Abstract
Human papillomavirus type 18 (HPV18) is a common cause of cervical cancer. To create a mouse model for this common neoplastic disease we used a human keratin 14 promoter to drive the HPV18 E7 oncogene to create transgenic mice. No mice up to a year of age developed cervical cancer. However, all transgenic mice and none of the controls developed progressive bilateral cortical cataracts. By 6 months of age the cortex liquefied leaving the lens nucleus. Proliferation of lens epithelium formed multifocal nodules and free floating lens epithelial cells within the liquefied cortex. These cells were hyperplastic not neoplastic. Other HPV transgenic stocks develop cataracts suggesting this virus may have a broad cellular tropism.
Keywords: Cataracts, human papillomavirus, transgenic mice, animal model
Introduction
Nearly 170 million individuals are blind or visually impaired, and cataracts are responsible for nearly 50% of the total.(Resnikoff et al., 2004) While congenital cataracts are less common than involutional cataracts in humans, the difficulty in treating children with cataracts is an important cause of amblyopia and blindness. Both congenital and adult-onset cataracts are often more common in families, but genetic defects and molecular mechanisms are not fully understood in many human cataracts. Recently, mutant loci on eleven different human chromosomes were associated with familial cataracts. The associated proteins include several alpha, beta, and gamma crystallins; the major intrinsic protein of the lens (MIP26); members of the connexin family responsible for gap junction formation; beaded filament structural protein-2 (BFSP2) and developmental regulators such as paired-like homeodomain transcription factor 3 (PITX3), avian musculoaponeurotic fibrosarcoma (v-maf) AS42 oncogene homolog (MAF), and heat shock transcription factor 4 (HSF4).(Reddy et al., 2004) Biochemical changes in human lens proteins are difficult to analyze for both ethical and scientific reasons. Mouse models provide a unique set of tools to analyze these biochemical changes and more importantly to help define the genetic and molecular bases for cataract formation. Mouse and human cataracts have 4 major forms: 1) nuclear, 2) cortical, 3) capsular-epithelial, and 4) lens extrusion for which many mouse models were described.(Smith et al., 1997) The number and variety of mouse models continue to expand.(Frederikse and Ren, 2002; Govindarajan et al., 2005; Graw et al., 2004; VanAgtmael et al., 2005)
Cutaneous and mucosal squamous cell carcinomas are common malignant neoplasms in human and other vertebrate species. Oncogenic papillomaviruses (PVs) cause more squamous cell carcinomas than all of the other known causes combined. Cervical cancer is the second leading cause of cancer deaths in women (500,000 per year) worldwide(Roden and Wu, 2006). Of the over 100 HPV types identified to date,(de Villiers et al., 2004) human papillomavirus type 18 (HPV-18) is responsible about 25% of cervical cancer case and is the most oncogenic of the known HPVs. Four “high risk” PVs (16, 18, 31 and 45) are associated with over 80% of cervical cancer; type 16 is responsible for at least 50%.(Munoz et al., 2004) Papillomatous (warty) growths caused by HPVs spontaneously regress, disseminate, or undergo malignant transformation depending on the biology of the virus and associated co-factors. These tiny DNA viruses (7 to 8 kilobase pairs in size) are highly species-specific and cannot be grown in vitro. Most, if not all, mammals and many lower vertebrates are susceptible to PV infections, and all PV types have the same genetic organization; up to 8 early genes and two late genes.(Sundberg et al., 1996)
Early genes E6 and E7 are always expressed in HPV-induced cancers and are required for maintaining the malignant state. E6 binds and degrades p53 whereas E7 binds to and deregulates pRb.(Helt and Galloway, 2003) Both exert an effect on cell cycle arrest. Their continuous expression can also result in accumulation of cellular change, including overexpression of cellular oncoproteins such as the myelocytomatosis oncogene (MYC) or the Harvey rat sarcoma virus oncogene 1 (HRAS1)(Wise-Draper and Wells, 2008). Progression of PV infected cells to malignancy involves mutations of host genes, such as inactivation or loss of tumor suppressor genes and genetic alterations at human Chromosomes 3p, 6p, 11q, 17p, and 18q. There are reports on the defects in antigen processing and presentation in cervical cancer and its cell lines. The best examples are loss of HLA and secretion of immune suppressive factors. Although the role of E7 protein in oncogenecity was the focal point of the studies, its functions have not yet been fully elucidated.(Kaufmann et al., 2002)
We report here the creation of a transgenic line of mice in which the human keratin 14 (KRT 1-14) promoter was used to drive the HPV18 E7 oncogene with the goal of developing a mouse model for cervical cancer. Systematic analysis revealed changes limited to the lens, where cataracts developed that were characterized by cortical liquefaction, cortical reabsorption, and lens epithelial metaplasia and proliferation. The severity of these changes progressed with age. Other ocular structures were normal, except for retinal degeneration that was due to the presence of retinal degeneration-1 (Pde6brd1), a mutation usually present in most FVB mouse strains.
Materials and Methods
Mice
All studies were approved by The Jackson Laboratory Institutional Animal Care and Use Committee (IACUC) and were performed in compliance with stipulations of that body. FVB/NTac mice (Taconic Farms, Germantown, NY) were used to create the transgenic mice. The transgenic mice were imported to The Jackson Laboratory (Bar Harbor, ME), Hysterectomy derivation and backcrossed onto FVB/NJ (N3) The transgenic mice are designated FVB/NJ-Tg(KRT14-HPV18E7)C/Sun by the International Mouse Genetic Nomenclature Committee and are hereafter abbreviated as K14HPV18E7 Tg mice. Mice were maintained in conventional barrier facilities at 24±2°C and 51±7% relative humidity, housed in 333.6 cm2 maxi-miser Duplex II cages (Thoren caging systems, Hazleton, PA) with pine shavings, exposed to a 14 h light/10 h dark cycle, allowed free access to sterilized acidified water (pH 2.8–3.2), and fed autoclaved NIH-31 Rat & Mouse 5K54 Lab Diet® (PMI Nutrition International, St. Louis, MO) ad libitum. The health status of each animal room is evaluated every 13 weeks. This includes necropsy of representative clinically normal mice, culture of feces for pathogenic bacteria, fecal examinations for parasitic ova, serological screening for major mouse pathogens, and culture of selected organs for pathogenic bacteria (current status of our colonies can be found at this web site address: http://jaxmice.jax.org/health/index.html). Preventive medicine programs also include microbiological monitoring of sterilizers, culture of environmental surfaces for coliforms, monitoring animal water for pH or residual chlorine, and culture of feed ingredients.
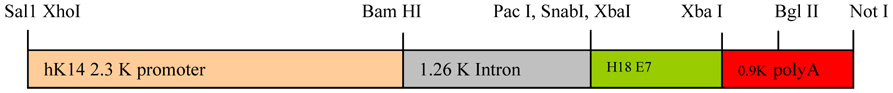
Construction of HPV-18 E7 transgene
A transgene transfer vector pBS-R3, which contains the human keratin 14 (KRT1-14) promoter upstream of multiple cloning sites, was kindly provided by Dr. Dennis Roop (Baylor College of Medicine, Houston, TX). The prototype HPV-18 E7 gene was amplified using a set of primers (P1: ATC ATC TCT AGA TGC ATG GAC CTA AGG CAA CAT TG, P2: ATG ATG TCT AGA TTA CTG CTG GGA TGC ACA CCA CGG) annealing to N-terminus and C-terminus of E7 by PCR, cloned into pCR2.1 according to the manufacturer’s instructions (Invitrogen, Carlsbad, CA), and subcloned successively into pBS-R3 at the Xba1 site (Fig. 1). The orientation and the absence of a mutation in insert were confirmed by multiple restriction enzyme digestions and DNA sequencing with two primers annealing to 1.26 intron (CCC CAC TCC TAA CAA TGA C) and to 0.9 K poly A site (CTA TGG TCA AGT TTT GTG GG), respectively. The transgene was digested with Xho1/Not1, gel-purified, and microinjected into FVB/NTac mouse blastocysts at the Transgenic Mouse Core Facility at the University of Louisville. These transgenic mice were genotyped by PCR using genomic DNA extracted from tail tips with primers used for the cloning of the E7 gene.
Figure 1.
Diagram of the recombinant transgene containing the K14 HPV18 E7 construct.
Tissue Processing, Histologic Analysis, and Immunohistochemistry
Mice were aged to over 165 days and representative groups of 2 each of male and female K14HPV18E7 transgenic and age and gender matched FVB/NJ +/+ mice (wildtype controls) used for the backcrosses were euthanized and complete systematic necropsies performed.(Seymour et al., 2004) The slides were reviewed by an experienced, board certified, veterinary anatomic pathologist (JPS). Lesions were only observed in the eyes. Subsequently, 2 males and 4 females were necropsied at 7 to 14 months of age and only the eyes were removed. These were all reviewed by both an ophthalmic pathologist (RSS) and anatomic pathologist (JPS).
Mice were euthanized by CO2 asphyxiation. Complete necropsies were performed initially(Seymour et al., 2004) after which eyes were manually removed, fixed overnight in Fekete’s acid-alcohol-formalin solution (61% ethanol, 3.2% formaldehyde, 0.75N acetic acid), transferred to 70% ethanol, processed routinely, embedded in paraffin, sectioned at 5–6 µm, placed on microscope slides (Superfrost/Plus Fisherbrand, Pittsburgh, PA) and stained with hematoxylin and eosin (H&E) for routine histopathologic analysis. Two transgenic mice and 2 controls were injected intraperitoneally with 50 ug/g body weight with bromodeoxyuridine (Sigma, St. Louis, MO) and euthanized one hour later.(Smith et al., 2000) The eyes were fixed in Fekete’s solution for routine histology. Immunohistochemistry, was done using the following primary antibodies: rat monoclonal anti-bromodeoxyuridine (1:50; Accurate Antibodies, Sera Labs, Westbury, NY), rabbit polyclonal mouse specific keratins 5, 6, and 14 (1:8000; Covance, Princeton, NJ). Routine procedures were used.(Mikaelian et al., 2004; Relyea et al., 2000) Immunohistochemistry was performed using Vectastain™ ABC kits (Vector, Burlingame, CA., USA). Slides were deparaffinized in xylene and dehydrated using graded ethanols. Endogenous peroxidase was blocked using 3% hydrogen peroxide in methanol for 30 min at room temperature. Slides were then washed and incubated for 30 min with blocking serum (10% normal fetal calf serum diluted in phosphate-buffer saline, PBS). Excess blocking serum was removed and the slides were incubated overnight at 4° C with primary antibodies diluted in PBS containing 1% fetal calf serum. Secondary biotinylated anti-immunoglobulin G antibody supplied with Vectastain™ kits (anti-guinea pig, anti-mouse, anti-rabbit, anti-rat and anti-goat) were applied for 30 minutes at room temperature followed by 45 minute incubation with the peroxidase labeled biotin and avidin complex. Diaminobenzidine (Sigma, St. Louis, MO., USA) and H2O2, which produces a brown precipitate, was used as the chromagen. Slides were counterstained with Mayer’s hematoxylin.
Results
Transgenic mice
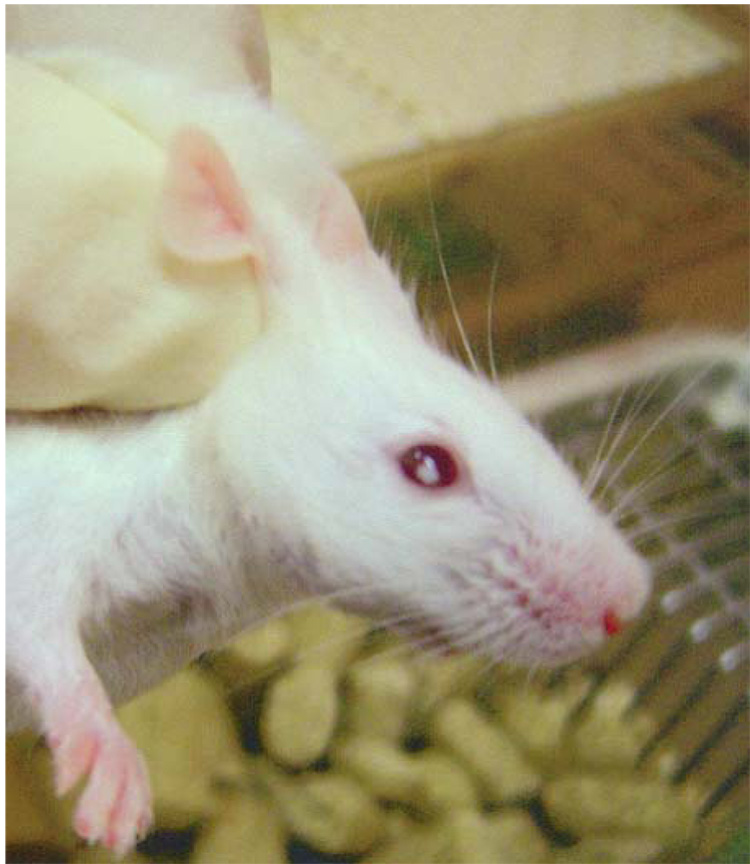
Six out of 22 mice were positive for the E7 gene by genotyping, and 5 of 6 produced reasonable number of pups positive for the E7 gene (Fig. 2). Of the 5 founders, mice from founder C had diffuse, bilateral, cortical cataracts on clinical examination (Fig. 3). Mice were otherwise fecund.
Figure 2.
Genotyping of 6 pups from an E7 positive founder mated with negative FVB mouse. First three lanes have a distinct band for the HPV18 E7 gene. The last three did not carry E7 gene.
Figure 3.
A centralized white area was grossly evident in both eyes of the transgenic mice within one month after the birth.
Routine histology
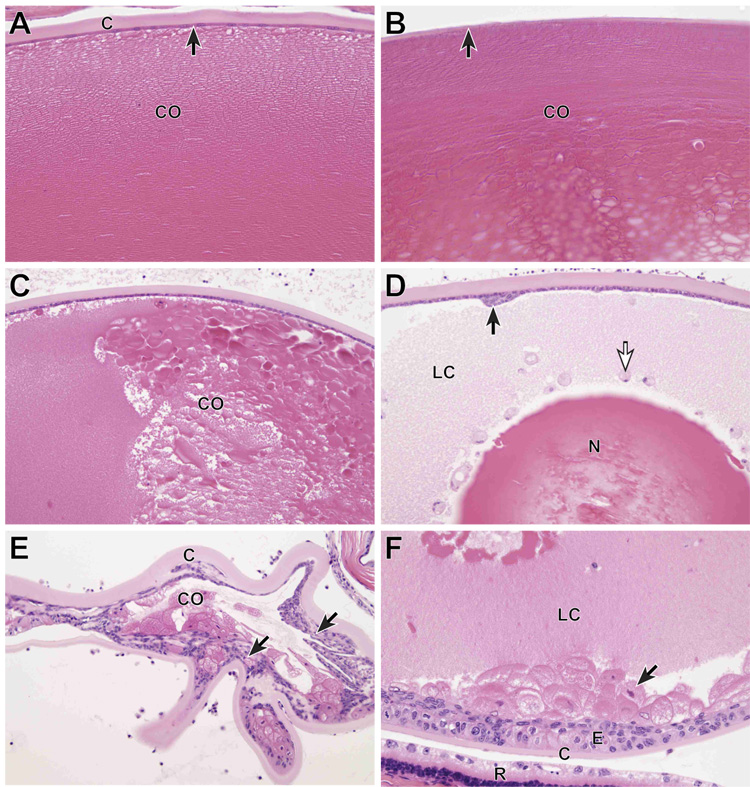
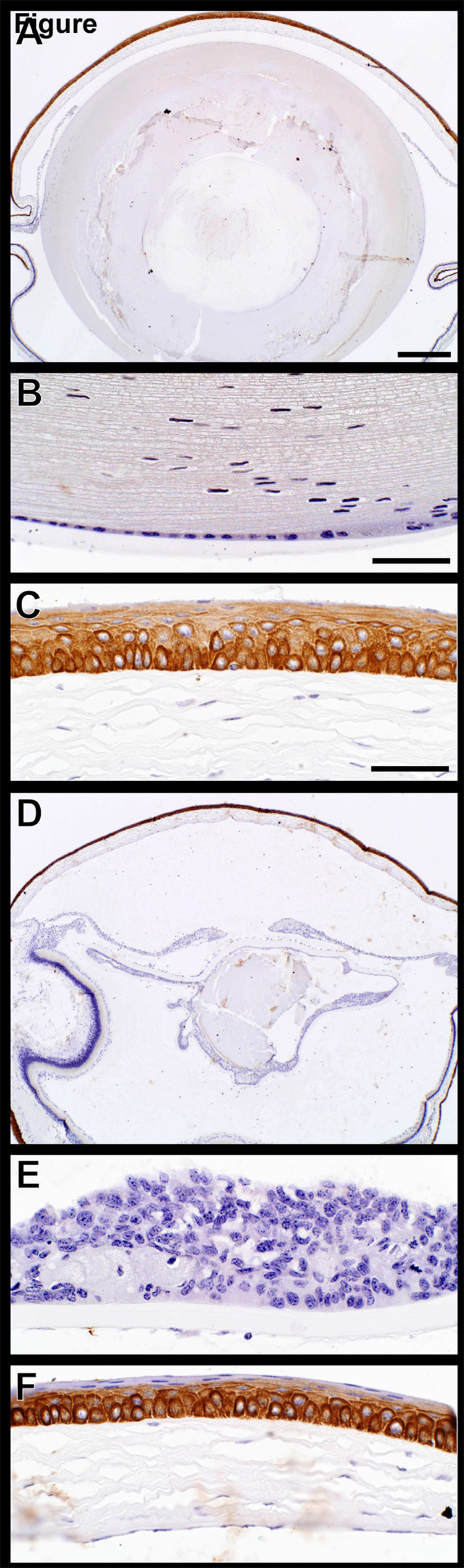
In +/+ (wildtype) mice, the lens was enclosed in an acellular eosinophilic lens capsule that is normally twice as thick at the anterior surface of the lens as it is at the posterior pole. Directly beneath the lens capsule, there was a monolayer of low cuboidal epithelium that extended from the anterior lens pole to the equator of the lens, but was normally absent behind the lens equator (Fig. 4A,B). At the equator of the lens (not shown), lens epithelial nuclei extended for a short distance into the lens cortex and then disappeared. The lens cortex consisted of lens “fibers” that are anucleate, very elongated lens cells arranged in an orderly hexagonal array (Fig. 4A).(Smith, 2002)
Figure 4.
Ocular histology. A) Normal adult FVB/NJ +/+ mouse. The anterior lens capsule (C) is acellular and eosinophilic. Directly beneath the capsule is a monolayer of cuboidal lens epithelium (arrow). The lens fibers in the anterior lens cortex (CO) are tightly packed and clearly delineated. The lens fibers are the remnants of lens cells and make up the bulk of the adult lens. B) Normal adult FVB/NJ +/+ mouse. The posterior lens capsule (arrow) is normally much thinner than the anterior lens capsule and lens epithelial cells are absent. The posterior lens cortex has a subtle lamellar structure and is more dense that the anterior cortex. C) K14HPV18E7 Tg mouse, 6 months old. The anterior lens capsule and underlying lens epithelium are normal, but the orderly structure seen in the WT mouse is absent and the cortex (CO) has developed a globular appearance, representing masses of degenerating lens fibers. D) K14HPV18E7 Tg mouse, 6 months old. The anterior lens capsule (C) is normal, but a focal patch of reactive proliferation of the lens epithelium is present (arrow). Much of the lens cortex has become liquefied (LC) and some of this degenerating cortical material is contained inside free-floating lens epithelial cells (white arrow). The lens nucleus (N) remains, but centrally shows signs of fragmentation. E) K14HPV18E7 Tg mouse, 8 months old. In this severely degenerated lens, the anterior capsule (C) is thickened and throughout the lens there is massive anterior and posterior proliferation of the lens epithelium (arrows). Small amounts of degenerating cortex (CO) remain. F) K14HPV18E7 Tg mouse, 6 months old. Although lens epithelium is normally absent beneath the posterior lens capsule (C), here there is extensive lens epithelial proliferation (E) of lens epithelium of normal appearance as well as a large number of “bladder” cells. The posterior lens cortex is liquefied (LC). A portion of the degenerated retina (R) with changes typical of Pde6brd1 is present.
In transgenic mice, the earliest change observed was breakdown of the orderly structure of the lens cortex into large and small globules of eosinophilic material (Fig. 1C). By six months of age, this had progressed in some mice to liquefaction of the cortex with loss of all structure except for the lens nucleus (most central portion). In addition, there was metaplastic proliferation of the lens epithelium to form focal nodules and free-floating lens epithelial cells were present in the liquefied cortex, often distended with ingested lens cortex (Fig. 4D).
In older mice there was a striking proliferation of the lens epithelium to form multiple layers of lens epithelial cells, present beneath both the anterior and posterior lens capsule. In some mice, lens epithelial cells distended with abnormal cortex were present beneath the posterior capsule (Fig. 4E,F). In humans, such cells are referred to as “bladder” cells and are thought to represent lens epithelial cells that are attempting to produce cortical material, but have lost the ability to do so.(Spencer, 1996) In other eyes, nearly all the cortex was reabsorbed and all that remained was a collapsed lens with a thickened anterior and posterior lens capsule that was partially filled with cortical remnants and proliferating lens epithelium.
Immunohistochemistry
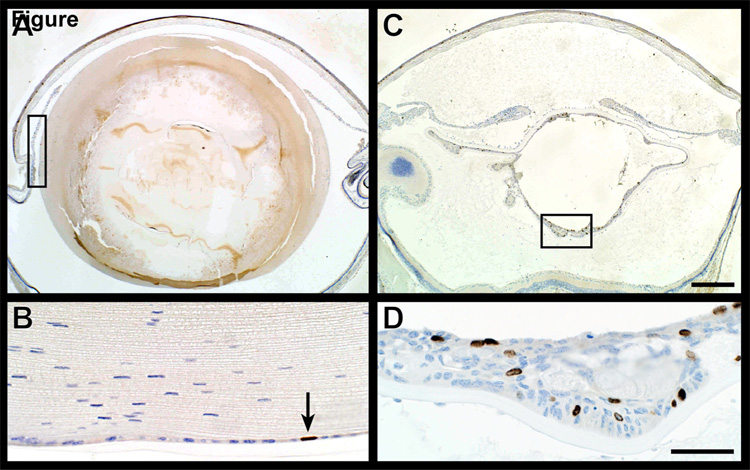
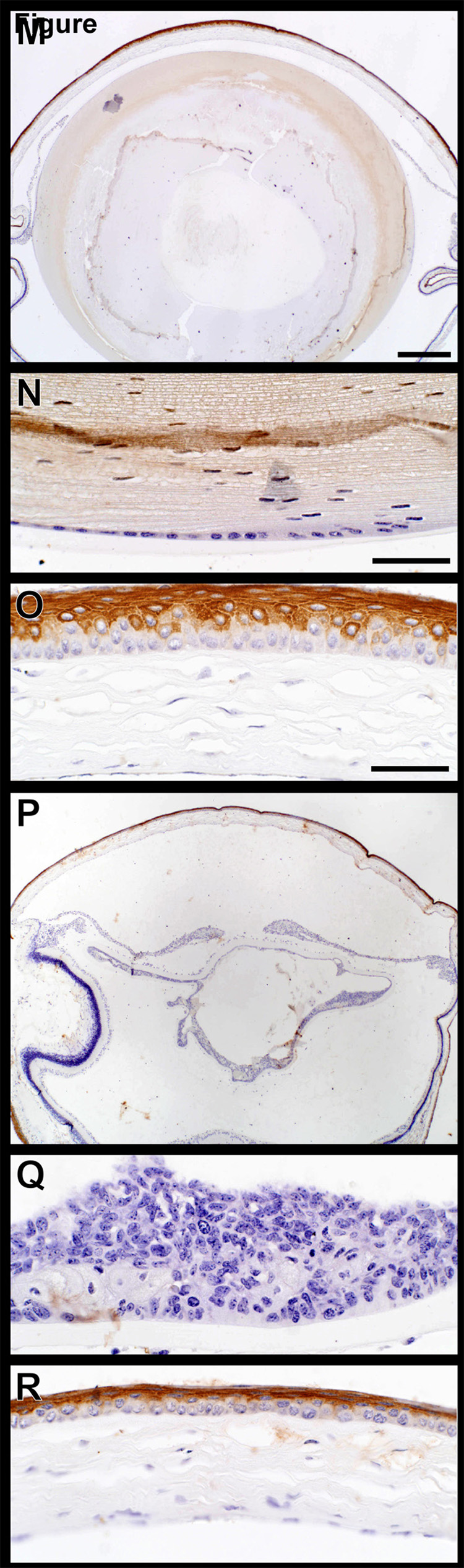
Regions of lens epithelium proliferation in older mice were highly cellular with numerous nuclei incorporating bromodeoxyuridine indicating high levels of DNA synthesis. This was in sharp contrast to normal lens epithelium where few, if any, nuclei were synthesizing DNA (Fig. 5). Similar changes were present in a partial trisomy mouse model with cataracts.(Smith et al., 1999)
Figure 5.
Bromodeoxyuridine (BRDU) uptake. Immunohistochemical evaluation of BRDU uptake, an indication of DNA synthesis rates, revealed very few positive (brown) nuclei in the cornea and lens epithelium (arrow, A,B). By contrast, numerous lens epithelial cells were positive in areas in which these cells were actively proliferating (C,D).
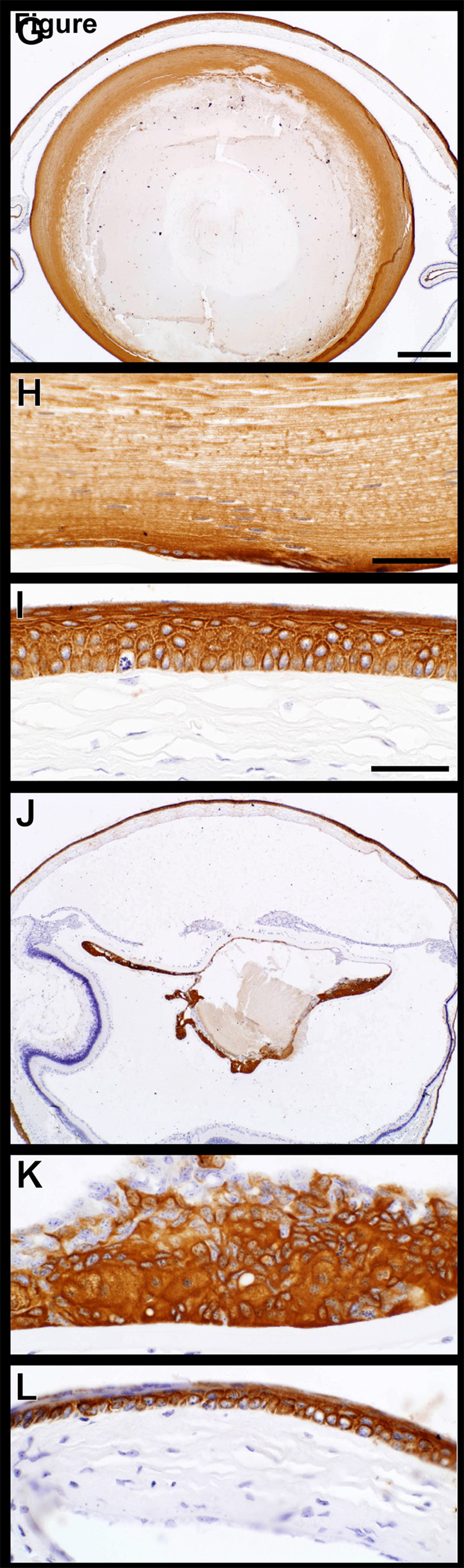
Mouse specific keratin expression was evaluated for keratins 5 and 14 which are the acid and base pair normally expressed in the basal cells of the epidermis.(Sundberg et al., 1994) The full thickness of corneal epithelium expressed both of these proteins (Fig. 6A–L). While expression patterns were similar in both wild type controls and K14HPV18E7 transgenic mice, it was surprising to find marked K5 expression throughout the lens while mouse specific K14 was not expressed there. Mouse specific keratin 6 is a nonspecific marker of epidermal hyperplasia.(Sundberg et al., 1994) Expression of keratin 6 was limited to the suprabasal keratinocytes of the cornea with no expression in the lens or within the proliferating lens epithelium from transgenic mouse eyes (Fig. 6M–R).
Figure 6.
Keratin expression in the eye. Mouse specific keratins 14 and 5 (A–J), the acid-base pair, were both expressed in all layers of the corneal epithelium (C,F,I). Keratin 14 was not expressed in the lens epithelium of +/+ (B) or K14HPV18E7 Tg mice (E) while keratin 5 was (H,K). Mouse specific keratin 6 (M–R), a nonspecific marker of epidermal hyperplasia, was expressed in the suprabasal cells of the cornea of both +/+ (O) and K14HPV18E7 Tg mice (R) but not the lens epithelial cells in either group (N,Q).
DISCUSSION
Keratin 14 driven papillomavirus gene based transgenic mice were first created in 1994 (Arbeit et al., 1994) and used as a model for studying malignant progression of PV-induced cancers. Transgenic mice expressing E6 or E7 from HPV16 under the control of the KRT1-14 promoter resulted in skin tumors and hyperplasia(Herber et al., 1996; Song et al., 1999). Mouse tumors caused by cervical cancer cell lines were used as a model system with very limited success since the data obtained could not be repeated in clinical trials.(Kaufmann et al., 2002) We hypothesized that a transgenic mouse line that expressed the most oncogenic HPV oncoproteins under conditions similar to those of cancer in humans might provide a more useful model. However, keratin 14 in mammals is expressed in many stratified squamous epithelia and glands, including purported stem cells in the mammary glands,(Mikaelian et al., 2006; Sundberg et al., 1994; Sundberg et al., 1991) such that while the goal was to create a mouse model for cervical cancer, any of a number of organs might develop abnormalities as a result of expression of this transgene. Detailed systematic histopathologic evaluation of all organ systems in aged mice determined lesions were limited to cataract formation in both eyes.
True congenital cataracts are unusual in mice. In most cases, an initially clear lens may develop focal opacities by 1–2 months of age. These opacities may progress and can eventually lead to total lens opacification,(Smith, 2002) as was the case in the transgenic mice. Aging mice of many strains may develop cataracts and some specific age-related lens opacities have been described.(Hosokawa et al., 1993; Kuck, 1990)
The lens of the eye consists primarily of lens fiber cells containing a variety of soluble and insoluble proteins that include lens crystallins. These are membrane-associated and cytoskeletal proteins.(Paterson and Delamere, 1992) Mutations in the lens gamma-crystallin genes (Cryga-Crygf) are associated with cataract formation in mice.(Klopp et al., 1998; Santhiya et al., 1995; Smith et al., 2000) Because the alpha A crystalline promoter is active in the lens from early embryonic development through adulthood (Overbeek et al., 1985) it was fused to the HPV16 E6 and E7 oncogenes in a transgenic mouse. These mice developed microphthalmia and cataracts in all cases and 40% developed lens tumors.(Griep et al., 1993)
The four basic cataract types in mice: capsular-epithelial, nuclear, cortical, and lens extrusion display a similar sequence of events. Initially, the lens cortex regular structure breaks up forming clumps of cortical material, often with vacuoles. This may progress to involve the entire lens with further protein breakdown, ending in a completely liquefied cortex. The degenerate cortical material leaks out of the lens capsule into the vitreous, leading to collapse of the lens capsule. As a cataract develops, one of the early changes is posterior migration of the lens epithelium with nuclei located beneath the posterior lens capsule. The lens epithelium may also undergo focal or diffuse proliferation. Because the lens epithelium is relatively undifferentiated, it retains the capacity to undergo metaplasia, and become phagocytic, produce focal or diffuse capsular thickening, or produce subcapsular deposits of collagen. In some instances, the posterior lens capture may undergo spontaneous rupture with extrusion of cortical fragments into the vitreous.(Smith, 2002)
In the K14HPV18E7 transgenic mice, early cortical breakdown was followed by focal and diffuse proliferation of lens epithelial cells. Subsequently, there was progressive liquefaction of the lens cortex, often leading to a collapse of the lens capsule. Even though the lens epithelium often produced multiple layers of cuboidal cells, they retained a benign appearance and mitotic activity was not excessive. These findings do not represent a malignant change and are consistent with anterior and posterior subcapsular cataracts both in humans (Spencer, 1996) and mice. (Reneker and Overbeek, 1996; Smith et al., 2000; Smith et al., 1999) Malignant tumors arising from lens epithelium are extremely rare in mice.(Gotz et al., 1991; Griep et al., 1993; Mahon et al., 1987; Pichel et al., 1993)
It may be highly significant that the only phenotypic effect of the transgene was on metaplastic squamous epithelium of mouse lens. Interestingly, the K14HPV16E6 and K14HPV16E7 mice also developed cataracts (Herber et al., 1996; Song et al., 1999) as well as overexpression of the endostatin domain of mouse Col18a1 by the same K14 promoter produced nuclear cataracts.(Elamaa et al., 2005) In the natural human host, two of the most susceptible sites of HPV-18 infection and E7 expression are metaplastic squamous epithelia of the cervix, anal area, and the tonsils. Since HPV does not appear to be transmitted by germline and does not appear to have a viremic stage, all natural HPV-18-induced lesions appear to occur by horizontal transmission involving contact. For some unknown reason, metaplastic epithelium of the lens of one of the founder lines has expressed the germline transmitted recombinant keratin 14 promoted E7 and caused proliferation of metaplastic squamous cells that eventually damaged surrounding tissue and induced cataracts of the eyes. Investigation of the mechanism of metaplastic squamous proliferation in such a tightly controlled environment as the mouse eye might offer some clues as to how human E7 is expressed by metaplastic squamous epithelial cells. Nevertheless, it represents a mouse model of how nuclear cataracts can be formed.
Acknowledgements
The authors thank B.J. Shirley, J. Miller, and L. Petell for their technical support. This work was supported in part by grants for the National Institutes of Health (RR00173) and facilities infrastructure by a Cancer Center Core Grant (CA34196).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arbeit JM, et al. Progressive squamous epithelial neoplasia in K14-human papillomavirus type 16 transgenic mice. J.Virol. 1994;68:4358–4368. doi: 10.1128/jvi.68.7.4358-4368.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Villiers EM, et al. Classification of papillomaviruses. Virology. 2004;324:17–27. doi: 10.1016/j.virol.2004.03.033. [DOI] [PubMed] [Google Scholar]
- Elamaa H, et al. Endostatin overexpression specifically in the lens and skin leads to cataract and ultrastructural alterations in basement membranes. Am J Pathol. 2005;166:221–229. doi: 10.1016/S0002-9440(10)62246-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederikse PH, Ren XO. Lens Defects and Age-Related Fiber Cell Degeneration in a Mouse Model of Increased AbetaPP Gene Dosage in Down Syndrome. Am J Pathol. 2002;161:1985–1990. doi: 10.1016/s0002-9440(10)64475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotz W, et al. Eye pathology in transgenic mice carrying a MSV-SV 40 large T-construct. Exp Eye Res. 1991;52:41–49. doi: 10.1016/0014-4835(91)90126-y. [DOI] [PubMed] [Google Scholar]
- Govindarajan V, et al. Intracorneal positioning of the lens in Pax6-GAL4/VP16 transgenic mice. Mol Vis. 2005;11:876–886. [PubMed] [Google Scholar]
- Graw J, et al. Genetic and allelic heterogeneity of Cryg mutations in eight distinct forms of dominant cataract in the mouse. Invest Ophthalmol Vis Sci. 2004;45:1202–1213. doi: 10.1167/iovs.03-0811. [DOI] [PubMed] [Google Scholar]
- Griep AE, et al. Tumorigenicity by human papillomavirus type 16 E6 and E7 in transgenic mice correlates with alterations in epithelial cell growth and differentiation. J.Virol. 1993;67:1373–1384. doi: 10.1128/jvi.67.3.1373-1384.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helt A-M, Galloway DA. Mechanisms by which DNA tumor virus oncoproteins target the Rb family of pocket proteins. Carcinogenesis. 2003;24:159–169. doi: 10.1093/carcin/24.2.159. [DOI] [PubMed] [Google Scholar]
- Herber R, et al. Squamous epithelial hyperplasia and carcinoma in mice transgenic for the human papillomavirus type 16 E7 oncogene. J Virol. 1996;70:1873–1881. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosokawa M, et al. Persistent hyaloid vascular system in age-related cataract in a SAM strain of mouse. Exp Eye Res. 1993;57:427–434. doi: 10.1006/exer.1993.1144. [DOI] [PubMed] [Google Scholar]
- Kaufmann AM, et al. HPV induced cervical carcinogenesis: molecular basis and vaccine development. Zentralbl Gynakol. 2002;124:511–524. doi: 10.1055/s-2002-39579. [DOI] [PubMed] [Google Scholar]
- Klopp N, et al. Three murine cataract mutants (Cat2) are defective in different gamma-crystallin genes Genomics. 1998;52:152–158. doi: 10.1006/geno.1998.5417. [DOI] [PubMed] [Google Scholar]
- Kuck JFR. Late onset hereditary cataract of the Emory mouse. A model for human senile cataract. Exp Eye Res. 1990;50:659–664. doi: 10.1016/0014-4835(90)90110-g. [DOI] [PubMed] [Google Scholar]
- Mahon KA, et al. Oncogenesis of the lens in transgenic mice. Science. 1987;235:1622–1628. doi: 10.1126/science.3029873. [DOI] [PubMed] [Google Scholar]
- Mikaelian I, et al. Expression of terminal differentiation proteins defines stages of mouse mammary gland development. Vet Pathol. 2006;43:36–49. doi: 10.1354/vp.43-1-36. [DOI] [PubMed] [Google Scholar]
- Mikaelian I, et al. Antibodies that label paraffin-embedded mouse tissues: a collaborative endeavor. Toxicol Pathol. 2004;32:1–11. doi: 10.1080/01926230490274335. [DOI] [PubMed] [Google Scholar]
- Munoz N, et al. Against which human papillomavirus types shall we vaccinate and screen? The international perspective. Int J Cancer. 2004;111:278–285. doi: 10.1002/ijc.20244. [DOI] [PubMed] [Google Scholar]
- Overbeek PA, et al. Lens-specific expression and developmental regulation of the bacterial chloramphenicol acetyltransferase gene driven by the murine alpha A crystallin promoter in transgenic mice. Proc Natl Acad Sci USA. 1985;82:7815–7819. doi: 10.1073/pnas.82.23.7815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson CA, Delamere NA. The lens. St Louis: Mosby-Yearbook Publishers; 1992. [Google Scholar]
- Pichel JG, et al. Timing of SV40 oncogene activation by site-specific recombination determines subsequent tumor progression during murine lens development. Oncogene. 1993;8:3333–3342. [PubMed] [Google Scholar]
- Reddy MA, et al. Molecular genetic basis of inherited cataract and associated phenotypes. Surv Ophthalmol. 2004;49:300–315. doi: 10.1016/j.survophthal.2004.02.013. [DOI] [PubMed] [Google Scholar]
- Relyea MJ, et al. Immunohistochemical and immunofluorescence methods. In: Sundberg JP, Boggess D, editors. Systematic approach to evaluation of mouse mutations. Boca Raton, FL: CRC Press; 2000. pp. 131–144. [Google Scholar]
- Reneker LW, Overbeek PA. Lens-specific expression of PDGF-A in transgenic mice results in retinal astrocytic hamartomas. Invest Ophthalmol Vis Sci. 1996;37:2455–2466. [PubMed] [Google Scholar]
- Resnikoff S, et al. Global data on visual impairment in the year 2002. Bull World Health Organ. 2004;82:844–851. [PMC free article] [PubMed] [Google Scholar]
- Roden R, Wu TC. How will HPV vaccines affect cervical cancer? Nat Rev Cancer. 2006;6:753–763. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhiya ST, et al. Reduced levels of gamma-crystallin transcripts during embryonic development of murine Cat2nop mutant lenses. Graefes Arch Clin Exp Ophthalmol. 1995;233:795–800. doi: 10.1007/BF00184093. [DOI] [PubMed] [Google Scholar]
- Seymour R, et al. Necropsy methods. In: Hedrich HJ, editor. Laboratory mouse. London: Academic Press; 2004. pp. 495–516. [Google Scholar]
- Smith RS. Choroid, lens, and vitreous. In: Smith RS, et al., editors. Systematic evaluation of the mouse eye: Anatomy, pathology, and biomethods. Boca Raton, FL: CRC Press; 2002. pp. 161–194. [Google Scholar]
- Smith RS, et al. Lop12, a mutation in mouse Crygd causing lens opacity similar to human Coppock cataract. Genomics. 2000;63:314–320. doi: 10.1006/geno.1999.6054. [DOI] [PubMed] [Google Scholar]
- Smith RS, et al. Lens epithelial proliferation cataract in segmental trisomy involving mouse Chromosomes 4 and 17. Mamm Genome. 1999;10:102–106. doi: 10.1007/s003359900952. [DOI] [PubMed] [Google Scholar]
- Smith RS, et al. Mouse mutations as models for studying cataracts. Pathobiology. 1997;65:146–154. doi: 10.1159/000164116. [DOI] [PubMed] [Google Scholar]
- Song S, et al. The human papillomavirus type 16 E6 gene alone is sufficient to induce carcinomas in transgenic animals. J Virol. 1999;73:5887–5893. doi: 10.1128/jvi.73.7.5887-5893.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer WH. Ophthalmic Pathology: An Atlas and Textbook. Philadelphia: W B Saunders Company; 1996. [Google Scholar]
- Sundberg JP, et al. Ornithine decarboxylase expression in cutaneous papillomas in SENCAR mice is associated with altered expression of keratins 1 and 10. Cancer Res. 1994;54:1344–1351. [PubMed] [Google Scholar]
- Sundberg JP, et al. Myoepitheliomas in inbred laboratory mice. Vet Pathol. 1991;28:313–323. doi: 10.1177/030098589102800408. [DOI] [PubMed] [Google Scholar]
- Sundberg JP, et al. The nonhuman (animal) papillomaviruses: host range, epitope conservation, and molecular diversity. In: Gross G, vonKrogh G, editors. Human papillomavirus infections in dermatology and venereology. Boca Raton: CRC Press, Inc; 1996. pp. 47–68. [Google Scholar]
- VanAgtmael T, et al. Dominant mutations of Col4a1 result in basement membrane defects which lead to anterior segment dysgenesis and glomerulopathy. Hum Mol Genet. 2005;14:3161–3168. doi: 10.1093/hmg/ddi348. [DOI] [PubMed] [Google Scholar]
- Wise-Draper TM, Wells SI. Papillomavirus E6 and E7 proteins and their cellular targets. Front Biosci. 2008;13:1003–1017. doi: 10.2741/2739. [DOI] [PubMed] [Google Scholar]