Summary
Background
Across many observational studies, herpes simplex virus type 2 (HSV-2) infection is associated with two-fold to three-fold increased risk for HIV-1 infection. We investigated whether HSV-2 suppression with aciclovir would reduce the risk of HIV-1 acquisition.
Methods
We undertook a double-blind, randomised, placebo-controlled phase III trial in HIV-negative, HSV-2 seropositive women in Africa and men who have sex with men (MSM) from sites in Peru and the USA. Participants were randomly assigned by block randomisation to twice daily aciclovir 400 mg (n=1637) or matching placebo (n=1640) for 12–18 months, and were seen monthly for dispensation of study drug, adherence counselling and measurement by pill count and self-reporting, and risk reduction counselling, and every 3 months for genital examination and HIV testing. The primary outcome was HIV-1 acquisition and secondary was incidence of genital ulcers. Analysis was by intention to treat. This study is registered with Clinicaltrials.gov, number NCT00076232.
Findings
3172 participants (1358 women, 1814 MSM) were included in the primary dataset (1581 in aciclovir group, 1591 in control group). The incidence of HIV-1 was 3.9 per 100 person-years in the aciclovir group (75 events in 1935 person-years of follow-up) and 3.3 per 100 person-years in the placebo group (64 events in 1969 person-years of follow-up; hazard ratio 1.16 [95% CI 0.83–1.62]). Incidence of genital ulcers on examination was reduced by 47% (relative risk 0.53 [0.46–0.62]) and HSV-2 positive genital ulcers by 63% (0.37 [0.31–0.45]) in the aciclovir group. Adherence to dispensed study drug was 94% in the aciclovir group and 94% in the placebo group, and 85% of expected doses in the aciclovir group and 86% in the placebo group. Retention was 85% at 18 months in both groups (1028 of 1212 in aciclovir group, 1030 of 1208 in placebo group). We recorded no serious events related to the study drug.
Interpretation
Our results show that suppressive therapy with standard doses of aciclovir is not effective in reduction of HIV-1 acquisition in HSV-2 seropositive women and MSM. Novel strategies are needed to interrupt interactions between HSV-2 and HIV-1.
Funding
US National Institute of Allergy and Infectious Diseases, US National Institute of Child Health and Human Development, US National Institute of Drug Abuse, US National Institute of Mental Health, US Office of AIDS Research, and GlaxoSmithKline.
Introduction
Herpes simplex virus type 2 (HSV-2) is the commonest cause of genital ulcers and a highly prevalent infection in sexually-active people worldwide. Seroprevalence rates of HSV-2 range from 22% of sexually-active adults in the USA, to up to 60% in HIV-negative women in sub-Saharan Africa and men who have sex with men (MSM) in Latin America, and to more than 80% in people infected with HIV.1,2 Despite high prevalence, HSV-2 infection is often unrecognised by people infected with HSV-2 and by health-care providers, partly because of mild, non-specific genital symptoms—such as itching or tingling—and an absence of classic genital lesions. However, HSV-2 reactivates frequently, as shown by the finding of HSV-2 on the genital epithelium by culture in 3% of days and by sensitive DNA PCR on about 20% of days, frequently without symptoms or lesions.3 Suppressive therapy is suggested for patients with frequent symptomatic recurrences (ie, six or more episodes in the past year) or for reducing the risk of transmission in couples who are HSV-2 discordant.
HSV-2 infection is associated with a 3.1-fold increased risk of HIV-1 acquisition in women, 2.8-fold increased risk in heterosexual men, and 1.8-fold increased risk in MSM, on the basis of longitudinal cohort studies showing HSV-2 infection preceding HIV-1 acquisition and adjusted for sexual behaviour.4,5 Studies have suggested an increased risk of HIV-1 acquisition in patients with recently acquired HSV-2 infection, some of whom could have acquired HSV-2 and HIV-1 concurrently from the same sexual partner.6,7 The biological mechanism for increased HIV-1 susceptibility because of HSV-2 is thought to be through disruption of the genital epithelium during HSV-2 reactivation and recruitment of activated target cells for HIV-1 (eg, CCR5-positive CD4 and immature dendritic cells) to the genital tract.8 Observational data suggest that HSV-2 might also increase HIV-1 transmission and disease progression.9,10 However, the proposed biological mechanism for this observation differs from that for HIV-1 susceptibility and probably results from up-regulation of HIV-1 during HSV replication and increased production of pro-inflammatory cytokines.11
Observational studies are limited by the potential for residual confounding in that HSV-2 infection might partly be an indicator for higher risk sexual behaviour that is not measured by self-reporting; thus, intervention trials are needed to show a causal relation between HSV-2 infection and increased HIV-1 acquisition. Aciclovir is a safe, effective, and widely available drug for suppression of genital herpes. A recent randomised, double-blind, placebo-controlled trial of aciclovir 400 mg given twice a day in 821 high-risk, HIV-1 negative, HSV-2 seropositive women in Tanzania showed no efficacy in reduction of HIV-1 acquisition (hazard ratio 1.08 [95% CI 0.64–1.83]); the absence of efficacy could have been partly due to suboptimal adherence, with median adherence of 90%.12
We hypothesised that a standard dose of aciclovir as daily suppressive therapy would reduce HIV-1 acquisition in men and women at high risk for HIV-1. We undertook a randomised, double-blind, placebo-controlled trial of aciclovir 400 mg given twice daily in HIV-1 negative, HSV-2 seropositive women in sub-Saharan Africa and MSM in North and Latin America to see if suppression of genital herpes could reduce risk of HIV-1 acquisition, through monthly visits to increase adherence and provide the best available services for HIV prevention.
Methods
Study population
We enrolled HIV-1 negative, HSV-2 seropositive women from sites in Johannesburg, South Africa; Harare, Zimbabwe; and Lusaka, Zambia; and MSM from sites in San Francisco (CA), Seattle (WA), New York (NY), USA; and Lima, Pucallpa, and Iquitos, Peru. Inclusion criteria were age 18 years or older (≥16 years in Zambia); HIV-1 seronegativity on the basis of rapid tests in the African sites and EIA in the US and Peruvian sites; HSV-2 seroZ-positivity on the basis of Focus HerpeSelect EIA (HerpeSelect-2 ELISA, Focus Technologies, Cypress, CA, USA); willingness to be randomly assigned and provide locator information; and with 2 or less months travel from the site's catchment area. Behavioural eligibility criteria were, for women, at least one episode of unprotected vaginal sex in the 6 months before screening and, for MSM, at least one episode of anal sex in the previous 6 months and not in a mutually monogamous relationship with a partner who is known to be HIV negative in the past year. We excluded participants with a previous adverse reaction to aciclovir; present or planned use of aciclovir, valaciclovir, or famciclovir; concurrent participation in trials for HIV-1 vaccination or prevention; and pregnant women.
Study design
Participants were recruited from voluntary counselling and testing, sexually transmitted infection (STI), family planning, and well-baby clinics, and through referrals from community-based organisations, printed media, radio, and community outreach. All participants received a comprehensive package for HIV-1 prevention consisting of counselling before and after the HIV-1 test, intensive risk reduction counselling, free condoms, and treatment of STIs according to guidelines from WHO and the US Centers for Disease Control and Prevention (CDC) for international and domestic US sites, respectively.
The study drug was manufactured by Carlsbad Laboratories (San Diego, CA, USA) with matched aciclovir 400 mg and placebo tablets, packaged in monthly bottles of 70 tablets to provide a 35-day supply, and stored between 15°C and 25°C. Placebo tablets were identical in appearance, taste, and weight to the aciclovir tablets. Participants were instructed to take one tablet in the morning and one in the evening, and to double the next dose if they missed a dose.
The randomisation method was developed and implemented by the Statistical Center for HIV Research Program (SCHARP) at Fred Hutchinson Cancer Research Center, Seattle, USA, and used block sizes of 4, 6, 8, and 10, stratified by site, and varied randomly with a pseudo-random number generator. The randomisation list was used to assemble sequentially numbered, identical sealed kits containing sufficient study drug or placebo for the entire follow-up period for every participant. All site staff and investigators were blinded to the randomisation code apart from an unblinded statistician at SCHARP. Data were electronically faxed from the clinics into a database system (DataFax, Clinical Datafax Systems, Hamilton, ON, Canada) at SCHARP. Investigators and study statisticians remained blinded to study assignment until completion of participant follow-up and adjudication of endpoints.
The study protocol was approved by the Division of AIDS Prevention Science Review Committee, Family Health International Regulatory Affairs, University of Washington institutional review board, and by institutional review boards at the local institutions and collaborating organisations. All participants provided written informed consent.
Procedures
Study screening included behavioural risk assessment, identification of HIV-1 and HSV-2 serostatus, and willingness to be randomly assigned to twice daily study drug and monthly visits for 12 months, which was extended to 18 months for participants who were willing to reconsent for an additional 6 months of follow-up. We undertook visits every month for provision of study drug and collection of unused drug from the preceding month. If participants failed to return unused study drug, they were asked to return it at their next monthly visit, which was used to compute adherence by pill count for the previous month. At monthly visits, we asked participants about self-reported adherence, the maximum number of consecutive missed doses, risk behaviours in that month, and symptoms of genital herpes in the previous 7 days. If symptoms were reported, we did a genital examination. Condoms and counselling for adherence and risk reduction were provided. At visits every 3 months, participants were interviewed about risk behaviour with their three most recent partners and STI symptoms, examined for genital herpes and other STIs, and tested for pregnancy and HIV-1.
STI testing was done at enrolment and, when symptomatic, at follow-up visits. We undertook syphilis serologies at enrolment and exit visits, with use of the rapid plasma reagin (RPR) with confirmation by microhaemagluttinin assay-Treponema pallidum. We obtained vaginal swabs to assess bacterial vaginosis by gram stain and Trichomonas vaginalis by In-pouch (BioMed Diagnostics, White City, OR, USA) at two women's sites or wet mount (one women's site), and we obtained a cervical swab for nucleic acid testing for Chlamydia trachomatis and Neisseria gonorrhoeae (two sites: Johannesburg and Harare) or by Neisseria gonorrhoeae culture and Chlamydia trachomatis rapid EIA testing (one site: Lusaka). MSM had a rectal swab obtained for Neisseria gonorrhoeae culture and urine obtained for leucocyte esterase testing as an indicator of urethritis, which was followed by nucleic acid testing for Chlamydia trachomatis and Neisseria gonorrhoeae if the result was 1 or higher (scale 1–4). Participants who had examination findings that were consistent with genital herpes were offered open-label aciclovir 400 mg three times every day for 5 days, which was to be taken in addition to their blinded regimen.13 Women with positive urine pregnancy tests were discontinued from study drug until pregnancy tests at follow-up were negative. As a surrogate for possible renal insufficiency related to aciclovir, participants were discontinued from aciclovir if they reported an unexplained weight gain of 2.27 kg (5 lb) and decreased urinary output.
To establish eligibility and at every 3-month visit, African sites undertook dual parallel rapid HIV-1 tests and the Peruvian and US sites used HIV-1 EIA; all reactive samples were confirmed by HIV-1 Western blot. Sera from enrolment were sent to the HIV Prevention Trials Network central laboratory, which undertook quality assurance testing on 418 (13%) samples, and confirmed all endpoints by HIV-1 EIA (Genetic Systems rLAV EIA, Bio-Rad laboratories, Hercules, CA, USA) and Western blot (Genetics Systems TM HIV-1). HSV-2 serostatus for eligibility was established by Focus HerpeSelect-2 EIA (Focus Technologies, Cypress, CA, USA), with a cut-off of 3.4 to improve specificity.14,15 Participants were eligible if their Focus EIA was greater than 3.4 or was between 1.1 and 3.4 and they were HSV-2 positive by Western blot. HSV-2 eligibility was confirmed by testing at the University of Washington Virology laboratory of sera from enrolment by Focus HerpeSelect-2 EIA and HSV Western blot;16 participants who did not have HSV-2 antibodies by Western blot were excluded from analyses.
We tested swabs of genital ulcers for HSV DNA at the University of Washington Virology laboratory; DNA was extracted from 200 μL of specimens with QIAamp 96 DNA blood kit (Qiagen, Santa Clarita, CA, USA) eluted into 100 μL of AE buffer. We used a real-time fluorescent probe-based PCR (TaqMan; Applied Biosystems) assay to quantitate HSV, with 10 μL of the extracted DNA for every PCR reaction, with primers and probe sequences and PCR conditions that have been described previously;17,18 more than 3 copies per μL (150 copies of HSV DNA per mL of fluid from genital ulcer swab) was considered positive. Vaginal slides taken at enrolment were sent to University of Pittsburgh (PA, USA), gram stained, and read by a laboratory technologist who was blinded to allocation; a score of 7 or greater by Nugent's criteria was classified as bacterial vaginosis.19
Monthly adherence to the study drug was assessed by pill count and self-report of 100%, or less than 100% adherence and maximum number of consecutive missed doses. Adherence was computed on the basis of monthly pill counts, and for the 1554 (3.5%) visits at which pill bottles were not returned, by self-reporting. If self-reported adherence was less than 100%, we set that month's adherence to missing since the number of missed doses was not ascertained. Monthly data were aggregated to quarters and categorised as less than 90%, more than 90%, or missing; these categories were selected on the basis of adherence levels that had been achieved in previous efficacy trials.20
Study endpoints
The primary outcome was HIV-1 acquisition; the secondary outcome was incidence of genital ulcers. Acquisition of HIV-1 infection was defined on the basis of a reactive HIV-1 EIA and positive Western blot in participants who had a previous HIV-1 antibody test that was negative. A second serum sample was obtained 2 weeks later to confirm seroconversion. For participants who tested positive at the first 3-month visit, serum samples obtained at enrolment were tested for HIV-1 RNA (Amplicor HIV Monitor Ultrasensitive version 1.5, Roche Molecular Systems, Indianapolis, IN, USA); if HIV-1 RNA PCR was positive at enrolment, the participant was excluded from the analysis because they were infected with HIV-1 at randomisation. An endpoints committee, the members of which were blinded to treatment assignment, reviewed all HIV-1 seroconversions. Genital ulcer disease was defined on the basis of ulcers with a clinical diagnosis of genital HSV based on examination at interim visits or visits every 3 months.
Statistical analysis
The study was originally designed to enrol 3682 individuals for 12 months follow-up. This design was projected to yield 93 seroconversions with an estimated HIV incidence of 3.5% in the placebo group and 90% power to detect a relative risk of 0.51, and a 0.025 false-positive error rate (two-sided type I error rate of 5%). On Sept 13, 2004, the protocol was revised to extend follow-up to 18 months because of slower enrolment than was expected, and participants were reconsented for 6 additional months of follow-up. To avoid potential bias, the 12–18 month data from reconsented participants were, by previous design, included only for sites that achieved 94% reconsent rates (all sites apart from those in Peru and South Africa).
An independent data safety and monitoring board met six times during the study and reviewed study enrolment, retention, and safety, and reviewed efficacy at four meetings. Criteria for statistical monitoring were based on the sequential method by the Lan-Demets group, with O'Brien-Fleming stopping boundaries to preserve the overall false-positive error rate at 2.5%.21,22
The primary analysis was intention to treat, on the basis of a proportional hazards regression model, stratified by site, to compare time to first positive HIV-1 test between the two intervention groups. We used the Kaplan-Meier method to estimate the cumulative probability of HIV-1 infection with time to first positive HIV-1 antibody test. Prespecified subgroup analyses included participants' sex and site. All tests for effect modification were based on likelihood ratio comparisons between models with and without appropriate interaction terms.
We undertook a prespecified secondary analysis, in which women were removed from the model when they discontinued study drug because of pregnancy and returned to the risk set following the first negative HIV-1 test after resumption of study drug. We undertook an exploratory, secondary per-protocol analysis, in which treatment group, study drug adherence averaged for each 3-month interval, and their interaction were included in a proportional hazards regression model. This analysis was stratified by site and adjusted for the following baseline variables: sex, age, history of genital ulcers in the 3 months before enrolment, and number of sexual partners in the past 12 months. Hazard ratios by level of adherence are reported. Adherence was a measurement after randomisation, thus the resulting hazard ratios represent non-randomised comparisons that could be biased.
To assess treatment effect on genital ulcers, we used the number of episodes of disease by examination over follow-up as the outcome. A log-linear regression with (log) duration of follow-up as the offset, negative binomial errors, and robust variances were used to estimate the intervention effect. Models with and without interaction terms were fit to test for effect modification.
All analyses used the primary database, which excluded participants who were inappropriately enrolled (ie, negative confirmatory testing for HSV-2 antibody, HIV PCR positive at enrolment in participants who seroconvertered at 3 months, and duplicate enrolments).
The trial is registered with Clinicaltrials.gov, number NCT00076232.
Role of the funding source
The sponsor of the study reviewed and approved the protocol and protocol revisions. The sponsor participated in study design and oversaw the monitoring of trial implementation, but had no role in data collection, data analysis, data interpretation, or writing of the report. All authors had full access to all the data in the study and contributed to the decision made by the corresponding author, who had full responsibility for the decision to submit for publication.
Results
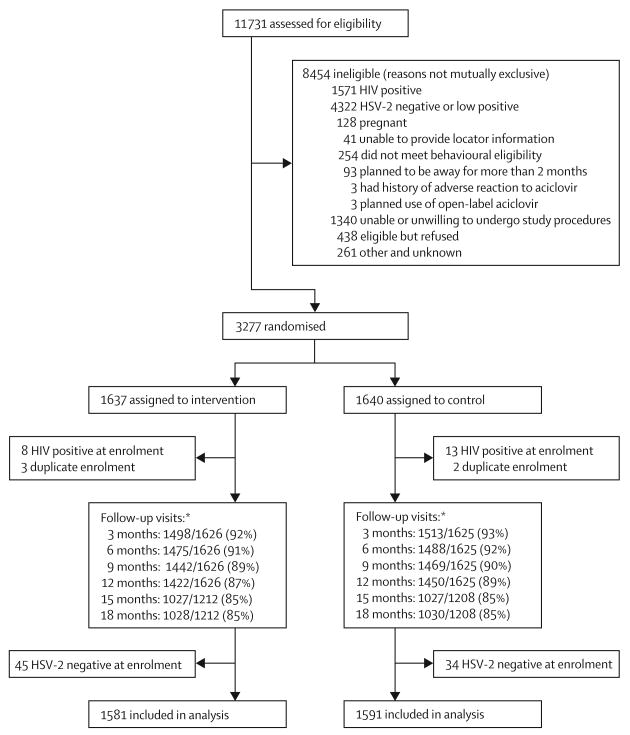
3277 HIV-negative, HSV-2 seropositive participants were randomly assigned (figure 1), of whom 1395 were women (625 from Lusaka, Zambia; 400 from Johannesburg, South Africa; and 370 from Harare, Zimbabwe) and 1882 were MSM (884 from Lima, 300 from Pucallpa, 200 from Iquitos, Peru; 270 from Seattle, 180 from San Francisco, and 48 from New York, USA). Of these randomised participants, 3172 (1814 men and 1358 women) were eligible for the primary analysis and 105 were excluded (figure 1). Excluding sites with low reconsent rates (Peru and South Africa), 264 (96%) of 276 participants enrolled under the original protocol in the placebo group and 262 (95%) of 273 participants enrolled in the aciclovir group reconsented to extending follow-up from 12 to 18 months. Retention was 85% at 18 months, with similar proportions of retention at 3-month assessments for both groups throughout follow-up (figure 1).
Figure 1. Trial profile.
*Denominator for follow-up at 12–18 months is lower than for previous follow-up assessments because of exclusion of data from two sites (Peru and South Africa) that had less than 95% reconsent rate for follow-up extension to avoid potential bias.
Age, education, household income, marital status, race, ethnic origin, number of sexual partners in the past 1 and 12 months, and proportion with unprotected sex in the 3 months before enrolment were similar at baseline in both treatment groups (table 1). We recorded heterogeneity between risk groups within treatment groups; African women had fewer sexual partners in the past 12 months, higher number of sexual acts, and a higher proportion of unprotected sex in the previous 3 months than did MSM (table 1). Male circumcision rates differed substantially between MSM from US and Peruvian sites (table 1). The baseline prevalence of other STIs was comparable between treatment groups and was low. The exceptions were the high prevalence of bacterial vaginosis in women, and syphilis seropositivity of low titre in MSM from Peru (table 1), indicating late latent syphilis or past treatment.
Table 1. Demographics and behavioural and clinical characteristics of participants at enrolment.
| Aciclovir group | Placebo group | |||||||
|---|---|---|---|---|---|---|---|---|
| US MSM (N=230) | Peru MSM (N=672) | African women (N=679) | Total (N=1581) | US MSM (N=229) | Peru MSM (N=683) | African women (N=679) | Total (N=1591) | |
| Demographics | ||||||||
| Age (years, median [min-max]) | 42 (18–75) | 27 (18–66) | 30 (17–66) | 30 (17–75) | 38 (20–79) | 28 (18–63) | 31 (16–61) | 30 (16–79) |
| Highest educational level | ||||||||
| Primary school or less | 1 (0.4%) | 32 (4.8%) | 283 (41.7%) | 316 (20.0%) | 0 | 25 (3.7%) | 252 (37.1%) | 277 (17.4%) |
| Secondary school (partial and completed) | 29 (12.6%) | 393 (58.5%) | 370 (54.5%) | 792 (50.1%) | 42 (18.3%) | 407 (59.6%) | 401 (59.1%) | 850 (53.4%) |
| College or greater | 200 (87.0%) | 247 (36.8%) | 26 (3.8%) | 473 (29.9%) | 187 (81.7%) | 251 (36.7%) | 26 (3.8%) | 464 (29.2%) |
| Any monthly income of own | 186 (80.9%) | 514 (76.5%) | 143 (21.1%) | 843 (53.3%) | 189 (82.5%) | 523 (76.6%) | 138 (20.3%) | 850 (53.4%) |
| Race/ethnic origin | ||||||||
| Hispanic (US only)* | 37 (16.1%) | .. | .. | 37 (16.1%) | 36 (15.7%) | .. | .. | 36 (15.7%) |
| White | 177 (77.0%) | 29 (4.3%) | 0 | 206 (13.0%) | 171 (74.7%) | 39 (5.7%) | 0 | 210 (13.2%) |
| Black | 22 (9.6%) | 14 (2.1%) | 678 (99.9%) | 714 (45.2%) | 25 (10.9%) | 17 (2.5%) | 674 (99.3%) | 716 (45.0%) |
| Mixed/other/missing | 28 (12.2%) | 629 (93.6%) | 1 (0.1%) | 658 (41.6%) | 33 (14.4%) | 627 (91.8%) | 5 (0.7%) | 665 (41.8%) |
| Behavioural characteristics | ||||||||
| Number of SP in: | ||||||||
| Past 1 month (median [IQR]) | 1 (0–2) | 2 (1–4) | 1 (1–1) | 1 (1–2) | 1 (0–2) | 2 (1–4) | 1 (1–1) | 1 (1–2) |
| Past 12 months (median [IQR]) | 6 (3–12) | 10 (5–30) | 1 (1–1) | 3 (1–10) | 6 (3–14) | 12 (5–30) | 1 (1–1) | 3 (1–12) |
| HIV serostatus of SP in past month | ||||||||
| Any HIV positive SP | 35 (15.2%) | 11 (1.6%) | 9 (1.3%) | 55 (3.5%) | 26 (11.4%) | 8 (1.2%) | 11 (1.6%) | 45 (2.8%) |
| Any HIV unknown SP | 62 (27.0%) | 489 (72.8%) | 417 (61.4%) | 968 (61.2%) | 64 (27.9%) | 484 (70.9%) | 403 (59.4%) | 951 (59.8%) |
| Any HIV negative SP | 110 (47.8%) | 179 (26.6%) | 215 (31.7%) | 504 (31.9%) | 120 (52.4%) | 201 (29.4%) | 227 (33.4%) | 548 (34.4%) |
| Sexual behaviours with last three SP in last 3 months | ||||||||
| Any unprotected anal receptive | 81 (35.2%) | 366 (54.5%) | .. | 447 (49.6%) | 81 (35.4%) | 386 (56.5%) | .. | 467 (51.2%) |
| Any protected anal receptive | 97 (42.2%) | 444 (66.1%) | .. | 541 (60.0%) | 107 (46.7%) | 448 (65.6%) | .. | 555 (60.9%) |
| Any unprotected anal insertive | 95 (41.3%) | 130 (19.3%) | .. | 225 (24.9%) | 97 (42.4%) | 159 (23.3%) | .. | 256 (28.1%) |
| Any protected anal insertive | 127 (55.2%) | 170 (25.3%) | .. | 297 (32.9%) | 118 (51.5%) | 167 (24.5%) | .. | 285 (31.3%) |
| Any unprotected vaginal | .. | .. | 610 (89.8%) | 610 (89.8%) | .. | 613 (90.3%) | 613 (90.3%) | |
| Any protected vaginal | .. | .. | 257 (37.8%) | 257 (37.8%) | .. | .. | 273 (40.2%) | 273 (40.2%) |
| Number of sexual acts with three most recent SP in past 3 months (median [IQR]) | ||||||||
| Anal sex | 5 (2–10) | 6 (3–14) | NA | 6 (3–13) | 4 (2–12) | 6 (3–14) | NA | 6 (3–14) |
| Vaginal sex | NA | NA | 24 (9–39) | 24 (9–39) | NA | NA | 24 (12–40) | 24 (12–40) |
| Alcohol use with sex | 94 (40.9%) | 345 (51.3%) | 14 (2.1%) | 453 (28.7%) | 87 (38.0%) | 354 (51.8%) | 18 (2.7%) | 459 (28.8%) |
| Drug use with sex | 91 (39.6%) | 37 (5.5%) | NA | 128 (14.2%) | 84 (36.7%) | 31 (4.5%) | NA | 115 (12.6%) |
| Exchange of money, drugs, or shelter for sex | 9 (3.9%) | 183 (27.2%) | 13 (1.9%) | 205 (13.0%) | 10 (4.4%) | 172 (25.2%) | 11 (1.6%) | 193 (12.1%) |
| Clinical characteristics | ||||||||
| Genital ulcers at enrolment | ||||||||
| Self-reported GUD in past 3 months | 56 (24.3%) | 114 (17.0%) | 211 (31.1%) | 381 (24.1%) | 75 (32.8%) | 113 (16.5%) | 232 (34.2%) | 420 (26.4%) |
| Self-reported GUD in past 7 days | 17 (7.4%) | 46 (6.8%) | 87 (12.8%) | 150 (9.5%) | 27 (11.8%) | 51 (7.5%) | 93 (13.7%) | 171 (10.8%) |
| Genital ulcers on examination | 6 (2.6%) | 25 (3.7%) | 96 (14.1%) | 127 (8.0%) | 9 (3.9%) | 14 (2.0%) | 129 (19.0%) | 152 (9.6%) |
| Male circumcision | 183 (79.6%) | 37 (5.5%) | .. | 220 (24.4%) | 192 (83.8%) | 41 (6.0%) | .. | 233 (25.5%) |
| STIs at enrolment | ||||||||
| Syphilis seropositive | 5 (2.2%) | 201 (29.9) | 27 (4.0%) | 233 (14.7%) | 14 (6.1%) | 220 (32.2%) | 28 (4.1%) | 262 (16.5%) |
| Titre (median [IQR]) | 4 (1–8) | 4 (2–8) | 8 (4–16) | 4 (2–8) | 2 (1–8) | 4 (2–8) | 4 (4–16) | 4 (2–8) |
| Gonorrhoea (rectal or cervical) | 2 (0.9%) | 3 (0.4%) | 6 (0.9%) | 11 (0.7%) | 2 (0.9%) | 1 (0.1%) | 5 (0.7%) | 8 (0.5%) |
| Chlamydia | NA | NA | 38 (5.6%) | 38 (5.6%) | NA | NA | 43 (6.3%) | 43 (6.3%) |
| Urine leucocyte esterase positive | 2 (0.9%) | 4 (0.6%) | NA | 6 (0.7%) | 7 (3.1%) | 4 (0.6%) | NA | 11 (1.2%) |
| Trichomonas | NA | NA | 60 (8.8%) | 60 (8.8%) | NA | NA | 48 (7.1%) | 48 (7.1%) |
| Bacterial vaginosis | .. | .. | 271 (40.2%) | 271 (40.2%) | .. | .. | 264 (39.1%) | 264 (39.1%) |
Data are number (%) unless otherwise specified. MSM=men who have sex with men. SP=sexual partners. NA=not available. GUD=genital ulcer disease. STI=sexually transmitted infection.
In the USA only, ethnic origin is categorised as Hispanic or not, in addition to racial categories of white, black/African-American, Asian, Pacific Islander, and other.
At enrolment, a small proportion of participants reported symptoms of genital herpes in the past 3 months or 7 days (table 1). The proportion diagnosed as having genital ulcers on examination at enrolment was similar between groups; higher proportions of women had genital ulcers that were diagnosed on examination at enrolment than did MSM (table 1).
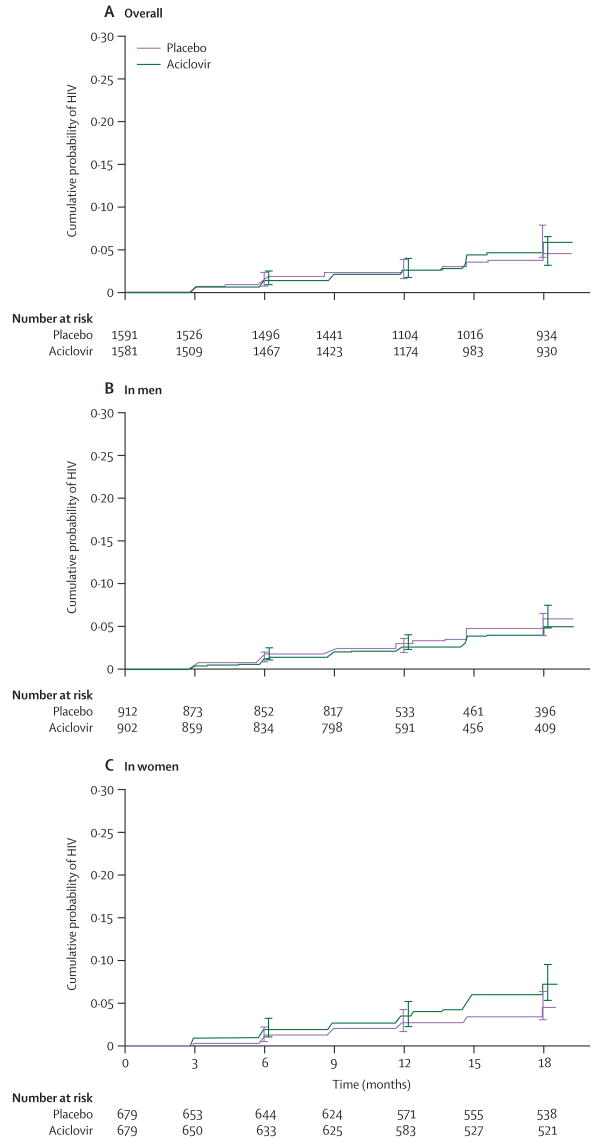
Although the enrolled sample size was lower than was originally specified in the protocol, this endpoint-driven trial had sufficient power with 139 endpoints compared with 93 based on the power calculations. The overall HIV-1 incidence in the primary analysis was 3.6 per 100 person-years, with 139 events and 3903 person-years of follow-up; we recorded 75 events in the aciclovir group (1935 person-years of follow-up) and 64 events in the placebo group (1969 person-years follow-up; hazard ratio [HR] 1.16 [95% CI 0.83–1.62]) with no significant differences by sex, site, or region (table 2). Figure 2 shows overall and sex-specific Kaplan-Meier estimates of the cumulative probability of HIV-1 infection by treatment group. We noted significant heterogeneity between the women's sites with regard to HIV-1 incidence—the Zambian and South African sites had 2.3-fold and 2.5-fold higher HIV-1 incidence, respectively, than did the Zimbabwean site (Zambia 4.7% per year; Zimbabwe 2.1% per year; South Africa 4.8% per year; p=0.01).
Table 2. Summary of HIV seroconversions, overall and by subgroups of sex, region, study drug adherence, and genital ulcer disease at enrolment.
| Aciclovir group | Placebo group | Total | |
|---|---|---|---|
| Overall study population | |||
| Sample size | 1581 (100%) | 1591 (100%) | 3172 |
| Seroconversions | 75 | 64 | 139 |
| Person-years of follow-up | 1935 | 1969 | 3903 |
| Rate per 100 person-years* | 3.9 | 3.3 | 3.6 |
| Hazard ratio (95% CI)* | .. | .. | 1.16 (0.83–1.62) |
| Women in African sites | |||
| Sample size | 679 (43%) | 679 (43%) | 1358 |
| Seroconversions | 44 | 28 | 72 |
| Person-years of follow-up | 893 | 905 | 1798 |
| Rate per 100 person-years† | 4.9 | 3.1 | 4.0 |
| Hazard ratio (95% CI) | .. | .. | 1.53 (0.95–2.46) |
| MSM in US sites | |||
| Sample size | 230 (15%) | 229 (14%) | |
| Seroconversions | 7 | 6 | 13 |
| Person-years of follow-up | 279 | 277 | 556 |
| Rate per 100 person-years | 2.5 | 2.2 | 2.3 |
| Hazard ratio (95% CI) | .. | .. | 1.09 (0.36–3.24) |
| MSM in Peruvian sites | |||
| Sample size | 672 (43%) | 683 (43%) | |
| Seroconversions | 24 | 30 | 54 |
| Person-years of follow-up | 762 | 786 | 1548 |
| Rate per 100 person-years | 3.2 | 3.8 | 3.5 |
| Hazard ratio (95% CI) | .. | .. | 0.82 (0.48–1.41) |
| <90% adherence‡ | |||
| Seroconversions | 22 | 14 | 36 |
| Person-years of follow-up | 402 | 401 | 803 |
| Rate per 100 person-years | 5.5 | 3.5 | 4.5 |
| Hazard ratio (95% CI) | .. | .. | 1.57 (0.80–3.08) |
| ≥90% adherence‡ | |||
| Seroconversions | 47 | 47 | 94 |
| Person-years of follow-up | 1375 | 1410 | 2785 |
| Rate per 100 person-years | 3.4 | 3.3 | 3.4 |
| Hazard ratio (95% CI) | .. | .. | 1.00 (0.67–1.50) |
| GUD at enrolment§ | |||
| Sample size | 440 (28%) | 489 (31%) | |
| Seroconversions | 24 | 18 | 42 |
| Person-years of follow-up | 560 | 613 | 1173 |
| Rate per 100 person-years | 4.3 | 2.9 | 3.58 |
| Hazard ratio (95% CI) | .. | .. | 1.36 (0.74–2.52) |
| No GUD at enrolment§ | |||
| Sample size | 1141 (72%) | 1102 (69%) | |
| Seroconversions | 51 | 46 | 97 |
| Person-years of follow-up | 1375 | 1355 | 2730 |
| Rate per 100 person-years | 3.7 | 3.4 | 3.55 |
| Hazard ratio (95% CI) | .. | .. | 1.08 (0.72–1.61) |
HIV seroconverions for overall study population are based on primary dataset, in which events and follow-up time is excluded from sites that did not achieve 94% consent rate for extension of follow-up from 12 to 18 months from participants enrolled in the original protocol. MSM=men who have sex with men. GUD=genital ulcer disease.
When protocol-specified time off study drug during pregnancy is excluded, 71 seroconversions were observed in the aciclovir group and 63 in the placebo group for HIV rates of 3.8 per 100 person-years and 3.3 per 100 person-years, respectively.
Based on Cox model, stratified by site.
Time dependent measure, so number of participants in each subgroup not given. Missing adherence category not shown.
GUD based on history of genital herpes symptoms in the past 3 months or genital ulcer on enrolment examination.
Figure 2. Cumulative probability of HIV infection (Kaplan-Meier estimates) by treatment.
(A) Overall (p=0.39). (B) In men (p=0.58). (C) In women (p=0.08). Error bars indicate 95% CIs.
In prespecified secondary analyses, the estimated relative risk of HIV-1 acquisition was similar by sex, region, and genital ulcers on examination at enrolment (table 2). We recorded a non-significant trend in difference in risk of HIV infection by sex and treatment group (MSM: HR 0.87 [95% CI 0.54–1.40]; women: 1.53 [0.95–2.46]; p=0.09). We noted no evidence of increased unprotected sexual activity in the intervention group (so-called risk compensation). Specifically, the probability and frequency of unprotected vaginal and anal sexual acts with the three most recent partners reported in the previous 3 months decreased over the course of the trial in both groups and did not differ by treatment group (probability of unprotected receptive/insertive anal sex 0.33/0.16 and 0.34/0.17 for aciclovir and placebo, respectively; RR 0.99/0.90 adjusted for time in study and age, p=0.34; probability of unprotected vaginal sex 0.76 and 0.72 for aciclovir and placebo, respectively; RR 1.19 adjusted for time in study and age, p=0.07).
Adherence to study drug was high overall, with a median of 94% of dispensed drug taken on the basis of pill count and self-reporting (equal in both groups). When we included non-adherence because of both missed visits and pill count, median adherence was 86% overall (86% in the placebo group and 85% in the aciclovir group). Based on monthly adherence data that were aggregated over quarters, 90% or higher adherence was recorded at 11 403 (73%) of the quarterly visits. In a subgroup analysis of high (≥90%) versus low (<90%) adherence, we recorded no significant difference in relative risk of HIV-1 incidence by adherence level (table 2). Notably, only 1958 (4.4%) visits indicated six or more consecutive missed doses, which would increase the likelihood of HSV reactivation. We noted good (77%) agreement between monthly pill count and self-reported adherence to study drug for participants with 100% self-reported adherence, but less agreement in those who reported missing some doses in the previous month (data not shown). The primary reasons for missed doses were forgetting (n=2656 [6%]) and being away from home (n=1326 [3%]). Incidence of pregnancy was 13.2 per 100 woman-years; pregnancy accounted for only 2% of missed study drug overall (4% at womens' sites) since only half of pregnancies continued to term. We recorded a significantly higher incidence of pregnancy in women in the aciclovir group than in the placebo group (129 [15% per year] vs 99 [11% per year], p=0.03).
Overall, 500 (31%) participants in the placebo group and 351 (22%) in the aciclovir group received open-label aciclovir treatment at some point during follow-up, but the rate of episodic treatment for genital ulcers was low (931 [4%] visits in the placebo group and 544 [3%] in the aciclovir group; relative risk 0.59 [95% CI 0.50–0.69]; p<0.0001). Thus, there is little indication of potential unblinding on the basis of rates of episodic treatment for genital ulcer disease, and loss to follow-up was identical in both groups (figure 1).
The overall incidence of genital ulcers diagnosed on examination decreased by 47% overall in the aciclovir group (table 3). We recorded significant differences in reductions in genital ulcers on examination by region (table 3). The difference between regions in intervention effect on genital ulcers was significant (p=0.0003), indicating that risk group or geographic region, or both, is an effect modifier. We noted heterogeneity in the womens' sites, with 1.4 and 1.7 higher likelihood of genital ulcers diagnosed at follow-up in Zambia than in South Africa and Zimbabwe, respectively (Zambia 73.4% per year; Zimbabwe 44.9% per year; South Africa 55.6% per year; p<0.0001).
Table 3. Relative risk of incidence of genital ulcers*.
| Aciclovir group | Placebo group | RR (95% CI) | p value | |||||
|---|---|---|---|---|---|---|---|---|
| Events | Person-years | Rate per 100 person-years | Events | Person-years | Rate per 100 person-years | |||
| GUD by examination | ||||||||
| Overall | 574 | 1936.05 | 29.65 | 1090 | 1971.00 | 55.30 | 0.53 (0.46–0.62) | <0.0001 |
| Africa | 409 | 893.18 | 45.79 | 679 | 905.94 | 74.95 | 0.61 (0.51–0.74) | <0.0001 |
| USA | 43 | 279.60 | 15.38 | 146 | 277.70 | 52.58 | 0.29 (0.18–0.47) | <0.0001 |
| Peru | 122 | 763.27 | 15.98 | 265 | 787.37 | 33.66 | 0.47 (0.36–0.62) | <0.0001 |
| HSV DNA positive GUD | ||||||||
| Overall | 231 | 1936.05 | 11.93 | 630 | 1971.00 | 31.96 | 0.37 (0.31–0.45) | <0.0001 |
| Africa | 148 | 893.18 | 16.57 | 348 | 905.94 | 38.41 | 0.43 (0.34–0.56) | <0.0001 |
| USA | 12 | 279.60 | 4.29 | 97 | 277.70 | 34.93 | 0.12 (0.05–0.29) | <0.0001 |
| Peru | 71 | 763.27 | 9.30 | 185 | 787.37 | 23.50 | 0.39 (0.28–0.56) | <0.0001 |
GUD=genital ulcer disease. RR=relative risk.
Genital ulcers based on examination findings and genital ulcers that were HSV DNA PCR positive.
The proportion of genital ulcers that had HSV DNA detected by PCR was significantly higher in swabs from the placebo group than in those from the aciclovir group (p<0.0001; table 3). All but seven of 870 swabs with detectable HSV PCR were positive for HSV-2 by typing. HSV-2 positive episodes of genital ulcers were significantly reduced by 63%, with significant differences by region (table 3). However, the quantity of HSV, as measured by copy number of HSV DNA PCR in genital ulcers, differed by only 0.4 log10 copies between participants receiving aciclovir versus placebo. The only population in which the quantity of HSV DNA was reduced in breakthrough ulcers in the aciclovir group was in MSM from US sites; median HSV DNA copy number was 4.3 log10 in ulcer swabs from participants in the aciclovir group compared with 6.3 log10 in the placebo group. Across all sites, we recorded no evidence of effect modification in relative risk of genital ulcers on examination or HSV-2 positive ulcers by low versus high (<90% vs ≥90%) adherence to study drug every 3 months. These results are not due to the potency of aciclovir used in the study; after storage of study drug in field conditions, testing of study drug by liquid chromatography confirmed presence of 400 mg aciclovir.
With respect to non-herpetic causes of genital ulcers, only 22 (2%) were classified by clinicians as likely to be caused by other aetiologies in women (two [0.2%] presumptive chancroid, six [0.6%] presumptive HSV and chancroid, and 14 [1.2%] other), and 41 (12.5%) were classified as non-herpetic in men (ten [3.0%] presumptive chancroid, 14 [4.3%] presumptive HSV and chancroid, and 17 [5.2%] other). Syphilis incidence by RPR conversion or greater than four-fold increase in titre during follow-up was 3% overall with no difference by treatment group (p=0.18; data not shown).
In this trial, serious adverse events were defined as death, admission to hospital, life-threatening illness, new onset seizures, or renal failure. A total of 138 serious adverse events occurred in the study population with 63 (4%) in the placebo group and 75 (5%) in the aciclovir group (p=0.28; table 4). All serious adverse events were considered unrelated to study drug, and most were caused by infections or trauma (table 4). Nine (0.3%) participants died in the study: two in the placebo group (one cardiac arrest after assault and one unknown cause) and seven in the aciclovir group (three trauma-related, one postoperative complication, one sepsis, one hyper tensive intracranial haemorrhage, and one of unknown cause; p=0.12 in death rates by group), and none of the deaths was considered related to study drug. Overall, 68 (4%) participants in the aciclovir group and 59 (4%) in the placebo group were admitted to hospital during the trial (p=0.40). A similar proportion of study participants (65 [4%] vs 58 [4%]) reported an unexplained weight gain of more than 2.27 kg or decreased urinary output, which is a surrogate indication of possible renal insufficiency, at any time during the follow-up. However, no participants reported both symptoms at the same visit to prompt assessment of renal insufficiency or discontinuation of study drug.
Table 4. Incidence of serious adverse events by study group.
| Placebo group (N=1591) | Aciclovir group (N=1581) | Total (N=3172) | |
|---|---|---|---|
| Participants with at least one SAE | 63 (4%) | 75 (5%) | 138 (4%) |
| Death | 2 (0.1%) | 7 (0.4%) | 9 (0.3%) |
| Admission to hospital | 59 (4%) | 68 (4%) | 127 (4%) |
| SAEs by type* | |||
| Infections and infestations | 21 (1%) | 22 (1%) | 43 (1%) |
| Injury, poisoning, and procedural complications | 12 (1%) | 10 (1%) | 22 (1%) |
| Pregnancy, puerperium, and perinatal disorders | 6 (0%) | 11 (1%) | 17 (1%) |
| Blood and lymphatic system disorders | 2 (0%) | 1 (0%) | 3 (0%) |
| Gastrointestinal disorders | 4 (0%) | 9 (1%) | 13 (0%) |
| Other | 27 (2%) | 28 (2%) | 55 (2%) |
Data are number (%). SAE=serious adverse event.
Total number of serious adverse events is greater than the number of individuals in both groups, since participants could have had more than one body system involved, as indicated by Medical Dictionary for Regulatory Activities coding of reports of serious adverse events.
Discussion
In HIV-1 negative, HSV-2 seropositive heterosexual women in three sub-Saharan Africa sites and MSM from six US and Peruvian sites, we recorded no reduction in HIV-1 incidence from suppression of HSV-2 infection with aciclovir 400 mg given twice daily. The study participants received monthly provision of best available services for HIV-1 prevention, including risk reduction counselling, voluntary counselling and testing, and diagnosis and treatment of curable STIs. We noted that the reported adherence to study drug, primarily measured by pill count, was high. Daily aciclovir was safe and well tolerated, and did not result in an increased rate of serious adverse events. Our primary results are consistent with the trial of high-risk HIV-negative women in Tanzania that also used aciclovir 400 mg given twice daily.12 Our trial was about four times larger than the Tanzania trial with more than double the number of endpoints, and extends the findings to MSM and women from the general population. The per-protocol analysis from our trial differed from the Tanzania trial in that the risk of HIV infection was similar in the aciclovir and placebo groups in participants with high adherence to the study drug, although subgroup analyses have to be interpreted cautiously. The results of these trials do not lend support to the use of standard doses of antiviral drugs for HSV-2 suppression to prevent HIV-1 infection.
This large study of herpes suppression was undertaken in international populations, thus providing invaluable data for aciclovir safety, efficacy, and the natural history of genital herpes in HSV-2 seropositive heterosexual women and MSM. A quarter of participants recognised symptoms of genital herpes in the 3 months before study entry, which is similar to the longitudinal observational studies3,4 indicating an association between prevalent HSV-2 infection and HIV-1 acquisition, most of whom had not previously recognised such symptoms. We selected this dose of aciclovir because of its widespread availability as a generic drug and since previous clinical trials indicated its effectiveness and safety in suppression of clinical and subclinical HSV-2 reactivation in immunocompetent participants.23,24 Aciclovir was modestly effective in suppression of symptomatic genital herpes, significantly reducing the incidence of genital ulcers and HSV DNA positive genital ulcers. Moreover, the quantity of HSV-2 DNA was not reduced in breakthrough ulcers in the aciclovir group other than in MSM in US sites, which differs from previous studies showing a relative reduction of 1.5–2 logs in HSV quantity in breakthrough ulcers in studies that were undertaken in North America and Europe.24
We have no evidence of overt unblinding in the trial on the basis of comparable rates of loss to follow-up by group. Although use of open-label aciclovir was modestly higher in the placebo group than in the aciclovir group, it is unlikely to account for the absence of efficacy against HIV-1 acquisition, in view of the short duration of effect and low rates of episodic aciclovir use in the two groups.
Although aciclovir was effective overall in reduction of the incidence of symptomatic genital herpes, we recorded less of an effect of the drug in reduction of visible genital ulcers and in HSV detection and quantity in breakthrough lesions in women from the African sites than in MSM from US sites. These clinical and virological differences by sex and region in the effect of aciclovir suggest that research is warranted to define potential differences in African women in the natural history of genital herpes, and population differences in aciclovir pharmacokinetics or aciclovir susceptibility of herpes isolates. However, even in MSM from the US sites in whom aciclovir had high virological efficacy, we noted no effect on HIV-1 acquisition.
In view of the abundance and consistency of epidemiological data and the biological data that lend support to the plausibility of HSV-2 increasing HIV-1 susceptibility,4,5,25 the results of our trial and the Tanzania trial12 are disappointing. Further studies are needed to establish whether the absence of efficacy of aciclovir suppression of HSV-2 on HIV-1 incidence in these trials is because the hypothesis that HSV-2 reactivation increases susceptibility to HIV-1 is incorrect due to residual confounding in the observational studies, or because aciclovir 400 mg given twice daily for HSV-2 suppression is inadequate to reduce HIV-1 susceptibility that is conferred by HSV-2 infection. Notably, with respect to whether other available antiviral drugs would have a different effect, valaciclovir—a drug that achieves higher concentrations of serum aciclovir because of increased bioavailability—results in similar reductions in clinical and subclinical HSV-2 reactivation rates as does standard doses (400 mg twice daily) of aciclovir.23,24
Our study has limitations. Some genital abnormalities could have been because of trauma or other infectious causes besides HSV-2. Diagnostic tests for STIs varied across sites which could have affected the sensitivity of non-HSV diagnoses of STIs. Our measures of adherence to study drug could have been overestimates, since pill counts would be inaccurate when study drug was shared or discarded; however, there is no perfect measure of adherence. Further, our results might not be generalisable to populations with frequent recurrences of symptomatic genital ulcers; three-quarters of participants had reported no symptomatic genital herpes in the past 3 months at enrolment. However, observational data suggest that HSV-2 seropositivity, not just symptomatic genital herpes, is a risk factor for HIV-1 infection. Our study enrolled people with prevalent HSV-2 infection, and some observational studies have suggested that recent HSV-2 infection might confer higher HIV-1 susceptibility,6,7 although the relative timing of HSV-2 and HIV-1 acquisition is not certain in these studies.
The absence of protective effect of herpes suppression from this trial is disappointing, since new prevention strategies for HIV-1 are urgently needed and in view of recent negative results from other HIV-1 vaccine, and non-vaccine prevention trials. However, the results emphasise the importance of undertaking well designed, randomised controlled trials of candidate prevention interventions for HIV-1 that are based on epidemiological observations, biological plausibility, and mathematical modelling. Our study has no bearing on whether HSV-2 suppression will decrease the number of HIV-1 transmissions from people co-infected with HIV-1 and HSV-2 and slow disease progression. Several proof-of-concept studies have shown that plasma and genital HIV-1 concentrations can be significantly reduced in people who are co-infected with HIV and HSV-2 through HSV-2 suppression.26–31 Research for herpes drugs with new targets, increased potency, and longer activity, as well as an effective preventive HSV vaccine, are urgently needed. Last, our study suggests that the interaction between HIV-1 and other organisms such as HSV-2 cohabiting the genital mucosa is complex, and more potent interventions might be needed to disrupt this interaction.
Acknowledgments
We thank the HPTN 039 study participants for their significant contributions; the HPTN 039 site coordinators, counsellors, clinicians, pharmacists, and recruitment, retention, data quality, and laboratory staff; Darcie Somera of University of Washington for fiscal and administrative support; Gray Davis, Ward Cates, Kathy Hinson, and Sam Griffith of Family Health International and Grace Chow of Division of AIDS, NIAID, for study implementation support; Alicia Young, Carol Antone, Karen Patterson, Jennifer Schille, and Thomas Fleming of the Statistical Center for HIV/AIDS Research Program at the Fred Hutchinson Cancer Research Center for study operations, data management, and statistical support; Estelle Piwowar-Manning of Johns Hopkins University; Lorna Rabe of Magee-Womens Research Institute; and Anne Cent, Rhoda Morrow, and Meei-Li Huang of University of Washington Virology Laboratory for laboratory operations support and confirmatory laboratory assays. This study was supported through R01 funding with University of Washington (converted to U01 AI52054) and by the HIV Prevention Trials Network (HPTN) under Cooperative Agreement # U01 AI46749, sponsored by the National Institute of Allergy and Infectious Diseases, National Institute of Child Health and Human Development, National Institute of Drug Abuse, National Institute of Mental Health, and Office of AIDS Research. The study drug was purchased with a grant provided by GlaxoSmithKline.
Footnotes
Contributors: CC was the overall principal investigator and provided scientific leadership on study design, implementation, data interpretation, and writing. AW was the co-chair, provided scientific and management leadership for the study, and contributed to the analysis and writing committees. JH was the lead statistician and oversaw the study analysis and contributed to the study report. JS, SRe, SD-M, FC, MC, AO, JF, SB, and BK were the site principal investigators, and they supervised study implementation at each research site and contributed to interpretation of data and review of the report. SZ was the medical officer at Division of AIDS, NIH, and contributed to the design and implementation of the protocol. SRo was the protocol manager who undertook protocol training, oversaw protocol implementation and operations, and contributed to review of the report. JW was the primary statistician who undertook the analyses and contributed to review of the report. LC was a co-investigator who contributed to the the scientific leadership, study management, analysis of the data, review of the report, and direction of the reference HSV laboratories. Please see webappendix for HPTN 039 Study Team.
Conflict of interest statement: CC has received research grant support from GlaxoSmithKline and has served on an advisory board for GlaxoSmithKline. JS has received grant support from GlaxoSmithKline. AW has received grant support from GlaxoSmithKline, Antigenics, 3M, Roche, Astellas, and Vical; has been a consultant for Novartis, Powdermed, and Medigene; and has been a speaker for Merck Vaccines. FC has received research grant support from GlaxoSmithKline. The University of Washington Virology Division Laboratories have received grant funding from GlaxoSmithKline and Novartis to undertake HSV serological assays and PCR assays for studies funded by these companies. LC directs these laboratories; he receives no salary support from these grants. All other authors declare that they have no conflict of interest.
References
- 1.Weiss H. Epidemiology of herpes simplex virus type 2 infection in the developing world. Herpes. 2004;11(suppl 1):24A–35A. [PubMed] [Google Scholar]
- 2.Lama JR, Lucchetti A, Suarez L, et al. Association of herpes simplex virus type 2 infection and syphilis with human immunodeficiency virus infection among men who have sex with men in Peru. J Infect Dis. 2006;194:1459–66. doi: 10.1086/508548. [DOI] [PubMed] [Google Scholar]
- 3.Wald A, Zeh J, Selke S, et al. Reactivation of genital herpes simplex virus type 2 infection in asymptomatic seropositive persons. N Engl J Med. 2000;342:844–50. doi: 10.1056/NEJM200003233421203. [DOI] [PubMed] [Google Scholar]
- 4.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 5.Wald A, Link K. Risk of human immunodeficiency virus infection in herpes simplex virus type 2-seropositive persons: a meta-analysis. J Infect Dis. 2002;185:45–52. doi: 10.1086/338231. [DOI] [PubMed] [Google Scholar]
- 6.Reynolds SJ, Risbud AR, Shepherd ME, et al. Recent herpes simplex virus type 2 infection and the risk of human immunodeficiency virus type 1 acquisition in India. J Infect Dis. 2003;187:1513–21. doi: 10.1086/368357. [DOI] [PubMed] [Google Scholar]
- 7.Brown EL, Wald A, Hughes JP, et al. High risk of human immunodeficiency virus in men who have sex with men with herpes simplex virus type 2 in the EXPLORE study. Am J Epidemiol. 2006;164:733–41. doi: 10.1093/aje/kwj270. [DOI] [PubMed] [Google Scholar]
- 8.Rebbapragada A, Wachihi C, Pettengell C, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589–98. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 9.Gray RH, Wawer MJ, Brookmeyer R, et al. Probability of HIV-1 transmission per coital act in monogamous, heterosexual, HIV-1-discordant couples in Rakai, Uganda. Lancet. 2001;357:1149–53. doi: 10.1016/S0140-6736(00)04331-2. [DOI] [PubMed] [Google Scholar]
- 10.Ioannidis JPA, Collier AC, Cooper DA, et al. Clinical efficacy of high-dose acyclovir in patients with human immunodeficiency virus infection: a meta-analysis of randomized individual patient data. J Infect Dis. 1998;178:349–59. doi: 10.1086/515621. [DOI] [PubMed] [Google Scholar]
- 11.Mosca JD, Bednarik DP, Raj NB, et al. Herpes simplex virus type-1 can reactivate transcription of latent human immunodeficiency virus. Nature. 1987;325:67–70. doi: 10.1038/325067a0. [DOI] [PubMed] [Google Scholar]
- 12.Watson-Jones D, Weiss HA, Rusizoka M, et al. Effect of Herpes Simplex Suppression on Incidence of HIV among Women in Tanzania. N Engl J Med. 2008;358:1560–71. doi: 10.1056/NEJMoa0800260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Centers for Disease Control and Prevention. Workowski KA, Berman SM. Sexually transmitted disease treatment guidelines. MMWR Recomm Rep. 2006;55:18. [PubMed] [Google Scholar]
- 14.Laeyendecker O, Henson C, Gray RH, et al. Performance of a commercial, type-specific enzyme-linked immunosorbent assay for detection of herpes simplex virus type 2-specific antibodies in Ugandans. J Clin Microbiol. 2004;42:1794–96. doi: 10.1128/JCM.42.4.1794-1796.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golden MR, Ashley-Morrow R, Swenson P, Hogrefe WR, Handsfield HH, Wald A. Herpes simplex virus type 2 (HSV-2) Western blot confirmatory testing among men testing positive for HSV-2 using the focus enzyme-linked immunosorbent assay in a sexually transmitted disease clinic. Sex Transm Dis. 2005;32:771–77. doi: 10.1097/01.olq.0000175377.88358.f3. [DOI] [PubMed] [Google Scholar]
- 16.Ashley RL, Militoni J, Lee F, Nahmias A, Corey L. Comparison of Western blot (immunoblot) and glycoprotein G-specific immunodot enzyme assay for detecting antibodies to herpes simplex virus types 1 and 2 in human sera. J Clin Microbiol. 1988;26:662–67. doi: 10.1128/jcm.26.4.662-667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jerome K, Huang M, Wald A, Selke S, Corey L. Quantitative stability of DNA after extended storage of clinical specimens as determined by real-time PCR. J Clin Microbiol. 2002;40:2609–11. doi: 10.1128/JCM.40.7.2609-2611.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wald A, Huang M, Carrell DS, S, Corey L. Polymerase chain reaction for detection of herpes simplex virus (HSV) DNA on mucosal surfaces: comparison with HSV isolation in cell culture. J Infect Dis. 2003;188:1345–51. doi: 10.1086/379043. [DOI] [PubMed] [Google Scholar]
- 19.Nugent R, Krohn M, Hillier S. Reliability of diagnosing bacterial vaginosis is improved by a standardized method of gram stain interpretation. J Clin Microbiol. 1991;2:297–301. doi: 10.1128/jcm.29.2.297-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mertz GJ, Jones CC, Mills J, et al. Long-term acyclovir suppression of frequently recurring genital herpes simplex virus infection. A multicenter double-blind trial. JAMA. 1988;260:201–06. [PubMed] [Google Scholar]
- 21.Lan K, Gordan K, DeMets DL. Discrete sequential boundaries for clinical trials. Biometrika. 1983;70:659–63. [Google Scholar]
- 22.O'Brien PC, Fleming TR. A multiple testing procedure for clinical trials. Biometrics. 1979;35:549–56. [PubMed] [Google Scholar]
- 23.Reitano M, Tyring S, Lang W, et al. Valaciclovir for the suppression of recurrent genital herpes simplex virus infection: a large-scale dose range-finding study. International Valaciclovir HSV Study Group. J Infect Dis. 1998;178:603–10. doi: 10.1086/515385. [DOI] [PubMed] [Google Scholar]
- 24.Gupta R, Wald A, Krantz E, et al. Valacyclovir and acyclovir for suppression of shedding of herpes simplex virus in the genital tract. J Infect Dis. 2004;190:1374–81. doi: 10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 25.Corey L, Wald A, Celum C, Quinn T. The effects of herpes simplex virus-2 on HIV-1 acquisition and transmission: a review of two overlapping epidemics. J Acquir Immune Defic Syndr. 2004;35:435–45. doi: 10.1097/00126334-200404150-00001. [DOI] [PubMed] [Google Scholar]
- 26.Nagot N, Ouedraogo A, Foulongne V, et al. Reduction of HIV-1 RNA levels with therapy to suppress herpes simplex virus. N Engl J Med. 2007;356:790–99. doi: 10.1056/NEJMoa062607. [DOI] [PubMed] [Google Scholar]
- 27.Zuckerman R, Lucchetti A, Whittington W, et al. Herpes simplex virus (HSV) suppression with valacyclovir reduces rectal and blood plasma HIV-1 levels in HIV-1/HSV-2-seropositive men: a randomized, double-blind, placebo-controlled crossover trial. J Infect Dis. 2007;196:1500–08. doi: 10.1086/522523. [DOI] [PubMed] [Google Scholar]
- 28.Delany S, Mayaud P, Clayton T, et al. Impact of HSV-2 suppressive therapy on genital and plasma HIV-1 RNA in HIV-1 and HSV-2-seropostive women not taking ART: a randomized, placebo-controlled trial in Johannesburg, South Africa. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA, USA. Foundation for Retrovirology and Human Health; 2007. abstract number 154LB. [Google Scholar]
- 29.Dunne E, Whitehead S, Sternberg M, et al. The effect of suppressive acyclovir therapy on HIV cervicovaginal shedding in HIV- and HSV-2-infected women, Chiang Rai, Thailand. 14th Conference on Retroviruses and Opportunistic Infections; Los Angeles, CA, USA. Foundation for Retrovirology and Human Health; 2007. abstract number 30. [Google Scholar]
- 30.Baeten J, Strick L, Lucchetti AW, et al. Herpes simplex virus suppressive treatment decreases plasma HIV-1 viral load in HSV-2/HIV-1 co-infected women: a randomized, placebo-controlled, cross-over trial. 15th Conference on Retroviruses and Opportunistic Infections; Boston, MA, USA. 2008. abstract number 676. [Google Scholar]
- 31.Ouedraogo A, Nagot N, Vergne L, et al. Impact of suppressive herpes therapy on genital HIV-1 RNA among women taking antiretroviral therapy: a randomized controlled trial. AIDS. 2006;20:2305–13. doi: 10.1097/QAD.0b013e328010238d. [DOI] [PubMed] [Google Scholar]