Abstract
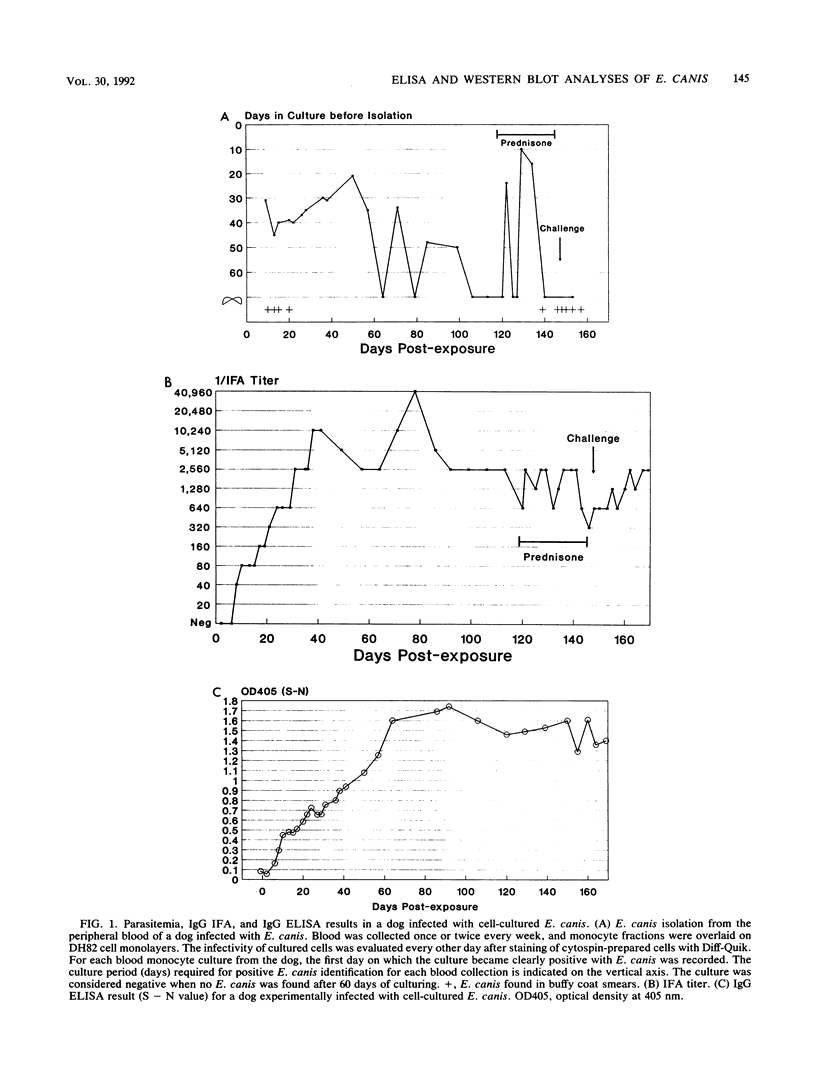
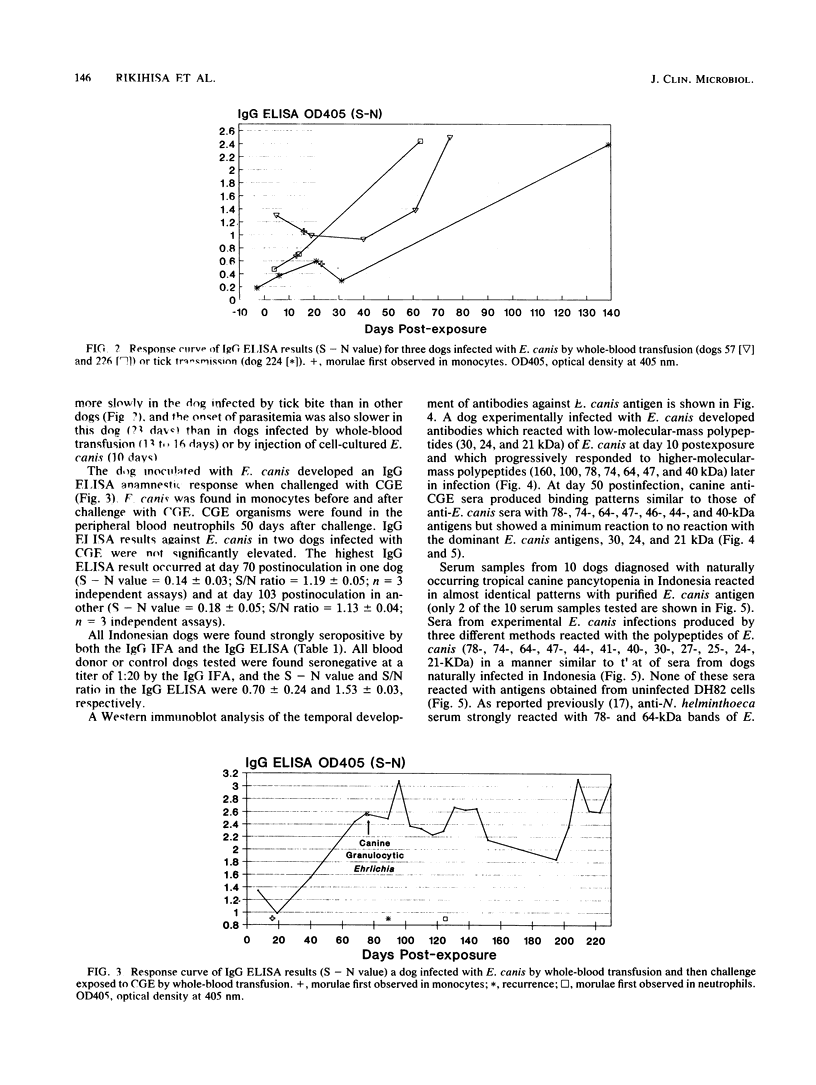
Ehrlichia canis and canine granulocytic Ehrlichia sp. (CGE) infect canine monocytes and granulocytes, respectively. E. canis has been cultured in vitro and used to develop an immunofluorescence assay. CGE has not been cultured, and a serologic assay is not available. The sera of dogs infected with CGE were reported to react with E. canis by immunofluorescence. In this study, the temporal response of immunoglobulin G (IgG) was determined by an enzyme-linked immunosorbent assay (ELISA) with purified E. canis antigen in four dogs experimentally infected with E. canis, in two dogs experimentally infected with CGE, and in one dog infected with E. canis and subsequently infected with CGE. E. canis-infected dogs developed an IgG ELISA result of 1.5 or greater for the optical density signal/noise ratio by 2 months postinfection. CGE challenge of a dog with a previous E. canis infection induced an anamnestic increase in the IgG ELISA result; however, CGE infection alone did not induce a significant IgG ELISA response. Western immunoblot analysis showed that dogs infected with E. canis developed antibodies initially that reacted with low-molecular-mass proteins (30, 24, and 21 kDa) and subsequently with higher-molecular-mass proteins (160, 100, 78, 64, 47, and 40 kDa). In contrast, CGE-infected dogs showed reactions with the same higher-molecular-mass proteins of E. canis but, unlike E. canis-infected dogs, not with the low-molecular-mass proteins of E. canis. Of 10 serum samples collected in the field of Indonesia from dogs with tropical canine pancytopenia, all had an optical density signal minus noise value of 2.54 or greater in the IgG ELISA and reacted with E. canis antigen in a pattern similar to that of serum samples from dogs experimentally infected with E. canis in Western immunoblotting. This study suggests that the IgG ELISA and Western immunoblotting with purified E. canis as the antigen are useful in distinguishing between E. canis and CGE infections in dogs.
Full text
PDF


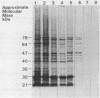
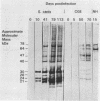
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellah J. R., Shull R. M., Selcer E. V. Ehrlichia canis-related polyarthritis in a dog. J Am Vet Med Assoc. 1986 Oct 15;189(8):922–923. [PubMed] [Google Scholar]
- Buhles W. C., Jr, Huxsoll D. L., Ristic M. Tropical canine pancytopenia: Clinical, hematologic, and serologic response of dogs to Ehrlichia canis infection, tetracycline therapy, and challenge inoculation. J Infect Dis. 1974 Oct;130(4):357–367. doi: 10.1093/infdis/130.4.357. [DOI] [PubMed] [Google Scholar]
- Cowell R. L., Tyler R. D., Clinkenbeard K. D., Meinkoth J. H. Ehrlichiosis and polyarthritis in three dogs. J Am Vet Med Assoc. 1988 Apr 15;192(8):1093–1095. [PubMed] [Google Scholar]
- Dawson J. E., Rikihisa Y., Ewing S. A., Fishbein D. B. Serologic diagnosis of human ehrlichiosis using two Ehrlichia canis isolates. J Infect Dis. 1991 Mar;163(3):564–567. doi: 10.1093/infdis/163.3.564. [DOI] [PubMed] [Google Scholar]
- EWING S. A. OBSERVATIONS ON LEUKOCYTIC INCLUSION BODIES FROM DOGS INFECTED WITH BABESIA CANIS. J Am Vet Med Assoc. 1963 Sep 1;143:503–506. [PubMed] [Google Scholar]
- Ewing S. A. Canine ehrlichiosis. Adv Vet Sci Comp Med. 1969;13:331–353. [PubMed] [Google Scholar]
- Ewing S. A., Roberson W. R., Buckner R. G., Hayat C. S. A new strain of Ehrlichia canis. J Am Vet Med Assoc. 1971 Dec 15;159(12):1771–1774. [PubMed] [Google Scholar]
- Lewis G. E., Jr, Huxsoll D. L., Ristic M., Johnson A. J. Experimentally induced infection of dogs, cats, and nonhuman primates with Ehrlichia equi, etiologic agent of equine ehrlichiosis. Am J Vet Res. 1975 Jan;36(1):85–88. [PubMed] [Google Scholar]
- Nyindo M. B., Ristic M., Huxsoll D. L., Smith A. R. Tropical canine pancytopenia: in vitro cultivation of the causative agent--Ehrlichia canis. Am J Vet Res. 1971 Nov;32(11):1651–1658. [PubMed] [Google Scholar]
- Pretzman C. I., Rikihisa Y., Ralph D., Gordon J. C., Bech-Nielsen S. Enzyme-linked immunosorbent assay for Potomac horse fever disease. J Clin Microbiol. 1987 Jan;25(1):31–36. doi: 10.1128/jcm.25.1.31-36.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikihisa Y. Cross-reacting antigens between Neorickettsia helminthoeca and Ehrlichia species, shown by immunofluorescence and Western immunoblotting. J Clin Microbiol. 1991 Sep;29(9):2024–2029. doi: 10.1128/jcm.29.9.2024-2029.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ristic M., Huxsoll D. L., Weisiger R. M., Hildebrandt P. K., Nyindo M. B. Serological diagnosis of tropical canine pancytopenia by indirect immunofluorescence. Infect Immun. 1972 Sep;6(3):226–231. doi: 10.1128/iai.6.3.226-231.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker J. S., Rundquist J. D., Taylor R., Wilson B. L., Andrews M. R., Barck J., Hogge A. L., Jr, Huxsoll D. L., Hildebrandt P. K., Nims R. M. Clinical and clinicopathologic findings in tropical canine pancytopenia. J Am Vet Med Assoc. 1970 Jul 1;157(1):43–55. [PubMed] [Google Scholar]