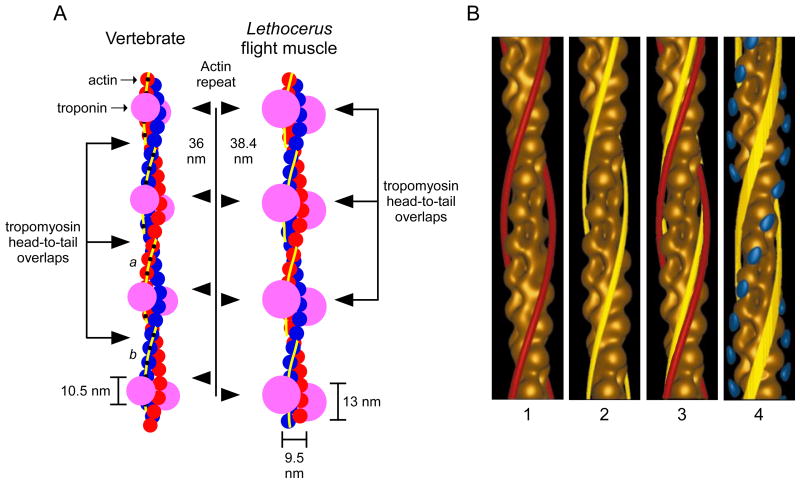
All muscles contain thin filaments and thick filaments. Muscle thin filaments (diameter 6–10 nm) are a double helix of polymerized actin monomers, and have, with minor variation, a common structure across Animalia (Fig. 1A; left filament vertebrate, right Lethocerus). The double helix repeats once every 28 monomers (red and blue circles) if the monomers from both strands are counted. Due to the helical nature of the filament, the molecule repeats every 14 monomers if the distinction between strands is ignored. Two important thin filament associated proteins in striated muscle are the globular protein troponin (large open purple circles) and the filamentous protein tropomyosin (yellow). Two troponin complexes (one for each helix) bind once every 14 monomers. Tropomyosin twists with the double helix and sterically blocks the myosin binding sites at rest but moves away from them in the presence of Ca++ (Fig. 1B). The rotation of the individual actin monomers along the helix results in thick filaments having staggered preferred binding sites (myosin binding sites are represented by black dots on the monomers). For instance, a thick filament lying above the thin filament could bind most easily to the actin monomers at positions a (on the red strand) and b (on the blue strand). For a more detailed description of this issue, see section 3.31.
Figure 1.
Thin filament structure. A. Schematic showing two actin helices (red, blue), tropomyosin (yellow), and troponin (pink) in vertebrate striated muscle and Lethocerus flight muscle. Modified from Wendt et al. (1997). B. Three dimensional electron microscopy reconstructions of thin filaments interacting with thick filaments at rest (1) and during contraction (2). Panel 3 compares tropomyosin position in panels 1 and 2. Panel 4 shows the likely myosin binding sites. Contour plot, actin; red and yellow helices, tropomyosin; blue dots, myosin binding sites. Modified from Craig and Lehman (2001); data from tarantula.
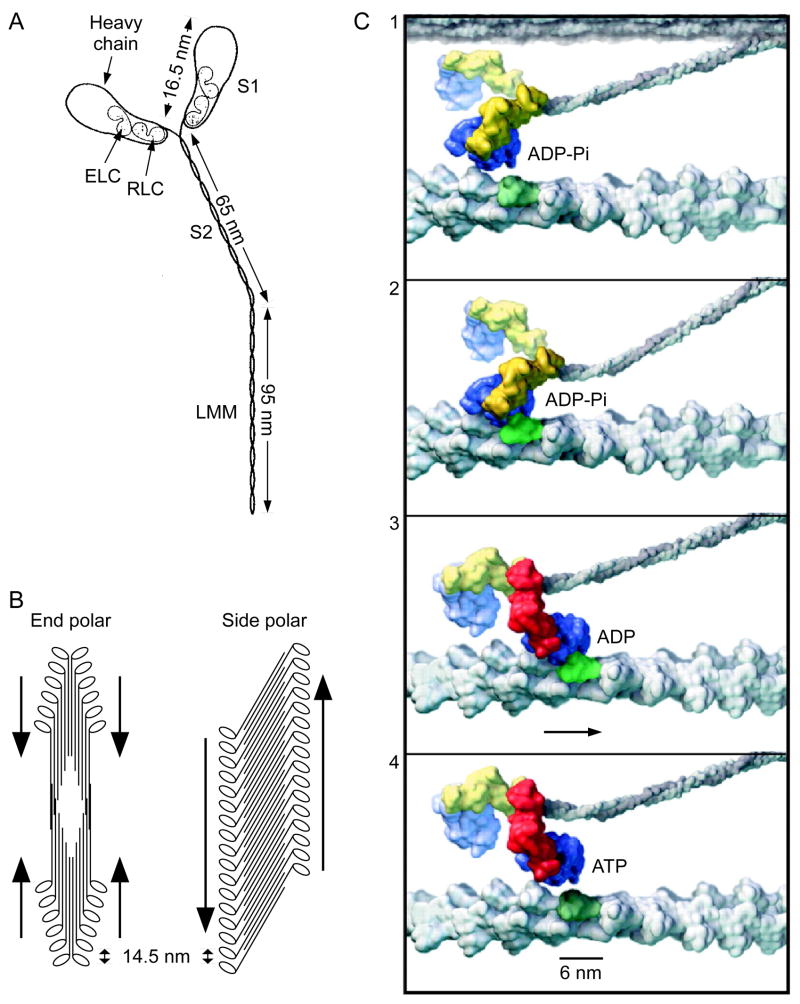
Muscle thick filaments are composed of myosin. Myosin is composed of three pairs of molecules, the heavy chain, the essential light chain, and the regulatory chain (Fig. 2A). The tails of the heavy chains form a coiled-coil tail and the other end of each heavy chain and one essential and one regulatory chain form one of the combined molecule’s two globular heads (which engage the actin filament to produce force) The extended tails bind together to form the thick filaments. All known thick filaments have two general organizations (Fig. 2B). The first, which occurs in vertebrate striated muscle and all known invertebrate muscle is end polarization, in which the myosin molecules are oriented in opposite directions at each end of the thick filament. This orientation results in a cylindrical filament (diameter 14–16 nm) with a central region without heads and two peripheral regions out of which heads protrude.
Figure 2.
Myosin, thick filament structure, and actomyosin power stroke. A. Myosin is composed of three paired molecules, the heavy chain and the essential and regulatory light chains. Part of the heavy chains form a coiled coil tail; the remainder of the heavy chains and the two light chains form two globular heads, each of which can independently bind the thin (actin) filament. Modified from Rayment and Holden (1994). B. Thick filaments can be end or side polarized. In end polarized filaments the heads on each half of the filament have the same orientation and the filament thus has a central zone bare of heads. As a result of this orientation, each end of the filament ‘pulls’ the actin filaments with which it interacts toward the central bare zone (arrows). In side polarized filaments the heads on each side of the filament all have the same orientation. Modified from Xu et al. (1996). C. The actomyosin power stroke. 1. A myosin head with bound ADP-Pi approaches an actin binding site. 2. The head become strongly bound. 3. The head rotates about a hinge, and the actin filament is displaced. During this step the Pi disassociates. 4. The ADP also disassociates, ATP binds to the myosin head, and the head dissociates from the actin filament, thus allowing the cycle to repeat. Blue is head catalytic core; yellow and red are, respectively, the pre and post stroke lever arm of the head. Modified from Vale and Milligan (2000).
The second, which has been only described in vertebrate smooth muscle, is side polarization (Small and Squire, 1972; Craig and Megerman, 1977; Cross et al., 1991; Xu et al., 1996; Rovner et al., 2002). The basic building block of these filaments is a flat sheet of myosin molecules oriented at a small angle to the filament long axis with the heads on each side of the sheet oriented in the same direction. These filaments have no central bare region, but instead a bare region at each end of the filament whose length depends on the angle between the individual myosin molecules and the filament’s long axis, and the overlap of the myosin molecules. The almost complete overlap shown in Fig. 2B is only illustrative; the overlap in real filaments is unknown. The fine detail of myosin packing in these filaments is not completely described. The filaments have a square cross section, and present evidence suggests that each filament is composed of two sheets lying one above the other.
2.2. Cross-bridge driven filament sliding underlies force production
In both types of filaments myosin heads possess an ATPase activity, and can bind to sites on the actin thin filaments. In their unbound state ADP-Pi is bound to the heads. The heads are in considerable disorder, but generally lie at obtuse angles relative to the myosin tail (Fig. 2C1). Initial binding of the myosin head to the thin filament is weak with the head having a 45° angle relative to the thin filament long axis (Fig. 4C2). As binding proceeds, the portion of the myosin heavy chain engaged with the actin (blue) retains its position and shape, but the region closer to the thick filament (yellow in Fig. 2C2, red in Fig. 2C3 and 2C4) rotates toward the Z-line, which produces an M-line directed force on the thin filament (Fig. 2C3). Force is thus not generated by rotation of the entire myosin head, but instead in a lever-like manner in which rotation of a more distant portion of the head uses the actin-binding portion to transfer force to the thin filament. At the end of the stroke the lever arm has a ~135° angle relative to the thin filament long axis and points to the Z-line (Fig. 2C3).
Figure 4.
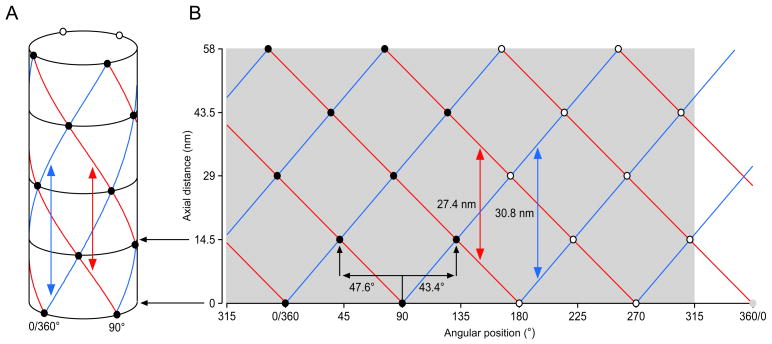
Conceptual explanation of repeating structures and X-ray analysis. A. A cylinder with objects that are equally spaced around the cylinder in axially repeating groups. Each group has four azimuthally equally spaced objects, each set of objects rotates as one moves axially along the cylinder. Circles linking the objects at each axial level (black circles) and two helices linking nearest objects on different axial levels (blue, red) can be drawn and the distances between them identified. These distances are measured by three of the reflections in an X-ray diffraction pattern. B. The cylinder sliced down the back side and unrolled to form a net. Filled circles are the objects that can be seen in panel A (the objects on the front of the cylinder), open circles are those that cannot be seen (those on the back). The circle at 0 axial distance and 360/0° is grey to indicate that it is a repetition of the object at 0 axial distance and 0/360°.
If this were the end of the process, the muscle would not contract further, and, furthermore, would become rigid, since the tight binding of the myosin head to the actin, and the inability of the myosin head to rotate back to its original angle, would lock the thin and thick filaments into a unyielding conformation (this is the basis of rigor mortis). However, myosin head rotation is accompanied by Pi unbinding and then ADP unbinding. ATP can then bind to the myosin head, which causes the head to detach from the thin filament (Fig. 2C4). The ATP is then dephosphorylated, at which time it can again bind the thin filament. This cross-bridge cycling is the fundamental mechanism for generating force in all muscles.
2.3. Regulation of cross-bridge cycling
Behaviorally relevant muscle contraction and relaxation requires that cross-bridge cycling be regulated. Three types of regulation exist in vertebrate muscle. The first is actin based and depends on tropomyosin and troponin (Fig. 1B). Tropomyosin binds along the thin filament (McLachlan and Stewart, 1975, 1976) and troponin controls tropomyosin’s position on it. When Ca++ binds to troponin the tropomyosin filament is displaced and the myosin heads can attach to the thin filament {Lehman, 1994 33489 /id;Lehman, 2001 35 /id;Ohtsuki, 1999 20 /id;Pirani, 2006 37634 /id;Xu, 1999 2430 /id}. In insect flight, Limulus, scallop, chicken, and rabbit muscle tropomyosin binding alone to the thin filament does not inhibit thin filament ability to stimulate myosin ATPase (Lehman and Szent-Györgyi, 1972; Lehman et al., 1974, 2000). Indeed, for insect flight and Limulus muscle, tropomyosin binding increases myosin ATPase activity. These data indicate that in the absence of troponin tropomyosin assumes a permissive configuration.
The three components of troponin, troponin I, C, and T, work together to regulate actomyosin activity (Farah and Reinach, 1995; Perry, 1999, 2003). Troponin I binding to the actin/tropomyosin chains results in the site on the actin with which the myosin heads interact becoming blocked. Adding troponin C, with or without Ca++, induces cross-bridge cycling due to troponin I binding to a high-affinity site on troponin C. In the absence of Ca++, troponin T prevents troponin C’s activating effect by weakening troponin I’s affinity for troponin C. Ca++ activates cross-bridge cycling by binding to troponin C and changing its conformation so that troponin T no longer masks troponin C’s high affinity site for troponin I.
A second mechanism for regulating cross-bridge cycling is myosin based. In vertebrates (see below for an alternative mechanism in invertebrates) in this type of control the myosin head cannot function as an ATPase or bind with high affinity to the thin filament unless the regulatory light chain is phosphorylated (Vorotnikov et al., 2002). The regulatory chain can be phosphorylated in two ways. The first is Ca++-dependent. When Ca++ levels rise Ca++ binds to calmodulin, and this complex activates a myosin light chain kinase that phosphorylates the regulatory chain. The second is by Ca++-independent changes of myosin light chain kinase {Deng, 2001 6 /id} or myosin light chain phosphatase (Somlyo and Somlyo, 2000) activity. Once phosphorylated by either mechanism, myosin binds with high affinity to the thin filament and undergoes repeated cross-bridge cycling as explained above.
Vertebrate smooth muscle contains a third thin filament regulatory system based not on troponin, but on the actin binding proteins caldesmon and calponin {Aidley, 1998 32 /id;Brading, 1999 27 /id;Gusev, 2001 24 /id;Hodgkinson, 1997 28 /id;Hodgkinson, 2000 1833 /id;Lehman, 1997 55 /id;Marston, 1991 36337 /id;Sobue, 1991 10 /id;Takahashi, 1991 34 /id;Winder, 1998 1 /id;Winder, 1990 36338 /id}. Calmodulin can interact with caldesmon {Krueger, 2000 25 /id;Gusev, 2001 24 /id;Zhou, 1997 26 /id}, and mitogen activated protein kinase phosphorylates caldesmon (Childs et al., 1992). Calponin does not bind to calmodulin with high affinity, but another Ca++ binding protein in vertebrate smooth muscle, caltropin, does and regulates calponin’s inhibition of the actomyosin ATPase (Wills et al., 1994). How calponin/caldesmon alter smooth muscle cross-bridge cycling is unclear, but phosphorylation alters caldesmon’s interaction with myosin and tropomyosin (Gusev, 2001), and calponin binding shifts where tropomyosin binds to the actin filament {Hodgkinson, 1997 28 /id}.
3. Invertebrate muscle
3.1. Thin filament
Although there was early controversy over repeat distances and numbers of actin monomers per repeat, biochemical, X-ray, and electron microscopy work indicates that nematode (Rosenbluth, 1967), crustacea (Wray et al., 1978; Maéda et al., 1979; Namba et al., 1980; Wray and Holmes, 1981), insect flight muscle (Hanson and Lowy, 1963; Rayns, 1972; Reedy et al., 1983b; Ruiz et al., 1998; Cammarato et al., 2004), mollusc (Bear, 1945; Selby and Bear, 1956; Worthington, 1959; Hanson and Lowy, 1963; Hanson, 1967; Lowy and Vibert, 1967; Tsuchiya et al., 1977a,b; Vibert and Craig, 1982; Egelman et al., 1983), sea urchin (Obinata et al., 1974), and annelid (Bear, 1945; Hanson and Lowy, 1963) thin filaments are very similar to vertebrate thin filaments, with the double helix repeating once every 35–40 nm in 13–15 monomers (if the fact that the two monomers in question are from different strands is ignored; Fig. 1A).
In vertebrates the actin filament repeats in 14 monomers every 36 nm, different from many of the measurements noted above on invertebrate thin filaments. However, it is unclear if all these differences (particularly the older data) are real. First, experimental preparation for X-ray diffraction (Reedy et al., 1983b) and electron microscopy introduces artifactual variations in filament structure, and the extent of these variations presumably depends on the tissue involved. Given the range of physiological ion concentrations present in invertebrates, and between vertebrates and invertebrates, some of the measured differences between the vertebrate and invertebrate data could thus be due to differing responses to experimental procedures. Second, actin filament repeat length depends on Ca++ concentration (Ruiz et al., 1998), whether the muscle is at rest or in rigor (Maéda et al., 1979), and the tension the muscle is experiencing (Tajima et al., 1994). Given the importance of thin filament structure in force generation, it would seem useful to repeat this work using modern techniques to determine definitively how much variation actually exists in invertebrate thin filaments.
Despite these caveats, in several instances true thin filament variation clearly exists. For instance, insect flight muscle has been extensively studied, and in this muscle the actin repeat distance is clearly about 38 nm (with the small differences in reported values being due to muscle state variations, e.g., Ca++ concentration) (Fig. 1A, right filament). Other examples of clear difference include 1) nematode actin depolymerizing factor/cofilin (see below) depolymerizes C. elegans, but not rabbit, thin filaments (Ono, 1999), 2) insect thin filaments contain a ubiquinated actin monomer, arthrin, every seventh subunit (Burgess et al., 2004), and 3) not all six Drosophila actins substitute for each other equally well (Röper et al., 2005).
Troponin binding to the thin filament has been studied in nematode (Kimura et al., 1987), Limulus (Lehman, 1982), crab (Maéda et al., 1979), scallop (Lehman, 1983a), and insect (Bullard et al., 1988; Newman et al., 1992; Reedy et al., 1994a; Wendt et al., 1997; Wendt and Leonard, 1999). This work has shown that troponin binds every 38–44 nm, as compared to 40 nm in vertebrates, but the large variety of preparation conditions again makes it difficult to interpret these differences. However, an unambiguous, qualitative difference exists in Lethocerus flight muscle, in which the head to tail overlap of the tropomyosin is at the troponin binding sites, instead of being halfway between the troponins as in vertebrates (Fig. 1A) (Wendt et al., 1997). Whether this difference is a general property of invertebrate thin filaments, or a special case related to this muscle being asynchronous, is unknown.
Thin filament length must be tightly regulated to produce and maintain sarcomere structure ({Carlier, 1998 36778 /id;Egelman, 1995 36783 /id;Littlefield, 1998 22862 /id;Schoenenberger, 1999 36639 /id;Zigmond, 2004 38753 /id}). A large number of proteins including tropomodulin (which blocks elongation and depolymerization of the slow-growing—pointed—end of tropomyosin-actin filaments), gelsolin (which severs actin filaments and caps the fast-growing—barbed—end), β-thymosins (which retard filament growth by binding to G actin), profilin (which both severs actin filaments and depolymerizes the filament at the pointed end), actin depolymerizing factor/cofilin, and tropomyosin (which stabilizes actin filaments against actin depolymerizing factor/cofilin activity) regulate actin polymerization.
These proteins regulate actin filament dynamics in the cytoskeleton of all cells. We cover here only work directly relevant to invertebrate muscle. Drosophila thin filaments elongate from their pointed ends during myofibril development and tropomodulin regulates thin filament growth (Mardahl-Dumesnil and Fowler, 2001). Gelsolin-like proteins are present in, or affect the development of, muscle in earthworm (D’Haese and Jinssen, 1987; Giebing et al., 1994, 1997), crustacea {Bock, 1994 36887 /id;Lück, 1995 36944 /id}, ascidia (Ohtsuka et al., 1994, 1998; Langer et al., 1998), and Drosophila, in which gelsolin is encoded by the flightless-1 gene (Campbell et al., 1993; de Couet et al., 1995). β-thymosins are present in sea urchin and scallop (Safer and Chowrashi, 1997). Three profilin homologues are present in C. elegans, with PFN-3 being specifically expressed in muscle (Polet et al., 2006).
C. elegans has two forms of actin depolymerizing factor/cofilin (UNC-60A and UNC-60B) generated by alternative splicing from a single gene (McKim et al., 1988, 1994). The forms differ in their filament severing and depolymerizing abilities and tissue locations. UNC-60A is required for proper early development and UNC-60B for muscle sarcomere structure (Ono and Benian, 1998; Ono et al., 1999, 2003; Ono, 2003; Yamashiro et al., 2005). The C-terminal portion of UNC-60B is critical for its interactions with filamentous actin (Ono et al., 2001). UNC-60B interacts with another actin severing protein, actin-interacting protein 1, coded for by the UNC-78 gene, that is also required for proper muscle thin filament assembly (Ono, 2001; Mohri and Ono, 2003; Ono et al., 2004; Mohri et al., 2006). This protein has two seven-blade propellers at each end of the protein that interact with the thin filament, and the UNC-60B actin depolymerizing factor/cofilin protein binds to the filament by wedging between the propellers (Ono, 2003; Mohri et al., 2004; Clark et al., 2006). Tropomyosin inhibits actin depolymerizing factor/cofilin activity (Ono and Ono, 2002; Yu and Ono, 2006). Actin depolymerizing factor/cofilin, tropomyosin, and myosin heavy chain are all required for proper muscle arm development (in C. elegans the muscles extend cytoplasmic arms to contact the motor nerves, see third review) (Dixon and Roy, 2005).
3.2. Thick filament
Invertebrate thick filaments show great ultrastructural variability. For instance, although some invertebrate thick filaments have ‘typical’ 20–30 nm diameters, others, particularly (but not only) molluscan smooth muscles, have very large (60–160 nm) thick filaments (Limulus telson (Levine et al., 1973), echinodermata (Baccetti and Rosati, 1968), amphioxus notochord (Flood et al., 1969; Yongshui and Zuxun, 1979), annelid (Lanzavecchia and de Eguileor, 1976; Camatini et al., 1976; Lanzavecchia, 1977), Nematomorpha (Swanson, 1971b; Lanzavecchia, 1977; Lanzavecchia et al., 1977), mollusc (Jakus et al., 1944; Philpott et al., 1960; Hanson and Lowy, 1961; Lowy and Hanson, 1962; Elliott, 1964a; Kalamkarova and Kriukova, 1966; Kryukova, 1968; Szent-Györgyi et al., 1971; Levine et al., 1976)). Thick filament structure varies even in single muscles—clam adductor (smooth) muscle contains both short (7.5 μm) thin (26.5 nm diameter) and long (13 μm) thick (42 nm diameter) thick filaments (Matsuno et al., 1993). Cross sections show similar variation, with some thick filaments having solid cores (Beinbrech et al., 1985) and others, particularly in insect flight muscle (Reedy et al., 1981; Beinbrech et al., 1985, 1988), crab (Franzini-Armstrong, 1970; Wakabayashi and Namba, 1981)} and C. elegans body wall and pharyngeal muscle (Epstein et al., 1974), being hollow tubes. Describing invertebrate thick filament structure has therefore been an enormous undertaking, and even today detailed understanding of their structure is available for only a few types of thick filament. Reviews with data on invertebrate thick filament structure include Harrington and Roger (1984), Warrick and Spudich (1987), Barral and Epstein (1999), Squire et al. (2005a) and Craig and Woodhead (2006).
3.2.1 Paramyosin
Invertebrate thick filament diversity arises from differences in both the protein complement of different invertebrate thick filaments, and how these proteins are packed in the filament. A protein that plays a central role in determining thick filament diameter is paramyosin. Paramyosin is present not only in large diameter thick filaments, but also in small diameter thick filaments in a large number of species including scallop striated adductor (Levine et al., 1976; Winkelman, 1976), insect flight and body (Bullard et al., 1973b; Levine et al., 1976; Winkelman, 1976; Reedy et al., 1981; Beinbrech et al., 1985; Hinkel-Aust et al., 1990), Limulus (Levine et al., 1976, 1983; Iwatsuki, 1981; Gaylinn and Dewey, 1986), tarantula (Levine et al., 1983), crustacea (Levine et al., 1976; Winkelman, 1976), and nematode (Waterston et al., 1974; Winkelman, 1976) muscle, with paramyosin:myosin mass ratios between 0.03 and 0.7 (Levine et al., 1976; Winkelman, 1976; Iwatsuki, 1981; Gaylinn and Dewey, 1986). However, paramyosin content is greatly increased in large diameter thick filaments (paramyosin:myosin ratios as great as 10:1, and paramyosin comprising up to half the muscle’s structural proteins, and 80% of thick filament weight) (Philpott et al., 1960; Rüegg, 1961b; Szent-Györgyi et al., 1971; Elliott, 1974; Levine et al., 1976; Winkelman, 1976; Margulis et al., 1979; Iwatsuki, 1981).
The history of understanding paramyosin is convoluted, but must be briefly summarized in order to be able to interpret the older literature and to understand best present knowledge of the structure of paramyosin containing thick filaments. Paramyosin was originally and clearly defined as a protein responsible for the unusual X-ray diffraction and electron microscopic characteristics of very large thick filaments (Hall et al., 1946; Schmitt et al., 1947). Nonetheless, for a considerable time many subsequent authors (e.g., Elliott, 1964a) used ‘paramyosin’ solely as a structural term, i.e., paramyosin filaments were ones that displayed these X-ray diffraction and electron microscopic characteristics, independent of any questions about protein composition. With the benefit of hindsight, this was not an unreasonable distinction, as very large thick filaments do have distinctive properties that separate them from smaller diameter paramyosin containing thick filaments.
However, this usage contains several pitfalls for modern readers. First, in this literature large diameter thick filaments are often called paramyosin ‘fibers’, ‘fibrils’, or ‘filaments’ (e.g., Bear and Selby, 1956; Elliott et al., 1957, 1968b; Hanson et al., 1957; Hanson and Lowy, 1961; Lowy and Hanson, 1962; Elliott, 1964b, 1971; Lanzavecchia, 1972), which could confuse modern readers with their knowledge that paramyosin is a protein and that sub-filaments composed of paramyosin could be components of thick filaments. Readers must always remember that in the older literature paramyosin fiber does not mean paramyosin subfilament, but is instead just a synonym for large diameter molluscan thick filament.
Second, in some older papers ‘paramyosin fiber’ refers both to myosin containing thick filaments and filaments from which myosin has been chemically extracted. Third, paramyosin had properties that reminded the early workers of vertebrate tropomyosin, and it was therefore (relatively briefly) originally called ‘tropomyosin A’ or ‘water insoluble tropomyosin’ and sometimes simply ‘tropomyosin’ (in which case in some papers it is impossible to be certain which protein is being examined) (Yoshimura, 1955; Bailey, 1956, 1957; Elliott et al., 1957; Hanson et al., 1957; Kominz et al., 1957, 1958; Laki, 1957; Mei-Hsuan and Tien-Chin, 1957; Kay, 1958, 1960; Laki et al., 1958; Rüegg, 1959, 1961b, 1961c, 1964; Kay and Bailey, 1959; Matsumoto, 1959; Bailey and Rüegg, 1960; Hanson and Lowy, 1961; Kubo, 1961; Milstein and Bailey, 1961; Lowy and Hanson, 1962; Lowy et al., 1964; Bailey et al., 1964; Milstein, 1966; Ikemoto and Kawaguti, 1967; Lanzavecchia, 1972).
Much of this early work was devoted to determining whether the sliding filament theory pertained to ‘paramyosin’ (and smooth and obliquely striated—another type of striation seen in invertebrate muscle, see third review) invertebrate muscles (e.g., Hanson and Lowy, 1959, 1961, 1964; Lowy and Hanson, 1962; Kalamkarova and Kriukova, 1966; Millman, 1967; Rüegg, 1968a; Szent-Györgyi et al., 1971; Lanzavecchia, 1977; Sugi and Tsuchiya, 1979). An important result from this work was that even in smooth invertebrate muscles the thick filaments taper at their ends, often have a central bare zone, and sometimes can be directly shown to be bipolar (Lowy and Hanson, 1962; Millman, 1967; Szent-Györgyi et al., 1971; Sobieszek, 1973; Lanzavecchia, 1977; Ishii and Takahashi, 1983; Yamada et al., 1989; Oiwa et al., 1998). These data indicate that these smooth muscle thick filaments are end polarized, and it has not been shown that any invertebrate muscle uses side polarized thick filaments.
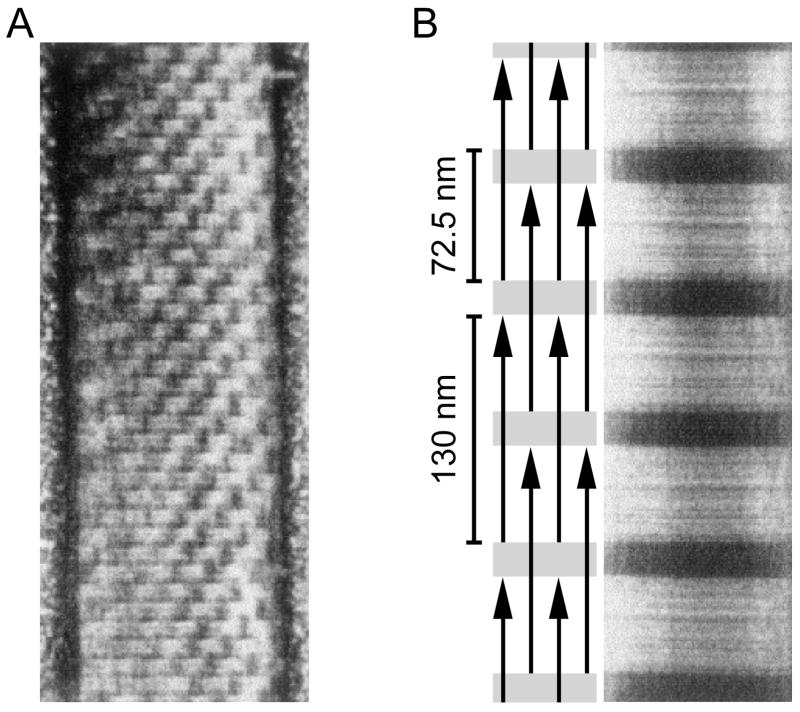
The structural characteristics of paramyosin containing thick filaments are the filament (or sometimes just its core) possessing one or more of 1) a prominent 14.5 nm periodicity (amphioxus: Flood et al., 1969; Yongshui and Zuxun, 1979; mollusc: Elliott et al., 1957; Philpott et al., 1960; Ishii and Takahashi, 1983), 2) a ribbon-like structure (Nematomorpha: Lanzavecchia et al., 1977), 3) long, typically 72 nm, periodicity, sometimes with a multiple of 14.5 nm substructure (Echinodermata: Baccetti and Rosati, 1968; annelid: Lanzavecchia, 1972; Camatini et al., 1976; Nematomorpha: Swanson, 1971b; Lanzavecchia et al., 1977; Deitiker and Epstein, 1993; Epstein et al., 1995; mollusc: Bear, 1944; Jakus et al., 1944; Hall et al., 1945; Schmitt et al., 1947; Bear and Selby, 1956; Hanson et al., 1957; Hodge, 1959; Kahn and Johnson, 1960; Elliott, 1964b, 1968b; Sobieszek, 1973; Heumann, 1973; Eshleman et al., 1982), or 4) a checkerboard pattern (a ‘Bear-Selby net’) with a repeat distance typically of 5 × 14.5 = 72 nm (Fig. 3A) (mollusc: Lanzavecchia, 1966, 1972; Szent-Györgyi et al., 1971; Heumann, 1973; Nonomura, 1974; Elliott, 1979; Castellani et al., 1983; Ishii and Takahashi, 1983; Bennett and Elliott, 1984; Panté, 1994; Cohen, 1998).
Figure 3.
Explanation of large scale (72 nm) structures often present in large diameter mollusc thick filaments. A. A mollusc thick filament with a checkerboard pattern. B. A reconstituted mollusc paramyosin filament with a simple light-dark banding pattern in which the distance of one repeat unit (one dark and one light band) is 72.5 nm. Schematic shows how an overlap-gap binding of individual paramyosin molecules explains the observed staining pattern (only the gap portions take up the stain). Modified from Cohen (1998).
Work on paramyosin as a protein showed that it was an α-helical rod approximately 130 nm in length, and that the basic building block of the filaments was likely a dimer of paramyosin molecules existing as a two-chain α-helical coiled-coil similar to the coiled-coil dimer formed by myosin tails {Allis, 1965 35387 /id;Allis, 1965 35386 /id;Chia-Mu, 1965 38775 /id;Cohen, 1963 29870 /id;Cowgill, 1968 34952 /id;Cowgill, 1972 34256 /id;Cowgill, 1974 34257 /id;Cowgill, 1975 34259 /id;Cowgill, 1975 34258 /id;Delaney, 1976 37063 /id;Crimmins, 1981 34130 /id;Edwards, 1977 34710 /id;Eshleman, 1982 36716 /id;Gal, 1979 41 /id;Halsey, 1971 35348 /id;Hanson, 1957 24161 /id;Johnson, 1959 35394 /id;Kay, 1958 29875 /id;Kay, 1959 29892 /id;Kay, 1960 35341 /id;Laki, 1958 29977 /id;Lowey, 1963 29871 /id;Lowey, 1965 35389 /id;Olander, 1967 29872 /id;Olander, 1971 36322 /id;Riddiford, 1962 35344 /id;Riddiford, 1962 35388 /id;Riddiford, 1966 35349 /id;Rosenheck, 1961 35391 /id;Simmons, 1961 35390 /id;Taylor, 1963 37352 /id;Tsuchiya, 1980 37069 /id;Weisel, 1975 35164 /id}, although data suggesting a different arrangement exist (Elliott et al., 1968a).
Purified paramyosin forms large filaments under appropriate conditions (Echinodermata: Obinata et al., 1975; annelid: Camatini et al., 1976; Castellani et al., 1978; amphioxus: Castellani-Ceresa and Lanzavecchia, 1982; mollusc: {Cohen, 1971 185 /id;Elliott, 1957 24159 /id;Hanson, 1957 24161 /id;Hodge, 1952 24202 /id;Hodge, 1959 38820 /id;Kendrick-Jones, 1969 24199 /id;Locker, 1957 29855 /id}. The filaments are bipolar (Kendrick-Jones et al., 1969). The N-termini of the molecules point toward the center of the filament (Cohen et al., 1971; Weisel, 1975; Panté, 1994), which agrees well with the end polarization (bipolarity) present in real thick filaments (Szent-Györgyi et al., 1971; Lanzavecchia, 1977; Ishii and Takahashi, 1983). Filament diameter increases with increased paramyosin content in both synthetic (Dufhues et al., 1991) and real thick filaments (references above).
Although not present in all synthetic filaments {Camatini, 1976 24200 /id;Castellani, 1978 32320 /id;Hodge, 1952 24202 /id;Locker, 1957 29855 /id;Obinata, 1975 30188 /id}, in many species these filaments have either 72.5 nm periodicity (Limulus: Ikemoto and Kawaguti, 1967; amphioxus: Castellani-Ceresa and Lanzavecchia, 1982; sea urchin: Fukushima and Murakami, 1985; mollusc: Hanson et al., 1957; Tien-Chin et al., 1965; Kendrick-Jones et al., 1969; Cohen et al., 1971; Fukushima and Murakami, 1985; crustacea: Tien-Chin et al., 1965), or a checkerboard pattern (annelid: Weisel, 1975; mollusc: Cohen et al., 1971; Weisel, 1975), which agrees well with the observations on real large diameter thick filaments.
These data and the known length of the paramyosin molecule were then used to explain the multiple types of paramyosin filament staining patterns as arising from variations in a common molecular structure (Kendrick-Jones et al., 1969; Cohen et al., 1971; Cohen, 1998). The fundamental building block of this model is that the paramyosin dimers bind with partial overlaps that result in gaps between axially adjacent molecules (Fig. 3B left, schematic); the overlap plus gap distance in all cases equals 72.5 nm. When the gaps are aligned across the entire filament, a single 72.5 nm banding pattern results (Fig. 3B right, electron micrograph). More complicated patterns, such as the checkerboard in Fig. 3A, would result from axially staggering the pattern in Fig. 3B. These staggers frequently occur at 14.5 nm, which explains both the strong 14.5 periodicity seen in many large thick filaments and the particular spacing seen in the checkerboard (note that in Fig. 3A five axial levels of the checkerboard are 72.5 nm). These staggers resumably arise, as with myosin, because of preferential binding opportunities when paramyosin dimers are displaced this distance (Cohen et al., 1987). As yet unexplained is the observation in annelid (Camatini et al., 1976) and mollusc (Miller, 1965, 1968; Morrison et al., 1970) muscle of a very short (3–6 nm) periodicity.
The larger scale order of paramyosin in large diameter thick filaments is also not well understood. Most early work favored a paracrystalline or stacked flat layer (ribbon) structure (Hall et al., 1945; Bear and Selby, 1956; Elliott et al., 1957; Elliott and Lowy, 1961; Elliott, 1964a; Lanzavecchia, 1966). This was followed by a period in which helical properties were believed to be present in large diameter thick filaments (Elliott and Lowy, 1969; Elliott, 1971), giving rise to models in which the paramyosin dimers were arranged to form a sheet that was then either rolled up like a rug to form the paramyosin filament (Elliott and Lowy, 1970; Lanzavecchia, 1977), or the filament was composed of concentrically nested cylinders of paramyosin sheets (Heumann, 1980). The data indicating helicity were later reinterpreted as being more likely due to the myosin in the filaments or otherwise not indicating a helical structure, and most recent (but still very old) data returned to the paramyosin in molluscan thick filaments having a layered or crystalline structure (Elliott, 1979; Bennett and Elliott, 1981; Castellani et al., 1983; Elliott and Bennett, 1984), with myosin also possibly playing a role in determining paramyosin organization (Castellani et al., 1983).
Despite some early confusion (Philpott et al., 1960), simultaneous work showed that ‘paramyosin’ fibers also contain myosin (Lajtha, 1947; Humphrey, 1949; Tonomura et al., 1955, 1956; Bailey, 1956; Worthington, 1959; Lowy and Hanson, 1962; Heumann and Zebe, 1966; Hardwicke and Hanson, 1971). With respect to how myosin is arranged on the filaments, myosin and paramyosin have α-helical coiled coil regions of similar length, and myosin binds to mollusc thick filaments from which the original myosin has been removed (Szent-Györgyi et al., 1971). Molluscan large diameter thick filaments are therefore believed to consist of a large paramyosin core filament whose surface is covered with myosin (Kahn and Johnson, 1960; Szent-Györgyi et al., 1971; Nonomura, 1974; Elliott, 1974; Cohen, 1982).
Consistent with this interpretation are experiments examining the ability of paramyosin to inhibit myosin’s ATPase activity (Szent-Györgyi et al., 1971; Epstein et al., 1976). This work shows that if myosin and paramyosin are co-precipitated under conditions that do not form thick filaments with normal periodicity (in which the myosin and paramyosin are believed to form an intermingled co-filament), myosin ATPase activity is blocked, but it is not when the co-precipitation occurs under conditions giving rise to what appear to be more normal filaments with a paramyosin core and myosin coat. The large diameter (up to 70 nm) thick filaments present in some annelid muscles appear to also be formed from a large paramyosin core whose outer surface is covered with myosin (Camatini et al., 1976).
Determining how myosin is arranged on the surface of the paramyosin core in large diameter thick filaments has been hampered by the strong paramyosin derived reflections in X-ray diffraction work (Schmitt et al., 1947) (see below for an explanation of X-ray diffraction) and the fact that the electron microscopy work that has been done was done before techniques for three dimensional electron microscopy were developed. Second-harmonic generation studies in mollusc show that the myosin tails continue to be in an α-helix when on the filament surface (Plotnikov et al., 2006), but this technique provides no information about their arrangement on it. Work in Pecten and Crassostrea indicates a helical arrangement of the heads, but does not provide information about how many helices are present or their spacing (Elliott, 1971, 1974). One early work in Mytilus, in which the thick filaments are relatively small (20–60 nm) and have relatively low paramyosin:myosin ratios and strong myosin-based X-ray diffraction, argued for a two stranded helix of myosin with a 72 nm interhelix repeat distance (Sobieszek, 1973). However, the correspondence of this distance with the paramyosin core repeat distance of 72 nm makes this interpretation suspect
This concern is heightened by later work in this muscle showing that the heads on its thick filament were instead arranged in 9 right-handed helices with a 17° slope relative to the thick filament long axis and the heads forming rings around the filament every 14.5 nm (Castellani et al., 1983). Whether the myosin exists as a uniform coat or a series of cables wrapped around the paramyosin core cannot be determined from the data. One other important result of this work is that it showed that the axial repeats of the paramyosin core and the myosin coat were incommensurate, and thus exact matching of the paramyosin and myosin lattices cannot occur. These data thus demonstrate that the arrangement of paramyosin in large diameter thick filament cores does not necessarily determine the arrangement of the myosin on its surface.
Before turning to smaller diameter thick filaments, it is important to comment on paramyosin’s function. Because of its early identification in mollusc muscles, which both develop great force and have a property called ‘catch’, in which the muscles maintain force in the absence of actomyosin cycling (Section 3.5), paramyosin was often posited to exist to subserve either or both of these functions. Modern work shows conclusively that paramyosin is not involved in catch. Furthermore, paramyosin’s presence in almost every invertebrate muscle (including those with relatively small thick filaments) suggests that paramyosin should not be considered a ‘special’ molecule whose presence needs explanation. Paramyosin is instead an everyday constituent of invertebrate muscles, similar, for instance, to the giant sarcomere associated proteins (see Hooper and Thuma, 2005). Indeed, a more salient question might be why vertebrates do not have paramyosin.
Nonetheless, paramyosin content is clearly greatly increased in the thick filaments of some muscles, many of which can generate great force (Lowy et al., 1964; Levine et al., 1976; Mukou et al., 2004). These two properties can be interrelated as follows. The amount of force a sarcomere develops is a function of how many crossbridges are active within it. This is the reason that force increases as thin:thick filament overlap increases, and that longer thick filaments can develop more force (since then at maximum overlap more cross-bridges can be engaged). In all known thick filaments the pairs of myosin heads are arranged in ‘crowns’ that repeat every 14.5 nm along the thick filament length. The number of pairs of heads per crown varies. In the large diameter thick filaments this number is unknown, but in the better-studied small diameter thick filaments it varies from 4 pairs per crown (chelicerate, crustacean abdominal and leg, insect flight muscles) to 7 (scallop), with the thick filaments that have larger numbers of heads per crown having larger diameters (see below and Fig. 5).
Figure 5.
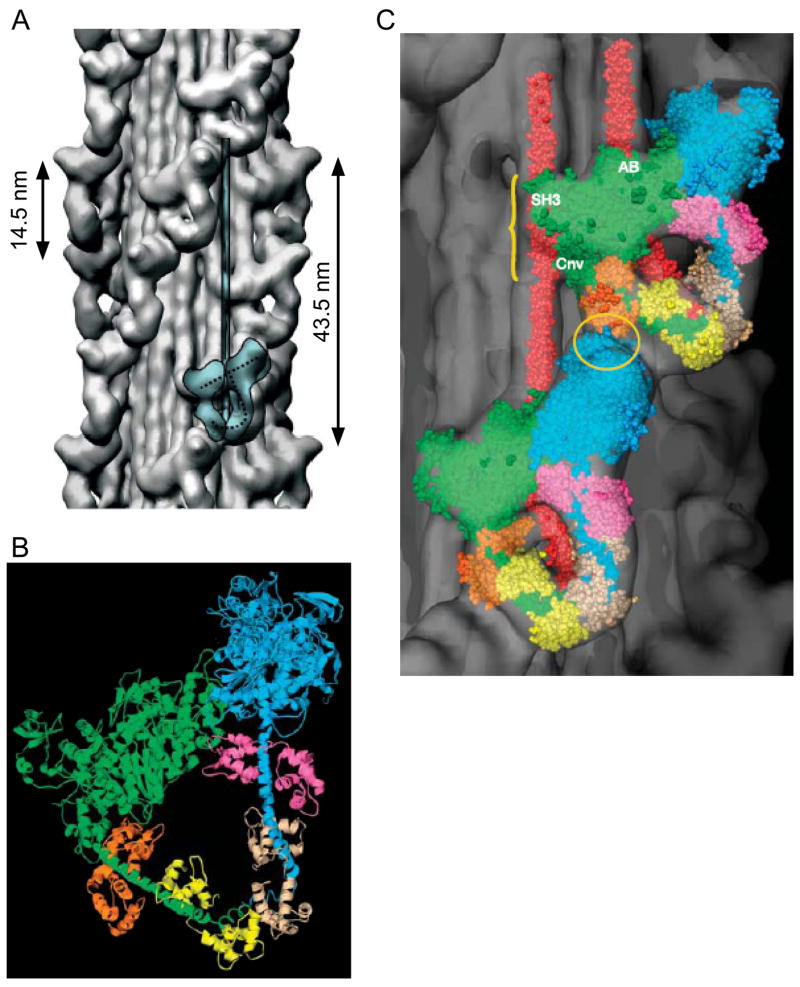
Thick filament strands. A. Surface rendering of three dimensional reconstruction from electron micrographs of tarantula leg muscle. Note helically ascending “hills” (strands) and “valleys”. Blue overlay shows the two myosin heads that form the repeating ‘J’ (dashed line in overlay) motif that forms the strands and a portion of the molecule’s rod in the thick filament body. B. Ribbon representation of two myosin heads (the ‘J’ in panel A) showing that one is free and the other is blocked by binding of its motor domain to the motor domain and essential light chain of the free head. Blue, pink, beige are motor domain, essential light chain, and regulatory light chain of the free head. Green, orange, and yellow are same domains for the blocked head. C. The free head of a crown below binds to the essential light chain of the bound head of the crown above (yellow ellipse). The motor domain of the bound head may also interact with the rod portion of the heads from the crown below (yellow curly bracket). Same color code as in B. Modified, with permission, from Woodhead et al. (2005)
A consequence of this arrangement (assuming that all heads can find thin filaments to bind to) is that each crown of a thick filament with 4 pairs of heads per crown can exert 8 head’s worth of force whereas each crown in a filament with 7 pairs of heads per crown can exert 14 head’s worth of force. If the number of pairs of heads per crown varies strictly with thick filament diameter, each crown of a 160 nm diameter thick filament could thus generate eight times more force than those of a 20 nm diameter thick filament. Assuming that sufficient thin filament binding sites are available, increasing thick filament diameter should thus in its own right increase force production. The number of thin filaments each thick filament interacts does increase with increased thick filament diameter (Lanzavecchia and de Eguileor, 1976; Lanzavecchia, 1977), although whether this increase is sufficient that the heads of large diameter thick filaments have the same chance of binding to a thin filament as do the heads in small diameter thick filaments is unknown.
As noted above, another way to increase the amount of force a sarcomere can produce is to increase thick filament length, and thick filament length does increase with paramyosin content (Levine et al., 1976). Both of these force-increasing effects would increase stress on the thick and thin filaments. Although it has not been shown that large diameter thick filaments are able to bear more tension, it is not unlikely that, as with woven strand steel cables, increased thick filament diameter would increase thick filament resistance to rupture. These observations are thus all consistent with thick filament diameter increasing so as to increase the amount of force a thick filament can produce and to increase thick filament resistance to rupture. Why paramyosin rather than myosin content increases is not known, but attractive hypotheses are that paramyosin:paramyosin binding may be stronger than myosin:myosin tail binding, or that its heads make myosin incompatible with serving as a structural element in very large diameter filaments.
A caveat to this hypothesis, however, is that increasing thick filament diameter increases the volume each filament occupies. Increasing thick filament diameter could thus so decrease thick filament number that this decrease overcomes the increases in per filament force production and tension resistance. This concern is heightened by an observation in mollusc that increased thick filament diameter is associated with decreased thick filament number (Margulis et al., 1979). A study that directly addresses this issue showed that tension per thick filament cross-sectional area was the same or less in mollusc muscles as in frog sartorius (Lowy et al., 1964). Given the greatly increased tension in the mollusc muscles, this work implies that thick filament number did not decrease sufficiently to negate the effects of the increased thick filament diameter. A study comparing strong and weak muscles with small diameter (15–20 nm) thick filaments showed that thick filament number, diameter, and length were all greater in the stronger muscle (Candia Carnevali and Saita, 1976).
3.2.2 Review of X-ray diffraction
Not all invertebrate thick filaments have large paramyosin contents, and myosin head placement is much better understood for these small diameter thick filaments. X-ray diffraction has been a key tool in investigating the structure of these thick filaments. This technique may be relatively little understood by many readers, and has a daunting terminology associated with it. To prepare readers for the original literature we therefore provide a brief background before proceeding to specific cases. Klug et al. (1958) provide a detailed mathematical description of the diffraction patterns expected from helical structures, Squire (1975) and Squire et al. (2005b) are excellent and accessible reviews of theory and application that include some invertebrate data, Al Khayat et al. (2004a) review some modern programs for analyzing X-ray diffraction from such structures, and Wray and Holmes (1981) is a detailed but dated review of X-ray diffraction work in invertebrates.
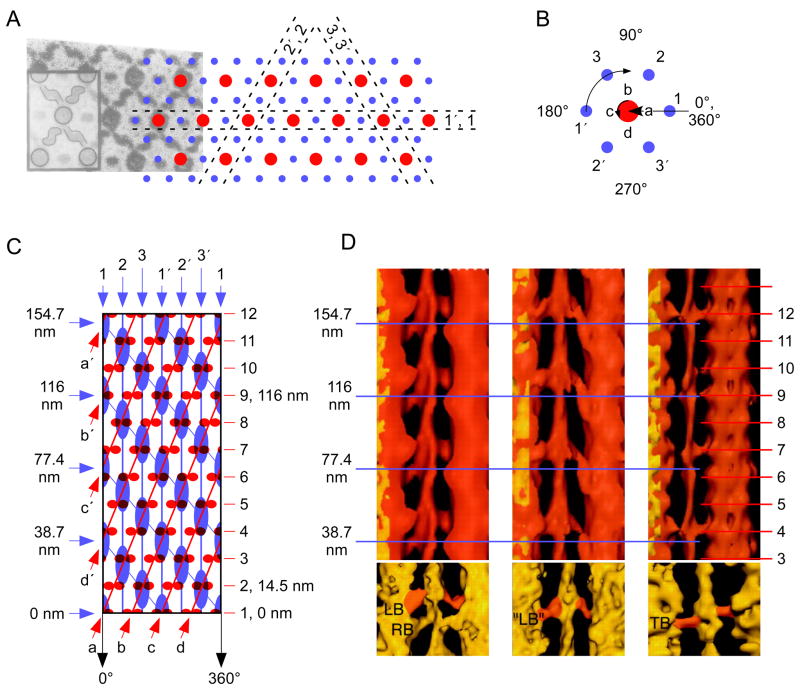
As a first step in this process, consider the cylinder in Fig. 4A. Every 14.5 nm it has on its surface 4 equally spaced (separated by 90°) objects, which could be, for instance, pairs of myosin heads on the surface of a thick filament. At each axial level the set of heads (called a crown) is rotated relative to those on the axial level below; in the example given the rotation is 43.4° to the right, or, equivalently, 47.6° to the left. The heads can be connected by three kinds of lines. The first are circles perpendicular to the cylinder’s long axis that connect the four heads present at a given axial level (black circles). The others are sets of helices formed by connecting a head on one axial level with either of the two closest heads above or below it. Because of the rotation that occurs at each head level, two types of helices can be drawn. In the first each pair of heads is connected to the pair on the level above that is to its right, resulting in a right handed helix (blue lines). In the second, each pair is connected to the pair on the level above that is to its left, resulting in a left-handed helix (red lines). Since there are four pairs of heads at each level, there are four of both the right and the left hand helices.
Each helix of a given type is separated from the helix of the same type above and below by a certain distance (in Fig. 4A, the blue helices are separated by 30.8 nm—double headed blue arrow—and the red helices by 27.4 nm—double headed red arrow), and since the pairs of heads that anchor the helices are equally spaced around the cylinder, all helices of a given type are separated by the same axial distance. Since there are four helices of each type, it follows that any given helix will go completely around the cylinder (from 0 to 360°) in four times the axial distance between each pair of that type of helix. That is, if we identify blue helix 1 as the one beginning at the marked ‘0/360’, blue helix 2 (the helix at the other end of the blue double headed arrow) is 30.8 nm axially above blue helix 1. Blue helix 3 (which is just visible at the far left top of the cylinder) is 30.8 nm above blue helix 2, blue helix 4 is 30.8 nm above blue helix 3, and blue helix 1 is 30.8 nm above blue helix 4. It thus takes 4 × 30.8 = 123.2 nm for a blue helix, and 4 × 27.4 = 109.6 nm for a red helix, to go 360° around the cylinder. From these numbers it is therefore possible to calculate how much each helix rotates per crown (each 14.5 nm axially along the cylinder): (360°/123.2 nm) × 14.5 nm = 43.4° right for the blue helices and (360°/109.6 nm) × 14.5 nm = 47.6° left for the red helices.
These relationships are often also plotted in a ‘net’ format in which one side of the cylinder is sliced axially (in the case at hand, down the hidden side of the cylinder) and the cylinder then laid flat (Fig. 4B). In the example here great care has been taken to retain all angular and distance relationships, and thus, for instance, the blue lines are angled 43.4° to the right of vertical, and the red lines 47.6 to the left of vertical. In most of the primary literature, however, these relationships are not maintained, and thus the angles of the net helix lines are not those present in the cylinders themselves.
This discussion is important because X-ray diffraction results in series of reflections that reveal distances of repeating motifs in the material being examined. Reflections on the meridian arise from repeating motifs that do not vary as a function of angular position on the cylinder (e.g., the circles in Fig. 4A) and non-meridional reflections arise from repeating motifs that do vary (e.g., the helices in Fig. 4A). As such, X-ray diffraction of the cylinder in Fig. 4A would result in a meridonal reflection at 14.5 nm and off-meriodonal reflections at 27.4 and 30.8 nm. As demonstrated above, this information coupled with knowledge of how many objects there are at each axially repeating motif (in Fig. 4, four) allows one to calculate how much each set of objects rotates per axial repeat. For instance, if there were 5 heads per crown instead of 4, it would take 5 × 30.8 for each right handed helix to turn 360°, and thus each set of heads would rotate (right) [360/(5 × 30.8)] × 14.5 = 33.9°.
This simple-minded explanation minimizes the difficulties of interpreting X-ray diffraction data. These diagrams contain information generated by all the repeating motifs in the specimen—the thin and thick filaments and their large-scale arrangement in the sarcomere—and thus contain many more than three reflections. Determining which reflections are due to which aspects of sarcomere structure can thus be difficult (e.g., Worthington, 1959; Hanson and Lowy, 1965; Tajima et al., 1999); for an exhaustive identification of the sources of all the reflections from insect flight muscle, see Reedy et al. (1992). As was shown by the calculation at the end of the above paragraph, another difficulty with these data is that a given set of X-ray diffraction distances are consistent with any number of heads per crown (of helices). The number of heads per crown must therefore be obtained from calculations of the amount of myosin present in thick filaments or direct visualization of the heads on the filaments, and many controversies in this field have stemmed from the difficulty of determining how many pairs of heads are present per crown.
This explanation also minimizes the amount of information provided by these data. Detailed analyses of X-ray diffraction data can reveal both larger scale patterns (e.g., that heads in sequential crowns are not identical) and smaller scale detail (e.g., the angle of the crossbridges relative to the surface of the thick filament) (Wray et al., 1975). However, this discussion is sufficient to read much of the invertebrate X-ray diffraction literature, and it is beyond the scope of this review to explain these more sophisticated techniques.
Before leaving this subject it is important to spend some time on terminology. ‘Subunit axial translation’ is the axial distance between each set of circumferentially linked objects—in Fig. 4A, each set of 4 heads (each crown). In all muscles this distance is about 14.5 nm. ‘Pitch’ is used in two ways. In the first (Elliott et al., 1968a; Sobieszek, 1973; Wakabayashi et al., 1984) it denotes the axial distance for a helix to rotate 360°. In Fig. 4A the blue and red helices have pitches of 123.2 and 109.6 nm, respectively. It is also sometimes used (Wray et al., 1975; Vibert and Craig, 1983; Vibert, 1992) as a synonym for ‘helical repeat’, the distance between sequential helices of the same type. In Fig. 4A the blue and red helices have helical repeats of 30.8 nm and 27.4 nm, respectively. Pitch is never used in the everyday sense of angle from the horizontal.
For all these terms there is no requirement that the objects that anchor the circles or helices be physically present at the distance being referred to. For instance, considering the red helix ‘helical repeat’, if the red double headed arrow is moved to start at any head, there will not be a head where the arrow ends. The distance required for a helix to both repeat and have an object again present is called the ‘axial repeat’ or ‘true repeat’ distance and can be very long. In Fig. 4A the heads rotate to the right 43.4° per head. For helix 1 have turned an integer multiple of 360° and a head to be also present at an angular position of exactly 0/360° thus takes 1800 crowns (the helix having rotated a total of 78,120°, 217 times). However, a reasonably close repeat occurs at 25 crowns and 3 complete helix rotations (helix total rotation, 1080°; head crown rotation 1085°, and thus the head is at 5° instead of 0/360°).
3.2.3 Reconstruction of small diameter thick filaments
A variety of reconstruction techniques (Dover and Elliott, 1979; Dover et al., 1980; Heuser, 1981; Crowther, 1984; Taylor et al., 1986; Taylor and Crowther, 1992; Lucic et al., 2005; and later references in this paragraph) to obtain three-dimensional data from electron micrographs have also been extremely important in defining thick filament structure. This work has shown that most (see below for the exception) thick filaments have prominent helical hills (called strands or tracks) (Fig. 5A). Because of the rotation that occurs going crown to crown, the strands helically ascend the filaments, and in all known cases the strands follow the right handed, longest pitch, helix (i.e., the blue helices in Fig. 4). These strands were long believed to be composed of splayed myosin heads in which one head ascended to interact with a descending head from the crown above, and the other head descended to interact with an ascending head from the crown below (tarantula: Offer and Elliott, 1978; Crowther et al., 1985; Padrón et al., 1995, 1998; scorpion: Stewart et al., 1985; Limulus: Stewart et al., 1985; Levine et al., 1988; mollusc: Vibert, 1992; Levine, 1993).
Recent work in tarantula, however, has shown that the two heads arising from one myosin molecule actually interact with each other, with one head being free and the other bound to the free head’s motor domain and essential light chain (Fig. 5B). The continuous strands result from the motor domain of the free head of one crown interacting with the essential light chain of the bound head on the crown above (Fig. 5C) (Woodhead et al., 2005). Although these data are from only tarantula, the higher resolution of this work, and the fact that earlier data from tarantula were also heretofore interpreted to indicate splayed heads, make the splayed interpretation in the other species highly suspect.
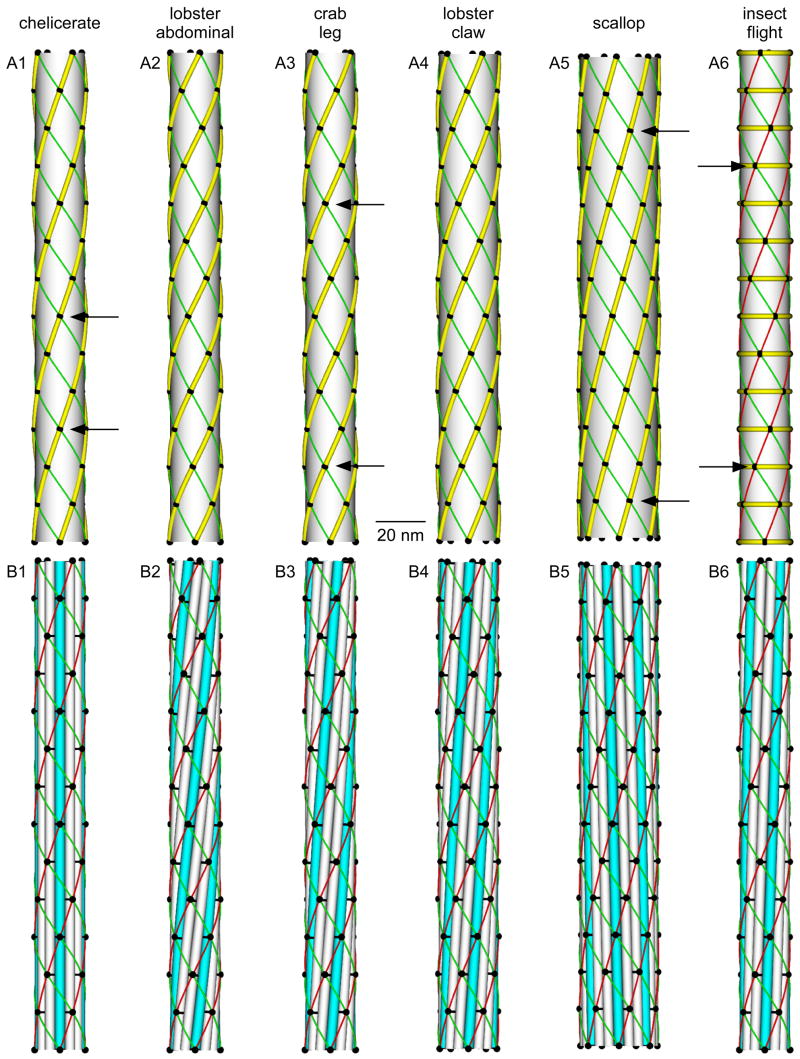
Putting these observations together allows three-dimensional reconstruction of a variety of thick filaments (Fig. 6). Since these reconstructions depend on independent measurements of the number of pairs of heads per crown, they will change if new data change these measurements. However, these changes would not alter the figure’s fundamental point, that small changes in the number and tilt of otherwise well-conserved subfilaments can produce a wide variety of thick filament types. The simplest thick filaments are those of tarantula leg (Offer and Elliott, 1978; Levine et al., 1983; Crowther et al., 1985; Padrón et al., 1992, 1993, 1995, 1998; Offer et al., 2000; Woodhead et al., 2005), scorpion tail (Stewart et al., 1985; Kensler et al., 1985), and Limulus telson (Schmitt et al., 1947; Stewart et al., 1981; Kensler and Levine, 1982a,b; Stewart et al., 1985; Levine and Kensler, 1985; Levine et al., 1988; Ménétret et al., 1990) (Fig. 6A1). These filaments are 20 to 35 nm in diameter (different sources give different diameters even for single species), although some Limulus thick filaments can have diameters as great as 150 nm (Levine et al., 1973). Every 14.5 nm four evenly spaced pairs of myosin heads emerge from the thick filament. The crowns rotate (right-handed) 30° every 14.5 nm (axial repeat distance 43.5 nm for the right handed helix and 21.75 nm for the left handed), and thus the heads come back into angular register every 3 crowns (43.5 nm). For instance, in Fig. 6A1 the two heads marked with arrows occupy the same angular position (are directly above one another). If the distinction between strands is observed, the repeat length (‘axial repeat’) is 12 crowns.
Figure 6.
Possible thick filament structures. Top panel (A1–A6). Strands and head origin placements of tarantula leg and Limulus telson (1), lobster abdominal flexor (striated) (2), crab striated (3), lobster smooth (4), scallop adductor (striated) (5), and Lethocerus flight (6) muscle. In each panel small black circles are head origins (where the heads leave the thick filament), yellow helices are strands (composed of interacting heads as shown in Fig. 5), green and red helices connect closest heads on different crowns (red helices in all but A6 hidden by the strands), and arrows indicate heads in angular register. Bottom panel (B1–B6). Possible subfilament organizations consistent with data in A1–A6. Black circles and red and green helices same as in panel A. Black horizontal lines on subfilaments mark 43.5 nm distances measured along the filaments. Every third subfilament colored blue to provide orientation. Scale bar applies to all dimensions (x, y, z) and to both panels.
Crustacean muscle shows a variety of off-meridonal reflections, with (largest) distances of 30, 30.8, 31, 31.5, and 31.8 nm in different fast crayfish and lobster muscles (Wray, 1979b). This variety results in a family of thick filaments that differ in only the amount of rotation that occurs with each crown. We show here two examples. Lobster abdominal flexor muscle has off-meridional reflections of 30.8 and 27.4 nm, and the filament again has 4 heads per crown (these were the data from which Fig. 4 was constructed). These data give the thick filament shown in Fig. 6A2. On a gross level this filament is very similar to the chelicerate thick filament shown in Fig. 6A1, differing only in small details of head placement. These small changes, however, have a large effect in one respect. In the chelicerate filament the heads come back into angular register every 3 crowns, or 43.5 nm, because the strands (and crowns) rotate exactly 30° every 14.5 nm. Alternatively, as noted in the discussion of Fig. 4, in the lobster muscle it takes 25 crowns for the heads on one strand (the right handed helices) to come into approximate angular register, and 1,800 to come into exact register.
This effect of small changes in crown rotation on how quickly heads come back into register is again shown by examining crab leg muscle (Fig. 6A3), which also has four pairs of myosin heads per crown and has off-meridional reflections of, depending on the source, 33.2 and 25.7 nm (Maéda, 1983) or 33.7 and 25.4 nm (Wakabayashi et al., 1984). Fig. 6A3 was drawn using the Wakabayashi values, which result in the myosin heads coming into register every 7 crowns (with the heads, as in the chelicerate thick filaments, belonging to different helices; it takes 28 crowns for the heads of a single helix to come again into register).
Not all thick filaments have four-fold rotational symmetry. Lobster crusher claw muscle thick filaments have a larger diameter (28 nm) and five-fold rotational symmetry (5 heads per crown, 5 helices in each direction, and 5 strands). Their off-meridional reflections are 35 and 24.8 nm, which result in the filament shown in Fig. 6A4 (Wray, 1979b). Scallop (Placopecten) striated adductor muscle thick filaments have a diameter of 27 nm and seven fold rotational symmetry (Vibert and Craig, 1983; Craig et al., 1991; Alamo et al., 1991). The filament has a surface array of myosin cross-bridges with a 14.5 nm axial period, heads (from different helices) coming into register every 10 crowns, and helical “tracks” with a interhelix spacing (helical repeat) of 48 nm (Fig. 6A5) (Vibert and Craig, 1983). The tracks are presumably the strands observed in other thick filaments.
There was early confusion about how many heads per crown were present in insect flight muscle (Worthington, 1961; Chaplain and Tregear, 1966; Reedy, 1967, 1968; Miller and Tregear, 1972; Bullard and Reedy, 1973; Reedy et al., 1973). Lethocerus flight muscle thick filaments (Fig. 6A6) are now known to have a four fold rotational symmetry and helix structure (off meriodonal reflections 38.5 and 23.3 nm) and diameter (23.5 nm) similar to those of chelicerate thick filaments (Fig. 6A1) (Reedy et al., 1981, 1992; Goody et al., 1985; Beinbrech et al., 1985; Hinkel-Aust et al., 1990; Ménétret et al., 1990; Morris et al., 1991; Schmitz et al., 1994b; Al Khayat et al., 2004a). The altered crown rotation results in this filament’s heads coming back into register every 8 crowns (116 nm) instead of every 3 (43.5 nm). Although enough head mass continues to point along the right handed helices that the filaments have a right handed appearance (Morris et al., 1991), most of the mass of the pairs of myosin heads extends circumferentially around the crowns. Instead of strong strands like the other filaments, this thick filament thus instead has rings around it (Levine, 1997).
The myosin head placements shown in Fig. 6A are consistent with a large number of myosin molecule packing arrangements inside the thick filament. Early modeling work (Squire, 1971, 1972, 1973) of myosin molecules alone suggested that the two packing arrangements most consistent with experimental data were 1) a hollow-cored “curved crystalline” arrangement in which the individual myosin molecules were not arranged in subfilaments and 2) a solid thick filament composed of subfilaments each composed (at any cross section) of three myosin molecules (see below). Squire favored the former model largely because in it all the myosin molecules are strictly equivalent, although some filament based packing schemes also preserve strict equivalence (Miroshnichenko et al., 2000).
However, a variety of later data suggests that many invertebrate thick filaments may nonetheless be composed of subfilaments. First, at least in nematode, thick filament assembly requires both chaperone (Liu et al., 1997; Hutagalung et al., 2002) and additional thick filament ‘core’ proteins (Epstein et al., 1986; Liu et al., 1997; Barral and Epstein, 1999), which suggests that strict molecular equivalence is not necessary for myosin assembly into thick filaments. Second, although (at least in Limulus) thick filaments are not rigid rods (Xu et al., 1991), studies of vertebrate and invertebrate thick filaments have shown that native thick filaments are less flexible than reconstituted thick filaments composed of myosin alone or rope-like filaments such as actin or DNA, and that this rigidity may be due to the thick filaments having tubular cores of non-myosin proteins (Schmid and Epstein, 1998; Barral and Epstein, 1999). Third, in several species (amphioxus, thick filament diameter 25–100 nm: Yongshui and Zuxun, 1979; Helix, thick filament diameter 10–55 nm: Schlote, 1968; Sobieszek, 1973; other species see below) direct evidence for subfilaments has been obtained.
A key element of the subfilament hypothesis was the recognition that the C. elegans myosin dimer coiled-coil tail (in some articles called the myosin rod) has alternating bands of positively and negatively charged residues along its entire length. If two tails are staggered by 14 residues, the positive residues of one are apposed to the negative residues of the other, and hence binding is enhanced. Detailed comparisons show that maximum electrostatic attraction occurs when the tails are displaced by 98 (7 × 14 residues), 294 (21 × 14), or 490 (35 × 14) residues, corresponding to distances of 14.5, 43.6, and 72.8 nm (McLachlan and Karn, 1982, 1983; McLachlan, 1983; Kagawa et al., 1989; Cohen, 1998; McLachlan, 1984 reviews the structural consequences of myosin’s amino acid sequence). All modern subfilament proposals are based on subfilaments composed of the myosin (dimer) molecules being staggered at the 43.5 (3 × 14.5) nm spacing. Since the portion of the myosin tail embedded in the thick filament is approximately 130.5 (3 × 43.5) nm long, this stagger means that at any axial position the subfilament would contain three intertwined myosin tails—the tail of any individual myosin interacts with 2/3 of the lengths of the tails of the myosins above and below it, and 1/3 of the lengths of the tails of the myosins above and below those—which results in a subfilament 4 nm in diameter from which heads emerge every 43.5 nm.
The fact that all thick filaments have crowns of heads every 14.5 nm means that thick filaments would have three times as many subfilaments as the thick filament has pairs of heads per crown, with the subfilaments being staggered axially 14.5 nm as one moves around the filament. That is, if one subfilament gave rise to a head on one crown, the next subfilament over, staggered 14.5 nm, would give rise to a head on the crown above (and the crown two crowns below), the next subfilament over, staggered 29 nm, would give rise to a head on the crown two crowns above (and the crown immediately below), and the third subfilament over, staggered 43.5 = 0 nm, would give rise to the next head around the filament on the same crown.
Figure 6B1 shows this arrangement for the chelicerate thick filament. This filament has 4 pairs of heads per crown, and thus requires 4 × 3 = 12 subfilaments. Twelve 4 nm diameter subfilaments arranged in a tube gives a 20 nm thick filament, consistent with the real thick filament backbone diameter. Each subfilament represents a 30° rotation around the thick filament and the pairs of heads on each crown occur every 3rd subfilament. Therefore, if the subfilaments are arranged parallel to the thick filament long axis, the pairs of heads on each crown emerge from the filament every 90°, each crown rotates 30° relative to the one beneath it, and pairs of heads come into angular register every third crown and perfectly repeat (register and helix the same) every 12. High resolution (2.5 nm) data (Woodhead et al., 2005) from tarantula show twelve 4 nm filaments on the surface of the thick filament running parallel to its long axis (Fig. 5A), in excellent agreement with the model shown. However, immunocytochemistry (Levine et al., 1972, 1986; Elfvin et al., 1979) and electron microscopy (Ikemoto and Kawaguti, 1967) suggest that in Limulus paramyosin forms an internal tube within the thick filament. Since the myosin subfilaments could be arrayed around such a core, these data do not contradict the model shown in Fig. 6B1, but they do indicate it is incomplete.
Figure 6B2 (bottom panel) shows the subfilament model for the lobster abdominal flexor thick filament (Wray, 1979b). This filament again has four pairs of heads per crown, and would therefore have 12 subfilaments. The rotation per crown for this thick filament, however, is 43.3°, not 30°. The 30° rotation of the crowns due to the pairs of heads of axially adjacent crowns arising from azimuthally adjacent subfilaments is thus insufficient for the observed crown rotation. In this model the subfilaments themselves must therefore be additionally tilted relative to the thick filament long axis. This tilt raises a potential difficulty with the subfilament model. In this model the 14.5 nm head placements result from the 43.5 nm head placement on the subfilaments and 14.5 nm staggers of the subfilaments. Since the 43.5 nm distances are along the subfilaments, when the subfilaments are tilted, the axial (vertical) distance between subfilament heads becomes less than 43.5 nm. The effect of this can be seen in the top crown in the figure, in which the helix net head (the center filled circle) is slightly above the subfilament head (horizontal black bar). This difficulty could be overcome by changing the subfilament stagger, but an alternative possibility is that it occurs in real thick filaments. The change in intercrown difference in the case at hand (the most severe of the well understood filaments) is only from 14.5 to 14.4 nm, and reported intercrown spacings do vary from 14.4 to 14.5 nm in different species.
The twelve subfilaments in the crab leg thick filament (Fig. 6B3) must be tilted to a different degree to fit the observed crown to crown rotation. Lobster slow crusher muscle, with its 5 pairs of heads per crown, requires 15 subfilaments (Fig. 6B4) (Wray, 1979b); this increased subfilament number is consistent with this fiber’s larger diameter. Fitting the observed head rotation going crown to crown again requires tilted subfilaments. Experimental work in multiple species is consistent with the hypothesis that crustacean thick filaments are indeed often composed of subfilaments arranged around a hollow or low density core (Gilëv, 1966; Zobel et al., 1967; Yagi and Matsubara, 1977; Ashton et al., 1987; Bard et al., 1987).
The seven fold symmetry of scallop (Placopectin) striated adductor muscle thick filaments requires 21 subfilaments if they continue to be arranged to form a tube (Fig 6B5), which would create a thick filament diameter of 32 nm with 4 nm diameter subfilaments. This is considerably larger than the observed 27 nm diameter (in the figure, the subfilament diameters have been reduced to give the proper thick filament diameter), which suggests that this simple tubular arrangement is incorrect. Incubation of scallop thick filaments in low ionic strength saline results in the filaments fraying into seven sub-filaments, each of which is considerably larger (up to 10 nm) than 4 nm in diameter (Vibert and Castellani, 1989; Castellani and Vibert, 1992), also inconsistent with the simple 21 subfilament model shown in Fig 6B5. Scallop striated adductor contains small amounts of paramyosin (paramyosin:myosin molecular ratio 1:8), which is not present as a separate core structure but is instead a component of each of the seven large diameter subfilaments (Castellani and Vibert, 1992). The molecular packing of paramyosin and myosin in the large diameter subfilaments is unknown, but one possibility is that each of the seven large diameter sub-filaments is composed of three 4 nm myosin subfilaments arranged around one to two paramyosin filaments, which gives a correct thick filament diameter.
Given its 4 fold head symmetry, Lethocerus flight muscle thick filament (Fig. 6B6) would also have 12 subfilaments. The head rotations per crown are such that the subfilaments would lie nearly parallel to the thick filament long axis. Considerable electron microscopic support for this arrangement has been obtained in fleshfly (Beinbrech et al., 1988, 1990, 1992; Schmitz et al., 1994a), housefly (Beinbrech et al., 1988), Lethocerus (Beinbrech et al., 1992; Schmitz et al., 1994a), and honeybee {Schmitz, 1993 22409 /id;Schmitz, 1994 22429 /id;Trombitás, 1986 38774 /id}. This work consistently shows that the 12 subfilaments are arranged into six pairs of closely associated subfilaments, and thus the thick filament has a six-fold symmetry. Early work (before the McLachlan and Wray work suggesting that myosin would form subfilaments composed of three myosin dimers) in Drosophila also found that the thick filament had six fold symmetry and argued for 36 myosin dimers arranged around the circumference of the thick filament, but arranged the dimers as six groups of six dimers, not twelve groups of three dimers (Goode, 1972). Very early work proposing a ‘9+2’ subfilament structure (similar to that seen for tubulin in flagella) for housefly flight (and annelid and human) muscle thick filaments (Baccetti, 1965) is similarly of only historical interest.
The thick filaments of all these species contain paramyosin. Its arrangement in Drosophila is unknown, but in the other species it forms subfilaments (3 in fleshfly, 5 in Lethocerus, and 6 in honeybee) that lie in the center of the thick filament (Beinbrech et al., 1992; Schmitz et al., 1994a). The three fleshfly paramyosin subfilaments are equally spaced around the interior of the thick filament, associate with three of the 6 myosin subfilament pairs, and ‘wobble’ between subfilament triads along the thick filament length. These transitions do not appear to be due to helical twisting of the paramyosin subfilaments. The five Lethocerus subfilaments closely associate with five of the six myosin subfilament pairs, and are only exposed to antibody binding in the H-zone (Bullard et al., 1977). Three of the six honeybee paramyosin subfilaments are equally spaced and associated with three myosin subfilaments; the other three are located eccentrically in the center of the thick filament.
A more complex arrangement has been described in C. elegans. C. elegans thick filaments consist of a tubular core composed of six proteins including α, β, and γ filagenin and one post-translationally modified paramyosin isoform (Epstein et al., 1985, 1988; Epstein, 1988; Anderson, 1989; Deitiker and Epstein, 1993; Liu et al., 1997, 1998, 2000; Barral and Epstein, 1999). Surrounding this core is a sheath of seven subfilaments composed of a second postranslationally modified paramyosin isoform. Each subfilament is most likely composed of 4 strands of paramyosin molecules staggered by 72 nm with respect to each other, resulting in a 22 nm gap between consecutive paramyosin molecules (Kagawa et al., 1989; Epstein et al., 1995; Liu et al., 1997; Barral and Epstein, 1999; Müller et al., 2001). Myosin surrounds this core plus sheath structure. The myosin A isoform is located in the central (M-line) region of the thick filaments, and the myosin B isoform on the distal regions of the filament (Miller III et al., 1983; Epstein, 1985, 1988; Barral and Epstein, 1999; Müller et al., 2001). The myosin A and B isoforms always form homodimers (Schachat et al., 1977, 1978). Despite this great knowledge of the core structure in this species, how the heads are arranged on the thick filament is unknown.
With respect to thick filament assembly, although C elegans myosin heavy chain protein and paramyosin form filaments with a paramyosin core and myosin coat in vitro (Harris and Epstein, 1977), these filaments are not identical to native thick filaments (Epstein et al., 1993). Proper thick filament assembly instead requires a chaperone protein (Liu et al., 1997; Hutagalung et al., 2002), which may also be a permanent, myosin B associated thick filament component (Ao and Pilgrim, 2000), and thick filament ‘core’ (Epstein et al., 1986; Liu et al., 1997) and other (Mercer et al., 2006) proteins including paramyosin (Epstein et al., 1987). Missense mutations in the globular head portion of the myosin heavy chain, including the ATP binding site, also disrupt thick filament structure. Thick filament assembly thus does not depend on the rod portion alone (Bejsovec and Anderson, 1990). Conserved regions in the C-terminals of the paramyosin and myosin rods important for paramyosin:myosin interaction have been identified in nematode and other species (Cohen et al., 1987; Cohen and Parry, 1998; Hoppe and Waterston, 2000). Single charge changes on the paramyosin rod can disrupt thick filament assembly (Gengyo-Ando and Kagawa, 1991).
Work in Drosophila shows that, although myosin molecules consisting only of the rod portion can form thick filaments, the filaments are of incorrect length, and result in aberrant myofibrils (Cripps et al., 1999). Work with synthetic thick filaments suggests that both projectin, a thick filament associated giant protein (see Hooper and Thuma, 2005), and paramyosin affect thick filament length (Kölsch et al., 1995). Similar results are obtained in locust and show that the effects depend on projectin phosphorylation state (Fährmann et al., 2002). The genetic advantages of C. elegans (Epstein, 1988, 1990) and Drosophila (Fyrberg and Beall, 1990; Vigoreaux, 2001) should eventually allow detailed understanding of thick filament assembly and myofibril structure in these species. Landsverk and Epstein (2005) review thick filament assembly in them.
3.3. Actin-myosin interaction and force generation
Very early work on muscle contraction used light microscopy to measure changes in whole muscle birefringence during contraction, stretch, etc. These changes stem ultimately from changes in thick:thin filament arrangement, but we find these papers so far from our training and experience that we cannot interpret them from a modern point of view. Most of this work was performed on vertebrate muscle, but invertebrate papers from this period include {Bozler, 1937 38708 /id;Fischer, 1936 38673 /id;Fischer, 1938 22794 /id;Fischer, 1938 34713 /id}.
Turning to the post sliding filament theory period, all invertebrate muscles contract via the typical sliding filament mechanism. The maintenance of a 14.5 nm intercrown distance in all thick filaments suggests that this spacing is functionally important for interaction with the thin filament, with its 38–44 nm helix repeat. The least common multiples of these distances is between 3 to 8 crowns and 1 to 3 actin helix repeats (for a 44 nm actin repeat, 3 × 14.5 = 43.5; for a 38 nm actin repeat, 5 × 14.5 = 72.5 and 2 × 38 = 76 or 8 × 14.5 = 116 nm and 3 × 38 = 114 nm). Although these seem odd multiples, it is important to realize that there are many more thin filaments than thick (6 or more in striated muscle, and as many as 10 to 20 in smooth), and all thick filament heads need not simultaneously engage the thin filament. Furthermore, the variety of head placements shown in Fig. 6 (even if the subfilament organization shown is incorrect, the head placement is constrained by experimental data), and the presence in some thick filaments of extremely long repeat distances, suggests that a variety of thin to thick filament helix staggers can create efficient thick/thin filament force generation. In many muscles it may thus suffice simply to have enough thin filaments that the thick filament heads can generally find a thin filament to bind to without the thin and thick filaments being arranged in some ‘ideal’ fashion with respect to their respective helix repeats. This conclusion is supported by work showing that physiologically relevant forces applied to thick filaments can lengthen them 23% in Mytilus and 66% in Limulus (Neumann et al., 1998). These length changes are presumably great enough, unless compensated for by matching changes in thin filament length, to alter thin:thick filament helix staggers.
3.3.1. Asynchronous flight muscle
Some muscle thin and thick filaments, however, are organized with great regularity. Insect asynchronous flight muscle is a particularly well studied example (Reedy, 1967, 1968; Reedy et al., 1973, 1981, 1993; Heuser, 1983; Morris et al., 1991). Great care must be taken in reading this literature. Due to early difficulties in estimating myosin mass in the sarcomere, some of these articles incorrectly identify the number of pairs of myosin heads present per shelf, helix number, or helix direction on the thick filament (Reedy, 1967; Reedy et al., 1973). Others, although technically correct in that they refer to the helices of the cross-bridges in rigor (see below), not the thick filament itself, as being left-handed, can still be confusing unless carefully read (Reedy, 1968; Heuser, 1983). An additional potentially confusing early observation (Zebe, 1966) of ATPase activity in insect flight muscle Z-lines after myosin extraction from the myofibril presumably results from the activity of Z-line located sarcomere accessory proteins (see Hooper and Thuma, 2005). General reviews of asynchronous muscle are listed in that section of this article. Cooke (1986), Highsmith and Cooke (1983), and Holmes and Goody (1984) are dated reviews of force generation in general that cover asynchronous muscle in part.
In asynchronous flight muscle the thick and thin filaments are arranged in hexagons with each thick filament surrounded by six thin filaments. The left portion of Fig. 7A shows an electron micrograph of a Lethocerus (asynchronous) flight muscle in rigor and the right portion is a continuing schematic identifying the thick (large red circles) and thin (small blue circles) filaments. The regular thin filament interfilament spacing is presumably determined by the Z-line structure. What determines thick filament spacing is unknown. Transverse stiffness varies as a function of contraction state (rigor > contracting > relaxed) (Nyland and Maughan, 2000), and thus one possibility is that interaction with the surrounding cage of thin filaments is a sufficient mechanism. Thick filament spacing (56 nm in Drosophila) does not vary during contraction (Irving and Maughan, 2000).
Figure 7.
Thin and thick filament organization and interactions in Lethocerus asynchronous flight muscle. A. Electron micrograph of cross section through muscle (left portion) merging into a schematic of thick (red circles) and thin (blue circles) filaments. Corridors defined by dashed lines mark thin filaments that are in helical register; all thick filaments are also in helical register. Cross bridges are apparent in electron micrograph, inset schematically shows different cross bridge shapes. B. Single thick filament (red circle) and its surrounding thin filaments (small circles). Letters on thick filament indicate head origins, arrow indicates that the filament is right handed. Numbers indicate pairs of thin filaments that are in helical register. Arrow indicates left handed helix of preferred binding sites, helix rotates 60° with each thin filament. C. Spatial relationship between myosin heads (small red ellipses) and thin filament preferred binding sites (large blue ellipses). ‘Unrolled’ and laid flat representation of the arrangement shown in panel B rotated so that the thin filaments (thick vertical blue lines, numbers and blue arrows on top of box) lie in the plane of the figure. Myosin heads leave the thick filament every 14.5 nm, actin helices (leftward slanted thin blue lines) repeat every 38.7 nm (blue arrows, numbers on left of box), preferred thin filament binding sites repeat every 12.9 nm. The thin rightward slanting red lines labeled ‘a–d’ are the right handed helices connecting myosin heads; letters with primes show continuation of helices that have ‘run off’ the right side of the box. D. Average (top panels) and representative individual (bottom panels) three dimensional electron micrograph reconstructions of two thick filaments and an interposed thin filament in rigor (left panels) and pharmacological treatments that reduce thick:thin filament binding (middle and right panels). Numbered red lines on right represent shelf positions, numbered blue lines on left preferred binding sites on the thin filament. In each case lines exactly correspond to those in panel C. Modified from Reedy and Reedy (1985) (A), Wray (1979a) and Schmitz et al. (1994a) (C), and Schmitz et al. (1997) (D).
Figure 7B shows a single thick filament and its surrounding six thin filaments. Each thick filament has four myosin molecules (marked a–d in panel B), each with two heads, equally spaced around the filament. The 8 heads repeat axially every 14.5 nm; in these muscles the heads do not form strands, but instead appear in relaxed muscles as shelves (red horizontal lines numbered 3–12 in Fig. 7D, right panel). Each shelf rotates 33.75° relative to the one beneath it in a right handed fashion (curved arrow on thick filament in panel B). The filament thus has four right-handed helices. In 8 shelves the helices rotate 8 × 33.75° = 270°. Since the helices are separated by 90°, this rotation brings the head of the strand that eight shelves below was at 90° into the 0° position (270° + 90° = 360°). If one ignores which helix the heads belong to the heads thus assume the same angular position every eight shelves. If this is unclear, note that in Fig. 7C on shelf 1 strand b has a pair of heads at 90°. By shelf 9 strand b has rotated 270°. Strand b’s pair of heads on this shelf is therefore directly above strand a’s 0/360° pair of heads on shelf 1. A complete 360° rotation of the same helix’s heads requires 4 × 8 = 32 shelves, because, although each helix repeats in 360/33.75 = 10.67 shelves (154.67 nm), since this is not an integer shelf number, no head occurs at this position. See Fig. 6A6 for a three dimensional view of this filament.
The thin filaments make a complete helix rotation every 38.7 nm, and so make three rotations in the 116 nm (8 shelves) it takes for the thick filament heads to return to the same angular position. Each thin filament pair on opposite sides of the thick filament is in helical register. Moving around the thin filament hexagon, each pair is rotated 60° relative to the preceding pair, but in the opposite direction to the thick filament’s rotation. That is, a line marking equivalent positions going from thin filament to thin filament would form a left handed helix (small curved arrow on thin filaments in Fig. 7B). Note that this left handed helicity has nothing to do with each thin filament’s intrinsic, right handed helical nature. It instead arises from rotation of entire thin filaments relative to each other. This rotation, coupled with the thin filament helix repeat of 38.7 nm, means that, moving axially along the filament array, adjacent thin filament pairs present identical configurations to the thick filament every 38.7/3 = 12.9 nm.
This arrangement can be difficult to visualize, and Fig. 7C is a two-dimensional representation (a radial projection) of a single thick filament (red) and its six surrounding thin filaments (blue) (Wray, 1979a; Schmitz et al., 1994b). In this representation one thin filament and half the thick filament are ‘cut’ through (horizontal arrow in Fig. 7B). The thin filaments and the thick filament are then ‘unrolled’ to form a row of thin filaments with the opposed portion of the thick filament circumference being adjusted so as to maintain the correct angular relationship between the thick filament surface and the surrounding thin filaments (so that ‘a’ on the thick filament in panel C continues to be opposite thin filament 1, ‘b’ continues to be between thin filaments 2 and 3, etc.). This array is then rotated so that the thin filaments lie vertically in the plane of the figure (the 1–3′ thin filament, a–d thick filament, and 0–360° angular labeling in panels B and C exactly correspond).
This representation allows easy visualization of the axial and azimuthal relationships between the thick (red) and thin (blue) filaments. The small paired red ovals represent the myosin heads and the right-ward slanting lines labeled a–d are the four thick filament helices. When each helix ‘runs off’ the right side of the unrolled filament it reappears on the left (helix d runs off and reappears, as d′, between shelves 3 and 4). A shelf of eight heads occurs every 14.5 nm, each rotated 33.75° relative to the one beneath it. On the ninth shelf (a 116 nm axial distance) the heads of helix b occupy the same angular position as did the heads of helix a on shelf one. The thin filaments are represented as vertical blue lines, with the ‘target areas’, positions on the thin filament where the thick filament heads can bind, shown as ellipses. In each thin filament these target areas recur axially every 38.7 nm. Since each thin filament pair on opposite sides of the thick filament (1, 1′; 2, 2′; 3, 3′) is in register, each pair’s target areas occur at the same axial position. The 60° rotation as one moves around the thin filament hexagon in Fig. 7B results in the target areas being displaced axially 12.9 nm between thin filaments 1 and 2, and again between 2 and 3, at which point the pattern repeats.
Consideration of this diagram raises three important issues. First, the combination of the four fold symmetry of the thick filament and the six fold symmetry of the surrounding thin filament cage means that thick filament binding opportunities are identical on opposite sides of the thick filament. For instance, considering shelf 2, the right head of the ‘a’ helix can just barely bind to the first target area of thin filament 2. The right head of the ‘c’ helix, which is on the opposite side of the thick filament, can similarly just barely bind to the first target area of thin filament 2′, located directly across the thick filament from thin filament 2. Second, with the exception of this symmetry across the thick filament, all binding opportunities differ for all pairs of heads inside the 116 nm (8 shelf) unit cell, as can be clearly seen by noting that the overlap between heads and target areas differs for every head on thin filaments 2, 3, and 1′ on shelves 2 through 9. Third, which heads and target areas overlap, and the total amount of overlap, varies as thin:thick filament stagger changes (e.g., with vertical translations of the thin filament array). This changing overlap may be a source of sarcomere length dependent variation in force production in these muscles (see Section 3.6).
All thick filaments, and all equivalent thin filament pairs (1, 1′; 2, 2′; 3, 3′), are in helical register across the entire myofibril (Reedy, 1968). How this extraordinary regularity arises is not completely understood. The simplest explanation would be that all the filaments have identical length and are perfectly lined up axially and azimuthally. Examination of thick filament backbones, however, shows that the filaments appear to be randomly oriented azimuthally (Freundlich and Squire, 1983; Beinbrech et al., 1990) and are clearly not in axial register, instead being randomly displaced one to another in multiples of 14.5 nm (Haselgrove and Reedy, 1984). These observations lead to the conclusion that thick filament helical registration across the myofibril occurs by thick filament axial translation and azimuthal rotation changing coordinately, so that if two thick filaments are displaced 14.5 nm (or multiples thereof) to one another they are also rotated ±33.75° (or multiples thereof) to bring them into helical registration (e.g., if the thick filament in Fig. 7C is translated downward 14.5 nm—one shelf—and also rotated 33.75° right or left, identical head positions occur) (Schmitz et al., 1994b).
Although this would not result in truly random thick filament azimuthal positions, the number of possible azimuthal/axial combinations (14 when the 4-fold symmetry and 8 shelf repeat of the thick filament are taken into account) is large enough that the underlying order would be undetectable with present techniques. What mechanism supports this coordination between translation and rotation is unknown. Two hypotheses are that it is imposed by interactions with the highly ordered thin filament lattice (Haselgrove and Reedy, 1984; Schmitz et al., 1994b) or from M-line thick filament interactions that couple 14.5 nm translations and 33.75° rotations (Schmitz et al., 1994b). The source of the helical order in the thin filament lattice is unknown, but presumably results from great regularity in the Z line structures from which the thin filaments originate.
This regularity allows for extremely detailed experimental analysis. For instance, because all the thick filaments and all equivalent thin filament pairs are in helical register across the sarcomere, in cross sections containing only one shelf the cross bridge pattern within the thin filament hexagons is identical across the section. For instance, assume that the head to target area overlaps on shelf 2 in Fig. 7C would both result in cross-bridge formation, and that the cross-bridges would have different shapes because of the different overlaps. In this case a cross section including only shelf 2 would show only two crossbridge shapes. The first would result from the strong overlap between the heads on helices b and d and the target areas on thin filaments 3 and 3′, and the second from the weak overlap between the heads on helices a and c and the target areas on thin filaments 2 and 2′.
The helical registration of the thin filaments across the sarcomere also means that transverse sections containing only a single row of thick filaments (myosin:actin or ‘myac’ slices; the dashed line pairs in Fig. 7A) will also contain exactly matched thin filament pairs (Taylor et al., 1989a). That is, if the horizontal slice in Fig. 7A contains only 1′ and 1 filaments, then the rightward slanting slice will contain only 2′ and 2 filaments and the left ward slanting slice only 3 and 3′ filaments. Due to the changing degree of head and target area overlap as one moves axially along the array, crossbridge shape should vary axially in such slices with a 116 nm period, which allows averaging as a function of position in the unit cell and thus great power in examining cross bridge shape. Taking the slice at a slightly different position will result in slices containing only actin filaments (Taylor et al., 1989b). Of course, such slices will contain only two of the three thin filament types; e.g., if the horizontal slice in Fig. 7B contained the 1 and 1′ thin filaments, an actin slice immediately above it would contain only 2 and 3 type thin filaments.
These advantages have resulted in asynchronous flight muscle being intensively studied. Techniques for preparing the muscles for electron microscopy and X-ray diffraction in relaxed, rigor, and nucleotide (e.g., ATP) attached states have been fully developed (Reedy et al., 1983b; McDowall et al., 1984; Ménétret et al., 1988). Techniques for identifying, averaging, and extracting three-dimensional information from the repeating motifs present in electron micrographs {Al Khayat, 2004 37678 /id;Winkler, 1999 23274 /id} and of X-ray diffraction patterns (Squire et al., 2003a) of asynchronous muscle are well-developed. Structural modeling began early in asynchronous muscle studies (Holmes et al., 1982), and protocols for fitting atomic models of actin and myosin head and neck regions to cross-bridge images are now also well-developed (Chen et al., 2001). Asynchronous muscle has been studied in four states: rigor, semi-relaxed by the addition of non-hydrolyzable ATP analogs or similar treatments, relaxed, and actively contracting.
Due to its great inherent order, by far the most work has been performed on rigor muscles. 70%–80%, 5.6–6.4 of the eight myosin heads at each crown, are attached in rigor (Miller and Tregear, 1970; Holmes et al., 1980; Thomas et al., 1983; Heuser, 1983; Goody et al., 1985); the estimate in Holmes et al. (1980) is low because they thought the myosin shelves had 6 heads instead of 4. X-ray diffraction (Reedy et al., 1965, 1983a; Miller and Tregear, 1972; Rodger and Tregear, 1974; Holmes et al., 1980), electron paramagnetic resonance (Thomas et al., 1983; Reedy et al., 1992), and electron microscopy of cross-sections (Reedy, 1968; Rayns, 1972; Reedy and Reedy, 1985; Heuser, 1987; Taylor et al., 1993) and myac (Reedy et al., 1965, 1983a; Reedy, 1967, 1968; Heuser, 1983, 1987; Taylor et al., 1984, 1989a, 1993; Reedy and Reedy, 1985; Schmitz et al., 1996) or only actin (Taylor et al., 1989b) layers all agree that in rigor rigid cross-bridges with relatively uniform angles relative to the thick filament long axis are uniformly present across the sarcomere.
This high level of myosin head binding, and the fact (see below) that both heads of single myosin bind to the same thin filament, means that at most shelves there will be cross-bridges to preferred binding sites on four of the surrounding thin filaments. The four cross bridges create a characteristic ‘flared-X’ motif (electron micrograph, Fig. 7A) in which two cross bridge shapes can be identified, one a ‘dog-leg’ (upper left, lower right in inset) and the other a ‘sigmoid’ (lower left, upper right in inset). Examining the cross-bridges in the micrograph shows that these shapes are present at every cross-bridge at this axial level. The cross-bridges project in the directions they do because the target areas of only the four thin filaments located to the upper and lower right and to the upper and lower left of the thick filaments, and not the target areas of the thin filaments directly to the right and left of the thick filaments, are in the appropriate configuration for myosin head binding. Using the labeling in Fig. 7b, the target areas of thin filaments 2′, 2, 3, and 3′, but not those of 1 and 1′, are available.
Considering Fig. 7C shows that equivalent target areas appear on the thin filaments every 12.9 nm, but rotated left 60°. Cross sections through asynchronous muscle at different axial levels show precisely this pattern, with the cross-bridges rotating leftward every 12.9 nm (Reedy and Reedy, 1985). For instance, on the next level up in Fig. 7A, the cross-bridges would bind to thin filaments 2 and 2′ (dogleg) and 1 and 1′ (sigmoid), but not 3 and 3′. However, Fig. 7C also shows that the amount of head overlap through the 116 nm unit cell varies on each 12.9 thin filament target layer, and thus one would predict that the shape or presence of all four cross-bridges would vary with axial position. Experimental difficulties prevent ‘stepping’ through the entire unit cell in this manner, but this prediction is borne out by the observation that the dog-leg cross-bridges are not present on all levels.
The top left panel of Fig. 7D shows an averaged three dimensional reconstruction (an averaged tomogram) of four cross-bridges between two thick filaments and an interposed thin filament from a myac layer of a rigor asynchronous muscle. Since there is only one thin filament here, cross-bridges only form every 38.7 nm (see Fig. 7C), with the thick filaments making one (right thick filament) or two (left thick filament) cross-bridges to the thin filament every 38.7 nm. If the reconstruction included the next thin filament to the right, this average cross bridge pattern would exactly repeat (i.e., the right thick filament would make two cross-bridges at each axial level to the next thin filament; put another way, under these conditions each thick filament made on average one cross-bridge to the thin filament on its left and two to the one on its right). It is important to note that this high regularity is only present in the average. In unaveraged tomograms crossbridge number varies from one to four along the filaments and instances in which two crossbridges are to the right and only one to the left are also present, although the shown configuration is of course most common.
The bottom left panel shows an unaveraged tomogram of a position in which both thick filaments made cross-bridges with the thin filament. Because of the slant of each cross-bridge relative to the thick filament (the slant is more evident in simple electron micrographs than in this three-dimensional reconstruction) double cross-bridges are also called double chevrons and single cross-bridges chevrons. The upper (closer to the M line) cross-bridge is called the lead cross-bridge (LB) and the lower the rear (RB). Troponin is visible in unaveraged electron micrographs as a small bead just below (Z-ward) the rear cross-bridge (Taylor et al., 1993; Reedy et al., 1994a). X-ray diffraction work shows that the myosin head shape in these rigor cross-bridges differs from its shape in resting muscle (Squire et al., 2003a).
It is important to make several observations about these data. First, note that all indications of 14.5 nm periodicity are completely lacking in the thick filaments (the numbered red horizontal lines in the right panel in Fig. 7D exactly correspond to the 14.5 nm shelf spacings in Fig. 7C) with the 38.7 nm periodicity of the thin filament target lattice instead completely dominating the figure (the blue horizontal lines in the panel exactly correspond to the 38.7 nm actin repeat spacings in Fig. 7C) (Taylor et al., 1993). This decrease in 14.5 nm periodicity is also seen in X-ray diffraction data {Beinbrech, 1972 18937 /id;Ménétret, 1988 37735 /id;McDowall, 1984 37736 /id;Reedy, 1965 2744 /id;Reedy, 1983 2743 /id;Reedy, 1983 2742 /id}. It is thus not an artifact of preparation of the myac slices or preparation for electron microscopy, as is also shown by the 14.5 nm periodicity being present in myac slices in relaxed muscle (right panel Fig. 7D). A similar loss of 14.5 nm periodicity occurs when isolated thick filaments are transferred from relaxing (ATP present) to rigor (ATP absent) conditions, and thus it does not depend on interaction with the thin filament lattice (Clarke et al., 1986).
Second, since this is a myac layer, only data about cross-bridges to a single thin filament class (say, 1 and 1′ in Fig. 7A) are obtained. However, given the symmetry of the thick filament and its surrounding thin filaments, there is no reason to expect that myac sections that included other thin filament classes would show different data. As such, 12.9 nm (38.7 nm divided by 3, note that in Fig. 7C the preferred binding sites of each matched pair of thin filaments is axially staggered by 12.9 nm) axially above each of the cross-bridges in the upper left panel of Fig. 7D there should be a set of cross-bridges angled ±60° to the plane of the figure attaching to thin filaments 3 and 3′, and 12.9 nm axially above that set of cross-bridges a set angled ±120 to the plane of the figure attaching to thin filaments 2 and 2′.
This recognition allows connecting the rigor myac and cross-section data as follows. The lower left panel of Fig. 7D shows that the rear bridges leave the thick filament some 10 to 12 nm below the lead bridges. This is approximately the same axial position as the position from which the lead bridges going to the target area 12.9 nm lower, the ones binding to the previous set of thin filaments angled ±120° to the plane of the figure, leave the thick filament. A cross-section thin enough to include only these two cross-bridges, the lead cross-bridge going to one target area and the rear cross-bridge going to the target area 12.9 nm above, would thus show four cross-bridges (four because both a lead and rear cross-bridge also leave the opposite sides of the thick filament). It is these four cross bridges that give rise to the arms of the flared X in cross sections, with the lead cross-bridges forming the sigmoid cross bridges and the rear cross-bridges the dog-leg cross-bridges (Reedy and Reedy, 1985).
Third, the lead and rear cross-bridges have very different shapes and angles (approximately 50° for the lead bridges and 80° for the rear) relative to the filament axis (Taylor et al., 1984, 1989a,Taylor et al., b; Reedy and Reedy, 1985; reports—Trombitás et al., 1986, 1988—of a wide range of angles being instead present being apparently in error). The shape differences arise because the lead bridges bind to the thin filament at sterically advantageous positions near the center of the target area whereas the rear bridges bind to the edge of the target area where considerable myosin bending and realignment is required (Reedy and Reedy, 1985; Schmitz et al., 1996). Most lead bridges consequently consist of two myosin heads but most rear bridges only one (Taylor et al., 1984, 1989a,Taylor et al., b, 1993; Reedy and Reedy, 1985; Schmitz et al., 1996). The difference in lead and rear cross bridge angle is believed to occur either because the rear cross-bridges cannot deliver their entire power stroke because of the already attached and fully rotated lead bridges, or because the rear bridges do fully rotate and in so doing rotate the lead bridges beyond the angle they would normally occupy after their power stroke (Taylor et al., 1984).
Modeling indicates that formation of the double chevron configuration requires target areas consisting of 3–5 actin monomers along each turn of one strand of the actin helix and that myosin molecules reach 10–14 nm axially and as much as 90° around the thin filament (Haselgrove and Reedy, 1978). That such bending actually occurs is supported by observations that 1) myosin heads are highly flexible (e.g., they can bind to thin filaments oriented in the ‘wrong’ direction) (Reedy et al., 1989) and 2) the S2 region of the myosin molecule, which links the myosin heads to the thick filament backbone, assumes angular ranges of 90° axially and 120° azimuthally in swollen rigor fibers (Liu et al., 2006). This bending is sufficient that under these conditions some of the cross-bridges likely generate drag rather than contraction promoting force. The modeling work also shows that the thin:thick filament stagger that leads to the greatest cross-bridge number (as occurs in rigor) would produce double chevron and flared X formations (Haselgrove and Reedy, 1978, 1984).
Fourth, in addition to the large scale average differences between lead and rear cross-bridges, one also expects systematic variation in cross-bridge shape within the 116 unit cell due to the variations in myosin head to target area overlap shown in Fig. 7C, a prediction also made by more realistic, detailed models (Haselgrove and Reedy, 1978, 1984). This prediction is exactly borne out by studies which look in close detail at the shape of the rigor cross-bridges across the unit cell, which show not only changes in cross-bridge shape (Reedy et al., 1983b; Morris et al., 1991; Taylor et al., 1993; Chen et al., 2002; Liu et al., 2004, 2006) and number (Taylor et al., 1993), but also that lead cross bridges containing only a single head, and rear cross-bridges containing two heads, are also present (Chen et al., 2002).
Relevant to this point it is important to stress again the degree to which in rigor the thin filament target zones overwhelm the thick filament’s intrinsic helical structure. In the 116 nm unit cell there are 8 thick filament shelves, each separated by 14.5 nm, and 9 thin filament target areas, each separated by 12.9 nm. Yet in rigor tomographs (upper left panel Fig. 7D) all sign of 14.5 nm rhythmicity is completely lost in the thick filaments, possibly due to the rotational orientation of the thick filament being altered in rigor (Beinbrech et al., 1990). Furthermore, for 8 shelves to result in cross-bridges to 9 target areas requires that some shelves extend cross-bridges to more than one target area. This apparent strong reorganization of the thick filament is also shown by the flared X structure, in which the cross-bridge origins no longer appear equally spaced at 90° around the thick filament, but the lead and rear (sigmoid and dogleg) cross-bridges instead appear to arise from a common origin (right schematic Fig. 7B) (Reedy, 1968). How shelf origin and cross-bridge target area varies across the unit cell is not yet known. One early hypothesis was that the two heads of individual myosin molecules might project to more than one thin filament (Offer and Elliott, 1978; Offer et al., 1981). Although the myosin heads and necks have sufficient flexibility and reach for this to be a theoretical possibility, it is now believed that in almost all cases in which both heads of a myosin molecule bind to a thin filament, they bind to the same thin filament (Freundlich et al., 1980; Taylor et al., 1984, 1989a,b, 1993).
Fifth, the extremely large cross-bridge number results in extremely strong binding between the thick and thin filaments, so strong that when stretched rigor muscle sarcomeres will rupture before filament sliding occurs (Reedy et al., 1993). Sixth, the strong triple binding in rigor induces a conformational change in the thin filaments, with them being nearly untwisted and widely separated near the lead crossbridge and normally spaced but overtwisted near the rear (Taylor et al., 1984, 1989a,Taylor et al., b, 1993; Reedy and Reedy, 1985).
Although these observations are highly suggestive, the non-physiological nature of rigor calls their physiological relevance into question. A first step in coupling these observations to physiological contractions was the use of pharmacological treatments that reduce rigor tension but not stiffness (pyrophosphate or the non-hydrolyzable ATP analog adenosine 5-[β,γ-imido]triphosphate (AMPPNP)) or that reduce both (AMPPNP with ethylene glycol). The pyrophosphate work showed that the tension reduction it induces (White, 1970; Kuhn et al., 1972) differs from that induced by ATP in that it does not promote thick and thin filament disaggregation (Winkelhahn and Beinbrech, 1974). Electron microscopy and X-ray diffraction showed that pyrophosphate increased the 14.5 nm layer line and decreased cross-bridge regularity, and thus induced a cross-bridge state intermediate between that of relaxed and rigor muscle {Beinbrech, 1972 18937 /id}.
Early work with AMPPNP showed that it slightly increased rigor muscle zero tension length (Barrington-Leigh et al., 1973; Beinbrech et al., 1976; Marston et al., 1976, 1979; Kuhn, 1978a) and changed the muscle’s X-ray diffraction pattern (Barrington-Leigh et al., 1973; Goody et al., 1975; Beinbrech et al., 1976; Marston et al., 1976, 1979) and electron micrograph appearance (Beinbrech et al., 1976; Marston et al., 1976) to a state intermediate between rigor and relaxation. These changes were explained either as arising from a change in the nature, but not the number, of the cross-bridges (Barrington-Leigh et al., 1973; Goody et al., 1975, 1976; Beinbrech et al., 1976; Marston et al., 1976, 1979) or from a change in the activity of previously unattached cross-bridges (Wray, 1984). Later work showed that these interpretations were incorrect (Reedy et al., 1983a, 1987, 1988; Tregear et al., 1990; Biosca et al., 1990; Schmitz et al., 1996; Winkler et al., 1996). AMPPNP actually causes the release of the rear cross-bridges while inducing only small changes in shape and attachment angle of the lead ones (middle top and bottom panels, Fig. 7D). Although not apparent in the averaged data shown here but consistent with X-ray diffraction data, AMPPNP also causes the reappearance of a 14.5 nm thick filament periodicity.
Addition of ethylene glycol to AMPPNP treated fibers causes a further reduction in muscle tension until a critical concentration at which the muscles are still stiff, but cannot bear any tension (Tregear et al., 1984, 1990; Clarke et al., 1984; Tregear and Clarke, 1984; Reedy et al., 1988). At this concentration cross-bridge and thick filament configuration again change (top and bottom right panels, Fig. 7D), with the thick filament showing a pronounced 14.5 nm rhythmicity and the cross-bridges now binding to the thin filament target areas at 90° and having a small size sufficient for only a single myosin head (Reedy et al., 1988; Tregear et al., 1990; Schmitz et al., 1997). In addition to this class of similar cross-bridges, unaveraged electron micrographs show large numbers of cross-bridges, with a wide variety of angles, attaching outside the thin filament target zones (Schmitz et al., 1997).
Electron microscopy and X-ray diffraction on relaxed muscle show a prominent 14.5 nm thick filament repeat with the (unbound) cross bridges at a uniform 90° angle to the filament axis (Reedy et al., 1965, 1983a, 1992; McDowall et al., 1984; Ménétret et al., 1988) (work reporting instead that electron micrographs of relaxed thick filaments show no periodicity (Heuser, 1983) presumably being in error). Electron paramagnetic resonance, however, shows that the nucleotide binding site is disordered in relaxed muscles, suggesting that only the bulk of the myosin is highly ordered (Crowder and Cooke, 1987; Reedy et al., 1992).
X-ray diffraction modeling provided a more detailed picture in which in relaxed muscle one head projects outward from the thick filament poised to bind the thin filament. These data were interpreted as showing that the second head wrapped around the thick filament and bound to the the neck of the adjacent, projecting myosin head where it left the thick filament surface (Al Khayat et al., 2003). This arrangement provided a compelling explanation both for the repeating thick filament shelves in relaxed muscle, and for their disappearance in rigor (in which the 5.6 to 6.4 average head binding per crown means that on average 1.6 to 2.4 of the ‘wrapped-around’ heads must leave their positions on the thick filament surface). The Woodhead (2005) work in tarantula requires that these data be reinterpreted so that the two heads of each pair interact with each other instead of acting separately.
Turning now to physiological methods of asynchronous muscle activation, early X-ray diffraction (Miller and Tregear, 1970; Chaplain and Honka, 1974a; Armitage et al., 1975; Rapp et al., 1991), tryptophan fluorescence (dos Romedios et al., 1972 and Steiger et al., 1972, but see Güth, 1980), and electron paramagnetic resonance (Crowder and Cooke, 1987) showed that Ca++ application and stretch-induced activation (see Section 3.6) increased myosin head binding to the thin filament and changed myosin head orientation relative to the rest state. Later X-ray diffraction (Tregear et al., 1998) and electron tomography (Taylor et al., 1999; Tregear et al., 2004) showed that during isometric contraction some 30% of the heads are bound, that the binding is to the target areas of the thin filaments, and that the binding is primarily single headed and is highest for heads within 8 nm axially of the target areas and low for heads more than 12 nm away. Most of the cross-bridges are nearly perpendicular to the filaments, implying that the cross-bridges can generate tension at this angle (presumably by flexing of the bridge).
Since in isometric contractions the bridges cannot reach other target zones, force is presumably produced by the bridges repeatedly cycling on and off the same actin target zone. Considerable axial and azimuthal tilting of the myosin head and neck regions is required to produce the observed cross-bridge angles. Taken together, these data suggest that force generation occurs in a two step process, in which weak binding is followed by catalytic domain rolling to a strong binding position, followed by a 5 nm lever arm swing of the light chain domain that results in a total interaction distance of 12–13 nm and a 4–6 nm working stroke (Taylor et al., 1999; Reedy, 2000). A recent technical advance that allows X-ray diffraction data to be obtained from flying Drosophila has shown that the changes in layer line intensity noted above when flight muscles were mechanically oscillated in vitro also occur in vivo (Dickinson et al., 2005).
Considerable work has been performed quantifying asynchronous myosin biochemical properties (rate constants and the like) (White et al., 1987; Yamakawa and Goldman, 1991; Webb et al., 1991; Swank et al., 2001, 2006b; Silva et al., 2003; Swank and Maughan, 2003; Littlefield et al., 2003). Asynchronous muscle myosin has one of the fastest reported sliding rates, and is 9-fold faster than the rate of the embryonic muscle myosin isoform (Swank et al., 2001). This rapid sliding rate is not due to the unitary cross-bridge step size (4 nm) differing in the two myosin isoforms, but instead to changes in cross-bridge cycling kinetics (Swank et al., 2001; Littlefield et al., 2003). This extremely rapid cycling is due to an extremely high rate of detachment of myosin from the thin filament (Swank et al., 2006b), asynchronous muscle having a very low affinity for MgATP (Swank et al., 2006b), and the rate limiting step in asynchronous muscle cross-bridge cycling likely being inorganic phosphate release (in contrast, for instance, with insect slow embryonic myosin, in which ADP release is the rate limiting step) (White et al., 1987; Yamakawa and Goldman, 1991; Swank and Maughan, 2003; Swank et al., 2006b). However, this last point is not certain, with Silva et al. (2003) arguing instead for ADP release being the rate limiting step.
Embryonic and asynchronous muscle myosins differ by alternative splicing at four exons—3, 7, 9, and 11 (Bernstein and Milligan, 1997). The effect of expressing ‘wrong’ exons in embryonic or asynchronous myosins has been investigated for exons 3, 7, and 11 (briefly reviewed in Murphy and Spudich, 2000). Substituting embryonic exon 3 into asynchronous myosin decreased ATPase rates, but did not affect actin sliding velocity. Substituting adult exon 3 into embryonic myosin increased actin sliding velocity, but not enough to restore flight to flies expressing the chimeric embryonic isoform in flight muscles (Swank et al., 2003). Substituting embryonic exon 7 into asynchronous myosin, or asynchronous exon 7 into embryonic myosin, in both cases increased myosin ATPase activity in the presence of actin {Miller, 2005 36626 /id}. Muscles containing the chimeric asynchronous myosin or native asynchronous myosin had identical performance. Asynchronous muscles containing embryonic myosin with an asynchronous exon 7 performed better than muscles containing embryonic myosin, although the animals were still unable to fly (Swank et al., 2006a). Substituting embryonic exon 11 (which is part of the converter domain that couples ATP hydrolysis to delivery of the power stroke) into asynchronous myosin decreased actin sliding velocity 2 fold, but the animals could still fly. Substituting asynchronous exon 11 into embryonic myosin increased actin sliding velocity to almost asynchronous values, but adults expressing this chimera in flight muscle nonetheless could not fly (Swank et al., 2002). The change in actin sliding velocity induced by substituting embryonic exon 11 into asynchronous myosin was not associated with changes in myosin step size, and thus the observed changes must be due to alterations is cross-bridge dynamics (Littlefield et al., 2003).
The role of actin in force generation has been investigated by comparing the sliding velocity of rabbit myosin on rabbit or Drosophila (asynchronous) actin (Molloy et al., 1995), and by studying the effects of various actin mutants (Drummond et al., 1990; Sparrow et al., 1991; Molloy et al., 1995; Razzaq et al., 1999). Rabbit myosin moves more slowly on asynchronous actin than on rabbit actin (Molloy et al., 1995), and slower still on an asynchronous actin mutant (E93K) (Molloy et al., 1995) that alters a glycine residue affecting myosin binding (Razzaq et al., 1999). These velocity reductions arise not from changes in myosin step size but reductions in cross-bridge force delivery (Molloy et al., 1995). Two other actin mutations that affect the kinetics of muscle fiber force generation but not sarcomere structure have been identified (Drummond et al., 1990; Sparrow et al., 1991). The molecular basis of these effects is unknown, but one of the mutations is distant from the myosin binding site, which suggests that long-range effects can alter actin function.
3.3.2. Bivalvia
Bivalvia, primarily scallop, is the other invertebrate system in which the molecular basis of force generation has been intensively studied. Early work showed that the sliding filament hypothesis (which was proposed on the basis of work in striated muscle) applied to molluscan smooth muscles as well (Dörr and Portzehl, 1954; Hanson and Lowy, 1959; Razumova et al., 1970; Millman and Elliott, 1972; Sugi and Tsuchiya, 1975). Molluscan muscle was also a preparation used in the extremely early X-ray diffraction studies of helical proteins (Astbury and Dickinson, 1940; Astbury, 1946; Fraser et al., 1965).
X-ray reflections arising from the thin and thick filaments in these muscles are very well separated (Vibert et al., 1972). X-ray diffraction has generally shown only an increase in actin layer line intensity, indicative of cross-bridge binding to thin filament target areas, during muscle activation (Lowy and Vibert, 1972; Vibert et al., 1972; Svendsen, 1981, 1982; Lowy and Poulsen, 1982; Tajima and Amemiya, 1991), although in one case a decrease in 14 nm intensity was also seen (Lowy and Poulsen, 1982). Given the large amount of paramyosin present in these thick filaments, and thus the relatively small proportion of thick filament mass that is myosin, the failure to see a diminished 14 nm line in most of this work is not surprising. In particular, it is not strong evidence that thick filament periodicity does not, as in insect muscles, decrease during muscle activation.
This work also showed that muscle activation is associated with a change in thin filament position (Svendsen, 1981, 1982), that cross-bridges are likely present every 6th actin monomer (Svendsen, 1981), and that the thin and thick filaments stretch during isometric contractions (Tajima et al., 1994). Electron microscopy is consistent with these and the asynchronous muscle data, showing that activation or rigor decrease thick filament 14.5 nm periodicity and cause the myosin heads to move away from the thick filaments (Vibert and Craig, 1985; Frado and Craig, 1989, 1992; Zhao and Craig, 2003a,b), that both heads attach to the thin filament at an acute angle, and that this binding is associated with elongation and bending of the lead head (Craig et al., 1980; Zhao and Craig, 2003b). Mg++ is required for the heads to return to their original configuration after rigor (Korchagin, 1995).
The movement of latex or polystyrene beads covered with molluscan myosin along thin filaments (Vale et al., 1984; Yamada et al., 1989; Ishii et al., 1997; Han and Sellers, 1998), and of thin filaments along thick filaments bound to a surface (Yamada et al., 1990; Sellers and Kachar, 1990; Yamada and Takahashi, 1992; West et al., 1996; Han and Sellers, 1998), can be visualized. The velocities of myosins from different species are consistent with their ATPase rates (Vale et al., 1984) and their force-velocity relationships are very similar to those of intact single muscle fibers (Ishii et al., 1997). Consistent with the data from asynchronous muscle showing great myosin head flexibility, these movements occur both when the thin and thick filaments are in their native configuration and when the filaments are in the ‘wrong’ orientation (the equivalent of a thin filament from one side of a sarcomere interacting with the portion of the thick filament on the opposite side of the M line). The velocities of (Yamada et al., 1990; Sellers and Kachar, 1990; West et al., 1996) and forces generated by (Yamada and Takahashi, 1992) wrongly oriented filaments are always less than those of correctly oriented ones. Comparing sliding velocity and myosin structure from different muscles and species has identified a part of the myosin molecule (loop 1) associated with changes in sliding velocity, and indicates that velocity differences are due to changes in ADP dissociation and affinity (Kurzawa-Goertz et al., 1998; Murphy and Spudich, 2000).
A variety of approaches have been used to investigate the molecular mechanism of force production in molluscan muscles. Relatively early work showed that mollusc myosin has the typical two headed structure of other myosins (Elliott et al., 1976), that in the rest state ADP binds to both heads (Marston and Lehman, 1974; Shibata-Sekiya, 1982), that ATP binding alters tryptophan fluorescence (Kondo et al., 1979; Wells et al., 1985), that a tryptophan present in the skeletal ATP binding site is replaced in scallop by an arginine (Kondo et al., 1979; Kerwin and Yount, 1992), and identified portions of the heavy chain and myosin head required for ATPase activity (Szentkiralyi, 1987) and actin binding (Castellani et al., 1987). Electron paramagnetic resonance showed that force generation (but not ATP hydrolysis) is associated with light chain rotation (Baker et al., 1998; Roopnarine et al., 1998; Cooke, 1998; Brust-Mascher et al., 1999; LaConte et al., 2003), and measurement of luminescence resonance energy transfer between the regulatory light chains showed that the light chains of both heads rotate together to act as a coordinated lever arm (Lidke and Thomas, 2002).
More detailed understanding of the molecular basis of force generation came with the description of the S1 (the head portion of the myosin molecule) subfragment on the atomic level (Houdusse et al., 1999) in the absence of nucleotide and in the presence of various nucleotides and analogs (MgADP, AMPPNP, ADP°BeFx) that induce different myosin head conformations (Houdusse et al., 2000; Himmel et al., 2002; Gourinath et al., 2003; Nitao et al., 2003; Risal et al., 2004). This work has identified the converter and lever arm domains of the molecule (Houdusse et al., 1999), shown that the heads can exist in a large number of different conformations as a result of rearrangements of the four domains of the head around three joints (Houdusse et al., 1999, 2000; Himmel et al., 2002; Gourinath et al., 2003), identified a hinge within the regulatory light chain domain of the lever arm that may be an important component of cross-bridge compliance (Gourinath et al., 2003), shown that the so-called SH1 helix may unwind to function as a clutch between the converter and lever arms (Himmel et al., 2002; Gourinath et al., 2003), shown that differences in the SH1 helix underlie some species-specific differences in myosin function (Nitao et al., 2003), been used in model search protocols to identify the lowest energy actin binding configurations of the head (Root, 2002a), and shown that the energy released by ATP hydrolysis spreads throughout the head and induces collective changes in the structure of the myosin neck and actin binding regions (Kawakubo et al., 2005).
An as yet unresolved discrepancy is that under isotonic conditions both insect flight myofibrils (Pollack et al., 1998, 2003; Blyakhman et al., 1999) and small ensembles (tens of myosin molecules) of mollusc myosin (Liu and Pollack, 2004) lengthen and shorten in 2.7 nm steps. These data suggest that the thin filaments translate over the thick filament in integer multiples of the actin monomer repeat unit. The mollusc working stroke is unknown, but these data in insect are incompatible with the preferential target area binding of insect flight muscle and its working stroke data. Although a number of explanations have been proposed to explain this difference, including that it arises from elastic properties of the connecting filaments in flight muscles, from high cooperativity across the myosin molecules, or from thick filament shortening (and has even been used to argue against the sliding filament theory) (Pollack et al., 1988; Blyakhman et al., 1999; Liu and Pollack, 2004), it remains as yet unexplained. Possible relevant to this issue is recent theoretical work suggesting that single molecule approaches likely underestimate stroke size (Brenner, 2006).
3.3.3. Other groups
Actomyosin ATPase rates correlate well with the contraction rates of several brachiopod muscles and the behaviors they help generate (Eshleman and Wilkens, 1979). Work studying the effect of vibration on tension production in Holothuria (sea cucumber) supports the sliding filament hypothesis (Kobayashi et al., 1994).
Work in Crustacea has provided support for the sliding filament theory (Baskin and Wiese, 1964; West et al., 1992), and showed that 1) crab and barnacle thin filaments undergo a conformational change during rigor (Yanagida et al., 1974; Borovikov and Chernogriadskaia, 1979; Maéda et al., 1979), 2) the Ca++-indicator dye antipyrylazo III appears to block barnacle muscle tension development by a direct effect on the actomyosin (Dubyak, 1985), 3) crayfish muscle can develop two rigor states, depending on the degree of muscle contraction present when rigor is induced (Kawai and Brandt, 1976), and 4) during crayfish muscle contraction myofilament lattice volume remains constant and myofilament spacing therefore increases {April, 1971 37724 /id;April, 1973 704 /id}. Increased myofilament spacing reduces force production in these muscles, and this increased spacing likely induces a small but significant reduction in force production (April and Maughan, 1986). Readers should discount an early report that crab actomyosin has a 60 nm working stroke (Yanagida et al., 1985), which resulted from a failure to appreciate the effects on actomyosin sliding velocity measurements of still attached myosin heads that had finished their power stroke (‘drag’ attachments) (Brenner, 2006).
X-ray diffraction and electron microscopy of crayfish and crab muscle in rigor and AMPPNP agrees well with asynchronous flight muscle data. In particular, 1) relaxed crab myosin heads are located between the surfaces of the thick and thin filaments and move toward the thin filament during rigor (Wakabayashi and Namba, 1981; Maéda, 1983; Wakabayashi et al., 1984), 2) in rigor the cross-bridges bind to 38 nm spaced thin filament target zones spatially locked to troponin position and thin filament periodicity overwhelms the myosin 14.5 nm repeat (Wray et al., 1978; Maéda et al., 1979; Maéda, 1979; Namba et al., 1980; Wakabayashi et al., 1984), 3) uniformly angled double chevrons, in which both lead and rear bridges are double headed, are present (Meisner and Beinbrech, 1979; Bard et al., 1987), and 4) AMPPNP treatment results in the cross-bridges assuming a perpendicular angle to the filaments (Meisner and Beinbrech, 1979).
Work in tarantula shows that during cross-bridge cycling myosin heads bind weakly to a peripheral site on actin before binding to a strong binding site (Craig and Lehman, 2001) and that maintaining myosin head helical order on the thick filaments requires that the heads be in a specific conformational state with the so-called γ phosphate pocket closed (Zoghbi et al., 2004)
A surprising but persistent observation in Limulus is that thick filament length changes with muscle shortening (de Villafranca, 1961; de Villafranca and Marchhaus, 1963; Stephens, 1965; Dewey et al., 1977; Walcott and Dewey, 1980; Huxley, 1985) (an early report of A-band shortening during contraction in stick insect muscle also exists, von Hehn, 1965). Isolated Limulus thick filaments also shorten when exposed to Ca++ and ATP, and shortened filaments lengthen when incubated with phosphatase (Brann et al., 1979). This observation was used in early work to contest the sliding filament hypothesis, but it is now certain that Limulus muscle contracts by this mechanism. Many of the procedures used to induce thick filament shortening may have been nonphysiological (Sugi and Gomi, 1981).
Several hypotheses have been advanced to explain this phenomenon, including that it arises from differences in A band protein organization in Limulus muscle (de Villafranca et al., 1959) or from thin:thick filament interactions of the wrong polarity in extremely contracted sarcomeres (Sydorenko and Klimov, 1994). The best supported hypothesis stems from observations that thick filament shortening 1) is not associated with changes in thick filament helical structure (Levine and Kensler, 1985) but 2) is associated with changes in thick filament charge (Brink and Dewey, 1984), phosphorylation state (Dewey et al., 1984), and the appearance of unattached thick filament end fragments (Levine et al., 1991b) and 3) regulatory light chain phosphorylation causes the release of similar end fragments in vitro (Levine et al., 1991a). These observations are consistent with thick filament shortening occurring via a reversible, phosphorylation dependent, disaggregation of thick filament ends.
X-ray diffraction in Limulus muscle in rigor shows a decrease in the thick filament (14.5 nm) reflection and an increase in the thin filament (38 nm) one, consistent with Limulus cross-bridges assuming an angled configuration and being bound to thin filament target areas as in insect and crustacean muscle. Rigor solutions applied to isolated Limulus thick filaments increase myosin head distance from the thick filament surface (Levine et al., 1986). ATP, ATP analogs, and Ca++ increase myosin head and/or neck movements in isolated Limulus thick filaments (Kubota et al., 1983; Fujime and Kubota, 1984; Fan et al., 1985a,b,c, 1987a,b,c, 1994); phenylmethylsulfonyl fluoride or deuterium oxide (once incorporated into the myosin) suppress these motions but not the myosin ATPase (Fan et al., 1985b,c).
3.4. Regulation of cross-bridge cycling
Much early work on actomyosin regulation is multi-topic papers that describe both techniques for isolating actomyosin (in much of which which proteins were being isolated is not always clear), and its dependence on various nucleotides (particularly ATP) and ions (particularly Ca++). Given these issues and the fact that this work uniformly showed that actomyosin activity depended on ATP and Ca++, we cover all this early work in this section. We do not exhaustively cover papers dealing with troponin and tropomyosin isolation and localization; for additional references on these topics see Hooper and Thuma (2005) and Section 3.1. The major conclusions of this work are 1) Ca++ is in all cases the intermediary between muscle depolarization and actomyosin activation, 2) in most species both troponin-tropomyosin actin based activation and thick filament activation via Ca++ binding to myosin light chain are present, 3) the relative importance of these two systems, and on what time scales they are used behaviorally, in different species is less well understood, and 4) regulation is by far best understood in molluscs.
We have organized this work phylogenetically. Before beginning the survey, however, three general issues should be covered. First, work in scallop has shown that lanthanides do not bind to Ca++ specific binding sites on myosin but instead activate myosin by releasing trace Ca++ from EGTA containing solutions (Chantler, 1983). Studies using lanthanides to activate actomyosin must be therefore judged with caution. Second, the clear distinction in vertebrates between smooth and striated muscle thin filament regulatory proteins, with troponin only in striated and calponin/caldesmon only in smooth, is not true of invertebrates. Troponin is present in smooth ascidian (Toyota et al., 1979; Endo and Obinata, 1981; Ohshima et al., 1988; Meedel and Hastings, 1993; Endo et al., 1996) and scallop (Ojima and Nishita, 1986a; Nishita et al., 1997) muscle. Calponin is present in Schistosoma cross-striated (Jones et al., 2001) and nematode obliquely striated (Castagnone-Sereno et al., 2001) muscle, caldesmon is present in Pecten and Sepia striated muscle (although not located in the sarcomeres, but instead in the muscle cell periphery) (Bartegi et al., 1989), and calponin and caldesomon are present in Helix obliquely striated buccal muscle (Royuela et al., 2000a) and Eisenia muscle intermediate between striated and smooth (Royuela et al., 1997). Complicating this issue further, a 160 kDa actin binding protein that induces actin filament aggregation similar to that induced by caldesmon, but which differs from caldesmon and α-actinin, has been identified in surf clam foot (smooth) muscle (Chiba et al., 1993).
Third, troponin C Ca++ binding shows strong phylogenetic variation (Nakamura et al., 1994). Vertebrate skeletal troponin C binds four Ca++. Vertebrate cardiac and amphioxus muscle bind three (Takagi et al., 1994). Ascidia (Endo and Obinata, 1981; Takagi and Konishi, 1983) and barnacle (Collins et al., 1991) bind two. Lobster and crayfish (Kobayashi et al., 1989; Wnuk, 1989; Garone et al., 1991) also bind two (Shima et al., 1984, apparently being in error in finding only one Ca++ being bound), but only one site is used for regulation (Regenstein and Szent-Györgyi, 1975; Wnuk et al., 1984; Kobayashi et al., 1989; Wnuk, 1989). Scallop (Lehman et al., 1980; Shima et al., 1984; Nishita et al., 1994, 1997; Ojima et al., 1994, 2000), squid (Shima et al., 1984; Ojima et al., 2001), and Limulus (Lehman et al., 1976) bind one. In insects with asynchronous muscle the multiple troponin isoforms divide into two classes, one of which is expressed only in asynchronous muscle and binds one Ca++, another of which is expressed in both synchronous and asynchronous muscle and binds two (Qiu et al., 2003; Fernandes, 2003). Drosophila troponin T is a Ca++ binding protein in its own right (Domingo et al., 1998).
Primarily vertebrate reviews of actomyosin regulation include {Adelstein, 1980 37370 /id;Brown, 2005 72 /id;Chalovich, 1992 37470 /id;Craig, 2002 37466 /id;Ebashi, 1980 29803 /id;Gordon, 2000 36067 /id;Weber, 1973 3355 /id}. Papers dealing with multiple invertebrate species and reviews covering regulation of invertebrate actomyosin to a larger extent include {Bagshaw, 1980 35366 /id;Bullard, 1983 18944 /id;Chantler, 1978 3051 /id;Craig, 2002 37466 /id;da Silva, 1991 35422 /id;Kalabokis, 1998 27187 /id;Kambara, 1990 2458 /id;Kendrick-Jones, 1976 9736 /id;Kendrick-Jones, 1981 29810 /id;Lehman, 1972 32783 /id;Lehman, 1975 165 /id;Lehman, 1976 32782 /id;Marston, 1995 1235 /id;Mehl, 1941 35334 /id;Milligan, 1996 37464 /id;Okada, 1954 34158 /id;Perry, 1998 37502 /id;Ruppel, 1995 23290 /id;Schädler, 1967 29861 /id;Scholey, 1981 22838 /id;Sellers, 1980 22836 /id;Silberstein, 1977 35453 /id;Szent-Györgyi, 1975 35133 /id;Szent-Györgyi, 1976 36114 /id;Szent-Györgyi, 1987 36349 /id;Szent-Györgyi, 1996 22661 /id;Szent-Györgyi, 1999 22820 /id;Szent-Györgyi, 2004 36261 /id;Trybus, 1994 30250 /id}. Care must be taken in reading this literature, as preservation of regulatory molecules in many species varies considerably depending on experimental procedures, and thus several systems originally thought to be singly controlled were later shown to have dual control mechanisms.
3.4. Radiata
A Ca++ activated actomyosin has been isolated from sea anemone (Maruyama, 1955, 1956a,Maruyama, b; Kanzawa et al., 1993).
3.4.2 Deuterostomia, Cephalochordata (amphioxus) and Urochordata (ascidia)
Amphioxus regulation is thin filament based (Lehman and Szent-Györgyi, 1975). Regulation in ascidia is very similar to that in skeletal muscle. The muscles contain tropomyosin and troponin, Ca++ has no effect on the ATPase activity of ascidian actomyosin lacking tropomyosin-troponin, and addition of these proteins restores Ca++ sensitivity to both ascidian and rabbit actomyosin (Toyota et al., 1979; Endo and Obinata, 1981; Obinata et al., 1983; Miyakawa and Konishi, 1984; Takito and Konishi, 1986; Ohshima et al., 1988). Ascidian smooth muscle troponin is a relatively weak inhibitor of cross-bridge cycling (in the absence of Ca++), and instead primarily functions as a Ca++-dependent activator of cross-bridge cycling (Endo and Obinata, 1981).
3.4.3 Deuterostomia, Echinodermata
Ca++ activated actomyosins were early isolated in starfish (Maruyama and Matsumiya, 1957), sea urchin (Obinata et al., 1974), and sea cucumber (Holothuria) (Mognoni and Lanzavecchia, 1969). Sea cucumber lantern retractor muscle is regulated by direct Ca++ binding to myosin (Lehman and Szent-Györgyi, 1975). However, although sea cucumber longitudinal (smooth) body wall muscles are also Ca++ activated (Suzuki, 1982), regulation is due to myosin light chain phosphorylation (Kerrick and Bolles, 1982).
3.4.4 Ecdysozoa, Nematoda
The physical and biochemical properties of nematode myosin are typical (Harris and Epstein, 1977) and regulation is primarily thin filament, troponin-tropomyson based (Harris et al., 1977; Martin et al., 1986; Kimura et al., 1987; Ono and Ono, 2004). Ascaris troponin and tropomyosin restore Ca++ sensitivity to rabbit actomyosin. Ascaris troponin I (with or without Ascaris or rabbit troponin T) with Ascaris tropomyosin inhibits rabbit ATPase regardless of Ca++. Addition of Ascaris or rabbit troponin C in all tropoinin I and T combinations removes the inhibition in a Ca++ dependent manner (Kimura et al., 1987). Both Ca++ binding sites of C. elegans troponin C are low affinity, fast dissociating, and Ca++ specific (Ueda et al., 2001). Parallel regulatory pathways via both direct Ca++ thick filament binding (Lehman and Szent-Györgyi, 1975; Harris et al., 1977) and myosin light chain phosphorylation (Martin et al., 1986) are also present. Ascaris actomyosin is active at low (relative to rabbit skeletal actomyosin) ATP concentrations (Yamaguchi et al., 1973).
3.4.5 Ecdysozoa, Chelicerata
Limulus actomyosin was early isolated (de Villafranca et al., 1959, 1968; Stanley, 1970) and requires Mg++ and Ca++ for activation (de Villafranca and Naumann, 1964; de Villafranca, 1967; de Villafranca and Campbell, 1969; de Villafranca and Waksmonski, 1970). The magnitude of Ca++’s effects on, and the amount of Ca++ bound by, isolated thick filaments show seasonal variation (Fan et al., 1992). Early work on which filament system was regulatory was contradictory, with some indicating that Limulus was only thin filament regulated (Lehman et al., 1972) and other that Limulus (and tarantula) muscle was dually regulated (Lehman and Szent-Györgyi, 1975; Chantler and Szent-Györgyi, 1978; Sellers et al., 1980). That there is a tropomyosin-troponin based thin filament regulatory system is unambiguous (Lehman and Szent-Györgyi, 1972; Lehman et al., 1976, 1994; Reedy et al., 1994b), although full movement of the tropomyosin to expose the entire myosin binding site on the thin filament requires both Ca++ and myosin head binding to the thin filament (Vibert et al., 1997). Limulus troponin C with rabbit troponin I and T restores full Ca++ sensitivity to rabbit actomyosin (Lehman, 1975).
The early observations of a parallel thick filament mediated regulation by direct Ca++ binding have not been further investigated. A controversy over the role of a third regulatory mechanism involving myosin light chain phosphorylation (Sellers, 1981; Kerrick and Bolles, 1981; Sellers and Harvey, 1984; Wang et al., 1993; Ritter et al., 1999) has been resolved with the recognition that, although Limulus regulatory light chains must be phosphorylated for actomyosin activity, under physiologically relevant conditions Limulus light chains are always phosphorylated and this mechanism is thus not used to regulate muscle contraction.
A method for rapid purification of tarantula muscle myosin is available (Martelo and Padrón, 1987). Myosin regulatory light chain must be phosphorylated in tarantula for activation (Hidalgo et al., 2001). As to the molecular mechanism of phosphorylation’s action, electron microscopy indicates that unphosphorylated myosin heads are highly ordered and likely held close to the thick filament backbone. Phosphorylation increases head disorder and induces a 6 nm increase in the average separation between the myosin heads and the surface of the thick filament, which may facilitate interactions with the thin filament (Craig et al., 1987; Panté et al., 1988; Padrón et al., 1991).
3.4.6 Ecdysozoa, Crustacea
Early work showed that crustacean actomyosin had a chemical composition roughly similar to that of other myosins (Bailey, 1937; Siemankowski and Zobel, 1976), showed typical Ca++ activated ATPase activity (Humphrey, 1948; Maruyama, 1958a, 1959a, 1968a; Portzehl et al., 1964, 1965, 1969; Tomioka et al., 1975; Siemankowski and Zobel, 1976; Orentlicher et al., 1977; Kawai and Brandt, 1977; Stephenson and Williams, 1980; Goblet and Mounier, 1987; Allhouse et al., 1999c; Shimada et al., 2000; Koenders et al., 2004), and that its temperature dependence matched species’ habitat temperature (Shimada et al., 2000).
Crustacean actomyosin has a thin filament regulatory system (Maruyama et al., 1968a; Lehman et al., 1972; Benzonana et al., 1974; Regenstein and Szent-Györgyi, 1975; Lehman and Szent-Györgyi, 1975; Chantler and Szent-Györgyi, 1978; Wnuk et al., 1984; Shima et al., 1984; Shinoda et al., 1988; Kobayashi et al., 1989; Wnuk, 1989; Nishita and Ojima, 1990; Kambara et al., 1990; Garone et al., 1991; Collins et al., 1991; Ashley et al., 1991; Miegel et al., 1992; Royuela et al., 1999; Allhouse et al., 1999c; Royuela et al., 2000b; Koenders et al., 2004). Early data suggested that some, but not all, crustacean muscles also have parallel myosin based regulation (Lehman and Szent-Györgyi, 1975; Lehman, 1977; Chantler and Szent-Györgyi, 1978; Sellers et al., 1980; Simmons and Szent-Györgyi, 1980; Watanabe et al., 1982; Ojima and Nishita, 1989). However, in interpreting the work showing a lack of myosin regulation, it is important to note that the myosin regulatory system in these muscles is highly sensitive to ionic conditions (Lehman, 1977). The negative work may thus have failed to find myosin based regulation because it was performed under the wrong conditions. Similar difficulties could also explain the inability of myosin regulatory chains from ‘only thin filament’ regulated crustacean muscles to restore regulation to scallop myosin from which one regulatory light chain had been removed with EDTA (Kendrick-Jones et al., 1976), particularly since this work did not test whether regulatory chains from ‘dually regulated’ crustacean muscles could do so. The most parsimonious interpretation of these data is thus that all crustacean muscles are thin filament regulated, some are clearly dually regulated, and the evidence that not all are dually regulated should be viewed with caution.
Crayfish (Benzonana et al., 1974), prawn (Ojima et al., 1995), and lobster (Regenstein and Szent-Györgyi, 1975; Nishita and Ojima, 1990; Miegel et al., 1992) troponin and tropomyosin restore Ca++ sensitivity to rabbit actomyosin. Lobster troponin I with lobster or rabbit tropomyosin inhibits rabbit actomyosin, and addition of lobster troponin C activates the actomyosin, but does not confer Ca++ sensitivity without the addition of lobster troponin T (Regenstein and Szent-Györgyi, 1975; Nishita and Ojima, 1990). Crayfish troponin I plus rabbit tropomyosin inhibits rabbit actomyosin (Shinoda et al., 1988). Crayfish troponin C reactivates troponin C depleted barnacle actomyosin as well as does barnacle troponin C (Ashley et al., 1991). Lobster and crayfish troponin C cannot activate vertebrate or scallop troponin C depleted myfibrils nor can vertebrate (either skeletal or cardiac) troponin C activate lobster or crayfish troponin C depleted actomyosin (Nakamura et al., 1994). However, rabbit skeletal troponin C can weakly restore Ca++ sensitivity and force generation to barnacle myofibrils from which native troponin C has been extracted (Ashley et al., 1991; Gordon et al., 1997). Bovine cardiac troponin C can bind and activate barnacle actomyosin, and barnacle troponin C rabbit skeletal actomyosin, but in each case only under non-physiologically acid conditions (Ashley et al., 1991).
In barnacle the role of specific motifs in troponin C in regulating contraction has been investigated by selective mutation. Troponin C’s Ca++ binding sites are not required for formation of the troponin I, C, T structure or binding to the thin filament (Allhouse et al., 1999a), mutations in troponin C’s central helix alter thin filament binding (Allhouse et al., 1999b), and inactivating one of troponin C’s Ca++ binding sites (site IV) increases the sensitivity of actomyosin activity to ionic strength changes (Allhouse et al., 2000).
3.4.7 Ecdysozoa, Insecta
A large number of early papers describe insect actomyosin isolation, its activation by and hydrolysis of ATP, that its activation requires both Mg++ and Ca++, and the effect of ADP on ATPase activity or muscle fiber tension (Hanson, 1952; Gilmour and Calaby, 1953; Maruyama, 1954b, 1956b, 1957a, 1958b, 1959b, 1966b, 1967; Gilmour and Robinson, 1964; Chaplain et al., 1965; Aidley, 1965; vom Brocke, 1966; Rüegg and Tregear, 1966; Schädler, 1967; Maruyama and Pringle, 1967; Chaplain, 1966a,b, 1967a,b; Maruyama et al., 1968b; Abbott and Mannherz, 1970; Pybus and Tregear, 1972; Griffiths et al., 1979; Wilson and White, 1983; Loxdale and Tregear, 1985). Early work also showed that insect actomyosin is dually regulated (Lehman et al., 1972, 1974; Lehman and Szent-Györgyi, 1975; Chantler and Szent-Györgyi, 1978; Sellers et al., 1980).
Further work on the myosin regulation has not been performed, but the existence of the troponin-tropomyosin thin filament system has been amply confirmed {Bing, 1998 22847 /id;Cammarato, 2004 36622 /id;Craig, 2001 22894 /id;de Nicola, 2004 37636 /id;Meinrenken, 1969 36317 /id;Qiu, 2003 QIU2003 /id;Royuela, 1996 363 /id;Ruiz, 1998 23184 /id}. Lethocerus troponin T and troponin H (with Lethocerus tropomyosin) inhibit rabbit actomysoin, and rabbit troponin C relieves the inhibition if Ca++ is present (Bullard et al., 1973a). Troponin H, although it replaces troponin I in asynchronous flight muscle, does not inhibit rabbit actomyosin ATPase activity (Bullard et al., 1988). Amino acids critical to Ca++-induced tropomyosin movement have been identified (Cammarato et al., 2005). Troponin phosphorylation increases troponin sensitivity to Ca++, and a thick filament associated giant protein, projectin, which possesses a kinase activity, may phosphorylate troponin (Weitkamp et al., 1998). An unusual aspect of the thin filament regulatory system in asynchronous flight muscle is that both the typical Ca++-activated troponin and a stretch-activated one are present (Agianian et al., 2004).
In addition to activation via direct Ca++ binding, insect actomyosin also has a third regulatory pathway involving myosin light chain phosphorylation (Takano-Ohmuro et al., 1986, 1990; Takahashi et al., 1990a,b; Sparrow, 1995; Tohtong et al., 1995). Drosophila mutations that block regulatory light chain phosphorylation show reduced flight muscle force production, likely because unphosphorylated heads remain close to the myosin backbone and thus do not interact with the thin filament (Irving and Maughan, 2000). However, as in Limulus, under normal conditions the myosin is always (at least once the animal becomes flight-capable) phosphorylated, and this pathway is thus not used to regulate muscle contraction (Takahashi et al., 1990a; Sparrow, 1995). A calmodulin based myosin light chain phosphorylation system regulates Locusta oviduct contraction (Nykamp et al., 1994).
3.4.8 Other Ecdysozoa
Pripulida (penis worms) body wall muscle is dually controlled (Lehman and Szent-Györgyi, 1975).
3.4.9 Lophotrochozoa, Brachiopoda
Lampshell muscle is myosin regulated (Lehman et al., 1972; Lehman and Szent-Györgyi, 1975).
3.4.10 Lophotrochozoa, Annelida and the near groups Nemertea, Sipuncula, and Echiura
Annelid (Godeaux, 1954) and Echiuroid (Maruyama, 1954a) actomyosin was early isolated and is Ca++ activated (Maruyama, 1954a; Maruyama and Kominz, 1959; Kanzawa et al., 1991). Despite the evolutionary closeness of the groups, Nemertean and Echiuran actomyosin has been identified as solely myosin regulated (Lehman et al., 1972; Lehman and Szent-Györgyi, 1975; Chantler and Szent-Györgyi, 1978) but annelid actomyosin as dually regulated (Lehman et al., 1972; Lehman and Szent-Györgyi, 1975; Royuela et al., 1996, 2000a). The annelid dual regulation is well confirmed, with annelid myosin being regulated by direct Ca++ binding (D’Haese, 1980; Carlhoff and D’Haese, 1987; Serwe et al., 1993; Ravaux et al., 2001) and skeletal muscle troponin regulating annelid tropomyosin in a Ca++ dependent manner (Ditgens et al., 1982). Alternatively, given the sensitivity of thin and thick filament regulatory systems to extraction conditions, it is unclear if the single regulation observed in the Annelid sister groups is real. This concern is heightened by variable results in Sipuncula, with one species identified as being both thick filament regulated (Lehman et al., 1972) and dually controlled (Lehman and Szent-Györgyi, 1975), and another as being thin filament regulated (Lehman and Szent-Györgyi, 1975).
3.4.11 Lophtrochozoa, Mollusca
Lophtrochozoa, Mollusca. A large number of papers deal with isolation and initial characterization (e.g., Ca++ and ATP dependence, heavy and light chain complement) of molluscan actomyosin (Lajtha, 1947; Humphrey, 1948, 1949; Weber, 1953; Migita and Matsumoto, 1954, 1957; Weber and Portzehl, 1954; de Villafranca, 1955; Tonomura et al., 1955, 1956; Bailey, 1956; Strelina et al., 1957; Matsumoto, 1957; Maruyama, 1957b, 1958a; Matsumoto, 1958a–d, 1959; Kishimoto, 1961; Bárány and Bárány, 1966; Schädler, 1967; Mognoni and Lanzavecchia, 1969; Twarog and Muneoka, 1972; Horie et al., 1975; Azuma et al., 1975; Azuma, 1976; Nishita, 1977; Nishita et al., 1977, 1979; Tsuchiya et al., 1978b–d; Asada et al., 1979; Toyo-Oka, 1979; Tanaka and Tanaka, 1979a,b; Ashiba et al., 1980, 1982; Kimura et al., 1980; Asakawa, 1980; Stephenson and Williams, 1980; Morita and Kondo, 1982; Szent-Györgyi and Niebieski, 1982; Yoshitomi and Konno, 1982; Krause and Munson, 1982; Kodama and Konno, 1983; Hikichi et al., 1983; Asakawa and Azuma, 1983; Yoshitomi et al., 1984; Kamiya et al., 1985; Shiraishi and Ohtsuki, 1989; Yamada et al., 1989; Dufhues et al., 1991; Han and Sellers, 1998). Two important issues with respect to isolating squid actomyosin are that Ca++ protects squid actomyosin from thermal denaturation (Konno, 1991b) and the muscle possesses a proteinase (Yoshioka et al., 2005) that splits myosin into heavy (head plus first 40% of the rod portion) and light (remaining 60% of the rod) meromyosin. One other general issue is that molluscan (and vertebrate smooth muscle) myosin can assume a ‘rolled’ up configuration in which the tail binds near the heads and the molecule becomes incapable of forming filaments or interacting with actin. This raised the question of whether such gross conformational change could be involved in myosin regulation in these muscles. However, kinetic studies show that regulation does not occur by this mechanism (Ankrett et al., 1991a).
With respect to which filament regulates the actomyosin, mollusc muscle was originally identified at the archetypal myosin regulated system (Kendrick-Jones et al., 1970; Lehman et al., 1972; Lehman and Szent-Györgyi, 1975; Azuma, 1976; Konno, 1978; Chantler and Szent-Györgyi, 1978; Sellers et al., 1980; Simmons and Szent-Györgyi, 1980). However, it rapidly became clear that mollusc muscles contain tropomyosin and all three troponin components (Bailey and Rüegg, 1960; Konno, 1978; Goldberg and Lehman, 1978; Lehman et al., 1980; Lehman, 1983a; Shima et al., 1984; Takahashi and Morita, 1986; Ojima and Nishita, 1986a,b, 1988a,; Ojima and Nishita, b) as well as a caldesmon-like protein (Bennett and Marston, 1990), that thin filament regulation was present in various molluscan muscles (Tsuchiya et al., 1978a; Lehman, 1981; Yazawa, 1985), and that the reason this regulatory system had been missed earlier is that high (but physiological for the organisms in question) Mg++ levels are required to prevent tropomyosin and troponin dissociation during actomyosin purification (Lehman, 1983b). Later work has verified these results (Kambara et al., 1990; Ojima and Nishita, 1992b; Nishita et al., 1994, 1997; Ojima et al., 1994, 1997, 2000, 2001; Nishimura et al., 1997; Shiraishi et al., 1999; Yumoto et al., 2003; Tanaka et al., 2005).
Scallop (Goldberg and Lehman, 1978; Ojima and Nishita, 1986a,b, 1988b, 1991; Nishita et al., 1997) and squid (Konno, 1978) troponin and tropomyosin restore Ca++ sensitivity to rabbit actomyosin. Scallop troponin I (with or without troponin T) with scallop or rabbit tropomyosin inhibits rabbit actomyosin (Lehman et al., 1980; Ojima and Nishita, 1986a, 1988a; Nishita et al., 1997). Scallop troponin C with rabbit or scallop tropomyosin relieves the scallop troponin I inhibition (Lehman et al., 1980; Ojima and Nishita, 1988a, 1991; Nishita et al., 1997). Rabbit troponin C can substitute for scallop troponin C (Ojima and Nishita, 1988a) and chicken troponin C or T can substitute for scallop troponin C or T (Goldberg and Lehman, 1978). However, scallop troponin C cannot replace lobster, crayfish, or vertebrate skeletal or cardiac troponin C (Ojima and Nishita, 1992a; Nakamura et al., 1994), nor can vertebrate skeletal or cardiac troponin C activate scallop troponin C depleted actomyosin (Nakamura et al., 1994). The binding of Ca++ to scallop troponin C has been characterized in some detail (Yumoto et al., 2001; Nara et al., 2004, 2006). Cyanogen bromide cleavage suggests that the molecule’s 17K C terminus is responsible for scallop troponin I’s inhibitory activity (Ojima et al., 1990).
In the one species (Ezo giant scallop) in which this issue has been investigated, which regulatory system is most active shifts with temperature, with thin filament regulation most active at the low temperatures the animal inhabits (Shiraishi et al., 1999). This temperature dependence may explain the anomalous observation that troponin I does not inhibit Ca++ activation of skinned muscle fibers of another scallop species, as these experiments were performed at 21°, at which myosin regulation would predominate if the shift in regulatory system importance has the same temperature dependence in both species (Kerrick et al., 1981). It is unclear which of the two molluscan actomyosin regulatory systems is of greatest physiological importance under behaviorally relevant conditions.
Myosin regulation has been extensively studied, particularly in scallop. Both regulatory light chains are required for the most robust myosin regulation (see below) (Szent-Györgyi et al., 1973; Kendrick-Jones et al., 1976; Simmons and Szent-Györgyi, 1978, 1985; Nishita et al., 1979; Chantler and Szent-Györgyi, 1980; Suzuki et al., 1980; Konno et al., 1981; Ojima and Nishita, 1983; Ojima et al., 1983b; Vale et al., 1984; Chantler, 1985). Similar results have been obtained in abalone and squid (in which the regulatory chain is called LC-2), and abalone and squid LC-2s exchange with scallop regulatory light chains (Asakawa and Azuma, 1983) and can restore Ca++ sensitivity to scallop myosin from which the regulatory chains have been removed (Konno et al., 1979; Asakawa et al., 1981; Kamiya et al., 1985). Additional support for the critical role of the regulatory light chains is provided by the ability to trifluoperazine, which is believed to bind to the regulatory chain, to lock scallop myosin in the off state (Patel et al., 2000). An important point to make about this work is that, due to the presence of unregulated myosins arising at least in part from damage to the proteins during extraction, there is significant background (in the absence of Ca++) actomyosin activity in extracted actomyosin preparations. This results in the true extent of actomyosin activation being greatly underestimated, as this background activity artificially inflates the baseline activity that Ca++ addition is augmenting (Wells and Bagshaw, 1984a, 1985; Simmons and Szent-Györgyi, 1985; Jackson et al., 1986; Ankrett et al., 1991b).
Scallop myosin regulatory light chains can be selectively removed by incubation with EDTA (and are hence in some articles called the EDTA chain, see Hooper and Thuma, 2005, for terminology issues and the light chain complement of various invertebrate actomyosins) (Kendrick-Jones et al., 1973; Bennett et al., 1984; Bennett and Bagshaw, 1986a). The missing chains can then be replaced with light chains from other organisms. This technique allows the ability of Ca++ to activate these myosins to be tested (Jakes et al., 1976; Sellers et al., 1980; Simmons and Szent-Györgyi, 1980; Ojima et al., 1983a; Kishimura et al., 1986; Ojima and Nishita, 1989; Kendrick-Jones et al., 1991; Kalabokis et al., 1994) and other aspects of their function to be examined (Reinach et al., 1986; Ojima and Nishita, 1987; Sweeney et al., 1994; Katoh and Morita, 1997; Ramachandran and Thomas, 1999). This work verified that light chain identity determines whether a myosin is Ca++ activated or not (i.e., substituting light chains from animals with thin filament regulation results in unregulated myosins, substituting light chains from animals with thick filament regulation results in regulated myosins). This work also showed that one rabbit light chain (the so-called DTNB chain) can functionally substitute for the EDTA light chain, which led to the suggestion that a parallel myosin regulation may be present in vertebrate striated muscle (Kendrick-Jones, 1974), a speculation that to our knowledge has not borne fruit.
Investigations of the physical relationships among the regulatory light chain, essential light chain, and heavy chain showed that the light chains are located near the neck region (Fig. 2A) and the three molecules are in close proximity and extensively interact (Craig et al., 1980; Wallimann and Szent-Györgyi, 1981; Hardwicke et al., 1982; Wallimann et al., 1982; Konno et al., 1983b; Szentkiralyi, 1984; Winkelmann et al., 1984; Ashiba and Szent-Györgyi, 1985; Konno and Watanabe, 1985a,b; Asakawa and Azuma, 1988; Chantler and Bower, 1988; Walker and Trinick, 1989; Chantler and Kensler, 1989; Park et al., 1991; Chantler et al., 1991; Konno, 1991a; Xie et al., 1994; Houdusse and Cohen, 1995; Offer and Knight, 1996). Thiol groups on the essential chain are likely involved in regulatory chain binding (Konno, 1991c). The two heads are close enough to interact (Stafford III et al., 1979; Wells and Bagshaw, 1983; Hardwicke and Szent-Györgyi, 1985; Vibert et al., 1985; Chantler and Tao, 1986; Bower et al., 1992). The initial portion of the rod is critical for Ca++ sensitivity, as S1 fragments, which contain only the head region (see Hooper and Thuma, 2005, for a description of the commonly used myosin fragments) are always active regardless of Ca++ concentration (Kamiya and Konno, 1984; Wells et al., 1985; Kamiya et al., 1985; Konno and Watanabe, 1985b; Asakawa and Azuma, 1988; Kalabokis and Szent-Györgyi, 1997).
These data are consistent with the neck portion of the heavy chain being required in its own right, or with it being required only because its presence allows the two heads to interact, and it is this interaction that allows regulation to occur. The literature on this issue is somewhat confusing. Early work showed that single headed myosin shows Ca++ dependent regulation (Stafford III et al., 1979). Despite this, later work consistently refers to regulation as depending on interactions between the two heads.
Resolution of this contradiction lies in three differences between the preparations. First, activation in the single headed case is only 3–4 fold, whereas activation in the two-headed case is 10–20 fold, a difference in Ca++ sensitivity of 70% (single) vs. 90–95% (double) (Stafford III et al., 1979; Kalabokis et al., 1996; Szent-Györgyi et al., 1999). Second, the half-life in the presence of EGTA for single turnover of the ATPase cycle of single headed myosin is ≤30 s, but for double headed myosin is ~3 min (Kalabokis et al., 1996; Szent-Györgyi et al., 1999). Third, the dependence of actomyosin activation on Ca++ concentration is linear in single headed myosin but shows high cooperativity in double headed mysosin (Chantler et al., 1981; Simmons and Szent-Györgyi, 1985; Kalabokis et al., 1996; Kalabokis and Szent-Györgyi, 1997; Szent-Györgyi et al., 1999). The importance of head-head interaction is further demonstrated by the observation that regulation is not restored to myosin lacking regulatory light chains until sufficient light chain is added that the myosin molecules begin to have two regulatory light chains apiece (Chantler and Szent-Györgyi, 1980). Thus, although single-headed myosin shows some regulation, full regulation requires both myosin heads.
The next issue to resolve was to determine where the Ca++ binding occurred. Scallop myosin binds two moles of divalent cations non-specifically and two moles of Ca++ specifically (Bagshaw and Kendrick-Jones, 1979; Bennett and Bagshaw, 1986b). Regulatory light chain re-association with myosins from which these chains have been removed depends on both Mg++ and Ca++ (Chantler and Szent-Györgyi, 1980; Konno et al., 1983a; Konno and Watanabe, 1985a; Bennett and Bagshaw, 1986b). The specific Ca++ and ATP binding sites are located at different sites on the myosin molecule (Wells et al., 1985) and different regions of the regulatory light chain are involved in Ca++ binding and in regulation (Goodwin et al., 1990). Domain 1 of the regulatory light chain is the non-specific cation binding site (Bagshaw and Kendrick-Jones, 1980; Xie et al., 1994). Sequencing work and comparison with other calcium binding proteins initially suggested that domain III on the essential light chain should be the specific Ca++ binding entity (Collins et al., 1986; Barouch et al., 1991). Later work showed that, although the essential light chain is indeed the Ca++ binding entity (Kwon et al., 1990), Ca++ binds instead to a novel sequence in it called domain I that had heretofore been considered unable to bind Ca++ (Xie et al., 1994; Fromherz and Szent-Györgyi, 1995). The regulatory light chains help stabilize this binding domain (Xie et al., 1994), and a glycine residue on the regulatory light chain critical for Ca++ binding has been identified (Jancsó and Szent-Györgyi, 1994).
Ca++ binding must induce changes in the regulatory complex formed from the two light chains and the heavy chain that in turn allow the motor domain of the myosin to function. What conformational changes Ca++ binding induces in the light chains is not well understood, with Ca++-induced motions of both the essential and the N terminal portion of the regulatory chain having been suggested (Hardwicke et al., 1983; Hardwicke and Szent-Györgyi, 1985). The difficulty in determining what changes occur at this level is not surprising, as work using a variety of techniques suggests that Ca++ induced changes in regulatory chain conformation are small (Chantler and Szent-Györgyi, 1978). With respect to how these changes alter actomyosin activity, present evidence suggests two parallel mechanisms are important.
The first hypothesis (Vibert and Craig, 1982, 1985; Vibert et al., 1986) is supported by data and modeling showing that 1) the head-rod junction (the neck) of the myosin molecule is flexible (Málnási-Csizmadia et al., 1998; Li et al., 2003), 2) Ca++ binding likely changes neck flexibility (Houdusse and Cohen, 1996; Málnási-Csizmadia et al., 1999) (although see Wells and Bagshaw, 1984b), 3) the two myosin heads can lie alongside one another (Offer and Knight, 1996), 4) the ATP and Ca++ binding sites of the two heads communicate in the ‘off’ state (Kalabokis and Szent-Györgyi, 1997; Azzu et al., 2006), EDTA increases the extent to which the two heads show correlated movement (Wells and Bagshaw, 1983), and 6) in the absence of Ca++ the heads are highly ordered and primarily extend towards the tail (Vibert and Craig, 1985; Frado and Craig, 1989, 1992; Stafford III et al., 2001; Zhao and Craig, 2003a). The idea is that in the absence of Ca++ the heads interact with one another and are thus prevented from interacting with the thin filament, and Ca++-induced changes in the flexibility of the myosin neck (where the regulatory complex is located) frees the heads to act independently. Work showing that mutations that would interfere with symmetric head-head interactions do not block regulation required Colegrave et al. (2003) to modify the hypothesis by proposing that the head-head interaction is asymmetric.
The second hypothesis is that activation occurs by Ca++ directly activating the myosin ATPase activity. Based primarily on observations that addition of actin did not change Ca++ activated ATPase activity, this hypothesis was proposed very early (Asada et al., 1979; Konno et al., 1981; Kamiya and Konno, 1984). Considerable modern and direct evidence also supports direct Ca++ activation of the myosin ATPase (Chalovich et al., 1984; Wells and Bagshaw, 1985; Jackson and Bagshaw, 1988a,b; Kerwin and Yount, 1993; Nyitrai et al., 2002). The most parsimonious interpretation of these data is thus that actomyosin activation occurs via both a Ca++ induced increase in cross-bridge formation and direct activation of myosin ATPase activity. Recent work has begun to investigate the effect of ionic interactions and ADP on scallop actomyosin regulation (Nyitrai et al., 2003a,b). Two observations that have not been incorporated into these mechanisms are that Ca++ induces large scale changes in monomeric myosin molecules (Takahashi et al., 1989) and that regulatory light chain removal changes the X-ray diffraction pattern of rigor scallop muscles (Vibert et al., 1978).
Scallop also has a myosin light chain kinase that phosphorylates both the regulatory light chain (Sohma et al., 1985, 1988a; Sohma and Morita, 1986, 1987) and the heavy chain (Sohma et al., 1988b). Molluscan twitchin also phosphorylates the regulatory light chain (Heierhorst et al., 1995). Scallop regulatory light chain can be dephosphorylated by an endogenous Ca++-activated phosphatase (Inoue et al., 1990). Scallop muscle also contains calmodulin {Yazawa, 1980 34900 /id} and at high Ca++ concentrations calmodulin can directly (i.e., in the absence of troponin and regulatory light chain) activate scallop actomyosin (Shiraishi and Morimoto, 1999). A depressing effect of shortening during isometric contraction in Mytilus anterior byssus retractor muscle has been hypothesized to arise from an effect of shortening on the thin or thick filament regulatory systems (Ekelund, 1983).
3.5. Unique properties due to acto-myosin interaction 1: catch
Catch is a state of extremely slow (hours to possibly days in some preparations) relaxation observed in bivalve shell closer muscles. Unraveling the mechanism underlying catch has been extremely difficult. However, this history is also an excellent demonstration of how tortuous scientific progress often is, and we therefore cover it in some detail here. Given the long history of this process, a number of reviews devoted to or that include catch exist (Evans, 1926; Johnson, 1962; Lowy and Millman, 1963; Lowy et al., 1964; Rüegg, 1965, 1968a, 1971; Millman, 1967; Hanson, 1968; Twarog and Muneoka, 1972; Heyer et al., 1973; Twarog, 1976; Hoyle, 1983; Cohen and Castellani, 1988; Bagshaw, 1988; Watabe and Hartshorne, 1990). However, readers of this literature must be always aware that before 1997 the mechanism underlying catch was completely not understood, and the mechanisms proposed in earlier work are therefore of primarily historical interest.
3.5.1. Pre-1997
Although the great resistance to opening of closed bivalve animals has been known throughout history, that these and muscles from some other molluscs as well produce unusually long-lasting contractions compared to vertebrate skeletal muscles was first noted in work dating from 1862 to 1912, of which we have been able to obtain only Biedermann, (1885), Pawlow, (1885), Grützner, (1904), Hofmann, (1907), Parnas, (1910), Bethe, (1911), and von Uexküll (1912), but the earlier papers are referenced in these and in Bayliss et al. (1930). This work is, of course, primitive by modern standards, but does show that electrical stimulation of the closer muscle of Anodonta causes a contraction that long outlasts the stimulation (Grützner, 1904).
There is then a gap, presumably due to World War I, that extends until a second burst of work from 1928 to 1943 on a variety of catch-producing molluscan muscles (von Uexküll, 1926; Bozler, 1928, 1930, 1931a, 1936; Bayliss et al., 1930; Jordan, 1931; van Overbeek, 1931; Herter, 1931a,b; Fletcher, 1937; Winton, 1937; van Dijk, 1937; Pumphrey, 1938; Singh, 1938a,b, 1943). These papers showed that these muscles could produce both rapidly and slowly relaxing contractions, depending on the details (alternating or direct current, respectively) of the electrical stimulation used. Given that the sliding filament theory was not yet proposed, however, the theories of contraction and catch proposed in them are again of only historical interest.
There is then another gap (presumably due to World War II) that lasts until 1953, after which the muscles (primarily the anterior byssal retractor of Mytilus edulis but also some work on the adductors of related bivalves and of Brachiopods) have been continually investigated until the present. The work at the beginning of this third period used recognizably modern techniques and was initially devoted to well defining the contractile responses of the muscles. This work showed that the muscles indeed produce, as a function of stimulation protocol, rapidly or very slowly relaxing contractions; that ACh and serotonin are present in the nerves innervating the muscle; that ACh application induces very slowly relaxing contractions; that serotonin application causes these slow relaxations to become rapid but does not induce contractions when applied alone; and that the nerves innervating the muscle contain both substances (Lowy, 1953, 1954; Twarog, 1954, 1967a, 1968; Bandmann and Reichel, 1954; Hoyle and Lowy, 1956; Welsh, 1957; Holgate and Cambridge, 1958; Abbott and Lowy, 1958a,b; Jewell, 1959; Takahashi, 1960; Twarog, 1960a,b; Rudwick, 1961; Baguet et al., 1962; Millman, 1964; Baguet and Gillis, 1964; Bullard, 1967; Hidaka et al., 1967; Leenders, 1967; Salánki and Hiripi, 1970; Twarog and Cole, 1972; Lowy and Vibert, 1972; York and Twarog, 1973; Nagahama et al., 1974; Sugi and Suzuki, 1978; Satchell and Twarog, 1978; Muneoka et al., 1978a–c, 1979). The muscle’s ability to produce both phasic and tonic contractions thus resulted from it having two innervations, one cholinergic and one serotonergic, with the differing stimulation protocols stimulating either only the cholinergic pathway or stimulating both pathways simultaneously. Later work showed that a number of other substances, including dopamine and several peptides, also cause rapid relaxation (Twarog and Cole, 1972; Muneoka et al., 1979; Yoshida et al., 1981; Takayanagi et al., 1981; Painter, 1982; Ishii and Takayanagi, 1982; Murakami et al., 1983, 1986; Gies, 1986; Hirata et al., 1986, 1987, 1989; Takemoto et al., 1986; Ohtani et al., 1995).
By this time how nerve activity induces muscle contraction was beginning to be understood, which gave rise to the first of the controversies in this field: were the slow relaxations due to continuous activity in the innervating pathways (the ‘tetanic’ hypothesis), as had been shown to be the basis of a sustained contraction in isolated crustacean limbs (Barnes, 1930), or to a sustained change intrinsic to the muscle itself (the ‘catch’ hypothesis) (Lowy, 1953; Jewell, 1959). A series of papers before 1960 showed that in the animal and in some in vitro preparations infrequent electrical events were indeed present in the muscle during catch, supporting the tetanic idea (Lowy, 1954, 1955; Hoyle and Lowy, 1956; Abbott and Lowy, 1958a,b). Three articles in 1960, however, unambiguously showed that catch could occur in the absence of nerve activity, and thus that a muscle-intrinsic catch state must exist (Johnson and Twarog, 1960; Takahashi, 1960; Twarog, 1960b).
During this period work in other muscles was also identifying calcium’s role in linking muscle membrane depolarization to contraction, which gave rise to the hypothesis that catch was due to ACh inducing a long-lasting increase in intramuscular Ca levels, which serotonin application reduced (Twarog, 1966, 1967a,Twarog, b). Although some immediately subsequent work on calcium fluxes during muscle contraction and catch development were interpreted as supporting this hypothesis (Bloomquist and Curtis, 1972, 1975a,Bloomquist and Curtis, b), it rapidly became clear (Atsumi, 1974; Marchand-Dumont and Baguet, 1975; Baguet and Marchand-Dumont, 1975; Atsumi and Sugi, 1976) and has been amply supported by later work (Cornelius, 1980, 1982; Pfitzer and Rüegg, 1982; Güth et al., 1984; Ishii et al., 1989; Tanaka et al., 1998) that although Ca concentrations rise during the muscle’s initial contraction (Kometani and Sugi, 1978), high Ca concentrations are neither needed for, nor present during, catch. Indeed, in modern parlance catch might be best defined as the ability to maintain resistance to stretch in the absence of high intramuscular Ca levels.
That catch is not due to continuous high actomyosin activation was further supported by work showing that 1) catch expends much less energy than active shortening (Parnas, 1910; Brecht et al., 1955; Nauss and Davies, 1966; Baguet and Gillis, 1967, 1968; Baguet et al., 1967; Yernaux and Baguet, 1971; Schumacher, 1972; Sugi and Suzuki, 1978; Ebberink et al., 1979; Zange et al., 1989; Ishii et al., 1991), 2) when catch muscles are appropriately vibrated catch is abolished but force is not regenerated when the vibration ends (Ljung and Hallgren, 1975), and 3) the ability of muscles to shorten and then re-develop tension when loading force is decreased (quick release experiments) is much reduced after catch has developed (Jewell, 1959; Johnson and Twarog, 1960; Lowy et al., 1964; Baguet and Marchand-Dumont, 1975). It is precisely this property, that muscles in catch strongly resist being stretched, but do not contract further if their load is decreased, that most distinguishes catch from more commonly observed contractile states, and which is responsible for the fact that, if a piece of wood is inserted between the valves and the animal stimulated to close, when the wood is removed after catch has been induced the shells do not close further (Hoyle, 1983)
These observations suggested that catch resulted from a process in the muscle that ‘locked’ it at whatever length it had when catch ensued. Supported in part by work in these very muscles (Dörr and Portzehl, 1954; Hanson and Lowy, 1959, 1961; Rüegg, 1961c, 1968a; Lowy and Hanson, 1962; Lowy et al., 1964; Heumann and Zebe, 1968; Sugi and Tsuchiya, 1979), that all muscles contract via a sliding filament mechanism had by this point been well established. One mechanism that could support such locking would thus be a ‘rigor’ state in which the myosin heads were permanently attached to the thin filaments, or at least only very slowly cycled, during catch (Lowy and Millman, 1959; Hanson and Lowy, 1961). However, it was also known at this time that the thick filaments of these muscles were unusually large and contained very large amounts of paramyosin. An alternative hypothesis was that the thick filaments formed interconnections among themselves, or non-actomyosin based interconnections to the thin filaments, that promoted muscle rigidity (Johnson et al., 1959; Rüegg, 1959; Philpott et al., 1960; Bailey and Rüegg, 1960). Resolution of this controversy was only achieved by a 1997 discovery that catch is instead determined by the phosphorylation state of the very large sarcomere associated protein, twitchin (see Hooper and Thuma, 2005, for twitchin references) (Siegman et al., 1997). We therefore consider the pre- and post-1997 eras separately.
3.5.1. Pre-1997
Pre-1997 experimental evidence interpreted as supporting the non-actomyosin hypothesis was the data mentioned above that catch required very little energy and evidence that catch continued even under conditions in which actomyosin cycling was chemically poisoned (Rüegg, 1961a,b, 1963, 1964; Rüegg et al., 1963), that catch was not associated with changes in ATP concentration (Rüegg and Strassner, 1963), that temperature affected contraction and catch differently (Reichel, 1953), that the birefringence of tonically and phasically contracting muscles was different (Zs-Nagy et al., 1965), and that vibration reduced catch but not muscle stiffness (on the belief that muscle stiffness was due to actomyosin linkages) (Kobayashi et al., 1985). As to what physical structure could mediate non-actomyosin based rigidity, the first proposal was that catch resulted from paramyosin ‘crystallization’ or similar large scale changes in thick filament structure (Johnson et al., 1959). X-ray diffraction studies quickly showed that such large changes did not occur (Millman and Elliott, 1965), but several ultrastructural papers did find small scale changes in thick filament interaction, aggregation, or diameter, each of which were proposed as possible thick filament based explanations for catch (Zs-Nagy et al., 1970, 1971; Schumacher, 1970; Wabnitz, 1975; Gilloteaux and Baguet, 1977; Gilloteaux, 1978; Chen and Cao, 1984; Hauck and Achazi, 1987; Chen et al., 1988). The discovery that caldesmon was present in catch muscles prompted an alternative (but still non-actomyosin based) thin and thick filament interaction hypothesis, that catch arose from caldesmon cross-linking the thick and thin filaments (Bennett and Marston, 1990), as it can in vertebrate smooth muscles. A thin filament based hypothesis was apparently (it is difficult to be certain, as the paper is in Russian with only an English abstract) also proposed based on the observation that catch muscle extracts form gels due to actin filament bundling (Podgornaya and Drozdov, 1981).
Opposed to these data (i.e., in support of the hypothesis that catch arose from a modification of the actomyosin force generating apparatus) was work arguing that the observed thick filament aggregations were a fixation artifact (Miller, 1968) and ultrastructural work showing either no thick filament fusion (Atsumi, 1978) or only a change in thin and thick filament association (interpreted as supporting the actomyosin hypothesis, although of course also consistent with the caldesmon hypothesis noted above) (Bennett and Elliott, 1989). Further data interpreted as supporting the actomyosin hypothesis were observations that 1) varying bathing solution tonicity affected catch (based on the belief that altering intracellular ionic strength would alter actomyosin interactions) (Tameyasu, 1978), 2) muscle force tension curves were similar during active contraction and catch (Tameyasu and Sugi, 1976; Tsuchiya and Takei, 1986) 3) ATPase activity and catch ability were correlated (Leenders, 1966, 1969), 4) catch maintenance does require some energy, and the amount required varies with applied load (Baguet and Gillis, 1968) and 4) relaxation from catch releases phosphate (Minihan and Davies, 1965, 1966; Nauss and Davies, 1966) (on the belief that unbinding myosin heads frozen onto the thin filaments would require ATP splitting but unbinding thick filaments would not). That contraction and catch had a common mechanism was also consistent with work showing that the rising portions of phasic and tonic contractions had the same energy costs (Devroede and Baguet, 1982) and that catch develops simultaneously with contraction (Leenders, 1967). Data showing that paramyosin reduced actin-activated ATPase activity of rabbit myosin was also interpreted as indicating that paramyosin was unlikely to play a role in catch (Epstein et al., 1975, 1976).
These data were clearly not strongly supportive of either hypothesis. Another way forward was to investigate the second messenger systems regulating catch. Work from 1972 to 1988 showed that agents that relaxed catch (e.g., serotonin) increased muscle cAMP levels, and that chemical treatments that increased cAMP relaxed catch {Achazi, 1979 36504 /id;Köhler, 1980 36506 /id;Achazi, 1974 35209 /id;Cole, 1972 449 /id;Gies, 1986 36300 /id;Gies, 1988 23435 /id;Marchand-Dumont, 1975 36296 /id;Matsuura, 1984 480 /id;Painter, 1982 54 /id;Pfitzer, 1982 23455 /id}. Subsequent work isolated and characterized the regulatory and catalytic subunits of the cAMP-dependent protein kinase (Cao et al., 1995a,b, 1996; Rodríguez et al., 1998; Díaz-Enrich et al., 2003; Bardales et al., 2004; Béjar and Villamarín, 2006). The importance of protein phosphorylation was further emphasized by the observation that a calcineuron type phosphatase was required for catch (Castellani and Cohen, 1992).
This work showed that the phosphorylation state of some 26 proteins, including myosin heavy and light chains, tropomyosin, and paramyosin, changed during catch (Achazi, 1979; Chen et al., 1988; Hauck and Achazi, 1991). This work resulted in two hypothesis for catch, both of which assumed that catch was due to changes in actomyosin cycling. The first was that actomyosin cycling was altered as a result of myosin light chain phosphorylation. Catch muscle regulatory light chains come in two forms, A and B (Kondo and Morita, 1981; Morita and Kondo, 1982; Morita et al., 1985; Miyanishi et al., 1985). Catch muscles are often composed of two anatomical parts, a translucent part and an opaque part, with the opaque portion showing both much greater catch (Rüegg, 1961b) and higher concentrations of the A form of the regulatory chain (Kondo and Morita, 1981; Morita and Kondo, 1982). The A form (and the myosin heavy chain) are phosphorylated by a cAMP dependent protein kinase (Sohma et al., 1985; Sohma and Morita, 1986, 1987; Sohma et al., 1988a,b). Work comparing actomyosin cycling with phosphorylated and unphosphorylated regulatory light chain A showed that, when unphosphorylated and in low calcium, the myosin heads remained bound to the thin filament (which would be the catch condition), and when the light chain was phosphorylated, the heads unbind (which would release catch) {Takahashi, 1988 23070 /id;Takahashi, 1989 23069 /id}. Catch would thus result from the actomyosin bridges assuming ‘several structural states with different internal mobilities’ as a function of calcium and ATP concentration and activation history (Tameyasu, 1990; Tameyasu and Tanaka, 1991).
The second hypothesis arose from the observation that myosin heavy chain and paramyosin were phosphorylated. These data were interpreted as supplying a new molecular basis for an old idea (Szent-Györgyi et al., 1971; Halsey and Harrington, 1971) that catch arose from changes in the rod portion of the thick filaments that in turn altered actomyosin cycling (Cooley et al., 1979; Cohen, 1982; Castellani and Cohen, 1987a,b; Castellani et al., 1988). The working hypothesis was that catch represented a slowly cycling actomyosin state, and that relaxation from catch occurred because myosin rod or paramyosin phosphorylation inhibited this slow cycling and resulted in myosin head detachment from the thin filament. Consistent with this hypothesis, paramyosin phosphorylation decreases muscle ATPase activity (Achazi, 1979; Chen et al., 1988; Watabe et al., 1989). A difficulty with this hypothesis is that the paramyosins of at least two catch muscles are already phosphorylated at rest (Watabe et al., 1990), although catch can increase paramyosin phosphorylation, and subsequent serotonin application can do so even more (Chen et al., 1988).
A final series of hypotheses involved pH and catch. Early work showed that catch duration varies as a function in pH (Baguet, 1973; Marchand-Dumont and Baguet, 1975), and serotonin application (but not catch itself nor the anoxia that occurs during prolonged shell closure) alters catch muscle internal pH (Ellington, 1983; Zange et al., 1989, 1990a,b; Ishii et al., 1991). The pH increase is slow, however, which argued against this mechanism being physiologically important in the control of catch.
3.5.2. Post-1997
The situation in 1996, after some 40 years of work in the modern era, was thus highly confused. Although the actomyosin hypothesis was in the ascendancy, in retrospect it is unclear this was due to any truly compelling data. Moreover, the actomyosin hypothesis had multiple competing variants, the field had multiple seemingly contradictory experimental results and, worse, it was not obvious how to proceed. This all changed in 1997 as a result of contemporaneously occurring work identifying sarcomere proteins other than myosin and actin. An important result of this work was the realization that, in addition to large scale structures such as the thin and thick filaments that are composed of large numbers of relatively low molecular weight subunits, there are also enormous single proteins so large that in vertebrates they span the entire sarcomere (see Hooper and Thuma, 2005, for references and a fuller description of these proteins). Although no invertebrate giant sarcomere protein is this large, invertebrate sarcomeres do contain proteins capable of spanning 30–50% of the sarcomere. One such protein is twitchin, originally discovered in C. elegans but later identified in a large number of invertebrates.
In 1997 Siegman et al. reported that a very large (~600 kDa) sarcomere protein, and only this protein, showed catch-correlated changes in phosphorylation. They identified this protein as twitchin, showed that protein kinase A phosphorylated it, and that its phosphorylation increased relaxation velocity (Siegman et al., 1998; Funabara et al., 2001, 2003; Funabara and Watabe, 2002). Parallel work by Yamada et al. (2002) showed that an in vitro assay containing only myosin, actin, and twitchin showed catch, and that catch depended only on twitchin phosphorylation state. These workers then showed that phosphatase 2B (the calcineuron sensitive phosphatase mentioned above) dephosphorylates twitchin, and this dephosphorylation initiates catch in vitro (Yamada et al., 2004). Phosphatase 2B is activated by calmodulin. Mytilus catch muscle contains large amounts of calmodulin and a calmodulin protein kinase system (Sailer et al., 1990), and scallop catch muscle contains an endogenous calcineuron type, Ca++/calmodulin-dependent phosphatase (Shiraishi and Morimoto, 1999).
Although this work clearly established the critical role played by twitchin in catch, it did not resolve the controversy over whether the long lasting resistance to stretch was due to persistent actomyosin interaction or some other stretch resisting structure. Early after the discovery of twitchin’s role some evidence was presented arguing that the stretch resistance was due to long lasting actomyosin cross bridges, with the actomyosin being unable to detach unless twitchin was phosphorylated (Butler et al., 1998, 2001; Funabara and Watabe, 2002). However, studies showing that 1) actively contracting muscles and muscles in catch have different load bearing abilities (Sugi et al., 1999; Mukou et al., 2004), 2) rigor cross-bridge detachment rates are too fast to explain catch time course (Galler et al., 1999), 3) treatments that alter or abolish cross-bridge formation do not alter catch (Galler et al., 2005; Andruchov et al., 2006; Höpflinger et al., 2006), and 4) twitchin phosphorylation does not affect myosin head detachment (Andruchova et al., 2005) gradually made that position untenable.
But if not myosin cross-bridges, what was the stretch resistant structure? The answer came in the demonstration that dephosphorylated, but not phosphorylated, twitchin bound the thin filament (Shelud’ko et al., 2004; Funabara et al., 2005; Tsutsui et al., 2005). Since twitchin is a thick filament protein, this would bind the two filaments and provide the necessary rigidity, and the phosphorylation dependence of the interaction explains how catch is controlled. However, this still left a nagging problem: although the actomyosin crossbridges were not the stress resistant structure in the catch state, various lines of evidence showed that when myosin is generating force catch force decreases, and when it is not, catch force increases, suggesting that the twitchin linkages and actomyosin force generating crossbridges interact in some way (Butler et al., 2006).
A hypothesis consistent with these and all other data to present is shown in Fig. 8. The first four rows (top to bottom) show idealized representations of muscle tension, Ca2+ concentration and phosphatase activation, cAMP levels and PKA activation, and twitchin phosphorylation in (left to right columns, dashed lines) control saline, ACh, a saline wash, 5-HT, 5-HT and ACh co-application, and a final wash. The cartoons at the bottom of the figure show twitchin and myosin interactions with the thin filament in the various conditions.
Figure 8.
Explanation of catch. Top four traces: muscle tension; Ca++ concentration and phosphatase activation; cAMP and PKA levels; and twitchin phosphorylation in saline, ACh, saline wash (catch), relaxation induced by 5-HT, ACh and 5-HT, and saline wash. Bottom cartoon, mechanism of catch development and relaxation. Thick filament is bottom thick line, the myosin head is the object resembling a microphone, the thin filament is the row of open circles, and twitchin is the object represented as a coil (unphosphorylated and unable to interact with the thin filament) and straight lines (interacting with the thin filament in alteration with myosin in ACh and continuously during catch in saline). Modified from Funabara et al. (2005) and Butler et al. (2006).
In control saline twitchin is phosphorylated (represented by the twitchin molecule being curled up) and does not interact with the thin filament. Myosin is also inactive because Ca2+ levels are low, and thus the muscle is relaxed. ACh application increases Ca2+ levels which activates myosin cycling, and the muscle initially contracts in the normal fashion. The increased calcium levels also activate the phosphatase and twitchin rapidly becomes dephosphorylated and can interact with the thin filament. During this phase of the contraction twitchin and myosin alternately bind the thin filament, twitchin being dislodged (or at least not preventing thin:thick filament sliding) when the myosin heads bind to deliver force and twitchin binding and resisting muscle stretch during the myosin head’s recovery phase. The next column shows the situation in catch, when calcium levels have dropped. The myosin heads now do not (or at least much less often) engage the thin filament, but the twitchin binding maintains resistance to stretch and prevents relaxation. 5-HT application increases cAMP and PKA levels and twitchin phosphorylation. Neither twitchin nor myosin now engage the thin filament, and so the muscle rapidly relaxes. Co-application of 5-HT and ACh results in myosin activation but does not induce sufficient phosphatase activity to overcome the PKA activity. Twitchin therefore remains sufficiently phosphorylated that catch does not develop, and thus in this case subsequent saline application results in rapid relaxation.
The data presented thus far appear to constitute a very good explanation for catch. They are consistent with the control of catch by phosphorylation, provide a mechanism for thin:thick filament interaction, and the strength of this interaction on the individual filament level is sufficient to explain force levels present in intact muscles (Yamada et al., 2001). They also explain the puzzling interaction between active and catch force noted above, the ability of catch muscles to ratchet (i.e., to show great resistance to applied stretch, and yet contract further in response to additional nerve stimulation or ACh application), and the contemporaneous time course of catch and active force generation (in that catch is always present, but can only be observed when actomyosin cycling falls below a certain level).
Although these data show that the twitchin mechanism is sufficient for catch, two arguments have been made that it may not be the only mechanism underlying catch. The first is that the protein myorod is a major component of catch muscle thick filaments and is also phosphorylated during catch release, and that these correlations indicate that myorod must be involved in catch (Sobieszek et al., 2006). However, this sort of argument by correlation must be viewed with great caution; many of the pre-1997 articles mentioned above similarly argued that paramyosin ‘must’ be involved in catch because it is so abundant in catch muscles, (references in Section 3.2, but see Tsuchiya et al., 1992). The second argument is electron microscopic work confirming the older ultrastructural work showing increased thick filament interconnections during catch (Takahashi et al., 2003). However, as was also true of older arguments of this sort, it is unclear how interconnections among thick filaments would result in catch force.
3.5.3. Terminology and presence in non bivalve muscle
The term ‘catch’ has been used to describe a number of phenomena that it is now clear are of very different natures. Given the fairly deep understanding we now have of the ‘catch’ described above, and the fact that a similar process probably exists in at least some other invertebrates, it might be better to reserve the term ‘catch’ for the process above, and to find other terminology for the other instances (see Hoyle, 1983, for an excellent discussion of this issue). Regardless, it is important for readers to be aware of these different usages. One such case is to use ‘catch’ to describe twitch temporal summation (the fact that if muscles are stimulated with spike trains whose interspike interval is too short to allow significant muscle relaxation, they develop large contractions because each individual twitch ‘builds’ upon the incompletely relaxed tail of its predecessor, Evans and Siegler, 1982). This process requires no special muscle properties and is nothing more than slow temporal filtering (see Morris and Hooper, 1997, 1998, 2001; Morris et al., 2000; Thuma et al., 2003 for detailed consideration of this issue and some of its functional consequences).
‘Catch’ has also been used to describe observations similar to those first made by Blaschko et al. (1931) in which single spikes or brief trains of high frequency spikes inserted into tonic low frequency spike trains result in a long-lasting increases in muscle contraction amplitude (invertebrate examples: Wilson and Larimer, 1968; Wilson et al., 1970; Wakabayashi and Kuroda, 1977; Burns and Usherwood, 1978; vertebrate examples: Lee et al., 1999a,b; Van Lunteren and Sankey, 2000). This clearly differs from molluscan catch in that nerve stimulation and actomyosin activation occur throughout the contraction. The mechanism underlying this phenomenon is not well understood, although in one invertebrate case may arise in part from non-uniform sarcomere lengths (Günzel and Rathmayer, 1994).
Catch similar to that observed in bivalves, at least to the extent that the contractions long out-last the nerve stimulations or pharmacological applications that induced them, has been observed in insect (Chesler and Fourtner, 1981; Hoyle and Field, 1983; Hoyle, 1984), crayfish (Hawkins and Bruner, 1979; Chesler and Fourtner, 1981; Hoyle and Field, 1983; Hoyle, 1984), nematode (Swanson, 1971a), earthworm (Hidaka et al., 1969), Sipunculus (von Uexküll, 1903), brachiopods (Wilkens, 1987), squid (Bozler, 1931b; Florey, 1966; Florey and Kriebel, 1969), and snail (Jordan, 1926; Masai, 1951). In two of these cases the contractions were shown to be not associated with contemporaneous muscle depolarization (Hoyle and Field, 1983; Hoyle, 1984), but in none of them is anything known about calcium concentration during the sustained contraction. Nonetheless, these data suggest that bivalve type catch may exist not only outside Bivalvia, but even outside Mollusca.
3.6. Unique properties due to acto-myosin interaction 2: asynchronous flight muscle
Muscles sometimes repeatedly contract and relax at extremely high frequency. It might be assumed that they do so because they are driven by motor neuron input synchronous with each contraction. Some very rapidly cycling muscles involved in sound production—rattlesnake, 90 Hz (Schaeffer et al., 1996; Rome and Lindstedt, 1998); lobster, 100 Hz (Mendelson, 1969); toadfish, 200 Hz (Fine, 1978; Fine and Mosca, 1989; Rome and Lindstedt, 1998); katydid stridulation, 200 Hz (Josephson and Halverson, 1971); the tymbal muscles of most cicadas, 50 to 550 Hz (Wakabayashi and Hagiwara, 1953; Hagiwara et al., 1954; Hagiwara, 1955; Aidley, 1969; Reid, 1971; Simmons, 1977; Josephson and Young, 1981, 1985; Young and Josephson, 1983a,b, 1984; Nahirney et al., 2006)—are indeed driven by motor neuron input contraction by contraction. The last three examples are well above what has at times been considered the upper limit of 100 Hz for synchronous muscle (Pringle, 1976; Smith, 1983), and thus asynchrony (see below) must not be considered a requirement for rapid repetitive muscle contraction. However, this requires extremely well developed and extensive sarcoplasmic reticulum to re-sequester Ca++ from the myofibril with each relaxation, which leaves less and less space for the myofibrils as operating frequency increases (Rosenbluth, 1969; Josephson and Young, 1981; Josephson et al., 2000; Nahirney et al., 2006). Furthermore, although actomyosin detachment rate has increased in such muscles, attachment rate has not, and therefore very few cross-bridges form during their contractions {Rome, 1999 37694 /id}. Rapidly oscillating synchronous muscles have therefore not been found in motor patterns requiring large force development.
A different asynchronous type of neuron to muscle control strategy is instead used to power insect flight, which requires high force and often high frequency, up to 500–1000 Hz in small midges (Sotavalta, 1947, 1953), although asynchronous muscle is also sometimes used in insects with low wingbeat frequency, belostomatid bugs, 20 Hz (Barber and Pringle, 1966) and some Coleoptera, 25 Hz (Pringle, 1981). In these muscles motor neuron spikes are at a much lower frequency than, and are not one-to-one with (i.e., are asynchronous with, although n:m phase locking can be present, Heide, 1979) the muscle contractions, with the ratio between spikes and muscle contractions ranging from 1:5–1:20 (Pringle, 1949; Roeder, 1951; Wilson and Wyman, 1963; Nachtigall and Wilson, 1967; Bastian and Esch, 1970). Another difference between these and synchronous muscles is that they contract only 3–4%, as opposed to the 8–15% commonly observed in insect muscle (Surholt et al., 1990; Gilmour and Ellington, 1993; Chan and Dickinson, 1996).
We cover here only articles relevant to the molecular mechanisms underlying force generation in these muscles. Articles about flight muscle genes and proteins in general are covered in Hooper and Thuma (2005); articles about sarcomere and gross anatomy of these muscles and their electrical properties are covered in the third and fourth reviews, respectively; and articles about thick and thin filament structure and force generation and regulation in asynchronous muscles in the other sections of this review. Reviews covering asynchronous muscle in whole or part include {Barrington-Leigh, 1976 37356 /id;Boettiger, 1952 384 /id;Boettiger, 1960 857 /id;Bullard, 2005 36430 /id;Bullard, 1983 18944 /id;Dickinson, 2006 36681 /id;Dickinson, 1997 1113 /id;Hanson, 1968 36528 /id;Josephson, 2000 1576 /id;Maughan, 1999 1859 /id;Peachey, 1968 478 /id;Pringle, 1967 2141 /id;Pringle, 1967 32510 /id;Pringle, 1968 36332 /id;Pringle, 1978 35095 /id;Pringle, 1981 2150 /id;Rüegg, 1967 22222 /id;Smith, 1965 21649 /id;Sparrow, 1995 551 /id;Squire, 2003 37409 /id;Svidersky, 1999 23396 /id;Syme, 2002 SYME2002A /id;Tregear, 1967 33978 /id;Tregear, 1983 37638 /id;Weber, 1973 3355 /id;White, 1973 7188 /id;Wilson, 1968 34683 /id;Zebe, 1960 37408 /id}.
How these muscles produce such rapid contraction/relaxation cycles, and do so without being triggered by one-to-one motor neuron input, has triggered considerable study. This work has shown that in many ways these muscles are very similar to synchronous muscles. For instance, calcium activates their actomyosin in the normal fashion (Maruyama and Sakagami, 1958; vom Brocke, 1966; Schädler, 1967; Chaplain, 1967b, 1969; Maruyama et al., 1968b; Abbott, 1973; Marston and Tregear, 1974; Peckham et al., 1990; Linari et al., 2004), although at matching calcium concentrations the activation is less than in typical muscle (vom Brocke, 1966; Maruyama et al., 1968b; Chaplain, 1969; Peckham et al., 1990). Magnesium, ATP, and ADP concentrations affect muscle fiber (Aronson, 1962) and actomyosin ATPase activity (vom Brocke, 1966; Jewell and Rüegg, 1966; Zieger, 1969; Abbott and Mannherz, 1970; White and Thorson, 1972; Kuhn et al., 1985), but not in any way that would obviously explain the muscles’ unusual properties. Actomyosin regulation is not unusual. Both thin and thick filament regulation are present (Section 3.4.7) with the thin filament system being used to start and stop flight activity but myosin light chain phosphorylation being required for normal activity (Takahashi et al., 1990a,b; Tohtong et al., 1995; Dickinson et al., 1997). Moreover, not only are asynchronous muscle isometric twitches not unusually fast, they are instead extremely long lasting (100 msec range) (see reviews referenced above and the fourth review of this series for references). The continuous, tonic motor neuron firing observed during flight (in the tens of Hz) is thus sufficient to induce a nearly smooth fused isometric tetanus.
How, then, do asynchronous muscles produce, in most cases very high frequency, oscillations? The large scale answer is that they do so by mechano-chemical coupling of the thorax and the muscles. When put under proper tension, the insect thorax and wings form a mechanical oscillator (much like a flaccid rubber band will not oscillate if plucked, but a stretched rubber band will); the tetanic contraction of the asynchronous flight muscles provide the necessary tension. [A note on nomenclature: since asynchronous muscles only indirectly (through the mechanical resonance noted above) power flight, they are called indirect flight muscles. Another set of smaller synchronous muscles, the direct flight muscles, that attach directly to the wing hinges, control wing movements for aerial maneuvering by altering how the mechanical energy produced by the direct flight muscles is transmitted to the wings.] The initial impetus to start the mechanical oscillator is provided by the thorax deformation induced by the jump from the substrate at flight beginning.
Although the indirect flight muscle tension results in the thorax/wing assembly being able to oscillate, without the addition of further energy over time the oscillation would end due to viscous energy losses and the energy the wings lose in accelerating the air. This energy is provided by indirect flight muscle contractions and relaxations induced by, and thus synchronous with, wingbeat. These contractions occur because the indirect flight muscles respond to stretch with a brief contraction after the stretch is over (delayed stretch activation). In a complimentary manner, they respond to being shortened with a delayed decrease in force production (Pringle, 1978; Josephson and Ellington, 1997).
These delayed responses support flight as follows. During flight the indirect muscles are subjected to alternating stretch and shortening due to the mechanical oscillation of the thorax. These stretches and shortenings induce delayed indirect muscle contractions and relaxations, respectively. The delays are such—i.e., are longer for insects with slower wing beat frequencies (Molloy et al., 1987; Peckham et al., 1990)—that the muscle contractions enhance the mechanical contractions, and thus transmit sufficient energy to the mechanical oscillator to maintain its activity. This oscillation induced force production can be measured in vitro by attaching the muscle to a vibrating device; when the vibrator is set within a narrow range of oscillation frequencies (which always includes the natural wing beat frequency) the muscle performs work on the vibrator (Machin, 1959; Machin and Pringle, 1959, 1960; Machin et al., 1962; Josephson, 1997). When appropriately loaded, the muscles are also capable of endogenous oscillation (Hanson, 1956a; Machin and Pringle, 1959).
Glycerinated asynchronous muscles (in which sarcoplasmic reticulum and cell membrane based Ca++ regulatory systems are absent) in the presence of ATP and elevated Ca++ continue to show delayed stretch activation and shortening inactivation (Rüegg and Tregear, 1966; Chaplain, 1967b, 1969; Schädler et al., 1969; Rüegg and Stumpf, 1969a; Rüegg, 1972; Kuhn, 1973; Breull et al., 1973; Pybus and Tregear, 1975; Abbott and Steiger, 1977; Güth et al., 1979; Rüegg et al., 1984; Kuhn et al., 1985; Peckham et al., 1990; Peckham and White, 1991; Linari et al., 2004) and oscillation (Rüegg and Tregear, 1966; Jewell and Rüegg, 1966; Abbott and Chaplain, 1966; Mannherz, 1968; Rüegg, 1968b; Steiger and Rüegg, 1969; Pringle and Tregear, 1969; Rüegg and Stumpf, 1969b; Mannherz, 1970; Schädler et al., 1971; Ulbrich and Rüegg, 1971; Abbott, 1973; Breull et al., 1973; Pybus and Tregear, 1975; Cuminetti and Rossmanith, 1980; Yamakawa and Goldman, 1991). This work also shows that both stretch and elevated Ca levels are required to achieve maximum ATPase activation (Rüegg and Tregear, 1966; Mannherz, 1968; Rüegg, 1968b; Steiger and Rüegg, 1969; Chaplain, 1969; Pringle and Tregear, 1969; Rüegg and Stumpf, 1969b; Breull, 1971; Breull et al., 1973; Chaplain and Honka, 1974b; Pybus and Tregear, 1975; Chaplain et al., 1976) and that AMPPNP (a non-hydrolyzable ATP analog) binding is stretch-dependent (Kuhn, 1978b).
The glycerin data show that delayed stretch activation and shortening inactivation arise at the level of the actomyosin itself. Like catch, the mechanisms underlying these phenomena have been long controversial. Two hypotheses, the helix match-mismatch hypothesis and strain sensor hypothesis, have been proposed. The match-mismatch hypothesis stems from the extraordinary regularity of asynchronous muscle sarcomeres (see section 3.3.1 and Fig. 7 for detailed explanation and references). In these muscles six actin filaments surround each thick filament. Moving clockwise around these six filaments, each is rotated clockwise 60° relative to the filament that precedes it. Comparing the thin filaments surrounding different thick filaments shows that all thin filaments with the same radial position (i.e., all the 12 o’clock thin filaments, all the 2 o’clock filaments, all the 4 o’clock filaments, etc.) are in perfect helical register across the sarcomere, as are also all the thick filaments.
This high order could explain stretch activation and shortening inactivation as follows. Myosin heads can bind only to certain target zones on the thin filaments. For any thick and thin filament pair, muscle stretch and shortening will move the thin filament target zones in and out of reach of the thick filament’s myosin heads. For a typical muscle, in which the thin and thick filaments are not in register across the sarcomere, these movements would not result in stretch activation/shortening inactivation because the random staggers of the various thin-thick filament pairs means that as muscle length changes different filament pairs come into best binding position. There are therefore no muscle lengths where all the thick and thin filaments simultaneously come into and go out of best binding position. In highly ordered muscles like asynchronous muscles, however, as muscle length changes the availability of the target zones to all the myosin heads changes synchronously. The idea is thus that in stretched muscles all the thick filaments are in positions with increased access to the target zones and so generate greater force, and in shortened muscles they all have less access and so generate less force (Wray, 1979a).
Some later work supported this concept (Abbott and Cage, 1984; Lund et al., 1988; Peckham and White, 1991), including observations that, among all the hypotheses proposed to explain stretch activation/shortening inactivation, only a lack of helix matching distinguishes synchronous and asynchronous muscle (Peckham and White, 1991) and power output is maximized when imposed oscillations have an amplitude equal to the 38 nm actin helix repeat distance (Abbott and Cage, 1984). A strong apparent argument against this mechanism was provided by the observation that in the M band (the center of the sarcomere, where only thick filaments are present) the thick filament backbones are arranged in one of three orientations 60° apart. Filaments with different orientations are randomly present across the sarcomere (Freundlich and Squire, 1983), which would seem to destroy the high order necessary for the match-mismatch hypothesis (Squire, 1992). The hypothesis was resurrected, however, by later data showing that in the A band (where the thin and thick filaments overlap), the myosin heads are arranged with high order across the sarcomere (see Section 3.3.1 for the explanation of how this occurs) (Schmitz et al., 1994b).
Three later papers have provided additional support. First, a study of isometrically active indirect flight muscle showed that the myosin heads indeed preferentially interact with repeating regions on the thin filament and translation of the two filaments by the amount expected during flight decreases thick:thin filament binding (Tregear et al., 2004). Second, a survey of 50 insects showed that all asynchronous muscles have extremely high regularity over the entire length of the myofibrils (>1000 sarcomeres in some species) but synchronous muscles do not, although more skilled flyers that use synchronous muscle have increased order relative to weaker flyers (Iwamoto et al., 2006). Third, Diptera asynchronous muscles have even greater order than that noted above, which was from Lecotherus (giant water bug), a weak flyer compared to flies and one that, unlike flies, requires a preflight warm-up period. This increased order is a superlattice in which adjacent thick filaments are axially shifted one third of the axial distance between the myosin heads, which modeling shows would increase the range of thick:thin filament displacements over which the heads can bind while still maintaining regions of increased and decreased head binding (Squire et al., 2006). Taken together, these data strongly suggest that match-mismatch likely plays a role in stretch activation and shortening inactivation (Squire et al., 2005b).
The strain sensor hypotheses argues that stress induced changes in thick or thin filament structure or regulatory molecule conformation (a molecular sensor) alters actomyosin attachment numbers or dynamics {Abbott, 1972 5059 /id;Chaplain, 1968 32694 /id;Chaplain, 1968 37435 /id;Granzier, 1993 1360 /id;Güth, 1981 33259 /id;Pringle, 1978 35095 /id;Smith, 1991 2354 /id;Thomas, 1996 36667 /id;Thorson, 1969 7037 /id;Thorson, 1983 303 /id}. Because it is likely that sarcomere strain is signaled by the thick filaments (see below), and because cross-linking the myosin heads results in oscillatory power production in vertebrate myofibrils (Tawada and Kawai, 1990), a first speculation might be that strain directly alters myosin activity. Two observations argue against this hypothesis. First, many properties of asynchronous muscle myosin do not significantly differ from those of vertebrate skeletal myosin (White et al., 1986, 1987; Silva et al., 2003), although, as expected given the high oscillation frequencies of the muscles, the asynchronous muscle myosin is exceptionally fast (Swank et al., 2001, 2006b; Silva et al., 2003). Second, expressing embryonic myosin heavy chains, or asynchronous myosin heavy chains in which parts have been replaced by embryonic sequences, in asynchronous muscles does not block stretch activation/shortening inactivation, although it does affect muscle power output and hence often results in flightless animals or ones that fly with altered wingbeat frequency (Swank et al., 2001, 2000, 2003, 2006a; Swank and Maughan, 2003; Littlefield et al., 2003).
Given that asynchronous muscles have a unique myosin light chain isoform (Falkenthal et al., 1987) and the importance of myosin light chain phosphorylation for flight production (Takahashi et al., 1990a,b), myosin light chains as a sensor was also an attractive speculation. Again, however, a variety of treatments that alter light chain structure show that, although these changes affect power output and the frequency at which maximum oscillatory work is produced, they do not destroy stretch activation/shortening inactivation (Tohtong et al., 1995; Moore et al., 2000; Irving and Maughan, 2000; Irving et al., 2001).
Another proposed sensor molecule (Reedy et al., 1994a) is a heavy form of troponin (tropomyosin in Diptera, Mateos et al., 2006) called troponin H. Considerable interspecies variation in troponin H sequence is present, but all asynchronous muscles contain a form of troponin H that retains the molecule’s hydrophobic region whereas relatively few synchronous muscle troponin H sequences do (Peckham et al., 1992). This molecule is also interesting because it binds glutathione S-transferase-2 in indirect flight muscle, and achieving correct stoichiometry of the latter requires both thick and thin filament systems to be intact (Clayton et al., 1998). However, the presence of a troponin H isoforms in synchronous muscles suggests that, although it may enhance stretch activation/shortening inactivation, it is alone insufficient for it (Peckham and White, 1991).
Another mechanism stems from early work showing that both Ca++ and stretch result in tropomyosin movement on the thin filament (Chaplain and Sacharjan, 1974). Furthermore, the Ca++ and stretch induced force increases are occluding in that the greater the Ca++ induced force, the smaller the additional force induced by stretch (Linari et al., 2004). These data indicate that the Ca++ and stretch activated pathways both use tropomyosin movement as a final common pathway, but do not directly identify the stretch activated pathway. An interesting speculation is that Ca++ causes some tropomyosin movement and myosin head binding and stretch-induced distortion of these cross-bridges causes additional tropomyosin movement and further target zone unmasking (Linari et al., 2004; Squire et al., 2005b).
A more direct mechanism has been proposed with the discovery of a troponin C isoform that stretch directly activates (Agianian et al., 2004). This isoform and the typical Ca++ activated isoform are both present in single asynchronous muscle myofibrils. When singly expressed in myofibrils, myofibrils containing only the Ca++-activated isoform show Ca++ responses but only minimal stretch activation and those containing only the stretch activated form show stretch activation but only minimal Ca++ responses. This troponin isoform may thus be the long-sought stretch sensor.
The above work thus suggests that both match-mismatch and molecular sensor mechanisms may play a role in asynchronous muscle stretch activation and shortening deactivation. A recent technical advance in the field that allows thick filament structure and actomyosin interactions to be visualized in flying insects on the sub millisecond time scale should greatly facilitate further work in resolving these mechanisms (Dickinson et al., 2005).
It is also important to stress that stretch activation (Aidley and White, 1969; Kawai and Brandt, 1980; Gagelmann et al., 1984), shortening deactivation (Aidley and White, 1969; Josephson and Stokes, 1999), and oscillatory work production (Kawai et al., 1977; Anazawa et al., 1992; Tameyasu, 1994; Yasuda et al., 1996) are all manifestations of the so-called ‘Fenn’ effect, in which the amount of work done by a muscle determines the amount of ATP splitting that takes place (Rüegg, 2005). These properties are present to some extent in all striated muscles, and just particularly pronounced in asynchronous insect flight muscle. The difference between asynchronous and ‘normal’ muscle may thus be one of degree rather than kind. In particular, many actomyosin models predict stretch activation and oscillatory behavior for certain ranges of parameter values {Chaplain, 1975 5398 /id;Cheung, 1983 37501 /id;Guo, 2002 36662 /id;Julian, 1969 37415 /id;Jülicher, 1997 37272 /id;Sicilia, 1991 23522 /id;Smith, 1991 2354 /id;Steiger, 1981 37497 /id;Thomas, 1998 36664 /id;Thorson, 1969 7037 /id;Vilfan, 2003 VILFAN2003 /id;Vilfan, 2005 36680 /id}.
Several of these models require high stiffness for oscillation {Julian, 1969 37415 /id;Sicilia, 1991 23522 /id;Smith, 1991 2354 /id;Thorson, 1983 303 /id}, and asynchronous muscles are extremely stiff (Machin and Pringle, 1959; Pringle, 1974; White, 1983; Peckham et al., 1990; Granzier and Wang, 1993; Josephson and Ellington, 1997; Josephson, 1997; Hao et al., 2004). This stiffness is appropriate both as a part of a high frequency resonator system and for coupling length changes to changes in force production. Consistent with this interpretation is that a lack of regulatory light chain phosphorylation decreases muscle stiffness (Tohtong et al., 1995), which provides an alternative explanation for the deleterious effects noted above of light chain non-phosphorylation on flight muscle performance. However, dragonfly (synchronous) flight muscles have high resting stiffness, but do not show significant stretch activation (Peckham and White, 1991), and thus high muscle stiffness alone cannot explain asynchronous muscle stretch activation.
This high stiffness does not arise from the muscle sarcolemma (Buchthal and Weis-Fogh, 1956), but instead from weak cross-bridge binding between the thin and thick filaments and from ‘C’ filaments that connect the thick filaments to the Z line (Pringle, 1974; Bullard et al., 1977; Maruyama et al., 1978; White, 1983; Granzier and Wang, 1993). These C filaments prevent asynchronous muscle from being stretched without damage more than a few (<10) percent of rest muscle length (Hanson, 1956b). Multiple proteins, including kettin (Bullard et al., 2000; Kulke et al., 2001), projectin (Moore et al., 1999; Ayme-Southgate et al., 2005), paramyosin (but not the myosin hinge region) (Liu et al., 2005; Hao et al., 2006), and flightin (Henkin et al., 2004; Barton et al., 2005) are likely involved. Direct evidence that projectin and flightin play a role in stretch activation is the observation that projectin and flightin mutants have reduced stretch activation and altered muscle kinetics (Moore et al., 1999; Vigoreaux et al., 2000; Barton et al., 2005).
Before leaving this section two additional issues should be addressed. The first is to note that asynchronous muscle is present in one non-flight muscle, the tymbal muscle of the cicada Platypleura (Pringle, 1953, 1954b; Hagiwara et al., 1954, 1955; Josephson and Young, 1981). Cicada song consists of a series of pulses, each in turn composed of subpulses. The subpulses occur because the tymbals have multiple ribs, and the buckling of one or a few ribs produces one sub-pulse (Morgan, 1886; Lucas, 1887; Pringle, 1954a; Reid, 1971; Simmons, 1977; Simmons and Young, 1978; Young and Josephson, 1983b; Nahirney et al., 2006; for detailed anatomy, see Myers, 1928; Pringle, 1954a, 1957). Each pulse is induced by a tymbal muscle contraction, with one pulse being produced when the muscle contracts (with subsequent sequential rib buckling producing the subpulses) and another when it relaxes (with subsequent sequential rib unbuckling producing another series of subpulses). Pulse frequency (and thus tymbal muscle contraction and relaxation) can be very high (500 Hz), yet in all other described cidadas is performed by synchronous muscle (see above references). Why asynchronous tymbal muscle evolved in Platypleura (pulse frequency 389 Hz) is unknown, as is any information about the mechanisms underlying the muscle’s (presumed) delayed stretch activation and shortening inactivation.
The second is two potentially confusing nomenclature issues. In most muscles the myofibrils cannot be manually disassociated due to the large amount of endoplasmic reticulum and t-tubules surrounding them. However, asynchronous muscle myofibrils are often not surrounded by endoplasmic reticulum (see third review) and can therefore be easily dissociated (Schäfer, 1891). Furthermore, in some cases the myofibrils are larger than vertebrate myofibrils, and can be observed at the light level. Early workers thought that these differences implied that these muscles were composed of fibrils (and that, in contrast, other muscles were not; in some of this early work the fibrils are also called sarcostyles or muscle columns), and they were therefore given a special name, fibrillar muscles (Kölliker, 1888).
All fibrillar muscles are asynchronous. However, not all asynchronous muscles are fibrillar regardless of whether fibrillar is defined by myofibril separability (in some asynchronous muscles the myofibrils are surrounded by sufficient sarcoplasmic reticulum that cannot be easily teased into fibrils) or by myofibril size (some asynchronous muscles have myofibrils in the normal diameter range) (Josephson and Young, 1981). Although the two terms are still sometimes used interchangeably, this imprecision should be abandoned (Josephson and Young, 1981), with asynchronous being used for muscles that display this type of coupling between nerve activity and muscle contraction and fibrillar used to describe muscle anatomy. The need for this distinction is shown by a controversy about whether whitefly (Aleyrodoidea) muscle is synchronous (because it does not have the classical fibrillar nature) despite the animals’ high (180 Hz) wingbeat frequency (Wootton and Newman, 1979). This issue was resolved by showing the muscles do have a more diagnostic characteristic of asynchronous muscle, reduced sarcoplasmic reticulum (Smith, 1983) (although, as noted above, the main point of the article, that 100 Hz is the upper limit of synchronous activity, is not true).
The second nomenclature problem is the use in some early literature of ‘myogenic’ for asynchronous muscle. Even though nervous activity and rather special conditions were required for the muscles to rhythmically contract, the lack of 1:1 coupling of nerve activity and muscle response was nonetheless a novel form of muscle activity that had aspects of autoactivity. Some authors therefore believed these muscles merited yet another special name, particularly since at the time it was not realized that muscles could possess the ion conductances required for electrical rhythmicity, and hence myogenicity as the term is typically used today. This use of ‘myogenic’ has decreased with the recognition that asynchronous muscles are not autorhythmic by virtue of their own membrane conductances, but readers of the early literature must take care to avoid confusion.
4. Summary and future directions
1) Thin filament structure is similar in vertebrates and invertebrates. There is good evidence for different arrangements of some accessory proteins, in particular tropomyosin, and some evidence for small differences in helix repeat lengths in different species. Given the importance of preferred thin filament binding sites in force production, determining whether these differences are real or the result of differing experimental conditions would be valuable.
2) Invertebrate thick filaments differ from vertebrate thick filaments, and among themselves show great similarity in some characteristics and great variability in others. Good progress has been made in defining head placement in small diameter thick filaments. All well-investigated small diameter thick filaments have crowns of heads every 14.5 nm along the filament. Depending on the muscle and species the crowns contain between 4 and 7 pairs of heads. Subsequent crowns along the filament rotate to different degrees, again in a muscle and species specific manner. In C. elegans thick filaments are composed of myosin and paramyosin subfilaments, and considerable data indicate that other small diameter thick filaments are also composed of myosin and paramyosin subfilaments, with the myosin subfilaments in some cases also containing paramyosin.
Large diameter thick filaments are composed of a paramyosin core and a myosin sheath. The paramyosin core is likely a para-crystal or composed of closely opposed paramyosin sheets. In the one case in which it is known (a moderate diameter thick filament) the myosin heads are arranged helically on the thick filament surface. The extremely high paramyosin content of large diameter thick filaments exists most likely to allow these muscles to exert relatively great force.
Unanswered questions that should be addressed (particularly since in several species much of the available work is quite old) include 1) reinvestigating the structure of large diameter thick filament cores, 2) determining head placement on large diameter thick filaments, 3) determining the C. elegans head placement, 4) further defining small diameter thick filament backbone structure, 5) proving that large diameter thick filaments can bear greater tensions, 5) determining if side polarized thick filaments are present in any invertebrate, and 6) increasing investigated species number with the goal of describing thick filament evolutionary history.
3) The molecular basis of force generation (a lever arm magnifying relatively small changes at its base) is similar in invertebrates and vertebrates. Less well understood is how the cross-bridges function as a collective. An exception is asynchronous flight muscle, in which the extraordinary helical synchrony of the thick and thin filaments across the sarcomere results in axially repeating ‘best’ thick:thin filament interaction sites that are mirrored at every thick filament. In many other muscles crown rotations are such that achieving similar structural order seems more difficult, and in these muscles cross bridge binding may occur in a ‘catch as catch can’ manner. However, there is X-ray diffraction evidence in Crustacea for preferred thin filament binding sites, and presumably with sufficient ingeniuity thin filament positions and thick to thin filament staggers could be arranged so that an orderly pattern of head placement vs. thin filament preferred binding sites was present even in synchronous muscles. It would seem important to investigate thin and thick filament positioning across the sarcomere in them in greater detail.
4) Actomyosin regulation in invertebrates is via both the standard vertebrate striated muscle troponin/tropomyosin based thin filament mechanism and through an invertebrate specific mechanism based on direct Ca++ binding to myosin, and in most species both mechanisms are present in single muscles. The more standard myosin based mechanism of myosin phosphorylation is also present in many species, but is seldom used for rapid control of contraction. Both forms of regulation are extremely well understood on the molecular level, in some cases approaching the atomic level. Much less well understood are the physiological and behavioral consequences of the dual regulation (is only one typically used to control muscle contraction, or does control mechanism vary depending on behavioral state). Given the importance of muscle in behavior, this issue should be better investigated.
5) The molecular basis of catch, a property of certain molluscan muscles that allows them to maintain resistance to stretch without continuous motor nerve stimulation, has been identified as the giant thick filament associated protein twitchin. Catch occurs because twitchin binds to the thin filaments when unphosphorylated, and thus can maintain muscle resistance to stretch in the absence of actomyosin cycling. Relaxation from catch occurs by twitchin phosphorylation. Although some data suggest that other mechanisms may exist in parallel, present data explain well all catch related phenomenon. This work resolves a nearly 150 year old problem in invertebrate muscle physiology. Interestingly, this resolution did not occur by any of the multiple early hypotheses being proved correct, but rather from work in a field (giant sarcomere accessory proteins) thought completely unrelated to catch.
6) The molecular basis underlying the pronounced mechanical activation of another type of invertebrate muscle, asynchronous muscle, however, remains unresolved. Two hypotheses, a match-mismatch in thin:thick filament crossbridge formation that occurs as muscle length changes, and a strain-sensor that increases and decreases actomyosin activity as a function of muscle tension, continue to vie to explain asynchronous muscle stretch activation and shortening inactivation.
Acknowledgments
This work would not have been possible without, and we express our deep gratitude to, C.A. Barcroft, H. Burstein, R.P. Harrison, R.R. Heck III, S. McKean, C. Quolke, B. Revill, and R. Thuma for help in creating and maintaining the reference database and to the Ohio University library system and OHIOLINK electronic journal center for providing several thousand article photocopies and PDFs. This work was supported by grants to S.L.H. from the National Institutes of Health, the Deutsche Forschungsgemeinschaft Mercator Guest Professor Program, and the University of Köln, Köln, Germany.
Abbreviation List
- ADP
adenosine diphosphate
- AMP
adenosine monophosphate
- AMPPNP
adenosine 5-[β,γ-imido]triphosphate
- ATP
adenosine triphosphate
- cAMP
cyclic adenosine monophosphate
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abbott BC, Lowy J. Contraction in molluscan smooth muscle. J Physiol. 1958a;141:385–397. doi: 10.1113/jphysiol.1958.sp005982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott BC, Lowy J. Mechanical properties of Helix and Mytilus muscle. J Physiol. 1958b;141:398–407. doi: 10.1113/jphysiol.1958.sp005983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott RH. An interpretation of the effects of fiber length and calcium on the mechanical properties of insect flight muscle. Cold Spring Harb Symp Quant Biol. 1972;37:647–654. [Google Scholar]
- Abbott RH. The effects of fibre length and calcium ion concentration on the dynamic response of glycerol extracted insect fibrillar muscle. J Physiol. 1973;231:195–208. doi: 10.1113/jphysiol.1973.sp010228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abbott RH, Cage PE. A possible mechanism of length activation in insect fibrillar flight muscle. J Muscle Res Cell Motil. 1984;5:387–397. doi: 10.1007/BF00818257. [DOI] [PubMed] [Google Scholar]
- Abbott RH, Chaplain RA. Preparation and properties of the contractile element of insect fibrillar muscle. J Cell Sci. 1966;1:311–330. doi: 10.1242/jcs.1.3.311. [DOI] [PubMed] [Google Scholar]
- Abbott RH, Mannherz HG. Activation by ADP and the correlation between tension and ATPase activity in insect fibrillar muscle. Pflügers Arch. 1970;321:223–232. doi: 10.1007/BF00588443. [DOI] [PubMed] [Google Scholar]
- Abbott RH, Steiger GJ. Temperature and amplitude dependence of tension transients in glycerinated skeletal and insect fibrillar muscle. J Physiol. 1977;266:13–42. doi: 10.1113/jphysiol.1977.sp011754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achazi RK. 5-HT induced accumulation of 3′,5′-AMP and the phosphorylation of paramyosin in the ABRM of Mytilus edulis. Malacologia. 1979;18:465–468. [PubMed] [Google Scholar]
- Achazi RK, Polling B, Haakshorst R. 5-HT induzierte Erschlaffung und cyclisches AMP bei einem glatten Molluskenmuskel. Pflügers Arch. 1974;349:19–27. doi: 10.1007/BF00587913. [DOI] [PubMed] [Google Scholar]
- Adelstein RS, Eisenberg E. Regulation and kinetics of the actin-myosin-ATP interaction. Annu Rev Biochem. 1980;49:921–956. doi: 10.1146/annurev.bi.49.070180.004421. [DOI] [PubMed] [Google Scholar]
- Agianian B, Krzic U, Qiu F, Linke WA, Leonard K, Bullard B. A troponin switch that regulates muscle contraction by stretch instead of calcium. EMBO J. 2004;23:772–779. doi: 10.1038/sj.emboj.7600097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aidley DJ. The effect of calcium ions on potassium contracture in a locust leg muscle. J Physiol. 1965;177:94–102. doi: 10.1113/jphysiol.1965.sp007577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aidley DJ. Sound production in Brazilian cicadas. J Exp Biol. 1969;51:325–337. [Google Scholar]
- Aidley DJ. The Physiology of Excitable Cells. Cambridge University Press; Cambridge: 1998. [Google Scholar]
- Aidley DJ, White DCS. Mechanical properties of glycerinated fibres from the tymbal muscles of a Brazilian cicada. J Physiol. 1969;205:179–192. doi: 10.1113/jphysiol.1969.sp008959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Khayat HA, Hudson L, Reedy MK, Irving TC, Squire JM. Myosin head configuration in relaxed insect flight muscle: X- ray modeled resting cross-bridges in a pre-powerstroke state are poised for actin binding. Biophys J. 2003;85:1063–1079. doi: 10.1016/S0006-3495(03)74545-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al Khayat HA, Hudson L, Reedy MK, Irving TC, Squire JM. Modeling oriented macromolecular assemblies from low-angle X-ray fibre diffraction data with the program MOVIE: insect flight muscle as example. Fibre Diffraction Review. 2004a;12:50–60. [Google Scholar]
- Al Khayat HA, Morris EP, Squire JM. Single particle analysis: a new approach to solving the 3D structure of myosin filaments. J Muscle Res Cell Motil. 2004b;25:635–644. doi: 10.1007/s10974-004-5333-5. [DOI] [PubMed] [Google Scholar]
- Alamo L, Padrón R, Craig R, Hidalgo C. Metodo para la determinacion directa de la simetria rotational de filamentos gruesos de musculo por procesamiento digital de imagenes. Acta Cient Venez. 1991;42:59–63. [PubMed] [Google Scholar]
- Allhouse LD, Guzman G, Miller T, Li Q, Potter JD, Ashley CC. Characterisation of a mutant of barnacle troponin C lacking Ca2+-binding sites at positions II and IV. Pflügers Arch - Eur J Physiol. 1999a;438:30–39. doi: 10.1007/s004240050876. [DOI] [PubMed] [Google Scholar]
- Allhouse LD, Li Q, Guzman G, Miller T, Lipscomb S, Potter JD, Ashley CC. Investigating the role of Ca2+-binding site IV in barnacle troponin C. Pflügers Arch. 2000;439:600–609. doi: 10.1007/s004249900216. [DOI] [PubMed] [Google Scholar]
- Allhouse LD, Miller T, Li Q, Guzman G, Potter JD, Mandveno A, Ashley CC. Investigation of a genetically engineered mutant of barnacle troponin C containing a central helix deletion. Pflügers Arch. 1999b;439:67–75. doi: 10.1007/s004249900133. [DOI] [PubMed] [Google Scholar]
- Allhouse LD, Potter JD, Ashley CC. A novel method of extraction of TnC from skeletal muscle myofibrils. Pflügers Arch. 1999c;437:695–701. doi: 10.1007/s004240050834. [DOI] [PubMed] [Google Scholar]
- Allis JW, Ferry JD. Dynamic viscoelastic behavior of dilute paramyosin solutions. Proc Natl Acad Sci USA. 1965a;54:369–371. doi: 10.1073/pnas.54.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allis JW, Ferry JD. Dynamic viscoelastic properties of solutions of paramyosin and bovine serum albumin. J Am Chem Soc. 1965b;87:4681–4687. doi: 10.1021/ja00949a004. [DOI] [PubMed] [Google Scholar]
- Amos LA, Cross RA. Structure and dynamics of molecular motors. Curr Op Struct Biol. 1997;7:239–246. doi: 10.1016/s0959-440x(97)80032-2. [DOI] [PubMed] [Google Scholar]
- Anazawa T, Yasuda K, Ishiwata S. Spontaneous oscillation of tension and sarcomere length in skeletal myofibrils. Microscopic measurement and analysis. Biophys J. 1992;61:1099–1108. doi: 10.1016/S0006-3495(92)81919-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson P. Molecular genetics of nematode muscle. Annu Rev Genet. 1989;23:507–525. doi: 10.1146/annurev.ge.23.120189.002451. [DOI] [PubMed] [Google Scholar]
- Andruchov O, Andruchova O, Galler S. The catch state of mollusc catch muscle is established during activation: experiments on skinned fibre preparations of the anterior byssus retractor muscle of Mytilus edulis L. using the myosin inhibitors orthovanadate and blebbistatin. J Exp Biol. 2006;209:4319–4328. doi: 10.1242/jeb.02501. [DOI] [PubMed] [Google Scholar]
- Andruchova O, Hopflinger MC, Andruchov O, Galler S. No effect of twitchin phosphorylation on the rate of myosin head detachment in molluscan catch muscle: are myosin heads involved in the catch state? Pflügers Arch. 2005;450:326–334. doi: 10.1007/s00424-005-1447-x. [DOI] [PubMed] [Google Scholar]
- Ankrett RJ, Rowe AJ, Cross RA, Kendrick-Jones J, Bagshaw CR. A folded (10 S) conformer of myosin from a striated muscle and its implications for regulation of ATPase activity. J Mol Biol. 1991a;217:323–335. doi: 10.1016/0022-2836(91)90546-i. [DOI] [PubMed] [Google Scholar]
- Ankrett RJ, Walmsley AR, Bagshaw CR. Kinetic analysis of regulated myosin ATPase activity using single and limited turnover assays. J Cell Sci. 1991b:1–5. doi: 10.1242/jcs.1991.supplement_14.1. [DOI] [PubMed] [Google Scholar]
- Ao WY, Pilgrim D. Caenorhabditis elegans unc-45 is a component of muscle thick filaments and colocalizes with myosin heavy chain B, but not myosin heavy chain A. J. Cell. Biol. 2000;148:375–384. doi: 10.1083/jcb.148.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April EW, Brandt PW. The myofilament lattice: studies on isolated fibers. III The effect of myofilament spacing upon tension. J Gen Physiol. 1973;61:490–508. doi: 10.1085/jgp.61.4.490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April EW, Brandt PW, Elliott GF. The myofilament lattice: studies on isolated fibers. I The constancy of the unit-cell volume with variation in sarcomere length in a lattice in which the thin-to-thick myofilament ratio is 6:1. J Cell Biol. 1971;51:72–82. doi: 10.1083/jcb.51.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- April EW, Maughan DW. Active force as a function of filament spacing in crayfish skinned muscle fibers. Pflügers Arch. 1986;407:456–460. doi: 10.1007/BF00652634. [DOI] [PubMed] [Google Scholar]
- Armitage PM, Tregear RT, Miller A. Effect of activation by calcium on the X-ray diffraction pattern from insect flight muscle. J Mol Biol. 1975;92:39–53. doi: 10.1016/0022-2836(75)90090-x. [DOI] [PubMed] [Google Scholar]
- Aronson JF. The elongation of myofibrils from the indirect flight muscle of Drosophila. J Cell Biol. 1962;13:33–41. doi: 10.1083/jcb.13.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada T, Ashiba G, Watanabe S. Calcium sensitivity of foot muscle myosin from clam (Meretrix lusoria) J Biochem (Tokyo) 1979;85:1543–1546. doi: 10.1093/oxfordjournals.jbchem.a132485. [DOI] [PubMed] [Google Scholar]
- Asakawa T. Enzymatic properties of myosin from scallop striated adductor muscle: comparative studies of native myosin and desensitized myosin. Hokkaido Kyoikudaigaku Kiyo (II-A) 1980;30:249–270. [Google Scholar]
- Asakawa T, Azuma N. Inhibitory effect of MgATP on the release of regulatory light chain from scallop myosin and light chain composition of scallop myosin hybridized with abalone light chain 2 at 30° C. J Biochem (Tokyo) 1983;94:395–401. doi: 10.1093/oxfordjournals.jbchem.a134368. [DOI] [PubMed] [Google Scholar]
- Asakawa T, Azuma N. Preparation of subfragment-1 from abalone smooth muscle myosin. J Biochem (Tokyo) 1988;103:667–671. doi: 10.1093/oxfordjournals.jbchem.a122326. [DOI] [PubMed] [Google Scholar]
- Asakawa T, Yazawa Y, Azuma N. Light chains of abalone myosin. UV absorption difference spectrum and resensitization of desensitized scallop myosin. J Biochem (Tokyo) 1981;89:1805–1814. doi: 10.1093/oxfordjournals.jbchem.a133381. [DOI] [PubMed] [Google Scholar]
- Ashiba G, Asada T, Watanabe S. Calcium regulation in clam foot muscle. Calcium sensitivity of clam foot myosin. J Biochem (Tokyo) 1980;88:837–846. doi: 10.1093/oxfordjournals.jbchem.a133038. [DOI] [PubMed] [Google Scholar]
- Ashiba G, Haraki K, Watanabe S. Effects of clam-foot paramyosin, tropomyosin, and rabbit skeletal light-meromyosin on the ATPase and superprecipitation activities of actomyosin. J Biochem (Tokyo) 1982;91:1795–1804. doi: 10.1093/oxfordjournals.jbchem.a133872. [DOI] [PubMed] [Google Scholar]
- Ashiba G, Szent-Györgyi AG. Essential light chain exchange in scallop myosin. Biochemistry. 1985;24:6618–6623. doi: 10.1021/bi00344a048. [DOI] [PubMed] [Google Scholar]
- Ashley CC, Lea TJ, Hoar PE, Kerrick WGL, Strang PF, Potter JD. Functional characterization of the two isoforms of troponin C from the arthropod Balanus nubilus. J Muscle Res Cell Motil. 1991;12:532–542. doi: 10.1007/BF01738441. [DOI] [PubMed] [Google Scholar]
- Ashton FT, Beibrech G, Pepe FA. Subfilament organization in myosin filaments of the fast abdominal muscles of the lobster, Homarus americanus. Tissue Cell. 1987;19:51–63. doi: 10.1016/0040-8166(87)90056-5. [DOI] [PubMed] [Google Scholar]
- Astbury WT. Croonian lecture. On the structure of biological fibres and the problem of muscle. Proc R Soc Lond Biol Sci. 1946;134:303–328. doi: 10.1098/rspb.1947.0016. [DOI] [PubMed] [Google Scholar]
- Astbury WT, Dickinson S. X-ray studies of the molecular structure of myosin. Proc R Soc Lond Biol Sci. 1940;129:307–332. [Google Scholar]
- Atsumi S. The role of calcium ions in the regulation of active and catch contractions in the anterior byssal retractor muscle of Mytilus edulis. Proc Jpn Acad. 1974;50:775–778. [Google Scholar]
- Atsumi S. Sarcoplasmic reticulum and intracellular calcium localization at rest and during contraction in Mytilus smooth muscle. Arch Histol Jpn. 1978;41:239–258. doi: 10.1679/aohc1950.41.239. [DOI] [PubMed] [Google Scholar]
- Atsumi S, Sugi H. Localization of calcium-accumulating structures in the anterior byssal retractor muscle of Mytilus edulis and their role in the regulation of active and catch contractions. J Physiol. 1976;257:549–560. doi: 10.1113/jphysiol.1976.sp011384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert X. L’influence du délai de relâchment sur la chaleur dégagée par un muscle soumis à un travail en cycle. C R Soc Biol. 1944;138:1048–1050. [Google Scholar]
- Ayme-Southgate A, Saide J, Southgate R, Bounaix C, Cammarato A, Patel S, Wussler C. In indirect flight muscles Drosophila projectin has a short PEVK domain, and its NH2-terminus is embedded at the Z-band. J Muscle Res Cell Motil. 2005;26:467–477. doi: 10.1007/s10974-005-9031-8. [DOI] [PubMed] [Google Scholar]
- Azuma N. Calcium sensitivity of abalone, Haliotis discus, myosin. J Biochem (Tokyo) 1976;80:187–189. doi: 10.1093/oxfordjournals.jbchem.a131250. [DOI] [PubMed] [Google Scholar]
- Azuma N, Asakura A, Koichi Y. Myosin from molluscan abalone, Haliotis discus. J Biochem (Tokyo) 1975;77:973–981. doi: 10.1093/oxfordjournals.jbchem.a130823. [DOI] [PubMed] [Google Scholar]
- Azzu V, Yadin D, Patel H, Fraternali F, Chantler PD, Molloy JE. Calcium regulates scallop muscle by changing myosin flexibility. Eur Biophys J. 2006;35:302–312. doi: 10.1007/s00249-005-0036-4. [DOI] [PubMed] [Google Scholar]
- Baccetti B. Nouvelles observations sur l’ultrastructure du myofilament. J Ultrastruct Res. 1965;13:245–256. doi: 10.1016/s0022-5320(65)80073-9. [DOI] [PubMed] [Google Scholar]
- Baccetti B, Rosati F. On the thick filaments of holothurian muscles. J Microsc (Paris) 1968;7:455–458. [Google Scholar]
- Bagshaw CR. Divalent metal ion binding and subunit interactions in myosins: a critical review. J Muscle Res Cell Motil. 1980;1:255–277. doi: 10.1007/BF00711931. [DOI] [PubMed] [Google Scholar]
- Bagshaw CR. Molluscan muscle. Where’s the catch? J Muscle Res Cell Motil. 1988;9:195–196. doi: 10.1007/BF01773741. [DOI] [PubMed] [Google Scholar]
- Bagshaw CR, Kendrick-Jones J. Characterization of homologous divalent metal ion binding sites of vertebrate and molluscan myosins using electron paramagnetic resonance spectroscopy. J Mol Biol. 1979;130:317–336. doi: 10.1016/0022-2836(79)90544-8. [DOI] [PubMed] [Google Scholar]
- Bagshaw CR, Kendrick-Jones J. Identification of the divalent metal ion binding domain of myosin regulatory light chains using spin-labelling techniques. J Mol Biol. 1980;140:411–433. doi: 10.1016/0022-2836(80)90392-7. [DOI] [PubMed] [Google Scholar]
- Baguet F. The catch-state in glycerol extracted fibres from a lamellibranch smooth muscle. Pflügers Arch. 1973;340:19–34. doi: 10.1007/BF00592194. [DOI] [PubMed] [Google Scholar]
- Baguet F, Gillis JM. Stimulation de la respiration des muscles de lamellibranches par la 5-hydroxytryptamine. Arch Int Physiol Biochim. 1964;72:351–352. [PubMed] [Google Scholar]
- Baguet F, Gillis JM. The respiration of the anterior byssus retractor muscle of Mytilus edulis (ABRM) after a phasic contraction. J Physiol. 1967;188:67–82. doi: 10.1113/jphysiol.1967.sp008124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguet F, Gillis JM. Energy cost of tonic contraction in a lamellibranch catch muscle. J Physiol. 1968;198:127–143. doi: 10.1113/jphysiol.1968.sp008597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baguet F, Gillis JM, Dainoff G. Energétique de relâchement phasique d’un muscle lisse de Lammellibranche. Arch Int Physiol. 1967;75:523–528. [PubMed] [Google Scholar]
- Baguet F, Marchand-Dumont G. The muscular membrane and calcium activation of the contractile system of a lamellibranch smooth muscle (ABRM) Pflügers Arch. 1975;354:75–85. doi: 10.1007/BF00584504. [DOI] [PubMed] [Google Scholar]
- Baguet F, Maréchal G, Aubert X. La thermogénèse d’un muscle lisse de Lamellibranche en contraction phasique. Arch Int Physiol Biochim. 1962;70:416–417. [PubMed] [Google Scholar]
- Baier RE, Zobel CR. Structure in protein films: myosin monolayers. Nature. 1966;212:351–352. doi: 10.1038/212351a0. [DOI] [PubMed] [Google Scholar]
- Bailey JT. Composition of the myosins and myogen of skeletal muscle. Biochem J. 1937;31:1406–1413. doi: 10.1042/bj0311406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey K. The proteins of adductor muscles. Pubbl Staz Zool Napoli. 1956;29:96–108. [Google Scholar]
- Bailey K. Invertebrate tropomyosin. Biochim Biophys Acta. 1957;24:612–619. doi: 10.1016/0006-3002(57)90255-x. [DOI] [PubMed] [Google Scholar]
- Bailey K, de Milstein CP, Kay CM, Smillie LB. Characterization of a tryptic fragment isolated from the insoluble tropomyosin of Pinna nobilis. Biochim Biophys Acta. 1964;90:503–520. doi: 10.1016/0304-4165(64)90230-2. [DOI] [PubMed] [Google Scholar]
- Bailey K, Rüegg JC. Further chemical studies on the tropomyosins of lamellibranch muscle with special reference to Pecten maximus. Biochim Biophys Acta. 1960;38:239–245. doi: 10.1016/0006-3002(60)91237-3. [DOI] [PubMed] [Google Scholar]
- Baker JE, Brust-Mascher I, Ramachandran S, LaConte LEW, Thomas DD. A large and distinct rotation of the myosin light chain domain occurs upon muscle contraction. Proc Natl Acad Sci USA. 1998;95:2944–2949. doi: 10.1073/pnas.95.6.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandmann HJ, Reichel H. Struktur und Mechanik des glatten Schliessmuskels von Pinna nobilis. Z Biol. 1954;107:67–80. [PubMed] [Google Scholar]
- Bárány M, Bárány K. Myosin from the striated adductor muscle of scallop (Pecten arradians) Biochemistry. 1966;345:37–56. [Google Scholar]
- Barber SB, Pringle JWS. Functional aspects of flight in belostomatid bugs (Heteroptera) Proc R Soc Lond Biol Sci. 1966;164:21–39. [Google Scholar]
- Bard F, Franzini-Armstrong C, Ip W. Rigor crossbridges are double headed in fast muscle from crayfish. J Cell Biol. 1987;105:2225–2234. doi: 10.1083/jcb.105.5.2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardales JR, az-Enrich MJ, Ibarguren I, Villamarín JA. Isoforms of cAMP-dependent protein kinase in the bivalve mollusk Mytilus galloprovincialis: activation by cyclic nucleotides and effect of temperature. Arch Biochem Biophys. 2004;432:71–78. doi: 10.1016/j.abb.2004.09.008. [DOI] [PubMed] [Google Scholar]
- Barnes TC. Peripheral tonus associated with impulse discharges in crustacean nerve. J Physiol. 1930;70:xxiv–xxv. [Google Scholar]
- Barouch WW, Breese KE, Davidoff SA, Leszyk J, Szent-Györgyi AG, Theibert JL, Collins JH. Amino acid sequences of myosin essential and regulatory light chains from two clam species: comparison with other molluscan myosin light chains. J Muscle Res Cell Motil. 1991;12:321–332. doi: 10.1007/BF01738587. [DOI] [PubMed] [Google Scholar]
- Barral JM, Epstein HF. Protein machines and self assembly in muscle organization. BioEssays. 1999;21:813–823. doi: 10.1002/(SICI)1521-1878(199910)21:10<813::AID-BIES3>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Barrington-Leigh J, Holmes KC, Mannherz HG, Rosenbaum G, Eckstein F, Goody RS. Effects of ATP analogs on the low-angle X-ray diffraction pattern of insect flight muscle. Cold Spring Harb Symp Quant Biol. 1973;37:443–447. [Google Scholar]
- Barrington-Leigh J, Rosenbaum G. Synchrotron X-ray sources: a new tool in biological structural and kinetic analysis. Annu Rev Biophys Bioeng. 1976;5:239–270. doi: 10.1146/annurev.bb.05.060176.001323. [DOI] [PubMed] [Google Scholar]
- Bartegi A, Fattoum A, Dagorn C, Gabrion J, Kassab R. Isolation, characterization, and immunocytochemical localization of caldesmon-like protein from molluscan striated muscle. Eur J Biochem. 1989;185:589–595. doi: 10.1111/j.1432-1033.1989.tb15154.x. [DOI] [PubMed] [Google Scholar]
- Barton B, Ayer G, Heymann N, Maughan DW, Lehmann FO, Vigoreaux JO. Flight muscle properties and aerodynamic performance of Drosophila expressing a flightin transgene. J Exp Biol. 2005;208:549–560. doi: 10.1242/jeb.01425. [DOI] [PubMed] [Google Scholar]
- Baskin RJ, Wiese GM. Contraction-band formation in barnicle myofibrils. Science. 1964;143:134–136. doi: 10.1126/science.143.3602.134. [DOI] [PubMed] [Google Scholar]
- Bastian J, Esch H. The nervous control of the indirect flight muscles of the honey bee. Z vergl Physiol. 1970;67:307–324. [Google Scholar]
- Bayliss LE, Boylane E, Ritchie AD. The adductor mechanism of Pecten. Proc R Soc Lond Biol Sci. 1930;106:363–376. [Google Scholar]
- Bear RS. X-ray diffraction studies on protein fibers. II Feather rachis, porcupine quill tip and clam muscle. J Am Chem Soc. 1944;66:2043–2050. [Google Scholar]
- Bear RS. Small angle X-ray diffraction studies on muscle. J Am Chem Soc. 1945;67:1625–1626. [Google Scholar]
- Bear RS, Selby CC. The structure of paramyosin fibrils according to X-ray diffraction. J Biophys Biochem Cytol. 1956;2:55–69. doi: 10.1083/jcb.2.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinbrech G, Ashton FT, Pepe FA. Invertebrate myosin filament: subfilament arrangement in the wall of tubular filaments of insect flight muscles. J Mol Biol. 1988;201:557–565. doi: 10.1016/0022-2836(88)90637-7. [DOI] [PubMed] [Google Scholar]
- Beinbrech G, Ashton FT, Pepe FA. Orientation of the backbone structure of myosin filaments in relaxed and rigor muscles of the housefly: evidence for nonequivalent crossbridge positions at the surface of thick filaments. Tissue Cell. 1990;22:803–810. doi: 10.1016/0040-8166(90)90045-b. [DOI] [PubMed] [Google Scholar]
- Beinbrech G, Ashton FT, Pepe FA. The invertebrate myosin filament: subfilament arrangement of the solid filaments of insect flight muscles. Biophys J. 1992;61:1495–1512. doi: 10.1016/S0006-3495(92)81955-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beinbrech G, Kuhn HJ, Herzig JW, Rüegg JC. Evidence for two attached myosin cross-bridge states of different potential energy. Cytobiologie. 1976;12:385–396. [Google Scholar]
- Beinbrech G, Kuhn HJ, Rüegg JC. Electron microscope and optical diffraction studies on glycerol-extracted insect flight muscle fibres relaxed by pyrophosphate. Experientia. 1972;28:511–513. doi: 10.1007/BF01931848. [DOI] [PubMed] [Google Scholar]
- Beinbrech G, Meller U, Sasse W. Paramyosin content and thick filament structure in insect muscles. Cell Tissue Res. 1985;241:607–614. [Google Scholar]
- Béjar P, Villamarín JA. Catalytic subunit of cAMP-dependent protein kinase from a catch muscle of the bivalve mollusk Mytilus galloprovincialis: purification, characterization, and phosphorylation of muscle proteins. Arch Biochem Biophys. 2006;450:133–140. doi: 10.1016/j.abb.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Bejsovec A, Anderson P. Functions of the myosin ATP and actin binding sites are required for C. elegans thick filament assembly. Cell. 1990;60:133–140. doi: 10.1016/0092-8674(90)90723-r. [DOI] [PubMed] [Google Scholar]
- Bennett AJ, Bagshaw CG. The mechanism of regulatory light chain dissociation from scallop myosin. Biochem J. 1986a;233:179–186. doi: 10.1042/bj2330179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AJ, Bagshaw CR. The kinetics of bivalent metal ion dissociation from myosin subfragments. Biochem J. 1986b;233:173–177. doi: 10.1042/bj2330173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett AJ, Patel N, Wells C, Bagshaw CR. 8-anilino-1-naphthalenesulphonate, a fluorescent probe for the regulatory light chain binding site of scallop myosin. J Muscle Res Cell Motil. 1984;5:165–182. doi: 10.1007/BF00712154. [DOI] [PubMed] [Google Scholar]
- Bennett PM, Elliott A. The structure of the paramyosin core in molluscan thick filaments. J Muscle Res Cell Motil. 1981;2:65–81. doi: 10.1007/BF00712062. [DOI] [PubMed] [Google Scholar]
- Bennett PM, Elliott A. Splicing” of paramyosin filaments. J Mol Biol. 1984;175:103–109. doi: 10.1016/0022-2836(84)90451-0. [DOI] [PubMed] [Google Scholar]
- Bennett PM, Elliott A. The ‘catch’ mechanism in molluscan muscle: an electron microscopy study of freeze-substituted anterior byssus retractor muscle of Mytilus edulis. J Muscle Res Cell Motil. 1989;10:297–311. doi: 10.1007/BF01758426. [DOI] [PubMed] [Google Scholar]
- Bennett PM, Marston SB. Calcium regulated thin filaments from molluscan catch muscles contain a caldesmon-like regulatory protein. J Muscle Res Cell Motil. 1990;11:302–312. doi: 10.1007/BF01766668. [DOI] [PubMed] [Google Scholar]
- Benzonana G, Kohler L, Stein EA. Regulatory proteins of crayfish tail muscle. Biochim Biophys Acta. 1974;368:247–258. doi: 10.1016/0005-2728(74)90153-4. [DOI] [PubMed] [Google Scholar]
- Bernstein SI, Milligan RA. Fine tuning a molecular motor: the location of alternative domains in the Drosophila myosin head. J Mol Biol. 1997;271:1–6. doi: 10.1006/jmbi.1997.1160. [DOI] [PubMed] [Google Scholar]
- Bethe A. Die Dauerverkürzung der Muskeln. Pflügers Arch. 1911;142:291–336. [Google Scholar]
- Biedermann W. Beiträge zur allgemeinen Nerven- und Muskel- physiologie. Über die elektrische Erregung des Schliessmuskels von Anodonta Sitz Akad Wissensch Wien. 1885;91:1–74. [Google Scholar]
- Bing W, Razzaq A, Sparrow J, Marston S. Tropomyosin and troponin regulation of wild type and E93K mutant actin filaments from Drosophila flight muscle - charge reversal on actin changes actin-tropomyosin from on to off state. J Biol Chem. 1998;273:15016–15021. doi: 10.1074/jbc.273.24.15016. [DOI] [PubMed] [Google Scholar]
- Biosca JA, Eisenberg E, Reedy MC, Reedy MK. Binding of ADP and adenosine 5′-[β,γ-imido]triphosphate to insect flight muscle fibrils. Eur J Biochem. 1990;189:395–399. doi: 10.1111/j.1432-1033.1990.tb15501.x. [DOI] [PubMed] [Google Scholar]
- Blaschko H, Cattell M, Kahn JL. On the nature of two types of response in the neuromuscular system of the crustacean claw. J Physiol. 1931;73:25–35. doi: 10.1113/jphysiol.1931.sp002793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist E, Curtis BA. The action of serotonin on calcium-45 efflux from the anterior byssal retractor muscle of Mytilus edulis. J Gen Physiol. 1972;59:476–485. doi: 10.1085/jgp.59.4.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloomquist E, Curtis BA. 45Ca efflux from anterior byssus retractor muscle in phasic and catch contraction. Am J Physiol. 1975a;229:1237–1243. doi: 10.1152/ajplegacy.1975.229.5.1237. [DOI] [PubMed] [Google Scholar]
- Bloomquist E, Curtis BA. Net calcium fluxes in anterior byssus retractor muscle with phasic and catch contraction. Am J Physiol. 1975b;229:1244–1248. doi: 10.1152/ajplegacy.1975.229.5.1244. [DOI] [PubMed] [Google Scholar]
- Blyakhman FA, Shklyar T, Pollack GH. Quantal length changes in single contracting sarcomeres. J Muscle Res Cell Motil. 1999;20:529–538. doi: 10.1023/a:1005590401721. [DOI] [PubMed] [Google Scholar]
- Bock D, Hinssen H, D’Haese J. A gelsolin-related actin-severing protein with fully reversible actin-binding properties from the tail muscle of crayfish, Astacus leptodactylus. Eur J Biochem. 1994;225:727–735. doi: 10.1111/j.1432-1033.1994.00727.x. [DOI] [PubMed] [Google Scholar]
- Boettiger EG. Insect flight muscles and their basic physiology. Annu Rev Entomol. 1960;5:1–16. [Google Scholar]
- Boettiger EG, Furshpan E. The mechanics of flight movements in diptera. Biol Bull. 1952;102:200–211. [Google Scholar]
- Borovikov YS, Chernogriadskaia NA. Studies on conformational changes in F-actin of glycerinated muscle fibers during relaxation by means of polarized ultraviolet fluorescence microscopy. Microsc Acta. 1979;81:383–392. [PubMed] [Google Scholar]
- Botts J, Cooke R, dos Remedios C, Duke J, Mendelson R, Morales MF, Tokiwa T, Veinegra G, Yount R. Does a myosin cross-bridge progress arm-over-arm on the actin filament? Cold Spring Harb Symp Quant Biol. 1972;37:195–200. [Google Scholar]
- Bower SM, Wang Y, Chantler PD. Regulatory light-chain Cys-55 sites on the two heads of myosin can come within 2Å of each other. FEBS Lett. 1992;310:132–134. doi: 10.1016/0014-5793(92)81313-b. [DOI] [PubMed] [Google Scholar]
- Bozler E. Über die Frage des Tonussubstrates. Z vergl Physiol. 1928;7:407–435. [Google Scholar]
- Bozler E. Untersuchungen zur Physiologie der Tonusmuskeln. Z vergl Physiol. 1930;12:579–602. [Google Scholar]
- Bozler E. Die mechanischen Eigenschaften des ruhenden Muskels, ihre experimentelle Beeinflussung und physiologische Bedeutung. Z vergl Physiol. 1931a;14:429–449. [Google Scholar]
- Bozler E. Über die Tätigkeit der einzelnen glatten Muskelfaser bei der Kontraktion. 3 Mitteilung: Registrierung der Kontraktionen der Chromatophorenmuskelzellen von Cephalopoden. J vergl Physiol. 1931b;13:762–772. [Google Scholar]
- Bozler E. An analysis of the properties of smooth muscle. Cold Spring Harb Symp Quant Biol. 1936;4:260–266. [Google Scholar]
- Bozler E, Cottrell CL. The birefringence of muscle and its variation during contraction. J Cell Comp Physiol. 1937;10:165–182. [Google Scholar]
- Brading A. The Autonomic Nervous System and its Effectors. Blackwell Science Ltd; Oxford: 1999. [Google Scholar]
- Brann L, Dewey MM, Baldwin EA, Brink P, Walcott B. Requirements for in vitro shortening and lengthening of isolated thick filaments of Limulus striated muscle. Nature. 1979;279:256–257. doi: 10.1038/279256a0. [DOI] [PubMed] [Google Scholar]
- Brecht K, Utz G, Lutz E. Über die Atmung guergestreifter und glatter Muskeln von Kalblütern in Ruhe, Dehnung, Kontraktion und Kontraktur. Pflügers Arch. 1955;260:524–537. doi: 10.1007/BF00363669. [DOI] [PubMed] [Google Scholar]
- Brenner B. The stroke size of myosins: a reevaluation. J Muscle Res Cell Motil. 2006;27:173–187. doi: 10.1007/s10974-006-9056-7. [DOI] [PubMed] [Google Scholar]
- Breull W. Myofibrillar ATP-splitting in the elementary contractile cycle of an insect flight muscle. Experientia. 1971;27:779–781. doi: 10.1007/BF02136861. [DOI] [PubMed] [Google Scholar]
- Breull W, Steiger G, Rüegg JC. ATP splitting in relation to isometric tension-oscillation and cross bridge cycling of insect fibrillar muscle. J Mechanochem Cell Motil. 1973;2:91–100. [PubMed] [Google Scholar]
- Brink P, Dewey MM. Change in fixed charge in the thick filament lattice of Limulus striated muscle with sarcomere shortening. Adv Exp Med Biol. 1984;170:353–357. doi: 10.1007/978-1-4684-4703-3_30. [DOI] [PubMed] [Google Scholar]
- Brown JH, Cohen C. Regulation of muscle contraction by tropomyosin and troponin: How structure illuminates function. Adv Prot Chem. 2005;71:121–159. doi: 10.1016/S0065-3233(04)71004-9. [DOI] [PubMed] [Google Scholar]
- Brust-Mascher I, LaConte LEW, Baker JE, Thomas DD. Myosin light-chain domain rotates upon muscle activation but not ATP hydrolysis. Biochemistry. 1999;38:12607–12613. doi: 10.1021/bi9905967. [DOI] [PubMed] [Google Scholar]
- Buchthal F, Weis-Fogh T. Contribution of the sarcolemma to the force exerted by resting muscle of insects. Acta Physiol Scand. 1956;35:345–364. doi: 10.1111/j.1748-1716.1955.tb01291.x. [DOI] [PubMed] [Google Scholar]
- Bullard B. The nervous control of the anterior byssus retractor muscle of Mytilus edulis. Comp Biochem Physiol. 1967;23:749–759. doi: 10.1016/0010-406x(67)90338-6. [DOI] [PubMed] [Google Scholar]
- Bullard B. Contractile proteins of insect flight muscle. Trends Biochem Sci. 1983;8:68–70. doi: 10.1042/bj1350277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard B, Burkart C, Labeit S, Leonard K. The function of elastic proteins in the oscillatory contraction of insect flight muscle. J Muscle Res Cell Motil. 2005;26:479–485. doi: 10.1007/s10974-005-9032-7. [DOI] [PubMed] [Google Scholar]
- Bullard B, Dabrowska R, Winkelman L. The contractile and regulatory proteins of insect flight muscle. Biochem J. 1973a;135:277–286. doi: 10.1042/bj1350277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bullard B, Goulding D, Ferguson C, Leonard K. Links in the chain: the contribution of kettin to the elasticity of insect muscles. Adv Exp Med Biol. 2000;481:207–220. doi: 10.1007/978-1-4615-4267-4_12. [DOI] [PubMed] [Google Scholar]
- Bullard B, Hammond KS, Luke BM. The site of paramyosin in insect flight muscle and the presence of an unidentified protein between myosin filaments and Z line. J Mol Biol. 1977;115:417–440. doi: 10.1016/0022-2836(77)90163-2. [DOI] [PubMed] [Google Scholar]
- Bullard B, Leonard K, Larkins A, Butcher G, Karlik CC, Fyrberg E. Troponin of asynchronous flight muscle. J Mol Biol. 1988;204:621–637. doi: 10.1016/0022-2836(88)90360-9. [DOI] [PubMed] [Google Scholar]
- Bullard B, Luke B, Winkelman L. The paramyosin of insect flight muscle. J Mol Biol. 1973b;75:359–367. doi: 10.1016/0022-2836(73)90026-0. [DOI] [PubMed] [Google Scholar]
- Bullard B, Reedy MK. How many myosins per cross-bridge? II Flight muscle myosin from the blowfly, Sarcophaga bullata. Cold Spring Harb Symp Quant Biol. 1973;37:423–428. [Google Scholar]
- Burgess S, Walker M, Knight PJ, Sparrow J, Schmitz S, Offer G, Bullard B, Leonard K, Holt J, Trinick J. Structural studies of arthrin: monoubiquitinated actin. J Mol Biol. 2004;341:1161–1173. doi: 10.1016/j.jmb.2004.06.077. [DOI] [PubMed] [Google Scholar]
- Burns MD, Usherwood PNR. Mechanical properties of locust extensor tibiae muscles. Comp Biochem Physiol. 1978;61A:85–95. [Google Scholar]
- Butler TM, Mooers SU, Li C, Narayan S, Siegman MJ. Regulation of catch muscle by twitchin phosphorylation: effects on force, ATPase, and shortening. Biophys J. 1998;75:1904–1914. doi: 10.1016/S0006-3495(98)77631-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TM, Mooers SU, Siegman MJ. Catch force links and the low to high force transition of myosin. Biophys J. 2006;90:3193–3202. doi: 10.1529/biophysj.105.077453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler TM, Narayan SR, Mooers SU, Hartshorne DJ, Siegman MJ. The myosin cross-bridge cycle and its control by twitchin phosphorylation in catch muscle. Biophys J. 2001;80:415–426. doi: 10.1016/S0006-3495(01)76024-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camatini M, Castellani LC, Franchi E, Lanzavecchia G, Paoletti L. Thick filaments and paramyosin of annelid muscles. J Ultrastruct Res. 1976;55:433–447. doi: 10.1016/s0022-5320(76)80098-6. [DOI] [PubMed] [Google Scholar]
- Cammarato A, Craig R, Sparrow JC, Lehman W. E93K charge reversal on actin perturbs steric regulation of thin filaments. J Mol Biol. 2005;347:889–894. doi: 10.1016/j.jmb.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Cammarato A, Hatch V, Saide J, Craig R, Sparrow JC, Tobacman LS, Lehman W. Drosophila muscle regulation characterized by electron microscopy and three-dimensional reconstruction of thin filament mutants. Biophys J. 2004;86:1618–1624. doi: 10.1016/S0006-3495(04)74229-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell HD, Schimansky T, Claudianos C, Ozsarac N, Kasprzak AB, Cotsell JN, Young IG, de Couet HG, Miklos GL. The Drosophila melanogaster flightless-I gene involved in gastrulation and muscle degeneration encodes gelsolin-like and leucine-rich repeat domains and is conserved in Caenorhabditis elegans and humans. Proc Natl Acad Sci USA. 1993;90:11386–11390. doi: 10.1073/pnas.90.23.11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia Carnevali MD, Saita A. Correlated structural and contractile properties in specialized fibers of a woodlouse Armadillidium vulgare (Latr. ) J Exp Zool. 1976;198:241–252. [Google Scholar]
- Cao J, Fernández M, Ramos-Martínez JI, Villamarín JA. Identification of RII-binding proteins in the mollusc Mytilus galloprovincialis. FEBS Lett. 1996;382:93–96. doi: 10.1016/0014-5793(96)00158-5. [DOI] [PubMed] [Google Scholar]
- Cao J, Fernández M, Vázquez-Illanes MD, Martínez JIR, Villamarín JA. Purification and characterization of the catalytic subunit of cAMP-dependent protein kinase from the bivalve mollusc Mytilus galloprovincialis. Comp Biochem Physiol. 1995a;111B:453–462. [Google Scholar]
- Cao J, Ramos-Martínez JI, Villamarín JA. Characterization of a cAMP-binding protein from the bivalve mollusc Mytilus galloprovincialis. Eur J Biochem. 1995b;232:664–670. [PubMed] [Google Scholar]
- Carlhoff D, D’Haese J. Slow type muscle cells in the earthworm gizzard with a distinct, Ca2+-regulated myosin isoform. J Comp Physiol. 1987;157B:589–597. [Google Scholar]
- Carlier MF. Control of actin dynamics. Curr Opin Cell Biol. 1998;10:45–51. doi: 10.1016/s0955-0674(98)80085-9. [DOI] [PubMed] [Google Scholar]
- Castagnone-Sereno P, Leroy F, Abad P. cDNA cloning and expression analysis of a calponin gene from the plant-parasitic nematode Meloidogyne incognita. Mol Biochem Parasitol. 2001;112:149–152. doi: 10.1016/s0166-6851(00)00353-4. [DOI] [PubMed] [Google Scholar]
- Castellani L, Cohen C. Myosin rod phosphorylation and the catch state of molluscan muscles. Science. 1987a;235:334–337. doi: 10.1126/science.3026049. [DOI] [PubMed] [Google Scholar]
- Castellani L, Cohen C. Rod phosphorylation favors folding in a catch muscle myosin. Proc Natl Acad Sci USA. 1987b;84:4058–4062. doi: 10.1073/pnas.84.12.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellani L, Cohen C. A calcineurin-like phosphatase is required for catch contraction. FEBS Lett. 1992;309:321–326. doi: 10.1016/0014-5793(92)80798-l. [DOI] [PubMed] [Google Scholar]
- Castellani L, Elliot BW, Winkelmann DA, Vibert P, Cohen C. Myosin binding to actin. Structural analysis using myosin fragments. J Mol Biol. 1987;196:955–960. doi: 10.1016/0022-2836(87)90420-7. [DOI] [PubMed] [Google Scholar]
- Castellani L, Elliott BW, Jr, Cohen C. Phosphorylatable serine residues are located in non-helical tailpiece of a catch muscle myosin. J Muscle Res Cell Motil. 1988;9:533–540. doi: 10.1007/BF01738758. [DOI] [PubMed] [Google Scholar]
- Castellani L, Vibert P. Location of paramyosin in relation to the subfilaments within the thick filaments of scallop striated muscle. J Muscle Res Cell Motil. 1992;13:174–82. doi: 10.1007/BF01874154. [DOI] [PubMed] [Google Scholar]
- Castellani L, Vibert P, Cohen C. Structure of myosin/paramyosin filaments from a molluscan smooth muscle. J Mol Biol. 1983;167:853–872. doi: 10.1016/s0022-2836(83)80115-6. [DOI] [PubMed] [Google Scholar]
- Castellani LC, Alberici A, Bezzi E, Landoni C. Structural and biochemical analysis of paramyosin from annelid muscles. J Submicrosc Cytol. 1978;10:315–325. [Google Scholar]
- Castellani-Ceresa L, Lanzavecchia G. Isolation and identification of paramyosin from Amphioxus notochord. J Muscle Res Cell Motil. 1982;3:75–85. doi: 10.1007/BF00711881. [DOI] [PubMed] [Google Scholar]
- Chalovich JM. Actin mediated regulation of muscle contraction. Pharmacol Ther. 1992;55:95–148. doi: 10.1016/0163-7258(92)90013-p. [DOI] [PubMed] [Google Scholar]
- Chalovich JM, Chantler PD, Szent-Györgyi AG, Eisenberg E. Regulation of molluscan actomyosin ATPase activity. J Biol Chem. 1984;259:2617–2621. [PMC free article] [PubMed] [Google Scholar]
- Chan WP, Dickinson MH. In vivo length oscillations of indirect flight muscles in the fruit fly Drosophila virilis. J Exp Biol. 1996;199:2767–2774. doi: 10.1242/jeb.199.12.2767. [DOI] [PubMed] [Google Scholar]
- Chantler PD. Lanthanides do not function as calcium analogues in scallop myosin. J Biol Chem. 1983;8:4702–4705. [PubMed] [Google Scholar]
- Chantler PD. Regulatory light chains and scallop myosin: form of light chain removal or reuptake is dependent on the presence of divalent cations. J Mol Biol. 1985;181:557–560. doi: 10.1016/0022-2836(85)90428-0. [DOI] [PubMed] [Google Scholar]
- Chantler PD, Bower SM. Cross-linking between translationally equivalent sites on the two heads of myosin. Relationship to energy transfer results between the same pair of sites. J Biol Chem. 1988;263:938–944. [PubMed] [Google Scholar]
- Chantler PD, Kensler RW. Position of Mercenaria regulatory light-chain Cys50 site on the surface of myosin visualized by electron microscopy. J Mol Biol. 1989;207:631–636. doi: 10.1016/0022-2836(89)90472-5. [DOI] [PubMed] [Google Scholar]
- Chantler PD, Sellers JR, Szent-Györgyi AG. Cooperativity in scallop myosin. Biochemistry. 1981;20:210–216. doi: 10.1021/bi00504a035. [DOI] [PubMed] [Google Scholar]
- Chantler PD, Szent-Györgyi AG. Spectroscopic studies on invertebrate myosins and light chains. Biochemistry. 1978;17:5440–5448. doi: 10.1021/bi00618a018. [DOI] [PubMed] [Google Scholar]
- Chantler PD, Szent-Györgyi AG. Regulatory light-chains and scallop myosin: full dissociation, reversibility and co-operative effects. J Mol Biol. 1980;138:473–492. doi: 10.1016/s0022-2836(80)80013-1. [DOI] [PubMed] [Google Scholar]
- Chantler PD, Tao T. Interhead fluorescence energy transfer between probes attached to translationally equivalent sites on the regulatory light chains of scallop myosin. J Mol Biol. 1986;192:87–99. doi: 10.1016/0022-2836(86)90466-3. [DOI] [PubMed] [Google Scholar]
- Chantler PD, Tao T, Stafford WF., III On the relationship between distance information derived from cross-linking and from resonance energy transfer, with specific reference to sites located on myosin heads. Biophys J. 1991;59:1242–1250. doi: 10.1016/S0006-3495(91)82339-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaplain RA. Control of insect myosin adenosinetriphosphatase by the binding of the substrate to an allosteric site. Biochem Biophys Res Commun. 1966a;25:514–517. [Google Scholar]
- Chaplain RA. The allosteric nature of substrate inhibition of insect actomyosin ATPase in presence of magnesium. Biochem Biophys Res Commun. 1966b;22:248–253. doi: 10.1016/0006-291x(66)90473-6. [DOI] [PubMed] [Google Scholar]
- Chaplain RA. Insect actomyosin ATPase - a regulatory protein. Arch Biochem Biophys. 1967a;121:154–168. doi: 10.1016/0003-9861(67)90020-3. [DOI] [PubMed] [Google Scholar]
- Chaplain RA. The effect of Ca2+ and fibre elongation on the activation of the contractile mechanism of insect fibrillar muscle. Biochim Biophys Acta. 1967b;131:385–392. doi: 10.1016/0005-2728(67)90152-1. [DOI] [PubMed] [Google Scholar]
- Chaplain RA. Changes of adenosine triphosphatase activity and tension with fibre elongation in glycerinated insect fibrillar flight muscle. Pflügers Arch. 1969;307:120–126. [PubMed] [Google Scholar]
- Chaplain RA. On the contractile mechanism of insect fibrillar flight muscle. IV A quantitative chemo-mechanical model. Biol Cybern. 1975;18:137–153. doi: 10.1007/BF00326685. [DOI] [PubMed] [Google Scholar]
- Chaplain RA, Abbott RH, White DCS. Indication for an allosteric effect of ADP on actomyosin gels and glycerinated fibres from insect fibrillar flight muscle. Biochem Biophys Res Commun. 1965;21:89–93. doi: 10.1016/0006-291x(65)90091-4. [DOI] [PubMed] [Google Scholar]
- Chaplain RA, Frommelt B. On the contractile mechanism of insect fibrillar flight muscle. I The dynamics and energetics of the linearised system. Kybernetik. 1968;5:1–17. doi: 10.1007/BF00288894. [DOI] [PubMed] [Google Scholar]
- Chaplain RA, Frommelt B, Honka B. The chemo-mechanical coupling relation in the oscillatory contraction-relaxation cycles of insect fibrillar muscle. J Mechanochem Cell Motil. 1976;3:253–264. [PubMed] [Google Scholar]
- Chaplain RA, Frommelt B, Pfister E. On the contractile mechanism of insect fibrillar flight muscle. II Passive muscle - a visco-elastic system. Kybernetik. 1968;5:61–70. doi: 10.1007/BF00272696. [DOI] [PubMed] [Google Scholar]
- Chaplain RA, Honka B. Changes in myosin cross-bridge attachment during oscillatory contraction of insect fibrillar muscle. FEBS Lett. 1974a;40:45–48. doi: 10.1016/0014-5793(74)80890-2. [DOI] [PubMed] [Google Scholar]
- Chaplain RA, Honka B. The velocity-dependence of myosin cross-bridge movement and tension development in oscillatory contractions of insect fibrillar muscle. Experientia. 1974b;30:501–504. doi: 10.1016/0014-5793(74)80890-2. [DOI] [PubMed] [Google Scholar]
- Chaplain RA, Sacharjan S. Calcium and tension-dependent changes in the actin filament structure of insect fibrillar muscle. FEBS Lett. 1974;42:50–53. doi: 10.1016/0014-5793(74)80276-0. [DOI] [PubMed] [Google Scholar]
- Chaplain RA, Tregear RA. The mass of myosin per cross-bridge in insect fibrillar flight muscle. J Mol Biol. 1966;21:275–279. doi: 10.1016/0022-2836(66)90098-2. [DOI] [PubMed] [Google Scholar]
- Chen GX, Cao TQ. The catch mechanism of molluscan smooth muscles. Sci Sin. 1984;27:583–589. [PubMed] [Google Scholar]
- Chen GX, Tan R, Gong Z, Huang Y, Wang S, Cao T. Paramyosin and the catch mechanism. Biophys Chem. 1988;29:147–153. doi: 10.1016/0301-4622(88)87034-0. [DOI] [PubMed] [Google Scholar]
- Chen LF, Blanc E, Chapman MS, Taylor KA. Real space refinement of acto-myosin structures from sectioned muscle. J Struct Biol. 2001;133:221–232. doi: 10.1006/jsbi.2000.4321. [DOI] [PubMed] [Google Scholar]
- Chen LF, Winkler H, Reedy MK, Reedy MC, Taylor KA. Molecular modeling of averaged rigor crossbridges from tomograms of insect flight muscle. J Struct Biol. 2002;138:92–104. doi: 10.1016/s1047-8477(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Chesler M, Fourtner CR. Mechanical properties of a slow muscle in the cockroach. J Neurobiol. 1981;12:391–402. doi: 10.1002/neu.480120408. [DOI] [PubMed] [Google Scholar]
- Cheung AS, Gray BF. Muscle tension response to sinusoidal length perturbation: a theoretical study. J Muscle Res Cell Motil. 1983;4:615–623. doi: 10.1007/BF00712156. [DOI] [PubMed] [Google Scholar]
- Chia-Mu P, Tsu-Hsün K, Li-Min H, Tien-Chin T. Electron microscopical studies of tropomyosin and paramyosin. Sci Sin. 1965;14:219–228. [PubMed] [Google Scholar]
- Chiba S, Ojima T, Tanaka H, Nishita K. Purification and characterization of a novel 160 kDa actin-binding protein from surf clam foot muscle. Nippon Suisan Gakk. 1993;59:1783–1791. [Google Scholar]
- Childs TJ, Watson M, Sanghera J, Campbell DL, Pelech S, Mak A. Phosphorylation of smooth muscle caldesmon by mitogen-activated protein (MAP) kinase and expression of MAP kinase in differentiated smooth muscle cells. J Biol Chem. 1992;267:22853–22859. [PubMed] [Google Scholar]
- Clark MG, Teply J, Haarer BK, Viggiano SC, Sept D, Amberg DC. A genetic dissection of Aip1p’s interactions leads to a model for Aip1p-cofilin cooperative activities. Mol Biol Cell. 2006;17:1971–1984. doi: 10.1091/mbc.E05-10-0956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke ML, Hofman W, Wray JS. ATP binding and crossbridge structure in muscle. J Mol Biol. 1986;191:581–585. doi: 10.1016/0022-2836(86)90153-1. [DOI] [PubMed] [Google Scholar]
- Clarke ML, Rodger CD, Tregear RT. Modification of cross-bridge states by ethylene glycol in insect flight muscle. J Muscle Res Cell Motil. 1984;5:81–96. doi: 10.1007/BF00713153. [DOI] [PubMed] [Google Scholar]
- Clayton JD, Cripps RM, Sparrow JC, Bullard B. Interaction of troponin-H and glutathione S-transferase-2 in the indirect flight muscles of Drosophila melanogaster. J Muscle Res Cell Motil. 1998;19:117–127. doi: 10.1023/a:1005304527563. [DOI] [PubMed] [Google Scholar]
- Cohen C. Matching molecules in the catch mechanism. Proc Natl Acad Sci USA. 1982;79:3176–3178. doi: 10.1073/pnas.79.10.3176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C. Why fibrous proteins are romantic. J Struct Biol. 1998;122:3–16. doi: 10.1006/jsbi.1998.3975. [DOI] [PubMed] [Google Scholar]
- Cohen C, Castellani L. New perspectives on catch. Comp Biochem Physiol. 1988;91C:31–33. doi: 10.1016/0742-8413(88)90165-x. [DOI] [PubMed] [Google Scholar]
- Cohen C, Holmes KC. X-ray diffraction evidence for alpha-helical coiled-coils in native muscle. J Mol Biol. 1963;6:423–432. doi: 10.1016/s0022-2836(63)80053-4. [DOI] [PubMed] [Google Scholar]
- Cohen C, Lanar DE, Parry DA. Amino acid sequence and structural repeats in schistosome paramyosin match those of myosin. Biosci Rep. 1987;7:11–16. doi: 10.1007/BF01122722. [DOI] [PubMed] [Google Scholar]
- Cohen C, Parry DAD. A conserved C-terminal assembly region in paramyosin and myosin rods. J Struct Biol. 1998;122:180–187. doi: 10.1006/jsbi.1998.3983. [DOI] [PubMed] [Google Scholar]
- Cohen C, Szent-Györgyi AG. Optical rotation and helical polypeptide chain configuration in alpha-proteins. J Am Chem Soc. 1957;79:248. [Google Scholar]
- Cohen C, Szent-Györgyi AG, Kendrick-Jones J. Paramyosin and the filaments of molluscan “catch” muscles. I Paramyosin: structure and assembly. J Mol Biol. 1971;56:223–227. doi: 10.1016/0022-2836(71)90461-x. [DOI] [PubMed] [Google Scholar]
- Cole RA, Twarog BM. Relaxation of catch in a molluscan smooth muscle. 1 Effects of drugs which act on adenyl cyclase system. Comp Biochem Physiol. 1972;43A:321–330. doi: 10.1016/0300-9629(72)90191-0. [DOI] [PubMed] [Google Scholar]
- Colegrave M, Patel H, Offer G, Chantler PD. Evaluation of the symmetric model for myosin-linked regulation: effect of site-directed mutations in the regulatory light chain on scallop myosin. Biochem J. 2003;374:89–96. doi: 10.1042/BJ20030404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins JH, Jakes R, Kendrick-Jones J, Leszyk J, Barouch W, Theibert JL, Spiegel J, Szent-Györgyi AG. Amino acid sequence of myosin essential light chain from the scallop Aquipecten irradians. Biochemistry. 1986;25:7651–7656. doi: 10.1021/bi00371a056. [DOI] [PubMed] [Google Scholar]
- Collins JH, Theibert JL, Francois JM, Ashley CC, Potter JD. Amino acid sequences and Ca2+-binding properties of two isoforms of barnacle troponin C. Biochemistry. 1991;30:702–707. doi: 10.1021/bi00217a017. [DOI] [PubMed] [Google Scholar]
- Cooke R. The mechanism of muscle contraction. CRC Crit Rev Biochem. 1986;21:53–118. doi: 10.3109/10409238609113609. [DOI] [PubMed] [Google Scholar]
- Cooke R. The actomyosin engine. FASEB J. 1995;9:636–642. doi: 10.1096/fasebj.9.8.7768355. [DOI] [PubMed] [Google Scholar]
- Cooke R. New angle on myosin. Proc Natl Acad Sci USA. 1998;95:2720–2722. doi: 10.1073/pnas.95.6.2720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke R. Milestone in physiology - the sliding filament model: 1972–2004. J Gen Physiol. 2004;123:643–656. doi: 10.1085/jgp.200409089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooley LB, Johnson WH, Krause S. Phosphorylation of paramyosin and its possible role in the catch mechanism. J Biol Chem. 1979;254:2195–2198. [PubMed] [Google Scholar]
- Cornelius F. The regulation of tension in a chemically skinned molluscan smooth muscle. Effect of Mg2+ on the Ca2+-activated tension generation. J Gen Physiol. 1980;75:709–725. doi: 10.1085/jgp.75.6.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius F. Tonic contraction and the control of relaxation in a chemically skinned molluscan smooth muscle. J Gen Physiol. 1982;79:821–834. doi: 10.1085/jgp.79.5.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowgill RW. Fluorescence and protein structure. XIV Tyrosine fluorescence in helical muscle proteins. Biochim Biophys Acta. 1968;168:417–430. doi: 10.1016/0005-2795(68)90175-x. [DOI] [PubMed] [Google Scholar]
- Cowgill RW. Susceptibility of paramyosin to proteolysis and its relationship to regions of different stability. Biochemistry. 1972;11:4532–4539. doi: 10.1021/bi00774a016. [DOI] [PubMed] [Google Scholar]
- Cowgill RW. Location and properties of sulfhydryl groups on the muscle protein paramyosin from Mercenaria mercenaria. Biochemistry. 1974;13:2467–2474. doi: 10.1021/bi00709a002. [DOI] [PubMed] [Google Scholar]
- Cowgill RW. Proteolysis of paramyosin from Mercenaria mercenaria and properties of its most stable segment. Biochemistry. 1975a;14:503–509. doi: 10.1021/bi00674a007. [DOI] [PubMed] [Google Scholar]
- Cowgill RW. Segments of paramyosin formed by cleavage at sites of cysteine residues. Biochemistry. 1975b;14:4277–4279. doi: 10.1021/bi00690a021. [DOI] [PubMed] [Google Scholar]
- Craig R, Lehman W. Crossbridge and tropomyosin positions observed in native, interacting thick and thin filaments. J Mol Biol. 2001;311:1027–1036. doi: 10.1006/jmbi.2001.4897. [DOI] [PubMed] [Google Scholar]
- Craig R, Lehman W. The ultrastructural basis of actin filament regulation. Results Probl Cell Differ. 2002;36:149–169. doi: 10.1007/978-3-540-46558-4_12. [DOI] [PubMed] [Google Scholar]
- Craig R, Megerman J. Assembly of smooth muscle myosin into side-polar filaments. J Cell Biol. 1977;75:990–996. doi: 10.1083/jcb.75.3.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R, Padrón R, Alamo L. Direct determination of myosin filament symmetry in scallop striated adductor muscle by rapid freezing and freeze substitution. J Mol Biol. 1991;220:125–132. doi: 10.1016/0022-2836(91)90386-k. [DOI] [PubMed] [Google Scholar]
- Craig R, Padrón R, Kendrick-Jones J. Structural changes accompanying phosphorylation of tarantula muscle myosin filaments. J Cell Biol. 1987;105:1319–1327. doi: 10.1083/jcb.105.3.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig R, Szent-Györgyi AG, Beese L, Flicker P, Vibert P, Cohen C. Electron microscopy of thin filaments decorated with a Ca2+-regulated myosin. J Mol Biol. 1980;140:35–55. doi: 10.1016/0022-2836(80)90355-1. [DOI] [PubMed] [Google Scholar]
- Craig R, Woodhead JL. Structure and function of myosin filaments. Curr Opin Struct Biol. 2006;16:204–212. doi: 10.1016/j.sbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- Crimmins DL, Holtzer A. Hetero-α-helical, two-chain coiled-coils. Clam-worm hybrid paramyosins. Biopolymers. 1981;20:925–950. [Google Scholar]
- Cripps RM, Suggs JA, Bernstein SI. Assembly of thick filaments and myofibrils occurs in the absence of the myosin head. EMBO J. 1999;18:1793–1804. doi: 10.1093/emboj/18.7.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross RA, Geeves MA, Kendrick-Jones J. A nucleation elongation mechansims for the self-assembly of side polar sheets of smooth-muscle myosin. EMBO J. 1991;10:747–756. doi: 10.1002/j.1460-2075.1991.tb08006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder MS, Cooke R. Orientation of spin-labeled nucleotides bound to myosin in glycerinated muscle fibers. Biophys J. 1987;51:323–333. doi: 10.1016/S0006-3495(87)83338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther RA. Three-dimensional reconstruction of a periodic specimen from a single view of an oblique section. Ultramicroscopy. 1984;13:295–304. [Google Scholar]
- Crowther RA, Padrón R, Craig R. Arrangement of the heads of myosin in relaxed thick filaments from tarantula muscle. J Mol Biol. 1985;184:429–439. doi: 10.1016/0022-2836(85)90292-x. [DOI] [PubMed] [Google Scholar]
- Cuminetti R, Rossmanith G. Small amplitude nonlinearities in the mechanical response of an asynchronous flight muscle. J Muscle Res Cell Motil. 1980;1:345–356. doi: 10.1007/BF00711935. [DOI] [PubMed] [Google Scholar]
- D’Haese J. Regulatory light chains of myosin from the obliquely striated body wall muscle of Lumbricus terristris. FEBS Lett. 1980;121:243–245. [Google Scholar]
- D’Haese J, Jinssen H. Isolation and characterization of a Ca2+ -activated actin-modulating protein from obliquely striated muscle. J Comp Physiol B. 1987;157:615–623. [Google Scholar]
- da Silva ACR, Reinach FC. Calcium binding induces conformational changes in muscle regulatory proteins. Trends Biochem Sci. 1991;16:53–57. doi: 10.1016/0968-0004(91)90024-p. [DOI] [PubMed] [Google Scholar]
- de Couet HG, Fong KSK, Weeds AG, Mclaughlin PJ, Miklos GLG. Molecular and mutational analysis of a gelsolin-family member encoded by the flightless I gene of Drosophila melanogaster. Genetics. 1995;141:1049–1059. doi: 10.1093/genetics/141.3.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Nicola G, Biekofsky R, Kelly G, Agianian B, Qiu F, Bullard B, Pastore A. Letter to the Editor: Assignment of the H-1, C-13, and N-15 resonances of holo isoform 4 of Lethocerus indicus troponin C. J Biomol NMR. 2004;29:461–462. doi: 10.1023/B:JNMR.0000032558.31512.c8. [DOI] [PubMed] [Google Scholar]
- de Villafranca GW. Adenosinetriphosphatase activity of squid mantle muscles (Loligo pealii) Biol Bull. 1955;108:113–119. [Google Scholar]
- de Villafranca GW. The A and I band lengths in stretched or contracted horseshoe crab skeletal muscle. J Ultrastruct Res. 1961;5:109–115. doi: 10.1016/s0022-5320(61)90008-9. [DOI] [PubMed] [Google Scholar]
- de Villafranca GW. The adenosinetriphosphatase activity of myofibrils from the horseshoe crab, Limulus polyphemus. Comp Biochem Physiol. 1967;21:259–271. doi: 10.1016/0010-406x(67)90786-4. [DOI] [PubMed] [Google Scholar]
- de Villafranca GW. Some physico-chemical properties of myosin B from the horseshoe crab, Limulus polyphemus. Comp Biochem Physiol. 1968;26:443–454. doi: 10.1016/0010-406x(68)90637-3. [DOI] [PubMed] [Google Scholar]
- de Villafranca GW, Campbell LK. Magnesium activation of natural actomyosin ATPase from horseshoe crab. Comp Biochem Physiol. 1969;29:775–783. doi: 10.1016/0010-406x(69)91628-4. [DOI] [PubMed] [Google Scholar]
- de Villafranca GW, Marchhaus CE. Contraction of the A band. J Ultrastruct Res. 1963;9:156–165. doi: 10.1016/s0022-5320(63)80043-x. [DOI] [PubMed] [Google Scholar]
- de Villafranca GW, Naumann DC. Some properties of the myosin B ATPase from Limulus. Comp Biochem Physiol. 1964;12:143–156. doi: 10.1016/0010-406x(64)90169-0. [DOI] [PubMed] [Google Scholar]
- de Villafranca GW, Scheinblum TS, Philpott DE. A study on the localization of contractile proteins in the muscle of the horseshoe crab (Limulus polyphemus) Biochim Biophys Acta. 1959;34:147–157. doi: 10.1016/0006-3002(59)90242-2. [DOI] [PubMed] [Google Scholar]
- de Villafranca GW, Waksmonski CA. Superprecipitation of horseshoe crab and rabbit myosin B. Int J Biochem. 1970;1:29–38. [Google Scholar]
- Deitiker PR, Epstein HF. Thick filament substructures in Caenorhabditis elegans: evidence for two populations of paramyosin. J Cell Biol. 1993;123:303–311. doi: 10.1083/jcb.123.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney DE, Krause S. Properties of monomeric paramyosin using transient electric birefringence techniques. Macromolecules. 1976;9:455–463. doi: 10.1021/ma60051a014. [DOI] [PubMed] [Google Scholar]
- Deng JT, Van Lierop JE, Sutherland C, Walsh MP. Ca2+-independent smooth muscle contraction. A novel function for integrin-linked kinase. J Biol Chem. 2001;276:16365–73. doi: 10.1074/jbc.M011634200. [DOI] [PubMed] [Google Scholar]
- Devroede J, Baguet F. Arginine, octopine et alanine durant la contraction tonique et phasique du muscle rétracteur antérieur du byssus (ABRM) de Mytilus edulis. J Physiol (Paris) 1982;78:485–491. [PubMed] [Google Scholar]
- Dewey MM, Brink P, Colflesh DE, Gaylinn B, Fan SF, Anapol F. Limulus striated muscle provides an unusual model for muscle contraction. Adv Exp Med Biol. 1984;170:67–87. doi: 10.1007/978-1-4684-4703-3_7. [DOI] [PubMed] [Google Scholar]
- Dewey MM, Walcott B, Colflesh DE, Terry H, Levine RJC. Changes in thick filament length in Limulus striated muscle. J Cell Biol. 1977;75:366–380. doi: 10.1083/jcb.75.2.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Díaz-Enrich MJ, Ibarguren I, Hellman U, Villamarín JA. Characterization of a type I regulatory subunit of cAMP-dependent protein kinase from the bivalve mollusk Mytilus galloprovincialis. Arch Biochem Biophys. 2003;416:119–127. doi: 10.1016/s0003-9861(03)00259-5. [DOI] [PubMed] [Google Scholar]
- Dickinson M. Insect flight. Curr Biol. 2006;16:R309–R314. doi: 10.1016/j.cub.2006.03.087. [DOI] [PubMed] [Google Scholar]
- Dickinson M, Farman G, Frye M, Bekyarova T, Gore D, Maughan D, Irving T. Molecular dynamics of cyclically contracting insect flight muscle in vivo. Nature. 2005;433:330–333. doi: 10.1038/nature03230. [DOI] [PubMed] [Google Scholar]
- Dickinson MH, Hyatt CJ, Lehmann FO, Moore JR, Reedy MC, Simcox A, Tohtong R, Vigoreaux JO, Yamashita H, Maughan DW. Phosphorylation dependent power output of transgenic flies: an integrated study. Biophys J. 1997;73:3122–3134. doi: 10.1016/S0006-3495(97)78338-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickinson MH, Tu MS. The function of dipteran flight muscle. Comp Biochem Physiol. 1997;116A:223–238. [Google Scholar]
- Ditgens A, D’Haese J, Small JV, Sobieszek A. Properties of tropomyosin from the dual-regulated obliquely striated body wall muscle of the earthworm (Lumbricus terrestris L.) J Muscle Res Cell Motil. 1982;3:57–74. doi: 10.1007/BF00711880. [DOI] [PubMed] [Google Scholar]
- Dixon SJ, Roy PJ. Muscle arm development in Caenorhabditis elegans. Development. 2005;132:3079–3092. doi: 10.1242/dev.01883. [DOI] [PubMed] [Google Scholar]
- Domingo A, Gonzalez-Jurado J, Maroto M, Diaz C, Vinós J, Carrasco C, Cervera M, Marco R. Troponin T is a calcium-binding protein in insect muscle: in vivo phosphorylation, muscle-specific isoforms, and developmental profile in Drosophila melanogaster. J Muscle Res Cell Motil. 1998;19:393–403. doi: 10.1023/a:1005349704790. [DOI] [PubMed] [Google Scholar]
- Dörr D, Portzehl H. Der kontraktile Myosinfaden aus glatter Muskulatur. Z Naturforsch. 1954;9b:550–555. [Google Scholar]
- dos Romedios CG, Millikan RGC, Morales MF. Polarization of tryptophan fluorescence from single striated muscle fibres. J Gen Physiol. 1972;59:103–120. doi: 10.1085/jgp.59.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover SD, Elliott A. Three-dimensional reconstruction of a paramyosin filament. J Mol Biol. 1979;132:340–341. doi: 10.1016/0022-2836(79)90264-x. [DOI] [PubMed] [Google Scholar]
- Dover SD, Elliott A, Kernaghan AK. Three-dimensional reconstruction from images of tilted specimens: the paramyosin filament. J Microsc. 1980;122:23–33. doi: 10.1111/j.1365-2818.1981.tb01239.x. [DOI] [PubMed] [Google Scholar]
- Drummond DR, Peckham M, Sparrow JC, White DCS. Alteration in crossbridge kinetics caused by mutations in actin. Nature. 1990;348:440–442. doi: 10.1038/348440a0. [DOI] [PubMed] [Google Scholar]
- Dubyak GR. Inhibition of tension development and actomyosin ATPase in barnacle muscle by the Ca2+ indicator antipyrylazo III. J Muscle Res Cell Motil. 1985;6:275–292. doi: 10.1007/BF00713170. [DOI] [PubMed] [Google Scholar]
- Dufhues G, Philipp L, Zielger C, Beinbrech G. The ATPase activity of actomysoin in the presence of C-protein and low paramyosin concentrations. Comp Biochem Physiol. 1991;99B:871–877. [Google Scholar]
- Ebashi S. The Croonian Lecture, 1979. Regulation of muscle contraction. Proc R Soc Lond Biol Sci. 1980;207:259–286. doi: 10.1098/rspb.1980.0024. [DOI] [PubMed] [Google Scholar]
- Ebberink RHM, Zurburg W, Zandee DI. Energy demand of the posterior adductor muscle of Mytilus edulis in catch during exposure to air. Mar Biol Lett. 1979;1:23–31. [Google Scholar]
- Edwards HH, Johnson WH, Merrick JP. Comparison of solubility properties of α-paramyosin, β-paramyosin, and acid-extracted paramyosin. Biochemistry. 1977;16:2255–2260. doi: 10.1021/bi00629a033. [DOI] [PubMed] [Google Scholar]
- Egelman EH, Francis N, de Rosier DJ. Helical disorder and the filament structure of F-actin are elucidated by the angle-layered aggregate. J Mol Biol. 1983;166:605–623. doi: 10.1016/s0022-2836(83)80286-1. [DOI] [PubMed] [Google Scholar]
- Egelman EH, Orlova A. New insights into actin filament dynamics. Curr Opin Struct Biol. 1995;5:172–180. doi: 10.1016/0959-440x(95)80072-7. [DOI] [PubMed] [Google Scholar]
- Ekelund MC. Depressant effect of active shortening in the anterior byssus retractor muscle of Mytilus edulis. Acta Physiol Scand. 1983;117:367–376. doi: 10.1111/j.1748-1716.1983.tb00008.x. [DOI] [PubMed] [Google Scholar]
- Elfvin MJ, Levine RJC, Pepe FA, Dewey MM. Antibody binding by native and denatured myosin and paramyosin. J Histochem Cytochem. 1979;27:1478–1482. doi: 10.1177/27.11.117049. [DOI] [PubMed] [Google Scholar]
- Ellington WR. The extent of intracellular acidification during anoxia in the catch muscle of two bivalve molluscs. J Exp Zool. 1983;227:313–317. [Google Scholar]
- Elliott A. Direct demonstration of the helical nature of paramyosin filaments. Philos Trans R Soc Lond B Biol Sci. 1971;261:197–199. [Google Scholar]
- Elliott A. The arrangement of myosin on the surface of paramyosin filaments in the white adductor muscle of Crassostrea angulata. Proc R Soc Lond Biol Sci. 1974;186:53–66. doi: 10.1098/rspb.1974.0034. [DOI] [PubMed] [Google Scholar]
- Elliott A. Structure of molluscan thick filaments: a common origin for diverse appearances. J Mol Biol. 1979;132:323–341. doi: 10.1016/0022-2836(79)90263-8. [DOI] [PubMed] [Google Scholar]
- Elliott A, Bennett PM. Molecular organization of paramyosin in the core of molluscan thick filaments. J Mol Biol. 1984;176:477–493. doi: 10.1016/0022-2836(84)90173-6. [DOI] [PubMed] [Google Scholar]
- Elliott A, Lowy J. Helicoidal structure of paramyosin. Nature. 1969;224:1105–1107. doi: 10.1038/2241105b0. [DOI] [PubMed] [Google Scholar]
- Elliott A, Lowy J. A model for the coarse structure of paramyosin filaments. J Mol Biol. 1970;53:181–203. doi: 10.1016/0022-2836(70)90294-9. [DOI] [PubMed] [Google Scholar]
- Elliott A, Lowy J, Parry DAD, Vibert PJ. Puzzle of the coiled coils in the alpha-protein paramyosin. Nature. 1968a;218:656–659. doi: 10.1038/218656a0. [DOI] [PubMed] [Google Scholar]
- Elliott A, Lowy J, Squire JM. Convolution camera to reveal periodicities in electron micrographs. Nature. 1968b;219:1224–1226. doi: 10.1038/2191224a0. [DOI] [PubMed] [Google Scholar]
- Elliott A, Offer G, Burridge K. Electron microscopy of myosin molecules from muscle and non-muscle sources. Proc R Soc Lond Biol Sci. 1976;193:45–53. doi: 10.1098/rspb.1976.0030. [DOI] [PubMed] [Google Scholar]
- Elliott GF. Electron microscope studies of the structure of the filaments in the opaque adductor muscle of the oyster Crassostrea angulata. J Mol Biol. 1964a;10:89–104. doi: 10.1016/s0022-2836(64)80030-9. [DOI] [PubMed] [Google Scholar]
- Elliott GF. X-ray diffraction studies on striated and smooth muscles. Proc R Soc Lond Biol Sci. 1964b;160:467–472. doi: 10.1098/rspb.1964.0057. [DOI] [PubMed] [Google Scholar]
- Elliott GF, Hanson J, Lowy J. Paramyosin elements in lamellibranch muscles. Nature. 1957;180:1291–1292. doi: 10.1038/1801291a0. [DOI] [PubMed] [Google Scholar]
- Elliott GF, Lowy J. Low-angle X-ray reflections from living molluscan muscles. J Mol Biol. 1961;3:41–46. doi: 10.1016/s0022-2836(61)80006-5. [DOI] [PubMed] [Google Scholar]
- Endo T, Matsumoto K, Hama T, Ohtsuka Y, Katsura G, Obinata T. Distinct troponin T genes are expressed in embryonic/larval tail striated muscle and adult body wall smooth muscle of ascidian. J Biol Chem. 1996;271:27855–27862. doi: 10.1074/jbc.271.44.27855. [DOI] [PubMed] [Google Scholar]
- Endo T, Obinata T. Troponin and its components from ascidian smooth muscle. J Biochem (Tokyo) 1981;89:1599–1608. doi: 10.1093/oxfordjournals.jbchem.a133355. [DOI] [PubMed] [Google Scholar]
- Epstein HF. Myosins A & B in the organization of myofilaments. Adv Exp Med Biol. 1985;182:215–222. doi: 10.1007/978-1-4684-4907-5_18. [DOI] [PubMed] [Google Scholar]
- Epstein HF. Modulation of myosin assembly. BioEssays. 1988;9:197–200. doi: 10.1002/bies.950090604. [DOI] [PubMed] [Google Scholar]
- Epstein HF. Genetic analysis of myosin assembly in Caenorhabditis elegans. Mol Neurobiol. 1990;4:1–25. doi: 10.1007/BF02935583. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Aronow BJ, Harris HE. Interaction of myosin and paramyosin. J Supramol Struct. 1975;3:354–360. doi: 10.1002/jss.400030407. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Aronow BJ, Harris HE. Myosin-paramyosin cofilaments: enzymatic interactions with F-actin. Proc Natl Acad Sci USA. 1976;73:3015–3019. doi: 10.1073/pnas.73.9.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein HF, Berliner GC, Casey DL, Ortiz I. Purified thick filaments from the nematode Caenorhabditis elegans: evidence for multiple proteins associated with core structures. J Cell Biol. 1988;106:1985–1995. doi: 10.1083/jcb.106.6.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein HF, Casey DL, Ortiz I. Myosin and paramyosin of Caenorhabditis elegans embryos assemble into nascent structures distinct from thick filaments and multi-filament assemblages. J Cell Biol. 1993;122:845–858. doi: 10.1083/jcb.122.4.845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein HF, Lu GY, Deitiker PR, Ortiz I, Schmid MF. Preliminary 3-dimensional model for nematode thick filament core. J Struct Biol. 1995;115:163–174. doi: 10.1006/jsbi.1995.1041. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Miller DM, III, Ortiz I, Berliner GC. Myosin and paramyosin are organized about a newly identified core structure. J Cell Biol. 1985;100:904–915. doi: 10.1083/jcb.100.3.904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein HF, Ortiz CL, Berliner GC. Assemblages of multiple thick filaments in nematode mutants. J Muscle Res Cell Motil. 1987;8:527–536. doi: 10.1007/BF01567911. [DOI] [PubMed] [Google Scholar]
- Epstein HF, Ortiz I, Mackinnon LA. The alteration of myosin isoform compartmentation in specific mutants of Caenorhabditis elegans. J Cell Biol. 1986;103:985–993. doi: 10.1083/jcb.103.3.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein HF, Waterston RH, Brenner S. A mutant affecting the heavy chain of myosin in Caenorhabditis elegans. J Mol Biol. 1974;90:291–300. doi: 10.1016/0022-2836(74)90374-x. [DOI] [PubMed] [Google Scholar]
- Eshleman WP, Wilkens JL. Actomyosin ATPase activities in the brachiopod Terebratalia transversa. Can J Zool. 1979;57:1944–1949. [Google Scholar]
- Eshleman WP, Wilkens JL, Cavey MJ. Electrophoretic and electron microscopic examination of the adductor and diductor muscles of an articulate brachiopod, Terebratalia transversa. Can J Zool. 1982;60:550–559. [Google Scholar]
- Evans CL. The physiology of plain muscle. Physiol Rev. 1926;6:358–398. [Google Scholar]
- Evans PD, Siegler MVS. Octopamine mediated relaxation of maintained and catch tension in locust skeletal muscle. J Physiol. 1982;324:93–112. doi: 10.1113/jphysiol.1982.sp014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fährmann M, Fonk I, Beinbrech G. The kinase activity of the giant protein projectin of the flight muscle of Locusta migratoria. Insect Biochem Molec Biol. 2002;32:1401–1407. doi: 10.1016/s0965-1748(02)00060-7. [DOI] [PubMed] [Google Scholar]
- Falkenthal S, Graham M, Wilkinson J. The indirect flight muscle of Drosophila accumulates a unique myosin alkali light chain isoform. Dev Biol. 1987;121:263–272. doi: 10.1016/0012-1606(87)90158-8. [DOI] [PubMed] [Google Scholar]
- Fan SF, Chen M. [The subfilament structure of the thick myofilament of the honey bee indirect flight muscle] [in Japanese] Acta Entomol Sin. 1986;29:139–142. [Google Scholar]
- Fan SF, Dewey MM, Chu B. Dynamic laser light scattering studies of the effects of pyrophosphate on cyclic motions of crossbridges in isolated thick myofilaments from Limulus striated muscle. Experientia. 1994;50:837–839. doi: 10.1007/BF01956466. [DOI] [PubMed] [Google Scholar]
- Fan SF, Dewey MM, Colflesh D. The flexibility of isolated Limulus thick filaments in relaxed and activated states. Biophys J. 1985a;48:859–861. doi: 10.1016/S0006-3495(85)83846-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan SF, Dewey MM, Colflesh D, Chu B. The effects of deuterium oxide on the active cross-bridge motion of thick filaments isolated from striated muscle of Limulus. Biochim Biophys Acta. 1985b;830:333–336. [Google Scholar]
- Fan SF, Dewey MM, Colflesh D, Chu B. Effect of ATP depletion on the isolated thick filament of Limulus striated muscle. Biophys J. 1987;52:859–866. doi: 10.1016/S0006-3495(87)83279-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan SF, Dewey MM, Colflesh D, Gaylinn B, Greguski R, Chu B. Suppression of active cross-bridge motions of isolated thick myofilaments in suspension by phenylmethlysulfonyl fluoride. Biochim Biophys Acta. 1985c;827:101–105. doi: 10.1016/0167-4838(85)90105-0. [DOI] [PubMed] [Google Scholar]
- Fan SF, Dewey MM, Gaylinn B, Chu B. Seasonal changes in the activation of crossbridge motions of isolated thick filament from Limulus striated muscle. J Comp Physiol. 1992;162B:508–512. doi: 10.1007/BF00264810. [DOI] [PubMed] [Google Scholar]
- Farah CS, Reinach FC. The troponin complex and regulation of muscle contraction. FASEB J. 1995;9:755–767. doi: 10.1096/fasebj.9.9.7601340. [DOI] [PubMed] [Google Scholar]
- Fernandes JMO. Asynchronicity: a job for troponin C. J Exp Biol. 2003;206:3307. [Google Scholar]
- Fine ML. Seasonal and geographical variation of the mating call of the oyster toadfish Opsanus tau L. Oecologia. 1978;36:45–57. doi: 10.1007/BF00344570. [DOI] [PubMed] [Google Scholar]
- Fine ML, Mosca PJ. Anatomical study of the innervation pattern of the sonic muscle of the oyster toadfish. Brain Behav Evol. 1989;34:265–272. doi: 10.1159/000116511. [DOI] [PubMed] [Google Scholar]
- Fischer E. The submicroscopical structure of muscle and its changes during contraction and stretch. Cold Spring Harb Symp Quant Biol. 1936;4:214–223. [Google Scholar]
- Fischer E. The birefringence of smooth muscle (Phascolosoma and Thyone) as related to muscle length, tension and tone. J Cell Comp Physiol. 1938a;12A:85–101. [Google Scholar]
- Fischer E. The changes in birefringence during contraction and stretch of a smooth muscle (retractor of Phacolosoma) J Cell Comp Physiol. 1938b;8:503–513. [Google Scholar]
- Fletcher CM. The relation between the mechanical and electrical activity of a molluscan unstriated muscle. J Physiol. 1937;91:172–185. doi: 10.1113/jphysiol.1937.sp003550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood PR, Guthrie DM, Banks JR. Paramyosin muscle in the notochord of Amphioxus. Nature. 1969;222:87–88. doi: 10.1038/222087a0. [DOI] [PubMed] [Google Scholar]
- Florey E. Nervous control and spontaneous activity of the chromatophores of a chephalopod, Loligo opalescens. Comp Biochem Physiol. 1966;18:305–324. doi: 10.1016/0010-406x(66)90189-7. [DOI] [PubMed] [Google Scholar]
- Florey E, Kriebel E. Electrical and mechanical responses of chromatophore muscle fibers of the squid, Loligo opalescens, to nerve stimulation and drugs. Z vergl Physiol. 1969;65:98–130. [Google Scholar]
- Frado LLY, Craig R. Structural changes induced in Ca2+-regulated myosin filaments by Ca2+ and ATP. J Cell Biol. 1989;109:529–538. doi: 10.1083/jcb.109.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frado LLY, Craig R. Structural changes induced in scallop heavy meromyosin molecules by Ca2+ and ATP. J Muscle Res Cell Motil. 1992;13:436–446. doi: 10.1007/BF01738038. [DOI] [PubMed] [Google Scholar]
- Franzini-Armstrong C. Natural variability in the length of thin and thick filaments in single fibres from a crab, Portunus depurator. J Cell Sci. 1970;6:559–592. doi: 10.1242/jcs.6.2.559. [DOI] [PubMed] [Google Scholar]
- Fraser RDB, MacRae TP, Miller A. X-ray diffraction patterns of α-fibrous proteins. J Mol Biol. 1965;14:432–442. doi: 10.1016/s0022-2836(65)80193-0. [DOI] [PubMed] [Google Scholar]
- Freundlich A, Luther PK, Squire JM. High-voltage electron microscopy of crossbridge interactions in striated muscle. J Muscle Res Cell Motil. 1980;1:321–343. doi: 10.1007/BF00711934. [DOI] [PubMed] [Google Scholar]
- Freundlich A, Squire JM. 3-dimensional structure of the insect (Lethocerus) flight-muscle M-band. J Mol Biol. 1983;169:439–453. doi: 10.1016/s0022-2836(83)80060-6. [DOI] [PubMed] [Google Scholar]
- Fromherz S, Szent-Györgyi AG. Role of essential light-chain EF hand domains in calcium binding and regulation of scallop myosin. Proc Natl Acad Sci USA. 1995;92:7652–7656. doi: 10.1073/pnas.92.17.7652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujime S, Kubota K. Effect of cross-bridge motion on the spectrum of light quasielastically scattered from Limulus thick myofilament suspensions. Macromolecules. 1984;17:441–445. [Google Scholar]
- Fukushima K, Murakami S. Structural changes of paramyosin paracrystals under evacuation in film-sealed environmental cell. J Electron Microsc (Tokyo) 1985;34:18–24. [Google Scholar]
- Funabara D. [Studies on the regulatory mechanisms involved in catch contraction of molluscan smooth muscles] [in Japanese] Nippon Suisan Gakk. 2004;70:508–511. [Google Scholar]
- Funabara D, Kanoh S, Siegman MJ, Butler TM, Hartshorne DJ, Watabe S. Twitchin as a regulator of catch contraction in molluscan smooth muscle. J Muscle Res Cell Motil. 2005;26:455–460. doi: 10.1007/s10974-005-9029-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funabara D, Kinoshita S, Watabe S, Siegman MJ, Butler TM, Hartshorne DJ. Phosphorylation of molluscan twitchin by the cAMP-dependent protein kinase. Biochemistry. 2001;40:2087–2095. doi: 10.1021/bi0022691. [DOI] [PubMed] [Google Scholar]
- Funabara D, Watabe S. The catch mechanism of anterior byssus retractor muscle in mussel. Nippon Suisan Gakk. 2002;68:913–914. [Google Scholar]
- Funabara D, Watabe S, Mooers SU, Narayan S, Dudas C, Hartshorne DJ, Siegman MJ, Butler TM. Twitchin from molluscan catch muscle - primary structure and relationship between site-specific phosphorylation and mechanical function. J Biol Chem. 2003;278:29308–29316. doi: 10.1074/jbc.M303272200. [DOI] [PubMed] [Google Scholar]
- Fyrberg EA, Beall C. Genetic approaches to myofibril form and function in Drosophila. Trends Genet. 1990;6:126–131. doi: 10.1016/0168-9525(90)90127-r. [DOI] [PubMed] [Google Scholar]
- Gagelmann M, Güth K, Rüegg JC. Stretch induced tension rise in a molluscan smooth muscle skinned by freeze drying. J Comp Physiol B. 1984;154:187–189. [Google Scholar]
- Gal V. Photon correlation measurements on paramyosin solutions. Periodicum Biologorum. 1979;81:619–624. [Google Scholar]
- Galler S, Hõpflinger MC, Andruchov O, Andruchova E, Grassberger H. Effects of vanadate, phosphate and 2,3-butanedione monoxime (BDM) on skinned molluscan catch muscle. Pflügers Arch. 2005;449:372–383. doi: 10.1007/s00424-004-1350-x. [DOI] [PubMed] [Google Scholar]
- Galler S, Kögler H, Ivemeyer M, Rüegg JC. Force responses of skinned molluscan catch muscle following photoliberation of ATP. Pflügers Arch - Eur J Physiol. 1999;438:525–530. doi: 10.1007/s004249900065. [DOI] [PubMed] [Google Scholar]
- Garone L, Theibert JL, Miegel A, Maeda Y, Murphy C, Collins JH. Lobster troponin C: amino acid sequences of three isoforms. Arch Biochem Biophys. 1991;291:89–91. doi: 10.1016/0003-9861(91)90108-u. [DOI] [PubMed] [Google Scholar]
- Gaylinn B, Dewey MM. Paramyosin and myosin content of the thick filament in the striated muscle of Limulus. J Muscle Res Cell Motil. 1986;7:467–473. doi: 10.1007/BF01753589. [DOI] [PubMed] [Google Scholar]
- Geeves MA, Holmes KC. The molecular mechanism of muscle contraction. Adv Prot Chem. 2005;71:161–193. doi: 10.1016/S0065-3233(04)71005-0. [DOI] [PubMed] [Google Scholar]
- Gengyo-Ando K, Kagawa H. Single charge change on the helical surface of the paramyosin rod dramatically disrupts thick filament assembly in Caenorhabditis elegans. J Mol Biol. 1991;219:429–441. doi: 10.1016/0022-2836(91)90184-8. [DOI] [PubMed] [Google Scholar]
- Giebing T, Hinssen H, D’Haese J. The complete sequence of a 40-Kda actin-modulating protein from the earthworm Lumbricus terrestris. Eur J Biochem. 1994;225:773–779. doi: 10.1111/j.1432-1033.1994.0773b.x. [DOI] [PubMed] [Google Scholar]
- Giebing T, Obermann WMJ, Furst D, D’Haese J. C-terminally deleted fragments of 40-kDa earthworm actin modulator still show gelsolin activities. FEBS Lett. 1997;417:191–195. doi: 10.1016/s0014-5793(97)01230-1. [DOI] [PubMed] [Google Scholar]
- Gies A. Serotonin and dopamine as regulators of adenylate cyclase and relaxation in a smooth muscle of the mussel Mytilus edulis. Comp Biochem Physiol. 1986;84C:61–66. doi: 10.1016/0742-8413(86)90165-9. [DOI] [PubMed] [Google Scholar]
- Gies A. Changes of nucleotide contents and of energy-charge induced by contraction, catch and relaxation in smooth molluscan muscle fibers. An analysis using reversed-phase ion-pair high-performance liquid chromatography. Comp Biochem Physiol. 1988;91B:483–487. doi: 10.1016/0305-0491(88)90009-0. [DOI] [PubMed] [Google Scholar]
- Gilëv VP. The fine structure of the ‘myosin’ protofibrils of cross striated muscle fibre. Biofizika. 1966;11:274–277. [PubMed] [Google Scholar]
- Gilloteaux J. 5-HT effect and the control of the relaxation of a molluscan smooth muscle (ABRM) of Mytilus edulis L. Cytobiologie. 1978;17:94–106. [PubMed] [Google Scholar]
- Gilloteaux J, Baguet F. Contractile filaments organization in functional states of the anterior byssus retractor muscle (ABRM) of Mytilus edulis L. Cytobiologie. 1977;15:192–220. [Google Scholar]
- Gilmour D, Calaby JH. Physical and enzymic properties of actomyosins from the femoral and thoracic muscles of an insect. Enzymologia. 1953;16:23–33. [PubMed] [Google Scholar]
- Gilmour D, Robinson PM. Contraction in glycerinated myofibrils of an insect (Orthopertera, Acrididae) J Cell Biol. 1964;21:385–396. doi: 10.1083/jcb.21.3.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmour KM, Ellington CP. In vivo muscle length changes in bumblebees and the in vitro effects on work and power. J Exp Biol. 1993;183:101–113. [Google Scholar]
- Goblet C, Mounier Y. Activation of skinned muscle fibers by calcium and strontium ions. Can J Physiol Pharmacol. 1987;65:642–647. doi: 10.1139/y87-107. [DOI] [PubMed] [Google Scholar]
- Godeaux J. Recherches electrophorétiques sur les protéines musculaires du Lombric. Bull Acad R Belg Cl Sci. 1954;40:948–961. [Google Scholar]
- Goldberg A, Lehman W. Troponin-like proteins from muscles of the scallop, Aequipecten irradians. Biochem J. 1978;171:413–418. doi: 10.1042/bj1710413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode MD. Ultrastructure and contractile properties of isolated myofibrils and myofilaments from Drosophila flight muscle. Trans Am Microsc Soc. 1972;91:182–194. [PubMed] [Google Scholar]
- Goodwin EB, Leinwand LA, Szent-Györgyi AG. Regulation of scallop myosin by mutant regulatory light chains. J Mol Biol. 1990;216:85–93. doi: 10.1016/S0022-2836(05)80062-2. [DOI] [PubMed] [Google Scholar]
- Goody RS, Barrington-Leigh J, Mannherz HG, Tregear RT, Rosenbaum G. X-ray titration of binding of β,γ-imido-ATP to myosin in insect flight muscle. Nature. 1976;262:613–615. doi: 10.1038/262613a0. [DOI] [PubMed] [Google Scholar]
- Goody RS, Holmes KC, Mannherz HG, Leigh JB, Rosenbaum G. Cross-bridge conformation as revealed by X-ray diffraction studies on insect flight muscles with ATP analogues. Biophys J. 1975;15:687–705. doi: 10.1016/S0006-3495(75)85848-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goody RS, Reedy MC, Hofmann W, Holmes KC, Reedy MK. Binding of myosin subfragment-1 to glycerinated insect flight muscle in the rigor state. Biophys J. 1985;47:151–169. doi: 10.1016/s0006-3495(85)83889-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AM, Homsher E, Regnier M. Regulation of contraction in striated muscle. Physiol Rev. 2000;80:853–924. doi: 10.1152/physrev.2000.80.2.853. [DOI] [PubMed] [Google Scholar]
- Gordon AM, Qian Y, Luo Z, Wang CK, Mondares RL, Martyn DA. Characterization of troponin-C interactions in skinned barnacle muscle: comparison with troponin-C from rabbit striated muscle. J Muscle Res Cell Motil. 1997;18:643–653. doi: 10.1023/a:1018631806182. [DOI] [PubMed] [Google Scholar]
- Gourinath S, Himmel DM, Brown JH, Reshetnikova L, Szent-Györgyi AG, Cohen C. Crystal structure of scallop myosin S1 in the pre-power stroke state to 2.6 Å resolution: flexibility and function in the head. Structure. 2003;11:1621–1627. doi: 10.1016/j.str.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Grabarek Z, Tao T, Gergely J. Molecular mechanism of troponin C function. J Muscle Res Cell Motil. 1992;13:383–393. doi: 10.1007/BF01738034. [DOI] [PubMed] [Google Scholar]
- Granzier HLM, Wang K. Interplay between passive tension and strong and weak binding cross-bridges in insect indirect flight muscle. A functional dissection by gelsolin-mediated thin filament removal. J Gen Physiol. 1993;101:235–270. doi: 10.1085/jgp.101.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths PJ, Kuhn HJ, Rüegg JC. Activation of the contractile system of insect fibrillar muscle at very low concentration of Mg2+ and Ca2+ Pflügers Arch. 1979;382:155–163. doi: 10.1007/BF00584217. [DOI] [PubMed] [Google Scholar]
- Grützner P. Die glatten Muskeln. Ergeb Physiol. 1904;3:12–88. [Google Scholar]
- Günzel D, Rathmayer W. Non-uniformity of sarcomere lengths can explain the ‘catch-like’ effect of arthropod muscle. J Muscle Res Cell Motil. 1994;15:535–46. doi: 10.1007/BF00121159. [DOI] [PubMed] [Google Scholar]
- Guo WS, Luo LF, Li QZ. A chemical kinetic theory on muscle contraction and spontaneous oscillation. Chem Phys Lett. 2002;363:471–478. [Google Scholar]
- Gusev NB. Some properties of caldesmon and calponin and the participation of these proteins in regulation of smooth muscle contraction and cytoskeleton formation. Biochem (Moscow) 2001;66:1112–1121. doi: 10.1023/a:1012480829618. [DOI] [PubMed] [Google Scholar]
- Güth K. Polarization of tryptophan fluorescence measurement in muscle. Biophys Struct Mech. 1980;6:81–93. doi: 10.1007/BF00535746. [DOI] [PubMed] [Google Scholar]
- Güth K, Gagelmann M, Rüegg JC. Skinned smooth muscle: time course of force and ATPase activity during contraction cycle. Experientia. 1984;40:174–176. doi: 10.1007/BF01963585. [DOI] [PubMed] [Google Scholar]
- Güth K, Kuhn HJ, Drexler B, Berberich W, Rüegg JC. Stiffness and tension during and after sudden length changes of glycerinated single insect fibrillar muscle fibers. Biophys Struct Mech. 1979;5:255–276. doi: 10.1007/BF02426662. [DOI] [PubMed] [Google Scholar]
- Güth K, Kuhn HJ, Tschuchiya T, Rüegg JC. Length dependent state of activation - length change dependent kinetics of cross bridges in skinned insect flight muscle. Biophys Struct Mech. 1981;7:139–169. [Google Scholar]
- Hagiwara S. Neuro-muscular mechanism of sound production in the cicada. Physiol Comp Oecol. 1955;4:142–153. [Google Scholar]
- Hagiwara S, Uchiyama H, Watanabe A. The mechanism of sound production in certain cicadas with special reference to the myogenic rhythm in insect muscles. Bull Tokyo Med Dent Univ. 1954;1:113–131. [Google Scholar]
- Hall CE, Jakus MA, Schmitt FO. The structure of certain muscle fibrils as revealed by the use of electron stains. J Appl Phys. 1945;16:459–465. [Google Scholar]
- Hall CE, Jakus MA, Schmitt FO. An investigation of cross striations and myosin filaments in muscle. Biol Bull. 1946;90:32–50. [PubMed] [Google Scholar]
- Halsey JF, Harrington WF. Substructure of paramyosin. Correlation of helix stability, trypsin digestion kinetics, and amino acid composition. Biochemistry. 1971;12:693–701. doi: 10.1021/bi00728a019. [DOI] [PubMed] [Google Scholar]
- Han YJ, Sellers JR. Motility assays on molluscan native thick filaments. Methods Enzymol. 1998;298:427–435. doi: 10.1016/s0076-6879(98)98038-7. [DOI] [PubMed] [Google Scholar]
- Hanson J. Changes in the cross-striation of myofibrils during contraction induced by adenosine triphosphate. Nature. 1952;169:530–533. doi: 10.1038/169530a0. [DOI] [PubMed] [Google Scholar]
- Hanson J. Elongation of cross-striated myofibrils. Biochim Biophys Acta. 1956a;20:289–292. doi: 10.1016/0006-3002(56)90287-6. [DOI] [PubMed] [Google Scholar]
- Hanson J. Studies on the cross-striation of the indirect flight myofibrils of the blowfly Calliphora. J Biophys Biochem Cytol. 1956b;2:691–710. doi: 10.1083/jcb.2.6.691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson J. Axial period of actin filaments. Electron microscope studies Nature. 1967;213:353–356. [Google Scholar]
- Hanson J. Recent X-ray diffraction studies of muscle. Quart Rev Biophys. 1968;1:177–216. doi: 10.1017/s0033583500000536. [DOI] [PubMed] [Google Scholar]
- Hanson J, Lowy J. Evidence for a sliding filament contractile mechanism in tonic smooth muscles of lamellibranch molluscs. Nature. 1959;184:286–287. [Google Scholar]
- Hanson J, Lowy J. The structure of the muscle fibres in the translucent part of the adductor of the oyster Crossostrea angulata. Proc R Soc Lond Biol Sci. 1961;154:173–196. [Google Scholar]
- Hanson J, Lowy J. The structure of F-actin and of actin filaments isolated from muscle. J Mol Biol. 1963;6:46–60. [Google Scholar]
- Hanson J, Lowy J. Comparative studies on the structure of contractile systems. Circ Res. 1964;14:II-4–II-13. [PubMed] [Google Scholar]
- Hanson J, Lowy J. Molecular basis of contractility in muscle. Br Med Bull. 1965;21:264–271. doi: 10.1093/oxfordjournals.bmb.a070407. [DOI] [PubMed] [Google Scholar]
- Hanson J, Lowy J, Huxley HE, Bailey K, Kay CM, Rüegg JC. Structure of molluscan tropomyosin. Nature. 1957;180:1134–1135. doi: 10.1038/1801134b0. [DOI] [PubMed] [Google Scholar]
- Hao YD, Bernstein SI, Pollack GH. Passive stiffness of Drosophila IFM myofibrils: a novel, high accuracy measurement method. J Muscle Res Cell Motil. 2004;25:359–366. doi: 10.1007/s10974-004-0684-5. [DOI] [PubMed] [Google Scholar]
- Hao YD, Miller MS, Swank DM, Liu HJ, Bernstein SI, Maughan DW, Pollack GH. Passive stiffness in Drosophila indirect flight muscle reduced by disrupting paramyosin phosphorylation, but not by embryonic myosin S2 hinge substitution. Biophys J. 2006;91:4500–4506. doi: 10.1529/biophysj.106.088492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwicke P, Hanson J. Separation of thick and thin myofilaments. J Mol Biol. 1971;59:509–516. doi: 10.1016/0022-2836(71)90314-7. [DOI] [PubMed] [Google Scholar]
- Hardwicke PMD, Szent-Györgyi AG. Proximity of regulatory light chains in scallop myosin. J Mol Biol. 1985;183:203–211. doi: 10.1016/0022-2836(85)90213-x. [DOI] [PubMed] [Google Scholar]
- Hardwicke PMD, Wallimann T, Szent-Györgyi AG. Regulatory and essential light chain interactions in scallop myosin. I Protection of essential light-chain thiol groups by regulatory light-chains. J Mol Biol. 1982;156:141–152. doi: 10.1016/0022-2836(82)90463-6. [DOI] [PubMed] [Google Scholar]
- Hardwicke PMD, Wallimann T, Szent-Györgyi AG. Light-chain movement and regulation in scallop myosin. Nature. 1983;301:478–482. doi: 10.1038/301478a0. [DOI] [PubMed] [Google Scholar]
- Harrington WF, Roger ME. Myosin. Annu Rev Biochem. 1984;53:35–73. doi: 10.1146/annurev.bi.53.070184.000343. [DOI] [PubMed] [Google Scholar]
- Harris HE, Epstein HF. Myosin and paramyosin of Caenorhabditis elegans: biochemical and structural properties of wild-type and mutant proteins. Cell. 1977;10:709–719. doi: 10.1016/0092-8674(77)90105-2. [DOI] [PubMed] [Google Scholar]
- Harris HE, Tso MY, Epstein HF. Actin and myosin-linked calcium regulation in the nematode Caenorhabditis elegans. Biochemical and structural properties of native filaments and purified proteins. Biochemistry. 1977;16:859–865. doi: 10.1021/bi00624a008. [DOI] [PubMed] [Google Scholar]
- Haselgrove JC, Reedy MK. Modeling rigor cross-bridge patterns in muscle. I Initial studies of the rigor lattice of insect flight muscle. Biophys J. 1978;24:713–728. doi: 10.1016/S0006-3495(78)85415-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haselgrove JC, Reedy MK. Geometrical constraints affecting crossbridge formation in insect flight muscle. J Muscle Res Cell Motil. 1984;5:3–24. doi: 10.1007/BF00713149. [DOI] [PubMed] [Google Scholar]
- Hauck R, Achazi RK. The ultrastructure of a molluscan catch muscle during a contraction-catch-relaxation cycle. Eur J Cell Biol. 1987;45:30–35. [Google Scholar]
- Hauck R, Achazi RK. In situ phosphorylation of contractile proteins of a molluscan (Mytilus edulis) catch muscle in different functional states. Comp Biochem Physiol. 1991;100B:237–242. [Google Scholar]
- Hawkins RD, Bruner J. Maintained contraction of the crayfish claw opener muscle in the absence of motor neuron activity. Brain Res. 1979;162:129–136. doi: 10.1016/0006-8993(79)90761-3. [DOI] [PubMed] [Google Scholar]
- Heide G. Proprioceptive feedback dominates the central oscillator in the patterning of the flight motoneuron output in Tipula (Diptera) J Comp Physiol A. 1979;134:177–189. [Google Scholar]
- Heierhorst J, Probst WC, Kohanski RA, Buku A, Weiss KR. Phosphorylation of myosin regulatory light chains by the molluscan twitchin kinase. Eur J Biochem. 1995;233:426–431. doi: 10.1111/j.1432-1033.1995.426_2.x. [DOI] [PubMed] [Google Scholar]
- Henkin JA, Maughan DW, Vigoreaux JO. Mutations that affect flightin expression in Drosophila alter the viscoelastic properties of flight muscle fibers. Am J Physiol Cell Physiol. 2004;286:C65–C72. doi: 10.1152/ajpcell.00257.2003. [DOI] [PubMed] [Google Scholar]
- Herter K. Galvanometrische Untersuchungen am Schneckenfuss. Z vergl Physiol. 1931a;14:609–628. [Google Scholar]
- Herter K. Untersuchungen über den Muskeltonus des Schneckenfusses. Z vergl Physiol. 1931b;13:709–739. [Google Scholar]
- Heumann HG. Substructure of paramyosin filaments prepared by freeze-substitution technique. Experientia. 1973;29:469–471. doi: 10.1007/BF01926788. [DOI] [PubMed] [Google Scholar]
- Heumann HG. Paramyosin structures in the thick filaments of the anterior byssus retractor muscle of Mytilus edulis. Eur J Cell Biol. 1980;22:780–788. [PubMed] [Google Scholar]
- Heumann HG, Zebe E. Zur Lokalisation des Myosins in den Muskelfasern aus dem Hautmuskelschlauch des Regenwurms. Z Naturforsch. 1966;21:62–65. [PubMed] [Google Scholar]
- Heumann HG, Zebe E. Über die Funktionsweise glatter Muskelfasern. Elektronenmikroskopische Untersuchungen am Byssusretractor (ABRM) von Mytilus edulis. Z Zellforsch. 1968;85:534–551. [PubMed] [Google Scholar]
- Heuser J. Quick-freeze, deep-etch preparation of samples for 3-D electron microscopy. Trends Biochem Sci. 1981;6:64–68. [Google Scholar]
- Heuser JE. Structure of the myosin crossbridge lattice in insect flight muscle. J Mol Biol. 1983;169:123–154. doi: 10.1016/s0022-2836(83)80178-8. [DOI] [PubMed] [Google Scholar]
- Heuser JE. Crossbridges in insect flight muscles of the blowfly (Sarcophaga bullata) J Muscle Res Cell Motil. 1987;8:303–321. doi: 10.1007/BF01568887. [DOI] [PubMed] [Google Scholar]
- Heyer CB, Kater SB, Karlson UL. Neuromuscular systems in molluscs. Am Zool. 1973;13:247–270. [Google Scholar]
- Hidaka T, Kuriyama H, Yamamoto T. The mechanical properties of the longitudinal muscle in the earthworm. J Exp Biol. 1969;50:431–443. doi: 10.1242/jeb.50.2.431. [DOI] [PubMed] [Google Scholar]
- Hidaka T, Osa T, Twarog BM. The action of 5-hydroxytryptamine on Mytilus smooth muscle. J Physiol. 1967;192:869–877. doi: 10.1113/jphysiol.1967.sp008337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo C, Craig R, Ikebe M, Padrón R. Mechanism of phosphorylation of the regulatory light chain of myosin from tarantula striated muscle. J Muscle Res Cell Motil. 2001;22:51–59. doi: 10.1023/a:1010388103354. [DOI] [PubMed] [Google Scholar]
- Highsmith S, Cooke R. Evidence for actomyosin conformational changes involved in tension generation. J Muscle Res Cell Motil. 1983;4:207–237. [PubMed] [Google Scholar]
- Hikichi S, Ojima T, Kakudate S, Nishita K. Biochemical characteristics of ATPase and Ca2+-sensitivity of myosin from Akazara smooth adductor and surf-clam foot muscles. Nippon Suisan Gakk. 1983;49:141–148. [Google Scholar]
- Himmel DM, Gourinath S, Reshetnikova L, Shen Y, Szent-Györgyi AG, Cohen C. Crystallographic findings on the internally uncoupled and near-rigor states of myosin: further insights into the mechanics of the motor. Proc Natl Acad Sci USA. 2002;99:12645–12650. doi: 10.1073/pnas.202476799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinkel-Aust S, Hinkel P, Beinbrech G. Four cross-bridge strands and high paramyosin content in the myosin filaments of honey bee flight muscles. Experientia. 1990;46:872–874. [Google Scholar]
- Hirata T, Kawahara A, Muneoka Y. Relaxing and inhibitory actions of pedal ganglion extracts on the anterior byssus retractor muscle of Mytilus. Hiroshima J Med Sci. 1986;35:397–402. [PubMed] [Google Scholar]
- Hirata T, Kubota I, Imada M, Muneoka Y. Pharmacology of relaxing response of Mytilus smooth muscle to the catch-relaxing peptide. Comp Biochem Physiol. 1989;92C:289–295. [Google Scholar]
- Hirata T, Kubota I, Takabatake I, Kawahara A, Shimamoto N, Muneoka Y. Catch-relaxing peptide isolated from Mytilus pedal ganglia. Brain Res. 1987;422:374–376. doi: 10.1016/0006-8993(87)90947-4. [DOI] [PubMed] [Google Scholar]
- Hodge AJ. A new type of periodic structure obtained by reconstitution of paramyosin from acid solutions. Proc Natl Acad Sci USA. 1952;38:850–855. doi: 10.1073/pnas.38.10.850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge AJ. Fibrous proteins of muscle. Rev Mod Physics. 1959;31:409–425. [Google Scholar]
- Hodgkinson JL. Actin and the smooth muscle regulatory proteins: a structural perspective. J Muscle Res Cell Motil. 2000;21:115–130. doi: 10.1023/a:1005697301043. [DOI] [PubMed] [Google Scholar]
- Hodgkinson JL, ElMezgueldi M, Craig R, Vibert P, Marston SB, Lehman W. 3-D image reconstruction of reconstituted smooth muscle thin filaments containing calponin: visualization of interactions between F-actin and calponin. J Mol Biol. 1997;273:150–159. doi: 10.1006/jmbi.1997.1307. [DOI] [PubMed] [Google Scholar]
- Hofmann FB. Über einen peripheren Tonus der Cephalopoden-Chromatophoren und über ihre Beeinflussung durch Gifte. Pflügers Arch. 1907;118:413–451. [Google Scholar]
- Holgate JA, Cambridge GW. Responses of the anterior retractor muscle of the byssus of Mytilus edulis. Nature. 1958;182:34–35. doi: 10.1038/182034a0. [DOI] [PubMed] [Google Scholar]
- Holmes KC. The actomyosin interaction and its control by tropomyosin. Biophys J. 1995;68:2S–5S. [PubMed] [Google Scholar]
- Holmes KC. The swinging lever-arm hypothesis of muscle contraction. Curr Biol. 1997;7:R112–R118. doi: 10.1016/s0960-9822(06)00051-0. [DOI] [PubMed] [Google Scholar]
- Holmes KC. A molecular model for muscle contraction. Acta Crystallogr A. 1998;54:789–797. doi: 10.1107/s0108767398010307. [DOI] [PubMed] [Google Scholar]
- Holmes KC, Geeves MA. The structural basis of muscle contraction. Philos Trans R Soc Lond B Biol Sci. 2000;355:419–431. doi: 10.1098/rstb.2000.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes KC, Goody RS. The nature of the actin cross-bridge interaction. Adv Exp Med Biol. 1984;170:373–384. doi: 10.1007/978-1-4684-4703-3_34. [DOI] [PubMed] [Google Scholar]
- Holmes KC, Goody RS, Amos LA. The structure of S1-decorated actin filaments calculated from x-ray diffraction data with phases derived from electron micrographs. Ultramicroscopy. 1982;9:37–44. doi: 10.1016/0304-3991(82)90227-3. [DOI] [PubMed] [Google Scholar]
- Holmes KC, Tregear RT, Barrington-Leigh J. Interpretation of the low angle x-ray diffraction from insect muscle in rigor. Proc R Soc Lond Biol Sci. 1980;207:13–33. [Google Scholar]
- Hooper SL, Thuma JB. Invertebrate muscles: muscle specific genes and proteins. Physiol Rev 85:1001–1060; Corrigenda, 85:1417. Physiol Rev. 2005;85:1001–1060. doi: 10.1152/physrev.00019.2004. [DOI] [PubMed] [Google Scholar]
- Höpflinger MC, Andruchova O, Andruchov O, Grassberger H, Galler S. Effect of pH on the rate of myosin head detachment in molluscan catch muscle: are myosin heads involved in the catch state? J Exp Biol. 2006;209:668–676. doi: 10.1242/jeb.02033. [DOI] [PubMed] [Google Scholar]
- Hoppe PE, Waterston RH. A region of the myosin rod important for interaction with paramyosin in Caenorhabditis elegans striated muscle. Genetics. 2000;156:631–643. doi: 10.1093/genetics/156.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horie N, Tsuchiya T, Matsumoto JJ. Studies on ATPase activity of actomysosin of squid mantle muscles. Nippon Suisan Gakk. 1975;41:1039–1045. [Google Scholar]
- Houdusse A, Cohen C. Target sequence recognition by the calmodulin superfamily: implications from light chain binding to the regulatory domain of scallop myosin. Proc Natl Acad Sci USA. 1995;92:10644–10647. doi: 10.1073/pnas.92.23.10644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houdusse A, Cohen C. Structure of the regulatory domain of scallop myosin at 2 Å resolution: implications for regulation. Structure. 1996;4:21–32. doi: 10.1016/s0969-2126(96)00006-8. [DOI] [PubMed] [Google Scholar]
- Houdusse A, Kalbokis VN, Himmel D, Szent-Györgyi AG, Cohen C. Atomic structure of scallop myosin subfragment S1 complexed with MgADP: a novel conformation of the myosin head. Cell. 1999;97:459–470. doi: 10.1016/s0092-8674(00)80756-4. [DOI] [PubMed] [Google Scholar]
- Houdusse A, Sweeney HL. Myosin motors: missing structures and hidden springs. Curr Op Struct Biol. 2001;11:182–194. doi: 10.1016/s0959-440x(00)00188-3. [DOI] [PubMed] [Google Scholar]
- Houdusse A, Szent-Györgyi AG, Cohen C. Three conformational states of scallop myosin S1. Proc Natl Acad Sci USA. 2000;97:11238–11243. doi: 10.1073/pnas.200376897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle G. Forms of modulatable tension in skeletal muscles. Comp Biochem Physiol. 1983;76A:203–210. doi: 10.1016/0300-9629(83)90316-x. [DOI] [PubMed] [Google Scholar]
- Hoyle G. Neuromuscular transmission in a primitive insect: modulation by octopamine, and catch-like tension. Comp Biochem Physiol. 1984;77C:219–32. doi: 10.1016/0742-8413(84)90005-7. [DOI] [PubMed] [Google Scholar]
- Hoyle G, Field LH. Elicitation and abrupt termination of behaviorally significant catchlike tension in a primitive insect. J Neurobiol. 1983;14:299–312. doi: 10.1002/neu.480140405. [DOI] [PubMed] [Google Scholar]
- Hoyle G, Lowy J. The paradox of Mytilus muscle. A new interpretation. J Exp Biol. 1956;33:295–310. [Google Scholar]
- Hozawa S. [On the sound organ of cicada] [in Japanese] Nipon Dobutsugaku. 1911;23:599–617. [Google Scholar]
- Humphrey GF. The adenosinetriphosphatase activity of myosins from marine animals. Physiol Comp Oecol. 1948;1:26–31. [PubMed] [Google Scholar]
- Humphrey GF. Adenosine triphosphatases in the adductor muscle of Saxstrea commercialis. Physiol Comp Oecol. 1949;1:366–375. [PubMed] [Google Scholar]
- Hutagalung AH, Landsverk ML, Price MG, Epstein HF. The UCS family of myosin chaperones. J Cell Sci. 2002;115:3983–3990. doi: 10.1242/jcs.00107. [DOI] [PubMed] [Google Scholar]
- Huxley AF. Cross-bridge action: present views, prospects, and unknowns. J Biomech. 2000a;33:1189–1195. doi: 10.1016/s0021-9290(00)00060-9. [DOI] [PubMed] [Google Scholar]
- Huxley AF. Mechanics and models of the myosin motor. Philos Trans R Soc Lond B Biol Sci. 2000b;355:433–440. doi: 10.1098/rstb.2000.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huxley HE. The crossbridge mechanism of muscular contraction and its implications. J Exp Biol. 1985;115:17–30. doi: 10.1242/jeb.115.1.17. [DOI] [PubMed] [Google Scholar]
- Huxley HE. Fifty years of muscle and the sliding filament hypothesis. Eur J Biochem. 2004;271:1403–1415. doi: 10.1111/j.1432-1033.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- Ikemoto N, Kawaguti S. Elongating effect of tropomyosin A on the thick myofilaments in the long-sarcomere muscle of the horse-shoe crab. Proc Jpn Acad. 1967;43:974–979. [Google Scholar]
- Inoue K, Sohma H, Morita F. Ca2+-dependent protein phosphatase which dephosphorylates regulatory light chain a in scallop smooth muscle myosin. J Biochem (Tokyo) 1990;107:872–878. doi: 10.1093/oxfordjournals.jbchem.a123141. [DOI] [PubMed] [Google Scholar]
- Irving T, Bhattacharya S, Tesic I, Moore J, Farman G, Simcox A, Vigoreaux J, Maughan D. Changes in myofibrillar structure and function produced by N-terminal deletion of the regulatory light chain in Drosophila. J Muscle Res Cell Motil. 2001;22:675–683. doi: 10.1023/a:1016336024366. [DOI] [PubMed] [Google Scholar]
- Irving TC, Maughan DW. In vivo X-ray diffraction of indirect flight muscle from Drosophila melanogaster. Biophys J. 2000;78:2511–2515. doi: 10.1016/S0006-3495(00)76796-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii N, Mitsumori F, Takahashi K. Changes in sarcoplasmic metabolite concentrations and pH associated with the catch contraction and relaxation of the anterior byssus retractor muscle of Mytilus edulis measured by P31 nuclear magnetic resonance. J Muscle Res Cell Motil. 1991;12:242–246. doi: 10.1007/BF01745113. [DOI] [PubMed] [Google Scholar]
- Ishii N, Simpson AW, Ashley CC. Free calcium at rest during “catch” in single smooth muscle cells. Science. 1989;243:1367–1368. doi: 10.1126/science.2922614. [DOI] [PubMed] [Google Scholar]
- Ishii N, Takahashi K. Polarity of myofilaments in molluscan smooth muscle. Cell Tissue Res. 1983;234:533–545. doi: 10.1007/BF00218649. [DOI] [PubMed] [Google Scholar]
- Ishii N, Tsuchiya T, Sugi H. An in vitro motility assay system retaining the steady-state force-velocity characteristics of muscle fibers under positive and negative loads. Biochim Biophys Acta. 1997;1319:155–162. [Google Scholar]
- Ishii Y, Takayanagi I. Effects of some ergot alkaloids on dopamine receptors of molluscan smooth muscle. Journal of Pharmacobio-Dynamics. 1982;5:748–750. doi: 10.1248/bpb1978.5.748. [DOI] [PubMed] [Google Scholar]
- Iwamoto H, Inoue K, Yagi N. Evolution of long-range myofibrillar crystallinity in insect flight muscle as examined by X-ray cryomicrodiffraction. Proc R Soc Lond Biol Sci. 2006;273:677–685. doi: 10.1098/rspb.2005.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwatsuki H. Modification of actomyosin ATPase by paramyosin: studies on the isolated myofilaments. Kawasaki Med J. 1981;7:1–17. [Google Scholar]
- Jackson AP, Bagshaw CR. Kinetic trapping of intermediates of the scallop heavy meromyosin adenosine triphosphatase reaction revealed by formycin nucleotides. Biochem J. 1988a;251:527–540. doi: 10.1042/bj2510527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AP, Bagshaw CR. Transient-kinetic studies of the adenosine triphosphatase activity of the scallop heavy meromyosin. Biochem J. 1988b;251:515–526. doi: 10.1042/bj2510515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AP, Warriner KE, Wells C, Bagshaw CR. The actin-activated ATPase of regulated and unregulated scallop heavy meromyosin. FEBS Lett. 1986;197:154–158. [Google Scholar]
- Jakes R, Northrop F, Kendrick-Jones J. Calcium binding regions of myosin ‘regulatory’ light chains. FEBS Lett. 1976;70:229–234. doi: 10.1016/0014-5793(76)80763-6. [DOI] [PubMed] [Google Scholar]
- Jakus MA, Hall CE, Schmitt FO. Electron microscopic observation of clam muscle fibrils. J Am Chem Soc. 1944;66:313–314. [Google Scholar]
- Jancsó A, Szent-Györgyi AG. Regulation of scallop myosin by the regulatory light chain depends on a single glycine residue. Proc Natl Acad Sci USA. 1994;91:8762–8766. doi: 10.1073/pnas.91.19.8762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell BR. The nature of the phasic and the tonic responses of the anterior byssal retractor muscle of Mytilus. J Physiol. 1959;149:154–177. doi: 10.1113/jphysiol.1959.sp006332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jewell BR, Rüegg JC. Oscillatory contraction of insect fibrillar muscle after glycerol extraction. Proc R Soc Lond Biol Sci. 1966;164:428–459. [Google Scholar]
- Johnson WH. Tonic mechanisms in smooth muscles. Physiol Rev. 1962;42(suppl 5):113–143. [PubMed] [Google Scholar]
- Johnson WH, Kahn JS. Titration of the protein paramyosin. Science. 1959;130:1190–1191. doi: 10.1126/science.130.3383.1190. [DOI] [PubMed] [Google Scholar]
- Johnson WH, Kahn JS, Szent-Györgyi AG. Paramyosin and contraction of “catch muscles”. Science. 1959;130:160–161. doi: 10.1126/science.130.3368.160. [DOI] [PubMed] [Google Scholar]
- Johnson WH, Twarog BM. The basis for prolonged contractions in molluscan muscles. J Gen Physiol. 1960;43:941–960. doi: 10.1085/jgp.43.5.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones MK, Yang W, McManus DP. Immunolocalization of the 38.3 kDa calponin-like protein in stratified muscles of the tail of Schistosoma japonicum cercariae. Parasitol Int. 2001;50:129–133. doi: 10.1016/s1383-5769(01)00060-5. [DOI] [PubMed] [Google Scholar]
- Jontes JD. Theories of muscle contraction. J Struct Biol. 1995;115:119–143. doi: 10.1006/jsbi.1995.1037. [DOI] [PubMed] [Google Scholar]
- Jordan HJ. Die Physiologie des Nerven-Muskelsystems bei den niederen Wirbellosen. Verhandlungsber Deutsch Zool Gesell. 1926;31:108–124. [Google Scholar]
- Jordan HJ. Die Funktion der glatten Muskeln bei Schnecken, verglichen mit den Funktionen des Protoplasmas bei Sarkodinen. Tijdschr nederl dierk Ver igg III. 1931;2:128–135. [Google Scholar]
- Josephson RK. Power output from a flight muscle of the bumblebee Bombus terrestris. II Characterization of the parameters affecting power output. J Exp Biol. 1997;200:1227–1239. doi: 10.1242/jeb.200.8.1227. [DOI] [PubMed] [Google Scholar]
- Josephson RK, Ellington C. Power output from a flight muscle of the bumblebee Bombus terrestris. I Some features of the dorso-ventral flight muscle. J Exp Biol. 1997;200:1215–26. doi: 10.1242/jeb.200.8.1215. [DOI] [PubMed] [Google Scholar]
- Josephson RK, Halverson RC. High frequency muscles used in sound production by a katydid. I Organization of the motor system. Biol Bull. 1971;141:411–433. [Google Scholar]
- Josephson RK, Malamud JG, Stokes DR. Asynchronous muscle: a primer. J Exp Biol. 2000;203:2713–2722. doi: 10.1242/jeb.203.18.2713. [DOI] [PubMed] [Google Scholar]
- Josephson RK, Stokes DR. Work-dependent deactivation of a crustacean muscle. J Exp Biol. 1999;202:2551–2565. doi: 10.1242/jeb.202.18.2551. [DOI] [PubMed] [Google Scholar]
- Josephson RK, Young D. Synchronous and asynchronous muscles in cicadas. J Exp Biol. 1981;91:219–237. [Google Scholar]
- Josephson RK, Young D. A synchronous insect muscle with an operating frequency greater than 500 Hz. J Exp Biol. 1985;118:185–208. [Google Scholar]
- Julian FJ. Activation in a skeletal muscle contraction model with a modification for insect fibrillar muscle. Biophys J. 1969;9:547–570. doi: 10.1016/S0006-3495(69)86403-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jülicher F, Prost J. Spontaneous oscillations of collective molecular motors. Phys Rev Lett. 1997;78:4510. [Google Scholar]
- Kagawa H, Gengyo K, McLachlan AD, Brenner S, Karn J. Paramyosin gene (unc15) of Caenorhabditis elegans. Molecular cloning, nucleotide sequence, and models for thick filament structure. J Mol Biol. 1989;207:311–333. doi: 10.1016/0022-2836(89)90257-x. [DOI] [PubMed] [Google Scholar]
- Kahn JS, Johnson WH. The localization of myosin and paramyosin in the myofilaments of the byssal retractor of Mytilus edulis. Arch Biochem Biophys. 1960;86:138–143. doi: 10.1016/0003-9861(60)90381-7. [DOI] [PubMed] [Google Scholar]
- Kalabokis VN, O’Neall-Hennessey E, Szent-Györgyi AG. Regulatory domains of myosins: influence of the heavy chain on Ca2+-binding. J Muscle Res Cell Motil. 1994;15:547–553. doi: 10.1007/BF00121160. [DOI] [PubMed] [Google Scholar]
- Kalabokis VN, Szent-Györgyi AG. Cooperativity and regulation of scallop myosin and myosin fragments. Biochemistry. 1997;36:15834–15840. doi: 10.1021/bi971932r. [DOI] [PubMed] [Google Scholar]
- Kalabokis VN, Szent-Györgyi AG. Regulation of scallop myosin by calcium. Cooperativity and the “off” state. Adv Exp Med Biol. 1998;453:235–240. doi: 10.1007/978-1-4684-6039-1_27. [DOI] [PubMed] [Google Scholar]
- Kalabokis VN, Vibert P, York ML, Szent-Györgyi AG. Single-headed scallop myosin and regulation. J Biol Chem. 1996;271:26779–26782. doi: 10.1074/jbc.271.43.26779. [DOI] [PubMed] [Google Scholar]
- Kalamkarova MB, Kriukova ME. Ultrastructural organization and features of protein components of the contractile apparatus of the Anodonta adductor. Biofizika. 1966;11:61–68. [PubMed] [Google Scholar]
- Kambara M, Shiraishi F, Ohtsuki I. Replacement of troponin C in fast skeletal myofibrils by troponin C from various muscles. Biomed Res. 1990;11:291–297. [Google Scholar]
- Kamiya S, Konno K. Calcium sensitivity of H-meromyosin and subfragment-1 from squid mantle muscle. Nippon Suisan Gakk. 1984;50:1889–1896. [Google Scholar]
- Kamiya S, Toshitomi B, Konno K, Watanabe S. Heavy meromyosin and subfragment-1 from squid mantle mysoin, and Ca-sensitivity of their Mg-ATPases. J Biochem (Tokyo) 1985;98:149–156. doi: 10.1093/oxfordjournals.jbchem.a135253. [DOI] [PubMed] [Google Scholar]
- Kanzawa N, Kawamura Y, Matsuno A, Maruyama K. Characterization of myosin isolated from bodywall smooth muscle of the annelid, Urechis unicinctus. Proc Jpn Acad. 1991;67B:176–180. [Google Scholar]
- Kanzawa N, Sato O, Takano-Ohmuro H, Maruyama K. Sea anemone (Actinia equina) myosin. Comp Biochem Physiol. 1993;104B:509–514. [Google Scholar]
- Katoh T. [Regulatory mechanism of smooth muscle myosin] [in Japanese] Seikagaku. 1999;71:290–294. [PubMed] [Google Scholar]
- Katoh T, Morita F. Conformation and activity of smooth muscle myosin probed by various essential light chains. J Biochem (Tokyo) 1997;121:56–62. doi: 10.1093/oxfordjournals.jbchem.a021570. [DOI] [PubMed] [Google Scholar]
- Kawai M, Brandt P, Orentlicher M. Dependence of energy transduction in intact skeletal muscles on the time in tension. Biophys J. 1977;18:161–172. doi: 10.1016/S0006-3495(77)85605-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Brandt PW. Two rigor states in skinned crayfish single muscle fibers. J Gen Physiol. 1976;68:267–280. doi: 10.1085/jgp.68.3.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Brandt PW. Effect of MgATP on stiffness measured at two frequencies in Ca2+-activated muscle fibers. Proc Natl Acad Sci USA. 1977;74:4073–4075. doi: 10.1073/pnas.74.9.4073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai M, Brandt PW. Sinusoidal analysis: a high resolution method for correlating biochemical reactions with physiological processes in activated skeletal muscles of rabbit, frog and crayfish. J Muscle Res Cell Motil. 1980;1:279–303. doi: 10.1007/BF00711932. [DOI] [PubMed] [Google Scholar]
- Kawakubo T, Okada O, Minami T. Molecular dynamics simulations of evolved collective motions of atoms in the myosin motor domain upon perturbation of the ATPase pocket. Biophys Chem. 2005;115:77–85. doi: 10.1016/j.bpc.2004.12.049. [DOI] [PubMed] [Google Scholar]
- Kay CM. Some physico-chemical properties of Pinna nobilis tropomyosin. Biochim Biophys Acta. 1958;27:469–477. doi: 10.1016/0006-3002(58)90374-3. [DOI] [PubMed] [Google Scholar]
- Kay CM. The partial specific volume of muscle proteins. Biochim Biophys Acta. 1960;38:420–427. doi: 10.1016/0006-3002(60)91277-4. [DOI] [PubMed] [Google Scholar]
- Kay CM, Bailey K. Further physico-chemical studies on Pinna nobilis tropomyosin. Biochim Biophys Acta. 1959;31:20–25. doi: 10.1016/0006-3002(59)90434-2. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J. Role of myosin light chains in calcium regulation. Nature. 1974;249:631– 634. doi: 10.1038/249631a0. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J, Cohen C, Szent-Györgyi AG, Longley W. Paramyosin: molecular length and assembly. Science. 1969;163:1196–1198. doi: 10.1126/science.163.3872.1196. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J, Dasilva ACR, Reinach FC, Messer N, Rowe T, McLaughlin P. Recombinant DNA approaches to study the role of the regulatory light chains (RLC) using scallop myosin as a test system. J Cell Sci. 1991;14:55–58. doi: 10.1242/jcs.1991.supplement_14.11. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J, Lehman W, Szent-Györgyi AG. Regulation in molluscan muscles. J Mol Biol. 1970;54:313–326. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- Kendrick-Jones J, Scholey JM. Myosin-linked regulatory systems. J Muscle Res Cell Motil. 1981;2:347–372. [Google Scholar]
- Kendrick-Jones J, Szentkiralyi EM, Szent-Györgyi AG. Myosin-linked regulatory systems: the role of the light chains. Cold Spring Harb Symp Quant Biol. 1973;37:47–53. [Google Scholar]
- Kendrick-Jones J, Szentkiralyi EM, Szent-Györgyi AG. Regulatory light chains in myosins. J Mol Biol. 1976;104:747–775. doi: 10.1016/0022-2836(76)90180-7. [DOI] [PubMed] [Google Scholar]
- Kensler RW, Levine RJC. An electron microscopic and optical diffraction analysis of the structure of Limulus telson muscle thick filaments. J Cell Biol. 1982a;92:443–451. doi: 10.1083/jcb.92.2.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kensler RW, Levine RJC. Determination of the handedness of the crossbridge helix of Limulus thick filaments. J Muscle Res Cell Motil. 1982b;3:349–361. doi: 10.1007/BF00713042. [DOI] [PubMed] [Google Scholar]
- Kensler RW, Levine RJC, Stewart M. Electron microscopic and optical diffraction analysis of the structure of scorpion muscle thick filaments. J Cell Biol. 1985;101:395–401. doi: 10.1083/jcb.101.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick WG, Bolles LL. Regulation of Ca2+-activated tension in Limulus striated muscle. Pflügers Arch. 1981;392:121–124. doi: 10.1007/BF00581259. [DOI] [PubMed] [Google Scholar]
- Kerrick WG, Hoar PE, Cassidy PS, Bolles L, Malencik DA. Calcium-regulatory mechanisms functional classification using skinned fibers. J Gen Physiol. 1981;77:177–190. doi: 10.1085/jgp.77.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerrick WGL, Bolles LL. Evidence that myosin light chain phosphorylation regulates contraction in the body wall muscles of the sea cucumbers. J Cell Physiol. 1982;112:307–315. doi: 10.1002/jcp.1041120302. [DOI] [PubMed] [Google Scholar]
- Kerwin BA, Yount RG. Photoaffinity labeling of scallop myosin with 2-[(4-azido-2-nitrophenyl)amino]ethyl diphosphate: identification of an active site arginine analogous to tryptophan-130 in skeletal muscle myosin. Bioconjugate Chem. 1992;3:328–336. doi: 10.1021/bc00016a012. [DOI] [PubMed] [Google Scholar]
- Kerwin BA, Yount RG. Photolabeling evidence for calcium-induced conformational changes at the ATP binding site of scallop myosin. Proc Natl Acad Sci USA. 1993;90:35–39. doi: 10.1073/pnas.90.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura I, Yoshitomi B, Konno K, Arai KI. Preparation of highly purified myosin from mantle muscle of squid, Ommastrephes sloani pacificus [in Japanese, English summary] Nippon Suisan Gakk. 1980;46:885–892. [Google Scholar]
- Kimura K, Tanaka T, Nakae H, Obinata T. Troponin from nematode: purification and characterization of troponin from Ascaris body wall muscle. Comp Biochem Physiol. 1987;88B:399–407. [Google Scholar]
- Kishimoto U. ATP-ase activities of adductor and byssus retractor muscles of marine molluscs, Mytilus and Modiolus. Comp Biochem Physiol. 1961;2:81–89. doi: 10.1016/0010-406x(61)90141-4. [DOI] [PubMed] [Google Scholar]
- Kishimura H, Ojima T, Nishita K. Hybridization experiments using fish myosin light chains and desensitized Akazara myosin [in Japanese, English summary] Nippon Suisan Gakk. 1986;52:847–851. [Google Scholar]
- Klug A, Crick FHC, Wyckoff HW. Diffraction by helical structures. Acta Crystallogr. 1958;11:199–213. [Google Scholar]
- Kobayashi T, Ichikawa C, Sugi H. Differential effects of sinusoidal vibrations on tension and stiffness in Mytilus smooth muscle during catch state. Jpn J Physiol. 1985;35:689–692. doi: 10.2170/jjphysiol.35.689. [DOI] [PubMed] [Google Scholar]
- Kobayashi T, Takagi T, Konishi K, Wnuk W. Amino acid sequences of the two major isoforms of troponin C from crayfish. J Biol Chem. 1989;264:18247–18259. [PubMed] [Google Scholar]
- Kobayashi T, Ushitani H, Wada H, Inoue J, Kawakubo T, Sugi H. Effect of mechanical vibration on active tension in the longitudinal retractor muscle of a sea cucumber Stichopus Japonicus. J Exp Biol. 1994;194:319–28. doi: 10.1242/jeb.194.1.319. [DOI] [PubMed] [Google Scholar]
- Kodama S, Konno K. Isolation and biochemical properties of myosin from squid brachial muscle [in Japanese, English summary] Nippon Suisan Gakk. 1983;49:437–442. [Google Scholar]
- Koenders A, Lamey TM, Medler S, West JM, Mykles DL. Two fast-type fibers in claw closer and abdominal deep muscles of the Australian freshwater crustacean, Cherax destructor, differ in Ca2+ sensitivity and troponin-I isoforms. J Exp Zool. 2004;301A:588–598. doi: 10.1002/jez.a.86. [DOI] [PubMed] [Google Scholar]
- Köhler G, Lindl T. Effects of 5-hydroxytryptamine, dopamine, and acetlycholine on accumulation of cyclic AMP and cyclic GMP in the anterior byssus retractor muscle of Mytilus edulis L. (mollusca) Pflügers Arch. 1980;383:257–262. doi: 10.1007/BF00587528. [DOI] [PubMed] [Google Scholar]
- Kölliker A. Zur Kenntnis der quergestreiften Muskelfasern. Z wiss Zool. 1888;47:689–710. [Google Scholar]
- Kölsch B, Ziegler C, Beinbrech G. Length determination of synthetic thick filaments by cooperation of two myosin associated proteins, paramyosin and projectin. Naturwissenschaften. 1995;82:239–241. doi: 10.1007/BF01133600. [DOI] [PubMed] [Google Scholar]
- Kometani K, Sugi H. Calcium transients in a molluscan smooth muscle. Experientia. 1978;34:1469–1470. [Google Scholar]
- Kominz DR, Maruyama K. Does native tropomyosin bind to myosin? J Biochem (Tokyo) 1967;61:269–271. doi: 10.1093/oxfordjournals.jbchem.a128542. [DOI] [PubMed] [Google Scholar]
- Kominz DR, Saad F, Laki K. Vertebrate and invertebrate tropomyosins. Nature. 1957;179:206–207. doi: 10.1038/179206b0. [DOI] [PubMed] [Google Scholar]
- Kominz DR, Saad F, Laki K. Chemical characterizations of annelid, mollusc and arthropod tropomyosins. Med Sci. 1958;9:66–76. [Google Scholar]
- Kondo S, Asakawa T, Morita F. Difference UV-absorption spectrums of scallop adductor myosin induced by ATP. J Biochem (Tokyo) 1979;86:1567–1571. doi: 10.1093/oxfordjournals.jbchem.a132674. [DOI] [PubMed] [Google Scholar]
- Kondo S, Morita F. Smooth muscle of scallop adductor contains at least two kinds of myosin. J Biochem (Tokyo) 1981;90:673–681. doi: 10.1093/oxfordjournals.jbchem.a133522. [DOI] [PubMed] [Google Scholar]
- Konno K. Two calcium regulation systems in squid (Ommastrephes sloani pacificus) muscle. Preparation of calcium-sensitive myosin and troponin-tropomyosin. J Biochem (Tokyo) 1978;84:1431–1440. doi: 10.1093/oxfordjournals.jbchem.a132265. [DOI] [PubMed] [Google Scholar]
- Konno K. Complex formation between regulatory and essential light chain on squid mantle myosin subfragment-1 revealed by thermal denaturation method. J Biochem (Tokyo) 1991a;109:816–821. doi: 10.1093/oxfordjournals.jbchem.a123464. [DOI] [PubMed] [Google Scholar]
- Konno K. Thermal denaturation of squid myofibrils: effects of calcium ion and EDTA. Nippon Suisan Gakk. 1991b;57:2145–2149. [Google Scholar]
- Konno K. Thiols in scallop (Patinopecten yessoensis) myosin and regulatory light chain binding. Comp Biochem Physiol. 1991c;99B:161–164. [Google Scholar]
- Konno K, Arai K, Watanabe S. Myosin-linked calcium regulation in squid mantle muscle. J Biochem (Tokyo) 1979;86:1639–1650. doi: 10.1093/oxfordjournals.jbchem.a132684. [DOI] [PubMed] [Google Scholar]
- Konno K, Arai K, Watanabe S. Calcium regulation in squid mantle and scallop adductor muscles. J Biochem (Tokyo) 1981;89:581–589. doi: 10.1093/oxfordjournals.jbchem.a133234. [DOI] [PubMed] [Google Scholar]
- Konno K, Arai KI, Watanabe S. Fluorescence intensity and UV absorption changes accompanying dissociation and association of regulatory light chain of scallop adductor myosin. J Biochem (Tokyo) 1983a;94:1061–1066. doi: 10.1093/oxfordjournals.jbchem.a134448. [DOI] [PubMed] [Google Scholar]
- Konno K, Kodama S, Arai K, Watanabe S. Changes in reactivities of scallop adductor myosin with 5,5′-dithiobis-(2-nitrobenzoate) and with 2,4,6-trinitrobenzene sulfonate accompanying dissociation and association of regulatory light chain. J Biochem (Tokyo) 1983b;94:1399–1407. doi: 10.1093/oxfordjournals.jbchem.a134486. [DOI] [PubMed] [Google Scholar]
- Konno K, Watanabe S. Effect of regulatory light chain on chymotryptic digestion of scallop adductor myosin. J Biochem (Tokyo) 1985a;97:1645–1651. doi: 10.1093/oxfordjournals.jbchem.a135222. [DOI] [PubMed] [Google Scholar]
- Konno K, Watanabe S. Two different preparations of subfragment-1 from scallop adductor myosin. J Biochem (Tokyo) 1985b;98:141–148. doi: 10.1093/oxfordjournals.jbchem.a135252. [DOI] [PubMed] [Google Scholar]
- Korchagin VP. Effect of Mg2+ on light transmission of a suspension of myofibrils from the phase portion of the Japanese scallop (Mizuhopecten yessoensis) adductor muscle under conditions of relaxation. Biochemistry (Moscow) 1995;60:1277–1283. [Google Scholar]
- Krause S, Munson NL. Preparation problems unique to Mercenaria paramyosin. Methods Enzymol. 1982;85:160–164. doi: 10.1016/0076-6879(82)85019-2. [DOI] [PubMed] [Google Scholar]
- Krueger JK, Gallagher SC, Wang CLA, Trewhella J. Calmodulin remains extended upon binding to smooth muscle caldesmon: a combined small-angle scattering and Fourier transform infrared spectroscopy study. Biochemistry. 2000;39:3979–3987. doi: 10.1021/bi992638x. [DOI] [PubMed] [Google Scholar]
- Kryukova MY. The structure of the thick protofibrils of the adductor of bivalves. Biophysics. 1968;13:1038–1042. [Google Scholar]
- Kubo S. Squid tropomyosins [in Japanese] Mem Fac Fisheries, Hokkaido Univ. 1961;9:57–83. [Google Scholar]
- Kubota K, Chu B, Fan SF, Dewey MM, Brink P, Colflesh DE. Quasi-elastic light scattering of suspensions of Limulus thick myofilaments in relaxed (long) activated and rerelaxed (short) states. J Mol Biol. 1983;166:329–340. doi: 10.1016/s0022-2836(83)80088-6. [DOI] [PubMed] [Google Scholar]
- Kuhn HJ. Transformation of chemical into mechanical energy by glycerol-extracted fibres of insect flight muscles in the absence of nucleosidetriphosphate-hydrolysis. Experientia. 1973;29:1086–1088. doi: 10.1007/BF01946734. [DOI] [PubMed] [Google Scholar]
- Kuhn HJ. Cross bridge slippage induced by the ATP-analogue AMP-PNP and stretch in glycerol extracted fibrillar muscle fibres. Biophys Struct Mech. 1978a;4:159–163. doi: 10.1007/BF00539229. [DOI] [PubMed] [Google Scholar]
- Kuhn HJ. Tension transients in fibrillar muscle fibres as affected by stretch-dependent binding of AMP-PNP: a teinochemical effect? Biophys Struct Mech. 1978b;4:209–222. doi: 10.1007/BF02426086. [DOI] [PubMed] [Google Scholar]
- Kuhn HJ, Bletz C, Guth K, Rüegg JC. The effect of MgATP on forming and breaking actin myosin linkages in contracted skinned insect flight muscle fibers. J Muscle Res Cell Motil. 1985;6:5–27. doi: 10.1007/BF00712308. [DOI] [PubMed] [Google Scholar]
- Kuhn HJ, Schröder H, Rüegg JC. Force generation in glycerinated insect-flight muscles without ATP. Experientia. 1972;28:510–511. doi: 10.1007/BF01931847. [DOI] [PubMed] [Google Scholar]
- Kulke M, Neagoe C, Kolmerer B, Minajeva A, Hinssen H, Bullard B, Linke WA. Kettin, a major source of myofibrillar stiffness in Drosophila indirect flight muscle. J Cell Biol. 2001;154:1045–1057. doi: 10.1083/jcb.200104016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurzawa-Goertz SE, Perreault-Micale CL, Trybus KM, Szent-Györgyi AG, Geeves MA. Loop I can modulate ADP affinity, ATPase activity, and motility of different scallop myosins. Transient kinetic analysis of S1 isoforms. Biochemistry. 1998;37:7517–7525. doi: 10.1021/bi972844+. [DOI] [PubMed] [Google Scholar]
- Kuschinski G, Turba F. Über den Chemismus von Zustandsänderungen des Aktomyosins. Experientia. 1950;6:103–106. [Google Scholar]
- Kwon H, Goodwin EB, Nyitray L, Berliner E, O’Neall-Hennessey E, Melandri FD, Szent-Györgyi AG. Isolation of the regulatory domain of scallop myosin: role of the essential light chain in calcium binding. Proc Natl Acad Sci USA. 1990;87:4771–4775. doi: 10.1073/pnas.87.12.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaConte LEW, Baker JE, Thomas DD. Transient kinetics and mechanics of myosin’s force-generating rotation in muscle: resolution of millisecond rotational transitions in the spin-labeled myosin light-chain domain. Biochemistry. 2003;42:9797–9803. doi: 10.1021/bi034288r. [DOI] [PubMed] [Google Scholar]
- Lajtha A. The muscle proteins of invertebrates. Pubbl Staz Zool Napoli. 1947;20:226–231. [Google Scholar]
- Laki K. A simple method for the isolation and crystallization of tropomyosin from the muscles of the clam, Venus mercenaria. Arch Biochem Biophys. 1957;67:240–242. doi: 10.1016/0003-9861(57)90263-1. [DOI] [PubMed] [Google Scholar]
- Laki K, Horváth B, Klatzo I. On the relationship between myosin and tropomyosin A. Biochim Biophys Acta. 1958;28:656–657. doi: 10.1016/0006-3002(58)90542-0. [DOI] [PubMed] [Google Scholar]
- Landsverk ML, Epstein HF. Genetic analysis of myosin II assembly and organization in model organisms. Cell Mol Life Sci. 2005;62:2270–2282. doi: 10.1007/s00018-005-5176-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer M, Giebing T, D’Haese J. Purification and functional characterization of an 85-kDa gelsolin from the ascidians Microcosmus sulcatus and Phallusia mammilata. Comp Biochem Physiol. 1998;119B:697–704. doi: 10.1016/s0305-0491(98)00045-5. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia G. Observazioni sull’ ultrastruttura del miofilamento paramiosinico nei Mulluschi. Lincei-Rend Sci Fis mat et nat. 1966;41:374–379. [Google Scholar]
- Lanzavecchia G. The «smooth» muscle of invertebrates. Boll Zool. 1972;39:159–172. [Google Scholar]
- Lanzavecchia G. Morphological modulations in helical muscles (Aschelminthes and Annelida) Int Rev Cytol. 1977;51:133–186. doi: 10.1016/s0074-7696(08)60227-2. [DOI] [PubMed] [Google Scholar]
- Lanzavecchia G, de Eguileor M. Studies on the helical and paramyosinic muscles. V Ultrastructural morphology and contraction speed of muscular fibres of Erpobcella octoculata and Erpobcella testacea (Annelida, Hirudina) J Submicrosc Cytol. 1976;8:69–88. [Google Scholar]
- Lanzavecchia G, Valvassori R, de Eguileor M. Bipolarity in thick filaments of Nematomorpha. J Mol Biol. 1977;111:371–374. doi: 10.1016/s0022-2836(77)80059-4. [DOI] [PubMed] [Google Scholar]
- Lee SCK, Becker CN, Binder-Macleod SA. Catchlike-inducing train activation of human muscle during isotonic contractions: burst modulation. J Appl Physiol. 1999a;87:1758–1767. doi: 10.1152/jappl.1999.87.5.1758. [DOI] [PubMed] [Google Scholar]
- Lee SCK, Gerdom ML, Binder-Macleod SA. Effects of length on the catchlike property of human quadriceps femoris muscle. Phys Ther. 1999b;79:738–748. [PubMed] [Google Scholar]
- Leenders HJ. Kontraktion und Spanningsruckstand an glycerinextrahierten Muskelfasern (ABRM) von Mytilus edulis. Naturwissenschaften. 1966;53:617. doi: 10.1007/BF00632287. [DOI] [PubMed] [Google Scholar]
- Leenders HJ. Der Einfluss der Sperrung auf die Kontraktion. Untersuchungen am ABRM von Mytilus edulis. L Pflügers Arch. 1967;295:127–135. [PubMed] [Google Scholar]
- Leenders HJ. Catch, peak tension and ATPase activity in glycerinated oyster adductor. Comp Biochem Physiol. 1969;31:187–196. doi: 10.1016/0010-406x(69)91646-6. [DOI] [PubMed] [Google Scholar]
- Lehman W. Hybrid troponin reconstituted from vertebrate and arthopod subunits. Nature. 1975;255:424–426. doi: 10.1038/255424a0. [DOI] [PubMed] [Google Scholar]
- Lehman W. Phylogenetic diversity of the proteins regulating muscular contraction. Int Rev Cytol. 1976;44:55–92. doi: 10.1016/s0074-7696(08)61647-2. [DOI] [PubMed] [Google Scholar]
- Lehman W. Calcium ion-dependent myosin from decapod crustacean muscles. Biochem J. 1977;163:291–296. doi: 10.1042/bj1630291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman W. Thin-filament-linked regulation in molluscan muscles. Biochim Biophys Acta. 1981;668:349–356. doi: 10.1016/0005-2795(81)90168-9. [DOI] [PubMed] [Google Scholar]
- Lehman W. The location and periodicity of a troponin-T-like protein in the myofibril of the horseshoe crab Limulus polyphemus. J Mol Biol. 1982;154:385–391. doi: 10.1016/0022-2836(82)90071-7. [DOI] [PubMed] [Google Scholar]
- Lehman W. The distribution of troponin-like proteins on thin filaments of the bay scallop, Aequipecten irradians. J Muscle Res Cell Motil. 1983a;4:379–389. doi: 10.1007/BF00712003. [DOI] [PubMed] [Google Scholar]
- Lehman W. The ionic requirements for regulation by molluscan thin filaments. Biochim Biophys Acta. 1983b;745:1–5. doi: 10.1016/0167-4838(83)90162-0. [DOI] [PubMed] [Google Scholar]
- Lehman W, Bullard B, Hammond K. Calcium-dependent myosin from insect flight muscles. J Gen Physiol. 1974;63:553–563. doi: 10.1085/jgp.63.5.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman W, Craig R, Vibert P. Ca2+-induced tropomyosin movement in Limulus thin filaments revealed by three-dimensional reconstruction. Nature. 1994;368:65–67. doi: 10.1038/368065a0. [DOI] [PubMed] [Google Scholar]
- Lehman W, Hatch V, Korman V, Rosol M, Thomas L, Maytum R, Geeves MA, Van Eyk JE, Tobacman LS, Craig R. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol. 2000;302:593–606. doi: 10.1006/jmbi.2000.4080. [DOI] [PubMed] [Google Scholar]
- Lehman W, Head JF, Grant PW. The stoichiometry and location of troponin I- and troponin C-like proteins in the myofibril of the bay scallop, Aequipecten irradians. Biochem J. 1980;187:447–456. doi: 10.1042/bj1870447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman W, Kendrick-Jones J, Szent-Györgyi AG. Myosin-linked regulatory systems: comparative studies. Cold Spring Harb Symp Quant Biol. 1972;37:319–330. [Google Scholar]
- Lehman W, Registein JM, Ransom AL. The stoichiometry of the components of arthropod thin filaments. Biochim Biophys Acta. 1976;434:215–222. doi: 10.1016/0005-2795(76)90053-2. [DOI] [PubMed] [Google Scholar]
- Lehman W, Rosol M, Tobacman LS, Craig R. Troponin organization on relaxed and activated thin filaments revealed by electron microscopy and three-dimensional reconstruction. J Mol Biol. 2001;307:739–744. doi: 10.1006/jmbi.2001.4514. [DOI] [PubMed] [Google Scholar]
- Lehman W, Szent-Györgyi AG. Activation of the adenosine triphosphatase of Limulus polyphemus actomyosin by tropomyosin. J Gen Physiol. 1972;59:375–387. doi: 10.1085/jgp.59.4.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman W, Szent-Györgyi AG. Regulation of muscular contraction. Distribution of actin control and myosin control in the animal kingdom. J Gen Physiol. 1975;66:1–30. doi: 10.1085/jgp.66.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehman W, Vibert P, Craig R. Visualization of caldesmon on smooth muscle thin filaments. J Mol Biol. 1997;274:310–317. doi: 10.1006/jmbi.1997.1422. [DOI] [PubMed] [Google Scholar]
- Levine RJC. Evidence for overlapping myosin heads on relaxed thick filaments of fish, frog, and scallop striated muscles. J Struct Biol. 1993;110:99–110. doi: 10.1006/jsbi.1993.1010. [DOI] [PubMed] [Google Scholar]
- Levine RJC. Differences in myosin head arrangement on relaxed thick filaments from Lethocerus and rabbit muscles. J Muscle Res Cell Motil. 1997;18:529–543. doi: 10.1023/a:1018611201639. [DOI] [PubMed] [Google Scholar]
- Levine RJC, Chantler PD, Kensler RW. Arrangement of myosin heads on Limulus thick filaments. J Cell Biol. 1988;107:1739–1747. doi: 10.1083/jcb.107.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJC, Chantler PD, Kensler RW, Woodhead JL. Effects of phosphorylation by myosin light chain kinase on the structure of Limulus thick filaments. J Cell Biol. 1991a;113:563–572. doi: 10.1083/jcb.113.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJC, Dewey MM, Colflesh DE. Unusual striated thick filaments in Limulus skeletal muscle. J Cell Biol. 1973;57:591–593. doi: 10.1083/jcb.57.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJC, Dewey MM, de Villafranca GW. Immunohistochemical localization of contractile proteins in Limulus striated muscle. J Cell Biol. 1972;55:221–235. doi: 10.1083/jcb.55.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJC, Elfvin M, Dewey MM, Walcott B. Paramyosin in invertebrate muscles. II Content in relation to structure and function. J Cell Biol. 1976;71:273–279. doi: 10.1083/jcb.71.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJC, Kensler RW. Structure of short thick filaments from Limulus muscle. J Mol Biol. 1985;182:347–352. doi: 10.1016/0022-2836(85)90351-1. [DOI] [PubMed] [Google Scholar]
- Levine RJC, Kensler RW, Levitt P. Crossbridge and backbone structure in invertebrate thick filaments. Biophys J. 1986;49:135–138. doi: 10.1016/S0006-3495(86)83624-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJC, Kensler RW, Reedy MC, Hofmann W, King HA. Structure and paramyosin content of tarantula thick filaments. J Cell Biol. 1983;97:186–195. doi: 10.1083/jcb.97.1.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine RJC, Woodhead JL, King HA. The effect of calcium activation of skinned fiber-bundles on the structure of Limulus thick filaments. J Cell Biol. 1991b;113:573–583. doi: 10.1083/jcb.113.3.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Brown JH, Reshetnikova L, Blazsek A, Farkas L, Nyitray L, Cohen C. Visualization of an unstable coiled coil from the scallop myosin rod. Nature. 2003;424:341–345. doi: 10.1038/nature01801. [DOI] [PubMed] [Google Scholar]
- Lidke DS, Thomas DD. Coordination of the two heads of myosin during muscle contraction. Proc Natl Acad Sci USA. 2002;99:14801–14806. doi: 10.1073/pnas.232161999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linari M, Reedy MK, Reedy MC, Lombardi V, Piazzesi G. Ca-activation and stretch-activation in insect flight muscle. Biophys J. 2004;87:1101–1111. doi: 10.1529/biophysj.103.037374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Littlefield KP, Swank DM, Sanchez BM, Knowles AF, Warshaw DM, Bernstein SI. The converter domain modulates kinetic properties of Drosophila myosin. Am J Physiol Cell Physiol. 2003;284:C1031–C1038. doi: 10.1152/ajpcell.00474.2002. [DOI] [PubMed] [Google Scholar]
- Littlefield R, Fowler VM. Defining actin filament length in striated muscle: Rulers and caps or dynamic stability? Annu Rev Cell Dev Biol. 1998;14:487–525. doi: 10.1146/annurev.cellbio.14.1.487. [DOI] [PubMed] [Google Scholar]
- Liu FZ, Barral JM, Bauer CC, Ortiz I, Cook RG, Schmid MF, Epstein HF. Assemblases and coupling proteins in thick filament assembly. Cell Struct Funct. 1997;22:155–162. doi: 10.1247/csf.22.155. [DOI] [PubMed] [Google Scholar]
- Liu FZ, Bauer CC, Ortiz I, Cook RG, Schmid MF, Epstein HF. β-filagenin, a newly identified protein coassembling with myosin and paramyosin in Caenorhabditis elegans. J Cell Biol. 1998;140:347–353. doi: 10.1083/jcb.140.2.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu FZ, Ortiz I, Hutagalung A, Bauer CC, Cook RG, Epstein HF. Differential assembly of α- and γ-filagenins into thick filaments in Caenorhabditis elegans. J Cell Sci. 2000;113:4001–4012. doi: 10.1242/jcs.113.22.4001. [DOI] [PubMed] [Google Scholar]
- Liu HJ, Miller MS, Swank DM, Kronert WA, Maughan DW, Bernstein SI. Paramyosin phosphorylation site disruption affects indirect flight muscle stiffness and power generation in Drosophila melanogaster. Proc Natl Acad Sci USA. 2005;102:10522–10527. doi: 10.1073/pnas.0500945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Reedy MC, Goldman YE, Franzini-Armstrong C, Sasaki H, Tregear RT, Lucaveche C, Winkler H, Baumann BAJ, Squire JM, Irving TC, Reedy MK, Taylor KA. Electron tomography of fast frozen, stretched rigor fibers reveals elastic distortions in the myosin crossbridges. J Struct Biol. 2004;147:268–282. doi: 10.1016/j.jsb.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Liu J, Wu SP, Reedy MC, Winkler H, Lucaveche C, Cheng YF, Reedy MK, Taylor KA. Electron tomography of swollen rigor fibers of insect flight muscle reveals a short and variably angled S2 domain. J Mol Biol. 2006;362:844–860. doi: 10.1016/j.jmb.2006.07.084. [DOI] [PubMed] [Google Scholar]
- Liu X, Pollack GH. Stepwise sliding of single actin and myosin filaments. Biophys J. 2004;86:353–358. doi: 10.1016/S0006-3495(04)74111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljung B, Hallgren P. On the mechanism of inhibitory action of vibrations as studied in a molluscan catch muscle and in vertebrate vascular smooth muscle. Acta Physiol Scand. 1975;95:424–430. doi: 10.1111/j.1748-1716.1975.tb10070.x. [DOI] [PubMed] [Google Scholar]
- Locker RH, Schmitt FO. Some chemical and structural properties of paramyosin. J Biophys Biochem Cytol. 1957;3:889–896. doi: 10.1083/jcb.3.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowey S. Comparative study of the α-helical muscle proteins. Tyrosyl titration and effect of pH on conformation. J Biol Chem. 1965;240:2421–2427. [PubMed] [Google Scholar]
- Lowey S, Kucera J, Holtzer A. On the structure of the paramyosin molecule. J Mol Biol. 1963;7:234–244. doi: 10.1016/s0022-2836(63)80003-0. [DOI] [PubMed] [Google Scholar]
- Lowy J. Contraction and relaxation in the adductor muscles of Mytilus edulis. J Physiol. 1953;120:129–140. doi: 10.1113/jphysiol.1953.sp004879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy J. Contraction and relaxation in the adductor muscles of Pecten maximus. J Physiol. 1954;124:100–105. doi: 10.1113/jphysiol.1954.sp005088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowy J. The lamellibranch muscle. Contractile mechanism. Nature. 1955;176:345–346. doi: 10.1038/176345a0. [DOI] [PubMed] [Google Scholar]
- Lowy J, Hanson J. Ultrastructure of invertebrate smooth muscles. Physiol Rev. 1962;42:34–47. [PubMed] [Google Scholar]
- Lowy J, Millman BM. Contraction and relaxation in smooth muscles of lamellibranch molluscs. Nature. 1959;183:1730–1731. doi: 10.1038/1831730a0. [DOI] [PubMed] [Google Scholar]
- Lowy J, Millman BM. The contractile mechanism of the anterior byssus retractor muscle of Mytilus edulis. Philos Trans R Soc Lond B Biol Sci. 1963;246:105–148. [Google Scholar]
- Lowy J, Millman BM, Hanson J. Structure and function in smooth tonic muscles of lamellibranch molluscs. Proc R Soc Lond Biol Sci. 1964;160:525–536. doi: 10.1098/rspb.1964.0068. [DOI] [PubMed] [Google Scholar]
- Lowy J, Poulsen FR. Time-resolved X-ray diffraction studies of the structural behavior of myosin heads in a living contracting unstriated muscle. Nature. 1982;299:308–312. doi: 10.1038/299308a0. [DOI] [PubMed] [Google Scholar]
- Lowy J, Vibert PJ. Structure and organization of actin in a molluscan smooth muscle. Nature. 1967;215:1254–1255. doi: 10.1038/2151254a0. [DOI] [PubMed] [Google Scholar]
- Lowy J, Vibert PJ. Studies of the low-angle X-ray pattern of a molluscan smooth muscle during tonic contraction and rigor. Cold Spring Harb Symp Quant Biol. 1972;37:353–359. [Google Scholar]
- Loxdale HD, Tregear RT. Dissociation between mechanical performance and the cost of isometric tension maintenance in Lethocerus flight muscle. J Muscle Res Cell Motil. 1985;6:163–175. doi: 10.1007/BF00713058. [DOI] [PubMed] [Google Scholar]
- Lucas AHS. On the sound organs of the green cicada, Cyclochila australasiae, Donovan sp. Trans Roy Soc Vict. 1887;23:173–178. [Google Scholar]
- Lucic V, Förster F, Baumeister W. Structural studies by electron tomography: from cells to molecules. Annu Rev Biochem. 2005;74:833–865. doi: 10.1146/annurev.biochem.73.011303.074112. [DOI] [PubMed] [Google Scholar]
- Lück A, D’Haese J, Hinssen H. A gelsolin-related protein from lobster muscle: cloning, sequence analysis and expression. Biochem J. 1995;305:767–775. doi: 10.1042/bj3050767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund J, Webb MR, White DCS. Changes in the ATPase activity of insect fibrillar flight muscle during sinusoidal length oscillation probed by phosphate-water oxygen exchange. J Biol Chem. 1988;263:5505–5511. [PubMed] [Google Scholar]
- Machin KE. The electronic simulation of the load applied to an insect muscle. Elect Eng. 1959;31:740–744. [Google Scholar]
- Machin KE, Pringle JWS. The physiology of insect fibrillar muscle. II Mechanical properties of a beetle flight muscle. Proc R Soc Lond Biol Sci. 1959;151:204–225. doi: 10.1098/rspb.1960.0041. [DOI] [PubMed] [Google Scholar]
- Machin KE, Pringle JWS. The physiology of insect fibrillar muscle. III The effect of sinusoidal changes of length on a beetle flight muscle. Proc R Soc Lond Biol Sci. 1960;152:311–330. doi: 10.1098/rspb.1960.0041. [DOI] [PubMed] [Google Scholar]
- Machin KE, Pringle JWS, Tamasige M. The physiology of insect fibrillar muscle. IV The effect of temperature on a beetle flight muscle. Proc R Soc Lond Biol Sci. 1962;155:493–499. doi: 10.1098/rspb.1960.0041. [DOI] [PubMed] [Google Scholar]
- Maéda Y. X-ray diffraction patterns from molecular arrangements with 38-nm periodicities around muscle thin filaments. Nature. 1979;277:670–672. doi: 10.1038/277670a0. [DOI] [PubMed] [Google Scholar]
- Maéda Y. The arrangement of myosin heads in relaxed crab muscle. Nature. 1983;302:69–72. doi: 10.1038/302069a0. [DOI] [PubMed] [Google Scholar]
- Maéda Y, Matsubara I, Yagi N. Structural changes in thin filaments of crab striated muscle. J Mol Biol. 1979;127:191–201. doi: 10.1016/0022-2836(79)90239-0. [DOI] [PubMed] [Google Scholar]
- Málnási-Csizmadia A, Hegyi G, Tolgyesi F, Szent-Györgyi AG, Nyitray L. Fluorescence measurements detect changes in scallop myosin regulatory domain. Eur J Biochem. 1999;261:452–458. doi: 10.1046/j.1432-1327.1999.00290.x. [DOI] [PubMed] [Google Scholar]
- Málnási-Csizmadia A, Shimony E, Hegyi G, Szent-Györgyi AG, Nyitray L. Dimerization of the head-rod junction of scallop myosin. Biochem Biophys Res Commun. 1998;252:595–601. doi: 10.1006/bbrc.1998.9603. [DOI] [PubMed] [Google Scholar]
- Mannherz HG. ATP-Spaltung und ATP-Diffusion in oscilleirenden extrahierten Muskelfasern. Pflügers Arch. 1968;303:230–248. doi: 10.1007/BF01890903. [DOI] [PubMed] [Google Scholar]
- Mannherz HG. On the reversibility of the biochemical reactions of muscular contraction during the absorption of negative work. FEBS Lett. 1970;10:233–236. doi: 10.1016/0014-5793(70)80636-6. [DOI] [PubMed] [Google Scholar]
- Marchand-Dumont G, Baguet F. The control mechanism of relaxation in molluscan catch-muscle (ABRM) Pflügers Arch. 1975;354:87–100. doi: 10.1007/BF00584505. [DOI] [PubMed] [Google Scholar]
- Mardahl-Dumesnil M, Fowler VM. Thin filaments elongate from their pointed ends during myofibril assembly in Drosophila indirect flight muscle. J Cell Biol. 2001;155:1043–1053. doi: 10.1083/jcb.200108026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis BA, Bobrova IF, Mashanski VF, Pinaev GP. Major myofibrillar protein content and the structure of mollusc adductor contractile apparatus. Comp Biochem Physiol. 1979;64A:291–298. [Google Scholar]
- Marston S. Ca2+-dependent protein switches in actomyosin based contractile systems. Int J Biochem Cell Biol. 1995;27:97–108. doi: 10.1016/1357-2725(94)00080-u. [DOI] [PubMed] [Google Scholar]
- Marston S, Lehman W. ADP binding to relaxed scallop myofibrils. Nature. 1974;252:38–39. doi: 10.1038/252038a0. [DOI] [PubMed] [Google Scholar]
- Marston S, Tregear RT. Calcium binding and the activation of fibrillar insect flight muscle. Biochim Biophys Acta. 1974;347:311–318. doi: 10.1016/0005-2728(74)90054-1. [DOI] [PubMed] [Google Scholar]
- Marston SB, Redwood CS. The molecular anatomy of caldesmon. Biochem J. 1991;279:1–16. doi: 10.1042/bj2790001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marston SB, Rodger CD, Tregear RT. Changes in muscle crossbridges when β,γ-imido-ATP binds to myosin. J Mol Biol. 1976;104:263–276. doi: 10.1016/0022-2836(76)90012-7. [DOI] [PubMed] [Google Scholar]
- Marston SB, Tregear RT, Rodger CD, Clarke ML. Coupling between the enzymatic site of myosin and the mechanical output of muscle. J Mol Biol. 1979;128:111–26. doi: 10.1016/0022-2836(79)90121-9. [DOI] [PubMed] [Google Scholar]
- Martelo MJ, Padrón R. Metodo rapido para el aislamiento y purificacion de miosina de musculo de tarantula. Acta Cient Venez. 1987;38:394–395. [PubMed] [Google Scholar]
- Martin RE, Masaracchia RA, Donahue MJ. Ascaris suum: regulation of myosin light chain phosphorylation from adult skeletal muscle. Exp Parasitol. 1986;61:114–119. doi: 10.1016/0014-4894(86)90141-4. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Adenosinetriphosphatase activity of the contractile protein from the body wall muscle of the echiuroid, Urechis unieinetus. Enzymologia. 1954a;17:90–94. [PubMed] [Google Scholar]
- Maruyama K. Studies on adenosinetriphosphatases of various insect muscles. J Fac Tokyo Univ. 1954b;7:231–272. [Google Scholar]
- Maruyama K. Apyrase action of an actomyosin-like protein from a sea-anemone. Biochim Biophys Acta. 1955;16:589–590. doi: 10.1016/0006-3002(55)90283-3. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Interaction of the contractile protein from a sea-anemone with adenosine nucleotides. Sci Pap Coll Gen Educ, Univ Tokyo. 1956a;4:96–111. [Google Scholar]
- Maruyama K. The effects of ethylenediaminetetraacetate on the enzymatic hydrolysis of adenosine triphosphate. Sci Pap Coll Gen Educ, Univ Tokyo. 1956b;6:183–185. [Google Scholar]
- Maruyama K. A further study of insect actomyosin. Sci Pap Coll Gen Educ, Univ Tokyo. 1957a;7:214–241. [Google Scholar]
- Maruyama K. Contractile proteins from adductors of Meretrix and Cristaria. Annot Zool Japon. 1957b;30:63–66. [Google Scholar]
- Maruyama K. Contractile protein from crayfish tail muscle. Biol Bull. 1958a;114:95–105. [Google Scholar]
- Maruyama K. Interaction of insect actomyosin with adenosine triphosphate. J Cell Comp Physiol. 1958b;51:173–187. doi: 10.1002/jcp.1030510204. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Flow birefringence studies of crayfish myosins. Arch Biochem Biophys. 1959a;82:422–430. doi: 10.1016/0003-9861(59)90138-9. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Insect actomyosin. A flow birefringence study. J Insect Physiol. 1959b;3:271–292. [Google Scholar]
- Maruyama K. Effect of magnesium on the adenosinetriphosphatase of insect actomyosin at low ionic strength. Comp Biochem Physiol. 1966;18:481–487. doi: 10.1016/0010-406x(66)90232-5. [DOI] [PubMed] [Google Scholar]
- Maruyama K. Effects of magnesium and calcium ions on the adenosinetriphosphatase activity of insect actomyosin at low ionic strength. Comp Biochem Physiol. 1967;21:713–718. doi: 10.1016/0010-406x(67)90466-5. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Cage PE, Bell JL. The role of connectin in elastic properties of insect flight muscle. Comp Biochem Physiol. 1978;61A:623–627. [Google Scholar]
- Maruyama K, Ishikawa Y. Effect of magnesium and calcium on the ATPase activity of actomyosin at low ionic strength. Biochim Biophys Acta. 1963;77:682–685. [Google Scholar]
- Maruyama K, Kominz DR. Earthworm myosin. Z vergl Physiol. 1959;42:17–19. [Google Scholar]
- Maruyama K, Matsumiya H. The contractile protein from the tube-feet of a starfish. J Biochem (Tokyo) 1957;44:537–542. [Google Scholar]
- Maruyama K, Nagashima S, Drabikowski W. The role of native tropomyosin in the calcium sensitivity of crayfish actomyosin. Comp Biochem Physiol. 1968a;25:1107–1112. doi: 10.1016/0010-406x(68)90596-3. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Pringle JWS. The effect of ADP on the ATPase activity of insect actomyosin at low ionic strength. Arch Biochem Biophys. 1967;120:225–227. doi: 10.1016/0003-9861(67)90619-4. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Pringle JWS, Tregear R. The calcium sensitivity of ATPase activity of myofibrils and actomyosins from insect flight and leg muscles. Proc R Soc Lond Biol Sci. 1968b;169:229–240. doi: 10.1098/rspb.1968.0008. [DOI] [PubMed] [Google Scholar]
- Maruyama K, Sakagami SF. Aktivität der Myofibrillen und Sarkosomen Adenosintriphosphatasen im Flugelmuskel der Bienenarbeiterinnen. Z vergl Physiol. 1958;40:543–548. [Google Scholar]
- Masai S. Tonic and phasic contractions of the retractor pharyngis of snail (Euhadra lubuana) Jpn J Physiol. 1951;2:60–68. doi: 10.2170/jjphysiol.2.60. [DOI] [PubMed] [Google Scholar]
- Mateos J, Herranz R, Domingo A, Sparrow J, Marco R. The structural role of high molecular weight tropomyosins in dipteran indirect flight muscle and the effect of phosphorylation. J Muscle Res Cell Motil. 2006;27:189–201. doi: 10.1007/s10974-005-9044-3. [DOI] [PubMed] [Google Scholar]
- Matsumoto JJ. A new contractile protein of squid muscle. A comparative study with carp actomyosin. Nippon Suisan Gakk. 1957;23:92–104. [Google Scholar]
- Matsumoto JJ. On actomyosin of squid muscle from salt-extract: preparation of actomyosin. Nippon Suisan Gakk. 1958a;24:125–132. [Google Scholar]
- Matsumoto JJ. On purified M-actomyosin of squid muscle. Nippon Suisan Gakk. 1958b;23:775–781. [Google Scholar]
- Matsumoto JJ. Some notes on M-actomyosin of squid muscle. Nippon Suisan Gakk. 1958c;24:29–36. [Google Scholar]
- Matsumoto JJ. The effect of ATP on the viscosity of squid actomyosin. Nippon Suisan Gakk. 1958d;24:355–362. [Google Scholar]
- Matsumoto JJ. An electrophoretic study of the squid actomyosin. Nippon Suisan Gakk. 1959;25:27–37. [Google Scholar]
- Matsuno A, Ishida H, Hori H. Two kinds of thick filament in smooth muscle cells in the adductor of a clam, Chlamys nobilis. Tissue Cell. 1993;25:325–332. doi: 10.1016/0040-8166(93)90074-u. [DOI] [PubMed] [Google Scholar]
- Matsuura M. Relaxation of Mytilus catch muscle by 8-bromo-cyclic GMP and related compounds. Comp Biochem Physiol. 1984;78C:111–116. [PubMed] [Google Scholar]
- Maughan DW, Vigoreaux JO. An integrated view of insect flight muscle: genes, motor molecules, and motion. News Physiol Sci. 1999;14:87–92. doi: 10.1152/physiologyonline.1999.14.3.87. [DOI] [PubMed] [Google Scholar]
- McDowall AW, Hofman W, LePault J, Adrian M, Dubochet J. Cryo-electron microscopy of vitrified insect flight muscle. J Mol Biol. 1984;178:105–111. doi: 10.1016/0022-2836(84)90233-x. [DOI] [PubMed] [Google Scholar]
- McKim KS, Heschl MF, Rosenbluth RE, Baillie DL. Genetic organization of the unc-60 region in Caenorhabditis elegans. Genetics. 1988;118:49–59. doi: 10.1093/genetics/118.1.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKim KS, Matheson C, Marra MA, Wakarchuk MF, Baillie DL. The Caenorhabditis elegans Unc-60 gene encodes proteins homologous to a family of actin-binding proteins. Mol Gen Genet. 1994;242:346–357. doi: 10.1007/BF00280425. [DOI] [PubMed] [Google Scholar]
- McLachlan AD. Analysis of gene duplication repeats in the myosin rod. J Mol Biol. 1983;169:15–30. doi: 10.1016/s0022-2836(83)80173-9. [DOI] [PubMed] [Google Scholar]
- McLachlan AD. Structural implications of the myosin amino acid sequence. Annu Rev Biophys Bioeng. 1984;13:167–189. doi: 10.1146/annurev.bb.13.060184.001123. [DOI] [PubMed] [Google Scholar]
- McLachlan AD, Karn J. Periodic charge distributions in the myosin rod amino acid sequence match cross-bridge spacings in muscle. Nature. 1982;299:226–231. doi: 10.1038/299226a0. [DOI] [PubMed] [Google Scholar]
- McLachlan AD, Karn J. Periodic features in the amino acid sequence of nematode myosin rod. J Mol Biol. 1983;164:605–626. doi: 10.1016/0022-2836(83)90053-0. [DOI] [PubMed] [Google Scholar]
- McLachlan AD, Stewart M. Tropomyosin coiled-coil interactions: evidence for an unstaggered structure. J Mol Biol. 1975;98:293–304. doi: 10.1016/s0022-2836(75)80119-7. [DOI] [PubMed] [Google Scholar]
- McLachlan AD, Stewart M. The 14-fold periodicity in α-tropomyosin and the interaction with actin. J Mol Biol. 1976;103:271–298. doi: 10.1016/0022-2836(76)90313-2. [DOI] [PubMed] [Google Scholar]
- Meedel TH, Hastings KEM. Striated muscle-type tropomyosin in a chordate smooth muscle, ascidian body-wall muscle. J Biol Chem. 1993;268:6755–6764. [PubMed] [Google Scholar]
- Mehl JW. Studies on the proteins of smooth muscle. II The myosins of the octopus, snail, sea cucumber and sea anemone. Biol Bull. 1941;79:488–497. [Google Scholar]
- Mehta AD, Finer JT, Spudich JA. Detection of single-molecule interactions using correlated thermal diffusion. Proc Natl Acad Sci USA. 1997;94:7927–7931. doi: 10.1073/pnas.94.15.7927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei-Hsuan J, Tien-Chin T. A comparative, chemical study of tropomyosins from different sources [translated from Acta Physiol Sin., 21:91–99, 1957] Sci Sin. 1957;6:317–326. [PubMed] [Google Scholar]
- Meinrenken W. Calciumionen-unabhängige Kontraction und ATPase bei glycerinierten Muskelfasern nach alkalischer Extraktion von Troponin. Pflügers Arch -Eur J Physiol. 1969;311:243–255. doi: 10.1007/BF00590528. [DOI] [PubMed] [Google Scholar]
- Meisner D, Beinbrech G. Alterations of crossbridge angle induced by β, γ-imido-adenosine-triphosphate. Electron microscope and optical diffraction studies on myofibrillar fragments of abdominal muscles of the crayfish Orconectes limosus. Eur J Cell Biol. 1979;19:189–195. [PubMed] [Google Scholar]
- Mendelson M. Electrical and mechanical characteristics of a very fast lobster muscle. J Cell Biol. 1969;42:548–563. doi: 10.1083/jcb.42.2.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ménétret JF, Hofmann W, LePault J. Cryo-electon microscopy of insect flight muscle thick filaments. An approach to dynamic electron microscope studies. J Mol Biol. 1988;202:175–178. doi: 10.1016/0022-2836(88)90530-x. [DOI] [PubMed] [Google Scholar]
- Ménétret JF, Schroeder RR, Hofmann W. Cryo-electron microscopic studies of relaxed striated muscle thick filaments. J Muscle Res Cell Motil. 1990;11:1–11. doi: 10.1007/BF01833321. [DOI] [PubMed] [Google Scholar]
- Mercer KB, Miller RK, Tinley TL, Sheth S, Qadota H, Benian GM. Caenorhabditis elegans UNC-96 is a new component of M-lines that interacts with UNC-98 and paramyosin and is required in adult muscle for assembly and/or maintenance of thick filaments. Mol Biol Cell. 2006;17:3832–3847. doi: 10.1091/mbc.E06-02-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miegel A, Kobayashi T, Maeda Y. Isolation, purification, and partial characterization of tropomyosin and troponin subunits from the lobster tail muscle. J Muscle Res Cell Motil. 1992;13:608–618. doi: 10.1007/BF01738250. [DOI] [PubMed] [Google Scholar]
- Migita M, Matsumoto JJ. On the nature of the streaming birefringence observed in the aqueous extracts of squid muscle. I An anomalous component in the aqueous extracts of squid muscle. Nippon Suisan Gakk. 1954;20:641–652. [Google Scholar]
- Migita M, Matsumoto JJ. On the extractability of muscle proteins of marine animals [in Japanese, English abstract] Nippon Suisan Gakk. 1957;22:561–568. [Google Scholar]
- Miller DM, III, Ortiz I, Berliner GC, Epstein HF. Differential localization of two myosins within nematode thick filaments. Cell. 1983;34:477–490. doi: 10.1016/0092-8674(83)90381-1. [DOI] [PubMed] [Google Scholar]
- Miller A. Short pseudo-repeat in paramyosin. Nature. 1965;207:524–525. [Google Scholar]
- Miller A. A short periodicity in the thick filaments of the anterior byssus retractor muscle of Mytilus edulis. J Mol Biol. 1968;32:687–688. doi: 10.1016/0022-2836(68)90352-5. [DOI] [PubMed] [Google Scholar]
- Miller A, Tregear RT. Evidence concerning crossbridge attachment during muscle contraction. Nature. 1970;226:1060–1061. doi: 10.1038/2261060a0. [DOI] [PubMed] [Google Scholar]
- Miller A, Tregear RT. Structure of insect fibrillar flight muscle in the presence and absence of ATP. J Mol Biol. 1972;70:85–104. doi: 10.1016/0022-2836(72)90165-9. [DOI] [PubMed] [Google Scholar]
- Miller BM, Zhang SX, Suggs JA, Swank DM, Littlefield KP, Knowles AF, Bernstein SI. An alternative domain near the nucleotide-binding site of Drosophila muscle myosin affects ATPase kinetics. J Mol Biol. 2005;353:14–25. doi: 10.1016/j.jmb.2005.08.012. [DOI] [PubMed] [Google Scholar]
- Milligan RA. Protein-protein interactions in the rigor actomyosin complex. Proc Natl Acad Sci USA. 1996;93:21–26. doi: 10.1073/pnas.93.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman BM. Contraction in the opaque part of the adductor muscle of the oyster (Crassostrea angulata) J Physiol. 1964;173:238–262. doi: 10.1113/jphysiol.1964.sp007455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millman BM. Mechanism of contraction in molluscan muscle. Am Zool. 1967;7:583–591. [Google Scholar]
- Millman BM, Elliott GF. X-ray diffraction from contracting molluscan muscle. Nature. 1965;206:824–825. doi: 10.1038/206824a0. [DOI] [PubMed] [Google Scholar]
- Millman BM, Elliott GF. An X-ray diffraction study of contracting molluscan smooth muscle. Biophys J. 1972;12:1405–1413. doi: 10.1016/S0006-3495(72)86171-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milstein CP. Tryptic digestion of tropomyosin A, tropomyosin B, and myosin. Nature. 1966;209:614–615. doi: 10.1038/209614a0. [DOI] [PubMed] [Google Scholar]
- Milstein CP, Bailey K. Isolation and characterisation of a tryptic core from the insoluble tropomyosin of Pinna nobilis. Biochim Biophys Acta. 1961;49:412–413. doi: 10.1016/0006-3002(61)90150-0. [DOI] [PubMed] [Google Scholar]
- Minihan K, Davies RE. Energy requirements for relaxation from tonic contractions (‘catch’) in an invertebrate muscle. Nature. 1965;208:1327–1329. doi: 10.1038/2081327a0. [DOI] [PubMed] [Google Scholar]
- Minihan K, Davies RE. Changes in inorganic phosphate and arginine during the development, maintenance and loss of tension in the anterior byssus retractor muscle of Mytilus edulis. Biochem Z. 1966;345:173–187. [Google Scholar]
- Miroshnichenko NS, Balanuk IV, Nozrenko DN. Packing of myosin molecules in muscle thick filaments. Cell Biol Int. 2000;24:327–333. doi: 10.1006/cbir.1999.0514. [DOI] [PubMed] [Google Scholar]
- Miyakawa I, Konishi K. Removal of troponin C and desensitization of myosin-B from ascidian smooth muscle by treatment with ethylene diamine tetraacetate. J Biochem (Tokyo) 1984;95:57–65. doi: 10.1093/oxfordjournals.jbchem.a134602. [DOI] [PubMed] [Google Scholar]
- Miyanishi T, Maita T, Morita F, Kondo S, Matsuda G. Amino acid sequences of the two kinds of regulatory light chains of adductor smooth muscle myosin from Patinopecten yessoensis. J Biochem (Tokyo) 1985;97:541–551. doi: 10.1093/oxfordjournals.jbchem.a135089. [DOI] [PubMed] [Google Scholar]
- Mognoni GA, Lanzavecchia G. Studi sulla muscolatura elicoidale e paramiosinca. III Osservazioni comparative sulle proteine muscolari di mitilo e oloturia Rend Lincei. Sci Fis Nat. 1969;46:610–619. [Google Scholar]
- Mohri K, Ono K, Yu R, Yamashiro S, Ono S. Enhancement of actin- depolymerizing factor/cofilin-dependent actin disassembly by actin-interacting protein 1 is required for organized actin filament assembly in the Caenorhabditis elegans body wall muscle. Mol Biol Cell. 2006;17:2190–2199. doi: 10.1091/mbc.E05-11-1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohri K, Ono S. Actin filament disassembling activity of Caenorhabditis elegans actin-interacting protein 1 (UNC-78) is dependent on filament binding by a specific ADF/cofilin isoform. J Cell Sci. 2003;116:4107–4118. doi: 10.1242/jcs.00717. [DOI] [PubMed] [Google Scholar]
- Mohri K, Vorobiev S, Federov AA, Almo SC, Ono S. Identification of functional residues on Caenorhabditis elegans actin-interacting protein 1 (UNC-78) for disassembly of actin depolymerizing factor/cofilin-bound actin filaments. J Biol Chem. 2004;279:31697–31707. doi: 10.1074/jbc.M403351200. [DOI] [PubMed] [Google Scholar]
- Molloy JE, Burns JE, Sparrow JC, Tregear RT, Kendrick-Jones J, White DCS. Single-molecule mechanics of heavy meromyosin and S1 interacting with rabbit or Drosophila actins using optical tweezers. Biophys J. 1995;68:298S–305S. [PMC free article] [PubMed] [Google Scholar]
- Molloy JE, Kyrtatas V, Sparrow JC, White DCS. Kinetics of flight muscles from insects with different wingbeat frequencies. Nature. 1987;328:449–451. [Google Scholar]
- Moore JR, Dickinson MH, Vigoreaux JO, Maughan DW. The effect of removing the N-terminal extension of the Drosophila myosin regulatory light chain upon flight ability and the contractile dynamics of the indirect flight muscle. Biophys J. 2000;78:1431–1440. doi: 10.1016/S0006-3495(00)76696-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JR, Vigoreaux JO, Maughan DW. The Drosophila projectin mutant, bentD, has reduced stretch activation and altered indirect flight muscle kinetics. J Muscle Res Cell Motil. 1999;20:797–806. doi: 10.1023/a:1005607818302. [DOI] [PubMed] [Google Scholar]
- Moos C. Actin activation of heavy meromyosin and subfragment-1 ATPases; steady state kinetics studies. Cold Spring Harb Symp Quant Biol. 1972;37:137–143. [Google Scholar]
- Morgan CL. On the sound-producing apparatus of the cicadas. Nature. 1886;33:368–369. [Google Scholar]
- Morita F, Kondo S. Regulatory light chain contents and molecular species of myosin in catch muscle of scallop. J Biochem (Tokyo) 1982;92:977–983. doi: 10.1093/oxfordjournals.jbchem.a134054. [DOI] [PubMed] [Google Scholar]
- Morita F, Kondo S, Tomari K, Minowa O, Ikura M, Hikichi K. Calcium binding and conformation of regulatory light chains of smooth muscle myosin of scallop. J Biochem (Tokyo) 1985;97:553–561. doi: 10.1093/oxfordjournals.jbchem.a135090. [DOI] [PubMed] [Google Scholar]
- Morris EP, Squire JM, Fuller GW. The 4-stranded helical arrangement of myosin heads on insect (Lethocerus) flight muscle thick filaments. J Struct Biol. 1991;107:237–249. [Google Scholar]
- Morris LG, Hooper SL. Muscle response to changing neuronal input in the lobster (Panulirus interruptus) stomatogastric system: spike number- versus spike frequency-dependent domains. J Neurosci. 1997;17:5956–5971. doi: 10.1523/JNEUROSCI.17-15-05956.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LG, Hooper SL. Muscle response to changing neuronal input in the lobster (Panulirus interruptus) stomatogastric system: slow muscle properties can transform rhythmic input into tonic output. J Neurosci. 1998;18:3433–3442. doi: 10.1523/JNEUROSCI.18-09-03433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris LG, Hooper SL. Mechanisms underlying stabilization of temporally summated muscle contractions in the lobster (Panulirus) pyloric system. J Neurophysiol. 2001;85:254–268. doi: 10.1152/jn.2001.85.1.254. [DOI] [PubMed] [Google Scholar]
- Morris LG, Thuma JB, Hooper SL. Muscles express motor patterns of non-innervating neural networks by filtering broad-band input. Nat Neurosci. 2000;3:245–250. doi: 10.1038/72955. [DOI] [PubMed] [Google Scholar]
- Morrison CM, Cameron ML, Odense PH. Periodicities in the thick filaments of the opaque and translucent parts of the adductor of the oyster, Crassostrea virginica. Can J Zool. 1970;48:608–609. [Google Scholar]
- Mukou M, Kishi H, Shirakawa I, Kobayashi T, Tominanga K, Imanishi H, Sugi H. Marked load-bearing ability of Mytilus smooth muscle in both active and catch states as revealed by quick increases in load. J Exp Biol. 2004;207:1675–1681. doi: 10.1242/jeb.00934. [DOI] [PubMed] [Google Scholar]
- Müller SA, Häner M, Ortiz I, Aebi U, Epstein HF. STEM analysis of Caenorhabditis elegans muscle thick filaments: evidence for microdifferentiated substructures. J Mol Biol. 2001;305:1035–1044. doi: 10.1006/jmbi.2000.4363. [DOI] [PubMed] [Google Scholar]
- Muneoka Y, Shiba Y, Kanno Y. Effect of propranolol on the relaxation of molluscan smooth muscle: possible inhibition of serotonin release. Hiroshima J Med Sci. 1978a;27:155–161. [PubMed] [Google Scholar]
- Muneoka Y, Shiba Y, Kanno Y. Effects of neuroleptic drugs on the relaxing action of various monoamines in molluscan smooth muscle. Hiroshima J Med Sci. 1978b;27:163–171. [PubMed] [Google Scholar]
- Muneoka Y, Shiba Y, Kanno Y. Relaxation of Mytilus smooth muscle by 5- hydroxytryptophan and DOPA. Hiroshima J Med Sci. 1979;28:123–132. [PubMed] [Google Scholar]
- Muneoka Y, Shiba Y, Maetani T, Kanno Y. Further study on the effect of mersalyl, an organic mercurial, on relaxing responses of a molluscan smooth muscle to monoamines. J Toxicol Sci. 1978c;3:117–126. doi: 10.2131/jts.3.117. [DOI] [PubMed] [Google Scholar]
- Murakami H, Ishikawa T, Sano M. The relaxing effect of SKF 38393 on the catch contraction of Mytilus smooth muscle. Gen Pharmacol. 1986;17:685–687. doi: 10.1016/0306-3623(86)90300-9. [DOI] [PubMed] [Google Scholar]
- Murakami H, Satoh T, Ishikawa T. Effects of bulbocapnine and butaclamol on the relaxation of catch contraction by some dopaminergic agonists in Mytilus smooth muscle. Comp Biochem Physiol. 1983;75C:227–229. [Google Scholar]
- Murphy CT, Spudich JA. Variable surface loops and myosin activity: accessories to a motor. J Muscle Res Cell Motil. 2000;21:139–151. doi: 10.1023/a:1005610007209. [DOI] [PubMed] [Google Scholar]
- Myers JG. The morphology of the Cicadidae (Homoptera) Proc Zool Soc Lond. 1928;(Part I):365–472. [Google Scholar]
- Nachtigall W, Wilson DM. Neuro-muscular control of dipteran flight. J Exp Biol. 1967;47:77–97. doi: 10.1242/jeb.47.1.77. [DOI] [PubMed] [Google Scholar]
- Nagahama H, Tanaka Y, Tazumi M. 1974. Mechanical responses of the anterior byssus retractor muscle of Mytilus edulis to direct-current stimulation. 25:309–325. [Google Scholar]
- Nahirney PC, Forbes JG, Morris HD, Chock SC, Wang K. What the buzz was all about: superfast song muscles rattle the tymbals of male periodical cicadas. FASEB J. 2006;20:2017–2026. doi: 10.1096/fj.06-5991com. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Shiraishi F, Ohtsuki I. The effect of troponin C substitution on the Ca2+-sensitive ATPase activity of vertebrate and invertebrate myofibrils by troponin Cs with various numbers of Ca2+-binding sites. Comp Biochem Physiol. 1994;108B:121–133. doi: 10.1016/0305-0491(94)90172-4. [DOI] [PubMed] [Google Scholar]
- Namba K, Wakabayashi K, Mitsui T. X-ray structure analysis of the thin filament of crab striated muscle in the rigor state. J Mol Biol. 1980;138:1–26. doi: 10.1016/s0022-2836(80)80002-7. [DOI] [PubMed] [Google Scholar]
- Nara M, Yumoto F, Nagata K, Tanokura M, Kagi H, Ojima T, Nishita K. Fourier transform infrared spectroscopic study on the binding of Mg2+ to a mutant akazara scallop troponin C (E142Q) Biopolymers. 2004;74:77–81. doi: 10.1002/bip.20048. [DOI] [PubMed] [Google Scholar]
- Nara M, Yumoto F, Nagata K, Tanokura M, Kagi H, Ojima T, Nishita K, Morii H. Infrared spectroscopic study on Ca2+ binding to Akazara scallop troponin C in comparison with peptide analogues of its Ca2+-binding site IV. Vibrational Spectroscopy. 2006;42:188–191. [Google Scholar]
- Nauss KM, Davies RE. Changes in inorganic phosphate and arginine during the development, maintenance and loss of tension in the anterior byssus retractor muscle of Mytilus edulis. Biochem Z. 1966;345:173–187. [Google Scholar]
- Neumann T, Fauver M, Pollack GH. Elastic properties of isolated thick filaments measured by nanofabricated cantilevers. Biophys J. 1998;75:938–947. doi: 10.1016/S0006-3495(98)77582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman R, Butcher GW, Bullard B, Leonard KR. A method for determining the periodicity of a troponin component in isolated insect flight muscle thin filaments by gold/Fab labelling. J Cell Sci. 1992;101:503–508. doi: 10.1242/jcs.101.3.503. [DOI] [PubMed] [Google Scholar]
- Nishimura Y, Ojima T, Nishita K. Bivalve tropomyosins exhibit strong inhibition of actomyosin Mg-ATPase and high viscosity. Fisheries Sci. 1997;63:802–806. [Google Scholar]
- Nishita K. Preparation and biochemical properties of actin from striated adductor muscle of scallop [in Japanese, English summary] Nippon Suisan Gakk. 1977;43:805–812. [Google Scholar]
- Nishita K. [Biological studies on muscular proteins from marine invertebrates] [in Japanese] Nippon Suisan Gakk. 1998;64:368–372. [Google Scholar]
- Nishita K, Fukuyama R, Ishihara Y. Comparative studies of biochemical properties of actomyosins from adductor muscles of sea-shells (in Japanese, English summary and figure legends) Nippon Suisan Gakk. 1977;43:229–235. [Google Scholar]
- Nishita K, Ojima T. American lobster troponin. J Biochem (Tokyo) 1990;108:677–683. doi: 10.1093/oxfordjournals.jbchem.a123262. [DOI] [PubMed] [Google Scholar]
- Nishita K, Ojima T, Takahashi A, Inoue A. Troponin from smooth adductor muscle of Ezo-giant scallop. J Biochem (Tokyo) 1997;121:419–424. doi: 10.1093/oxfordjournals.jbchem.a021605. [DOI] [PubMed] [Google Scholar]
- Nishita K, Ojima T, Watanabe S. Myosin from striated adductor muscle of Chlamys nipponensis akazara. J Biochem (Tokyo) 1979;86:663–673. doi: 10.1093/oxfordjournals.jbchem.a132570. [DOI] [PubMed] [Google Scholar]
- Nishita K, Tanaka H, Ojima T. Amino acid sequence of troponin C from scallop striated adductor muscle. J Biol Chem. 1994;269:3464–3468. [PubMed] [Google Scholar]
- Nitao LK, Loo RRO, O’Neall-Hennessey E, Loo JA, Szent-Györgyi AG, Reisler E. Conformation and dynamics of the SH1-SH2 helix in scallop myosin. Biochemistry. 2003;42:7663–7674. doi: 10.1021/bi027312u. [DOI] [PubMed] [Google Scholar]
- Nonomura Y. Fine structure of the thick filament in molluscan catch muscle. J Mol Biol. 1974;88:445–455. doi: 10.1016/0022-2836(74)90494-x. [DOI] [PubMed] [Google Scholar]
- Nyitrai M, Stafford WF, III, Szent-Györgyi AG, Geeves MA. Ionic interactions play a role in the regulatory mechanism of scallop heavy meromyosin. Biophys J. 2003a;85:1053–1062. doi: 10.1016/S0006-3495(03)74544-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyitrai M, Szent-Györgyi AG, Geeves MA. A kinetic model of the co-operative binding of calcium and ADP to scallop (Argopecten irradians) heavy meromyosin. Biochem J. 2002;365:19–30. doi: 10.1042/BJ20020099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyitrai M, Szent-Györgyi AG, Geeves MA. Interactions of the two heads of scallop (Argopecten irradians) heavy meromyosin with actin: influence of calcium and nucleotides. Biochem J. 2003b;370:839–848. doi: 10.1042/BJ20021519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nykamp DA, Lydan MA, Oday DH, Lange AB. Calmodulin mediates contraction of the oviducts of Locusta migratoria. Insect Biochem Molec Biol. 1994;24:507–516. [Google Scholar]
- Nyland LR, Maughan DW. Morphology and transverse stiffness of Drosophila myofibrils measured by atomic force microscopy. Biophys J. 2000;78:1490–1497. doi: 10.1016/S0006-3495(00)76702-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obinata T, Ikeda M, Hayashi T. The native actin filaments from sea urchin muscle. Int J Biochem. 1974;5:875–884. [Google Scholar]
- Obinata T, Ooi A, Takano-Ohmuro H. Myosin and actin from ascidian smooth muscle and their interaction. Comp Biochem Physiol. 1983;76:437–442. doi: 10.1016/0305-0491(83)90272-9. [DOI] [PubMed] [Google Scholar]
- Obinata T, Shirao T, Murakami S. Sea urchin paramyosin. Int J Biochem. 1975;6:569–574. [Google Scholar]
- Offer G. Fifty years on: where have we reached? J Muscle Res Cell Motil. 2006;27:205–213. doi: 10.1007/s10974-006-9062-9. [DOI] [PubMed] [Google Scholar]
- Offer G, Couch J, O’Brien E, Elliott A. Arrangement of cross-bridges in insect flight muscle in rigor. J Mol Biol. 1981;151:663–702. doi: 10.1016/0022-2836(81)90429-0. [DOI] [PubMed] [Google Scholar]
- Offer G, Elliott A. Can a myosin molecule bind to two actin filaments? Nature. 1978;271:325–329. doi: 10.1038/271325a0. [DOI] [PubMed] [Google Scholar]
- Offer G, Knight P. The structure of the head-tail junction of the myosin molecule. J Mol Biol. 1996;256:407–416. doi: 10.1006/jmbi.1996.0096. [DOI] [PubMed] [Google Scholar]
- Offer G, Knight PJ, Burgess SA, Alamo L, Padrón R. A new model for the surface arrangement of myosin molecules in tarantula thick filaments [Erratum, J. Mol. Biol 302:521–522, 2000] J Mol Biol. 2000;298:239–260. doi: 10.1006/jmbi.2000.3664. [DOI] [PubMed] [Google Scholar]
- Ogawa Y. Calcium binding to troponin C and troponin: effects of Mg2+, ionic strength, and pH. J Biochem (Tokyo) 1985;97:1011–1023. doi: 10.1093/oxfordjournals.jbchem.a135143. [DOI] [PubMed] [Google Scholar]
- Ohshima S, Komiya T, Takeuchi K, Endo T, Obinata T. Generation of multiple troponin T isoforms is a common feature of the muscles in various chordate animals. Comp Biochem Physiol. 1988;90B:779–784. doi: 10.1016/0305-0491(88)90334-3. [DOI] [PubMed] [Google Scholar]
- Ohtani M, Muneoka Y, Kanemoto N, Ogino K, Masui Y, Aimoto S. Structure and action of MIP (Mytilus inhibitory peptide)-related tetrapeptides synthesized with a multiple synthesizer. Acta Biol Hung. 1995;46:445–448. [PubMed] [Google Scholar]
- Ohtsuka Y, Nakae H, Abe H, Obinata T. Immunochemical studies of an actin-binding protein in ascidian body wall smooth muscle. Zool Sci. 1994;11:407–412. [Google Scholar]
- Ohtsuka Y, Nakae H, Abe H, Obinata T. Functional characteristics and the complete primary structure of ascidian gelsolin. Biochim Biophys Acta. 1998;1383:219–231. doi: 10.1016/s0167-4838(97)00211-2. [DOI] [PubMed] [Google Scholar]
- Ohtsuki I. Calcium ion regulation of muscle contraction: the regulatory role of troponin T. Mol Cell Biochem. 1999;190:33–38. [PubMed] [Google Scholar]
- Oiwa K, Yamaga T, Yamada A. Direct observation of a central bare zone in a native thick filament isolated from the anterior byssus retractor muscle of Mytilus edulis using fluorescent ATP analogue. J Biochem (Tokyo) 1998;123:614–618. doi: 10.1093/oxfordjournals.jbchem.a021981. [DOI] [PubMed] [Google Scholar]
- Ojima T. [Studies on structure and function of molluscan troponin] [in Japanese] Nippon Suisan Gakk. 2003;69:326–329. [Google Scholar]
- Ojima T, Koizumi N, Ueyama K, Inoue A, Nishita K. Functional role of Ca2+- binding site IV of scallop troponin C. J Biochem (Tokyo) 2000;128:803–809. doi: 10.1093/oxfordjournals.jbchem.a022818. [DOI] [PubMed] [Google Scholar]
- Ojima T, Maita M, Inoue A, Nishita K. Bacterial expression, purification, and characterization of Akazara scallop troponin C. Fisheries Sci. 1997;63:137–141. [Google Scholar]
- Ojima T, Nishita K. Reversible dissociation of the regulatory light chain from Akazara striated adductor myosin by heat treatment [in Japanese, English summary] Nippon Suisan Gakk. 1983;49:1257–1264. [Google Scholar]
- Ojima T, Nishita K. Isolation of troponins from striated and smooth adductor muscles of Akazara scallop. J Biochem (Tokyo) 1986a;100:821–824. doi: 10.1093/oxfordjournals.jbchem.a121777. [DOI] [PubMed] [Google Scholar]
- Ojima T, Nishita K. Troponin from Akazara scallop striated adductor muscles. J Biol Chem. 1986b;261:16749–16754. [PubMed] [Google Scholar]
- Ojima T, Nishita K. Dissociation of regulatory light chains from hybrid myosin [in Japanese, English summary] Nippon Suisan Gakk. 1987;53:93–98. [Google Scholar]
- Ojima T, Nishita K. Biochemical characteristics of the Mr 52,000 component of Akazara scallop troponin. J Biochem (Tokyo) 1988a;104:207–210. doi: 10.1093/oxfordjournals.jbchem.a122443. [DOI] [PubMed] [Google Scholar]
- Ojima T, Nishita K. Separation of Akazara scallop and rabbit troponin components by a single step chromatography on CM-toyopearl. J Biochem (Tokyo) 1988b;104:9–11. doi: 10.1093/oxfordjournals.jbchem.a122429. [DOI] [PubMed] [Google Scholar]
- Ojima T, Nishita K. Biochemical properties of Akazara scallop myosin hybridized with the foreign regulatory light chains. Nippon Suisan Gakk. 1989;55:1857–1863. [Google Scholar]
- Ojima T, Nishita K. A binary complex of troponin-I and troponin-T from Akazara scallop striated adductor muscle. J Biochem (Tokyo) 1991;110:847–850. doi: 10.1093/oxfordjournals.jbchem.a123675. [DOI] [PubMed] [Google Scholar]
- Ojima T, Nishita K. Akazara scallop troponin C: Ca2+ induced conformational change and interaction with rabbit troponin subunits. Arch Biochem Biophys. 1992a;299:344–349. doi: 10.1016/0003-9861(92)90285-5. [DOI] [PubMed] [Google Scholar]
- Ojima T, Nishita K. Comparative studies on biochemical characteristics of troponins from Ezo-giant scallop (Patinopecten yessoensis) and Akazara scallop (Chlamys nipponensis akazara) Comp Biochem Physiol. 1992b;103:727–732. [Google Scholar]
- Ojima T, Nishita K, Watanabe S. Akazara scallop myosins hybridized with DTNB-light chains of skeletal myosin and with regulatory light chains of gizzard myosin. J Biochem (Tokyo) 1983a;94:307–310. doi: 10.1093/oxfordjournals.jbchem.a134345. [DOI] [PubMed] [Google Scholar]
- Ojima T, Nishita K, Watanabe S. Heat (30°C)-desensitization of Akazara striated adductor myosin, and its resensitization. J Biochem (Tokyo) 1983b;93:607–613. doi: 10.1093/oxfordjournals.jbchem.a134216. [DOI] [PubMed] [Google Scholar]
- Ojima T, Ohta T, Nishita K. Amino acid sequence of squid troponin C. Comp Biochem Physiol. 2001;129B:787–796. doi: 10.1016/s1096-4959(01)00397-9. [DOI] [PubMed] [Google Scholar]
- Ojima T, Tanaka H, Nishita K. Cyanogen bromide fragments of Akazara scallop Mr 52,000 troponin-I. J Biochem (Tokyo) 1990;108:519–521. doi: 10.1093/oxfordjournals.jbchem.a123234. [DOI] [PubMed] [Google Scholar]
- Ojima T, Tanaka H, Nishita K. Cloning and sequence of a cDNA encoding Akazara scallop troponin C. Arch Biochem Biophys. 1994;311:272–276. doi: 10.1006/abbi.1994.1237. [DOI] [PubMed] [Google Scholar]
- Ojima T, Toyoguchi T, Nishita K. Isolation and characterization of troponin from abdominal muscle of prawn Penaeus japonicus. Fisheries Sci. 1995;61:871–875. [Google Scholar]
- Okada M, Tada S. Streaming birefringence in extracts of muscles of aquatic animals. Nippon Suisan Gakk. 1954;20:224–231. [Google Scholar]
- Olander J. Substructure of the paramyosin molecules. Biochemistry. 1971;10:601–609. doi: 10.1021/bi00780a009. [DOI] [PubMed] [Google Scholar]
- Olander J, Emerson M, Holtzer A. On the dissociation and reassociation of the polypeptide chains of tropomyosin and paramyosin. J Am Chem Soc. 1967;89:3058–3059. doi: 10.1021/ja00988a051. [DOI] [PubMed] [Google Scholar]
- Ono K, Ono S. Tropomyosin and troponin are required for ovarian contraction in the Caenorhabditis elegans reproductive system. Mol Biol Cell. 2004;15:2782–2793. doi: 10.1091/mbc.E04-03-0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K, Parast M, Alberico C, Benian GM, Ono S. Specific requirement for two ADF/cofilin isoforms in distinct actin-dependent processes in Caenorhabditis elegans. J Cell Sci. 2003;116:2073–2085. doi: 10.1242/jcs.00421. [DOI] [PubMed] [Google Scholar]
- Ono S. Purification and biochemical characterization of actin from Caenorhabditis elegans: its difference from rabbit muscle actin in the interaction with nematode ADF cofilin. Cell Motil Cytoskeleton. 1999;43:128–136. doi: 10.1002/(SICI)1097-0169(1999)43:2<128::AID-CM4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Ono S. The Caenorhabditis elegans unc-78 gene encodes a homologue of actin-interacting protein 1 required for organized assembly of muscle actin filaments (correction J. Cell. Biol 153:889, 2001) J Cell Biol. 2001;152:1313–1319. doi: 10.1083/jcb.152.6.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S. Regulation of actin filament dynamics by actin depolymerizing factor/cofilin and actin-interacting protein 1: new blades for twisted filament. Biochemistry. 2003;42:13363–13370. doi: 10.1021/bi034600x. [DOI] [PubMed] [Google Scholar]
- Ono S, Baillie DL, Benian GM. UNC-60B, an ADF/cofilin family protein, is required for proper assembly of actin into myofibrils in Caenorhabditis elegans body wall muscle. J Cell Biol. 1999;145:491–502. doi: 10.1083/jcb.145.3.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono S, Benian GM. Two Caenorhabditis elegans actin depolymerizing factor/cofilin proteins, encoded by the unc-60 gene, differentially regulate actin filament dynamics. J Biol Chem. 1998;273:3778–3783. doi: 10.1074/jbc.273.6.3778. [DOI] [PubMed] [Google Scholar]
- Ono S, McGough A, Pope BJ, Tolbert VT, Bui A, Pohl J, Benian GM, Gernert KM, Weeds AG. The C-terminal tail of UNC-60B (actin depolymerizing factor/cofilin) is critical for maintaining its stable association with F-actin and is implicated in the second actin-binding site. J Biol Chem. 2001;276:5952–5958. doi: 10.1074/jbc.M007563200. [DOI] [PubMed] [Google Scholar]
- Ono S, Mohri K, Ono K. Microscopic evidence that actin-interacting protein 1 actively disassembles actin-depolymerizing factor/cofilin-bound actin filaments. J Biol Chem. 2004;279:14207–14212. doi: 10.1074/jbc.M313418200. [DOI] [PubMed] [Google Scholar]
- Ono S, Ono K. Tropomyosin inhibits ADF/cofilin-dependent actin filament dynamics. J Cell Biol. 2002;156:1065–1076. doi: 10.1083/jcb.200110013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orentlicher M, Brandt PW, Reuben JP. Regulation of tension in skinned muscle fibers: effect of high concentrations of Mg-ATP. Am J Physiol. 1977;233:C127–C134. doi: 10.1152/ajpcell.1977.233.5.C127. [DOI] [PubMed] [Google Scholar]
- Padrón R, Alamo L, Guerrero JR, Granados M, Uman P, Craig R. Three-dimensional reconstruction of thick filaments from rapidly frozen, freeze-substituted tarantula muscle. J Struct Biol. 1995;115:250–257. doi: 10.1006/jsbi.1995.1049. [DOI] [PubMed] [Google Scholar]
- Padrón R, Alamo L, Murgich J, Craig R. Towards an atomic model of the thick filaments of muscle. J Mol Biol. 1998;275:35–41. doi: 10.1006/jmbi.1997.1448. [DOI] [PubMed] [Google Scholar]
- Padrón R, Granados M, Alamo L, Guerrero JR, Craig R. Visualization of myosin helices in sections of rapidly frozen relaxed tarantula muscle. J Struct Biol. 1992;108:269–276. doi: 10.1016/1047-8477(92)90027-8. [DOI] [PubMed] [Google Scholar]
- Padrón R, Guerrero JR, Alamo L, Granados M, Gherbesi N, Craig R. Direct visualization of myosin filament symmetry in tarantula striated muscle by electron microscopy. J Struct Biol. 1993;111:17–21. doi: 10.1006/jsbi.1993.1031. [DOI] [PubMed] [Google Scholar]
- Padrón R, Panté N, Sosa H, Kendrick-Jones J. X-ray diffraction study of the structural changes accompanying phosphorylation of tarantula muscle. J Muscle Res Cell Motil. 1991;12:235–241. doi: 10.1007/BF01745112. [DOI] [PubMed] [Google Scholar]
- Painter SD. FMRFamide catch contractures of a mollscan smooth muscle: pharmacology, ionic dependence and cyclic nucleotides. J Comp Physiol. 1982;148:491–501. [Google Scholar]
- Panté N. Paramyosin polarity in the thick filament of molluscan smooth muscles. J Struct Biol. 1994;113:148–163. doi: 10.1006/jsbi.1994.1047. [DOI] [PubMed] [Google Scholar]
- Panté N, Sosa H, Padrón R. Estudio por difraccion de rayos-X de los cambios estructurales que acompañan la fosforilacion de los filamentos gruesos de musculo de tarantula. Acta Cient Venez. 1988;39:230–236. [PubMed] [Google Scholar]
- Park HS, Tao T, Chantler PD. Proximity relationships between sites on myosin and actin. Resonance energy transfer determination of the following distances, using a hybrid myosin: those between Cys-55 on the Mercenaria regulatory light chain, SH-1 on the Aequipecten myosin heavy chain, and Cys-374 of actin. Biochemistry. 1991;30:3189–3195. doi: 10.1021/bi00227a005. [DOI] [PubMed] [Google Scholar]
- Parnas J. Energetik glatter Muskeln. Pflügers Arch. 1910;134:441–495. [Google Scholar]
- Patel H, Margossian SS, Chantler PD. Locking regulatory myosin in the off-state with trifluoperazine. J Biol Chem. 2000;275:4880–4888. doi: 10.1074/jbc.275.7.4880. [DOI] [PubMed] [Google Scholar]
- Pawlow IP. Wie die Muschel ihre Schale öffnet. Pflügers Arch. 1885;37:6–31. [Google Scholar]
- Peachey LD. Muscle. Annu Rev Physiol. 1968;30:401–440. doi: 10.1146/annurev.ph.30.030168.002153. [DOI] [PubMed] [Google Scholar]
- Peckham M, Cripps RM, White DCS, Bullard B. Mechanics and protein content of insect flight muscles. J Exp Biol. 1992;168:57–76. [Google Scholar]
- Peckham M, Molloy JE, Sparrow JC, White DCS. Physiological properties of the dorsal longitudinal flight muscle and the tergal depressor of the trochanter muscle of Drosophila melanogaster. J Muscle Res Cell Motil. 1990;11:203–215. doi: 10.1007/BF01843574. [DOI] [PubMed] [Google Scholar]
- Peckham M, White DCS. Mechanical properties of demembranated flight muscle fibers from a dragonfly. J Exp Biol. 1991;159:135–147. [Google Scholar]
- Perry SV. Troponin T: genetics, properties and functions. J Muscle Res Cell Motil. 1998;19:575–602. doi: 10.1023/a:1005397501968. [DOI] [PubMed] [Google Scholar]
- Perry SV. Troponin I: inhibitor or facilitator. Mol Cell Biochem. 1999;190:9–32. [PubMed] [Google Scholar]
- Perry SV. What is the role of tropomyosin in the regulation of muscle contraction? J Muscle Res Cell Motil. 2003;24:593–596. doi: 10.1023/b:jure.0000009811.95652.68. [DOI] [PubMed] [Google Scholar]
- Pfitzer G, Rüegg JC. Molluscan catch muscle: regulation and mechanics in living and skinned anterior byssus retractor muscle of Mytilus edulis. J Comp Physiol. 1982;147B:137–142. [Google Scholar]
- Philpott DE, Kahlbrock M, Szent-Györgyi AG. Filamentous organization of molluscan muscles. J Ultrastruct Res. 1960;3:254–269. doi: 10.1016/s0022-5320(60)80013-5. [DOI] [PubMed] [Google Scholar]
- Philpott DE, Szent-Györgyi AG. The structure of light-meromyosin: an electron microscopic study. Biochim Biophys Acta. 1954;15:165–173. doi: 10.1016/0006-3002(54)90056-6. [DOI] [PubMed] [Google Scholar]
- Pirani A, Vinogradova MV, Curmi PMG, King WA, Fletterick RJ, Craig R, Tobacman LS, Xu C, Hatch V, Lehman W. An atomic model of the thin filament in the relaxed and Ca2+-activated states. J Mol Biol. 2006;357:707–717. doi: 10.1016/j.jmb.2005.12.050. [DOI] [PubMed] [Google Scholar]
- Plotnikov SV, Millard AC, Campagnola PJ, Mohler WA. Characterization of the myosin-based source for second-harmonic generation from muscle sarcomeres. Biophys J. 2006;90:693–703. doi: 10.1529/biophysj.105.071555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgornaya OI, Drozdov AL. Investigation of gels of sarcoplasmic proteins of the catch muscles in the scallop Patinopecten yessoensis. Biologiya Morya [Marine Biology, Russian] 1981;2:65–68. [Google Scholar]
- Polet D, Lambrechts A, Ono K, Mah A, Peelman F, Vandekerckhove J, Baillie DL, Ampe C, Ono S. Caenorhabditis elegans expresses three functional profilins in a tissue-specific manner. Cell Motil Cytoskeleton. 2006;63:14–28. doi: 10.1002/cm.20102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollack GH, Blyakhman F, Shklyar T, Tourovskaya A, Tameyasu T, Yang P. Implications of quantal motor action in biological systems. Adv Exp Med Biol. 1998;453:361–371. doi: 10.1007/978-1-4684-6039-1_41. [DOI] [PubMed] [Google Scholar]
- Pollack GH, Granzier HLM, Mattiazzi A, Trombitás K, Perisamy A, Baatsen PHWW, Burns DH. Pauses, steps, and the mechanism of contraction. Adv Exp Med Biol. 1988;226:617–642. [PubMed] [Google Scholar]
- Pollack GH, Liu XM, Yakovenko O, Blyakhman FA. Translation step size measured in single sarcomeres and single filament pairs. Adv Exp Med Biol. 2003;538:129–141. doi: 10.1007/978-1-4419-9029-7_12. [DOI] [PubMed] [Google Scholar]
- Portzehl H, Caldwell PC, Rüegg JC. The dependence of contraction and relaxation of muscle fibers from the crab Maja squinado on the internal concentration of free calcium ions. Biochim Biophys Acta. 1964;79:581–591. doi: 10.1016/0926-6577(64)90224-4. [DOI] [PubMed] [Google Scholar]
- Portzehl H, Zaoralek P, Gaudin H. The activation by Ca2+ of the ATPase of extracted muscle fibrils with variation of ionic strength, pH and concentration of MgATP. Biochim Biophys Acta. 1969;189:440–448. doi: 10.1016/0005-2728(69)90175-3. [DOI] [PubMed] [Google Scholar]
- Portzehl H, Zaorelek P, Greider A. Der Calcium-Spiegel in lebenden unisolierten Muskelfibrillen van Maia squinado und seine Regulierung durch die sarkoplasmatischen Vesikel. Pflügers Arch. 1965;286:44–56. [PubMed] [Google Scholar]
- Pringle JWS. The excitation and contraction of the flight muscles of insects. J Physiol. 1949;108:226–232. doi: 10.1113/jphysiol.1949.sp004326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle JWS. Physiology of song in cicadas. Nature. 1953;172:248–249. doi: 10.1038/172248b0. [DOI] [PubMed] [Google Scholar]
- Pringle JWS. A physiological analysis of cicada song. J Exp Biol. 1954a;31:525–560. [Google Scholar]
- Pringle JWS. The mechanism of the myogenic rhythm of certain insect striated muscle. J Physiol. 1954b;124:269–291. doi: 10.1113/jphysiol.1954.sp005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle JWS. The structure and evolution of the organs of sound-production in cicadas. Proc Linn Soc Lond. 1957;167:144–159. [Google Scholar]
- Pringle JWS. Evidence from insect fibrillar muscle about the elementary contractile process. J Gen Physiol. 1967a;50:139–156. doi: 10.1085/jgp.50.6.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringle JWS. The contractile mechanism of insect fibrillar muscle. Prog Biophys Mol Biol. 1967b;17:1–60. doi: 10.1016/0079-6107(67)90003-x. [DOI] [PubMed] [Google Scholar]
- Pringle JWS. Mechano-chemical transformation in striated muscle. Symp Soc Exp Biol. 1968;22:67–86. [PubMed] [Google Scholar]
- Pringle JWS. The resting elasticity of insect flight muscle. Symp Biol Hung. 1974;17:67–78. [Google Scholar]
- Pringle JWS. The muscles and sense organs involved in insect flight. Symp R Ent Soc Lond. 1976;7:3–15. [Google Scholar]
- Pringle JWS. The Croonian lecture, 1977. Stretch activation of muscle: function and mechanism. Proc R Soc Lond Biol Sci. 1978;201:107–130. doi: 10.1098/rspb.1978.0035. [DOI] [PubMed] [Google Scholar]
- Pringle JWS. The Bidder lecture, 1980. The evolution of fibrillar muscle in insects. J Exp Biol. 1981;94:1–14. [Google Scholar]
- Pringle JWS, Tregear RT. Mechanical properties of insect fibrillar muscle at large amplitudes of oscillation. Proc R Soc Lond Biol Sci. 1969;174:33–50. doi: 10.1098/rspb.1969.0079. [DOI] [PubMed] [Google Scholar]
- Pumphrey RJ. The double innervation of muscles in the clam (Mya arenaria) J Exp Biol. 1938;15:500–505. [Google Scholar]
- Pybus J, Tregear R. Estimates of force and time of actomyosin interaction in active muscle and the number interacting at any one time. Cold Spring Harb Symp Quant Biol. 1972;37:655–660. [Google Scholar]
- Pybus J, Tregear RT. The relationship of adenosine triphosphatase activity to tension and power output of insect flight muscle. J Physiol. 1975;247:71–89. doi: 10.1113/jphysiol.1975.sp010921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Lakey A, Agianian B, Hutchings A, Butcher GW, Labeit S, Leonard K, Bullard B. Troponin C in different insect muscle types: identification of two isoforms in Lethocerus, Drosophila, and Anopheles that are specific to asynchronous flight muscle in the adult insect. Biochem J. 2003;371:811–821. doi: 10.1042/BJ20021814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran S, Thomas DD. Rotational dynamics of the regulatory light chain in scallop muscle detected by time-resolved phosphorescence anisotropy. Biochemistry. 1999;38:9097–9104. doi: 10.1021/bi9902945. [DOI] [PubMed] [Google Scholar]
- Rapp G, Guth K, Maeda Y, Poole KJ, Goody RS. Time-resolved X-ray diffraction studies on stretch-activated insect flight muscle. J Muscle Res Cell Motil. 1991;12:208–215. doi: 10.1007/BF01774040. [DOI] [PubMed] [Google Scholar]
- Ravaux J, Hassanin A, Deutsch J, Gaill F, Markmann-Mulisch U. Sequence analysis of the myosin regulatory light chain gene of the vestimentiferan Riftia pachyptila. Gene. 2001;263:141–149. doi: 10.1016/s0378-1119(00)00581-3. [DOI] [PubMed] [Google Scholar]
- Rayment I. Structure of the actin-myosin complex and its implications for muscle contraction. Science. 1993;261:58–65. doi: 10.1126/science.8316858. [DOI] [PubMed] [Google Scholar]
- Rayment I, Holden HM. The 3-dimensional structure of a molecular motor. Trends Biochem Sci. 1994;19:129–134. doi: 10.1016/0968-0004(94)90206-2. [DOI] [PubMed] [Google Scholar]
- Rayns DG. Myofilaments and cross bridges as demonstrated by freeze-fracturing and etching. J Ultrastruct Res. 1972;40:103–121. doi: 10.1016/s0022-5320(72)80025-x. [DOI] [PubMed] [Google Scholar]
- Razumova LL. [X-ray diffraction studies of muscles] [in Russian] Biofizika. 1975;20:171–184. [PubMed] [Google Scholar]
- Razumova LL, Ianchuk K, Lemazhikhin BK, Mel’nikov LA, Frank GM. [On wing muscles in insects (electron microscopic and roentgenographic study)] [in Russian] Biofizika. 1966;11:818–824. [PubMed] [Google Scholar]
- Razumova LL, Kriukova ME, Mel’nikov LA, Lemazhikhin BK. [Some X-ray data on molluscan constrictor muscle] [in Russian] Biofizika. 1968;13:892–894. [PubMed] [Google Scholar]
- Razumova LL, Mel’nikov LA, Frank GM. X-ray findings on the mechanism of contraction of the adductor of Anadonta [translated from original Russian, Biofizika 15:915–917, 1970] Biophysics. 1970;15:951–953. [PubMed] [Google Scholar]
- Razumova LL, Mel’nikov LA, Gilëv VP. [Structural changes in striated muscle fixed with glutaraldehyde] [in Russian] Biofizika. 1973a;18:170–172. [PubMed] [Google Scholar]
- Razumova LL, Veretennikova AA, Mel’nikov LA. [X-ray study of the changes in the structure of glycerinated muscle fibers] [in Russian] Biofizika. 1972;15:915–916. [PubMed] [Google Scholar]
- Razumova LL, Veretennikova AA, Mel’nikov LA. [Characteristics of the changes in molecular orientation in molluscan constrictor muscles during contraction] [in Russian] Biofizika. 1973b;18:569–571. [PubMed] [Google Scholar]
- Razzaq A, Schmitz S, Veigel C, Molloy JE, Geeves MA, Sparrow JC. Actin residue Glu93 is identified as an amino acid affecting myosin binding. J Biol Chem. 1999;274:28321–28328. doi: 10.1074/jbc.274.40.28321. [DOI] [PubMed] [Google Scholar]
- Reedy MC. Visualizing myosin’s power stroke in muscle contraction. J Cell Sci. 2000;113:3551–3562. doi: 10.1242/jcs.113.20.3551. [DOI] [PubMed] [Google Scholar]
- Reedy MC, Beall C, Fyrberg E. Formation of reverse rigor chevrons by myosin heads. Nature. 1989;339:481–483. doi: 10.1038/339481a0. [DOI] [PubMed] [Google Scholar]
- Reedy MC, Reedy MK, Goody RS. Co-ordinated electron microscopy and X-ray studies of glycerinated insect flight muscle. II Electron microscopy and image reconstruction of muscle fibres fixed in rigor, in ATP and in AMPPNP. J Muscle Res Cell Motil. 1983a;4:55–81. doi: 10.1007/BF00711958. [DOI] [PubMed] [Google Scholar]
- Reedy MC, Reedy MK, Goody RS. The structure of insect flight muscle in the presence of AMPPNP. J Muscle Res Cell Motil. 1987;8:473–503. doi: 10.1007/BF01567908. [DOI] [PubMed] [Google Scholar]
- Reedy MC, Reedy MK, Leonard KR, Bullard B. Gold/Fab immuno electron microscopy localization of troponin H and troponin T in Lethocerus flight muscle. J Mol Biol. 1994a;239:52–67. doi: 10.1006/jmbi.1994.1350. [DOI] [PubMed] [Google Scholar]
- Reedy MC, Reedy MK, Tregear RT. Two attached non-rigor crossbridge forms in insect flight muscle. J Mol Biol. 1988;204:357–383. doi: 10.1016/0022-2836(88)90582-7. [DOI] [PubMed] [Google Scholar]
- Reedy MK. Cross-bridges and periods in insect flight muscle. Am Zool. 1967;7:465–481. [Google Scholar]
- Reedy MK. Ultrastructure of insect flight muscle. I Screw sense and structural grouping in the rigor cross-bridge lattice. J Mol Biol. 1968;31:155–176. doi: 10.1016/0022-2836(68)90437-3. [DOI] [PubMed] [Google Scholar]
- Reedy MK, Bahr GF, Fischman DA. How many myosins per cross-bridge? I Flight muscle myofibrils from the blowfly Sarcophaga bullata. Cold Spring Harb Symp Quant Biol. 1973;37:397–421. [Google Scholar]
- Reedy MK, Goody RS, Hofmann W, Rosenbaum G. Co-ordinated electron microscopy and X-ray studies of glycerinated insect flight muscle. I X-ray diffraction monitoring during preparation for electron microscopy of muscle fibres fixed in rigor, in ATP and in AMPPNP. J Muscle Res Cell Motil. 1983b;4:25–53. doi: 10.1007/BF00711957. [DOI] [PubMed] [Google Scholar]
- Reedy MK, Holmes KC, Tregear RT. Induced changes in orientation of the cross- bridges of glycerinated insect flight muscle. Nature. 1965;207:1276–1280. doi: 10.1038/2071276a0. [DOI] [PubMed] [Google Scholar]
- Reedy MK, Leonard KR, Freeman R, Arad T. Thick myofilament mass determination by electron scattering measurements with the scanning transmission electron microscope. J Muscle Res Cell Motil. 1981;2:45–64. doi: 10.1007/BF00712061. [DOI] [PubMed] [Google Scholar]
- Reedy MK, Lucaveche C, Naber N, Cooke R. Insect crossbridges, relaxed by spin-labeled nucleotide, show well-ordered 90° state by X-ray diffraction and electron microscopy, but spectra of electron paramagnetic resonance probes report disorder. J Mol Biol. 1992;227:678–697. doi: 10.1016/0022-2836(92)90217-8. [DOI] [PubMed] [Google Scholar]
- Reedy MK, Lucaveche C, Reedy MC, Somasundaram B. Experiments on rigor crossbridge action and filament sliding in insect flight muscle. Adv Exp Med Biol. 1993;332:33–44. doi: 10.1007/978-1-4615-2872-2_4. [DOI] [PubMed] [Google Scholar]
- Reedy MK, Reedy MC. Rigor crossbridge structure in tilted single filament layers and flared-X formations from insect flight muscle. J Mol Biol. 1985;185:145–176. doi: 10.1016/0022-2836(85)90188-3. [DOI] [PubMed] [Google Scholar]
- Reedy MK, Reedy MC, Schachat F. Tropomyosin. Does resolution lead to reconciliation? Curr Biol. 1994b;4:624–626. doi: 10.1016/s0960-9822(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Regenstein JM, Szent-Györgyi AG. Regulatory proteins of lobster striated muscle. Biochemistry. 1975;14:917–925. doi: 10.1021/bi00676a007. [DOI] [PubMed] [Google Scholar]
- Reichel H. Über die Temeraturabhängigkeit der Spannung im Zustand des Tonus und der Kontraktur im glatten Schliessmuskel von Pinna nobilis. Pubbl Staz Zool Napoli. 1953;27:73–79. [Google Scholar]
- Reid KH. Periodical cicada: mechanism of sound production. Science. 1971;172:949–951. doi: 10.1126/science.172.3986.949. [DOI] [PubMed] [Google Scholar]
- Reinach FC, Nagai K, Kendrick-Jones J. Site-directed mutagenesis of the regulatory light chain Ca2+/Mg2+ binding site and its role in hybrid myosins. Nature. 1986;322:80–83. doi: 10.1038/322080a0. [DOI] [PubMed] [Google Scholar]
- Riddiford LM. Solvent perturbation and ultraviolet optical rotatory dispersion studies of paramyosin. J Biol Chem. 1966;241:2792–2802. [PubMed] [Google Scholar]
- Riddiford LM, Scheraga HA. Structural studies of paramyosin. I Hydrogen ion equilibria. Biochemistry. 1962a;1:95–107. doi: 10.1021/bi00907a015. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Scheraga HA. Structural studies of paramyosin. II Conformational changes. Biochemistry. 1962b;1:108–114. doi: 10.1021/bi00907a016. [DOI] [PubMed] [Google Scholar]
- Risal D, Gourinath S, Himmel DM, Szent-Györgyi AG, Cohen C. Myosin subfragment 1 structures reveal a partially bound nucleotide and a complex salt bridge that helps couple nucleotide and actin binding. Proc Natl Acad Sci USA. 2004;101:8930–8935. doi: 10.1073/pnas.0403002101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritter O, Haase H, Morano I. Regulation of Limulus skeletal muscle contraction. FEBS Lett. 1999;446:233–235. doi: 10.1016/s0014-5793(99)00224-0. [DOI] [PubMed] [Google Scholar]
- Rodger CD, Tregear RT. Crossbridge angle when ADP is bound to myosin. J Mol Biol. 1974;86:495–497. doi: 10.1016/0022-2836(74)90033-3. [DOI] [PubMed] [Google Scholar]
- Rodríguez JL, Barcia R, Ramos-Martínez JI, Villamarín JA. Purification of a novel isoform of the regulatory subunit of cAMP-dependent protein kinase from the bivalve mollusk Mytilus galloprovincialis. Arch Biochem Biophys. 1998;359:57–62. doi: 10.1006/abbi.1998.0879. [DOI] [PubMed] [Google Scholar]
- Roeder KD. Movements of the thorax and potential changes in the thoracic muscles of insects during flight. Biol Bull. 1951;100:95–106. doi: 10.2307/1538681. [DOI] [PubMed] [Google Scholar]
- Rome LC, Cook C, Syme DA, Connaughton MA, Ashley-Ross M, Klimov A, Tikunov B, Goldman YE. Trading force for speed: why superfast crossbridge kinetics leads to superlow forces. Proc Natl Acad Sci USA. 1999;96:5826–5831. doi: 10.1073/pnas.96.10.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rome LC, Lindstedt SL. The quest for speed: muscles built for high-frequency contractions. News Physiol Sci. 1998;13:261–268. doi: 10.1152/physiologyonline.1998.13.6.261. [DOI] [PubMed] [Google Scholar]
- Roopnarine O, Szent-Györgyi AG, Thomas DD. Microsecond rotational dynamics of spin-labeled myosin regulatory light chain induced by relaxation and contraction of scallop muscle. Biochemistry. 1998;37:14428–14436. doi: 10.1021/bi9808363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root DD. A computational comparison of the atomic models of the actomyosin interface. Cell Biochem Biophys. 2002a;37:97–110. doi: 10.1385/CBB:37:2:097. [DOI] [PubMed] [Google Scholar]
- Root DD. The dance of actin and myosin - a structural and spectroscopic perspective. Cell Biochem Biophys. 2002b;37:111–139. doi: 10.1385/CBB:37:2:111. [DOI] [PubMed] [Google Scholar]
- Röper K, Mao YL, Brown NH. Contribution of sequence variation in Drosophila actins to their incorporation into actin-based structures in vivo. J Cell Sci. 2005;118:3937–3948. doi: 10.1242/jcs.02517. [DOI] [PubMed] [Google Scholar]
- Rosenbluth J. Obliquely striated muscle. III Contraction mechanism of Ascaris body muscle. J Cell Biol. 1967;34:15–33. doi: 10.1083/jcb.34.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbluth J. Sarcoplasmic reticulum of an unusually fast-acting crustacean muscle. J Cell Biol. 1969;42:534–547. doi: 10.1083/jcb.42.2.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenheck K, Doty P. The far ultraviolet absorption spectra of polypeptide and protein solutions and their dependence on conformation. Proc Natl Acad Sci USA. 1961;47:1775–1785. doi: 10.1073/pnas.47.11.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovner AS, Fagnant PM, Lowey S, Trybus KM. The carboxyl-terminal isoforms of smooth muscle myosin heavy chain determine thick filament assembly properties. J Cell Biol. 2002;156:113–123. doi: 10.1083/jcb.200107131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royuela M, Fraile B, Arenas MI, Paniagua R. Characterization of several invertebrate muscle cell types: a comparison with vertebrate muscles. Microsc Res Tech. 2000a;48:107–115. doi: 10.1002/(SICI)1097-0029(20000115)48:2<107::AID-JEMT6>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Royuela M, Fraile B, Paniagua R, Meyer-Rochow VB. Immunocytochemical observations on muscle cell proteins in the antarctic mussel shrimp Acetabulastoma sp (Crustacea, Ostracoda) Invert Biol. 1999;118:184–189. [Google Scholar]
- Royuela M, Fraile B, Picazo ML, Paniagua R. Immunocytochemical electron microscopic study and Western blot analysis of caldesmon and calponin in striated muscle of the fruit fly Drosophila melanogaster and in several muscle cell types of the earthworm Eisenia foetida. Eur J Cell Biol. 1997;72:90–94. [PubMed] [Google Scholar]
- Royuela M, Garcia-Anchuelo R, Paz de Miguel M, Arenas MI, Fraile B, Paniagua R. Immunocytochemical electron microscopic study and Western blot analysis of troponin in striated muscle of the fruit fly Drosophila melanogaster and in several muscle cell types of the earthworm Eisenia foetida. Anat Rec. 1996;244:148–154. doi: 10.1002/(SICI)1097-0185(199602)244:2<148::AID-AR2>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Royuela M, Meyer-Rochow VB, Fraile B, Paniagua R. Muscle cells in the tiny marine Antarctic mite Halacarellus thomasi: an ultrastructural and immunocytochemical study. Polar Biol. 2000b;23:759–765. [Google Scholar]
- Rudwick MJS. “Quick” and “catch” adductor muscles in brachiopods. Nature. 1961;191:1021. [Google Scholar]
- Rüegg JC. Tropomyosin and tonus in lamellibranch adductor muscles. Biochim Biophys Acta. 1959;35:278–279. doi: 10.1016/0006-3002(59)90370-1. [DOI] [PubMed] [Google Scholar]
- Rüegg JC. On the effect of inhibiting actin-myosin interaction on the viscous tone of a lamellibranch catch muscle. Biochem Biophys Res Commun. 1961a;6:24–28. doi: 10.1016/0006-291x(61)90178-4. [DOI] [PubMed] [Google Scholar]
- Rüegg JC. On the tropomyosin-paramyosin system in relation to the viscous tone of lamellibranch ‘catch’ muscle. Proc Natl Acad Sci USA. 1961b;154:224–249. [Google Scholar]
- Rüegg JC. The proteins associated with contraction in lamellibranch ‘catch’ muscle. Proc R Soc Lond Biol Sci. 1961c;154:209–223. [Google Scholar]
- Rüegg JC. Actomysosin inactivation by thiourea and the nature of the viscous tone in a molluscan smooth muscle. Proc R Soc Lond Biol Sci. 1963;158:177–195. [PubMed] [Google Scholar]
- Rüegg JC. Tropomyosin-paramyosin system and ‘prolonged contraction’ in a molluscan smooth muscle. Proc R Soc Lond Biol Sci. 1964;160:536–542. [PubMed] [Google Scholar]
- Rüegg JC. Physiologie und Biochemie des Sperrtonus. Experimentelle Untersuchung mit besonderer Berücksichtigung des M. retractor byssi von Mytilus edulis. Helv Physiol Pharmacol Acta. 1965;16(Supp):1–76. [PubMed] [Google Scholar]
- Rüegg JC. ATP-driven oscillation of glycerol-extracted insect fibrillar muscle: mechano-chemical coupling. Am Zool. 1967;7:457–464. [Google Scholar]
- Rüegg JC. Contractile mechanisms of smooth muscle. Symp Soc Exp Biol. 1968a;22:45–66. [PubMed] [Google Scholar]
- Rüegg JC. Oscillatory mechanism in fibrillar insect flight muscle. Experientia. 1968b;24:529–536. doi: 10.1007/BF02153756. [DOI] [PubMed] [Google Scholar]
- Rüegg JC. Smooth muscle tone. Physiol Rev. 1971;51:201–248. doi: 10.1152/physrev.1971.51.1.201. [DOI] [PubMed] [Google Scholar]
- Rüegg JC. Die Funktionsweise myogen oszillierender Insektenmuskeln. Verhandlungsber Deutsch Zool Gesell. 1972;65:285–296. [Google Scholar]
- Rüegg JC. Comparative aspects of crossbridge function - skinned fibre studies. Adv Exp Med Biol. 2005;565:331–340. doi: 10.1007/0-387-24990-7_25. [DOI] [PubMed] [Google Scholar]
- Rüegg JC, Kuhn HJ, Guth K, Pfitzer G, Hofmann F. Tension transients in skinned muscle fibres of insect flight muscle and mammalian cardiac muscle: effects of substrate concentration and treatment with myosin light chain kinase. Adv Exp Med Biol. 1984;170:605–615. doi: 10.1007/978-1-4684-4703-3_56. [DOI] [PubMed] [Google Scholar]
- Rüegg JC, Strassner E. Sperrtonus und Nucleosidtriphosphate. Z Naturforsch. 1963;18:133–138. [Google Scholar]
- Rüegg JC, Straub RW, Twarog BM. Inhibition of contraction in a molluscan smooth muscle by thiourea, an inhibitor of the actomyosin contractile mechanism. Proc R Soc Lond Biol Sci. 1963;158:156–176. doi: 10.1098/rspb.1963.0040. [DOI] [PubMed] [Google Scholar]
- Rüegg JC, Stumpf H. Activation of the myofibrillar ATPase activity by extension of glycerol extracted insect fibrillar muscle. Pflügers Arch. 1969a;305:34–46. doi: 10.1007/BF00586394. [DOI] [PubMed] [Google Scholar]
- Rüegg JC, Stumpf H. The coupling of power output and myofibrillar ATPase activity in glycerol-extracted insect fibrillar muscle at varying amplitude of ATP-driven oscillation. Pflügers Arch. 1969b;305:21–33. doi: 10.1007/BF00586393. [DOI] [PubMed] [Google Scholar]
- Rüegg JC, Tregear RT. Mechanical factors affecting the ATPase activity of glycerol-extracted insect fibrillar flight muscle. Proc R Soc Lond Biol Sci. 1966;165:497–512. doi: 10.1098/rspb.1966.0080. [DOI] [PubMed] [Google Scholar]
- Rüegg JC, Veigel C, Molloy JE, Schmitz S, Sparrow JC, Fink RHA. Molecular motors: force and movement generated by single myosin II molecules. News Physiol Sci. 2002;17:213–218. doi: 10.1152/nips.01389.2002. [DOI] [PubMed] [Google Scholar]
- Ruiz T, Bullard B, LePault J. Effects of calcium and nucleotides on the structure of insect flight muscle thin filaments. J Muscle Res Cell Motil. 1998;19:353–364. doi: 10.1023/a:1005341502973. [DOI] [PubMed] [Google Scholar]
- Ruppel KM, Lorenz M, Spudich JA. Myosin structure-function - a combined mutagenesis-crystallography approach. Curr Opin Struct Biol. 1995;5:181–186. doi: 10.1016/0959-440x(95)80073-5. [DOI] [PubMed] [Google Scholar]
- Ruppel KM, Spudich JA. Structure-function analysis of the motor domain of myosin. Annu Rev Cell Dev Biol. 1996;12:543–573. doi: 10.1146/annurev.cellbio.12.1.543. [DOI] [PubMed] [Google Scholar]
- Safer D, Chowrashi PK. β-thymosins from marine invertebrates: primary structure and interaction with actin. Cell Motil Cytoskeleton. 1997;38:163–171. doi: 10.1002/(SICI)1097-0169(1997)38:2<163::AID-CM5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Sailer M, Reuzelselke A, Achazi RK. The calmodulin protein-kinase system of Mytilus edulis catch muscle. Comp Biochem Physiol. 1990;96B:533–541. [Google Scholar]
- Salánki J, Hiripi L. Increase of serotonin in the adductors of Anodonta cygnea L. (Pelecypoda) relaxed by nerve stimulation and in relation to the periodic activity. Comp Biochem Physiol. 1970;32:629–636. doi: 10.1016/0010-406x(70)90817-0. [DOI] [PubMed] [Google Scholar]
- Samosudova NV, Frank GM. [On structural reorganization of striated muscle during its contraction] [in Russian] Biofizika. 1962;7:411–416. [PubMed] [Google Scholar]
- Satchell DG, Twarog BM. Identification of 5-hydroxytryptamine (serotonin) released from the anterior byssus retractor muscle of Mytilus californianus in response to nerve stimulation. Comp Biochem Physiol. 1978;59C:81–85. doi: 10.1016/0306-4492(78)90015-1. [DOI] [PubMed] [Google Scholar]
- Schachat F, Garcea RL, Epstein HF. Myosins exist as homodimers of heavy chains: demonstration with specific antibody purified by nematode mutant myosin affinity chromatography. Cell. 1978;15:405–411. doi: 10.1016/0092-8674(78)90009-0. [DOI] [PubMed] [Google Scholar]
- Schachat FH, Harris HE, Epstein HF. Two homogeneous myosins in body-wall muscle of Caenorhabditis elegans. Cell. 1977;10:721–728. doi: 10.1016/0092-8674(77)90106-4. [DOI] [PubMed] [Google Scholar]
- Schädler M. Proportionale Aktivierung von ATPase Aktivität und Kontraktionsspannung durch Calciumionen in isolierten contractilen Strukturen verschiedener Muskelarten. Pflügers Arch. 1967;296:70–90. [PubMed] [Google Scholar]
- Schädler M, Steiger GJ, Rüegg JC. Tension transients in glycerol-extracted fibres of insect fibrillar muscle. Experientia. 1969;25:942–943. doi: 10.1007/BF01898077. [DOI] [PubMed] [Google Scholar]
- Schädler M, Steiger GJ, Rüegg JC. Mechanical activation and isometric oscillation in insect fibrillar muscle. Pflügers Arch. 1971;330:217–229. doi: 10.1007/BF00588613. [DOI] [PubMed] [Google Scholar]
- Schaeffer P, Conley K, Lindstedt S. Structural correlates of speed and endurance in skeletal muscle: the rattlesnake tailshaker muscle. J Exp Biol. 1996;199:351–358. doi: 10.1242/jeb.199.2.351. [DOI] [PubMed] [Google Scholar]
- Schäfer EA. On the minute structure of the muscle-columns or sarcostyles which form the wing-muscles of insects. Preliminary note. Proc R Soc Lond Biol Sci. 1891;49:280–286. [Google Scholar]
- Schlote FW. Die dicken Myofilamente der glatten Muskelfasern von Helix pomatia. Z Zellforsch. 1968;92:503–508. [PubMed] [Google Scholar]
- Schmid MF, Epstein HF. Muscle thick filaments are rigid coupled tubules, not flexible ropes. Cell Motil Cytoskeleton. 1998;41:195–201. doi: 10.1002/(SICI)1097-0169(1998)41:3<195::AID-CM1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Schmitt FO, Bear RS, Hall CE, Jakus MA. Electron microscope and X-ray diffraction studies of muscle structure. Ann NY Acad Sci. 1947;47:799–809. doi: 10.1111/j.1749-6632.1947.tb31737.x. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Ashton FT, Pepe FA, Beinbrech G. Invertebrate myosin filament: parallel subfilament arrangement in the wall of solid filaments from the honeybee, Apis mellifica. Tissue Cell. 1993;25:111–119. doi: 10.1016/0040-8166(93)90068-v. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Ashton FT, Pepe FA, Beinbrech G. Substructures in the core of thick filaments: arrangement and number in relation to the paramyosin content of insect flight muscles. Tissue Cell. 1994a;26:83–100. doi: 10.1016/0040-8166(94)90085-x. [DOI] [PubMed] [Google Scholar]
- Schmitz H, Lucaveche C, Reedy MK, Taylor KA. Oblique section 3-D reconstruction of relaxed insect flight muscle reveals the cross-bridge lattice in helical registration. Biophys J. 1994b;67:1620–1633. doi: 10.1016/S0006-3495(94)80635-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz H, Reedy MC, Reedy MK, Tregear RT, Taylor KA. Tomographic three-dimensional reconstruction of insect flight muscle partially relaxed by AMPPNP and ethylene glycol. J Cell Biol. 1997;139:695–707. doi: 10.1083/jcb.139.3.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz H, Reedy MC, Reedy MK, Tregear RT, Winkler H, Taylor KA. Electron tomography of insect flight muscle in rigor and AMPPNP at 23° C. J Mol Biol. 1996;264:279–301. doi: 10.1006/jmbi.1996.0641. [DOI] [PubMed] [Google Scholar]
- Schoenenberger CA, Steinmetz MO, Stoffler D, Mandinova A, Aebi U. Structure, assembly, and dynamics of actin filaments in situ and in vitro. Microsc Res Tech. 1999;47:38–50. doi: 10.1002/(SICI)1097-0029(19991001)47:1<38::AID-JEMT4>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Scholey JM, Taylor KA, Kendrick-Jones J. The role of myosin light chains in regulating actin-myosin interaction. Biochimie. 1981;63:255–271. doi: 10.1016/s0300-9084(81)80115-0. [DOI] [PubMed] [Google Scholar]
- Schumacher T. Paramyosin-Struktur und Sperrtonus, Untersuchungen am Byssusretractor von Mytilus edulis mit dem Interferenz-Kontrast-Mikroskop. Experientia. 1970;26:631–633. doi: 10.1007/BF01898730. [DOI] [PubMed] [Google Scholar]
- Schumacher T. Zum Mechanismus der ökonomischen Halteleistung eines glatten Muskels (Byssus retractor anterior, Mytilus edulis) Pflügers Arch. 1972;331:77–89. doi: 10.1007/BF00587193. [DOI] [PubMed] [Google Scholar]
- Selby CC, Bear RS. The structure of actin-rich filaments of muscles according to X ray diffraction. J Biophys Biochem Cytol. 1956;2:71–85. doi: 10.1083/jcb.2.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellers JR. Phosphorylation-dependent regulation of Limulus myosin. J Biol Chem. 1981;256:9274–9278. [PubMed] [Google Scholar]
- Sellers JR. Fifty years of contractility research post sliding filament hypothesis. J Muscle Res Cell Motil. 2004;25:475–482. doi: 10.1007/s10974-004-4239-6. [DOI] [PubMed] [Google Scholar]
- Sellers JR, Chantler PD, Szent-Györgyi AG. Hybrid formation between scallop myofibrils and foreign regulatory light-chains. J Mol Biol. 1980;144:223–245. doi: 10.1016/0022-2836(80)90088-1. [DOI] [PubMed] [Google Scholar]
- Sellers JR, Harvey EV. Purification of myosin light chain kinase from Limulus muscle. Biochemistry. 1984;23:5821–5826. doi: 10.1021/bi00319a022. [DOI] [PubMed] [Google Scholar]
- Sellers JR, Kachar B. Polarity and velocity of sliding filaments: control of direction by actin and of speed by myosin. Science. 1990;249:406–408. doi: 10.1126/science.2377894. [DOI] [PubMed] [Google Scholar]
- Serwe M, Meyer HE, Craig AG, Carlhoff D, D’Haese J. Complete amino acid sequence of the regulatory light chain of obliquely striated muscle myosin from earthworm, Lumbricus terrestris. Eur J Biochem. 1993;211:341–346. doi: 10.1111/j.1432-1033.1993.tb19903.x. [DOI] [PubMed] [Google Scholar]
- Shechter E, Blout ER. An analysis of the optical rotatory dispersion of polypeptides and proteins. Proc Natl Acad Sci USA. 1964;51:695–702. doi: 10.1073/pnas.51.4.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelud’ko NS, Matusovskaya GG, Permyakova TV, Matusovsky OS. Twitchin, a thick-filament protein from molluscan catch muscle, interacts with F-actin in a phosphorylation-dependent way. Arch Biochem Biophys. 2004;432:269–277. doi: 10.1016/j.abb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- Sheng PK, Tsao TC, Peng CM. [The electrophoretic behaviour of nucleotropomyosins from different sources and the nuclear base composition of their pentose nucleic acid components] [in Chinese] Acta Physiol Sin. 1956;20:152–168. [Google Scholar]
- Sheterline P, Clayton J, Sparrow J. Actin. Protein Profile. 1995;2:1–103. [PubMed] [Google Scholar]
- Shibata-Sekiya K. Reaction intermediates of myosin ATPase from scallop adductor muscles: non-identical two-headed structure of striated adductor muscle myosin. J Biochem (Tokyo) 1982;92:1151–1162. doi: 10.1093/oxfordjournals.jbchem.a134031. [DOI] [PubMed] [Google Scholar]
- Shima Y, Tsuchiya T, Lehman W, Matsumoto JJ. The characterization of invertebrate troponin C. Comp Biochem Physiol. 1984;79B:525–529. doi: 10.1016/0305-0491(84)90360-2. [DOI] [PubMed] [Google Scholar]
- Shimada R, Ushio H, Yamanaka H. Effects of temperature on myofibrillar ATPase activities of two lobster species. Fisheries Sci. 2000;66:379–383. [Google Scholar]
- Shinoda Y, Yamada A, Yagi K. Identification of troponin I of crayfish myofibrils. J Biochem (Tokyo) 1988;103:636–640. doi: 10.1093/oxfordjournals.jbchem.a122320. [DOI] [PubMed] [Google Scholar]
- Shiraishi F, Morimoto S. Calmodulin substitutes the activating action of troponin C and myosin regulatory light chain on the Ca2+-sensitive ATPase activity of myofibrils from scallop striated muscle. Biomed Res. 1999;20:353–356. doi: 10.1093/oxfordjournals.jbchem.a022545. [DOI] [PubMed] [Google Scholar]
- Shiraishi F, Morimoto S, Nishita K, Ojima T, Ohtsuki I. Effects of removal and reconstitution of myosin regulatory light chain and troponin C on the Ca2+-sensitive ATPase activity of myofibrils from scallop striated muscle. J Biochem (Tokyo) 1999;126:1020–1024. doi: 10.1093/oxfordjournals.jbchem.a022545. [DOI] [PubMed] [Google Scholar]
- Shiraishi F, Ohtsuki I. Ca2+ and Sr2+-sensitivity of natural actomyosin prepared from various kinds of muscles. Biomed Res. 1989;10:509–515. [Google Scholar]
- Sicilia S, Smith DA. Theory of asynchronous oscillations in loaded insect flight muscle. Math Biosci. 1991;106:159–201. doi: 10.1016/0025-5564(91)90076-u. [DOI] [PubMed] [Google Scholar]
- Siegman MJ, Funabara D, Kinoshita S, Watabe S, Hartshorne DJ, Butler TM. Phosphorylation of a twitchin related protein controls catch and calcium sensitivity of force production in invertebrate smooth muscle. Proc Natl Acad Sci USA. 1998;95:5383–5388. doi: 10.1073/pnas.95.9.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegman MJ, Mooers SU, Li C, Narayan S, Trinkle-Mulcahy L, Watabe S, Hartshorne DJ, Butler TM. Phosphorylation of a high molecular weight (~600 kDa) protein regulates catch in invertebrate smooth muscle. J Muscle Res Cell Motil. 1997;18:655–670. doi: 10.1023/a:1018683823020. [DOI] [PubMed] [Google Scholar]
- Siemankowski RF, Zobel CR. Comparative studies on the structure and aggregative properties of the myosin molecule. I The structure of the lobster myosin molecule. J Mechanochem Cell Motil. 1976;3:171–184. [PubMed] [Google Scholar]
- Silberstein L, Lowey S. Investigation of immunological relationships among myosin light chains and troponin C. Biochemistry. 1977;16:4403–4408. doi: 10.1021/bi00639a012. [DOI] [PubMed] [Google Scholar]
- Silva R, Sparrow JC, Geeves MA. Isolation and kinetic characterisation of myosin and myosin S1 from the Drosophila indirect flight muscles. J Muscle Res Cell Motil. 2003;24:489–498. doi: 10.1023/b:jure.0000009809.69829.74. [DOI] [PubMed] [Google Scholar]
- Simmons NS, Cohen C, Szent-Györgyi AG, Wetlaufer DB, Blout ER. A conformation-dependent cotton effect in α-helical polypeptides and proteins. J Am Chem Soc. 1961;83:4766–4769. [Google Scholar]
- Simmons P, Young D. The tymbal mechanism and song patterns of the bladder cicada, Cystosoma saundersii. J Exp Biol. 1978;76:27–45. [Google Scholar]
- Simmons PJ. Neuronal generation of singing in a cicada. Nature. 1977;270:243–245. doi: 10.1038/270243a0. [DOI] [PubMed] [Google Scholar]
- Simmons RM, Szent-Györgyi AG. Reversible loss of calcium control of tension in scallop striated muscle associated with the removal of regulatory light chains. Nature. 1978;273:62–64. doi: 10.1038/273062a0. [DOI] [PubMed] [Google Scholar]
- Simmons RM, Szent-Györgyi AG. Control of tension development in scallop muscle fibres with foreign regulatory light chains. Nature. 1980;7:626–628. doi: 10.1038/286626a0. [DOI] [PubMed] [Google Scholar]
- Simmons RM, Szent-Györgyi AG. A mechanical study of regulation in the striated adductor muscle of the scallop. J Physiol. 1985;358:47–64. doi: 10.1113/jphysiol.1985.sp015539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I. Properties of tonic contractions produced by electrical stimulation of the anterior retractor of the byssus of Mytilus edulis. J Physiol. 1938a;94:1–12. doi: 10.1113/jphysiol.1938.sp003658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I. The effect of adaptation to electrical and chemical stimulation on the excitability of the anterior retractor of the byssus of Mytilus edulis. J Physiol. 1938b;92:241–248. doi: 10.1113/jphysiol.1938.sp003599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh I. Isotonic extension of unstriated muscle. Proc Indian Acad Sci B. 1943;17:20–27. [Google Scholar]
- Small JV, Squire JM. Structural basis of contraction in vertebrate smooth muscle. J Mol Biol. 1972;67:117–149. doi: 10.1016/0022-2836(72)90390-7. [DOI] [PubMed] [Google Scholar]
- Smith DA. Quantitative model for Schädler’s isometric oscillations in insect flight and cardiac muscle. J Muscle Res Cell Motil. 1991;12:455–465. doi: 10.1007/BF01738330. [DOI] [PubMed] [Google Scholar]
- Smith DS. The flight muscles of insects. Sci Am. 1965;212:77–87. [PubMed] [Google Scholar]
- Smith DS. 100 Hz remains upper limit of synchronous muscle contraction - an anomaly resolved. Nature. 1983;303:539–540. doi: 10.1038/303539a0. [DOI] [PubMed] [Google Scholar]
- Sobieszek A. The fine structure of the contractile apparatus of the anterior byssus retractor muscle of Mytilus edulis. J Ultrastruct Res. 1973;43:313–343. doi: 10.1016/s0022-5320(73)80041-3. [DOI] [PubMed] [Google Scholar]
- Sobieszek A, Matusovsky OS, Permyakova TV, Sarg B, Lindner H, Shelud’ko NS. Phosphorylation of myorod (catchin) by kinases tightly associated to molluscan and vertebrate smooth muscle myosins. Arch Biochem Biophys. 2006;454:197–205. doi: 10.1016/j.abb.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Sobue K, Sellers JR. Caldesmon, a novel regulatory protein in smooth muscle and nonmuscle actomyosin systems. J Biol Chem. 1991;266:12115–12118. [PubMed] [Google Scholar]
- Sohma H, Inoue K, Morita F. A cAMP-dependent regulatory protein for RLC-a myosin kinase catalyzing the phosphorylation of scallop smooth muscle myosin light chain. J Biochem (Tokyo) 1988a;103:431–435. doi: 10.1093/oxfordjournals.jbchem.a122287. [DOI] [PubMed] [Google Scholar]
- Sohma H, Morita F. Purification of a protein kinase phosphorylating myosin regulatory light chain-a (RLC-a) from smooth muscle of scallop, Patinopecten yessoensis. J Biochem (Tokyo) 1986;100:1155–1163. doi: 10.1093/oxfordjournals.jbchem.a121819. [DOI] [PubMed] [Google Scholar]
- Sohma H, Morita F. Characterization of regulatory light chain-a myosin kinase from smooth muscle of scallop, Patinopecten yessoensis. J Biochem (Tokyo) 1987;101:497–502. [PubMed] [Google Scholar]
- Sohma H, Sasada H, Inoue K, Morita F. Regulatory light chain-a myosin kinase (aMK) catalyzes phosphorylation of smooth muscle myosin heavy chains of scallop, Patinopecten yessoensis. J Biochem (Tokyo) 1988b;104:889–893. doi: 10.1093/oxfordjournals.jbchem.a122578. [DOI] [PubMed] [Google Scholar]
- Sohma H, Yazawa M, Morita F. Phosphorylation of regulatory light chain a (RLC-a) in smooth muscle myosin of scallop, Patinopecten yessoensis. J Biochem (Tokyo) 1985;98:569–572. doi: 10.1093/oxfordjournals.jbchem.a135311. [DOI] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol 522 Pt. 2000;2:177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotavalta O. The flight-tone (wing-stroke frequency) of insects. Acta Ent Fenn. 1947;4:1–117. doi: 10.1038/1701057a0. [DOI] [PubMed] [Google Scholar]
- Sotavalta O. Recordings of high wing-stroke and thoracic vibration frequency in some midges. Biol Bull. 1953;104:439–444. [Google Scholar]
- Sparrow J, Drummond D, Peckham M, Hennessey E, White D. Protein engineering and the study of muscle contraction in Drosophila flight muscles. J Cell Sci. 1991;14(Suppl):73–78. doi: 10.1242/jcs.1991.supplement_14.15. [DOI] [PubMed] [Google Scholar]
- Sparrow JC. Flight and phosphorylation. Nature. 1995;374:592–593. doi: 10.1038/374592a0. [DOI] [PubMed] [Google Scholar]
- Spudich JA. The myosin swinging cross-bridge model. Nat Rev Mol Cell Biol. 2001;2:387–392. doi: 10.1038/35073086. [DOI] [PubMed] [Google Scholar]
- Spudich JA, Finer J, Simmons B, Ruppel K, Patterson B, Uyeda T. Myosin structure and function. Cold Spring Harb Symp. 1995;60:783–791. doi: 10.1101/sqb.1995.060.01.084. [DOI] [PubMed] [Google Scholar]
- Squire JM. General model for the structure of all myosin-containing filaments. Nature. 1971;233:457–462. doi: 10.1038/233457a0. [DOI] [PubMed] [Google Scholar]
- Squire JM. General model of myosin filament structure. II Myosin filaments and cross-bridge interactions in vertebrate striated and insect flight muscles. J Mol Biol. 1972;72:125–138. doi: 10.1016/0022-2836(72)90074-5. [DOI] [PubMed] [Google Scholar]
- Squire JM. General model of myosin filament structure. III Molecular packing arrangements in myosin filaments. J Mol Biol. 1973;77:291–323. doi: 10.1016/0022-2836(73)90337-9. [DOI] [PubMed] [Google Scholar]
- Squire JM. Muscle filament structure and muscle contraction. Annu Rev Biophys Bioeng. 1975;4:137–163. doi: 10.1146/annurev.bb.04.060175.001033. [DOI] [PubMed] [Google Scholar]
- Squire JM. Muscle filament lattices and stretch-activation: the match-mismatch model reassessed. J Muscle Res Cell Motil. 1992;13:183–189. doi: 10.1007/BF01874155. [DOI] [PubMed] [Google Scholar]
- Squire JM, Al Khayat HA, Harford JJ, Hudson L, Irving T, Knupp C, Reedy MK. Modelling muscle motor conformations using low-angle X-ray diffraction. Proc Bio -Nanotechnol. 2003a;150:103–110. doi: 10.1049/ip-nbt:20031094. [DOI] [PubMed] [Google Scholar]
- Squire JM, Al Khayat HA, Harford JJ, Hudson L, Irving TC, Knupp C, Mok NS, Reedy MK. Myosin filament structure and myosin crossbridge dynamics in fish and insect muscles. Adv Exp Med Biol. 2003b;538:251–266. doi: 10.1007/978-1-4419-9029-7_24. [DOI] [PubMed] [Google Scholar]
- Squire JM, Al Khayat HA, Knupp C, Luther PK. Molecular architecture in muscle contractile assemblies. Adv Protein Chem. 2005a;71:17–87. doi: 10.1016/S0065-3233(04)71002-5. [DOI] [PubMed] [Google Scholar]
- Squire JM, Bekyarova T, Farman G, Gore D, Rajkumar G, Knupp C, Lucaveche C, Reedy MC, Reedy MK, Irving TC. The myosin filament superlattice in the flight muscles of flies: A-band lattice optimisation for stretch-activation? J Mol Biol. 2006;361:823–838. doi: 10.1016/j.jmb.2006.06.072. [DOI] [PubMed] [Google Scholar]
- Squire JM, Knupp C, Roessle M, Al Khayat HA, Irving TC, Eakins F, Mok NS, Harford JJ, Reedy MK. X-ray diffraction studies of striated muscles. Adv Exp Med Biol. 2005b;565:45–60. doi: 10.1007/0-387-24990-7_5. [DOI] [PubMed] [Google Scholar]
- Stafford WF, III, Jacobsen MP, Woodhead J, Craig R, O’Neall-Hennessey E, Szent-Györgyi AG. Calcium-dependent structural changes in scallop heavy meromyosin. J Mol Biol. 2001;307:137–147. doi: 10.1006/jmbi.2000.4490. [DOI] [PubMed] [Google Scholar]
- Stafford WF, III, Szentkiralyi EM, Szent-Györgyi AG. Regulatory properties of single-headed fragments of scallop myosin. Biochemistry. 1979;18:5273–5280. doi: 10.1021/bi00591a002. [DOI] [PubMed] [Google Scholar]
- Stanley DW. Actomyosin solubility and skeletal muscle cell emptying of horseshoe crab. Comp Biochem Physiol. 1970;36:279–284. doi: 10.1016/0010-406x(70)90007-1. [DOI] [PubMed] [Google Scholar]
- Steiger GJ, Abbott RH. Biochemical interpretation of tension transients produced by a four-state mechanical model. J Muscle Res Cell Motil. 1981;2:245–260. doi: 10.1007/BF00713264. [DOI] [PubMed] [Google Scholar]
- Steiger GJ, Rüegg JC. Energetics and “efficiency” in the isolated contractile machinery of an insect fibrillar muscle at various frequencies of oscillation. Pflügers Arch. 1969;307:1–21. doi: 10.1007/BF00589455. [DOI] [PubMed] [Google Scholar]
- Steiger GJ, Rüegg JC, Boldt KM, Lübbers DW, Breull W. Changes in the polarization of tryptophan fluorescence in the actomyosin system of working muscle fibers. Cold Spring Harb Symp Quant Biol. 1972;37:377–378. [Google Scholar]
- Stephens RE. Anomalous contraction of invertebrate striated muscle. J Cell Biol. 1965;27:639–649. doi: 10.1083/jcb.27.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson DG, Williams DA. Activation of skinned arthropod muscle fibres by Ca2+ and Sr2+ J Muscle Res Cell Motil. 1980;1:73–87. doi: 10.1007/BF00711926. [DOI] [PubMed] [Google Scholar]
- Stewart M, Kensler RW, Levine RJC. Structure of Limulus telson muscle thick filaments. J Mol Biol. 1981;153:781–790. doi: 10.1016/0022-2836(81)90418-6. [DOI] [PubMed] [Google Scholar]
- Stewart M, Kensler RW, Levine RJC. Three-dimensional reconstruction of thick filaments from Limulus and scorpion muscle. J Cell Biol. 1985;101:402–411. doi: 10.1083/jcb.101.2.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelina AV, Ivanow II, Zhukow EK. Some properties of contractile proteins in different types of striated muscle fibers [in Russian, English summary] J Physiol (USSR) 1957;43:351–357. [Google Scholar]
- Sugi H, Gomi S. Changes in the A band width during contraction in horseshoe crab striated muscle. Experientia. 1981;37:65–67. [Google Scholar]
- Sugi H, Iwamoto H, Shimo M, Shirakawa I. Evidence for load-bearing structures specialized for the catch state in Mytilus smooth muscle. Comp Biochem Physiol. 1999;122A:347–353. [Google Scholar]
- Sugi H, Suzuki S. The nature of potassium- and acetylcholine-induced contractures in the anterior byssal retractor muscle of Mytilus edulis. Comp Biochem Physiol. 1978;61C:275–279. doi: 10.1016/0306-4492(78)90053-9. [DOI] [PubMed] [Google Scholar]
- Sugi H, Tsuchiya T. Effect of isotonic shortening on the load-sustaining ability and the instantaneous elasticity in molluscan smooth muscle. Proc Jpn Acad. 1975;51:712–715. [Google Scholar]
- Sugi H, Tsuchiya T. The change in the load-sustaining ability and in the series elasticity in Mytilus smooth muscle during isotonic shortening. J Physiol. 1979;288:635–648. [PMC free article] [PubMed] [Google Scholar]
- Surholt B, Bertsch A, Baal T, Greive H. Non-shivering thermogenesis in asynchronous flight muscles of bumblebees? Comparative studies on males of Bombus terrestris, Xylocopa sulcatipes and Acherontia atropos. Comp Biochem Physiol. 1990;97A:493–499. [Google Scholar]
- Suzuki H, Konno K, Arai K, Watanabe S. ATP-induced tension development in glycerinated fibers of scallop adductor striated muscle. J Biochem (Tokyo) 1980;88:909–911. doi: 10.1093/oxfordjournals.jbchem.a133047. [DOI] [PubMed] [Google Scholar]
- Suzuki S. Physiological and cytochemical studies on activator calcium in contraction by smooth muscle of a sea cucumber, Isostichopus badionotus. Cell Tissue Res. 1982;222:11–24. doi: 10.1007/BF00218285. [DOI] [PubMed] [Google Scholar]
- Svendsen KH. Actin filament organization and crossbridge decoration in a contracting molluscan smooth muscle. Int J Biol Macromol. 1981;3:253–258. [Google Scholar]
- Svendsen KH. Ultrastructure of a molluscan smooth muscle during phasic contractions and the relaxed state. Int J Biol Macromol. 1982;4:43–49. [Google Scholar]
- Svidersky VL. Are there stereotypes of evolution? Skeletal muscles of insects and mammals. J Evol Biochem Physiol. 1999;35:343–359. [PubMed] [Google Scholar]
- Swank DM, Bartoo ML, Knowles AF, Iliffe C, Bernstein SI, Molloy JE, Sparrow JC. Alternative exon-encoded regions of Drosophila myosin heavy chain modulate ATPase rates and actin sliding velocity. J Biol Chem. 2001;276:15117–15124. doi: 10.1074/jbc.M008379200. [DOI] [PubMed] [Google Scholar]
- Swank DM, Braddock J, Brown W, Lesage H, Bernstein SI, Maughan DW. An alternative domain near the ATP binding pocket of Drosophila myosin affects muscle fiber kinetics. Biophys J. 2006a;90:2427–2435. doi: 10.1529/biophysj.105.075184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swank DM, Knowles AF, Kronert WA, Suggs JA, Morrill GE, Nikkhoy M, Manipon GG, Bernstein SI. Variable N-terminal regions of muscle myosin heavy chain modulate ATPase rate and actin sliding velocity. J Biol Chem. 2003;278:17475–17482. doi: 10.1074/jbc.M212727200. [DOI] [PubMed] [Google Scholar]
- Swank DM, Knowles AF, Suggs JA, Sarsoza F, Lee A, Maughan DW, Bernstein SI. The myosin converter domain modulates muscle performance. Nat Cell Biol. 2002;4:312–316. doi: 10.1038/ncb776. [DOI] [PubMed] [Google Scholar]
- Swank DM, Maughan DW. Rates of force generation in Drosophila fast and slow muscle types have opposite responses to phosphate. Adv Exp Med Biol. 2003;538:459–468. doi: 10.1007/978-1-4419-9029-7_42. [DOI] [PubMed] [Google Scholar]
- Swank DM, Vishnudas VK, Maughan DW. An exceptionally fast actomyosin reaction powers insect flight muscle. Proc Natl Acad Sci USA. 2006b;103:17543–17547. doi: 10.1073/pnas.0604972103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson CJ. Isometric responses of the paramyosin smooth muscle of Paragordius varius (Leidy) (Aschelminthes, Nematomorpha) Z vergl Physiol. 1971a;74:403–410. [Google Scholar]
- Swanson CJ. Occurrence of paramyosin among the nematomorpha. Nat New Biol. 1971b;232:122–123. doi: 10.1038/newbio232122a0. [DOI] [PubMed] [Google Scholar]
- Sweeney HL, Yang Z, Zhi G, Stull JT, Trybus KM. Charge replacement near the phosphorylatable serine of the myosin regulatory light chain mimics aspects of phosphorylation. Proc Natl Acad Sci USA. 1994;91:1490–1494. doi: 10.1073/pnas.91.4.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sydorenko NP, Klimov AA. Thick filament shortening as a corollary of the filament sliding [in Russian, English summary] Biofizika. 1994;39:153–155. [PubMed] [Google Scholar]
- Syme DA, Josephson RK. How to build fast muscles: synchronous and asynchronous designs. Integr Comp Biol. 2002;42:762–770. doi: 10.1093/icb/42.4.762. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi AG. Meromyosins, the subunits of myosin. Arch Biochem Biophys. 1953;42:305–320. doi: 10.1016/0003-9861(53)90360-9. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi AG. Calcium regulation of muscle contraction. Biophys J. 1975;15:707–723. doi: 10.1016/S0006-3495(75)85849-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szent-Györgyi AG. Comparative survey of the regulatory role of calcium in muscle. Symp Soc Exp Biol. 1976;30:335–347. [Google Scholar]
- Szent-Györgyi AG. Regulation of molluscan myosin by light chains. Acta Biochim Biophys Hung. 1987;22:377–389. [PubMed] [Google Scholar]
- Szent-Györgyi AG. Regulation of contraction by calcium binding myosins. Biophys Chem. 1996;59:357–363. doi: 10.1016/0301-4622(95)00128-x. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi AG. Milestone in physiology. The early history of the biochemistry of muscle contraction. J Gen Physiol. 2004;123:631–641. doi: 10.1085/jgp.200409091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szent-Györgyi AG, Borbiro M. Depolymerization of light meromyosin by urea. Arch Biochem Biophys. 1956;60:180–197. doi: 10.1016/0003-9861(56)90410-6. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi AG, Cohen C. Role of proline in polypeptide chain configuration of proteins. Science. 1957;126:697–698. doi: 10.1126/science.126.3276.697. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi AG, Cohen C, Kendrick-Jones J. Paramyosin and the filaments of molluscan “catch” muscles. II Native filaments: isolation and characterization J Mol Biol. 1971;56:239–258. doi: 10.1016/0022-2836(71)90462-1. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi AG, Cohen C, Philpott DE. Light meromyosin fraction. I: A helical molecule from myosin. J Mol Biol. 1960;2:133–142. [Google Scholar]
- Szent-Györgyi AG, Kalabokis VN, Perreault-Micale CL. Regulation by molluscan myosins. Mol Cell Biochem. 1999;190:55–62. [PubMed] [Google Scholar]
- Szent-Györgyi AG, Niebieski R. Preparation of light chains from scallop myosin. Methods Enzymol. 1982;85:81–84. doi: 10.1016/0076-6879(82)85011-8. [DOI] [PubMed] [Google Scholar]
- Szent-Györgyi AG, Szentkiralyi EM, Kendrick-Jones J. The light chains of scallop myosin as regulatory subunits. J Mol Biol. 1973;74:179–203. doi: 10.1016/0022-2836(73)90106-x. [DOI] [PubMed] [Google Scholar]
- Szentkiralyi EM. Tryptic digestion of scallop S1: evidence for a complex between the two light-chains and a heavy-chain peptide. J Muscle Res Cell Motil. 1984;5:147–164. doi: 10.1007/BF00712153. [DOI] [PubMed] [Google Scholar]
- Szentkiralyi EM. An intact heavy chain at the actin-subfragment 1 interface is required for the ATPase activity of scallop myosin. J Muscle Res Cell Motil. 1987;8:349–357. doi: 10.1007/BF01568891. [DOI] [PubMed] [Google Scholar]
- Tajima Y, Amemiya Y. X-ray studies of thin filaments in a tonically contracting mollucan smooth muscle. Adv Biophys. 1991;27:77–88. doi: 10.1016/0065-227x(91)90009-3. [DOI] [PubMed] [Google Scholar]
- Tajima Y, Makino K, Hanyuu T, Wakabayashi K, Amemiya Y. X-ray evidence for the elongation of thin and thick filaments during isometric contraction of a molluscan smooth muscle. J Muscle Res Cell Motil. 1994;15:659–671. doi: 10.1007/BF00121073. [DOI] [PubMed] [Google Scholar]
- Tajima Y, Makino K, Hanyuu T, Wakabayashi K, Amemiya Y. The overlap between the thin- and thick-filament reflections in the small-angle X-ray diffraction pattern from a molluscan smooth muscle. J Synchrotron Radiat. 1999;6:93–100. [Google Scholar]
- Takagi T, Konishi K. Amino acid sequence of troponin C obtained from ascidian (Halocynthia roretzi) body wall muscle. J Biochem (Tokyo) 1983;94:1753–1760. doi: 10.1093/oxfordjournals.jbchem.a134526. [DOI] [PubMed] [Google Scholar]
- Takagi T, Petrova T, Comte M, Kuster T, Heizmann CW, Cox JA. Characterization and primary structure of Amphioxus troponin C. Eur. J Biochem. 1994;221:537–546. doi: 10.1111/j.1432-1033.1994.tb18766.x. [DOI] [PubMed] [Google Scholar]
- Takahashi I, Shimada M, Akimoto T, Kishi T, Sugi H. Electron microscopic evidence for the thick filament interconnections associated with the catch state in the anterior byssal retractor muscle of Mytilus edulis. Comp Biochem Physiol. 2003;134A:117–122. doi: 10.1016/s1095-6433(02)00225-8. [DOI] [PubMed] [Google Scholar]
- Takahashi K. Nervous control of contraction and relaxation in the anterior byssus retractor muscle of Mytilus edulis. Annot Zool Japon. 1960;33:67–84. [Google Scholar]
- Takahashi K, Nadalginard B. Molecular cloning and sequence analysis of smooth muscle calponin. J Biol Chem. 1991;266:13284–13288. [PubMed] [Google Scholar]
- Takahashi M, Fukushima Y, Inoue K, Hasegawa Y, Morita F, Takahashi K. Ca2+-sensitive transition in the molecular conformation of molluscan muscle myosins. J Biochem (Tokyo) 1989;105:149–151. doi: 10.1093/oxfordjournals.jbchem.a122628. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Morita F. An activating factor (tropomyosin) for the superprecipitation of actomyosin prepared from scallop adductor muscles. J Biochem (Tokyo) 1986;99:339–347. doi: 10.1093/oxfordjournals.jbchem.a135488. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Morita F. Myosin may stay in EADP species during the catch contraction in scallop smooth muscle. J Biochem (Tokyo) 1989;106:868–871. doi: 10.1093/oxfordjournals.jbchem.a122944. [DOI] [PubMed] [Google Scholar]
- Takahashi M, Sohma H, Morita F. The steady state intermediate of scallop smooth muscle myosin ATPase and effect of light chain phosphorylation. A molecular mechanism for catch contraction. J Biochem (Tokyo) 1988;104:102–107. doi: 10.1093/oxfordjournals.jbchem.a122402. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Takano-Ohmuro H, Maruyama K. Regulation of Drosophila myosin ATPase activity by phosphorylation of myosin light chains. I Wild-type fly. Comp Biochem Physiol. 1990a;95B:179–181. doi: 10.1016/0305-0491(90)90267-w. [DOI] [PubMed] [Google Scholar]
- Takahashi S, Takano-Ohmuro H, Maruyama K, Hotta Y. Regulation of Drosophila myosin ATPase activity by phorphorylation of myosin light chains. II Flightless mfd- fly. Comp Biochem Physiol. 1990b;95B:183–185. doi: 10.1016/0305-0491(90)90268-x. [DOI] [PubMed] [Google Scholar]
- Takano-Ohmuro H, Takahashi S, Hirose G, Maruyama K. Phosphorylated and dephosphorylated myosin light chains of Drosophila fly and larva. Comp Biochem Physiol. 1990;95B:171–177. doi: 10.1016/0305-0491(90)90266-v. [DOI] [PubMed] [Google Scholar]
- Takano-Ohmuro H, Tanikawa M, Maruyama K. Phosphorylation of cricket myosin light chain and Mg2+-activated actomyosin ATPase activity. Zool Sci. 1986;3:715–717. [Google Scholar]
- Takayanagi I, Murakami H, Iwayama Y, Yoshida Y, Miki S. Dopamine receptor in anterior byssus retractor muscle of Mytilus edulis. Jpn J Pharmacol. 1981;31:249–252. doi: 10.1254/jjp.31.249. [DOI] [PubMed] [Google Scholar]
- Takemoto M, Saitoh H, Muneoka Y. Relaxing action of Trp-Nle-Arg-Phe-NH2 on the anterior byssus retractor muscle of Mytilus. Hiroshima J Med Sci. 1986;35:381–388. [PubMed] [Google Scholar]
- Takito J, Konishi K. Enzymatic properties of myosin from ascidian body-wall smooth muscle. Comp Biochem Physiol. 1986;84B:59–62. [Google Scholar]
- Tameyasu T. The effect of hypertonic solutions on the rate of relaxation of contracture tension in Mytilus smooth muscle. J Exp Biol. 1978;74:197–210. doi: 10.1242/jeb.74.1.197. [DOI] [PubMed] [Google Scholar]
- Tameyasu T. Regulation of cross bridge detachment by Ca ions at high ionic strength in molluscan catch muscle. Experientia. 1990;46:677–679. doi: 10.1007/BF01939931. [DOI] [PubMed] [Google Scholar]
- Tameyasu T. Oscillatory contraction of single sarcomere in single myofibril of glycerinated, striated adductor muscle of scallop. Jpn J Physiol. 1994;44:295–318. doi: 10.2170/jjphysiol.44.295. [DOI] [PubMed] [Google Scholar]
- Tameyasu T, Sugi H. The series elastic component and the force-velocity relation in the anterior byssal retractor muscle of the Mytilus edulis during active and catch contractions. J Exp Biol. 1976;64:497–510. doi: 10.1242/jeb.64.2.497. [DOI] [PubMed] [Google Scholar]
- Tameyasu T, Tanaka M. Effects of calcium and ADP on tension responses to step length increases in glycerinated Mytilus smooth muscle. Jpn J Physiol. 1991;41:413–427. doi: 10.2170/jjphysiol.41.413. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Takeya Y, Doi T, Yumoto F, Tanokura M, Ohtsuki I, Nishita K, Ojima T. Comparative studies on the functional roles of N- and C-terminal regions of molluskan and vertebrate troponin-I. FEBS J. 2005;272:4475–4486. doi: 10.1111/j.1742-4658.2005.04866.x. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Tanaka M. Dependence of tension development on calcium and magnesium adenosinetriphosphates in chemically skinned molluscan smooth muscle fibers. J Biochem (Tokyo) 1979a;85:713–717. [PubMed] [Google Scholar]
- Tanaka M, Tanaka H. Extraction and functional reformation of thick filaments in chemically skinned molluscan catch muscle fibers. J Biochem (Tokyo) 1979b;85:535–540. doi: 10.1093/oxfordjournals.jbchem.a132361. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Ishida H, Ohtsu K, Matsuno A. Ultrastructural studies of calcium location during the “catch” contraction of clam smooth adductor muscle cells. Zool Sci. 1998;15:855–859. [Google Scholar]
- Tawada K, Kawai M. Covalent cross-linking of single fibers from rabbit psoas increases oscillatory power. Biophys J. 1990;57:643–7. doi: 10.1016/S0006-3495(90)82582-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor EA, Cramer C. Birefringence of protein solutions and biological systems: II. Studies on TMV, tropocollagen, and paramyosin. Biophys J. 1963;3:143–154. doi: 10.1016/s0006-3495(63)86810-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KA, Crowther RA. 3D reconstruction from the Fourier transform of a single superlattice image of an oblique section. Ultramicroscopy. 1992;41:153–167. doi: 10.1016/0304-3991(92)90105-s. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Reedy MC, Cordova L, Reedy MK. Three-dimensional reconstruction of rigor insect flight muscle from tilted thin sections. Nature. 1984;310:285–291. doi: 10.1038/310285a0. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Reedy MC, Cordova L, Reedy MK. Image reconstruction using electron micrographs of insect flight muscle: use of thick transverse sections to supplement data from tilted thin longitudinal sections. Biophys J. 1986;49:353–364. doi: 10.1016/S0006-3495(86)83648-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KA, Reedy MC, Cordova L, Reedy MK. Three-dimensional image reconstruction of insect flight muscle. I The rigor myac layer. J Cell Biol. 1989a;109:1085–1102. doi: 10.1083/jcb.109.3.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KA, Reedy MC, Cordova L, Reedy MK. Three-dimensional image reconstruction of insect flight muscle. II The rigor actin layer. J Cell Biol. 1989b;109:1103–1123. doi: 10.1083/jcb.109.3.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KA, Reedy MC, Reedy MK, Crowther RA. Crossbridges in the complete unit cell of rigor insect flight muscle imaged by three-dimensional reconstruction from oblique sections. J Mol Biol. 1993;233:86–108. doi: 10.1006/jmbi.1993.1487. [DOI] [PubMed] [Google Scholar]
- Taylor KA, Schmitz H, Reedy MC, Goldman YE, Franzini-Armstrong C, Sasaki H, Tregear RT, Poole K, Lucaveche C, Edwards RJ, Chen LF, Winkler H, Reedy MK. Tomographic 3D reconstruction of quick-frozen, Ca2+-activated contracting insect flight muscle. Cell. 1999;99:421–431. doi: 10.1016/s0092-8674(00)81528-7. [DOI] [PubMed] [Google Scholar]
- Thomas DD, Cooke R, Barnett VA. Orientation and rotational mobility of spin-labeled myosin heads in insect flight muscle in rigor. J Muscle Res Cell Motil. 1983;4:367–378. doi: 10.1007/BF00712002. [DOI] [PubMed] [Google Scholar]
- Thomas N, Thornhill RA. Stretch activation and nonlinear elasticity of muscle cross-bridges. Biophys J. 1996;70:2807–2818. doi: 10.1016/S0006-3495(96)79850-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas N, Thornhill RA. The physics of biological molecular motors. Journal of Physics D-Applied Physics. 1998;31:253–266. [Google Scholar]
- Thorson J, White DCS. Distributed representations for actin-myosin interaction in the oscillatory contraction of muscle. Biophys J. 1969;9:360–391. doi: 10.1016/S0006-3495(69)86392-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorson J, White DCS. Role of cross-bridge distortion in the small-signal mechanical dynamics of insect and rabbit striated muscle. J Physiol. 1983;343:59–84. doi: 10.1113/jphysiol.1983.sp014881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thuma JB, Morris LG, Weaver AL, Hooper SL. Lobster (Panulirus interruptus) pyloric muscles express the motor patterns of three neural networks, only one of which innervates the muscles. J Neurosci. 2003;23:8911–20. doi: 10.1523/JNEUROSCI.23-26-08911.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien-Chin T, Tsu-Hsün K, Chia-Mu P, Yu-Shang C, Yüng-Shui T. Electron microscopical studies of tropomyosin and paramyosin. I Crystals [translated from Chinese from Acta Biochim et Biophys Sin 3:206–219, 1963] Sci Sin. 1965;14:91–92. [PubMed] [Google Scholar]
- Tohtong R, Yamashita H, Graham M, Haeberle J, Simcox A, Maughan D. Impairment of muscle function caused by mutations of phosphorylation sites in myosin regulatory light chain. Nature. 1995;374:650–3. doi: 10.1038/374650a0. [DOI] [PubMed] [Google Scholar]
- Tomioka H, Yamaguchi K, Hashimoto K, Matsuura F. Studies on myosin of spiny lobster. II Enzymatic properties. B Jpn Soc Sci Fish. 1975;41:51–58. [Google Scholar]
- Tonomura Y, Yagi K, Matsumiya H. Contractile proteins from adductors of pecten. I Some enzymic and physicochemical properties. Arch Biochem Biophys. 1955;59:76–89. doi: 10.1016/0003-9861(55)90464-1. [DOI] [PubMed] [Google Scholar]
- Tonomura Y, Yaki K, Matsumiya H. Contractile proteins from adductors of pecten. II Interaction with adenosine triphosphate. Arch Biochem Biophys. 1956;64:466–479. doi: 10.1016/0003-9861(56)90289-2. [DOI] [PubMed] [Google Scholar]
- Toyo-Oka T. Effects of various concentrations of MgATP on the superprecipitation and ATPase activity of scallop striated muscle myosin B1. J Biochem (Tokyo) 1979;85:871–877. [PubMed] [Google Scholar]
- Toyota N, Obinata R, Terakado K. Isolation of troponin-tropomyosin-containing thin filaments from ascidian smooth muscle. Comp Biochem Physiol. 1979;62B:433–441. [Google Scholar]
- Tregear RT. The oscillation of insect flight muscle. Curr Top Bioenerg. 1967;2:269–286. [Google Scholar]
- Tregear RT. Physiology of insect flight muscle. Handbook of Physiology. 1983;10:487–506. [Google Scholar]
- Tregear RT, Clarke ML. On the possibility of interaction between neighbouring crossbridges. Adv Exp Med Biol. 1984;170:177–184. doi: 10.1007/978-1-4684-4703-3_15. [DOI] [PubMed] [Google Scholar]
- Tregear RT, Edwards RJ, Irving TC, Poole KJ, Reedy MC, Schmitz H, Towns-Andrews E, Reedy MK. X-ray diffraction indicates that active cross-bridges bind to actin target zones in insect flight muscle. Biophys J. 1998;74:1439–1451. doi: 10.1016/S0006-3495(98)77856-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregear RT, Reedy MC, Goldman YE, Taylor KA, Winkler H, Franzini-Armstrong C, Sasaki H, Lucaveche C, Reedy MK. Cross-bridge number, position, and angle in target zones of cryofixed isometrically active insect flight muscle. Biophys J. 2004;86:3009–3019. doi: 10.1016/S0006-3495(04)74350-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregear RT, Terry CS, Sayers AJ. The process of muscle relaxation by the combined action of MgAMPPNP and ethylene glycol. J Muscle Res Cell Motil. 1984;5:687–696. doi: 10.1007/BF00713927. [DOI] [PubMed] [Google Scholar]
- Tregear RT, Wakabayashi K, Tanaka H, Iwamoto H, Reedy MC, Reedy MK, Sugi H, Amemiya Y. X-ray diffraction and electron microscopy from Lethocerus flight muscle partially relaxed by adenylylimidodiphosphate and ethylene glycol. J Mol Biol. 1990;214:129–141. doi: 10.1016/0022-2836(90)90152-C. [DOI] [PubMed] [Google Scholar]
- Trombitás K, Baatsen P, Pollack GH. Rigor bridge angle: effects of applied stress and preparative procedure. J Ultrastruct Mol Struct Res. 1986;97:39–49. [Google Scholar]
- Trombitás K, Baatsen PH, Pollack GH. Effect of tension on the rigor cross-bridge angle. Adv Exp Med Biol. 1988;226:17–30. [PubMed] [Google Scholar]
- Trombitás K, Tigyi-Sebes A. Structure of thick filaments from insect flight muscle. Acta Biochim Biophys Hung. 1986;21:115–128. [PubMed] [Google Scholar]
- Trybus KM. Role of myosin light chains. J Muscle Res Cell Motil. 1994;15:587–594. doi: 10.1007/BF00121066. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Fukuhara S, Matsumoto JJ. Physico-chemical properties of squid paramyosin. B Jpn Soc Sci Fish. 1980;46:197–200. [Google Scholar]
- Tsuchiya T, Kaneko T, Matsumoto JJ. Calcium sensitivity of mantle muscle of squid. J Biochem (Tokyo) 1978a;83:1191–1193. doi: 10.1093/oxfordjournals.jbchem.a132009. [DOI] [PubMed] [Google Scholar]
- Tsuchiya T, Obinata T, Sato M, Mori T, Suzuki E, Amemiya S. Non-catch contraction in paramyosin containing muscle in an echinothuriid sea urchin Asthenosoma ijjimai. J Exp Biol. 1992;162:361–365. [Google Scholar]
- Tsuchiya T, Suzuki H, Matsumoto JJ. Isolation and purification of squid actin. B Jpn Soc Sci Fish. 1977a;43:877–884. [Google Scholar]
- Tsuchiya T, Suzuki H, Matsumoto JJ. Physicochemical and biochemical properties of squid actin. B Jpn Soc Sci Fish. 1977b;43:1233–1240. [Google Scholar]
- Tsuchiya T, Takei N. Time course of tension decay after stretch and unloaded shortening velocity in molluscan catch muscle at various physiological states. Comp Biochem Physiol. 1986;83A:519–523. [Google Scholar]
- Tsuchiya T, Yamada N, Matsumoto JJ. Extraction and purification of squid myosin. B Jpn Soc Sci Fish. 1978b;44:175–179. [Google Scholar]
- Tsuchiya T, Yamada N, Matsumoto JJ. Physico-chemical properties of squid myosin. B Jpn Soc Sci Fish. 1978c;44:181–184. [Google Scholar]
- Tsuchiya T, Yamada N, Mori H, Matsumoto JJ. Adenosinetriphosphatase activity of squid myosin. B Jpn Soc Sci Fish. 1978d;44:203–207. [Google Scholar]
- Tsutsui Y, Yoshio M, Oiwa K, Yamada A. Twitchin purified from molluscan catch muscles regulates interactions between actin and myosin filaments at rest in a phosphorylation-dependent manner. J Muscle Res Cell Motil. 2005;26:461–465. doi: 10.1007/s10974-005-9030-9. [DOI] [PubMed] [Google Scholar]
- Twarog BM. Responses of a molluscan smooth muscle to acetylcholine and 5-hydroxytryptamine. J Cell Comp Physiol. 1954;44:141–164. doi: 10.1002/jcp.1030440112. [DOI] [PubMed] [Google Scholar]
- Twarog BM. Effects of acetylcholine and 5-hydroxytryptamine on the contraction of a molluscan smooth muscle. J Physiol. 1960a;152:236–242. doi: 10.1113/jphysiol.1960.sp006484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twarog BM. Innervation and activity of a molluscan smooth muscle. J Physiol. 1960b;152:220–235. doi: 10.1113/jphysiol.1960.sp006483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twarog BM. Catch and the mechanism of action of 5-hydroxytryptamine on molluscan muscle: a speculation. Life Sci. 1966;5:1201–1213. doi: 10.1016/0024-3205(66)90042-7. [DOI] [PubMed] [Google Scholar]
- Twarog BM. Factors influencing contraction and catch in Mytilus smooth muscle. J Physiol. 1967a;192:847–856. doi: 10.1113/jphysiol.1967.sp008335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twarog BM. The regulation of catch in molluscan muscle. J Gen Physiol. 1967b;50:157–169. doi: 10.1085/jgp.50.6.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twarog BM. Possible mechanism of action of serotonin on molluscan muscle. Adv Pharmacol. 1968;6:5–15. doi: 10.1016/s1054-3589(08)60289-6. [DOI] [PubMed] [Google Scholar]
- Twarog BM. Aspects of smooth muscle function in molluscan catch muscle. Physiol Rev. 1976;56:829–838. doi: 10.1152/physrev.1976.56.4.829. [DOI] [PubMed] [Google Scholar]
- Twarog BM, Cole RA. Relaxation of catch in a molluscan smooth muscle. II Effects of serotonin, dopamine and related compounds. Comp Biochem Physiol. 1972;43A:331–335. doi: 10.1016/0300-9629(72)90192-2. [DOI] [PubMed] [Google Scholar]
- Twarog BM, Muneoka Y. Calcium and the control of contraction and relaxation in a molluscan catch muscle. Cold Spring Harb Symp Quant Biol. 1972;37:489–503. [Google Scholar]
- Ueda T, Katsuzaki H, Terami H, Ohtsuka H, Kagawa H, Murase T, Kajiwara Y, Yoshioka O, Iio T. Calcium-bindings of wild type and mutant troponin Cs of Caenorhabditis elegans. Biochim Biophys Acta. 2001;1548:220–228. doi: 10.1016/s0167-4838(01)00234-5. [DOI] [PubMed] [Google Scholar]
- Ulbrich M, Rüegg JC. Stretch-induced formation of ATP-P32 in glycerinated fibres of insect flight muscle. Experientia. 1971;27:45–46. doi: 10.1007/BF02137732. [DOI] [PubMed] [Google Scholar]
- Vale RD, Milligan RA. The way things move: looking under the hood of molecular motor proteins. Science. 2000;288:88–95. doi: 10.1126/science.288.5463.88. [DOI] [PubMed] [Google Scholar]
- Vale RD, Szent-Györgyi AG, Sheetz MP. Movement of scallop myosin on Nitella actin filaments: regulation by calcium. Proc Natl Acad Sci USA. 1984;81:6775–6778. doi: 10.1073/pnas.81.21.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Dijk JA. Some points in the physiology and pharmacology of the adductor muscle of pecten and their relation to tonus in vertebral muscle. Arch Neerland Physiol. 1937;22:419–456. [Google Scholar]
- Van Lunteren E, Sankey CB. Catchlike property of rat diaphragm: subsequent train frequency effects in variable-train stimulation. J Appl Physiol. 2000;88:586–598. doi: 10.1152/jappl.2000.88.2.586. [DOI] [PubMed] [Google Scholar]
- van Overbeek J. Über Tonuserzeugung unter dem Einfluss von Muskeldehnung (bei Anodonta cygnea) Z vergl Physiol. 1931;15:784–797. [Google Scholar]
- Vibert P. Helical reconstruction of frozen hydrated scallop myosin filaments. J Mol Biol. 1992;223:661–671. doi: 10.1016/0022-2836(92)90982-p. [DOI] [PubMed] [Google Scholar]
- Vibert P, Castellani L. Substructure and accessory proteins in scallop myosin filaments. J Cell Biol. 1989;109:539–547. doi: 10.1083/jcb.109.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert P, Cohen C, Hardwicke PM, Szent-Györgyi AG. Electron microscopy of cross-linked scallop myosin. J Mol Biol. 1985;183:283–286. doi: 10.1016/0022-2836(85)90221-9. [DOI] [PubMed] [Google Scholar]
- Vibert P, Craig R. Three-dimensional reconstruction of thin filaments decorated with a Ca2+-regulated myosin. J Mol Biol. 1982;157:299–319. doi: 10.1016/0022-2836(82)90236-4. [DOI] [PubMed] [Google Scholar]
- Vibert P, Craig R. Electron microscopy and image analysis of myosin filaments from scallop striated muscle. J Mol Biol. 1983;165:303–320. doi: 10.1016/s0022-2836(83)80259-9. [DOI] [PubMed] [Google Scholar]
- Vibert P, Craig R. Structural changes that occur in scallop myosin filaments upon activation. J Cell Biol. 1985;101:830–837. doi: 10.1083/jcb.101.3.830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert P, Craig R, Lehman W. Steric model for activation of muscle thin filaments. J Mol Biol. 1997;266:8–14. doi: 10.1006/jmbi.1996.0800. [DOI] [PubMed] [Google Scholar]
- Vibert P, Szent-Györgyi AG, Craig R, Wray J, Cohen C. Changes in crossbridge attachment in a myosin-regulated muscle. Nature. 1978;273:64–66. doi: 10.1038/273064a0. [DOI] [PubMed] [Google Scholar]
- Vibert P, Szentkiralyi E, Hardwicke P, Szent-Györgyi AG, Cohen C. Structural models for the regulatory switch of myosin. Biophys J. 1986;49:131–133. doi: 10.1016/S0006-3495(86)83622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vibert PJ, Haselgrove JC, Lowy J, Poulsen FR. Structural changes in actin- containing filaments of muscle. J Mol Biol. 1972;71:757–767. doi: 10.1016/s0022-2836(72)80036-6. [DOI] [PubMed] [Google Scholar]
- Vigoreaux JO. Genetics of the Drosophila flight muscle myofibril: a window into the biology of complex systems. BioEssays. 2001;23:1047–1063. doi: 10.1002/bies.1150. [DOI] [PubMed] [Google Scholar]
- Vigoreaux JO, Moore JR, Maughan DW. Role of the elastic protein projectin in stretch activation and work output of Drosophila flight muscles. Adv Exp Med Biol. 2000;481:237–250. doi: 10.1007/978-1-4615-4267-4_14. [DOI] [PubMed] [Google Scholar]
- Vilfan A, Duke T. Synchronization of active mechanical oscillators by an inertial load. Phys Rev Lett. 2003;91:114101-1–114101-4. doi: 10.1103/PhysRevLett.91.114101. [DOI] [PubMed] [Google Scholar]
- Vilfan A, Frey E. Oscillations in molecular motor assemblies. Journal of Physics-Condensed Matter. 2005;17:S3901–S3911. doi: 10.1088/0953-8984/17/47/018. [DOI] [PubMed] [Google Scholar]
- Volkmann N, Hanein D. Actomyosin: law and order in motility. Curr Opin Cell Biol. 2000;12:26–34. doi: 10.1016/s0955-0674(99)00053-8. [DOI] [PubMed] [Google Scholar]
- vom Brocke HH. The activating effects of calcium ions on the contractile system of insect fibrillar flight muscle. Pflügers Arch. 1966;290:70–79. doi: 10.1007/BF00362620. [DOI] [PubMed] [Google Scholar]
- von Hehn G. Verkürzung der A-Zone während der Kontraktion lebender Muskelfasern des Eileiters von Carausius morosus. Exp Cell Res. 1965;37:699–701. doi: 10.1016/0014-4827(65)90222-3. [DOI] [PubMed] [Google Scholar]
- von Uexküll J. Studien über den Tonus. I Der biologische Bauplan von Sipunculus nudus. Z Biol. 1903;44:269–344. [Google Scholar]
- von Uexküll J. Studien über den Tonus. VI Die Pilger-Muschel. Z Biol. 1912;58:305–332. [Google Scholar]
- von Uexküll J. Die Sperrmuskulatur von Holthurien. Pflügers Arch. 1926;212:1–14. [Google Scholar]
- Vorotnikov AV, Krymsky MA, Shirinsky VP. Signal transduction and protein phosphorylation in smooth muscle contraction. Biochem (Moscow) 2002;67:1309–1328. doi: 10.1023/a:1021835924335. [DOI] [PubMed] [Google Scholar]
- Wabnitz RW. Functional states and fine structure of the contractile apparatus of the penis retractor muscle (PRM) of Helix pomatia L. Cell Tissue Res. 1975;156:253–265. doi: 10.1007/BF00221808. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Namba K. X-ray equatorial analysis of crab striated muscle in the relaxed and rigor states. Biophys Chem. 1981;14:111–122. doi: 10.1016/0301-4622(81)85012-0. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Namba K, Mitsui T. Configurations of myosin heads in the crab striated muscle as studied by X-ray diffraction. Adv Exp Med Biol. 1984;170:237–250. doi: 10.1007/978-1-4684-4703-3_21. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Hagiwara S. Mechanical and electrical events in the main sound muscle of cicada. Jpn J Physiol. 1953;3:249–253. doi: 10.2170/jjphysiol.3.249. [DOI] [PubMed] [Google Scholar]
- Wakabayashi T, Kuroda T. Responses of crayfish muscle preparations to nerve stimulation with various patterns of impulse sequence. Effects of intermittent, intercalated and adaptational types of impulse sequence Tohoku. J Exp Med. 1977;121:207–218. doi: 10.1620/tjem.121.207. [DOI] [PubMed] [Google Scholar]
- Walcott B, Dewey MM. Length-tension relation in Limulus striated muscle. J Cell Biol. 1980;87:204–208. doi: 10.1083/jcb.87.1.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker M, Trinick J. Electron microscopy of negatively stained scallop myosin molecules. Effect of regulatory light chain removal on head structure. J Mol Biol. 1989;208:469–475. doi: 10.1016/0022-2836(89)90510-x. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Hardwicke PMD, Szent-Györgyi AG. Regulatory and essential light-chain interactions in scallop myosin. II Photochemical cross-linking of regulatory and essential light-chains by heterobifunctional reagents. J Mol Biol. 1982;156:153–173. doi: 10.1016/0022-2836(82)90464-8. [DOI] [PubMed] [Google Scholar]
- Wallimann T, Szent-Györgyi AG. An immunological approach to myosin light-chain function in thick filament linked regulation. 2 Effects of anti-scallop myosin light-chain antibodies Possible regulatory role for the essential light chain. Biochemistry. 1981;20:1188–1197. doi: 10.1021/bi00508a021. [DOI] [PubMed] [Google Scholar]
- Wang F, Martin BM, Sellers JR. Regulation of actomyosin interactions in Limulus muscle proteins. J Biol Chem. 1993;268:3776–3780. [PubMed] [Google Scholar]
- Warrick HM, Spudich JA. Myosin structure and function in cell motility. Ann Rev Cell Biol. 1987;3:379–421. doi: 10.1146/annurev.cb.03.110187.002115. [DOI] [PubMed] [Google Scholar]
- Warshaw DM. Lever arms and necks: a common mechanistic theme across the myosin superfamily. J Muscle Res Cell Motil. 2004;25:467–474. doi: 10.1007/s10974-004-1767-z. [DOI] [PubMed] [Google Scholar]
- Watabe S, Hartshorne DJ. Paramyosin and the catch mechanism. Comp Biochem Physiol. 1990;96B:639–646. doi: 10.1016/0305-0491(90)90207-a. [DOI] [PubMed] [Google Scholar]
- Watabe S, Kantha SS, Hashimoto K, Kagawa H. Phosphorylation and immunological cross-reactivity of paramyosin: a comparative study. Comp Biochem Physiol. 1990;96B:81–88. [Google Scholar]
- Watabe S, Tsuchiya T, Hartshorne DJ. Phosphorylation of paramyosin. Comp Biochem Physiol. 1989;94B:813–821. doi: 10.1016/0305-0491(89)90171-5. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Kitaura T, Yamaguchi M. Crayfish myosin has no Ca2+-dependent regulation in actomyosin. J Biochem (Tokyo) 1982;92:1635–1641. doi: 10.1093/oxfordjournals.jbchem.a134089. [DOI] [PubMed] [Google Scholar]
- Waterston RH, Epstein HF, Brenner S. Paramyosin of Caenorhabditis elegans. J Mol Biol. 1974;90:285–290. doi: 10.1016/0022-2836(74)90373-8. [DOI] [PubMed] [Google Scholar]
- Webb MR, Lund J, Hunter JL, White DCS. Kinetics of ATP release and Pi binding during the ATPase cycle of Lethocerus flight muscle fibers, using phosphate- water oxygen exchange. J Muscle Res Cell Motil. 1991;12:254–261. doi: 10.1007/BF01745115. [DOI] [PubMed] [Google Scholar]
- Weber A, Murray J. Molecular control mechanisms in muscle contraction. Physiol Rev. 1973;53:612–673. doi: 10.1152/physrev.1973.53.3.612. [DOI] [PubMed] [Google Scholar]
- Weber HH. Muskelkontraktion, Zellmotilität und ATP. Biochim Biophys Acta. 1953;12:150–162. doi: 10.1016/0006-3002(53)90134-6. [DOI] [PubMed] [Google Scholar]
- Weber HH, Portzehl H. The transference of the muscle energy in the contraction cycle. Prog Biophys. 1954;4:60–111. [Google Scholar]
- Weisel JW. Paramyosin segments: molecular orientation and interactions in invertebrate muscle thick filaments. J Mol Biol. 1975;98:675–681. doi: 10.1016/s0022-2836(75)80003-9. [DOI] [PubMed] [Google Scholar]
- Weisel JW, Szent-Györgyi AG. The coiled-coil structure: identity of the two chains of Mercenaria paramysosin. J Mol Biol. 1975;98:665–673. doi: 10.1016/s0022-2836(75)80002-7. [DOI] [PubMed] [Google Scholar]
- Weitkamp B, Jurk K, Beinbrech G. Projectin-thin filament interactions and modulation of the sensitivity of the actomyosin ATPase to calcium by projectin kinase. J Biol Chem. 1998;273:19802–19808. doi: 10.1074/jbc.273.31.19802. [DOI] [PubMed] [Google Scholar]
- Wells C, Bagshaw CR. Segmental flexibility and head-head interaction in scallop myosin. A study using saturation transfer electron paramagnetic resonance spectroscopy. J Mol Biol. 1983;164:137–157. doi: 10.1016/0022-2836(83)90090-6. [DOI] [PubMed] [Google Scholar]
- Wells C, Bagshaw CR. The Ca2+ sensitivity of the actin-activayted ATPase of scallop heavy meromyosin. FEBS Lett. 1984a;168:260–264. [Google Scholar]
- Wells C, Bagshaw CR. The characterization of vanadate-trapped nucleotide complexes with spin-labelled myosins. J Muscle Res Cell Motil. 1984b;5:97–112. doi: 10.1007/BF00713154. [DOI] [PubMed] [Google Scholar]
- Wells C, Bagshaw CR. Calcium regulation of molluscan myosin ATPase in the absence of actin. Nature. 1985;313:696–697. doi: 10.1038/313696a0. [DOI] [PubMed] [Google Scholar]
- Wells C, Warriner KE, Bagshaw CR. Fluorescence studies on the nucleotide- and Ca2+-binding domains of molluscan myosin. Biochem J. 1985;231:31–38. doi: 10.1042/bj2310031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh JH. Serotonin as a possible neurohumoral agent: evidence obtained in lower animals. Ann New York Acad Sci. 1957;66:618–630. doi: 10.1111/j.1749-6632.1957.tb40752.x. [DOI] [PubMed] [Google Scholar]
- Wendt T, Guenebaut V, Leonard KR. Structure of the Lethocerus troponin-tropomyosin complex as determined by electron microscopy. J Struct Biol. 1997;118:1–8. doi: 10.1006/jsbi.1996.3834. [DOI] [PubMed] [Google Scholar]
- Wendt T, Leonard K. Structure of the insect troponin complex. J Mol Biol. 1999;285:1845–1856. doi: 10.1006/jmbi.1998.2414. [DOI] [PubMed] [Google Scholar]
- West JM, Higuchi H, Ishijima A, Yanagida T. Modification of the bi-directional sliding movement of actin filaments along native thick filaments isolated from a clam. J Muscle Res Cell Motil. 1996;17:637–646. doi: 10.1007/BF00154058. [DOI] [PubMed] [Google Scholar]
- West JM, Humphris DC, Stephenson DG. Differences in maximal activation properties of skinned short- and long-sarcomere muscle fibres from the claw of the freshwater crustacean Cherax destructor. J Muscle Res Cell Motil. 1992;13:668–684. doi: 10.1007/BF01738256. [DOI] [PubMed] [Google Scholar]
- White DCS. Rigor contraction and the effect of various phosphate compounds on glycerinated insect flight and vertebrate muscle. J Physiol. 1970;208:583–605. doi: 10.1113/jphysiol.1970.sp009138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DCS. The elasticity of relaxed insect fibrillar flight muscle. J Physiol. 1983;343:31–57. doi: 10.1113/jphysiol.1983.sp014880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DCS, Ricigliano JW, Webb MR. Analysis of the ATPase mechanism of myosin subfragment 1 from insect fibrillar flight muscle in the presence and absence of actin, using phosphate water oxygen exchange measurements. J Muscle Res Cell Motil. 1987;8:537–540. doi: 10.1007/BF01567912. [DOI] [PubMed] [Google Scholar]
- White DCS, Thorson J. Phosphate starvation and the nonlinear dynamics of insect fibrillar flight muscle. J Gen Physiol. 1972;60:307–336. doi: 10.1085/jgp.60.3.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White DCS, Thorson J. The kinetics of muscle contraction. Prog Biophys Mol Biol. 1973;27:173–255. [Google Scholar]
- White DCS, Zimmerman RW, Trentham DR. The ATPase kinetics of insect fibrillar flight muscle myosin subfragment 1. J Muscle Res Cell Motil. 1986;7:179–192. doi: 10.1007/BF01753419. [DOI] [PubMed] [Google Scholar]
- Wilkens JL. Tonic force maintenance after decay of active state in brachiopod smooth adductor muscle. J Comp Physiol. 1987;157B:651–658. [Google Scholar]
- Wills FL, McCubbin WD, Kay CM. Smooth muscle calponin-caltropin interaction - effect on biological activity and stability of calponin. Biochemistry. 1994;33:5562–5569. doi: 10.1021/bi00184a027. [DOI] [PubMed] [Google Scholar]
- Wilson DM. The nervous control of insect flight and related behavior. Adv Insect Physiol. 1968;5:289–338. [Google Scholar]
- Wilson DM, Larimer JL. The catch property of ordinary muscle. Proc Natl Acad Sci USA. 1968;61:909–916. doi: 10.1073/pnas.61.3.909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson DM, Smith DO, Dempster P. Length and tension hysteresis during sinusoidal and step function stimulation of arthropod muscle. Am J Physiol. 1970;218:916–922. doi: 10.1152/ajplegacy.1970.218.3.916. [DOI] [PubMed] [Google Scholar]
- Wilson DM, Wyman RJ. Phasically unpatterned nervous control of dipteran flight. J Insect Physiol. 1963;9:859–865. [Google Scholar]
- Wilson MGA, White DCS. The role of magnesium adenosine triphosphate in the contractile kinetics of insect fibrillar flight muscle. J Muscle Res Cell Motil. 1983;4:283–306. doi: 10.1007/BF00711997. [DOI] [PubMed] [Google Scholar]
- Winder SJ, Allen BG, Clement-Chomienne O, Walsh MP. Regulation of smooth muscle actin-myosin interaction and force by calponin. Acta Physiol Scand. 1998;164:415–26. doi: 10.1111/j.1365-201x.1998.tb10697.x. [DOI] [PubMed] [Google Scholar]
- Winder SJ, Walsh MP. Smooth muscle calponin. Inhibition of actomyosin MgATPase and regulation by phosphorylation. J Biol Chem. 1990;265:10148–10155. [PubMed] [Google Scholar]
- Winkelhahn JM, Beinbrech G. Electron microscope studies on the dissociation of actomyosin by pyrophosphate. Experientia. 1974;30:350–352. doi: 10.1007/BF01921657. [DOI] [PubMed] [Google Scholar]
- Winkelman L. Comparative studies of paramyosins. Comp Biochem Physiol. 1976;55B:391–397. doi: 10.1016/0305-0491(76)90310-2. [DOI] [PubMed] [Google Scholar]
- Winkelmann DA, Almeda S, Vibert P, Cohen C. A new myosin fragment: visualization of the regulatory domain. Nature. 1984;307:758–760. doi: 10.1038/307758a0. [DOI] [PubMed] [Google Scholar]
- Winkler H, Reedy MC, Reedy MK, Tregear RT, Taylor KA. Three-dimensional structure of nucleotide bearing crossbridges in situ: oblique section reconstruction of insect flight muscle in aqueous AMPPNP. J Mol Biol. 1996;264:302–322. doi: 10.1006/jmbi.1996.0642. [DOI] [PubMed] [Google Scholar]
- Winkler H, Taylor KA. Multivariate statistical analysis of three-dimensional cross-bridge motifs in insect flight muscle. Ultramicroscopy. 1999;77:141–152. [Google Scholar]
- Winton FR. The changes in viscosity of an unstriated muscle (Mytilus edulis) during and after stimulation with alternating, interrupted and uninterrupted direct currents. J Physiol. 1937;88:492–511. doi: 10.1113/jphysiol.1937.sp003455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wnuk W. Resolution and calcium-binding properties of the two major isoforms of troponin C from crayfish. J Biol Chem. 1989;264:18240–18246. [PubMed] [Google Scholar]
- Wnuk W, Schoechlin M, Stein EA. Regulation of actomyosin ATPase by a single calcium-binding site on troponin C from crayfish. J Biol Chem. 1984;259:9017–9023. [PubMed] [Google Scholar]
- Woodhead JL, Zhao FQ, Craig R, Egelman EH, Alamo L, Padrón R. Atomic model of a myosin filament in the relaxed state. Nature. 2005;436:1195–1199. doi: 10.1038/nature03920. [DOI] [PubMed] [Google Scholar]
- Wootton RJ, Newman DJS. Whitefly have the highest contraction frequencies yet recorded in non-fibrillar flight muscles. Nature. 1979;280:402–403. [Google Scholar]
- Worthington CR. Large axial spacings in striated muscle. J Mol Biol. 1959;1:398–401. [Google Scholar]
- Worthington CR. X-ray diffraction studies on the large-scale molecular structure of insect muscle. J Mol Biol. 1961;3:618–633. doi: 10.1016/s0022-2836(61)80025-9. [DOI] [PubMed] [Google Scholar]
- Wray J, Vibert P, Cohen C. Actin filaments in muscle: pattern of myosin and tropomyosin/troponin attachments. J Mol Biol. 1978;124:501–521. doi: 10.1016/0022-2836(78)90184-5. [DOI] [PubMed] [Google Scholar]
- Wray JS. Filament geometry and the activation of insect flight muscle. Nature. 1979a;280:325–326. [Google Scholar]
- Wray JS. Structure of the backbone in myosin filaments of muscle. Nature. 1979b;277:37–40. doi: 10.1038/277037a0. [DOI] [PubMed] [Google Scholar]
- Wray JS. Cross bridge states in invertebrate muscles. Adv Exp Med Biol. 1984;170:185–192. doi: 10.1007/978-1-4684-4703-3_16. [DOI] [PubMed] [Google Scholar]
- Wray JS, Holmes KC. X-ray diffraction studies of muscle. Annu Rev Physiol. 1981;43:553–565. doi: 10.1146/annurev.ph.43.030181.003005. [DOI] [PubMed] [Google Scholar]
- Wray JS, Vibert PJ, Cohen C. Diversity of cross-bridge configurations in invertebrate muscles. Nature. 1975;257:561–564. doi: 10.1038/257561a0. [DOI] [PubMed] [Google Scholar]
- Xie X, Harrison DH, Schlichting I, Sweet RM, Kalabokis VN, Szent-Györgyi AG, Cohen C. Structure of the regulatory domain of scallop myosin at 2.8 Å resolution. Nature. 1994;368:306–312. doi: 10.1038/368306a0. [DOI] [PubMed] [Google Scholar]
- Xu C, Craig R, Tobacman L, Horowitz R, Lehman W. Tropomyosin positions in regulated thin filaments revealed by cryoelectron microscopy. Biophys J. 1999;77:985–992. doi: 10.1016/S0006-3495(99)76949-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu JQ, Harder BA, Uman P, Craig R. Myosin filament structure in vertebrate smooth muscle. J Cell Biol. 1996;134:53–66. doi: 10.1083/jcb.134.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R, Fan SF, Maeda T, Chu B. Isolated thick filaments of Limulus striated muscle in suspension. Macromolecules. 1991;24:610–613. [Google Scholar]
- Yagi N, Matsubara I. The equatorial X-ray diffraction patterns of crustacean striated muscles. J Mol Biol. 1977;117:797–803. doi: 10.1016/0022-2836(77)90070-5. [DOI] [PubMed] [Google Scholar]
- Yamada A, Ishii N, Shimmen T, Takahashi K. Mg-ATPase activity and motility of native thick filaments isolated from the anterior byssus retractor muscle of Mytilus edulis. J Muscle Res Cell Motil. 1989;10:124–134. doi: 10.1007/BF01739968. [DOI] [PubMed] [Google Scholar]
- Yamada A, Ishii N, Takahashi K. Direction and speed of actin filaments moving along thick filaments isolated from molluscan smooth muscle. J Biochem (Tokyo) 1990;108:341–343. doi: 10.1093/oxfordjournals.jbchem.a123203. [DOI] [PubMed] [Google Scholar]
- Yamada A, Takahashi K. Sudden increase in speed of an actin filament moving on myosin cross-bridges of “mismatched” polarity observed when its leading end begins to interact with cross-bridges of “matched” polarity. J Biochem (Tokyo) 1992;111:676–680. doi: 10.1093/oxfordjournals.jbchem.a123817. [DOI] [PubMed] [Google Scholar]
- Yamada A, Yoshio M, Kojima H, Oiwa K. An in vitro assay reveals essential protein components for the ‘catch’ state of invertebrate smooth muscle. Proc Natl Acad Sci USA. 2001;98:6635–6640. doi: 10.1073/pnas.111585098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada A, Yoshio M, Nakamura A, Kohama K, Oiwa K. Protein phosphatase 2B dephosphorylates twitchin, initiating the catch state of invertebrate smooth muscle. J Biol Chem. 2004;279:40762–40768. doi: 10.1074/jbc.M405191200. [DOI] [PubMed] [Google Scholar]
- Yamaguchi H, Nakamura T, Oya H, Sekine T. Preparation of myosin A from body wall musculature of Ascaris lumbricoides. Biochim Biophys Acta. 1973;317:312–315. doi: 10.1016/0005-2795(73)90226-2. [DOI] [PubMed] [Google Scholar]
- Yamakawa M, Goldman YE. Mechanical transients initiated by photolysis of caged ATP within fibers of insect fibrillar flight muscle. J Gen Physiol. 1991;98:657–679. doi: 10.1085/jgp.98.4.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashiro S, Mohri K, Ono S. The two Caenorhabditis elegans actin depolymerizing factor/cofilin proteins differently enhance actin filament severing and depolymerization. Biochemistry. 2005;44:14238–14247. doi: 10.1021/bi050933d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagida T, Arata T, Oosawa F. Sliding distance of actin filament induced by a myosin cross-bridge during one ATP hydrolysis cycle. Nature. 1985;316:366–369. doi: 10.1038/316366a0. [DOI] [PubMed] [Google Scholar]
- Yanagida T, Taniguchi N, Oosawa F. Conformational changes of F-actin in the thin filaments of muscle induced in vivo and in vitro by calcium ions. J Mol Biol. 1974;90:509–522. doi: 10.1016/0022-2836(74)90231-9. [DOI] [PubMed] [Google Scholar]
- Yasuda K, Shindo Y, Ishiwata S. Synchronous behavior of spontaneous oscillations of sarcomeres in skeletal myofibrils under isotonic conditions. Biophys J. 1996;70:1823–1829. doi: 10.1016/S0006-3495(96)79747-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yazawa M, Sakura M, Yagi K. Calmodulins from muscle of marine invertebrates, scallop and sea anemone. Comparison with calmodulins from rabbit skeletal muscle and pig brain. J Biochem (Tokyo) 1980;87:1313–1320. doi: 10.1093/oxfordjournals.jbchem.a132869. [DOI] [PubMed] [Google Scholar]
- Yazawa M, Yoshida M. [title translation not available] [in Japanese] Seibutsu- Butsuri. 1979;19:158–163. [Google Scholar]
- Yazawa Y. Actin linked regulation in scallop striated muscle. Proc Jpn Acad Phys Biol Sci. 1985;61:497–500. [Google Scholar]
- Yernaux M, Baguet F. Effets de l’acide bromolysergique diéthylamide (BOL 148) sur la dépense d’énergie d’un muscle lisse de Lamellibranche en contraction phasique. Arch Int Physiol Biochim. 1971;79:811–813. [PubMed] [Google Scholar]
- Yongshui Z, Zuxun G. Paramyosin filaments of amphioxus. Sci Sin. 1979;22:1329–1332. [Google Scholar]
- York B, Twarog BM. Evidence for the release of serotonin by relaxing nerves in molluscan muscle. Comp Biochem Physiol. 1973;44A:423–430. [Google Scholar]
- Yoshida Y, Takayanagi I, Murakami H. Dopamine and its antagonists on molluscan smooth muscle. J Pharmacobio-Dynamics. 1981;4:226–228. doi: 10.1248/bpb1978.4.226. [DOI] [PubMed] [Google Scholar]
- Yoshimura K. Studies on the tropomyosin of squid. Mem Fac Fisheries, Hokkaido Univ. 1955;3:159–176. [Google Scholar]
- Yoshioka T, Kinoshita Y, Kato S, Cho YJ, Konno K. Preparation of heavy meromyosin from the autolyzed squid mantle muscle homogenate. Fisheries Sci. 2005;71:213–219. [Google Scholar]
- Yoshitomi B, Kamiya S, Konno K. Heavy meromyosin from squid mantle muscle. Nippon Suisan Gakk. 1984;50:1555–1559. [Google Scholar]
- Yoshitomi B, Konno K. Enzymatic properties of myosin ATPase from squid Todarodes pacificus mantle muscle. Nippon Suisan Gakk. 1982;48:581–586. [Google Scholar]
- Young D, Josephson RK. Mechanisms of sound-production and muscle contraction kinetics in cicadas. J Comp Physiol. 1983a;152A:183–195. [Google Scholar]
- Young D, Josephson RK. Pure-tone songs in cicadas with special reference to the genus Magicicada. J Comp Physiol. 1983b;152A:197–207. [Google Scholar]
- Young D, Josephson RK. 100 Hz is not the upper limit of synchronous muscle contraction. Nature. 1984;309:286–287. doi: 10.1038/309286b0. [DOI] [PubMed] [Google Scholar]
- Yu R, Ono S. Dual roles of tropomyosin as an F-actin stabilizer and a regulator of muscle contraction in Caenorhabditis elegans body wall muscle. Cell Motil Cytoskeleton. 2006;63:659–672. doi: 10.1002/cm.20152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumoto F, Nagata K, Adachi K, Nemoto N, Ojima T, Nishita K, Ohtsuki I, Tanokura M. NMR structural study of troponin C C-terminal domain complexed with troponin I fragment from Akazara scallop. Adv Exp Med Biol. 2003;538:195–201. doi: 10.1007/978-1-4419-9029-7_18. [DOI] [PubMed] [Google Scholar]
- Yumoto F, Nara M, Kagi H, Iwasaki W, Ojima T, Nishita K, Nagata K, Tanokura M. Coordination structures of Ca2+ and Mg2+ in Akazara scallop troponin C in solution. FTIR spectroscopy of side-chain COO- groups. Eur J Biochem. 2001;268:6284–6290. doi: 10.1046/j.1432-1327.2001.02583.x. [DOI] [PubMed] [Google Scholar]
- Zange J, Grieshaber MK, Jans AW. The regulation of intracellular pH estimated by 31P-NMR spectroscopy in the anterior byssus retractor muscle of Mytilus edulis L. J Exp Biol. 1990a;150:95–109. [Google Scholar]
- Zange J, Portner HO, Grieshaber MK. The anaerobic energy metabolism in the anterior byssus retractor muscle of Mytilus edulis during contraction and catch. J Comp Physiol. 1989;159B:349–358. [Google Scholar]
- Zange J, Portner HO, Jans AWH, Grieshaber MK. The intracellular pH of a molluscan smooth muscle during a contraction-catch-relaxation cycle estimated by the distribution of [14C]DMO and by 31P-NMR spectroscopy. J Exp Biol. 1990b;150:81–93. [Google Scholar]
- Zebe E. Die Muskeln der Insekten. Ein Beispiel für extreme Spezialisierung in Funktion und Strukture Umschau. 1960;60:40–43. [Google Scholar]
- Zebe E. Zur Spaltung von Adenosintriphosphat durch die Z-Scheiben der indirekten Flugmuskeln von Phormia regina (Diptera) Experientia. 1966;22:96–97. doi: 10.1007/BF01900172. [DOI] [PubMed] [Google Scholar]
- Zhao FQ, Craig R. Ca2+ causes release of myosin heads from the thick filament surface on the milliseconds time scale. J Mol Biol. 2003a;327:145–158. doi: 10.1016/s0022-2836(03)00098-6. [DOI] [PubMed] [Google Scholar]
- Zhao FQ, Craig R. Capturing time-resolved changes in molecular structure by negative staining. J Struct Biol. 2003b;141:43–52. doi: 10.1016/s1047-8477(02)00546-4. [DOI] [PubMed] [Google Scholar]
- Zhou N, Yuan T, Mak AS, Vogel HJ. NMR studies of caldesmon-calmodulin interactions. Biochemistry. 1997;36:2817–2825. doi: 10.1021/bi9625713. [DOI] [PubMed] [Google Scholar]
- Zieger WW. Magnesium und die Hemmung der Actin-Myosin-Interaktion in isolierten contractilen Strukturen der fibrillären Insektenmuskeln. Pflügers Arch. 1969;311:256–264. doi: 10.1007/BF00590529. [DOI] [PubMed] [Google Scholar]
- Zigmond SH. Beginning and ending an actin filament: control at the barbed end. Curr Top Dev Biol. 2004;63:145–188. doi: 10.1016/S0070-2153(04)63005-5. [DOI] [PubMed] [Google Scholar]
- Zobel CR, Baskin RJ, Wolf SL. Electron microscope observations on thick filaments isolated from striated muscle. J Ultrastruct Res. 1967;18:637–650. doi: 10.1016/s0022-5320(67)80209-0. [DOI] [PubMed] [Google Scholar]
- Zoghbi ME, Woodhead JL, Craig R, Padrón R. Helical order in tarantula thick filaments requires the “closed” conformation of the myosin head. J Mol Biol. 2004;342:1223–1236. doi: 10.1016/j.jmb.2004.07.037. [DOI] [PubMed] [Google Scholar]
- Zs-Nagy I, Romhanyi G, Salánki J. Polarization optical investigations on the adductors of pelecypods (mollusca) Acta Biol Hung. 1965;21:321–330. [PubMed] [Google Scholar]
- Zs-Nagy I, Romhanyi G, Salánki J. Polarization optical observations on the adductors of pelecypoda (mollusca) Acta Biol Hung. 1970;21:321–330. [PubMed] [Google Scholar]
- Zs-Nagy I, Salánki J, Garamvölgyi N. The contractile apparatus of the adductor muscles in Anodonta cygnea L. (Mollusca, pelecypoda) J Ultrastruct Res. 1971;37:1–16. doi: 10.1016/s0022-5320(71)80036-9. [DOI] [PubMed] [Google Scholar]