Since the development of albuterol >4 decades ago (1), agonists selective for the β2-adrenergic receptor (β2AR) have been the drug of choice for relief of life-threatening bronchospasm experienced by asthmatics. Both short- and long-acting β2AR agonists (SABAs and LABAs, respectively) have also been used extensively for the prophylactic management of asthma symptoms. The therapeutic efficacy of β2AR agonists relates to their ability to directly relax airway smooth muscle to cause airways to dilate and conduct greater airflow. Yet increasing concerns that chronic use of β2AR agonists actually increases mortality in asthmatics have culminated in a recent (and controversial) recommendation by an FDA advisory panel that the risks of two widely-prescribed LABAs outweigh their benefits. Why such risks may exist has puzzled both researchers and clinicians. Some have pointed to tolerance to β-agonists caused by desensitization of airway β2ARs (2). Others have asserted that β-agonists have no direct toxic effects yet their ability to provide symptomatic relief masks an increasing level of airway inflammation (3). In this issue of PNAS, Nguyen et al. (4) shed new light onto the role of β2AR agonism in asthma and suggest the paradoxical notion that blocking β2ARs may be a more effective strategy for managing asthma (see Fig. 1).
Fig. 1.
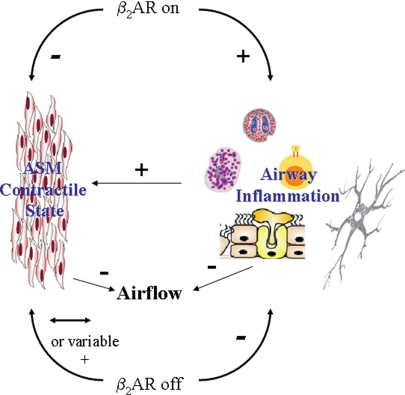
β2AR agonism in the asthmatic airway, a potential model suggested by findings of Nguyen et al. (4). β2AR activation (“β2AR on”), resulting from constitutive β2AR activity, endogenous catecholamines, or inhaled β-agonists, has a permissive effect on airway inflammation that generates contractile agents causing airway smooth muscle (ASM) contraction and promotes airway mucus secretion that increases airway resistance. Direct effects of β-agonists on ASM antagonize effects of contractile agents to prevent/reverse bronchoconstriction. Airflow conductance is affected by the competitive actions of procontractile and prorelaxant effects on ASM, and by impedance caused by airway mucus. Alternatively, β2AR inverse agonists (“β2AR off”) inhibit all β2AR activity, resulting in inhibition of allergic inflammation and null or modest inhibition the bronchoprotective/relaxing effect of β2AR activity in ASM. ASM contractile state and airflow are effectively managed by the anti-inflammatory effects that reduce levels of bronchoactive agents and mucus.
An analogous paradigm shift in the management of congestive heart failure (CHF) was proposed in 1975 when Waagstein et al. (5) demonstrated that chronic administration of β-blockers to CHF patients improved their clinical condition and reversed maladaptive heart remodeling. It had been previously presumed that the pump function of the weak, failing heart could be treated by stimulation with synthetic β-agonists such as dobutamine. Since then we have learned that although β1-Adrenergic receptor (β1AR) agonism can acutely increase cardiac contractility, it is also central to the pathogenesis of heart failure. By blocking β1ARs with β-blockers the heart is spared from the excessive work and various biochemical mechanisms that cause a large, hypodynamic heart (6).
Similar to studies demonstrating the role of β1AR agonism in CHF, Nguyen et al. (4) suggest that β2AR agonism is pathogenic in asthma. Experiments used both genetic and pharmacological strategies to examine the effect of blocking β2ARs on the development of antigen-induced, asthma-like properties in the mouse. After a period of sensitization and challenge with antigen, mice lacking the gene for the β2AR developed significantly lesser airway hyperresponsiveness (AHR) and airway inflammation than did control mice. Chronic administration to control mice of nadolol, which has properties of a β2AR “inverse” agonist, produced results qualitatively similar to those observed with β2AR gene knockout. Inverse agonists are a class of antagonists that not only can block the ability of agonists to bind and activate a receptor but also can lock a receptor in a “closed” conformation and thereby block any (unliganded) constitutive or spontaneous activity of the receptor. This mechanism is in contrast to that of neutral antagonists, which can only block access of agonists to the receptor (7). Interestingly, chronic administration of the β2AR antagonist alprenolol failed to affect either AHR or airway inflammation induced by antigen in control mice. These findings suggest that inverse agonism of the β2AR is key to eliminating the permissive effect of β2ARs on antigen-induced asthma properties.
These novel data identify the β2AR as the key target mediating the effects of inverse β-agonists in this model, and extend previous studies by Bond and coworkers (8–10) suggesting a possible therapeutic role for β2AR inverse agonists in asthma. Asthma is a complex disease in which an exaggerated immune response to inhaled antigen results in airway inflammation that causes the smooth muscle surrounding the airways to contract excessively. For most asthmatics this bronchospasm can be rapidly reversed by inhalation of SABAs such as albuterol. Some LABAs can also provide rapid relief, although LABAs are used primarily as maintenance prophylactic drugs to prevent the occurrence of bronchospasm. Although SABAs/LABAs are effective in managing this critical asthma symptom, their ability to address the primary cause of bronchospasm—airway inflammation—is unclear. Some studies have suggested that β2AR agonists have anti-inflammatory properties with respect to inflammatory cell functions, whereas others have ascribed pro-inflammatory effects of β2AR agonists on inflammatory cells or airway inflammation (reviewed in ref. 11). An underlying rationale for the use of combined inhaled LABA and corticosteroid therapy in asthma is that corticosteroids are highly effective in controlling airway inflammation.
Interestingly, the findings of Nguyen et al. (4) suggest that minimal β2AR agonism is sufficient to enable significant antigen-induced airway inflammation and AHR. Alprenolol, which lacks inverse agonist properties but can function as a β2AR antagonist, was ineffective in attenuating either indices of airway inflammation or AHR. Although interpretation of these results is confounded by the potential of alprenolol to function as a partial agonist, it appears safe to conclude that β2AR agonism caused by either constitutive activity of the β2AR or a very low level of agonist-induced receptor activation permits the inflammatory effects of antigen in this model. Numerous basic science and clinical questions are prompted by this pro-inflammatory effect of β2AR agonism and the use of β2AR inverse agonists in asthmatics.
(i) Through which cells does β2AR agonism promote airway inflammation? Induction of airway inflammation in asthma is a complex process involving the participation of numerous cell types. Some inflammatory cells, such as type 2 T cells, exhibit β2AR-agonist-promoted pro-inflammatory properties when examined in vitro (11). However, such properties cannot be readily extrapolated to the in vivo condition, and experimental approaches for testing in vivo relevance, especially in humans, are technically challenging.
(ii) What signals, leading to what cell functions, do β2ARs transduce to promote airway inflammation? Classical β2AR signaling involves events leading to the activation of the cAMP-dependent protein kinase. However, there is a growing appreciation that β2ARs signal through alternative pathways, including those dependent on other cAMP effectors, Gi G proteins, or arrestins (7). Should specific β2AR signaling events be associated with pro-inflammatory effects of β-agonists, the possibility exists of targeting these downstream events for inhibition, or alternatively using specific β2AR ligands that induce qualitatively different signaling (12).
(iii) Can β2AR inverse agonists be safely administered to asthmatics? As was the case when β-blockers were originally proposed for the treatment of CHF, major concerns exist regarding the safety of β2AR inverse agonists as a treatment for asthma. β-Blockers are generally contraindicated in asthma because of early studies demonstrating that β-blockers caused bronchospasm in asthmatics (13). Similarly, β-blockers had been contraindicated in CHF because short-term treatment with β-blockers resulted in a worsening of CHF symptoms. Whether treatment of asthmatics with β2AR inverse agonists results in a brief period of adverse effects followed by improved asthma control remains to be determined. A recent pilot study examining nadolol effects in 10 mild asthmatics reported 4 subjects as experiencing a moderate drop in expired airflow after their first dose of nadolol (9). However, after 9 weeks of treatment, mean values for expired airflow were greater than those measured at baseline, and a significant reduction in the sensitivity to the bronchoconstricting agent methacholine was observed.
Major concerns exist regarding the safety of β2AR inverse agonists as a treatment for asthma.
Additional safety questions involve the asthmatic population(s) suited for treatment with β2AR inverse agonists, and which rescue medications are most appropriate for asthmatics on β2AR inverse agonists. β1AR–selective β-blockers are relatively safe in patients with mild asthma (14), and based on the pilot study results described above safety concerns may be limited in mild asthmatics taking β2AR inverse agonists. However, severe asthmatics appear more susceptible to bronchospasm induced by β-blockers (13). Given the increased likelihood of an adverse event during initial treatment with β2AR inverse agonists, rescue medications will assume greater importance. Anticholinergics are one rescue medication option because their efficacy in reversing bronchospasm induced by β-blockers has been demonstrated (15). Another interesting possibility is high-affinity β2AR agonists, which could effectively compete with β2AR inverse agonists for occupancy of β2ARs on airway smooth muscle to promote bronchorelaxation. Moreover, the signaling capacity of agonist-activated β2ARs is predicted to be increased as a result of chronic treatment with β2AR inverse agonists, which causes an up-regulation of β2AR density in the lung (16).
Ultimately, numerous clinical issues will need to be resolved in human studies to establish β2AR inverse agonists as viable asthma drugs. Equally challenging are the basic science questions regarding the complexity of β2AR signaling and function in the lung.
Acknowledgments.
Work on G protein-coupled receptor function in my laboratory is currently supported by National Institutes of Health Grants HL58506 and AI59755.
Footnotes
The author declares no conflict of interest.
See companion article on page 2435.
References
- 1.Brittain RT, Farmer JB, Jack D, Martin LE, Simpson WT. Alpha-[(t-butylamino)methyl]-4-hydroxy-m-xylene-alpha1,alpha3-diol (AH.3365): A selective beta-adrenergic stimulant. Nature. 1968;219:862–863. doi: 10.1038/219862a0. [DOI] [PubMed] [Google Scholar]
- 2.Sears MR. Adverse effects of beta-agonists. J Allergy Clin Immunol. 2002;110:S322–S328. doi: 10.1067/mai.2002.129966. [DOI] [PubMed] [Google Scholar]
- 3.Nelson HS. Is there a problem with inhaled long-acting beta-adrenergic agonists? J Allergy Clin Immunol. 2006;117:3–16. doi: 10.1016/j.jaci.2005.10.013. [DOI] [PubMed] [Google Scholar]
- 4.Nguyen LP, et al. β2-Adrenoceptor signaling is required for the development of an asthma phenotype in a murine model. Proc Natl Acad Sci USA. 2009;106:2435–2440. doi: 10.1073/pnas.0810902106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Waagstein F, Hjalmarson A, Varnauskas E, Wallentin I. Effect of chronic beta-adrenergic receptor blockade in congestive cardiomyopathy. Br Heart J. 1975;37:1022–1036. doi: 10.1136/hrt.37.10.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liggett SB. β-Adrenergic receptors in the failing heart: the good, the bad, and the unknown. J Clin Invest. 2001;107:947–948. doi: 10.1172/JCI12774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Penn RB. Embracing emerging paradigms of G protein-coupled receptor agonism and signaling to address airway smooth muscle pathobiology in asthma. Naunyn Schmiedebergs Arch Pharmacol. 2008;378:149–169. doi: 10.1007/s00210-008-0263-1. [DOI] [PubMed] [Google Scholar]
- 8.Callaerts-Vegh Z, et al. Effects of acute and chronic administration of β-adrenoceptor ligands on airway function in a murine model of asthma. Proc Natl Acad Sci USA. 2004;101:4948–4953. doi: 10.1073/pnas.0400452101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanania NA, et al. The safety and effects of the beta-blocker, nadolol, in mild asthma: An open-label pilot study. Pulm Pharmacol Ther. 2007;21:134–141. doi: 10.1016/j.pupt.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nguyen LP, et al. Chronic exposure to beta-blockers attenuates inflammation and mucin content in a murine asthma model. Am J Respir Cell Mol Biol. 2008;38:256–262. doi: 10.1165/rcmb.2007-0279RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Loza MJ, Peters SP, Foster S, Khan IU, Penn RB. β-Agonist enhances type 2 T-cell survival and accumulation. J Allergy Clin Immunol. 2007;119:235–244. doi: 10.1016/j.jaci.2006.09.019. [DOI] [PubMed] [Google Scholar]
- 12.Wisler JW, et al. A unique mechanism of β-blocker action: Carvedilol stimulates β-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zaid G, Beall GN. Bronchial response to beta-adrenergic blockade. N Engl J Med. 1966;275:580–584. doi: 10.1056/NEJM196609152751103. [DOI] [PubMed] [Google Scholar]
- 14.Salpeter S, Ormiston T, Salpeter E. Cardioselective beta-blockers for reversible airway disease. Cochrane Database Syst Rev. 2002 doi: 10.1002/14651858.CD002992. CD002992. [DOI] [PubMed] [Google Scholar]
- 15.Ind PW, Dixon CM, Fuller RW, Barnes PJ. Anticholinergic blockade of beta-blocker-induced bronchoconstriction. Am Rev Respir Dis. 1989;139:1390–1394. doi: 10.1164/ajrccm/139.6.1390. [DOI] [PubMed] [Google Scholar]
- 16.Lin R, et al. Changes in beta2-adrenoceptor and other signaling proteins produced by chronic administration of ‘beta-blockers’ in a murine asthma model. Pulm Pharmacol Ther. 2007;21:115–124. doi: 10.1016/j.pupt.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]