Epstein-Barr virus (EBV), a gammaherpesvirus infecting >90% of the world's population, is a recognized oncogenic agent. First discovered in a case of endemic (African) Burkitt's lymphoma (BL) (1), it has since been linked to a list of other human malignancies, including nasopharyngeal carcinoma, diffuse large cell lymphoma in immunocompromised individuals, in particular AIDS patients and transplant recipients, approximately half of all cases of Hodgkin's lymphoma, and, more recently, a significant percentage of gastric cancer (reviewed in refs. 2 and 3). Although considerable progress has been made in our understanding of how EBV causes cancer, and why such a widely distributed carcinogen does so in only a comparatively small number of infected individuals, many facets remain only poorly understood. In this issue of PNAS, Gruhne et al. (4) report observations that make an old acquaintance appear in a new light.
It is thought that EBV plays a different role in different tumors: EBV immortalizes primary B cells in tissue culture (5). In these cells, EBV displays a viral gene expression profile, referred to as latency III, which involves the expression of 8 viral genes encoding 5 nuclear proteins (the EBNAs), 2 latent membrane proteins (LMP1, LMP2A), and 2 untranslated RNAs (EBERs) (2, 3). LMP1, EBNA2, and EBNA-LP are thought to directly contribute to immortalization, LMP2A enhances its efficiency, and others also contribute (3, 6, 7, 20). This latency III gene expression pattern is also found in some lymphomas of immunosuppressed individuals. Because several of the EBNAs are targets of EBV-specific cytotoxic B lymphocytes (8), it is thought that their expression is only tolerated in an individual with severely compromised T cell immunity (as is the case in transplant recipients and AIDS patients) and that these tumors may therefore result from the outgrowth of EBV-immortalized B cells.
In nasopharyngeal carcinoma and EBV-associated cases of Hodgkin's disease a much more limited repertoire of EBV genes is used (referred to as latency II). In particular, LMP1 and LMP2A, but also EBNA1 (a protein required for the episomal persistence of latent circular EBV genomes) and the EBERs are expressed. LMP1 triggers intracellular signaling pathways such as NF-κB, JNK, and p38 and mimics the effect of activating CD40 in B cells, whereas LMP2A activates pathways normally engaged by the B cell antigen receptor (reviewed in ref. 3). LMP1 and LMP2A are considered the most likely culprits in EBV-associated nasopharyngeal carcinoma and Hodgkin's disease. However, because these tumors occur only in a small minority of EBV-infected individuals (EBV infects >95% of the adult population worldwide), there is a need for cofactors and/or additional explanations. In the case of nasopharyngeal carcinoma, its characteristic geographic distribution (Southeast China, the Mediterranean Coast of Northern Africa, some Inuit populations) has long suggested the existence of additional regional cofactors, most likely dietary carcinogens, although none of them have ever been positively identified. In the case of EBV-associated Hodgkin's disease, recent interesting findings have suggested that certain HLA combinations are associated with an increased severity of infectious mononucleosis (the clinical manifestation of primary EBV infection), and also an increased risk for EBV-associated Hodgkin's disease (9, 10). This result could suggest that an inefficient clearance of EBV-infected B cells by cytotoxic T cells during primary EBV infection may increase the risk for the emergence of rare B cell clones, in which EBV failed to switch off the expression of LMP1 and LMP2A.
Finally, in EBV-positive BL EBV mostly adopts a gene expression pattern termed latency I (although different latency patterns are sometimes found), which is characterized by the exclusive expression of EBNA-1 and the EBERs. The essential step in the pathogenesis of BL appears to be the translocation of the c-myc gene into the vicinity of 1 of the 3 Ig gene loci on chromosomes 2, 14, and 22 (11). A recent study from Alan Rickinson's laboratory in Birmingham, United Kingdom, also published in PNAS (12), suggests that in BL the main role of EBV may be to protect c-myc translocation harboring B cells against apoptosis associated with over expression of c-myc.
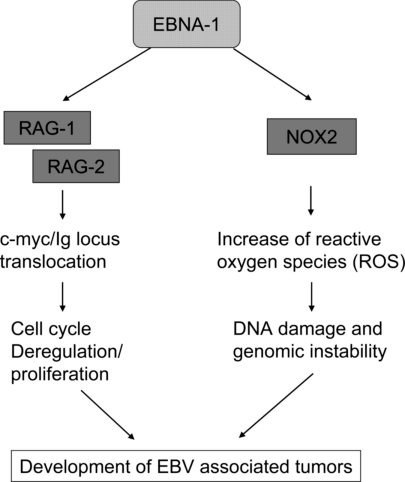
Gruhne et al. (4) have now discovered that EBNA-1, the only EBV protein to be expressed in all EBV-associated tumors, induces genomic instability and a DNA damage response. Having shown in an earlier article (13) that EBNA-1 induces chromosomal abnormalities, they now provide a clue as to the mechanisms involved: EBNA-1 activates the transcription of the catalytic subunit of the NADPH oxidase, NOX2/gp91Phox, thereby increasing the production of reactive oxygen species (ROS) (Fig. 1). They could link the EBNA-1–induced increase in ROS to the development of genomic instability and 2 chemical inhibitors of NADPH oxidase, and shRNA-mediated silencing of NOX2 reduced ROS levels seen in EBNA-1-expressing cells. These findings strongly suggest that EBNA-1 promotes genomic instability as a consequence of raising intracellular ROS and may thus contribute to the multistep process of tumor development (Fig. 1). Because EBNA-1 is expressed in all EBV-associated tumors, it could represent a common mechanism in EBV-induced oncogenesis.
Fig. 1.
Suggested mechanisms for a role of EBNA-1 in the development of EBV-associated tumors.
What other evidence is there to support an oncogenic role for EBNA-1 and is it possible to estimate how “strong” an oncogene EBNA-1 is likely to be? There is only little evidence from classical transformation assays, although Kube et al. (14) observed the development of lymphoma in approximately one-third of NOD/SCID mice injected with an EBNA-1-transfected Hodgkin's disease cell line (L428). More than 10 years ago, Wilson et al. (15) reported the development of B cell lymphoma in 2 of 4 lines of transgenic mice, in which a polyomavirus early promoter and Ig heavy chain intronic enhancer had been used to direct EBNA-1 expression in B cells (expression of the transgene was also noted in other organs). The lymphoma phenotype was seen in backcrosses into several mouse strains (C57BL/6, DBA/2J, and BALB/cAnN) (15) and expression of EBNA-1 mRNA (but not protein) levels correlated with the speed of lymphoma development in the 2 lymphoma-prone transgenic lines (15). In contrast, Kang et al. (16) did not observe any lymphoma in 3 transgenic FVB mouse lineages, which expressed EBNA-1 in B cells at a higher level than found in most EBV-transformed human B cell lines (LCLs). Together, these reports suggest that transgenic expression of EBNA-1 in murine B cells appears to lead to B cell lymphoma only inconsistently. Although an effect of the polyomavirus promoter used by Wilson et al. (15) cannot be ruled out (16), it is tempting to speculate, in the light of the findings by Gruhne et al. (4), that increased ROS production caused by EBNA-1 and the ensuing genomic instability may have contributed to the emergence of lymphoma in some of the transgenic lineages reported by Wilson et al. (15).
If EBNA-1 does have oncogenic properties as a result of inducing genomic instability, as observed by Gruhne et al. (4) and Kamranvar et al. (13), is the production of ROS the only mechanism? Earlier studies by Srinivas and Sixbey (17) and Kuhn-Hallek et al. (18) had shown that EBV, and EBNA-1 on its own, can induce the expression of the V(D)J recombinases RAG-1 and RAG-2, suggesting that EBNA-1 might facilitate genomic recombination events and could thereby contribute to the c-myc/Ig locus translocation that is crucial for the development of BL (see Fig. 1). Increased expression of RAG-1 and RAG-2 was also noted in preneoplastic samples of EBNA-1 transgenic mice (19).
These findings strongly suggest that EBNA-1 promotes genomic instability.
What about the role of EBNA-1 in B lymphocyte immortalization? A few years ago Humme et al. (6) showed that deletion of EBNA-1 from a recombinant EBV genome did not compromise immortalization of B lymphocytes per se and lymphocytes immortalized by an EBV lacking EBNA-1 still grew in SCID mice (6). However, the efficiency of immortalization was markedly reduced, most likely because of the essential role of EBNA-1 in establishing a stable episomal persistence of EBV genomes in the infected B cells: the (much fewer) immortalized B cell lines obtained with the EBNA-1–deleted EBV all had the EBV genome integrated into host DNA (6). In stark contrast to this phenotype, deletion of EBNA-2, EBNA-LP, or EBNA-3C destroys the ability of EBV to transform B cells, and deletion of LMP1 allows the emergence of immortalized B cells only at reduced frequency and in the presence of irradiated feeder cells (6, 7, 20). As far as can be detected by such genetic approaches, the contribution of EBNA-1 to B cell immortalization appears therefore to be limited, at least in comparison with that of some other latent EBV proteins.
Still, the observations reported by Gruhne et al. (4) add additional weight to the suggestion that EBNA-1 may contribute to EBV-associated oncogenesis, in particular in those tumors like endemic BL, where it is the only EBV protein to be expressed consistently, or in nasopharyngeal carcinoma, where good epidemiological evidence exists for a contributory role of environmental carcinogens. The latter could conceivably compound the genomic damage caused by the increased ROS levels that are caused by EBNA-1, as elegantly shown by Gruhne et al. (4).
Acknowledgments.
S.C. is supported by Deutsche Forschungsgemeinschaft IRTG 1273.
Footnotes
The authors declare no conflict of interest.
See companion article on page 2313.
References
- 1.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;15:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 2.Klein E, Kis LL, Klein G. Epstein–Barr virus infection in humans: From harmless to life endangering virus–lymphocyte interactions. Oncogene. 2007;26:1297–1305. doi: 10.1038/sj.onc.1210240. [DOI] [PubMed] [Google Scholar]
- 3.Young LS, Rickinson AB. Epstein–Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 4.Gruhne B, et al. The Epstein–Barr virus nuclear antigen-1 promotes genetic instability via induction of reactive oxygen species. Proc Natl Acad Sci USA. 2009;106:2313–2318. doi: 10.1073/pnas.0810619106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pope JH, Horne MK, Scott W. Transformation of foetal human leukocytes in vitro by filtrates of a human leukaemic cell line containing herpes-like virus. Int J Cancer. 1968;3:857–866. doi: 10.1002/ijc.2910030619. [DOI] [PubMed] [Google Scholar]
- 6.Humme S, et al. The EBV nuclear antigen 1 (EBNA1) enhances B cell immortalization several thousandfold. Proc Natl Acad Sci USA. 2003;100:10989–10994. doi: 10.1073/pnas.1832776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dirmeier U, et al. Latent membrane protein 1 is critical for efficient growth transformation of human B cells by Epstein–Barr virus. Cancer Res. 2003;63:2982–2989. [PubMed] [Google Scholar]
- 8.Burrows SR, Sculley TB, Misko IS, Schmidt C, Moss DJ. An Epstein–Barr virus-specific cytotoxic T cell epitope in EBV nuclear antigen 3 (EBNA 3) J Exp Med. 1990;171:345–349. doi: 10.1084/jem.171.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Niens M, et al. HLA-A*02 is associated with a reduced risk and HLA-A*01 with an increased risk of developing EBV+ Hodgkin lymphoma. Blood. 2007;110:3310–3315. doi: 10.1182/blood-2007-05-086934. [DOI] [PubMed] [Google Scholar]
- 10.McAulay KA, et al. HLA class I polymorphisms are associated with development of infectious mononucleosis upon primary EBV infection. J Clin Invest. 2007;117:3042–3048. doi: 10.1172/JCI32377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Polack A, et al. C-myc activation renders proliferation of Epstein–Barr virus (EBV)-transformed cells independent of EBV nuclear antigen 2 and latent membrane protein 1. Proc Natl Acad Sci USA. 1996;93:10411–10416. doi: 10.1073/pnas.93.19.10411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly GL, Milner AE, Baldwin GS, Bell AI, Rickinson AB. Three restricted forms of Epstein–Barr virus latency counteracting apoptosis in c-myc-expressing Burkitt lymphoma cells. Proc Natl Acad Sci USA. 2006;103:14935–14940. doi: 10.1073/pnas.0509988103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kamranvar SA, Gruhne B, Szeles A, Masucci MG. Epstein–Barr virus promotes genomic instability in Burkitt's lymphoma. Oncogene. 2007;26:5115–5123. doi: 10.1038/sj.onc.1210324. [DOI] [PubMed] [Google Scholar]
- 14.Kube D, et al. Expression of Epstein–Barr virus nuclear antigen 1 is associated with enhanced expression of CD25 in the Hodgkin cell line L428. J Virol. 1999;73:1630–1636. doi: 10.1128/jvi.73.2.1630-1636.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson JB, Bell JL, Levine AJ. Expression of Epstein–Barr virus nuclear antigen-1 induces B cell neoplasia in transgenic mice. EMBO J. 1996;15:3117–3126. [PMC free article] [PubMed] [Google Scholar]
- 16.Kang MS, et al. Epstein–Barr virus nuclear antigen 1 does not induce lymphoma in transgenic FVB mice. Proc Natl Acad Sci USA. 2005;102:820–825. doi: 10.1073/pnas.0408774102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srinivas SK, Sixbey JW. Epstein–Barr virus induction of recombinase-activating genes RAG1 and RAG2. J Virol. 1995;69:8155–8158. doi: 10.1128/jvi.69.12.8155-8158.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuhn-Hallek I, Sage DR, Stein L, Groelle H, Fingeroth JD. Expression of recombination activating genes (RAG-1 and RAG-2) in Epstein–Barr virus-bearing B cells. Blood. 1995;85:1289–1299. [PubMed] [Google Scholar]
- 19.Tsimbouri P, Drotar ME, Coy JL, Wilson JB. bcl-xL and RAG genes are induced and the response to IL-2 enhanced in EmuEBNA-1 transgenic mouse lymphocytes. Oncogene. 2002;21:5182–5187. doi: 10.1038/sj.onc.1205490. [DOI] [PubMed] [Google Scholar]
- 20.Hammerschmidt W, Sugden B. Genetic analysis of immortalizing functions of Epstein–Barr virus in human B lymphocytes. Nature. 1989;340:393–397. doi: 10.1038/340393a0. [DOI] [PubMed] [Google Scholar]