Abstract
Objective
Biomarkers are increasingly important to diagnose and test treatments of neurodegenerative diseases such as Parkinson disease (PD). This study compared neuroimaging, neurochemical, and olfactory potential biomarkers to detect central dopamine (DA) deficiency and distinguish PD from multiple system atrophy (MSA).
Methods
In 77 PD, 57 MSA, and 87 control subjects, radioactivity concentrations in the putamen (PUT), caudate (CAU), occipital cortex (OCC), and substantia nigra (SN) were measured 2 hours after 6-[18F]fluorodopa injection, septal myocardial radioactivity measured 8 minutes after 6-[18F]fluorodopamine injection, CSF and plasma catechols assayed, or olfaction tested (University of Pennsylvania Smell Identification Test (UPSIT)). Receiver operating characteristic curves were constructed, showing test sensitivities at given specificities.
Results
PUT:OCC, CAU:OCC, and SN:OCC ratios of 6-[18F]fluorodopa-derived radioactivity were similarly low in PD and MSA (p<0.0001, p<0.0001, p=0.003 compared to controls), as were CSF dihydroxyphenylacetic acid (DOPAC) and DOPA concentrations (p<0.0001 each). PUT:SN and PUT:CAU ratios were lower in PD than in MSA (p=0.004; p=0.005). CSF DOPAC correlated positively with PUT:OCC ratios (r=0.61, p<0.0001). Myocardial 6-[18F]fluorodopamine-derived radioactivity distinguished PD from MSA (83% sensitivity at 80% specificity, 100% sensitivity among patients with neurogenic orthostatic hypotension (NOH)). Only PD patients were anosmic; only MSA patients had normal olfaction (61% sensitivity at 80% specificity).
Conclusions
PD and MSA feature low PUT:OCC ratios of 6-[18F]fluorodopa-derived radioactivity and low CSF DOPAC and DOPA concentrations, cross-validating the neuroimaging and neurochemical approaches but not distinguishing the diseases. PUT:SN and PUT:OCC ratios of 6-[18F]fluorodopa-derived radioactivity, cardiac 6-[18F]fluorodopamine-derived radioactivity, and olfactory testing separate PD from MSA.
Key words or phrases: Parkinson, multiple system atrophy, fluorodopa, fluorodopamine, DOPAC, PET, biomarker
Biomarkers are becoming increasingly important for early diagnosis and for testing potential treatments of neurodegenerative diseases such as Parkinson disease (PD). Two challenges are validating indices of central dopamine (DA) deficiency and distinguishing PD from multiple system atrophy (MSA).
Because nigrostriatal DA deficiency characterizes PD, a variety of means to visualize abnormalities of DA uptake or storage have been developed, such as 6-[18F]fluorodopa positron emission tomographic (PET) scanning [1]. The occipital cortex (OCC) contains scant dopaminergic innervation; one can quantify striatal DA deficiency from the PUT:OCC ratio of 6-[18F]fluorodopa-derived radioactivity [2, 3]. Spatial resolution of conventional PET scanning is inadequate to visualize the substantia nigra (SN) in individuals. Computerized registration of PET with magnetic resonance images, supplemented by a high resolution research tomograph (HRRT), enables quantification of SN 6-[18F]fluorodopa-derived radioactivity [4].
CSF profiles of DA and its metabolites are potential neurochemical biomarkers. CSF concentrations of homovanillic acid, the main end-product of DA metabolism, are not consistently decreased in PD [5]. CSF levels of dihydroxyphenylacetic acid (DOPAC), a major deaminated metabolite of DA, can be decreased [6] but rarely have been studied. Low CSF DOPA levels might indicate decreased DA synthesis in PD; however, one study did not find low CSF DOPA in PD [6].
Even if CSF neurochemistry cross-validated 6-[18F]fluorodopa PET scanning in detecting central DA deficiency, neither modality might identify PD specifically, because other diseases entail central DA deficiency. The parkinsonian form of MSA (MSA-P) can be difficult to distinguish from PD [7]. MSA entails loss of nigrostriatal neurons [8], decreased striatal 6-[18F]fluorodopa-derived radioactivity [9], and low CSF levels of homovanillic acid [10]. Most patients with MSA have neurogenic orthostatic hypotension (NOH). In patients with parkinsonism and NOH as an early, prominent disease manifestation, MSA is the favored diagnosis [11]; however, in about 60% of patients with PD+NOH, orthostatic intolerance or hypotension develop before, concurrent with, or within 1 year after onset of the movement disorder [12].
Diffusion weighted magnetic resonance imaging, midbrain ultrasound, and combinations of metabolic, DA transporter, and DA receptor imaging can separate PD from MSA. Diffusion weighted imaging of the PUT is abnormal in MSA but does not sensitively detect PD [13, 14], and nigral echogenicity may be increased in PD but does not sensitively detect MSA [15]. Neither approach therefore efficiently identifies central DA deficiency. MSA patients have been reported to have low striatal binding of DA-2 receptor ligands, consistent with decreased populations of cells expressing this receptor; however, the ligands may bind to both dopaminergic and non-dopaminergic cells [16, 17].
More than 50 sympathetic neuroimaging studies have reported evidence for loss of cardiac noradrenergic nerves in PD. Post-mortem histopathologic analyses have confirmed profound loss of tyrosine hydroxylase immunoreactivity in cardiac nerves [18-20]. In contrast, most (but not all) patients with MSA have normal cardiac sympathetic innervation [21].
Loss of sense of smell in PD has been associated with neuroimaging evidence of both central DA deficiency [22] and cardiac noradrenergic denervation [23]. Decreased olfaction also can occur in MSA [24, 25], and it has been unclear how efficiently olfactory testing separates PD from MSA.
The main purpose of this study was to identify biomarkers that detect central DA deficiency and distinguish PD from MSA. Because of the importance of distinguishing PD+NOH from MSA-P, the study included subgroups of PD patients with or without NOH and subgroups of MSA patients with MSA-P or cerebellar failure without parkinsonism (MSA-C). To compare efficiencies of potential biomarkers, we constructed receiver operating characteristic (ROC) curves, relating test sensitivity to specificity across a range of criteria for defining positive and negative test results.
Ideally, a biomarker should track disease progression. The present report includes 5-year follow-up data about myocardial 6-[18F]fluorodopamine-derived radioactivity in subgroups of PD patients who had 6-[18F]fluorodopamine-derived radioactivity either more than or within 2 standard deviations below the normal mean at the time of initial evaluation.
METHODS
A total of 77 PD, 57 MSA, and 87 control subjects were studied at the NIH Clinical Center. Each gave informed written consent before participating in protocols approved by the NINDS Institutional Review Board. Different numbers of subjects were tested for the various biomarkers, as noted in the Results. Normal data for CSF levels of catechols in geriatric subjects were also obtained from assaying samples kindly provided by E. Peskind (Univ. of Washington) under an approved protocol amendment and material transfer agreement.
MSA was diagnosed based on previously published consensus statements [26, 27]. MSA-P was identified by symptoms or signs of autonomic failure, coupled with rigidity and bradykinesia, with or without clinical evidence of cerebellar failure; and MSA-C by autonomic and cerebellar failure without parkinsonism.
All patients were tested while on their usual medications. To ensure that treatment with levodopa/carbidopa did not influence the neurochemical results, CSF and plasma neurochemical data were included only for patients in whom the plasma DOPA level was less than 2,500 pg/mL (less than about 12.7 nmol/L) during supine rest.
Orthostatic hypotension was defined by a decrease in systolic blood pressure of at least 20 mm Hg and in diastolic pressure at least 10 mm Hg between supine rest for at least 15 minutes and upright posture for 5 minutes (unless symptomatic or rapid hypotension necessitated return to the supine position before 5 minutes upright). NOH was defined by orthostatic hypotension coupled with abnormal beat-to-beat blood pressure associated with performance of the Valsalva maneuver [28].
For 6-[18F]fluorodopa brain PET scanning, the subject was placed supine head-first in a GE Advance scanner. Seven mCi of 6-[18F]fluorodopa was injected i.v. over 3 minutes using an automated syringe pump. Carbidopa pre-treatment was not used. In subjects undergoing measurements of arterial plasma concentrations of 6-[18F]fluorodopa, a brachial arterial catheter was inserted after local anesthesia of the overlying skin. In most subjects, after the initial static scan was obtained, the head was scanned in an HRRT scanner. The HRRT produces 207 slices per 3-D data frame, compared to 35 on the GE Advance scanner, and reconstructed image resolution is 2.5-3 mm, compared with 6-7 mm. Emission data in the HRRT were obtained for 15 minutes. A final 15-minute static scan was obtained in the GE Advance scanner, ending about 120 minutes from the time of injection of 6-[18F]fluorodopa.
For 6-[18F]fluorodopamine PET scanning, 1 mCi of the imaging agent was injected i.v. over 3 minutes. Dynamic data were obtained for 30 minutes, as described previously [29]. Follow-up 6-[18F]fluorodopamine scans were obtained in 20 PD patients at about 2 years and 11 patients at about 5 years after initial testing.
Lumbar puncture was done under fluoroscopic guidance. Six 1-mL aliquots of CSF were obtained and frozen immediately in cryotubes placed in dry ice and stored at -70 °C or colder until thawed for assay. The sixth 1-mL aliquot was assayed for catechols in our laboratory [30].
Blood was obtained through an indwelling i.v. catheter, after the subject was supine for at least 15 minutes after catheter insertion. Plasma levels of catechols were assayed in our laboratory as described previously [31, 32].
Patients filled out the 4-booklet University of Pennsylvania Smell Identification Test (UPSIT), scored using a template provided with the test (The Smell Identification Test Administration Manual: Third Edition, Sensonics, Inc., Haddon Heights, NJ).
6-[18F]Fluorodopa brain PET and MRI scans were fused using PMOD (PMOD Technologies Ltd., Zurich, Switzerland). Regions of interest were placed manually at the perimeters of the SN, PUT, head of the CAU, and occipital (OCC) cortex in the MRI scans. The sizes of the regions of interest therefore were not fixed. Tissue concentrations of 6-[18F]fluorodopa-derived radioactivity (in nCi/cc) were adjusted for the dose per unit body mass and expressed in units of nCi-kg/cc-mCi.
Data were analyzed by factorial analyses of variance, with Fisher’s PLSD post-hoc test, and Pearson correlation coefficients, using StatView 5.0.1 (SAS Institute, Inc., Cary, NC) and KaleidaGraph 4.0.1 (Synergy Software, Reading, PA). T-tests of ANOVAs were used to independent groups. Frequency data were analyzed by χ2 calculation.
RESULTS
As expected, the PD group had a higher prevalence of levodopa responsiveness and positive family history of a neurodegenerative disease than did the MSA group, and the MSA group had a higher prevalence of slurred speech and of erectile failure in men (Table 1). The PD group was somewhat older in mean age at the time of study and was studied after a longer interval from the time of onset of motor symptoms, whereas the groups had similar mean ages of onset of the movement disorder. The PD group also had higher prevalences of treatment with a dopamine receptor agonist, monoamine oxidase inhibitor, or serotonin reuptake inhibitor. The groups did not differ significantly in mean UPDRS scores or frequencies of orthostatic hypotension, urinary symptoms, constipation, decreased sweating, depression, or dementia.
Table 1.
Clinical Characteristics of Patient Groups
| PD | MSA | |
|---|---|---|
| Levodopa Responsiveness | 93% | 36%*** |
| Positive Family History | 31% | 2%*** |
| Slurred Speech | 21% | 79%*** |
| Interval from Onset to Study (years) | 9 ± 1 | 5 ± 1*** |
| Age (years) | 65 ± 1 | 61 ± 1* |
| Erectile Failure in Men | 75% | 94%* |
| Dopamine Agonist Treatment | 38% | 18%* |
| MAO Inhibitor | 16% | 0%* |
| Serotonin Reuptake Inhibitor | 20% | 7%* |
| Male/Female | 57/21 | 36/21 |
| Age at Onset of Motor Symtpoms (years) | 56 ± 1 | 56 ± 1 |
| UPDRS (Off Levodopa) | 40 ± 8 | 41 ± 6 |
| Orthostatic Hypotension | 65 | 77% |
| Urinary Symptoms | 78% | 94% |
| Constipation | 62% | 80% |
| Decreased Sweating | 29% | 33% |
| Depression | 49% | 50% |
| Dementia | 25% | 6% |
| COMT Inhibitor | 15% | 15% |
Notes:
significant group difference, p<0.05;
significant group difference, p<0.001.
Abbreviations: MAO=monoamine oxidase; UPDRS=Unified Parkinson’s Disease Rating Scale; COMT=catechol-O-methyltransferase.
At all time points after 6-[18F]fluorodopa injection, the PD, MSA, and control groups did not differ in arterial plasma concentrations of 6-[18F]fluorodopa. The groups also did not differ in 6-[18F]fluorodopa-derived radioactivity in the PUT, CAU, SN, or OCC within the first 30 minutes after 6-[18F]fluorodopa injection.
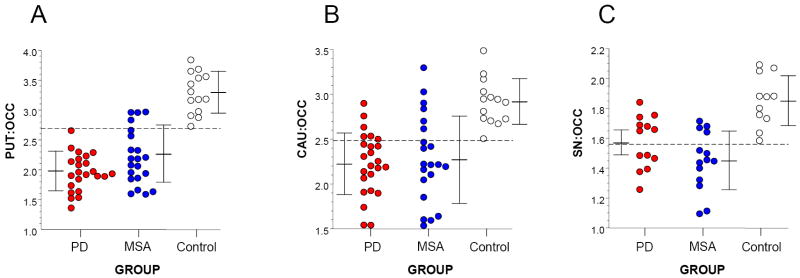
In all groups the PUT:OCC ratio of 6-[18F]fluorodopa-derived radioactivity increased linearly over time. In the PD and MSA groups, decreased rates of increase in mean PUT:OCC ratios became apparent after about 40 minutes. Smaller differences in CAU:OCC and SN:OCC ratios emerged by 2 hours. At 2 hours after 6-[18F]fluorodopa injection, all PD patients had a PUT:OCC ratio below the range of the controls (p<0.0001), whereas the distributions of CAU:OCC and SN:OCC values overlapped with those in the controls (Figure 1). The MSA-P subgroup had a lower mean PUT:OCC ratio than did the MSA-C subgroup (F=10.7, p=0.004). For given amounts of CAU and SN radioactivity, all PD and most MSA patients had less than the expected amount of PUT radioactivity.
Figure 1.
Relationships among individual values for 6-[18F]fluorodopa-derived radioactivity in (A) the putamen (PUT) with respect to occipital cortex (OCC), (B) head of the caudate (CAU) with respect to OCC, and (C) substantia nigra (SN) with respect to OCC, in patients with Parkinson disease (PD, red), multiple system atrophy (MSA, blue), and control subjects (white). Horizontal bars show mean values ± 1 standard deviation. Note that all PD patients had a PUT:OCC ratio below the range of the control subjects (horizontal dashed line).
For given amounts of SN or CAU 6-[18F]fluorodopa-derived radioactivity, PD patients had lower PUT radioactivity than did MSA patients or controls. The PD group had a lower mean value for the PUT:SN ratio (1.32 ± 0.05) and PUT:CAU ratio (0.87 ± 0.02 than did the MSA group (1.59 ± 0.07, F=10.2, p=0.004; 0.97 ± 0.02, F=8.78, p=0.005).
The PD and MSA groups had lower mean CSF levels of DOPA and DOPAC than did the controls (p<0.0001 each), whereas the three groups did not differ in mean levels of DA. The mean ratio of CSF DA:DOPAC was higher in the PD and MSA groups than in the control group (p=0.0004, p=0.006) and tended to be higher in the PD than in the MSA group (p=0.09).
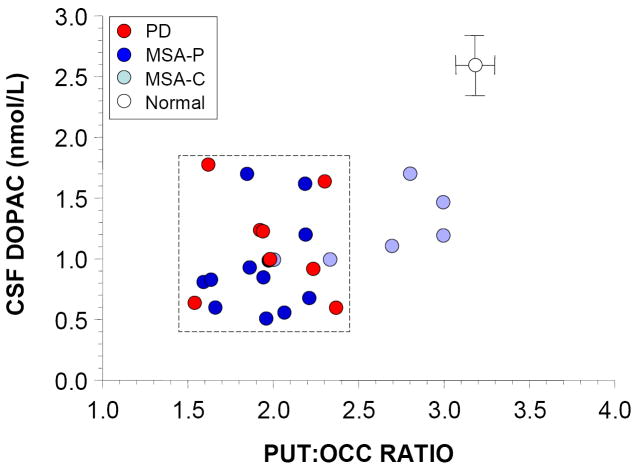
Individual values for PUT 6-[18F]fluorodopa-derived radioactivity and the PUT:OCC ratio correlated positively with CSF DOPAC (r=0.64, r=0.66, p<0.01 each; Figure 2. Individual values for CAU radioactivity and CAU:OCC ratios correlated less strongly with CSF DOPAC (r=0.49, r=0.40, p<0.05 each), and SN 6-[18F]fluorodopa-derived radioactivity radioactivity and SN:OCC ratios did not correlate significantly with CSF DOPAC.
Figure 2.
Individual values for cerebrospinal fluid (CSF) levels of dihydroxyphenylacetic acid (DOPAC) as a function of the putamen:occipital cortex (PUT:OCC) ratio of 6-[18F]fluorodopa-derived radioactivity, in patients with Parkinson disease (PD, red), multiple system atrophy (MSA, blue), and healthy control subjects (white). Light blue circles show data for patients with the cerebellar form of MSA (MSA-C) and dark blue MSA with parkinsonism (MSA-P). Error bars show healthy control mean values (± SEM). Dashed lines placed to highlight that patients with PD or MSA-P had similarly low PUT:OCC ratios and low CSF DOPAC concentrations.
The PD group had low interventricular septal concentrations of 6-[18F]fluorodopamine-derived radioactivity, compared to the MSA and control groups (p<0.0001 each). All PD+NOH patients had a septal-chamber difference in 6-[18F]fluorodopamine-derived radioactivity below the range of values in MSA patients. There was no relationship between the PUT:OCC ratio of 6-[18F]fluorodopa-derived radioactivity and cardiac 6-[18F]fluorodopamine-derived radioactivity, across all subjects (r=0.29) or among PD patients (r=0.32).
PD+NOH patients had lower plasma DHPG and NE levels than did MSA patients (p=0.007, p=0.05) and control subjects (p<0.0001, p=0.03). Plasma DHPG correlated positively with plasma NE (r=0.52, p<0.0001). For given NE levels, PD+NOH patients had lower DHPG levels than expected, in contrast with MSA patients (χ2=11.3, p=0.0008, for the difference between the PD+NOH and MSA groups). Among PD patients, plasma norepinephrine was positively correlated with cardiac 6-[18F]fluorodopamine-derived radioactivity.
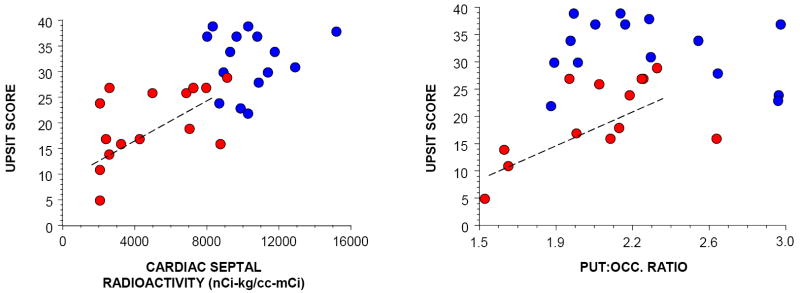
The PD group had a lower mean UPSIT score (20±2) than did the MSA group (32±2, p=0.0002). About ½ of MSA patients tested had normal olfaction, which was not observed in any PD patients; and about ½ of PD patients tested had anosmia, which was not observed in any MSA patients (χ2=12.1, p=0.002). Among PD patients, UPSIT scores correlated positively with cardiac 6-[18F]fluorodopamine-derived radioactivity (r=0.65, p=0.03) and the PUT:OCC ratio of 6-[18F]fluorodopa-derived radioactivity (r=0.91, p=0.002; Figure 3). Most PD patients had both an UPSIT score less than 30 and cardiac 6-[18F]fluorodopamine-derived radioactivity less than 7500 nCi-kg/cc-mCi, a combination not seen in any MSA patients, and most MSA patients had both an UPSIT score of at least 30 and cardiac 6-[18F]fluorodopamine-derived radioactivity more than 7500 nCi-kg/cc-mCi, a combination not seen in any PD patients (χ2=27, p<0.0001).
Figure 3.
University of Pennsylvania Smell Identification Test (UPSIT) results in patients with Parkinson disease (PD, red) or multiple system atrophy (MSA, blue). Left panel shows individual UPSIT scores, expressed as a function of septal myocardial 6-[18F]fluorodopamine-derived radioactivity; and right panel shows individual UPSIT scores, expressed as a function of the putamen:occipital cortex (PUT:OCC) ratio of 6-[18F]fluorodopa-derived radioactivity. Dashed lines indicate line of best fit among PD patients.
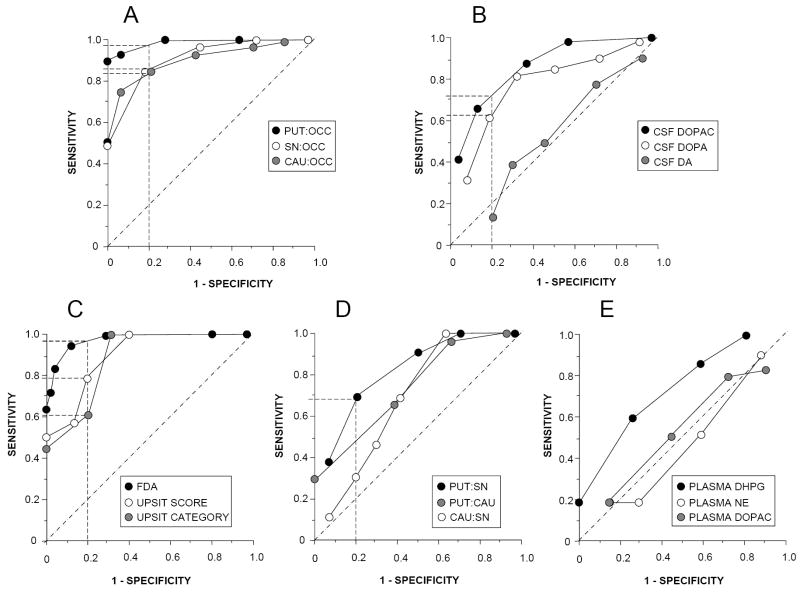
At any given specificity, the PUT:OCC ratio was more sensitive than the CAU:OCC or SN:OCC ratio in separating patients with PD or MSA from control sujbects (Figure 4). Among the CSF neurochemical measures, DOPAC was more sensitive than DOPA, and DA was of no value. The neuroimaging measures were more sensitive than the neurochemical measures.
Figure 4.
Receiver operating characteristic curves for various potential biomarkers of central dopamine (DA) deficiency. (A) Data from putamen:occipital cortex (PUT:OCC), caudate (CAU):OCC, and substantia nigra (SN):OCC ratios of 6-[18F]fluorodopa-derived radioactivity; (B) Data from cerebrospinal fluid neurochemistry (dihydroxyphenylacetic acid (DOPAC), DOPA, and dopamine (DA)). (C) Data for cardiac 6-[18F]fluorodopamine-derived radioactivity (FDA), University of Pennsylvania Smell Identification Test (UPSIT) scores, and UPSIT categories; (D) Date for putamen (PUT):substantia nigra (SN) and caudate (CAU):SN ratios of 6-[18F]fluorodopa-derived radioactivity; (E) Data for plasma levels of dihydroxyphenylglycol (DHPG), norepinephrine (NE), and dihydroxyphenylacetic acid (DOPAC). Dashed lines show sensitivities more than 50% at 1 – specificity of 20%.
Interventricular septal myocardial 6-[18F]fluorodopamine-derived radioactivity was highly sensitive and specific in separating PD from MSA. Among patients with NOH, septum minus chamber 6-[18F]fluorodopamine-derived radioactivity distinguished PD from MSA with sensitivity of 100% at 100% specificity. UPSIT scores and categories also distinguished PD from MSA, but with lower sensitivity at any specificity. Among neurochemical measures, plasma DHPG was of some value, whereas plasma DOPAC was not. The PUT:SN ratio of 6-[18F]fluorodopa-derived radioactivity distinguished PD from MSA, with sensitivity 68% at specificity 80%; the PUT:CAU and CAU:SN ratios were less efficient.
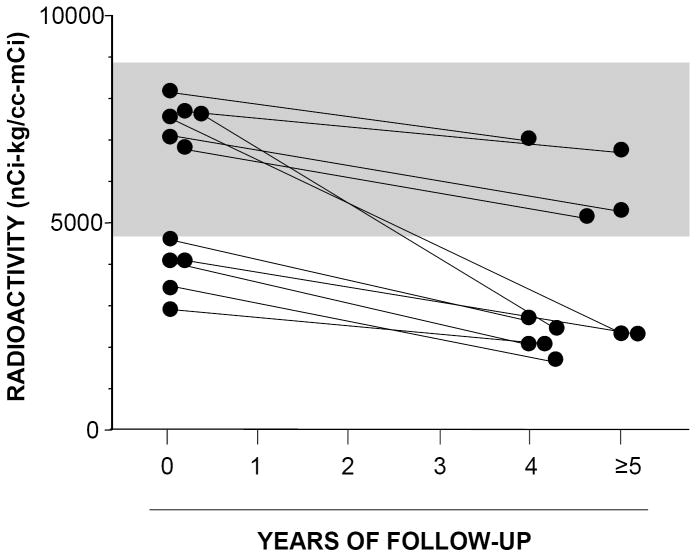
Among 20 PD patients with follow-up 6-[18F]fluorodopamine scans at 2 or 5 years, septal myocardial 6-[18F]fluorodopamine-derived radioactivity declined by a mean of 8.7% per year (t=3.7, p<0.001). Among 11 patients with 5-year follow-up data, all had a decrease in septal 6-[18F]fluorodopamine-derived radioactivity (p<0.001 by sign test), regardless of whether initial radioactivity was more than or within 2 standard deviations of the normal mean (Figure 5).
Figure 5.
Individual values for interventricular septal myocardial 6-[18F]fluorodopamine-derived radioactivity upon initial testing and follow-up after at least 4 years in patients with Parkinson disease. All patients had a decrease in 6-[18F]fluorodopamine-derived radioactivity.
DISCUSSION
In this study, PD and MSA involved similarly low PUT:OCC, CAU:OCC, and SN:OCC ratios of 6-[18F]fluorodopa-derived radioactivity, suggesting similar extents of nigrostriatal DA deficiency. Previous reports described inadequate sensitivity of 6-[18F]fluorodopa PET scanning in PD [33]; however, in the present study all patients with PD had low PUT:OCC ratios. Measurements at the relatively late time point of 2 hours after 6-[18F]fluorodopa injection, registration of PET with MRI scans, and use of the HRRT may have improved sensitivity of 6-[18F]fluorodopa PET scanning.
Reports about low 6-[18F]fluorodopa-derived radioactivity in the PUT and CAU in PD and MSA have disagreed about the relative extents of decreased CAU radioactivity [3, 9, 34]. In this study, PD patients had lower mean PUT:CAU and PUT:SN ratios of 6-[18F]fluorodopa-derived radioactivity than did MSA patients, suggesting that PD involves more severe loss of dopaminergic innervation in the PUT than in other parts of the nigrostriatal system. Post-mortem neurochemical studies by Hornykiewicz [35-37] showed more severely decreased PUT than CAU DA concentrations in PD; however, Spokes et al. also found lower PUT than CAU DA concentrations in MSA patients [38].
Among MSA patients, those with parkinsonism (MSA-P) had lower PUT:OCC ratios than did those with cerebellar failure and no parkinsonism (MSA-C). Therefore, in MSA, neuroimaging evidence of nigrostriatal DA deficiency seems linked with parkinsonism.
The finding of low CSF DOPAC levels in PD confirms that in a previous report [6]; the findings of low CSF DOPAC levels in MSA and of low CSF DOPA levels in PD and MSA seem new. The correlated decreases in CSF DOPA and DOPAC suggest correlated decreases in synthesis and turnover of DA, as one would expect from denervation. CSF levels of DA itself, however, were not decreased, possibly because of compensatorily increased nigrostriatal pathway traffic, decreased reuptake of released DA, or decreased aldehyde dehydrogenase activity. Previous studies did not attempt to correlate neuroimaging with neurochemical indices of central DA deficiency. The finding of similar abnormalities of neuroimaging and neurochemical test results in the same patients provides important cross-validation of the two testing modalities.
6-[18F]Fluorodopamine scanning was especially efficient in distinguishing PD from MSA in patients with NOH, since virtually all patients with PD+NOH had 6-[18F]fluorodopamine-derived radioactivity more than 2 standard deviations below the means of control subjects and of patients with MSA. The finding of low plasma DHPG for given NE levels in PD+NOH but not in MSA suggests that PD+NOH is associated with decreased extra-cardiac norepinephrine stores, whereas MSA is not.
All PD patients had a further decrease in 6-[18F]fluorodopamine-derived radioactivity at 5 years of follow-up. This finding suggests that 6-[18F]fluorodopamine scanning may be useful in tracking progression of catecholaminergic denervation in PD.
Mild to moderate decreases in sense of smell can occur in MSA [24, 25, 39]. In distinguishing PD from MSA, the UPSIT was surprisingly robust for a relatively simple, non-invasive test, because about half of PD patients and none of the MSA patients were anosmic, and about half of MSA patients and none of the PD patients had normal olfaction. Individual UPSIT scores correlated positively with the magnitude of cardiac 6-[18F]fluorodopamine-derived radioactivity, confirming a report using another sympathoneural imaging agent, 123I-metaiodobenzylguanidine [23]. Thus, in alpha-synucleinopathies the loss of sense of smell seems to be related to the loss of cardiac sympathetic nerves. UPSIT scores also correlated positively with the PUT:OCC ratio in PD patients, confirming a previously reported positive correlation between olfactory testing scores and PUT [99mTc]TRODAT-1-derived radioactivity in early PD [22].
Since individual values for the PUT:OCC ratio of 6-[18F]fluorodopa-derived radioactivity were unrelated to septal 6-[18F]fluorodopamine-derived radioactivity, the extent of loss of nigrostriatal DA innervation in PD seems unrelated to the extent of loss of cardiac noradrenergic innervation, even though both types of abnormality occur in this disease. The finding that the PD and MSA groups had similarly low mean values for PUT:OCC, CAU:OCC, and SN:OCC ratios, despite decreased 6-[18F]fluorodopamine-derived radioactivity in PD and generally normal radioactivity in MSA, also fits with the concept of independence of nigrostriatal DA deficiency from cardiac NE deficiency. The mechanism of the movement disorder in PD therefore seems to differ from that of the dysautonomia.
Among a variety of biomarkers based on brain 6-[18F]fluorodopa scanning to detect central DA deficiency, ROC curves showed that the most efficient was the PUT:OCC ratio (more than 95% sensitivity at 80% specificity). PUT:SN ratios distinguished PD from MSA fairly well (68% sensitivity at 80% specificity), UPSIT scores more efficiently (78% sensitivity at 80% specificity), and cardiac 6-[18F]fluorodopamine scanning most efficiently (more than 95% sensitivity at 80% specificity).
Particular MRI procedures, such as diffusion weighted imaging, are currently more widely available in Europe and the US than is cardiac sympathetic neuroimaging and can efficiently distinguish PD from MSA [14, 40]. In general, PD does not entail abnormal MRI results. Since in the present study PD and MSA-P featured virtually identically severe decreases in 6-[18F]fluorodopa-derived radioactivity, differences in MRI findings between the two diseases seem to reflect pathophysiologic changes independent of striatal dopamine deficiency.
From the present results we propose that clinical laboratory testing to diagnose PD differentially from MSA should begin with the UPSIT. Anosmia supports PD, and normal or mildly decreased olfaction supports MSA. About half of patients can be diagnosed by this means. In patients with moderately or severely decreased olfaction, cardiac sympathetic imaging can separate PD+NOH from MSA, since the finding of cardiac noradrenergic denervation supports a diagnosis of PD+NOH, and the finding of normal cardiac innervation supports a diagnosis of MSA.
6-[18F]Fluorodopamine scanning is currently done routinely only at the National Institutes of Health Clinical Center; however, 6-[18F]fluorodopamine can be synthesized readily from 18F-fluorodopa, by incubation with L-aromatic-amino-acid decarboxylase and pyridoxal phosphate, followed by preparative liquid chromatography [41]. Cardiac 6-[18F]fluorodopamine scanning therefore is feasible in centers that do brain 6-[18F]fluorodopa scanning.
Cross-validation of neuroimaging and neurochemical indices of central DA deficiency and delineation of specific, pathophysiologic patterns of central and peripheral catecholamine systems in alpha-synucleinopathies set the stage for testing in de novo PD or individuals at risk for developing the disease; such testing is under way.
Acknowledgments
This research was supported by the Intramural Research Program of the NIH, National Institute of Neurological Disorders and Stroke.
We thank Dr. Nicholas Patronas and other members of the neuroradiology staff of the NIH Diagnostic Imaging Department, for carrying out lumbar punctures under fluoroscopic guidance. Dr. Peter Herscovitch acted as the Authorized User for administration of 6-[18F]fluorodopa and 6-[18F]fluorodopamine to humans. The PET Department of the NIH Clinical Center also provided technical assistance in the neuroimaging techniques. Richard Carson, PhD, W. Craig Barker, PhD, and Peter Herscovitch, MD supervised development and implementation of HRRT scanning at the NIH Clinical Center. Ms. Tereza Jenkins coordinated patient travel. Sandra Pechnik, RN, assisted with clinical procedures and scheduling. Dr. Elaine Peskind provided several CSF samples from normal control subjects.
Financial support: Division of Intramural Research, NINDS, NIH
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ravina B, Eidelberg D, Ahlskog JE, Albin RL, Brooks DJ, Carbon M, et al. The role of radiotracer imaging in Parkinson disease. Neurology. 2005;64:208–15. doi: 10.1212/01.WNL.0000149403.14458.7F. [DOI] [PubMed] [Google Scholar]
- 2.Hoshi H, Kuwabara H, Leger G, Cumming P, Guttman M, Gjedde A. 6-[18F]fluoro-L-dopa metabolism in living human brain: a comparison of six analytical methods. J Cereb Blood Flow Metab. 1993;13:57–69. doi: 10.1038/jcbfm.1993.8. [DOI] [PubMed] [Google Scholar]
- 3.Otsuka M, Kuwabara Y, Ichiya Y, Hosokawa S, Sasaki M, Yoshida T, et al. Differentiating between multiple system atrophy and Parkinson’s disease by positron emission tomography with 18F-dopa and 18F-FDG. Ann Nucl Med. 1997;11:251–7. doi: 10.1007/BF03164771. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein DS, Imrich R, Peckham E, Holmes C, Lopez G, Crews C, et al. Cardiac sympathetic denervation, baroreflex failure, and nigrostriatal dopamine deficiency in Parkinson disease from LRRK2 gene mutation. Neurology. doi: 10.1212/01.wnl.0000268696.57912.64. in press. [DOI] [PubMed] [Google Scholar]
- 5.LeWitt PA, Galloway MP, Matson W, Milbury P, McDermott M, Srivastava DK, et al. Markers of dopamine metabolism in Parkinson’s disease. The Parkinson Study Group. Neurology. 1992;42:2111–7. doi: 10.1212/wnl.42.11.2111. [DOI] [PubMed] [Google Scholar]
- 6.Eldrup E, Mogensen P, Jacobsen J, Pakkenberg H, Christensen NJ. CSF and plasma concentrations of free norepinephrine, dopamine, 3,4-dihydroxyphenylacetic acid (DOPAC), 3,4-dihydroxyphenylalanine (DOPA), and epinephrine in Parkinson’s disease. Acta Neurol Scand. 1995;92:116–21. doi: 10.1111/j.1600-0404.1995.tb01023.x. [DOI] [PubMed] [Google Scholar]
- 7.Litvan I, Goetz CG, Jankovic J, Wenning GK, Booth V, Bartko JJ, et al. What is the accuracy of the clinical diagnosis of multiple system atrophy? A clinicopathologic study. Arch Neurol. 1997;54:937–44. doi: 10.1001/archneur.1997.00550200007003. [DOI] [PubMed] [Google Scholar]
- 8.Wenning GK, Ben-Shlomo Y, Magalhaes M, Daniel SE, Quinn NP. Clinicopathological study of 35 cases of multiple system atrophy. J Neurol Neurosurg Psychiatry. 1995;58:160–6. doi: 10.1136/jnnp.58.2.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Antonini A, Leenders KL, Vontobel P, Maguire RP, Missimer J, Psylla M, et al. Complementary PET studies of striatal neuronal function in the differential diagnosis between multiple system atrophy and Parkinson’s disease. Brain. 1997;120(Pt 12):2187–95. doi: 10.1093/brain/120.12.2187. [DOI] [PubMed] [Google Scholar]
- 10.Polinsky RJ, Brown RT, Burns RS, Harvey-White J, Kopin IJ. Low lumbar CSF levels of homovanillic acid and 5-hydroxyindoleacetic acid in multiple system atrophy with autonomic failure. J Neurol Neurosurg Psychiatry. 1988;51:914–9. doi: 10.1136/jnnp.51.7.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Senard JM, Brefel-Courbon C, Rascol O, Montastruc JL. Orthostatic hypotension in patients with Parkinson’s disease: pathophysiology and management. Drugs Aging. 2001;18:495–505. doi: 10.2165/00002512-200118070-00003. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein DS. Orthostatic hypotension as an early finding in Parkinson disease. Clin Auton Res. 2006;16:46–64. doi: 10.1007/s10286-006-0317-8. [DOI] [PubMed] [Google Scholar]
- 13.Schocke MF, Seppi K, Esterhammer R, Kremser C, Jaschke W, Poewe W, et al. Diffusion-weighted MRI differentiates the Parkinson variant of multiple system atrophy from PD. Neurology. 2002;58:575–80. doi: 10.1212/wnl.58.4.575. [DOI] [PubMed] [Google Scholar]
- 14.Kollensperger M, Seppi K, Liener C, Boesch S, Heute D, Mair KJ, et al. Diffusion weighted imaging best discriminates PD from MSA-P: A comparison with tilt table testing and heart MIBG scintigraphy. Mov Disord. 2007 doi: 10.1002/mds.21614. [DOI] [PubMed] [Google Scholar]
- 15.Behnke S, Berg D, Naumann M, Becker G. Differentiation of Parkinson’s disease and atypical parkinsonian syndromes by transcranial ultrasound. J Neurol Neurosurg Psychiatry. 2005;76:423–5. doi: 10.1136/jnnp.2004.049221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baldessarini RJ, Marsh ER, Kula NS, Zong RS, Gao YG, Neumeyer JL. Effects of isomers of hydroxyaporphines on dopamine metabolism in rat brain regions. Biochemical pharmacology. 1990;40:417–23. doi: 10.1016/0006-2952(90)90538-v. [DOI] [PubMed] [Google Scholar]
- 17.Hurd YL, Suzuki M, Sedvall GC. D1 and D2 dopamine receptor mRNA expression in whole hemisphere sections of the human brain. Journal of chemical neuroanatomy. 2001;22:127–37. doi: 10.1016/s0891-0618(01)00122-3. [DOI] [PubMed] [Google Scholar]
- 18.Orimo S, Ozawa E, Oka T, Nakade S, Tsuchiya K, Yoshimoto M, et al. Different histopathology accounting for a decrease in myocardial MIBG uptake in PD and MSA. Neurology. 2001;57:1140–1. doi: 10.1212/wnl.57.6.1140. [DOI] [PubMed] [Google Scholar]
- 19.Orimo S, Oka T, Miura H, Tsuchiya K, Mori F, Wakabayashi K, et al. Sympathetic cardiac denervation in Parkinson’s disease and pure autonomic failure but not in multiple system atrophy. J Neurol Neurosurg Psychiatry. 2002;73:776–7. doi: 10.1136/jnnp.73.6.776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amino T, Orimo S, Takahashi A, Uchihara T, Mizusawa H. Profound cardiac sympathetic denervation occurs in Parkinson disease. Brain Path. 2005;15:29–34. doi: 10.1111/j.1750-3639.2005.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orimo S, Kanazawa T, Nakamura A, Uchihara T, Mori F, Kakita A, et al. Degeneration of cardiac sympathetic nerve can occur in multiple system atrophy. Acta Neuropathol (Berl) 2007;113:81–6. doi: 10.1007/s00401-006-0160-y. [DOI] [PubMed] [Google Scholar]
- 22.Siderowf A, Newberg A, Chou KL, Lloyd M, Colcher A, Hurtig HI, et al. [99mTc]TRODAT-1 SPECT imaging correlates with odor identification in early Parkinson disease. Neurology. 2005;64:1716–20. doi: 10.1212/01.WNL.0000161874.52302.5D. [DOI] [PubMed] [Google Scholar]
- 23.Lee PH, Yeo SH, Kim HJ, Youm HY. Correlation between cardiac 123I-MIBG and odor identification in patients with Parkinson’s disease and multiple system atrophy. Mov Disord. 2006;21:1975–7. doi: 10.1002/mds.21083. [DOI] [PubMed] [Google Scholar]
- 24.Abele M, Riet A, Hummel T, Klockgether T, Wullner U. Olfactory dysfunction in cerebellar ataxia and multiple system atrophy. J Neurol. 2003;250:1453–5. doi: 10.1007/s00415-003-0248-4. [DOI] [PubMed] [Google Scholar]
- 25.Nee LE, Scott J, Polinsky RJ. Olfactory dysfunction in the Shy-Drager syndrome. Clin Auton Res. 1993;3:281–2. doi: 10.1007/BF01829019. [DOI] [PubMed] [Google Scholar]
- 26.Kaufmann H. Consensus statement on the definition of orthostatic hypotension, pure autonomic failure and multiple system atrophy. Clin Auton Res. 1996;6:125–6. doi: 10.1007/BF02291236. [DOI] [PubMed] [Google Scholar]
- 27.Gilman S, Low P, Quinn N, Albanese A, Ben-Shlomo Y, Fowler C, et al. Consensus statement on the diagnosis of multiple system atrophy. Clin Auton Res. 1998;8:359–62. doi: 10.1007/BF02309628. [DOI] [PubMed] [Google Scholar]
- 28.Goldstein DS, Tack C. Non-invasive detection of sympathetic neurocirculatory failure. Clin Auton Res. 2000;10:285–91. doi: 10.1007/BF02281111. [DOI] [PubMed] [Google Scholar]
- 29.Goldstein DS, Holmes C, Li ST, Bruce S, Metman LV, Cannon RO., 3rd Cardiac sympathetic denervation in Parkinson disease. Ann Intern Med. 2000;133:338–47. doi: 10.7326/0003-4819-133-5-200009050-00009. [DOI] [PubMed] [Google Scholar]
- 30.Goldstein DS, Holmes C, Patronas N, Kopin IJ. Cerebrospinal fluid levels of catechols in patients with neurogenic orthostatic hypotension. Clin Sci (Lond) 2003;104:649–54. doi: 10.1042/CS20020315. [DOI] [PubMed] [Google Scholar]
- 31.Holmes C, Eisenhofer G, Goldstein DS. Improved assay for plasma dihydroxyphenylacetic acid and other catechols using high-performance liquid chromatography with electrochemical detection. J Chromatog B Biomed Applic. 1994;653:131–8. doi: 10.1016/0378-4347(93)e0430-x. [DOI] [PubMed] [Google Scholar]
- 32.Goldstein DS, Holmes C. Metabolic fate of the sympathoneural imaging agent 6-[18F]fluorodopamine in humans. Clin Exper Hypertens. 1997;19:155–61. doi: 10.3109/10641969709080812. [DOI] [PubMed] [Google Scholar]
- 33.Lang AE, Garnett ES. Dopa-responsive parkinsonism with normal 6[18F]-fluorodopa positron emission tomography scans. Ann Neurol. 1990;28:592–3. doi: 10.1002/ana.410280427. [DOI] [PubMed] [Google Scholar]
- 34.Brooks DJ, Salmon EP, Mathias CJ, Quinn N, Leenders KL, Bannister R, et al. The relationship between locomotor disability, autonomic dysfunction, and the integrity of the striatal dopaminergic system in patients with multiple system atrophy, pure autonomic failure, and Parkinson’s disease, studied with PET. Brain. 1990;113(Pt 5):1539–52. doi: 10.1093/brain/113.5.1539. [DOI] [PubMed] [Google Scholar]
- 35.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J Neurol Sci. 1973;20:415–55. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 36.Wilson JM, Levey AI, Rajput A, Ang L, Guttman M, Shannak K, et al. Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson’s disease. Neurology. 1996;47:718–26. doi: 10.1212/wnl.47.3.718. [DOI] [PubMed] [Google Scholar]
- 37.Kish SJ, Shannak K, Hornykiewicz O. Uneven pattern of dopamine loss in the striatum of patients with idiopathic Parkinson’s disease. Pathophysiologic and clinical implications. N Engl J Med. 1988;318:876–80. doi: 10.1056/NEJM198804073181402. [DOI] [PubMed] [Google Scholar]
- 38.Spokes EGS, Bannister R, Oppenheimer DR. Multiple system atrophy with autonomic failure: clinical, histological and neurochemical observations on four cases. J Neurol. 1979;43:59–82. doi: 10.1016/0022-510x(79)90073-x. [DOI] [PubMed] [Google Scholar]
- 39.Muller A, Mungersdorf M, Reichmann H, Strehle G, Hummel T. Olfactory function in Parkinsonian syndromes. J Clin Neurosci. 2002;9:521–4. doi: 10.1054/jocn.2001.1071. [DOI] [PubMed] [Google Scholar]
- 40.Seppi K, Schocke MF, Donnemiller E, Esterhammer R, Kremser C, Scherfler C, et al. Comparison of diffusion-weighted imaging and [123I]IBZM-SPECT for the differentiation of patients with the Parkinson variant of multiple system atrophy from those with Parkinson’s disease. Mov Disord. 2004;19:1438–45. doi: 10.1002/mds.20229. [DOI] [PubMed] [Google Scholar]
- 41.Goldstein DS, Eisenhofer G, Dunn BB, Armando I, Lenders J, Grossman E, et al. Positron emission tomographic imaging of cardiac sympathetic innervation using 6-[18F]fluorodopamine: initial findings in humans. J Am Coll Cardiol. 1993;22:1961–71. doi: 10.1016/0735-1097(93)90786-z. [DOI] [PubMed] [Google Scholar]